Abstract
Objective:
To assess the role of registries in the postmarketing surveillance of surgical meshes.
Background:
To date, surgical meshes are classified as group II medical devices. Class II devices do not require premarket clearance by clinical studies. Ethicon initiated a voluntary market withdrawal of Physiomesh for laparoscopic use after an analysis of unpublished data from the 2 large independent hernia registries—Herniamed German Registry and Danish Hernia Database. This paper now presents the relevant data from the Herniamed Registry.
Methods:
The present analysis compares the prospective perioperative and 1-year follow-up data collected for all patients with incisional hernia who had undergone elective laparoscopic intraperitoneal onlay mesh repair either with Physiomesh (n = 1380) or with other meshes recommended in the guidelines (n = 3834).
Results:
Patients with Physiomesh repair had a markedly higher recurrence rate compared with the other recommended meshes (12.0% vs 5.0%; P < 0.001). In the multivariable analysis, the recurrence rate was highly significantly influenced by the mesh type used (P < 0.001). If Physiomesh was used, that led to a highly significant increase in the recurrence rate on 1-year follow-up (odds ratio 2.570, 95% CI 2.057, 3.210). The mesh type used also had a significant influence on chronic pain rates.
Conclusions:
The importance of real-world data for postmarketing surveillance of surgical meshes has been demonstrated in this registry-based study. Randomized controlled trials are needed for premarket approval of new devices. The role of sponsorship of device studies by the manufacturing company must be taken into account.
Keywords: hernia mesh, hernia registries, incisional hernia, laparoscopic IPOM, postmarketing surveillance
In incisional hernia repair, laparoscopic intraperitoneal onlay mesh (IPOM) has a lower rate of wound infections compared with open techniques.1–7 Recurrence rates and postoperative pain are similar for the 2 techniques during mid-term follow-up.1–7 Identified risk factors for recurrence are as follows8–13: defect sizes greater than 10 cm, fixation techniques, mesh overlap, and defect closure. The intraperitoneal placement of a composite mesh, specifically produced for laparoscopic ventral hernia repair, seemed to be safe.14,15
During a planned interim analysis for safety, a randomized controlled trial (RCT) was stopped after enrolment of 25 patients, because a 20% recurrence rate was observed in the first 6 months in the Physiomesh/Securestrap group and none in the Ventralight ST/SorbaFix group.16
To date, the conditions under which surgical meshes are brought to market are similar in the United States and the European Union (EU). So far, surgical meshes are classified as group II medical devices under the US Food and Drug Administration (FDA) and the EU Medical Device Directive (CE mark) clearance regulations. Class II products need premarket notification 510 (k), requiring only demonstration of substantial equivalence to another device legally marketed in the United States or EU. Substantial equivalence means that the new device is at least as safe and effective as the predicate. In contrast to class III devices, class II devices do not require premarket clearance by clinical studies.
On May 25, 2016, Ethicon initiated a voluntary market withdrawal of PHYSIOMESH Flexible Composite Mesh for laparoscopic use after an analysis of unpublished data from 2 large independent hernia registries—Herniamed German Registry and Danish Hernia Database.17
This paper now presents the Herniamed data to compare Physiomesh with the other meshes recommended in the guidelines8–10 for incisional hernia repair in laparoscopic IPOM technique, while taking account of how the outcome was impacted by all the influence variables reported in the literature.
METHODS
Herniamed is a multicenter, internet-based hernia registry18 into which 537 participating hospitals and surgeons engaged in private practice in Germany, Austria, and Switzerland (status: October 10, 2016) have entered data prospectively on their patients who had undergone routine hernia repair and signed an informed consent agreeing to participate. As part of the information provided to patients regarding participation in the Herniamed Registry and signing the informed consent declaration, all patients were informed that the treating hospital or medical practice would like to be informed about any problems occurring after the operation and that the patient has the opportunity to attend for clinical examination. All postoperative complications occurring up to 30 days after surgery were recorded. On 1-year follow-up, postoperative complications were once again reviewed when the general practitioners and patients completed a questionnaire. On 1-year follow-up, general practitioners and patients were also asked about any recurrences, bulging, pain at rest, pain on exertion, and chronic pain requiring treatment. If recurrences or chronic pain were reported by the general practitioner or patient, patients could be requested to attend clinical examination or radiologic tests. A recent publication has provided impressive evidence of the role of patient-reported outcomes for both recurrence and chronic pain.19
The present analysis compares the prospective data collected for all patients with incisional hernias who had undergone elective laparoscopic IPOM either with Physiomesh or other meshes recommended in the guidelines. Inclusion criteria were minimum age of 18 years, incisional hernia, elective operation, mesh fixation with sutures, tacks or sutures and tacks, and availability of data on 1-year follow-up (Fig. 1). In all, 5214 patients were enrolled between September 1, 2009 and September 1, 2015 (Fig. 1). Of these patients, 1380 (26.5%) had a laparoscopic IPOM repair with Physiomesh and 3834 (73.5%) with other meshes. The other most commonly used meshes (≥1.8%) were Parietex Composite (n = 1460/5214; 28.0%), Parietene Composite (n = 399/5214; 7.7%), Symbotex Composite (n = 93/5214; 1.8%), Dynamesh IPOM (n = 808/5214; 15.5%), TiMesh (n = 201/5214; 3.9%), Ventralight ST (n = 241/5214; 4.6%), and other meshes (n = 632/5214; 12.0%).
FIGURE 1.
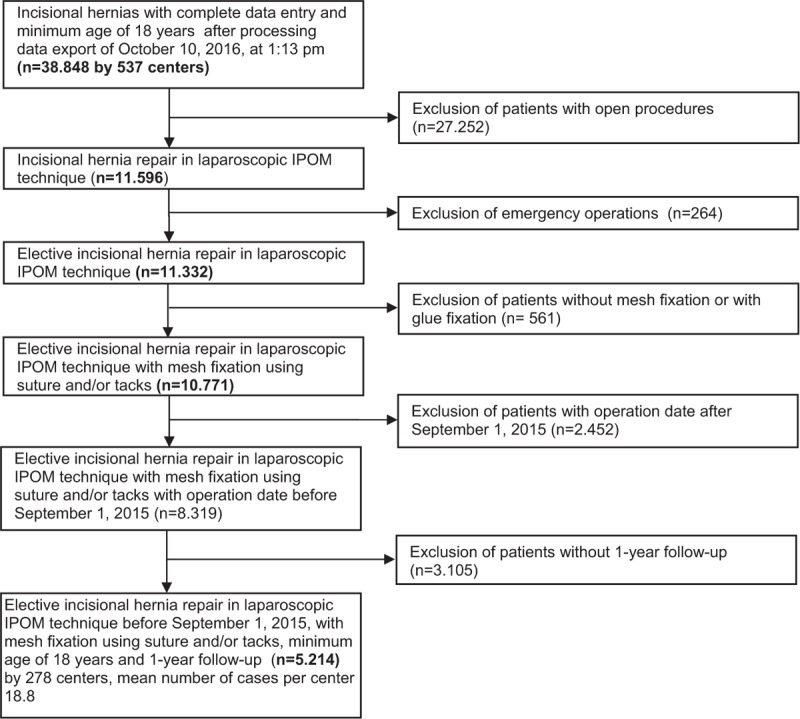
Flowchart of patient inclusion.
