Abstract
A system characterized by redundancy has various elements that are able to act in the same biological or dynamic manner, where the inhibition of one of those elements has no significant effect on the global biological outcome or on the system’s dynamic behavior. Methods that aim to predict the effectiveness of cancer therapies must include evolutionary and dynamic features that would change the static view that is widely accepted. Here, we explore several important issues about mechanisms of redundancy, heterogeneity, biological importance and drug resistance and describe methodological challenges that, if overcome, would significantly contribute to cancer research.
Keywords: systems biology, cancer dynamics, redundancy, drug resistance, therapy
Introduction
For more than a decade, researchers have accepted the fact that studying the various components of a system alone is not sufficient to understand the complexity involved. A more holistic system perspective has now been widely adopted to the biology and cancer research (1–4). Computational approaches that span the fields of mathematics, statistics, computer science, and theoretical physics, offer models to describe or to predict unknown features of a system, either as data- or theory-driven methods (5–8). According to George E. P. Box, a British mathematician and professor of statistics at the University of Wisconsin, “All models are wrong, but some are useful”(9). This assessment is especially relevant in cancer studies, due to the dynamic behavior of such a quickly evolving and adapting system. To develop a useful model that optimizes predictions concerning cancer treatments, an integrated approach that includes the strengths and conclusions from various relevant disciplines should be considered.
Choosing drugs for cancer therapy is a challenging task. Several targeted drugs are currently available or have been tested in clinical trials. Some, target a specific altered gene, such as everolimus, which targets mammalian target of rapamycin (mTOR). While others target several genes and pathways, such as sorafenib that targets BRAF, FLT3, PDGFRB tyrosine kinase, VEGFR2 tyrosine kinase, VEGFR3 tyrosine kinase, and c-KIT tyrosine kinase. However, many cancer drugs fail or underperform due to the evolving redundancy mechanisms related to their targeted genes or pathways (10, 11). In addition, efficiently targeting pathways is problematic, because it is unclear whether it is better to identify pathway targets by level of expression or by their location in the pathway (e.g., upstream elements). One of the major obstacles to improving cancer therapy is the cell ability to compensate a targeted gene/pathway. There are many examples indicating that redundancy serves as a resistance mechanism with clinical implications. For instance, several different ABC transporters can confer resistance to the same drugs, so inhibitors, such as cyclosporin, must target all of these transporters to be effective in reversing transporter-related multidrug resistance (12). Another example of treatment is traditional chemotherapy with broad-spectrum cytotoxic drugs. Those cytotoxic drugs aim to increase the apoptosis rate through accumulation of damage to DNA and other cell structures such as membranes and microtubules. Although in general chemotherapy has the most positive results as a therapy for disseminated cancer, it increases the tumor-cell rates of genetic and epigenetic alterations, and can be predicted to increase the heterogeneity and evolution of resistance in the remaining cells (13). A third type of promising therapy is immunotherapy, which in many cases is used to treat advanced cancers with high alteration rates. For certain cases this type of treatment should be the first treatment and not the last {Snyder, 2014 #86}. Developing treatment protocols that incorporate these three main treatment approaches to capture the network structure with its random and/or acquired dynamic changes would be valuable tools. Here, we explore several underlying problems and suggest approaches to overcome those obstacles.
The underlying Problems
A Robust Cellular System
The cellular system has an amazing capacity to survive and adapt to numerous extreme conditions, even in its normal state. There are many examples of robust cellular behaviors involving reverse or irreversible changes induced by different signals (such as temperature shocks, and amino acid starvation (14), or by gene knockouts (15–17)). However, one of the most impressive complex behaviors of a cell can be seen in cancer, where tumor cells survive through different resistance mechanisms given diverse treatments (18). In general, there are two key homeostasis mechanisms by which even normal cells can robustly regulate a perturbation or a stress signal: redundancy and a specific dynamic structure that is constructed by feedback loops. Understanding these two systematic mechanisms is a critical step in cancer study due to their fundamental functions as the central system regulators. Both mechanisms are common to serve the purpose of homeostasis mechanisms, however the redundancy (and compensation), in its global definition, is a more systematic way to overcome perturbation problems, and thus will be the subject of this perspective.
