Abstract
Objectives:
To compare the effects of adding electrical dry needling into a manual therapy (MT) and exercise program on pain, stiffness, function, and disability in individuals with painful knee osteoarthritis (OA).
Materials and Methods:
In total, 242 participants (n=242) with painful knee OA were randomized to receive 6 weeks of electrical dry needling, MT, and exercise (n=121) or MT and exercise (n=121). The primary outcome was related-disability as assessed by the Western Ontario and McMaster Universities (WOMAC) Osteoarthritis Index at 3 months.
Results:
Individuals receiving the combination of electrical dry needling, MT, and exercise experienced significantly greater improvements in related-disability (WOMAC: F=35.504; P<0.001) than those receiving MT and exercise alone at 6 weeks and 3 months. Patients receiving electrical dry needling were 1.7 times more likely to have completely stopped taking medication for their pain at 3 months than individuals receiving MT and exercise (OR, 1.6; 95% confidence interval, 1.24-2.01; P=0.001). On the basis of the cutoff score of ≥5 on the global rating of change, significantly (χ2=14.887; P<0.001) more patients (n=91, 75%) within the dry needling group achieved a successful outcome compared with the MT and exercise group (n=22, 18%) at 3 months. Effect sizes were large (standardized mean differences >0.82) for all outcome measures in favor of the electrical dry needling group at 3 months.
Discussion:
The inclusion of electrical dry needling into a MT and exercise program was more effective for improving pain, function, and related-disability than the application of MT and exercise alone in individuals with painful knee OA.
Level of Evidence:
Level 1b—therapy. Prospectively registered February 10, 2015 on www.clinicaltrials.gov (NCT02373631).
Key Words: knee osteoarthritis, dry needling, manual therapy, exercise, clinical trial
Osteoarthritis (OA) of the knee affects up to 37% of adults in the United States between 45 and 60 years of age.1 A recent meta-analysis found that the crude prevalence of knee OA was 25% in patients aged above 20 years and 39% in people aged above 30 years.2 In addition, hip and knee OA are ranked as the 11th highest contributors to global disability in patients with chronic pain.3 Physiological changes in OA are characterized by degeneration of articular cartilage with osteophyte formation, microfractures, subchondral sclerosis and plate thickening, and exposure of the articular end of the bone.4–6 The clinical manifestations of knee OA are joint pain, stiffness in the morning or after rest, limited joint motion, night pain, and/or joint deformity. The clinical diagnosis of knee OA is typically made using the American College of Rheumatology clinical criteria developed by Altman, which has been found to be 89% sensitive and 88% specific.7,8 The pathogenesis and temporal relationship of anatomic lesions is largely unknown, and there are currently no curative treatments for OA. Long-term use of oral nonsteroidal anti-inflammatory drugs has been discouraged, and many patients with chronic pain seek for nonpharmacological management options.9,10 Exercise11–16 and acupuncture17–23 are 2 nonpharmacological interventions recommended for individuals with knee OA in recent meta-analyses and international clinical guidelines.18,24
A Cochrane review found a statistically significant benefit, with moderate effect sizes for pain (standardized mean differences [SMD], 0.49) and physical function (SMD, 0.52) immediately after treatment, and small effect sizes (pain, SMD, 0.24; physical function, SMD, 0.15) at 2 to 6 months follow-up for various forms of exercise in individuals with moderate knee OA.11 Another recent systematic review reported that exercise plus manual therapy (MT) for joint mobilization showed a moderate effect size (SMD, 0.69) that was significantly higher than the effect sizes observed for exercise alone (SMD, 0.34).12
Pain may be a potential barrier leading to underdosage of strength training and aerobic exercise stimulus in individuals with painful knee OA; therefore, needling therapies may be a reasonable nonpharmacologic adjunct intervention for the reduction of chronic pain in individuals participating in exercise programs for knee OA.17,18,20,21 Needling therapy refers to the insertion of thin monofilament needles, as used in the practice of acupuncture, without the use of injectate.25–29 Dry needling is typically used to stimulate muscles, ligaments, tendons, subcutaneous fascia, scar tissue, or peripheral nerves for the management of pain and disability associated with neuromusculoskeletal disorders.25,28–30 Interestingly, the most common term used to describe dry needling is “acupuncture,” that is, “acu” literally translates to needle and “puncture” to penetration.29
The terminology, theoretical constructs, and philosophies may differ; however, dry needling and acupuncture overlap in terms of needling technique with the use of thin monofilament needles.31 Notably, several previous meta-analyses and literature reviews have chosen to consider “acupuncture and dry needling” as one category of interventions.32–36 Therefore, from a procedural and technical perspective, and for the purpose of evaluating and comparing efficacy and effect sizes within the broader literature on the use of needling without injectate in patients with knee OA published by acupuncturists, western medical physicians, and physical therapists alike, “electroacupuncture” and “electrical dry needling” will be considered interchangeable terms, and in this context do not rely on diagnoses from oriental medicine (eg, bi syndrome, blood stagnation, or kidney yang deficiency37,38) or theoretical movement of qi along traditional Chinese acupuncture meridians.39,40 Importantly, none of the knee OA studies cited herein used injectate in conjunction with their needling procedure; therefore, all studies fit within the strict definition of dry needling, acupuncture, or “noninjection needling” (as opposed to “injection needling” or “wet needling”), regardless of the differing terminologies, theoretical constructs, or philosophies.25,29,31
The current body of evidence seems to support the use of dry needling therapies without injectate, that is, acupuncture for treating the pain, stiffness, and related-disability associated with knee OA.17,21,23,29,41–43 Zhang et al44 cited a 69% consensus following a Delphi study recommending the use of acupuncture for the symptomatic treatment of OA and reported a moderate effect size for this needling modality (ie, acupuncture). The OARSI guidelines20 for hip and knee OA reported acupuncture to have a moderate effect size for pain (0.51), stiffness (0.41), and function (0.51). In addition, based on the individual effect sizes of 11 trials reported by Manheimer et al,45 Zhang et al44 concluded that acupuncture was superior to usual care and wait list controls with a pooled effect size of 0.58 for pain relief. Although it is not always appropriate to compare effect sizes among various treatments,20 to our knowledge, a pooled standard effect size for pain relief of 0.58 for acupuncture in patients with knee OA is higher than most other conservative treatments applied to this pain population, including nonsteroidal anti-inflammatory drugs (0.32), muscle strengthening exercises (0.32), and aerobic exercises (0.52).20,44
Electrical dry needling and the combination of MT and exercise, when applied separately, have been found to be moderately effective for knee OA. Although 3 previous studies46–48 investigated the combined effects of acupuncture and exercise in patients with knee OA, they used manual acupuncture rather than electroacupuncture. No previous study has investigated the combination of the effectiveness of electrical dry needling in addition to MT and exercise in patients with knee OA. Therefore, the purpose of this multicenter randomized clinical trial was to compare the effects of adding electrical dry needling, into a MT and exercise program on pain, stiffness, function, and disability in individuals with painful knee OA. We hypothesized that individuals receiving electrical dry needling combined with MT and exercise would exhibit greater improvements in pain, stiffness, function, and disability than those receiving only MT and exercise.