The demographic and patient-related parameters included age (years), sex, American Society of Anesthesiologist (ASA) score (I, II, III, IV), World Health Organization (WHO) obesity classification [body mass index (BMI) <18.5 = underweight, 18.5–24.9 = normal weight, 25.0–29.9 = overweight, ≥30 = obesity),20 and risk factors (chronic obstructive pulmonary disease, diabetes, aneurysms, cortisone, immunosuppression, etc).
The second group of surgery-related categorical variables influencing the outcome included the hernia defect size based on European Hernia Society (EHS) classification (W1 <4 cm, W2 ≥4–10 cm, W3 >10 cm),21 hernia localization, fixation technique, defect closure, mesh type, recurrent operation, preoperative pain, and mesh size. Hernia width was recorded during surgery based on intraoperative measurements.
The dependent variables were intraoperative and postoperative, and general complication rates, complication-related reoperation rates, recurrence rates and rates of pain at rest, pain on exertion, and chronic pain requiring treatment.
All analyses were performed with the software SAS 9.4 (SAS Institute Inc. Cary, NC) and intentionally calculated to a full significance level of 5%, that is, they were not corrected in respect to multiple tests, and each P value ≤0.05 represents a significant result. To discern differences between the groups in unadjusted analyses, Fisher's exact test was used for categorical outcome variables, and the robust t test (Satterthwaite) for continuous variables. For mesh size (cm2), a logarithmic transformation was applied, and re-transformed mean and range of dispersion were given. In an attempt to rule out confounding of data caused by different patient characteristics, the results of univariable analyses were verified via multivariable analyses in which, in addition to mesh type, other influence parameters were simultaneously reviewed.
To access influence factors in multivariable analyses, the binary logistic regression model for dichotomous outcome variables was used. Estimates for odds ratio (OR) and the corresponding 95% confidence interval (CI) based on the Wald test are given. In addition, all pair-wise ORs with a corresponding 95% confidence interval are given. For age (years), the 10-year OR estimate, and for mesh size, the 10-point OR estimate are given. The results are presented in tabular form, sorted by descending impact.
RESULTS
Univariable Analyses
Univariable analysis merely provides a first hint of the heterogeneity between the mesh groups used, which is then taken into account in the models. Thus, the focus is on the appropriate multivariable models.
The descriptive statistics, and also the results for the continuous variables age (mean) (Physiomesh: 62.4 vs other meshes: 62.6) and mesh size (cm2) (Physiomesh: 319.6 vs other meshes: 279.1) identified only a significant difference in the mesh size (P = 0.001).
The descriptive statistics, and also the results for the categorical variables, are given in Table 1. Significant differences between the meshes used were identified for defect closure (P < 0.001), fixation technique (P < 0.001), and ASA score (P = 0.004). As regards the recurrent operations, no significant difference was detected between the 2 groups in respect of the proportion of recurrent operations, number of prior surgeries, or the repair technique used for the most recent previous operation. Overall assessment of the risk factors, that is, presence of at least 1 risk factor, did not reveal any significant difference between the 2 groups.
TABLE 1.
Demographic, Patient and Procedure-related Parameters, Risk Factors, and Mesh Type
| PhysioMesh | Other Meshes | |||||
| n | % | n | % | P | ||
| Sex | Male | 671 | 48.62 | 1945 | 50.73 | 0.179 |
| Female | 709 | 51.38 | 1889 | 49.27 | ||
| BMI | Underweight | 10 | 0.73 | 16 | 0.42 | 0.203 |
| Normal weight | 231 | 16.76 | 697 | 18.26 | ||
| Overweight | 517 | 37.52 | 1472 | 38.55 | ||
| Obesity | 620 | 44.99 | 1633 | 42.77 | ||
| Defect size | W1 (<4 cm) | 474 | 34.35 | 1366 | 35.63 | 0.177 |
| W2 (≥4–10 cm) | 723 | 52.39 | 1904 | 49.66 | ||
| W3 (≥10 cm) | 183 | 13.26 | 564 | 14.71 | ||
| ASA score | I | 111 | 8.04 | 424 | 11.06 | 0.004 |
| II | 829 | 60.07 | 2177 | 56.78 | ||
| III/IV | 440 | 31.88 | 1233 | 32.16 | ||
| Defect closure | 364 | 26.38 | 841 | 21.94 | <0.001 | |
| Fixation | Suture | 42 | 3.04 | 178 | 4.64 | <0.001 |
| Nonabsorbable tacks | 69 | 5.00 | 181 | 4.72 | ||
| Nonabsorbable tacks + suture | 53 | 3.84 | 175 | 4.56 | ||
| Absorbable tacks | 813 | 58.91 | 2009 | 52.40 | ||
| Absorbable tacks + suture | 403 | 29.20 | 1291 | 33.67 | ||
| Recurrent operation | 289 | 20.94 | 802 | 20.92 | 0.985 | |
| EHS classification | Combined | 132 | 9.57 | 365 | 9.52 | 0.724 |
| Lateral | 208 | 15.07 | 613 | 15.99 | ||
| Medial | 1040 | 75.36 | 2856 | 74.49 | ||
| Preoperative pain | Yes | 781 | 56.59 | 2188 | 57.07 | 0.534 |
| No | 468 | 33.91 | 1320 | 34.43 | ||
| Unknown | 131 | 9.49 | 326 | 8.50 | ||
| Risk factors | Total | 551 | 39.93 | 1574 | 41.05 | 0.465 |
| COPD | 149 | 10.80 | 416 | 10.85 | 0.957 | |
| Diabetes | 194 | 14.06 | 550 | 14.35 | 0.794 | |
| Aortic aneurysm | 20 | 1.45 | 56 | 1.46 | 0.976 | |
| Immunosuppression | 23 | 1.67 | 74 | 1.93 | 0.535 | |
| Corticoids | 22 | 1.59 | 80 | 2.09 | 0.257 | |
| Smoking | 152 | 11.01 | 460 | 12.00 | 0.330 | |
| Coagulopathy | 25 | 1.81 | 62 | 1.62 | 0.629 | |
| Antiplatelet medication | 152 | 11.01 | 382 | 9.96 | 0.269 | |
| Anticoagulation therapy | 36 | 2.61 | 113 | 2.95 | 0.517 | |
ASA indicates American Society of Anesthesiologist; COPD, chronic obstructive pulmonary disease; EHS, European Hernia Society.