Redundancy as an Evolutionary Mechanism of Robustness
The cellular system is a spatiotemporal nonlinear dynamic system that has an underlying architecture guided by universal principles. One such principle is that this system includes redundant elements that can compensate for one another. Redundancy has been widely addressed in cellular research (19–25). In its global description, redundancy describes a scenario where different elements may potentially act in the same biological or dynamic manner, where the inhibition of one of these elements has no significant effect on the global biological outcome or on the system dynamic behavior. Elements may refer to the fundamental variables or subsystems that construct the system. The elements are redundant with respect to a given function, and not necessarily concerning all of their activities. The redundant elements can compensate for one another either because they functionally active simultaneously or act as a backup mechanism that is activated only given a stress signal. In order to maintain a regulated cellular system that functions consistently despite routine noisy signals, the cellular system must intrinsically include redundancy mechanisms at many levels. It is clear that not all redundant elements act in the same exact way, but they may compensate for one another to some extent, even at the cost of inaccuracy concerning timing or concentration of their targets. However, to maintain its controlled growth in its normal setting, the cellular system also includes key elements that can stop the cell cycle or in certain extreme cases even initiate death, depending on the stress intensity.
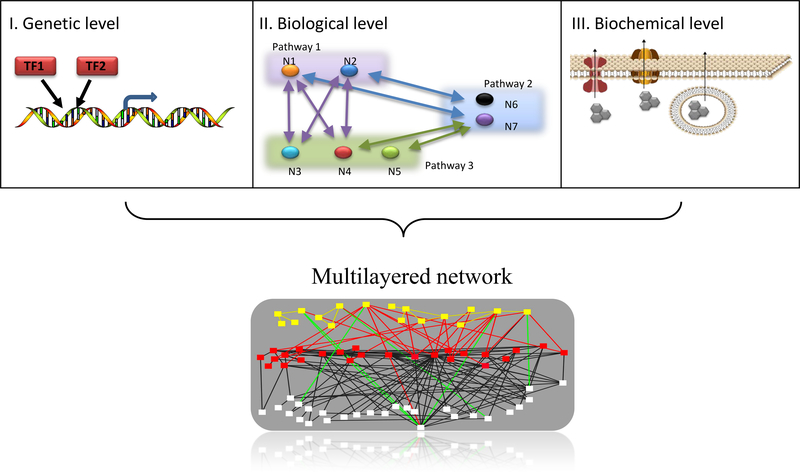
There are many examples of redundancy in biology such as in developmental biology (25, 26) and immunology (21). Redundancy occurs naturally at many cellular levels (Figure 1). The most studied level is the genetic level (22, 23). One example is when two or more transcription factors (TFs) share targeted genes, such as E2F1–3. The E2F family regulates a wide range of cellular processes, including DNA replication, mitosis, the function of DNA damage checkpoints, DNA repair, differentiation and autophagy (27). It was shown that only the combined loss of the E2F1–3 TFs eliminates the ability of mouse embryonic fibroblasts to enter S phase (28). A second well-known level concerns biological function, where different elements participate in the same pathway, with shared effects on the pathway. Experiments in a mouse germ line that perturbed all cyclins and cyclin-dependent kinases (Cdks) that govern the G1 phase of the cell cycle have revealed that much of fetal development occurs normally in their absence. Thus, most of these elements can be compensated for by others, such as when Cdk2 compensates for Cdk4, 6 and 1, due to their redundant functions in cell-cycle feedback loops (29). A third level of redundancy concerns biochemical function, where at least two elements share a common biochemical feature. For instance, the multidrug transporters ABCB1, ABCC1, and ABCG2 share many substrates (30). Even elements from different genetic families may share a biochemical feature, such as ABCG2 and RLIP76 (31).
Figure 1. Mechanisms of redundancy.
Redundancy, in general, describes a scenario in which different elements may potentially act in the same biological or dynamic manner, where the inhibition of one of these elements has no significant effect on the global biological outcome or on the system’s dynamic behavior. The redundant elements may refer to the fundamental variables, paths or subsystems that construct the system. Redundancy in biology is a problem known and considered for many years; however, it is essentially unsolved. Unfortunately, this familiar problem is still at the core of many cancer-related open questions that must be addressed. Redundancy occurs at many levels, such as at the genetic level (e.g., two TFs bind to a shared promoter), at the level of biochemical function (e.g., crosstalk among pathways composed of several redundant interactions and variables), and at the level of biological function (e.g. three different ways to efflux a drug from a cell). These different levels have not previously been well-enough defined to be addressed, mainly due to the lack of data and an inadequate understanding of how cancer cells compensate in each of these levels. Today, with advances in technology that enable the collection of different types of high-throughput data sets from a patient, and with new computational methods that have been designed to integrate ‘big-data’ sets into a meaningful representation of a patient’s information, we have a good opportunity to make a renewed effort to find creative ways to solve this problem.