MATERIALS AND METHODS
Study Design
This randomized, single-blinded, multicenter, parallel-group trial compared 2 treatment protocols for the management of knee OA: MT and exercise versus MT and exercise plus electrical dry needling. The primary outcome was related-disability as assessed by the Western Ontario and McMaster Universities (WOMAC total score) Osteoarthritis Index at 3 months. Secondary outcomes included knee pain intensity as measured by the Numeric Pain Rating Scale (NPRS), all WOMAC subscales (pain: WOMAC-P; stiffness: WOMAC-S; physical function: WOMAC-PF), medication intake, and the global rating of change (GROC). The current clinical trial was conducted following the Consolidated Standards of Reporting Trials (CONSORT) extension for pragmatic clinical trials.49 The study was approved by the ethics committee at Universidad Rey Juan Carlos, Madrid, Spain (URJC-DPTO 31-2014) and the trial was prospectively registered (ClinicalTrials.gov: NCT02373631).
Participants
Consecutive individuals with painful knee OA from 18 outpatient physical therapy clinics in 10 different states (Arizona, Florida, Georgia, Illinois, New Hampshire, New York, North Carolina, Rhode Island, South Carolina, Virginia) were screened for eligibility criteria and recruited over a 24-month period (from February 2015 to 2017).
For patients to be eligible, they had to have met the American College of Rheumatology criteria for the diagnosis of knee OA7,8 and have had chronic pain in the knee joint for >3 months. Patients had to have at least 3 of the following criteria7,8,49 to be included in the study: (1) above 50 years of age; (2) <30 minutes of morning stiffness; (3) crepitus on active motion; (4) bony tenderness; (5) bony enlargement; and (6), no palpable warmth of synovium.7 In addition, participants had to have a minimum knee pain intensity score of 2 points and be older than 18 years of age.
Patients were excluded if they exhibited: (1) a history of surgery to the painful knee; (2) a history of surgery to either of the lower extremities in the last 6 months; (3) any red flags to MT, dry needling, or exercise; (4) had received physical therapy, acupuncture, massage therapy, chiropractic, or intra-articular injections for the painful knee in the last 3 months; (5) presented with ≥2 positive neurological signs; or (6) had involvement in litigation or worker’s compensation regarding their knee pain. Patients were also excluded if they were pregnant. All participants signed an informed consent before their participation in the study. All participants were naïve to the use of dry needling procedures and had not previously experienced needling without injectate for their knee pain.
Treating Therapists
In total, 18 physical therapists (mean age, 38.4 y; SD, 10.44) participated in the delivery of treatment for patients in this study. They had an average of 12.5 (SD, 9.54) years of clinical experience, an average of 4.3 (SD, 1.88) years using dry needling, and all had completed a 54-hour postgraduate certification program that included practical training in electrical dry needling for knee OA. All participating physical therapists were required to study a manual of standard operating procedures and participate in a 6-hour training session with the principal investigator.
Randomization and Blinding
Following the baseline examination, patients were randomly assigned to receive MT and exercise alone or in combination with electrical dry needling. Concealed allocation was conducted using a computer-generated randomized table of numbers created by a statistician who was not otherwise involved in the trial and did not participate in analysis or interpretation of the results. Individual and sequentially numbered index cards with the random assignment were prepared for each of the 18 data collection sites. The index cards were folded and placed in sealed opaque envelopes. Blinded to the baseline examination, the treating therapist opened the envelope and proceeded with treatment according to the group assignment. The examining therapist remained blind to the patient’s treatment group assignment at all times; however, based on the nature of the interventions it was not possible to blind patients or treating therapists.
Interventions
All participants received between 8 and 10 treatment sessions at a frequency of 1 to 2 times per week over a 6-week period. Both groups received MT (passive joint mobilizations and muscle stretching) and exercise (riding a stationary bicycle, range of motion, and strengthening exercises to the lower extremity) on each session. In addition, the dry needling group also received electrical dry needling using a standardized 9-point protocol for 20 to 30 minutes on each treatment session.
Although specific recommendations cannot be made regarding the type of exercise12 or the optimal exercise dosage in patients with knee OA,11 patients received the following interventions at all treatment sessions: 30 minutes of lower extremity strengthening (weight bearing, non–weight-bearing, concentric, eccentric), range of motion (riding a stationary bicycle), stretching exercises (static muscle stretching), and passive accessory and physiological joint mobilizations.50 The exercise program was taught to the patient by an experienced physical therapist on the first session and supervised on subsequent sessions. Strengthening, range of motion, and stretching exercises were gradually progressed according to tolerance of each individual patient. That is, progression only occurred if patients reported a decrease in symptoms and in the absence of excessive soreness. Details regarding the exercise and MT program have previously been described by Deyle et al.50
All patients in both groups were asked to complete a daily home exercise program.50 The home exercise program consisted of the same strengthening, range of motion, and stretching exercises that were prescribed and supervised in the clinic.50 Patients were asked to complete the home exercise program during all days that they did not receive supervised physical therapy in the clinic. Patients were asked to monitor their compliance with the home exercise program by maintaining a home exercise program logbook.
In addition to MT and exercise, patients allocated to the dry needling group also received 8 to 10 sessions of periosteal electrical dry needling at a frequency of 1 to 2 times per week over 6 weeks. Electric dry needling included a 9-point standardized protocol as depicted in Figure 1. Each needle insertion site and anatomic target is summarized within Appendix 1. In addition to the obligatory 9-point standardized protocol, clinicians were also permitted to insert needles at up to 4 additional locations based on the presence of the symptoms.
FIGURE 1.
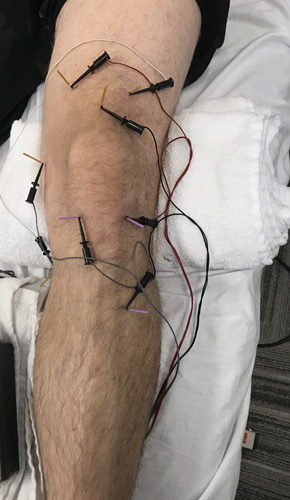
Standardized 9-point protocol of periosteal electrical dry needling for knee osteoarthritis.