As regards the outcome parameters (Table 2), only for the recurrences, pain on exertion and chronic pain requiring treatment was a significant difference identified between the patient groups using Physiomesh versus other meshes for repair. For example, patients with Physiomesh repair had a markedly higher recurrence rate (12.0% vs 5.0%p; P < 0.001). To discount the possibility that the significant difference in the recurrence rate to the advantage of the comparator meshes was due to the high performance of 1 of the meshes, the recurrence rates of meshes accounting for a proportion of at least 1.8% of all meshes were also calculated. This revealed that for all other meshes recommended in the guidelines, a highly significantly (P < 0.001) lower recurrence rate was identified compared with Physiomesh (Fig. 2). Likewise, the rate of pain on exertion (22.1% vs 19.0%; P = 0.013) and chronic pain requiring treatment (9.7% vs 7.3%; P = 0.005) were significantly higher in patients with Physiomesh repair.
TABLE 2.
Unadjusted Perioperative and 1-year Follow-up Outcomes and Mesh Type
| PhysioMesh | Other Meshes | ||||||
| n | % | n | % | P | |||
| Intraoperative complications | Total | 37 | 2.68 | 95 | 2.48 | 0.680 | |
| Bleeding | 16 | 1.16 | 43 | 1.12 | 0.909 | ||
| Organ injuries | 27 | 1.96 | 74 | 1.93 | 0.951 | ||
| Injuries | Vascular | 7 | 0.51 | 18 | 0.47 | 0.862 | |
| Bowel | 18 | 1.30 | 44 | 1.15 | 0.645 | ||
| Bladder | 2 | 0.14 | 5 | 0.13 | 0.900 | ||
| Stomach | 0 | 0.00 | 1 | 0.03 | 0.549 | ||
| Spleen | 0 | 0.00 | 1 | 0.03 | 0.549 | ||
| General complications | Total | 37 | 2.68 | 110 | 2.87 | 0.718 | |
| Fever | 3 | 0.22 | 8 | 0.21 | 0.952 | ||
| Urinary tract infection | 4 | 0.29 | 17 | 0.44 | 0.440 | ||
| Diarrhea | 2 | 0.14 | 6 | 0.16 | 0.925 | ||
| Gastritis | 1 | 0.07 | 2 | 0.05 | 0.787 | ||
| Thrombosis | 1 | 0.07 | 1 | 0.03 | 0.451 | ||
| Pulmonary embolism | 2 | 0.14 | 2 | 0.05 | 0.286 | ||
| Pleural effusion | 1 | 0.07 | 3 | 0.08 | 0.947 | ||
| Pneumonia | 6 | 0.43 | 12 | 0.31 | 0.508 | ||
| COPD | 3 | 0.22 | 11 | 0.29 | 0.669 | ||
| Cardiac insufficiency | 3 | 0.22 | 1 | 0.03 | 0.028 | ||
| Coronary heart disease | 2 | 0.14 | 2 | 0.05 | 0.286 | ||
| Myocardial infarction | 0 | 0.00 | 1 | 0.03 | 0.549 | ||
| Renal insufficiency | 1 | 0.07 | 4 | 0.10 | 0.743 | ||
| Hypertensive crisis | 4 | 0.29 | 4 | 0.10 | 0.131 | ||
| Postoperative complication | Total | 72 | 5.22 | 160 | 4.17 | 0.107 | |
| Bleeding | 16 | 1.16 | 27 | 0.70 | 0.109 | ||
| Seroma | 33 | 2.39 | 95 | 2.48 | 0.859 | ||
| Infection | 4 | 0.29 | 16 | 0.42 | 0.511 | ||
| Bowel injury/anastomotic insufficiency | 5 | 0.36 | 19 | 0.50 | 0.531 | ||
| Wound healing disorder | 7 | 0.51 | 11 | 0.29 | 0.231 | ||
| Ileus | 13 | 0.94 | 10 | 0.26 | 0.001 | ||
| Complication-related reoperation | 32 | 2.32 | 67 | 1.75 | 0.182 | ||
| Recurrence on 1-year follow-up | 166 | 12.03 | 190 | 4.96 | <0.001 | ||
| Pain on exertion on 1-year follow-up | 305 | 22.10 | 728 | 18.99 | 0.013 | ||
| Pain at rest on 1-year follow-up | 161 | 11.67 | 379 | 9.89 | 0.063 | ||
| Pain requiring treatment on 1-year follow-up | 134 | 9.71 | 281 | 7.33 | 0.005 | ||
COPD indicates chronic obstructive pulmonary disease.
FIGURE 2.
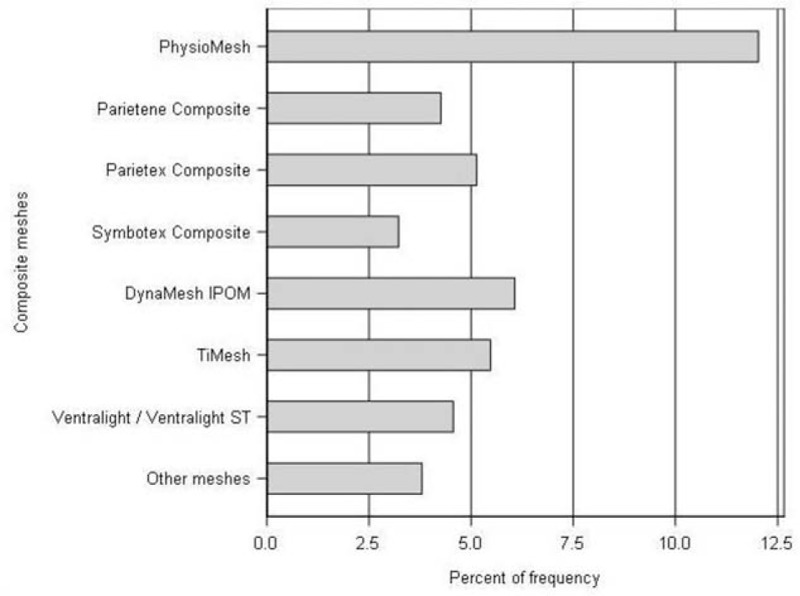
Recurrence rate and mesh type (P < 0.001).