Cancer and Drug Resistance
Cancer cells are characterized by numerous alterations related to processes critical to their survival. These alterations deregulate the cell-cycle checkpoints, growth, and apoptosis. There are many mechanisms of drug resistance, where a cell avoids or overcomes a drug. Among them are decreased drug uptake, increased drug efflux, activation of detoxifying systems, activation of DNA repair mechanisms, evasion of drug-induced apoptosis, etc (18). Moreover, the heterogeneity of cancer cells due to biological redundancy mechanisms assures that many perturbed cells already have acquired resistance before treatment. Treatments of advanced disease are largely ineffective, mainly due to the lack of understanding of mechanisms responsible for the development of the cancer and the basis of therapy-resistance mechanisms. Having a system that can robustly function to promote survival despite the existence of many redundant elements, with a dysfunctional cell-cycle regulation mechanism, makes the development of multidrug resistance in a cancer cell a very likely event.
New Methodological Directions for Finding Better Solutions
Given a mathematical description, one can reveal the therapy-related redundancy mechanisms, and uncover ways to overcome those obstacles. Here, we explore several important issues, presenting open methodological questions and challenges that, if answered, would contribute to cellular studies, and specifically to cancer research.
Emphasizing Redundancy Mechanisms in Modeling
1. How to systematically find and characterize a redundant element?
In general, there are several computational and mathematical approaches to directly/indirectly address redundancy mechanisms, given a specific dataset type, where each emphasizes different aspects of the problem. Using those methods, one can determine the redundancy level of an element, and find the types or circumstances of biological elements that are more likely to be redundant or that could develop redundancy. Moreover, natural cell-to-cell variations fluctuate among cells, in many cases due to the regulatory mechanisms affecting the redundant elements. Using these methods one can reveal the different types of regulation mechanisms that limit these cellular differences in a population of cells. Here, there are mentioned three types of analysis and propose to expand the study of redundancy mechanisms using these methods: 1) Networks and graph theory help to describe relationships between every pair of components, where the components could be genes, proteins, metabolites, etc. The relationships could include physical interactions, correlations, mutual information, etc. The network could be as complex as we need it to be, e.g., a weighted multilayered network that describes the flow of information between the different layers. Redundancy in a network, for instance, can be expressed by the redundant paths that start at one node and end at another, by the redundant nodes in a process that are part of several layers in a multilayered network, by redundant feedback loops or even modules (32). The main strength in this description is the ability to study the global and local properties of a network, to reveal the redundant components, and to study their impact on those network properties. 2) Any spatiotemporal process can also be described by dynamic system theory, e.g., by a system of differential equations, where its elements change as a function of time and space. The dynamics of a very complex system, such as the cell cycle, can include many redundant feedback loops or even redundant dynamic sub-systems that act on the same variable (33). The major strength in such a modeling approach is to define the global dynamic structure of the system based on its dynamic sub-systems, and to understand the dynamic significance of each redundant component. A component could be a sub-system, not just a specific variable, depending on the level of simplicity. 3) Redundancy is also hidden in multiple data sources. There are many computational methods (mainly, machine learning techniques) that reformulate the system high dimensionality such that all of its elements can be mathematically described by fewer essential elements. For instance, a commonly used linear method is principal component analysis.
Current advances in whole-genome sequencing and other “omics” technologies, along with the developments of many new computational and statistical integration methods, have resulted in some success in translating cancer “omics” data into therapeutics and diagnostics (34–36). Clearly, the integration of different data types that incorporate redundancy mechanisms should be included in the design of new experimental, computational, and mathematical models in order to have a better description of the various systems that contribute to the cancer evolution, progress and survival.
2. How can one predict an effective combination therapy in an evolving system?
An important concept in combination therapy is drug synergy (39–41). One of the most common definitions of drug synergy is that the effects of several drugs in combination are greater than those of a single drug. Although in general, synergy is difficult to predict, predicting synergy in drug-resistant cells adds another level of complexity to cancer modeling. In drug-resistant cells, synergy is not only a function of the connectivity in known static cellular networks, but also includes the evolution of new crosstalk that forms an evolving resistance network based on the alteration rate of those cells. The robustness characteristic of such a network could be based on the degree of redundancy. Redundant elements compensate for mutated or targeted genes and create new and unexpected pathway crosstalk that gives a cancer cell the ability to overcome the effects of treatment. Given the robust survival mechanisms of a cell, and the known mechanisms of multidrug resistance, what would be a better approach to predict an efficient combination therapy? Most system-level studies on this issue have focused on developing methods to maximize the damage in the cancer-cell network, mainly by studying the network topology and dynamics, neglecting the impact of evolving redundancy and its relation to cancer progression and resistance development (42, 43). Therapeutic strategies that can be applied to minimize the effectiveness of evolving redundancy mechanisms should yield better survival outcomes.