Sterilized disposable stainless steel acupuncture needles were used with 3 sizes: 0.25 mm×30 mm, 0.30 mm×40 mm, and 0.30 mm×50 mm. The depth of needle insertion ranged from 15 to 45 mm and depended on the point selected (intramuscular, periosteal, joint line, intra/periarticular) and the patient’s physical constitution. Following topical skin cleansing with sterile alcohol prep pads, all needles were inserted and then manipulated bidirectionally to illicit a sensation of aching, tingling, deep pressure, heaviness, or warmth.51,52 In addition, at least 3 of the 9 obligatory needles (ie, over the posteromedial aspect of the medial tibial condyle, within the depression posterior to the femoral epicondyle, and over the anterolateral crest of the tibia 1 fingerbreadth lateral to the tibial tuberosity) were repeatedly thrusted and tapped on to the respective bone using a “periosteal stimulation” technique.53 Notably, with the exception of the 2 obligatory needles inserted at the level of the tibiofemoral joint margin within the medial or lateral infrapatellar sulcus, and depending on the patient’s physical constitution, the needle length selected by the practitioner and the patient’s tolerance to such, the remaining obligatory needles were also advanced toward the underlying bone to facilitate direct mechanical and electrical “periosteal stimulation.”53 The needles were then left in situ for 20 to 30 minutes41–43,54–56 with electric stimulation (ES-160 electrostimulator ITO co.) in pairs (crossing through the knee joint in a superior-inferior and diagonal orientation) using 4 channels to 8 of the needles using a low frequency (2 Hz), moderate pulse duration (250 μs), biphasic continuous waveform at a maximum tolerable intensity.55,56 In cases of bilateral knee OA, both knees were treated, but only the most painful side at baseline was recorded and analyzed throughout the study to satisfy the assumption of independent data.57
Outcome Measures
Participants received a standardized physical examination during which the affected knees were examined for conditions other than OA; that is, referred pain from the hip joint or lumbopelvic region were ruled out. The physical examination included, but was not limited to, measurements of passive and active knee range of motion.
The primary outcome was related-disability as assessed with the WOMAC total index score, whereas each WOMAC subscale (WOMAC-P, WOMAC-S, and WOMAC-PF) were considered as secondary outcomes. The WOMAC is a valid and reliable instrument and has been used extensively to evaluate 3 dimensions (pain, stiffness, and physical function) in patients with hip or knee OA.58–60 In patients with OA of the lower extremities participating in rehabilitation programs, the minimum clinically important difference (MCID) for the WOMAC has been calculated to range from 9% to 12% of the baseline score.61–63 However, in our study, we used 36% change in the WOMAC (ie, triple the value of the 12% MCID) to represent a successful outcome.
Secondary outcomes included knee pain intensity, the 3 WOMAC subscales, medication intake and the GROC. A NPRS measured knee pain intensity. Patients were asked to indicate the average intensity of knee pain over the past week using an 11-point scale ranging from 0 (no pain) to 10 (worst pain imaginable) at baseline, 2 weeks, 6 weeks, and 3 months following the initial treatment session.64 The NPRS is a reliable and valid instrument to assess pain intensity.65–67 The MCID for the NPRS has been shown to be 1.74 in patients with chronic pain conditions67; however, the MCID for knee-related pain has not yet been established. Nevertheless, a change of 2 points or a 30% decrease in pain from baseline can be considered as a MCID in patients with chronic musculoskeletal pain.67,68
Medication intake was measured as the number of times the patient had taken prescription or over-the-counter analgesic or anti-inflammatory medication in the past week for their knee pain, with 5 options: (1) not at all, (2) once a week, (3) once every couple of days, (4) once or twice a day, or (5) ≥3 times a day. Medication intake was assessed at baseline and at 3 months after the first treatment session.
At 2 weeks, 6 weeks, and 3 months following the initial treatment session, patients completed a 15-point GROC question based on a scale described by Jaeschke et al69 to rate their self-perceived improved function. The MCID for the GROC has not been specifically reported but scores of +4 and +5 have typically been indicative of moderate changes in patient status.69
Treatment Side Effects
Patients were asked to report adverse events that they experienced during any part of the study. In the current study, an adverse event was defined as a sequelae of 1-week duration with any symptom perceived as distressing and unacceptable to the patient that required further treatment.70 Particular attention was given to the presence of ecchymosis and postneedling soreness within the group receiving electrical dry needling.
Sample Size Determination
The sample size calculations were based on detecting a between-groups moderate effect size of 0.4 at 3 months, assuming a 2-tailed test, an alpha level (α) of 0.05 and a desired power (β) of 90%. The estimated desired sample size was calculated to be at least 105 patients per group. A dropout percentage of 15% was expected, so 120 patients were included on each group.
Statistical Analysis
Statistical analysis was performed using SPSS software, version 24.0 (Chicago, IL) and it was conducted according to intention-to-treat analysis. We performed Little’s Missing Completely at Random (MCAR) test71 to determine whether missing data points associated with dropouts were missing at random or missing for systematic reasons. Intention-to-treat analysis was performed by using expectation-maximization whereby missing data were computed using regression equations.
The effects of treatment on pain, stiffness, physical function, and related-disability were each examined with a 2-by-4 mixed-model analysis of covariance (ANCOVA) with treatment group as the between-subjects factor, time as the within-subjects factor, and adjusted for baseline data. Separate ANCOVAs were performed with each outcome as the dependent variable. For each ANCOVA, the main hypothesis of interest was the 2-way interaction (group by time) with a Bonferroni-corrected α level of 0.0125 (4 timepoints). We used χ2 tests to compare self-perceived improvement with GROC and changes in medication intake. To enable comparison of between-group effect sizes, SMDs were calculated by dividing mean score differences between groups by the pooled SD. Numbers needed to treat (NNT) and 95% confidence intervals (CI) were also calculated at the 3-month follow-up period using each definition for a successful outcome.
RESULTS
Between February 2015 and 2017, 431 consecutive patients with knee pain were screened for possible eligibility criteria. In total, 242 (56.15%) satisfied all the inclusion criteria, agreed to participate, and were randomly allocated into the MT and exercise (n=121) or MT and exercise plus electrical dry needling (n=121) group. Randomization resulted in similar baseline characteristics for all variables (Table 1). The reasons for ineligibility are found in Figure 2, which provides a flow diagram of patient recruitment and retention. There was no significant difference (P=0.468) between the mean number of completed treatment sessions for the MT, exercise plus electrical dry needling group (mean, 8.7±1.8) and the MT and exercise group (mean, 8.9±1.9). In total, 235 of the 242 patients completed all outcome measures through 3 months (97% follow-up). Of the 7 patients that dropped out or failed to complete outcome measures, 3 were from the electrical dry needling group and 4 were from the MT and exercise group.
TABLE 1.
Baseline Characteristics by Treatment Assignment
FIGURE 2.
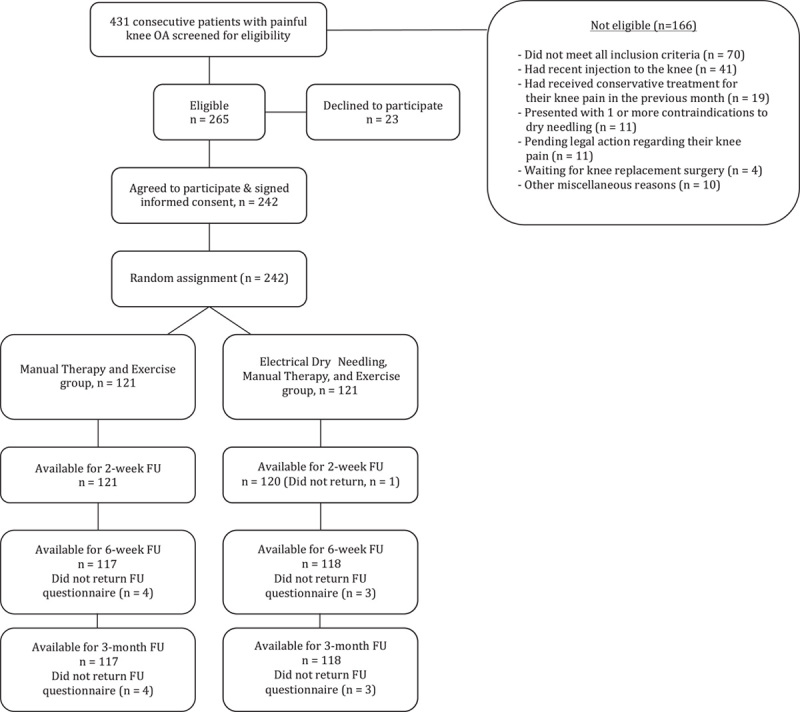
Flow diagram of patient recruitment and retention. FU indicates follow-up; OA, osteoarthritis.