Multivariable Analyses
The results of the model fit used to investigate the influence exerted by patient and procedure-related variables (mesh type, sex, age, WHO obesity classification, ASA score, defect size, defect localization, defect closure, mesh size, fixation technique, recurrent operation, preoperative pain, and risk factors) on the outcome variables are presented. The multivariable analyses of intraoperative, postoperative surgical, and postoperative general complications, and also complication-related re-operations and pain at rest, showed no influence of the mesh type on the outcome.
Recurrence
Table 3 shows the multivariable analysis results of the factors impacting onset of recurrences on 1-year follow-up (model fit: P < 0.001). The recurrence rate was highly significantly influenced by the mesh type used (P < 0.001). If Physiomesh was used, it led to an increase in the recurrence rate on 1-year follow-up (OR 2.570, 95% CI 2.057, 3.210). A recurrent operation (OR 1.499, 95% CI 1.166, 1.926, P = 0.002), and also larger defect sizes (W3 vs W1: OR 1.831, 95% CI 1.245; 2.692, P = 0.002; W2 vs W1: OR 1.471, 95% CI 1.117, 1.937, P = 0.006) were significantly associated with a higher recurrence risk. Differences were also observed with regard to the EHS classification, with the majority of recurrences found for lateral hernias (lateral vs combined: OR 1.657, 95% CI 1.070, 2.567, P = 0.024; lateral vs medial: OR 1.546, 95% CI 1.166, 2.051, P = 0.002). Likewise, a larger mesh (10-point: OR 1.006, 95% CI 1.000, 1.011, P = 0.040) was conducive to recurrence and obesity versus normal weight (OR 1.621, 95% CI 1.138, 2.309, P = 0.007) was also associated with a higher recurrence risk although BMI classification was not be found to be a significant predictor of recurrence.
TABLE 3.
Multivariable Analysis of Recurrences on 1-year Follow-up
| Parameter | P | Category | P Paired | OR Estimate | 95% CI | |
| Mesh type | <0.001 | PhysioMesh vs other meshes | 2.570 | 2.057 | 3.210 | |
| Recurrent operation | 0.002 | Yes vs no | 1.499 | 1.166 | 1.926 | |
| Defect size | 0.005 | W3 (≥10 cm) vs W2 (≥4–10 cm) | 0.173 | 1.244 | 0.909 | 1.704 |
| W3 (≥10 cm) vs W1 (<4 cm) | 0.002 | 1.831 | 1.245 | 2.692 | ||
| W2 (≥4–10 cm) vs W1 (<4 cm) | 0.006 | 1.471 | 1.117 | 1.937 | ||
| EHS classification | 0.007 | Lateral vs combined | 0.024 | 1.657 | 1.070 | 2.567 |
| Lateral vs medial | 0.002 | 1.546 | 1.166 | 2.051 | ||
| Combined vs medial | 0.724 | 0.933 | 0.636 | 1.370 | ||
| Mesh size [10-point OR] | 0.040 | 1.006 | 1.000 | 1.011 | ||
| WHO obesity classification | 0.052 | Obesity vs overweight | 0.117 | 1.220 | 0.951 | 1.565 |
| Obesity vs normal weight | 0.007 | 1.621 | 1.138 | 2.309 | ||
| Obesity vs underweight | 0.949 | |||||
| Overweight vs normal weight | 0.122 | 1.329 | 0.927 | 1.904 | ||
| Overweight vs underweight | 0.950 | |||||
| Normal weight vs underweight | 0.952 | |||||
| ASA score | 0.076 | III/IV vs II | 0.100 | 1.235 | 0.960 | 1.588 |
| III/IV vs I | 0.036 | 1.705 | 1.036 | 2.808 | ||
| II vs I | 0.168 | 1.381 | 0.872 | 2.185 | ||
| Fixation | 0.103 | Suture vs nonabsorbable tacks + suture | 0.524 | 0.797 | 0.396 | 1.604 |
| Suture vs absorbable tacks + suture | 0.251 | 1.401 | 0.788 | 2.492 | ||
| Suture vs nonabsorbable tacks | 0.759 | 1.121 | 0.539 | 2.332 | ||
| Suture vs absorbable tacks | 0.920 | 1.029 | 0.592 | 1.788 | ||
| Nonabsorbable tacks + suture vs absorbable tacks + suture | 0.028 | 1.759 | 1.064 | 2.906 | ||
| Nonabsorbable tacks + suture vs nonabsorbable tacks | 0.321 | 1.408 | 0.716 | 2.765 | ||
| Nonabsorbable tacks + suture vs absorbable tacks | 0.292 | 1.291 | 0.802 | 2.078 | ||
| Absorbable tacks + suture vs nonabsorbable tacks | 0.426 | 0.800 | 0.462 | 1.385 | ||
| Absorbable tacks + suture vs absorbable tacks | 0.020 | 0.734 | 0.566 | 0.952 | ||
| Non-absorbable tacks vs absorbable tacks | 0.746 | 0.917 | 0.544 | 1.547 | ||
| Age [10-yr OR] | 0.337 | 0.954 | 0.867 | 1.050 | ||
| Sex | 0.379 | Female vs male | 0.904 | 0.721 | 1.133 | |
| Defect closure | 0.508 | Yes vs no | 0.912 | 0.696 | 1.197 | |
| Risk factors | 0.664 | Yes vs no | 0.949 | 0.750 | 1.201 | |
ASA indicates American Society of Anesthesiologist; EHS, European Hernia Society; WHO, World Health Organization.
Pain on Exertion
The multivariable analysis results of pain on exertion on 1-year follow-up are shown in Table 4 (model fit: P < 0.001). Likewise, pain on exertion was primarily and negatively influenced by preoperative pain (preoperative pain yes vs no: OR 1.808, 95% CI 1.531, 2.133, P < 0.001). Female sex (female vs male: OR 1.557, 95% CI 1.345, 1.801, P < 0.001), lateral EHS classification (lateral vs medial: OR 1.587, 95% CI 1.319, 1.908, P < 0.001), the use of Physiomesh (Physiomesh vs other meshes: OR 1.194, 95% CI 1.022, 1.395, P = 0.026), mesh size (10-point: OR 1.004, 95% CI 1.000, 1.008, P = 0.037), and recurrent operation (yes vs no: OR 1.187, 95% CI 1.004, 1.402, P = 0.044) were also associated with a higher risk of pain on exertion. A higher age reduced the risk of pain on exertion (10-year: OR 0.787, 0.742, 0.835, P < 0.001).
