While anti-cancer responses in host immunity have been identified, this baseline immune surveillance has been inadequate to prevent cancer in 1.5 million individuals in the U.S. each year and many fold more around the globe. The immune modulations caused by cytotoxic chemotherapies are varied and understanding the resulting interactions of the host against cancer will be critical for applying additional immunotherapies to patients. In the case of pediatric cancers, any approach that has the potential for reducing exposure to cytotoxic chemotherapy or radiation during childhood development is worth investigating.
Immune responses are divided into innate and adaptive immunity. Innate immunity provides a general response that physically targets microorganisms in order to remove them from the host. Adaptive immunity utilizes a complex set of interactions that allows the immune system to selectively target affected cells by examining them for disease. In the case of cancer, both types of immunity have roles to play but the cytolytic activity of the adaptive response may have the most potential for manipulation. Once an antigen presenting cell (APC) is primed, effector cells are expanded, which then seek out and destroy cells bearing that identifying fragment. Because cancers arise from host tissue, identification of tumor antigens can be difficult and as a result, harnessing the adaptive immune response can be challenging.
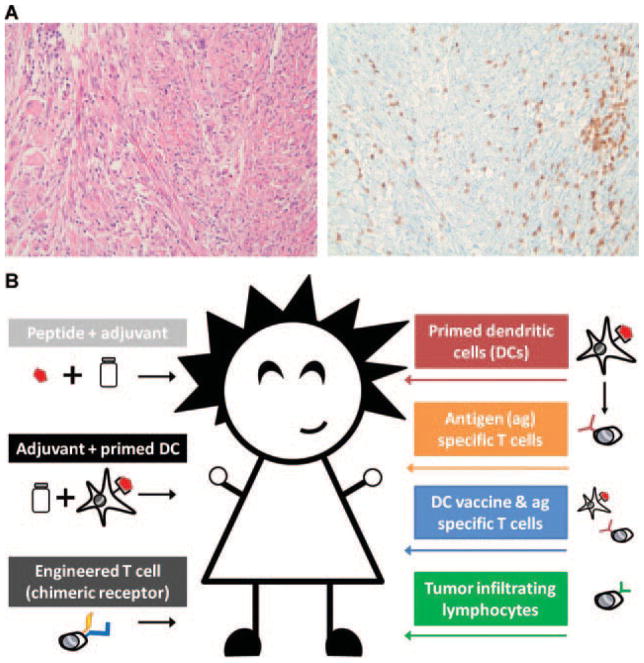
Conventional wisdom has held that chemotherapy causes immune suppression, which leaves patients not only with diminished capacity to defend against pathogens but also without natural anti-tumor responses that might alter the course of disease in their favor. Additionally, negative regulation from T regulatory cells (Tregs) have been shown to reduce the natural immune response against cancer [1], despite the high number of T cells that can be found in tumors (Fig. 1A); Meadors et al. demonstrate this phenomena to occur in their preclinical RMS model in that mice partially depleted of Tregs have improved anti-tumor response to vaccination. The implication is that a clinical reduction in Treg number can aid the patient in mounting a response. Ultimately, chemotherapy agents have many known and yet to be identified differential direct effects on subsets of cells and impact host responses based on how tumors are destroyed and antigens are released for recognition and presentation. Despite the immunosuppressive effects of cytotoxic chemotherapy, however, it is worth noting that cancer vaccines are seeing increasingly positive results in some solid tumors such as prostate cancer even if patients had received limited prior chemotherapy [2], although this success has not been seen in clinical trials for chemotherapy-treated rhabdomyosarcoma (RMS) patients [3].
Fig. 1.