In total, 87 patients assigned to the MT and exercise plus electrical dry needling group (71.9%) experienced postneedling muscle soreness and 57 (47.1%) experienced mild bruising (ecchymosis) that most commonly resolved spontaneously within 48 hours and 2 to 4 days, respectively. In addition, 6 patients (4.9%) in the electrical dry needling group experienced drowsiness, headache, or nausea, which spontaneously resolved within several hours. No other adverse events were reported.
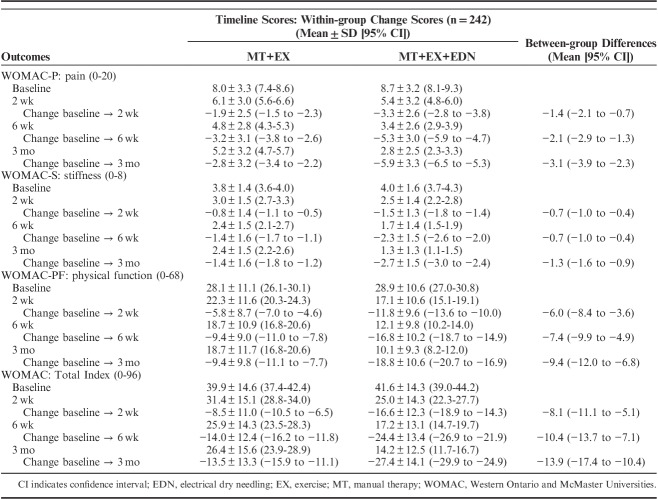
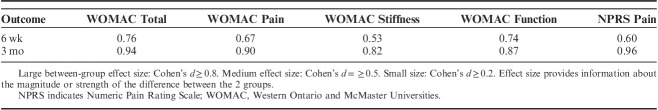
Adjusting for baseline outcomes, the mixed-model ANCOVA revealed a significant group×time interaction for the primary outcome (WOMAC: F=35.504; P<0.001): patients receiving electrical dry needling experienced significantly greater improvements in related-disability at 6 weeks (Δ, −10.4; 95% CI, −13.7 to −7.1; P<0.001) and 3 months (Δ, −13.9; 95% CI, −17.4 to −10.4; P<0.001) than those receiving MT and exercise alone (Fig. 3). Similarly, significant group×time interactions were also found for all WOMAC subscales (WOMAC-P: F=30.131, P<0.001; WOMAC-S: F=29.665, P<0.001; WOMAC-PF: F=30.114, P<0.001) in favor of the dry needling group (Table 2). For the WOMAC and all subscales, between-groups effect sizes were moderate (0.53<SMD<0.76) at 6 weeks and large (0.82<SMD<0.94) at 3 months after the first treatment session in favor of the dry needling group (Table 3). Within-group percentage change from baseline to 3 months for the primary outcome (WOMAC) was 67.0% and 32.9% for the electrical dry needling group and nondry needling group, respectively.
FIGURE 3.
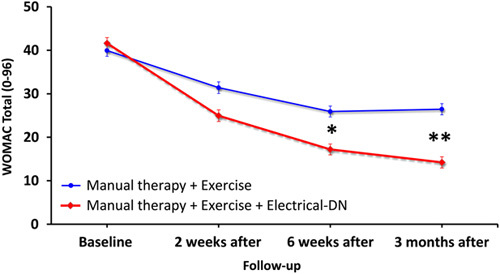
Evolution of the WOMAC Osteoarthritis Index throughout the course of the study stratified by randomized treatment assignment. Data are means (SE). DN indicates dry needling; WOMAC, Western Ontario and McMaster Universities.
TABLE 2.
WOMAC Osteoarthritis Index at Baseline, 2 Weeks, 6 Weeks, and 3 Months After the First Treatment Sessions as Well as Within-group and Between-groups Mean Scores by Randomized Treatment Assignment
TABLE 3.
Between-group Effect Sizes (Standardized Mean Difference) in Favor of the Dry Needling Group When Compared With the Combination Manual Therapy and Exercise
The intention-to-treat analysis also revealed a significant group×time interaction for knee pain (NPRS) intensity (F=29.094; P<0.001): individuals receiving electrical dry needling experienced significantly greater decrease in knee pain at 6 weeks (Δ, −1.2; 95% CI, −1.7 to −0.7; P<0.001) and 3 months (Δ, −2.7; 95% CI, −3.4 to −2.0; P<0.001) than those receiving MT and exercise alone (Fig. 4). For knee pain intensity (NPRS), between-groups effect sizes were moderate (SMD, 0.60) at 6 weeks and large (SMD, 0.96) at 3 months in favor of the dry needling group (Table 3). Within-group percentage change from baseline to 3 months for knee pain intensity (NPRS) was 67.2% and 28.9% for the electrical dry needling group and nondry needling group, respectively.
FIGURE 4.
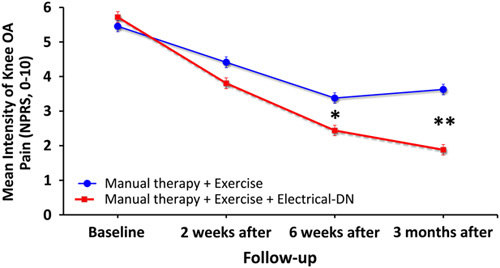
Evolution of knee pain intensity (NPRS, 0 to 10) throughout the course of the study stratified by randomized treatment assignment. Data are means (SE). NPRS indicates Numeric Pain Rating Scale.