TABLE 4.
Multivariable Analysis of Pain on Exertion on 1-year Follow-up
| Parameter | P | Category | P Paired | OR Estimate | 95% CI | |
| Age [10-yr OR] | <0.001 | 0.787 | 0.742 | 0.835 | ||
| Preoperative pain | <0.001 | Unknown vs yes | 0.542 | 1.077 | 0.848 | 1.367 |
| Unknown vs no | <0.001 | 1.947 | 1.499 | 2.528 | ||
| Yes vs no | <0.001 | 1.808 | 1.531 | 2.133 | ||
| Sex | <0.001 | Female vs male | 1.557 | 1.345 | 1.801 | |
| EHS classification | <0.001 | Lateral vs combined | 0.003 | 1.537 | 1.156 | 2.042 |
| Lateral vs medial | <0.001 | 1.587 | 1.319 | 1.908 | ||
| Combined vs medial | 0.799 | 1.033 | 0.807 | 1.322 | ||
| Mesh type | 0.026 | PhysioMesh vs other meshes | 1.194 | 1.022 | 1.395 | |
| Mesh size [10-point OR] | 0.037 | 1.004 | 1.000 | 1.008 | ||
| Recurrent operation | 0.044 | Yes vs no | 1.187 | 1.004 | 1.402 | |
| Fixation | 0.164 | Suture vs nonabsorbable tacks + suture | 0.466 | 0.845 | 0.537 | 1.329 |
| Suture vs absorbable tacks + suture | 0.549 | 1.113 | 0.785 | 1.578 | ||
| Suture vs nonabsorbable tacks | 0.199 | 1.361 | 0.850 | 2.177 | ||
| Suture vs absorbable tacks | 0.285 | 1.204 | 0.857 | 1.691 | ||
| Nonabsorbable tacks + suture vs absorbable tacks + suture | 0.111 | 1.317 | 0.939 | 1.847 | ||
| Nonabsorbable tacks + suture vs nonabsorbable tacks | 0.043 | 1.611 | 1.015 | 2.556 | ||
| Nonabsorbable tacks + suture vs absorbable tacks | 0.035 | 1.425 | 1.026 | 1.979 | ||
| Absorbable tacks + suture vs nonabsorbable tacks | 0.278 | 1.223 | 0.850 | 1.759 | ||
| Absorbable tacks + suture vs absorbable tacks | 0.327 | 1.082 | 0.924 | 1.266 | ||
| Nonabsorbable tacks vs absorbable tacks | 0.497 | 0.885 | 0.621 | 1.260 | ||
| Defect size | 0.442 | W3 (≥10 cm) vs W2 (≥4–10 cm) | 0.605 | 1.061 | 0.848 | 1.326 |
| W3 (≥10 cm) vs W1 (<4 cm) | 0.255 | 1.162 | 0.897 | 1.504 | ||
| W2 (≥4–10 cm) vs W1 (<4 cm) | 0.278 | 1.095 | 0.929 | 1.291 | ||
| ASA score | 0.538 | III/IV vs II | 0.677 | 1.036 | 0.877 | 1.224 |
| III/IV vs I | 0.270 | 1.170 | 0.885 | 1.547 | ||
| II vs I | 0.327 | 1.129 | 0.885 | 1.441 | ||
| WHO obesity classification | 0.800 | Obesity vs overweight | 0.688 | 1.034 | 0.879 | 1.216 |
| Obesity vs normal weight | 0.828 | 1.023 | 0.836 | 1.250 | ||
| Obesity vs underweight | 0.348 | 1.694 | 0.563 | 5.102 | ||
| Overweight vs normal weight | 0.918 | 0.989 | 0.805 | 1.215 | ||
| Overweight vs underweight | 0.381 | 1.639 | 0.543 | 4.952 | ||
| Normal weight vs underweight | 0.373 | 1.657 | 0.546 | 5.032 | ||
| Defect closure | 0.853 | Yes vs no | 0.984 | 0.831 | 1.165 | |
| Risk factors | 0.984 | Yes vs no | 1.002 | 0.861 | 1.164 | |
ASA indicates American Society of Anesthesiologist; EHS, European Hernia Society; WHO, World Health Organization.
Chronic Pain Requiring Treatment
The multivariable results of pain requiring treatment are illustrated in Table 5 (model fit: P < 0.001). Chronic pain requiring treatment was primarily influenced by female sex (female vs male: OR 1.706, 95% CI 1.374, 2.118, P < 0.001). Likewise, preoperative pain (yes vs no: OR 1.651, 95% CI 1.272, 2.067, P < 0.001), the use of Physiomesh (Physiomesh vs other meshes: OR 1.321, 95% CI 1.060, 1.648, P = 0.013), lateral vs medial EHS classification (lateral vs medial: OR 1.410, 95% CI 1.083, 1.837, P = 0.011), and the mesh size (10-point: OR 1.005, 95% CI 1.000, 1.011, P = 0.045) increased the risk of chronic pain requiring treatment. By contrast, a higher age reduced the risk of chronic pain requiring treatment (10-year: OR 0.818, 95% CI 0.751, 0.890, P < 0.001).
TABLE 5.
