Abstract
Background:
Postoperative hemorrhage following total-knee arthroplasty (TKA) remains an important topic. The objective of the meta-analysis is to assess the effectiveness of oral antifibrinolytics for blood management in patients undergoing TKA.
Methods:
We searched Medline (1966 to August 2018), PubMed (1966 to August 2018), Embase (1980 to August 2018), ScienceDirect (1985 to August 2018), and the Web of Science (1995 to August 2018) for randomized control trials (RCTs). To assess the heterogeneity of study trial and determine the model for analysis (random-effect model or fixed-effect model), I2 tests and Chi-squared were conducted. We utilized the STATA 12.0 (StataCorp, College Station, TX) to perform all statistical analyses.
Results:
A total of 5 RCTs met our inclusion criteria. This meta-analysis shows that there are significant differences between the 2 groups regarding total blood loss, hemoglobin reduction, and transfusion rates. In addition, no adverse effects were identified in treatment groups.
Conclusion:
The oral form of antifibrinolytics in TKA is able to significantly decrease blood loss, postoperative hemoglobin reduction, as well as transfusion requirements. No increased risk of postoperative complications was observed. Higher quality RCTs is necessary to confirm our finding.
Keywords: antifibrinolytics, blood loss, meta-analysis, oral, total-knee arthroplasty
1. Introduction
Total-knee arthroplasty (TKA) is a common surgical procedure for treatment of the degenerative disorders and rheumatic arthritis.[1] It has been estimated that more than 700,000 TKAs were performed throughout the United States annually.[2] However, arthroplasties were associated with substantial bleeding. Previous articles have reflected that there was an estimated 750 to 1800 mL of calculated total blood loss during TKA.[3–5] Although various methods such as hemostatic agent administration, hypothermic anesthesia, tourniquet, and minimally invasive procedures have been used, blood transfusion rates remain relatively high which may cause hemolysis, infection, and allergic reactions.[6,7]
In recent years, antifibrinolytics have been well-documented in the field of surgery. Tranexamic acid (TXA) is a synthetic analog of the amino acid lysine and it serves as an antifibrinolytic.[8] Recently, it was widely used in major orthopedic surgery.[9–11] Previous articles have demonstrated that it was effective and safe for patients who received intravenous administration or topical application of TXA. Alshryda et al[12] indicted that topical use of TXA was effective in decreasing blood transfusion rates after TKA. Jaszczyk et al[13] reported that 15 mg/kg of TXA could significantly decrease the need for postoperative transfusion of allogeneic blood due to a significant reduction in intra-, post-, and perioperative blood loss. Indeed, the oral form of TXA was cheap and easy to use and did not require specific equipment for administration.
However, the relatively small amount of patients meant the results of the present studies were inconclusive and there has been no systematic evaluation of the literature. Consequently, we applied a systematic and meta-analytical approach to determine the evidence for the safety and efficacy of oral TXA.
2. Methods
This systematic review and meta-analysis were conducted following the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) Statement. No primary personal data will be collected; therefore no additional ethical approval needs to be obtained.
2.1. Search strategy
The electronic databases of PubMed, Embase, ScienceDirect, and the Web of Science were searched from the inception of the database to August 2018, without language restriction. The following key words were used in combination with Boolean operators AND or OR: “oral antifibrinolytics OR tranexamic acid,” “total-knee replacement OR arthroplasty,” and “blood loss OR bleeding OR transfusion.” Additionally, unpublished studies or gray literature were also searched. The selection process of all studies used in the literature was presented in a flow diagraph (Fig. 1).
Figure 1.

Search results and the selection procedure. RCT = randomized controlled trial.
2.2. Inclusion and exclusion criteria
The inclusion criteria were: Participants: patients with a diagnosis of end-stage knee osteoarthritis and prepared for TKA; Interventions: the intervention group received the oral form of antifibrinolytics for postoperative blood management after TKA; Comparisons: the control group received a placebo; Outcomes: total blood loss, hemoglobin reduction, transfusion requirements, and postoperative complications, such as deep vein thrombosis (DVT) and pulmonary embolism (PE); Study design: randomized control trials (RCTs).
The exclusion criteria were: abstracts, case reports, letters, editorials, conference articles, repeated studies, and retrospective studies.
2.3. Selection criteria and date extraction
Two authors independently assessed the potentially eligible studies. Firstly, the titles and abstracts were screened to exclude the duplicated and apparently irrelevant ones or those that do not meet our inclusion criteria. After then, the remaining potential studies were full-text downloaded and reviewed. Any disagreement between 2 above authors was sent and discussed with the 3rd independent author. Two reviewers independently extracted data. A standard form was used; the extracted items included the following: the general study information, for example, the authors, publishing date, study design, case number, age, gender, and follow-up term. Whenever necessary, we contacted the authors of the studies for the missing data and additional information. Primary outcomes included total blood loss, hemoglobin reduction, and transfusion rate. Secondary outcomes were postoperative complications such as DVT and PE.