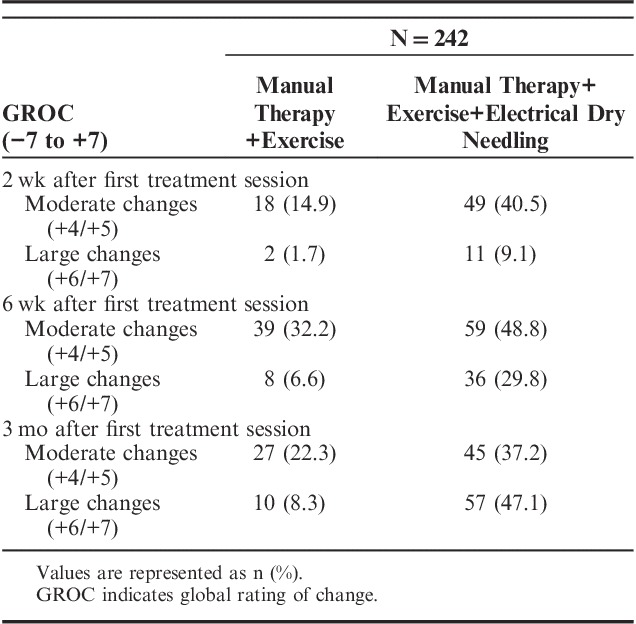
Patients receiving electrical dry needling were 1.7 times more likely to have completely stopped taking medication for their pain at 3 months than individuals receiving MT and exercise alone (OR, 1.6; 95% CI, 1.24-2.01; P=0.001). On the basis of the cutoff score of ≥+5 on the GROC, significantly (χ2=14.887; P<0.001) more patients (n=91, 75%) in the dry needling group achieved a successful outcome compared with the nondry needling group (n=23, 19%) at 3 months (Table 4). Therefore, based on the cutoff score of ≥+5 on the GROC, the NNT was 1.78 (95% CI, 1.50-2.18) in favor of the electrical dry needling group at 3-month follow-up. Likewise, based on the cutoff score of 36% improvement (ie, triple the MCID) on the WOMAC, the NNT was 2.37 (95% CI, 1.89-3.19) in favor of the electrical dry needling group at 3-month follow-up.
TABLE 4.
Self-perceived Improvement With GROC in Both Groups
DISCUSSION
To our knowledge, this study is the first randomized clinical trial comparing the effectiveness of MT and exercise plus electrical dry needling to MT and exercise alone in patients with painful knee OA. The results suggest that a mean of 9 sessions of MT and exercise plus electrical dry needling, using a 9-point standardized protocol targeting the knee locally at a frequency of 1 to 2 times per week over 6 weeks, resulted in greater improvements in pain, stiffness, function, related-disability, and medication intake than MT and exercise alone. For the primary outcome of related-disability (WOMAC), between-groups effect sizes were moderate at 6 weeks and large at 3 months in favor of the dry needling group. The between-groups difference for change in related-disability, as measured by the WOMAC (34.1%; 95% CI, 26.6-41.4) exceeded the reported MCID (ie, 12%61) at 3 months. In addition, for knee pain intensity, the point estimate for the between-groups change (3.23 points; 95% CI, 2.4-4.0) also exceeded the reported MCID (ie, 1.74 points67,68) at 3 months. Finally, the NNT suggests for every 2 patients treated with electrical dry needling, rather than MT and exercise alone, 1 additional patient with knee OA achieves clinically important reductions in related-disability at 3-month follow-up.
Three previous studies found nonsuperior results when adding acupuncture as an adjunct therapy to exercise-based physical therapy in knee OA.46–48 Notably, Foster et al47 reported no statistically significant between-groups difference in WOMAC pain subscale scores after adding a course of acupuncture to exercise in knee OA. Nevertheless, in the Foster et al47 trial, the acupuncture points were not standardized but selected based on the “clinical opinion” of 67 different physiotherapists at different centers. Considering the recent findings regarding the influence of acupuncture on cartilage repair72 and the efficacy of periosteal stimulation53 in knee OA, it is also possible that the needles in these previous studies (0.2 to 3.5 cm) were not inserted deep enough.46–48 In addition, a recent meta-analysis73 and a separate secondary analysis that pooled data from the Cochrane review19,74 concluded that electroacupuncture is superior to manual acupuncture in knee OA; however, neither the Foster et al47 nor Chen et al46 trials used electrical stimulation with the needles.
Mechanisms of Periosteal Electrical Dry Needling
The underlying mechanisms as to why the electrical dry needling group in the current study experienced greater improvements than the MT and exercise group remains to be elucidated. However, appropriate needle depth may be an important component to consider when using dry needling therapies for joint OA. A number of studies have shown that periosteal needling, that is, getting the needle close to the bone, cartilage or joint line, or tapping the needle repeatedly on to the bone, leads to significant and clinically meaningful improvements in pain and disability in hip and knee OA.53,75,76 Zhang et al72 recently reported significantly lower T2 values on magnetic resonance imaging at the anteromedial and anterolateral tibial subregions of 100 knees following 20 minute sessions over 4 weeks of 7-point, low-frequency electroacupuncture; that is, electroacupuncture seems to play a role in cartilage repair in individuals with knee OA.72 Moreover, acupuncture has been shown to reduce interleukin-6 mRNA expression in bone marrow, thereby limiting inflammation and inhibiting myelogenic osteoclast activity driving degeneration.77
Electroacupuncture to local points at the knee has been found to modulate knee joint microcirculation, significantly increase endogenous opioid levels, and significantly reduce plasma cortisol levels.78,79 In addition, electroacupuncture has been found to block the local release of inflammatory cytokines (ie, interleukin-1 β and tumor necrosis factor-α) in the synovia of osteoarthritic joints80 and the systemic release of inflammatory factors in the periaqueductal gray of the brain stem.81 Acupuncture may also stimulate an increase in hyaluronic acid, allowing the synovial fluid to better lubricate the joint.82
Strengths and Limitations
Major strengths of the current study include the inclusion of a large sample size with 18 treating physical therapists from 18 clinics in 10 different geographical states, and the use of the same standardized 9-point needling protocol and dosage parameters. However, we only assessed mid-term follow-up; thus, we do not know if the significant between-groups differences observed at 3 months would be sustained in the long term. We also cannot be certain that the results are generalizable to other dry needling protocols, dosages, techniques, or needle placements. In addition, we did not include a dry needling placebo group; which should be included in future studies. Finally, therapist and patient treatment preferences were not collected and could potentially affect the results.
CONCLUSIONS
The results of the current randomized clinical trial demonstrated that patients with painful knee OA who received MT and exercise plus electrical dry needling experienced significantly greater improvements in pain intensity, stiffness, physical function, related-disability, and medication intake as compared with the group that received MT and exercise alone. Future studies should examine the effectiveness of different types and dosages of electrical dry needling and include a long-term follow-up.
ACKNOWLEDGMENTS
The authors thank all the participants of the study.
APPENDIX 1: DESCRIPTION OF PERIOSTEAL ELECTRICAL DRY NEEDLING INTERVENTION
Technique
9-point electrical dry needling protocol for knee OA.
Technique Description
The technique is performed with the patient supine with the treated knee slightly flexed over a towel roll. Sterilized disposable stainless steel Seirin J-type acupuncture needles were used with 3 sizes: 0.25 mm×30 mm, 0.30 mm×40 mm, and 0.30 mm×50 mm. The depth of needle insertion ranged from 10 mm to 45 mm and depended on the point (intramuscular, periosteal, joint line, intra/periarticular) and the patient’s constitution (ie, size and bone depth, muscle and/or connective tissue thickness). The following 9 needles were inserted:
Superolateral and anterior insertion within the popliteus, with periosteal stimulation over the posteromedial aspect of the medial tibial condyle.