Considerations for rhabdomyosarcoma immunotherapy. A: Histology (left) and immunohistochemistry using the pan-T cell marker CD3 (right) of human embryonal rhabdomyosarcoma demonstrating widespread, naturally infiltrating T lymphocytes prior to any form of treatment. B: Common approaches to immunotherapy. [Color figure can be seen in the online version of this article, available at http://wileyonlinelibrary.com/journal/pbc]
Approaches to tumor targeting using host immune responses have taken many forms (Fig. 1B). Tumor antigens can be administered along with an adjuvant in order to stimulate host APCs into expanding tumor specific T cells endogenously. Alternatively, APCs can be loaded exogenously and then transferred into patients or T cells can be expanded using artificial APCs or with a bead stimulation system. Autologous T cells engineered to express a chimeric antigen receptor are an approach that has shown promise in neuroblastoma [4] [ClinicalTrials.gov Identifier NCT00085930]. One particularly interesting approach is the expansion of tumor infiltrating lymphocytes for subsequent transfer into patients [5]. A precedent has also been set for repurposing banked antigen-specific T cells which were expanded in vivo and then used for multiple human recipients [6]. Meadors et al. pursue an autologous adoptive transfer approach of isolating and priming APCs, expanding T cells in vitro and then reintroducing APCs and tumor specific T cells into mice. Based on the results seen in their model of RMS, a combination of expanded tumor specific T cells and a primed dendritic cell vaccine are most able to slow tumor growth.
The outcome for metastatic childhood RMS has been virtually unchanged for decades. Alveolar and embryonal subtypes represent the majority of pediatric RMS cases. However, as was highlighted in our recent Cancer Cell publication, the variability of tumor biology (i.e., cell of origin with respect to mutation or mutations found) within a given subtype are complex, especially for embryonal RMS [7]. The inherent challenge of any preclinical model of RMS is to identify the human patient subtype to which the results might apply. Credentialing the preclinical model at the histological, immunohistochemical, and molecular level are key. Meadors et al. have employed a transgenic HGF+ p53+/− tumor [8] orthotopically injected into syngeneic C57BL/6 mice. The histology of these tumors has previously been reported to be consistent with embryonal RMS [8], but by the authors’ metagene projection analysis these tumors classify intermediately between alveolar RMS and embryonal RMS. HGF is the ligand for the MET receptor tyrosine kinase whose expression is generally elevated in alveolar RMS more so than embryonal RMS [9], perhaps not surprisingly since MET is a transcriptional target of PAX3:FKHR [10]. This HGF-driven preclinical RMS model is therefore very interesting but its applicability to subsets of specific patients is subject to additional characterization.
A number of tumor specific antigens have been identified and are being investigated. For RMS, the only consistent surface antigen demonstrated is fetal acetylcholine receptor, AChRγ [11]. In one in vitro study of chimeric T cells, however, AChRγ specific T cells are not able to target RMS cells indicating that multiple avenues of modification may need to be addressed at once in order to generate a functional anti-tumor response [12]. Hope for sarcomas in general remains, however, as sarcoma specific antigens categorized as cancer testis antigens (CTAs) have been identified as having immunogenic potential. Use of CTAs in patients with synovial sarcoma, liposarcoma, leiomyosarcoma, and skeletal sarcomas (osteosarcoma) have results ranging from mixed responses and stabilization of disease to complete responses with long duration [13].
As more RMS specific antigens are discovered, it becomes more likely that adoptive cell transfer in any of the aforementioned forms will be a viable option for treating patients. A clinical study similar to work presented by Meadors et al. and being conducted by the same principal investigator is currently recruiting patients as a Phase I/II study to examine the ability to use patient tumor to prime cells for embryonal or alveolar RMS treatment [ClinicalTrial.gov identifier NCT00923351]. The results that are presented by Meadors et al. in this edition of Pediatric Blood & Cancer lend enthusiasm to this clinical trial with the caveat that correlating the preclinical model to human RMS subtypes would have better determined the applicability of these findings to specific patient cases.
The prospect of using host responses to generate long-term successful treatment of solid tumors is promising. Harnessing the immune systems of pediatric cancer patients to prevent relapse and metastasis has the potential for reducing dependence on cytotoxic chemotherapy and/or radiation that can be harmful to development and which affect the quality of life for patients in the short and long term. The challenges that face adoptive transfer as a treatment are numerous, but evidence from preclinical studies and select clinical trials indicates that enhancing the immune system to eradicate residual tumor cells following surgery can be a reality.