Multivariable Analysis of Pain Requiring Treatment on 1-year Follow-up
| Parameter | P | Category | P Paired | OR Estimate | 95% CI | |
| Sex | <0.001 | Female vs male | 1.706 | 1.374 | 2.118 | |
| Age [10-yr OR] | <0.001 | 0.818 | 0.751 | 0.890 | ||
| Preoperative pain | <0.001 | Unknown vs yes | 0.145 | 0.753 | 0.514 | 1.103 |
| Unknown vs no | 0.344 | 1.221 | 0.807 | 1.848 | ||
| Yes vs no | <0.001 | 1.621 | 1.272 | 2.067 | ||
| Mesh type | 0.013 | PhysioMesh vs other meshes | 1.321 | 1.060 | 1.648 | |
| EHS classification | 0.019 | Lateral vs combined | 0.022 | 1.651 | 1.076 | 2.533 |
| Lateral vs medial | 0.011 | 1.410 | 1.083 | 1.837 | ||
| Combined vs medial | 0.414 | 0.854 | 0.586 | 1.247 | ||
| Mesh size [10-point OR] | 0.045 | 1.005 | 1.000 | 1.011 | ||
| Fixation | 0.169 | Suture vs nonabsorbable tacks + suture | 0.650 | 0.867 | 0.468 | 1.606 |
| Suture vs absorbable tacks + suture | 0.175 | 1.399 | 0.862 | 2.273 | ||
| Suture vs nonabsorbable tacks | 0.196 | 1.566 | 0.794 | 3.088 | ||
| Suture vs absorbable tacks | 0.179 | 1.379 | 0.863 | 2.202 | ||
| Nonabsorbable tacks + suture vs absorbable tacks + suture | 0.043 | 1.614 | 1.016 | 2.565 | ||
| Nonabsorbable tacks + suture vs nonabsorbable tacks | 0.081 | 1.806 | 0.930 | 3.509 | ||
| Nonabsorbable tacks + suture vs absorbable tacks | 0.042 | 1.591 | 1.018 | 2.486 | ||
| Absorbable tacks + suture vs nonabsorbable tacks | 0.688 | 1.119 | 0.647 | 1.934 | ||
| Absorbable tacks + suture vs absorbable tacks | 0.901 | 0.985 | 0.781 | 1.243 | ||
| Nonabsorbable tacks vs absorbable tacks | 0.640 | 0.881 | 0.517 | 1.499 | ||
| ASA score | 0.285 | III/IV vs II | 0.330 | 1.127 | 0.886 | 1.432 |
| III/IV vs I | 0.118 | 1.397 | 0.919 | 2.124 | ||
| II vs I | 0.256 | 1.240 | 0.856 | 1.797 | ||
| Recurrent operation | 0.333 | Yes vs no | 1.127 | 0.885 | 1.435 | |
| Defect closure | 0.645 | Yes vs no | 1.059 | 0.830 | 1.351 | |
| WHO obesity classification | 0.709 | Obesity vs overweight | 0.319 | 1.130 | 0.889 | 1.436 |
| Obesity vs normal weight | 0.916 | 0.985 | 0.738 | 1.313 | ||
| Obesity vs underweight | 0.681 | 1.362 | 0.312 | 5.944 | ||
| Overweight vs normal weight | 0.371 | 0.872 | 0.645 | 1.177 | ||
| Overweight vs underweight | 0.804 | 1.206 | 0.275 | 5.296 | ||
| Normal weight vs underweight | 0.669 | 1.384 | 0.313 | 6.116 | ||
| Defect size | 0.711 | W3 (≥10 cm) vs W2 (≥4–10 cm) | 0.427 | 1.137 | 0.828 | 1.560 |
| W3 (≥10 cm) vs W1 (<4 cm) | 0.451 | 1.150 | 0.799 | 1.655 | ||
| W2 (≥4–10 cm) vs W1 (<4 cm) | 0.922 | 1.012 | 0.797 | 1.284 | ||
| Risk factors | 0.767 | Yes vs no | 0.967 | 0.777 | 1.204 | |
ASA indicates American Society of Anesthesiologist; EHS, European Hernia Society; WHO, World Health Organization.
DISCUSSION
Univariable and multivariable analyses of the Herniamed data showed that the mesh used in laparoscopic IPOM had no detectable impact on the intraoperative, postoperative surgical, and postoperative general complications, complication-related reoperations, and pain at rest, but did have an influence on recurrence, pain on exertion, and chronic pain requiring treatment.
Furthermore, multivariable analysis of the recurrence rate revealed that Physiomesh compared with the other meshes recommended in the guidelines did present a highly significantly higher risk of onset of a recurrence on 1-year follow-up, with a P value <0.001 and an OR of 2.570. Other variables revealed by multivariable analysis to have had a significant influence on the recurrence rate were recurrent operation, larger defect size, lateral EHS classification, obesity, and larger mesh size. Whereas there was evidence that the use of Physiomesh impacted the risk of pain on exertion and of chronic pain requiring treatment, with an OR of 1.194 and 1.321, respectively, this was lower than the influence exerted by preoperative pain, female sex, and lateral EHS classification.
The Laparoscopic Intraperitoneal Onlay Mesh Augmentation trial, sponsored by Ethicon, was designed to standardize surgical technique for laparoscopic IPOM, that is, mesh fixation with absorbable tacks in double crown technique and transfascial sutures at the edges of mesh, along with the use of Physiomesh alone in incisional hernia repair. Compliance with specified criteria by the participating study sites was strictly monitored.22 Using this standard operative procedure, the recurrence rate after 1 year in 85 enrolled patients, of whom 75 presented for 1-year follow-up examination, was 4.1% (95% CI 0.9–11.9).23
A single-arm observational study by the International Hernia Mesh Registry sponsored by Ethicon did not find any evidence of an increased recurrence rate for laparoscopic IPOM repair of ventral hernias with Physiomesh.24,25 The role of the sponsor must, however, be taken into account with regard to that finding since both studies23,25 were sponsored by Ethicon and this may have impacted the findings. A Cochrane review suggests the existence of an industry bias that cannot be explained by standard “risk of bias” assessment. Sponsorship of device studies by the manufacturing company merely leads to favorable efficacy results.26
One possible explanation for the significantly higher recurrence rate associated with Physiomesh could be its burst strength. Measurements of the burst strength of the nonabsorbable portion of the Physiomesh yielded the sufficient value of 696 mm Hg.27–29 To design the ideal mesh for intraperitoneal placement, the paradoxical requirements of tissue separation on the visceral surface and tissue integration on the parietal surface need to be addressed.30 Physiomesh is a composite mesh consisting of a macroporous, warp-knitted polypropylene sandwiched between 2 tissue-separating layers of a bioabsorbable coating (poliglecaprone 25). Polydioxanone is used as glue to keep all layers together.30 The poliglecaprone-25 layers are comprised of a copolymer of eta-caprolactone and glycolide which degrade through hydrolysis and are expected to be fully absorbed within approximately 240 days.31
In a preclinical porcine model, Deeken and Matthews32 found for Ventralight ST/SorbaFix more favorable strength of tissue ingrowth and histologic response, and similar mesh contracture and adhesion characteristics compared with Physiomesh/Securestrap over a short-term 14-day implantation period. Vogels et al33 also reported in an experimental study in rats after 90 days significantly lower incorporation strengths for Physiomesh compared with all other mesh groups. They hypothesized that the reason for this delay of tissue integration was the application of an anti-adhesive coating on both sides of the mesh.33
Another important risk factor for recurrence after Physiomesh implantation could be the significant loss of cranio-caudal mesh size up to 30% after 90 days.33
In yet another experimental study, seroma was often observed with Physiomesh, whereby seromas were found trapped between the 2 poliglecaprone films.34
In a preclinical study conducted by Ethicon using rabbits as experimental models, Physiomesh was found to be superior to other composite meshes in preventing adhesions.35 The tissue integration, migration, and contraction characteristics of Physiomesh were also evaluated in another preclinical porcine study.36 At 28, 56, and 91 days postimplantation, Physiomesh had adequate tissue fixation and excellent tissue integration.