Inferolateral insertion angle within the distal adductor magnus, with periosteal stimulation within the depression posterosuperior to the femoral epicondyle.
Perpendicular insertion within the tibialis anterior, with periosteal stimulation over the anterolateral crest of the tibia one fingerbreadth lateral to the tibial tuberosity.
Perpendicular insertion within the quadriceps tendon, one fingerbreadth proximal to the superior border of the patella.
Perpendicular insertion within the vastus lateralis, 3 fingerbreadths proximal to the superolateral border of the patella.
Perpendicular insertion within the vastus medialis, 3 fingerbreadths proximal to the superomedial border of the patella.
Perpendicular insertion at the level of the tibiofemoral joint margin within the medial infrapatellar sulcus.
Perpendicular insertion at the level of the tibiofemoral joint margin within the lateral infrapatellar sulcus.
Perpendicular insertion within the extensor digitorum longus, one thumb width distal and anterior to the fibula head. Unlike the other 8 needles that were electrically connected in pairs, and for the purpose of standardization, the ninth needle was not paired with 1 of the 4 electrical channels; nevertheless, it was manually manipulated and left in situ for the duration of the treatment (Fig. 1).
Footnotes
J.D., R.B., and C.F.-d.-l.-P.: participated in the conception, design, data acquisition, statistical analyses, data interpretation, drafting, and revision of the manuscript. I.Y.: was involved in the statistical analysis, data interpretation, drafting, and revision of the manuscript. F.M.: was involved in the revision of the manuscript. V.G., P.B., and M.T.: were involved in data collection and revision of the manuscript.
The authors declare no conflict of interest.
REFERENCES
- 1.Zhang Y, Jordan JM. Epidemiology of osteoarthritis. Clin Geriatr Med. 2010;26:355–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kobayashi S, Pappas E, Fransen M, et al. The prevalence of patellofemoral osteoarthritis: a systematic review and meta-analysis. Osteoarthritis Cartilage. 2016;24:1697–1707. [DOI] [PubMed] [Google Scholar]
- 3.Cross M, Smith E, Hoy D, et al. The global burden of hip and knee osteoarthritis: estimates from the global burden of disease 2010 study. Ann Rheum Dis. 2014;73:1323–1330. [DOI] [PubMed] [Google Scholar]
- 4.Martel-Pelletier J. Pathophysiology of osteoarthritis. Osteoarthritis Cartilage. 1999;7:371–373. [DOI] [PubMed] [Google Scholar]
- 5.Carter DR, Beaupre GS, Wong M, et al. The mechanobiology of articular cartilage development and degeneration. Clin Orthop Relat Res. 2004;427(suppl):S69–S77. [DOI] [PubMed] [Google Scholar]
- 6.Ene R, Sinescu RD, Ene P, et al. Synovial inflammation in patients with different stages of knee osteoarthritis. Rom J Morphol Embryol. 2015;56:169–173. [PubMed] [Google Scholar]
- 7.Altman R, Asch E, Bloch D, et al. Development of criteria for the classification and reporting of osteoarthritis. Classification of osteoarthritis of the knee. Diagnostic and Therapeutic Criteria Committee of the American Rheumatism Association. Arthritis Rheum. 1986;29:1039–1049. [DOI] [PubMed] [Google Scholar]
- 8.Altman RD. Criteria for classification of clinical osteoarthritis. J Rheumatol Suppl. 1991;27:10–12. [PubMed] [Google Scholar]
- 9.Hsu C, Sherman KJ, Eaves ER, et al. New perspectives on patient expectations of treatment outcomes: results from qualitative interviews with patients seeking complementary and alternative medicine treatments for chronic low back pain. BMC Complement Altern Med. 2014;14:276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pound P, Britten N, Morgan M, et al. Resisting medicines: a synthesis of qualitative studies of medicine taking. Soc Sci Med. 2005;61:133–155. [DOI] [PubMed] [Google Scholar]
- 11.Fransen M, McConnell S, Harmer AR, et al. Exercise for osteoarthritis of the knee. Cochrane Database Syst Rev. 2015;1:CD004376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jansen MJ, Viechtbauer W, Lenssen AF, et al. Strength training alone, exercise therapy alone, and exercise therapy with passive manual mobilisation each reduce pain and disability in people with knee osteoarthritis: a systematic review. J Physiother. 2011;57:11–20. [DOI] [PubMed] [Google Scholar]
- 13.Smidt N, de Vet HC, Bouter LM, et al. Effectiveness of exercise therapy: a best-evidence summary of systematic reviews. Aust J Physiother. 2005;51:71–85. [DOI] [PubMed] [Google Scholar]
- 14.van Baar ME, Assendelft WJ, Dekker J, et al. Effectiveness of exercise therapy in patients with osteoarthritis of the hip or knee: a systematic review of randomized clinical trials. Arthritis Rheum. 1999;42:1361–1369. [DOI] [PubMed] [Google Scholar]
- 15.Thomas KS, Miller P, Doherty M, et al. Cost effectiveness of a two-year home exercise program for the treatment of knee pain. Arthritis Rheum. 2005;53:388–394. [DOI] [PubMed] [Google Scholar]
- 16.Hay EM, Foster NE, Thomas E, et al. Effectiveness of community physiotherapy and enhanced pharmacy review for knee pain in people aged over 55 presenting to primary care: pragmatic randomised trial. Bmj. 2006;333:995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Corbett MS, Rice SJ, Madurasinghe V, et al. Acupuncture and other physical treatments for the relief of pain due to osteoarthritis of the knee: network meta-analysis. Osteoarthritis Cartilage. 2013;21:1290–1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hochberg MC, Altman RD, April KT, et al. American College of Rheumatology 2012 recommendations for the use of nonpharmacologic and pharmacologic therapies in osteoarthritis of the hand, hip, and knee. Arthritis Care Res (Hoboken). 2012;64:465–474. [DOI] [PubMed] [Google Scholar]
- 19.Manheimer E, Cheng K, Linde K, et al. Acupuncture for peripheral joint osteoarthritis. Cochrane Database Syst Rev. 2010;1:CD001977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang W, Moskowitz RW, Nuki G, et al. OARSI recommendations for the management of hip and knee osteoarthritis, Part II: OARSI evidence-based, expert consensus guidelines. Osteoarthritis Cartilage. 2008;16:137–162. [DOI] [PubMed] [Google Scholar]
- 21.Lin X, Huang K, Zhu G, et al. The effects of acupuncture on chronic knee pain due to osteoarthritis: a meta-analysis. J Bone Joint Surg Am. 2016;98:1578–1585. [DOI] [PubMed] [Google Scholar]