Acknowledgments
Author Charles Keller is funded by NIH/NCI grant number 5R01CA133229-04.
References
- 1.Thistlethwaite FC, Elkord E, Griffiths RW, et al. Adoptive transfer of T(reg) depleted autologous T cells in advanced renal cell carcinoma. Cancer Immunol Immunother. 2008;57:623–634. doi: 10.1007/s00262-007-0400-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kantoff PW, Higano CS, Shore ND, et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med. 2010;363:411–422. doi: 10.1056/NEJMoa1001294. [DOI] [PubMed] [Google Scholar]
- 3.Dagher R, Long LM, Read EJ, et al. Pilot trial of tumor-specific peptide vaccination and continuous infusion interleukin-2 in patients with recurrent Ewing sarcoma and alveolar rhabdomyosarcoma: An inter-institute NIH study. Med Pediatr Oncol. 2002;38:158–164. doi: 10.1002/mpo.1303. [DOI] [PubMed] [Google Scholar]
- 4.Pule MA, Savoldo B, Myers GD, et al. Virus-specific T cells engineered to coexpress tumor-specific receptors: Persistence and antitumor activity in individuals with neuroblastoma. Nat Med. 2008;14:1264–1270. doi: 10.1038/nm.1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.June CH. Adoptive T cell therapy for cancer in the clinic. J Clin Invest. 2007;117:1466–1476. doi: 10.1172/JCI32446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barker JN, Doubrovina E, Sauter C, et al. Successful treatment of EBV-associated posttransplantation lymphoma after cord blood transplantation using third-party EBV-specific cytotoxic T lymphocytes. Blood. 2010;116:5045–5049. doi: 10.1182/blood-2010-04-281873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rubin BP, Nishijo K, Chen HI, et al. Evidence for an unanticipated relationship between undifferentiated pleomorphic sarcoma and embryonal rhabdomyosarcoma. Cancer Cell. 2011;19:177–191. doi: 10.1016/j.ccr.2010.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sharp R, Recio JA, Jhappan C, et al. Synergism between INK4a/ARF inactivation and aberrant HGF/SF signaling in rhabdomyosarcomagenesis. Nat Med. 2002;8:1276–1280. doi: 10.1038/nm787. [DOI] [PubMed] [Google Scholar]
- 9.Chen Y, Takita J, Mizuguchi M, et al. Mutation and expression analyses of the MET and CDKN2A genes in rhabdomyosarcoma with emphasis on MET overexpression. Genes Chromosomes Cancer. 2007;46:348–358. doi: 10.1002/gcc.20416. [DOI] [PubMed] [Google Scholar]
- 10.Relaix F, Polimeni M, Rocancourt D, et al. The transcriptional activator PAX3-FKHR rescues the defects of Pax3 mutant mice but induces a myogenic gain-of-function phenotype with ligand-independent activation of Met signaling in vivo. Genes Dev. 2003;17:2950–2965. doi: 10.1101/gad.281203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gattenloehner S, Vincent A, Leuschner I, et al. The fetal form of the acetylcholine receptor distinguishes rhabdomyosarcomas from other childhood tumors. Am J Pathol. 1998;152:437–444. [PMC free article] [PubMed] [Google Scholar]
- 12.Simon-Keller K, Paschen A, Eichmuller S, et al. Adoptive T-cell therapy of rhabdomyosarcoma. Pathologe. 2010;31:215–220. doi: 10.1007/s00292-010-1344-8. [DOI] [PubMed] [Google Scholar]
- 13.Pollack SM, Loggers ET, Rodler ET, et al. Immune-based therapies for sarcoma. Sarcoma. 2011;2011:438940. doi: 10.1155/2011/438940. [DOI] [PMC free article] [PubMed] [Google Scholar]