In 2017, the Medical Device Regulation (MDR) will replace the EU's current Medical Device Directive. Under the MDR, surgical meshes will, in future, be classified as risk class III medical devices. This means that clinical data will have to be gathered and evaluated before new meshes are placed on the market. The most appropriate means of doing so is likely to be RCTs. Through patient selection on the basis of exclusion and inclusion criteria, the quality of a medical device can be evaluated relatively quickly in not too large patient groups. This has been demonstrated in the RCT by Pawlak et al.16 Furthermore, once new surgical meshes have been placed on the market, manufacturers must submit, on a yearly basis, clinical data on the safety of their products. Registries can play a crucial role during this postmarketing surveillance process for surgical meshes. Since in a registry all patients are enrolled, registries need a correspondingly larger sample size to determine through multivariable analyses how the various influence factors affect the outcome. Accordingly, registries need more time than randomized studies to generate reliable statements. The new regulations for placement of medical devices on the market will, no doubt, be welcomed by surgeons as they help to reinforce their confidence in the medical devices placed on the market. Fostering awareness of these inter-relationships should be an important component of surgical training.
The lack of follow-up for 3105 out of 8319 patients (37.3%), and hence their exclusion from analysis, represents the main limitation of the present study. In a best-case worse-case scenario, we assumed that the recurrence rate was halved for the patients with no follow-up in the Physiomesh group, that is, 6% instead of 12%, and that this was doubled for the non-Physiomesh group, that is, 10% instead of 5%. Even given that assumption, there was still a highly significant difference in the recurrence rate to the disadvantage of Physiomesh (9.9% vs 6.9%; P < 0.001). Apart from data completeness, another principal concern with registries is that of making inferences without regard to the quality of the data. The best safeguard is to match the data against another registry, if possible, and literature data.37 In the present case of Physiomesh, the data from the Herniamed and Danish Hernia Registries and a RCT showed comparable results and led to the voluntary recall by the manufacturer.
CONCLUSIONS
In summary, the present multivariable analysis of data from the Herniamed Registry in laparoscopic IPOM revealed a significantly higher recurrence and pain rate when using Physiomesh compared with the other composite meshes recommended in the guidelines. The different findings obtained from animal experimental and clinical studies suggest that the Physiomesh product characteristics, and also patient and procedure-related factors, may have had a potential effect. Since, to date, the reasons have not been fully ascertained, the manufacturer initiated voluntary recall of Physiomesh from the market because the Danish Hernia Database had also revealed similar clinical findings. The importance of real-world data (registry-based, population-based) for postmarketing surveillance of surgical meshes has been demonstrated in this registry-based study, with similar findings in the Danish Hernia Database. Together, this has led to the decision taken by the manufacturer of Physiomesh. The experiences gathered over the years since the placement of Physiomesh on the market and the amount of validated data now collected in registries for evaluation of the quality of this mesh demonstrate that registries tend to be more suitable for long-term evaluation of surgical meshes for all patients operated on with this medical device. This is due to the fact that no patient with other factors that could impact the outcome is excluded from registries. Therefore, large sample sizes are needed for multivariable analysis. The sample size can be smaller in prospective randomized trials since several potential influence factors can be controlled on the basis of inclusion and exclusion criteria. Therefore, based on the new marketing authorization procedures, before surgical meshes are first placed on the market in the future, it is likely that RCTs will be carried out, whereas registry studies will be used to collect clinical data for postmarketing surveillance of surgical meshes.
Footnotes
The authors report no conflicts of interest.
REFERENCES
- 1.Sauerland S, Walgenbach M, Habermalz B, et al. Laparoscopic versus open surgical techniques for ventral or incisional hernia repair. Cochrane Database Syst Rev 2011; CD007781. [DOI] [PubMed] [Google Scholar]
- 2.Zhang Y, Zhou H, Chai Y, et al. Laparoscopic versus open incisional and ventral hernia repair: a systematic review and meta-analysis. World J Surg 2014; 38:2233–2240. [DOI] [PubMed] [Google Scholar]
- 3.Al Chalabi H, Larkin J, Mehigan B, et al. A systematic review of laparoscopic versus open abdominal incisional hernia repair, with meta-analysis of randomized controlled trials. Int J Surg 2015; 20:65–74. [DOI] [PubMed] [Google Scholar]
- 4.Awaiz A, Rahman F, Hossain MB, et al. Meta-analysis and systematic review of laparoscopic versus open mesh repair for elective incisional hernia. Hernia 2015; 19:449–463. [DOI] [PubMed] [Google Scholar]
- 5.Jensen KK, Jorgensen LN. Comment to: Meta-analysis and systematic review of laparoscopic versus open mesh repair for elective incisional hernia [Awaiz A et al. Hernia 2015; 19: 449–463]. Hernia 2015; 19:1025–1026. [DOI] [PubMed] [Google Scholar]
- 6.Awaiz A, Rahman F, Hossain MB, et al. Reply to comment to Meta-analysis and systematic review of laparoscopic versus open mesh repair for elective incisional hernia. Jensen K, Jorgensen LN. Hernia 2015; 19:1027–1029. [DOI] [PubMed] [Google Scholar]
- 7.Earle D, Roth JS, Saber A, et al. SAGES guidelines for laparoscopic ventral hernia repair. Surg Endosc 2016; 30:3163–3183. [DOI] [PubMed] [Google Scholar]