- 22.Ezzo J, Hadhazy V, Birch S, et al. Acupuncture for osteoarthritis of the knee: a systematic review. Arthritis Rheum. 2001;44:819–825. [DOI] [PubMed] [Google Scholar]
- 23.White A, Foster NE, Cummings M, et al. Acupuncture treatment for chronic knee pain: a systematic review. Rheumatology (Oxford). 2007;46:384–390. [DOI] [PubMed] [Google Scholar]
- 24.Brosseau L, Taki J, Desjardins B, et al. The Ottawa panel clinical practice guidelines for the management of knee osteoarthritis. Part two: strengthening exercise programs. Clin Rehabil. 2017;31:596–611. [DOI] [PubMed] [Google Scholar]
- 25.Casanueva B, Rivas P, Rodero B, et al. Short-term improvement following dry needle stimulation of tender points in fibromyalgia. Rheumatol Int. 2014;34:861–866. [DOI] [PubMed] [Google Scholar]
- 26.Manheimer E, White A, Berman B, et al. Meta-analysis: acupuncture for low back pain. Ann Intern Med. 2005;142:651–663. [DOI] [PubMed] [Google Scholar]
- 27.Gunn CC, Milbrandt WE, Little AS, et al. Dry needling of muscle motor points for chronic low-back pain: a randomized clinical trial with long-term follow-up. Spine (Phila Pa 1976). 1980;5:279–291. [DOI] [PubMed] [Google Scholar]
- 28.Lewit K. The needle effect in the relief of myofascial pain. Pain. 1979;6:83–90. [DOI] [PubMed] [Google Scholar]
- 29.Dunning J, Butts R, Mourad F, et al. Dry needling: a literature review with implications for clinical practice guidelines. Phys Ther Rev. 2014;19:252–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Neal BS, Longbottom J. Is there a role for acupuncture in the treatment of tendinopathy? Acupunct Med. 2012;30:346–349. [DOI] [PubMed] [Google Scholar]
- 31.Zhou K, Ma Y, Brogan MS. Dry needling versus acupuncture: the ongoing debate. Acupunct Med. 2015;33:485–490. [DOI] [PubMed] [Google Scholar]
- 32.Furlan AD, van Tulder M, Cherkin D, et al. Acupuncture and dry-needling for low back pain: an updated systematic review within the framework of the Cochrane Collaboration. Spine (Phila Pa 1976). 2005;30:944–963. [DOI] [PubMed] [Google Scholar]
- 33.Furlan AD, van Tulder MW, Cherkin DC, et al. Acupuncture and dry-needling for low back pain. Cochrane Database Syst Rev. 2005;1:CD001351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tough EA, White A. Effectiveness of acupuncture/dry needling for myofascial trigger point pain—a systematic review. Phys Ther Rev. 2011;16:147–154. [Google Scholar]
- 35.Tough EA, White AR, Cummings TM, et al. Acupuncture and dry needling in the management of myofascial trigger point pain: a systematic review and meta-analysis of randomised controlled trials. Eur J Pain. 2009;13:3–10. [DOI] [PubMed] [Google Scholar]
- 36.Rathnayake T. Back pain (low): acupuncture and dry needling. Evidence Summaries-Joanna Briggs Institute; 2009. [Google Scholar]
- 37.Brinkhaus B, Witt C, Jena S, et al. Interventions and physican characteristics in a randomized multicenter trial of acupuncture in patients with low-back pain. J Altern Complement Med. 2006;12:649–657. [DOI] [PubMed] [Google Scholar]
- 38.Moffet H. Traditional acupuncture theories yield null outcomes: a systematic review of clinical trials. J Clin Epidemiol. 2008;61:741–747. [DOI] [PubMed] [Google Scholar]
- 39.Deadman P, Al-Khafaji M, Baker K. A Manual of Acupuncture, 2nd ed Hove, East Sussex, UK: Journal of Chinese Medicine Publications; 2011. [Google Scholar]
- 40.O’Conner J, Bensky D. Acupuncture: A Comprehensive Text. Seattle, WA: Eastland Press; 1981. [Google Scholar]
- 41.Mavrommatis CI, Argyra E, Vadalouka A, et al. Acupuncture as an adjunctive therapy to pharmacological treatment in patients with chronic pain due to osteoarthritis of the knee: a 3-armed, randomized, placebo-controlled trial. Pain. 2012;153:1720–1726. [DOI] [PubMed] [Google Scholar]
- 42.Vas J, Mendez C, Perea-Milla E, et al. Acupuncture as a complementary therapy to the pharmacological treatment of osteoarthritis of the knee: randomised controlled trial. Bmj. 2004;329:1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Witt C, Brinkhaus B, Jena S, et al. Acupuncture in patients with osteoarthritis of the knee: a randomised trial. Lancet. 2005;366:136–143. [DOI] [PubMed] [Google Scholar]
- 44.Zhang W, Nuki G, Moskowitz RW, et al. OARSI recommendations for the management of hip and knee osteoarthritis: part III: changes in evidence following systematic cumulative update of research published through January 2009. Osteoarthritis Cartilage. 2010;18:476–499. [DOI] [PubMed] [Google Scholar]
- 45.Manheimer E, Linde K, Lao L, et al. Meta-analysis: acupuncture for osteoarthritis of the knee. Ann Intern Med. 2007;146:868–877. [DOI] [PubMed] [Google Scholar]
- 46.Chen LX, Mao JJ, Fernandes S, et al. Integrating acupuncture with exercise-based physical therapy for knee osteoarthritis: a randomized controlled trial. J Clin Rheumatol. 2013;19:308–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Foster NE, Thomas E, Barlas P, et al. Acupuncture as an adjunct to exercise based physiotherapy for osteoarthritis of the knee: randomised controlled trial. Bmj. 2007;335:436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Scharf HP, Mansmann U, Streitberger K, et al. Acupuncture and knee osteoarthritis: a three-armed randomized trial. Ann Intern Med. 2006;145:12–20. [DOI] [PubMed] [Google Scholar]
- 49.Zwarenstein M, Treweek S, Gagnier JJ, et al. Improving the reporting of pragmatic trials: an extension of the CONSORT statement. Bmj. 2008;337:a2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Deyle GD, Allison SC, Matekel RL, et al. Physical therapy treatment effectiveness for osteoarthritis of the knee: a randomized comparison of supervised clinical exercise and manual therapy procedures versus a home exercise program. Phys Ther. 2005;85:1301–1317. [PubMed] [Google Scholar]
- 51.Kong J, Gollub R, Huang T, et al. Acupuncture de qi, from qualitative history to quantitative measurement. J Altern Complement Med. 2007;13:1059–1070. [DOI] [PubMed] [Google Scholar]
- 52.Zhou W, Benharash P. Significance of “Deqi” response in acupuncture treatment: myth or reality. J Acupunct Meridian Stud. 2014;7:186–189. [DOI] [PubMed] [Google Scholar]
- 53.Weiner DK, Moore CG, Morone NE, et al. Efficacy of periosteal stimulation for chronic pain associated with advanced knee osteoarthritis: a randomized, controlled clinical trial. Clin Ther. 2013;35:1703.e1705–1720.e1705. [DOI] [PubMed] [Google Scholar]
- 54.Zhang Q, Yue J, Lu Y. Acupuncture treatment for chronic knee pain: study by Hinman et al underestimates acupuncture efficacy. Acupunct Med. 2015;33:170. [DOI] [PubMed] [Google Scholar]