In addition, intratumoral heterogeneity increases the complexity of the cancer problem. Intratumoral heterogeneity refers to genetic and non-genetic differences between cancer cells originating within the same tumor. In many cases, cells with different alterations can achieve the same drug sensitivities and cellular rates. Thus, an open question is how to reduce cell-to-cell variations in a way that includes information about alterations that have a direct impact on the resistance level and survival of a cell. This type of study can include the effects of the development of redundancy mechanisms, and deepen our understanding by studying the roles of different mutations/epimutations in the evolving redundancy mechanisms. Methods that aim to predict the effectiveness of combination therapies must include some evolutionary and dynamic features that would change the static view that we currently hold.
3. Can functional importance of an element be correlated to redundancy level?
Interesting evolutionary theories related to redundancy mechanisms have been proposed. For example, genetic redundancy, on the one hand, might be considered as suppressing evolvability, but on the other hand, it also promotes adaptation by allowing duplicated genes to evolve distinct functions (37). If an element is known to be redundant, does that imply biological importance? Studies of E. coli and yeast reveal opposite relationships between the functional importance and redundancy level of a reaction in a metabolic network, concluding that redundant reactions in their network are probably not kept as backups (20, 38). A study of the plasticity of genetic interactions in metabolic networks of yeast also supported this view, and concluded that functional redundancy offers a unified framework for the evolution of environmental adaptation and mutational robustness (24). All of these studies were performed only on metabolic networks, with specific biological and computational assumptions and limitations, where the ‘importance’ was mainly reflected by the level of the network connectivity. As mentioned above, there are different system descriptions that possible to model a cellular process (e.g., dynamical system), to define redundancy level (e.g., genetic, biological function, or biochemical function), and to estimate the importance of an element in that system. Specifically concerning cancer cells, the functional importance of a protein could also be defined by its different contributions to the network structure and to the system dynamics in a cancer cell at different levels of resistance, or in a cell at different stages of cancer progression. Thus, an open question remains, if ‘importance’ refers to the element’s contribution to cancer progression or evolution, can functional importance of an element be correlated to redundancy level?
Conclusions
One of the greatest challenges in studying the biology of cancer and in formulating an effective treatment for cancer is not just acknowledging the disease heterogeneity, but also evaluating and predicting its rapid changes to different heterogeneous distributions over time. The cancer research community has already taken initial steps towards interdisciplinary research, yet the remaining gaps between the disciplines call for a revolutionary re-thinking of the approach. Several computational approaches can be applied to describe and to quantify biological regulations, heterogeneity, redundancy, drug resistance, and functional importance. It would be useful to define and study each of these terms based on their contribution to the network structure, in addition to the system dynamics that includes the cellular rates. Moreover, treatment protocols that schedule and optimize different treatment approaches (e.g., targeted drugs, chemotherapy, immunotherapy) as a holistic personalized approach based on the evolving network structure with its random and/or acquired dynamic changes, would potentially lead to better responses in the clinic.
Highlights.
Methods that aim to predict the effectiveness of cancer therapies must include evolutionary and dynamic features that would change the static view that is widely accepted.
Redundancy should be viewed as one of the most important multidrug resistance mechanisms in cancer, as it is one of the intrinsic building blocks of cellular architecture.
Defining the heterogeneity, redundancy, drug resistance, and functional importance based on their contribution to the evolving network structure, in addition to the system dynamics would result in focusing on the important cell-to-cell variations.
Acknowledgments
I would like to thank Drs. Michael M. Gottesman (LCB, NCI, NIH) and Doron Levy (Mathematics, University of Maryland), for their critical comments, helpful guidance, and tremendous support. Also, I would like to thank George Leiman for editorial assistance. This work was supported by the Intramural Research Program of the National Institutes of Health, the National Cancer Institute.
Footnotes
The author declares no conflicts of interest.