2.4. Quality assessment
Quality assessment for the included articles was independently evaluated by 2 reviewers which used the Cochrane Collaboration's tool. We conducted a “risk of bias” table including the following key points: random sequence generation, allocation concealment, blinding, incomplete outcome data, free of selective reporting and other bias, and each item was recorded by “Yes,” “No,” or “Unclear.” Each risk of bias item was then presented as a percentage across all included studies. The percentage indicated the proportion of different levels bias risk for each item. If there was disagreement between 2 reviewers, consensus was reached through discussion.
The qualities of evidence in the main outcomes of the present meta-analysis were evaluated using the Recommendations Assessment, Development and Evaluation (GRADE) system including the following items: risk of bias, inconsistency, indirectness, imprecision, and publication bias. The GRADE evidence was divided into the following categories: high-quality evidence, which indicated that further research was unlikely to change the confidence in an estimate of effect; (2) moderate-quality evidence, which indicated that further research was likely to have an important impact on confidence in an estimate of effect and may change the estimate; (3) low-quality evidence, which indicated that further research was likely to have an important impact on confidence in an estimate of effect and was likely to change the estimate; and (4) very low-quality evidence, which indicated that we were very uncertain about the results.
2.5. Data analysis and statistical methods
The data were collected and input into the STATA software (version 12.0; StataCorp, College Station, TX) for meta-analysis. Risk difference (RD) with a 95% confidence interval (CI) or weighted mean difference (WMD) with 95% CI were assessed for dichotomous outcomes or continuous outcomes, respectively. A random-effects model was applied when heterogeneity was detected or the statistical heterogeneity was high (P < .05 or I2 > 50%). Otherwise, a fixed-effects model was used (P ≥ .05 or I2 ≤ 50%).
3. Results
3.1. Search result
A total of 459 studies were preliminarily reviewed. About 314 articles were removed as duplicates and 138 were excluded due to irrelevant content. Two studies were removed for non-RCTs. No gray reference was included. Finally, 5 RCTs[14–18] which had been published between 2004 and 2017 were included in our study. There were 307 participates in the intervention group and 301 patients in the control group.
3.2. Study characteristics
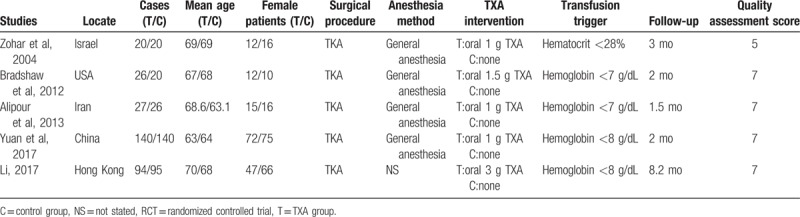
The characteristics of included studies are presented in Table 1. The sample size ranged from 40 to 280 and the mean age ranged from 63 to 70. All articles assessed the oral TXA for blood management in TKA. Four studies performed general anesthesia.[14–17] The intervention group received oral form TXA, while control groups received placebo or none. All articles indicated that TKA was performed by the same group of surgeon. Blood transfusions were performed following postoperative level of hemoglobin level or symptoms of anemia. The follow-up period ranged from 1 to 8.2 months.
Table 1.
Cohort characteristics.
3.3. Risk of bias assessment
A standardized assessment of the risk of bias in the 5 RCTs is summarized in Figure 2. All RCTs detailed the methods of randomization and stated allocate concealment was achieved by sealed envelope. Four RCTs reported the blinding of participants and personnel[15–18] and 3[15–17] of them had attempted to blind assessors. All of them suggest the outcomes for at least 95% of the participants. Each risk of bias item is presented as the percentage across all included studies, which indicates the proportion of different levels of risk of bias for each item (Fig. 3).
Figure 2.

Methodologic quality of the randomized controlled trials.
Figure 3.
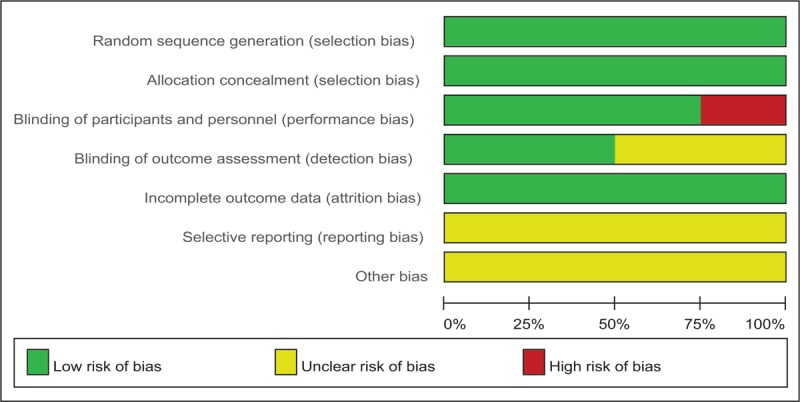
Risk of bias.
3.4. Outcomes for meta-analysis
3.4.1. Total blood loss
Five articles[15–18] reported the total blood loss following TKA. There was significant heterogeneity among the studies (χ2 = 31.89, df = 4, I2 = 87.5%, P = .000); therefore, a random-effects model was used. Pooled results demonstrated there was significant difference regarding total blood loss between groups (WMD = −159.53, 95% CI: −256.95 to 62.11, P = .001; Fig. 4).