- 55.Mata J, Cabrera S, Sanchis P, et al. Electro-acupuncture for treatment of knee pain from osteoarthritis and the possible endocrinology changes: a study protocol for a randomized controlled trial. Trials. 2015;16:248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sangdee C, Teekachunhatean S, Sananpanich K, et al. Electroacupuncture versus diclofenac in symptomatic treatment of osteoarthritis of the knee: a randomized controlled trial. BMC Complement Altern Med. 2002;2:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Menz HB. Analysis of paired data in physical therapy research: time to stop double-dipping? J Orthop Sports Phys Ther. 2005;35:477–478. [DOI] [PubMed] [Google Scholar]
- 58.Sun Y, Sturmer T, Gunther KP, et al. Reliability and validity of clinical outcome measurements of osteoarthritis of the hip and knee—a review of the literature. Clin Rheumatol. 1997;16:185–198. [DOI] [PubMed] [Google Scholar]
- 59.Bellamy N, Buchanan WW, Goldsmith CH, et al. Validation study of WOMAC: a health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. J Rheumatol. 1988;15:1833–1840. [PubMed] [Google Scholar]
- 60.McConnell S, Kolopack P, Davis AM. The Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC): a review of its utility and measurement properties. Arthritis Rheum. 2001;45:453–461. [DOI] [PubMed] [Google Scholar]
- 61.Angst F, Aeschlimann A, Stucki G. Smallest detectable and minimal clinically important differences of rehabilitation intervention with their implications for required sample sizes using WOMAC and SF-36 quality of life measurement instruments in patients with osteoarthritis of the lower extremities. Arthritis Rheum. 2001;45:384–391. [DOI] [PubMed] [Google Scholar]
- 62.Williams VJ, Piva SR, Irrgang JJ, et al. Comparison of reliability and responsiveness of patient-reported clinical outcome measures in knee osteoarthritis rehabilitation. J Orthop Sports Phys Ther. 2012;42:716–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Abbott JH, Hobbs C, Moa Trial T. The ShortMAC: minimum important change of a reduced version of the WOMAC Osteoarthritis Index. J Orthop Sports Phys Ther. 2017:1–16. [DOI] [PubMed] [Google Scholar]
- 64.Jensen MP, Karoly P, Braver S. The measurement of clinical pain intensity: a comparison of six methods. Pain. 1986;27:117–126. [DOI] [PubMed] [Google Scholar]
- 65.Cleland JA, Childs JD, Whitman JM. Psychometric properties of the Neck Disability Index and Numeric Pain Rating Scale in patients with mechanical neck pain. Arch Phys Med Rehabil. 2008;89:69–74. [DOI] [PubMed] [Google Scholar]
- 66.Young IA, Cleland JA, Michener LA, et al. Reliability, construct validity, and responsiveness of the Neck Disability Index, patient-specific functional scale, and numeric pain rating scale in patients with cervical radiculopathy. Am J Phys Med Rehabil. 2010;89:831–839. [DOI] [PubMed] [Google Scholar]
- 67.Farrar JT, Young JP, Jr, LaMoreaux L, et al. Clinical importance of changes in chronic pain intensity measured on an 11-point numerical pain rating scale. Pain. 2001;94:149–158. [DOI] [PubMed] [Google Scholar]
- 68.Salaffi F, Stancati A, Silvestri CA, et al. Minimal clinically important changes in chronic musculoskeletal pain intensity measured on a numerical rating scale. Eur J Pain. 2004;8:283–291. [DOI] [PubMed] [Google Scholar]
- 69.Jaeschke R, Singer J, Guyatt GH. Measurement of health status. Ascertaining the minimal clinically important difference. Control Clin Trials. 1989;10:407–415. [DOI] [PubMed] [Google Scholar]
- 70.Carlesso LC, Macdermid JC, Santaguida LP. Standardization of adverse event terminology and reporting in orthopaedic physical therapy: application to the cervical spine. J Orthop Sports Phys Ther. 2010;40:455–463. [DOI] [PubMed] [Google Scholar]
- 71.Rubin LH, Witkiewitz K, Andre JS, et al. Methods for handling missing data in the behavioral neurosciences: don’t throw the baby rat out with the bath water. J Undergrad Neurosci Educ. 2007;5:A71–A77. [PMC free article] [PubMed] [Google Scholar]
- 72.Zhang Y, Bao F, Wang Y, et al. Influence of acupuncture in treatment of knee osteoarthritis and cartilage repairing. Am J Transl Res. 2016;8:3995–4002. [PMC free article] [PubMed] [Google Scholar]
- 73.Chen N, Wang J, Mucelli A, et al. Electro-acupuncture is beneficial for knee osteoarthritis: the evidence from meta-analysis of randomized controlled trials. Am J Chin Med. 2017;45:965–985. [DOI] [PubMed] [Google Scholar]
- 74.Langevin HM, Schnyer R, MacPherson H, et al. Manual and electrical needle stimulation in acupuncture research: pitfalls and challenges of heterogeneity. J Altern Complement Med. 2015;21:113–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Weiner DK, Rudy TE, Morone N, et al. Efficacy of periosteal stimulation therapy for the treatment of osteoarthritis-associated chronic knee pain: an initial controlled clinical trial. J Am Geriatr Soc. 2007;55:1541–1547. [DOI] [PubMed] [Google Scholar]
- 76.Mcindoe A. A comparison of acupunture with intra-articular steroids injection as analgesia for osteoarthritis of the hip. Acupunct Med. 1995;13:67–70. [Google Scholar]
- 77.Liu X, Shen L, Wu M, et al. Effects of acupuncture on myelogenic osteoclastogenesis and IL-6 mRNA expression. J Tradit Chin Med. 2004;24:144–148. [PubMed] [Google Scholar]
- 78.Ahsin S, Saleem S, Bhatti AM, et al. Clinical and endocrinological changes after electro-acupuncture treatment in patients with osteoarthritis of the knee. Pain. 2009;147:60–66. [DOI] [PubMed] [Google Scholar]
- 79.Loaiza LA, Yamaguchi S, Ito M, et al. Electro-acupuncture stimulation to muscle afferents in anesthetized rats modulates the blood flow to the knee joint through autonomic reflexes and nitric oxide. Auton Neurosci. 2002;97:103–109. [DOI] [PubMed] [Google Scholar]
- 80.Huang J, Zhuo LS, Wang YY, et al. Effects of electroacupuncture on synovia IL-1beta and TNF-alpha contents in the rabbit with knee osteoarthritis. Zhen Ci Yan Jiu. 2007;32:115–118. [PubMed] [Google Scholar]
- 81.Zhang R, Lao L, Ren K, et al. Mechanisms of acupuncture-electroacupuncture on persistent pain. Anesthesiology. 2014;120:482–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Li ZD, Cao LH, Wang SC. Effect of moxibustion in treating knee joint osteoarthritis and its relation with contents of hyaluronic acid in serum and synovial fluid. Zhongguo Zhong Xi Yi Jie He Za Zhi. 2009;29:883–885. [PubMed] [Google Scholar]