References
- 1.Alon U An Introduction to Systems Biology: Design Principles of Biological Circuits London, UK: CRC Press; 2006. [Google Scholar]
- 2.Hastie T, Tibshirani R, Friedman J. The Elements of Statistical Learning: Data Mining, Inference, and Prediction, Second Edition New York: Springer Science; 2009. [Google Scholar]
- 3.Murray JD. Mathematical Biology: I An Introduction (Interdisciplinary Applied Mathematics) (Pt. 1) Berlin: Springer-Verlag; 2002. [Google Scholar]
- 4.Wang EE. Cancer Systems Biology. Boca Raton, FL: CRC Press; 2010. [Google Scholar]
- 5.Ding L, Wendl MC, McMichael JF, Raphael BJ. Expanding the computational toolbox for mining cancer genomes. Nat Rev Genet. 2014;15:556–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lavi O, Gottesman MM, Levy D. The dynamics of drug resistance: a mathematical perspective. Drug Resist Updat. 2012;15:90–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pe’er D, Hacohen N. Principles and strategies for developing network models in cancer. Cell. 2011;144:864–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Du W, Elemento O. Cancer systems biology: embracing complexity to develop better anticancer therapeutic strategies. Oncogene. 2014. [DOI] [PubMed] [Google Scholar]
- 9.Box GEP, Draper NR. Empirical Model-Building and Response Surfaces. New York: John Wiley & Sons.; 1987. [Google Scholar]
- 10.Jia J, Zhu F, Ma X, Cao Z, Li Y, Chen YZ. Mechanisms of drug combinations: interaction and network perspectives. Nat Rev Drug Discov. 2009;8:111–28. [DOI] [PubMed] [Google Scholar]
- 11.Smalley KS, Haass NK, Brafford PA, Lioni M, Flaherty KT, Herlyn M. Multiple signaling pathways must be targeted to overcome drug resistance in cell lines derived from melanoma metastases. Mol Cancer Ther. 2006;5:1136–44. [DOI] [PubMed] [Google Scholar]
- 12.Qadir M, O’Loughlin KL, Fricke SM, Williamson NA, Greco WR, Minderman H, et al. Cyclosporin A is a broad-spectrum multidrug resistance modulator. Clin Cancer Res. 2005;11:2320–6. [DOI] [PubMed] [Google Scholar]
- 13.Lavi O, Greene JM, Levy D, Gottesman MM. Simplifying the complexity of resistance heterogeneity in metastasis. Trends Mol Med. 2014;20:129–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gasch AP, Spellman PT, Kao CM, Carmel-Harel O, Eisen MB, Storz G, et al. Genomic expression programs in the response of yeast cells to environmental changes. Mol Biol Cell. 2000;11:4241–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ideker T, Thorsson V, Ranish JA, Christmas R, Buhler J, Eng JK, et al. Integrated genomic and proteomic analyses of a systematically perturbed metabolic network. Science. 2001;292:929–34. [DOI] [PubMed] [Google Scholar]
- 16.Fong SS, Palsson BO. Metabolic gene-deletion strains of Escherichia coli evolve to computationally predicted growth phenotypes. Nat Genet. 2004;36:1056–8. [DOI] [PubMed] [Google Scholar]
- 17.Daran-Lapujade P, Jansen ML, Daran JM, van Gulik W, de Winde JH, Pronk JT. Role of transcriptional regulation in controlling fluxes in central carbon metabolism of Saccharomyces cerevisiae. A chemostat culture study. J Biol Chem. 2004;279:9125–38. [DOI] [PubMed] [Google Scholar]
- 18.Gillet JP, Gottesman MM. Mechanisms of multidrug resistance in cancer. Methods Mol Biol. 2010;596:47–76. [DOI] [PubMed] [Google Scholar]
- 19.Kitano H Biological robustness. Nat Rev Genet. 2004;5:826–37. [DOI] [PubMed] [Google Scholar]
- 20.Wang Z, Zhang J. Abundant indispensable redundancies in cellular metabolic networks. Genome Biol Evol. 2009;1:23–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Taniguchi T Cytokine signaling through nonreceptor protein tyrosine kinases. Science. 1995;268:251–5. [DOI] [PubMed] [Google Scholar]
- 22.Nowak MA, Boerlijst MC, Cooke J, Smith JM. Evolution of genetic redundancy. Nature. 1997;388:167–71. [DOI] [PubMed] [Google Scholar]
- 23.Brookfield JF. Genetic redundancy. Adv Genet. 1997;36:137–55. [DOI] [PubMed] [Google Scholar]