- 8.Bittner R, Bingener-Casey J, Dietz U, et al. Guidelines for laparoscopic treatment of ventral and incisional abdominal wall hernias (International Endohernia Society [IEHS]): part 1. Surg Endosc 2014; 28:2–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bittner R, Bingener-Casey J, Dietz U, et al. Guidelines for laparoscopic treatment of ventral and incisional abdominal wall hernias International Endohernia Society [IEHS]): part 2. Surg Endosc 2014; 28:353–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bittner R, Bingener-Casey J, Dietz U, et al. Guidelines for laparoscopic treatment of ventral and incisional abdominal wall hernias (International Endohernia Society [IEHS]): part III. Surg Endosc 2014; 28:380–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Christoffersen MW, Brandt E, Helgstrand F, et al. Recurrence rate after absorbable tack fixation of mesh in laparoscopic incisional hernia repair. BJS 2015; 102:541–547. [DOI] [PubMed] [Google Scholar]
- 12.Tandon A, Pathak S, Lyons NJR, et al. Meta-analysis of closure of the fascial defect during laparoscopic incisional and ventral hernia repair. Br J Surg 2016; 103:1598–1607. [DOI] [PubMed] [Google Scholar]
- 13.LeBlanc K. Proper mesh overlap is a key determinant in hernia recurrence following laparoscopic ventral and incisional hernia repair. Hernia 2016; 20:85–99. [DOI] [PubMed] [Google Scholar]
- 14.Silecchia G, Campanile FC, Sanchez L, et al. Laparoscopic ventral/incisional hernia repair: updated guidelines from the EAES and EHS endorsed Consensus Development Conference. Surg Endosc 2015; 29:2463–2484. [DOI] [PubMed] [Google Scholar]
- 15.Moreno-Egea A, Carrillo-Alcaraz A, Soria-Aledo V. Randomized clinical trial of laparoscopic hernia repair comparing titanium-coated lightweight mesh and medium-weight composite mesh. Surg Endosc 2013; 27:231–239. [DOI] [PubMed] [Google Scholar]
- 16.Pawlak M, Hilgers RD, Bury K, et al. Comparison of two different concepts of mesh and fixation technique in laparoscopic ventral hernia repair: a randomized controlled trial. Surg Endosc 2016; 30:1188–1197. [DOI] [PubMed] [Google Scholar]
- 17.Voluntary Product Recall of Ethicon Physiomesh™ Flexible Composite Mesh. Available at: www.swissmedic.ch/recalllists_dl/13779/Vk_20160525_11_e1.pdf Accessed May 25, 2016. [Google Scholar]
- 18.Stechemesser B, Jacob DA, Schug-Paß C, et al. Herniamed: an Internet-based registry for outcome research in hernia surgery. Hernia 2012; 16:269–276. [DOI] [PubMed] [Google Scholar]
- 19.Baucom RB, Ousley J, Feurer ID, et al. Patient reported outcomes after incisional hernia repair: establishing the ventral hernia recurrence inventory. Am J Surg 2016; 212:81–88. [DOI] [PubMed] [Google Scholar]
- 20.WHO Technical Report Series 894. Obesity: Preventing and Managing the Global Epidemic. 2000; Geneva: World Health Organization, ISBN 92 4 120894 5. [PubMed] [Google Scholar]
- 21.Muysoms FE, Miserez M, Berrevoet F, et al. Classification of primary and incisional abdominal wall hernias. Hernia 2009; 13:407–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hellinger A, Wotzlaw F, Fackeldey V, et al. Development of an open prospective observational multicentre cohort study to determine the impact of standardization of laparoscopic intraoeritoneal onlay mesh repair (IPOM) for incisional hernia on clinical outcome and quality of life (LIPOM-Trial). Contemp Clin Trials Commun 2016; 4:118–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hellinger A, Wotzlaw F, Fackeldey V, et al. Results of LIPOM-Trial 2016 (data on file). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.International Hernia Mesh Registry (IHMR). Clinical Trials.gov Identifier: NCT00622583. Study ID Numbers: 200-06-007. https://clinicaltrials.gov/ct2/show/NCT00622583. [Google Scholar]
- 25.Bradley JF, Williams KB, Wormer BA, et al. Preliminary results of surgical and quality of life outcomes of Physiomesh in an international, prospective study. Surg Technol Int 2012; 22:113–119. [PubMed] [Google Scholar]
- 26.Lundh A, Lexchin J, Mintzes B, et al. Industry sponsorship and research outcome. Cochrane Database Syst Rev 2017; 2:MR000023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cobb WS, Burns JM, Kercher KW, et al. Normal intra-abdominal pressure in healthy adults. J Surg Res 2005; 129:231–235. [DOI] [PubMed] [Google Scholar]
- 28.Iqbal A, Haider M, Stadlhuber RJ, et al. A study of intragastric and intravesicular pressure changes during rest, coughing, weight lifting, retching, and vomiting. Surg Endosc 2008; 22:2571–2575. [DOI] [PubMed] [Google Scholar]
- 29. Ethicon Inc. Design Comparison of Physiomesh to leading Competitors: Burst Strength (mmHg) Parietex vs Symbotex vs Physiomesh (data on file). [Google Scholar]
- 30.Tollens T, Maxime E, Anthony B, et al. Retrospective study on the use of a composite mesh [Physiomesh®] in laparoscopic ventral hernia repair. Surg Technol Int 2012; 22:141–145. [PubMed] [Google Scholar]
- 31.Deeken CR, Faucher KM, Matthews BD. A review of the composition, characteristics, and effectiveness of barrier mesh prostheses utilized for laparoscopic ventral hernia repair. Surg Endosc 2012; 26:566–575. [DOI] [PubMed] [Google Scholar]
- 32.Deeken CR, Matthews BD. Ventralight ST and SorbaFix versus Physiomesh and Securestrap in a porcine model. JSLS 2013; 17:549–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vogels RRM, van Barneveld KWY, Bosmans JWAM, et al. Long-term evaluation of adhesion formation and foreign boy response to three new meshes. Surg Endosc 2015; 29:2251–2259. [DOI] [PubMed] [Google Scholar]
- 34.Pascual G, Sotomayor S, Rodriguez M, et al. Tissue integration and inflammatory reaction in full-thickness abdominal wall repair using an innovative composite mesh. Hernia 2016; 20:607–622. [DOI] [PubMed] [Google Scholar]
- 35. Ethicon Inc. Holste JL, Muench T, Shnoda P, et al. An evaluation of Ethicon Physiomesh™ Flexible composite mesh in the prevention of adhesions in a rabbit model of abdominal hernia repair: a comparative study. PHYSM-335-10 (data on file). [Google Scholar]
- 36. Ethicon Inc. Holste JL, Muench T, Shnoda P, et al. A Preclinical evaluation of the tissue separation and abdominal wall integration properties of Ethicon Physiomesh™ Flexible Composite Mesh. PHYSM 336-10-8/12 (data on file). [Google Scholar]
- 37.Hannan EL, Cozzens K, King SB, et al. The New York State Cardiac Registries: history, contributions, limitations, and lessons for future efforts to assess and publicly report healthcare outcomes. JACC 2012; 59:2309–2316. [DOI] [PubMed] [Google Scholar]