- 24.Harrison R, Papp B, Pal C, Oliver SG, Delneri D. Plasticity of genetic interactions in metabolic networks of yeast. Proc Natl Acad Sci U S A. 2007;104:2307–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Laney JD, Biggin MD. Redundant control of Ultrabithorax by zeste involves functional levels of zeste protein binding at the Ultrabithorax promoter. Development. 1996;122:2303–11. [DOI] [PubMed] [Google Scholar]
- 26.Saga Y, Yagi T, Ikawa Y, Sakakura T, Aizawa S. Mice develop normally without tenascin. Genes Dev. 1992;6:1821–31. [DOI] [PubMed] [Google Scholar]
- 27.Polager S, Ginsberg D. p53 and E2f: partners in life and death. Nat Rev Cancer. 2009;9:738–48. [DOI] [PubMed] [Google Scholar]
- 28.Wu L, Timmers C, Maiti B, Saavedra HI, Sang L, Chong GT, et al. The E2F1–3 transcription factors are essential for cellular proliferation. Nature. 2001;414:457–62. [DOI] [PubMed] [Google Scholar]
- 29.Sherr CJ, Roberts JM. Living with or without cyclins and cyclin-dependent kinases. Genes Dev. 2004;18:2699–711. [DOI] [PubMed] [Google Scholar]
- 30.Szakacs G, Paterson JK, Ludwig JA, Booth-Genthe C, Gottesman MM. Targeting multidrug resistance in cancer. Nat Rev Drug Discov. 2006;5:219–34. [DOI] [PubMed] [Google Scholar]
- 31.Singhal SS, Singhal J, Nair MP, Lacko AG, Awasthi YC, Awasthi S. Doxorubicin transport by RALBP1 and ABCG2 in lung and breast cancer. Int J Oncol. 2007;30:717–25. [PubMed] [Google Scholar]
- 32.Milo R, Shen-Orr S, Itzkovitz S, Kashtan N, Chklovskii D, Alon U. Network motifs: simple building blocks of complex networks. Science. 2002;298:824–7. [DOI] [PubMed] [Google Scholar]
- 33.Lavi O, Ginsberg D, Louzoun Y. Regulation of modular Cyclin and CDK feedback loops by an E2F transcription oscillator in the mammalian cell cycle. Math Biosci Eng. 2011;8:445–61. [DOI] [PubMed] [Google Scholar]
- 34.Eberlin LS, Tibshirani RJ, Zhang J, Longacre TA, Berry GJ, Bingham DB, et al. Molecular assessment of surgical-resection margins of gastric cancer by mass-spectrometric imaging. Proc Natl Acad Sci U S A. 2014;111:2436–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Budczies J, Brockmoller SF, Muller BM, Barupal DK, Richter-Ehrenstein C, Kleine-Tebbe A, et al. Comparative metabolomics of estrogen receptor positive and estrogen receptor negative breast cancer: alterations in glutamine and beta-alanine metabolism. J Proteomics. 2013;94:279–88. [DOI] [PubMed] [Google Scholar]
- 36.Cavill R, Kamburov A, Ellis JK, Athersuch TJ, Blagrove MS, Herwig R, et al. Consensus-phenotype integration of transcriptomic and metabolomic data implies a role for metabolism in the chemosensitivity of tumour cells. PLoS Comput Biol. 2011;7:e1001113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lenski RE, Barrick JE, Ofria C. Balancing robustness and evolvability. PLoS Biol. 2006;4:e428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gibson G, Wagner G. Canalization in evolutionary genetics: a stabilizing theory? Bioessays. 2000;22:372–80. [DOI] [PubMed] [Google Scholar]
- 39.Lehar J, Krueger AS, Avery W, Heilbut AM, Johansen LM, Price ER, et al. Synergistic drug combinations tend to improve therapeutically relevant selectivity. Nat Biotechnol. 2009;27:659–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Greco WR, Bravo G, Parsons JC. The search for synergy: a critical review from a response surface perspective. Pharmacol Rev. 1995;47:331–85. [PubMed] [Google Scholar]
- 41.Cokol M, Chua HN, Tasan M, Mutlu B, Weinstein ZB, Suzuki Y, et al. Systematic exploration of synergistic drug pairs. Mol Syst Biol. 2011;7:544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Huang S, Kauffman S. How to escape the cancer attractor: rationale and limitations of multi-target drugs. Semin Cancer Biol. 2013;23:270–8. [DOI] [PubMed] [Google Scholar]
- 43.Jerby L, Ruppin E. Predicting drug targets and biomarkers of cancer via genome-scale metabolic modeling. Clin Cancer Res. 2012;18:5572–84. [DOI] [PubMed] [Google Scholar]