Figure 4.

Forest plot diagram showing effect of oral tranexamic acid on total blood loss. CI = confidence interval, WMD = weighted mean difference.
3.4.2. Hemoglobin decline
Five studies[15–18] showed the postoperative hemoglobin decline following TKA. There was significant heterogeneity (χ2 = 9.06, df = 4, I2 = 55.9%, P = .060); therefore, a random-effects model was used. Significant difference was identified regarding the postoperative hemoglobin decline between groups (WMD = −0.492, 95% CI: −0.696 to −0.288, P = .000; Fig. 5).
Figure 5.
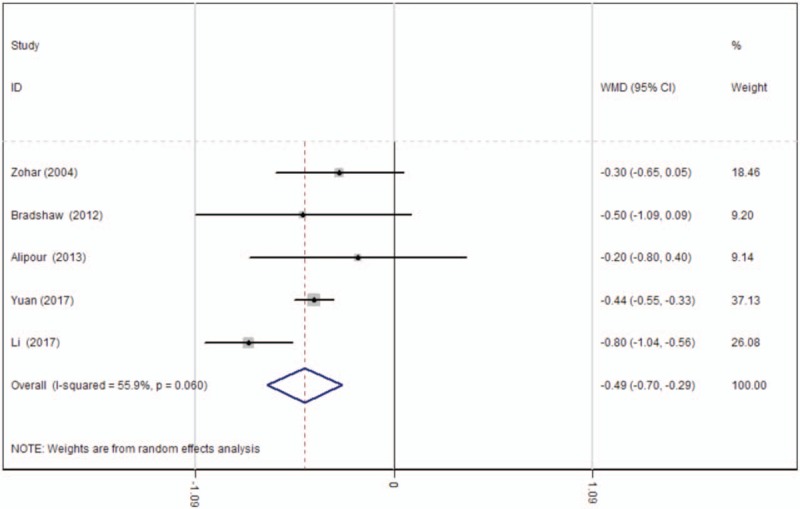
Forest plot diagram showing effect of oral tranexamic acid on hemoglobin decline. CI = confidence interval, WMD = weighted mean difference.
3.4.3. Transfusion rates
Four studies[15–18] reported data regarding the transfusion rates following TKA. There was significant heterogeneity (χ2 = 14.87, df = 4, I2 = 73.1%, P = .005) and a random-effects model was applied. The present meta-analysis indicated that there was significant difference between the TXA and control groups in terms of transfusion rates (RD = −0.108, 95% CI: −0.214 to −0.001, P = .048; Fig. 6).
Figure 6.
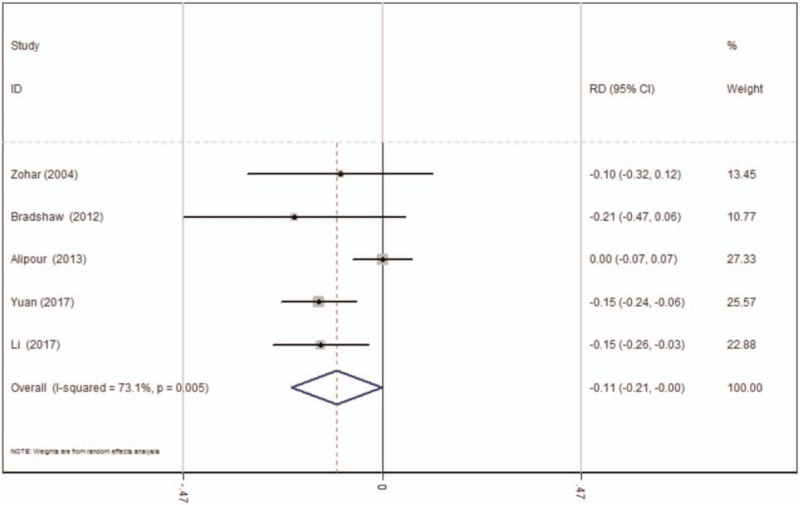
Forest plot diagram showing effect of oral tranexamic acid on transfusion rate. CI = confidence interval, RD = risk difference.
3.4.4. Deep vein thrombosis
The occurrence of DVT was provided in 4 studies.[15–18] No significant heterogeneity among these studies was found; therefore, a fixed-effects model was used (χ2 = 1.07, df = 3, I2 = 0%, P = .784). There was no significant difference between the 2 groups (RD = −0.001, 95% CI: −0.024 to 0.022, P = .942; Fig. 7).
Figure 7.

Forest plot diagram showing effect of oral tranexamic acid on the risk of deep vein thrombosis. CI = confidence interval, RD = risk difference.
3.4.5. Pulmonary embolism
The occurrence of PE was shown in 4 articles.[15–18] There was no significant heterogeneity among these studies; therefore, a fixed-effects model was used (χ2 = 0.70, df = 4, I2 = 0%, P = .952). There was no significant difference between the 2 groups (RD = −0.004, 95% CI: −0.024 to 0.017, P = .728; Fig. 8).
Figure 8.
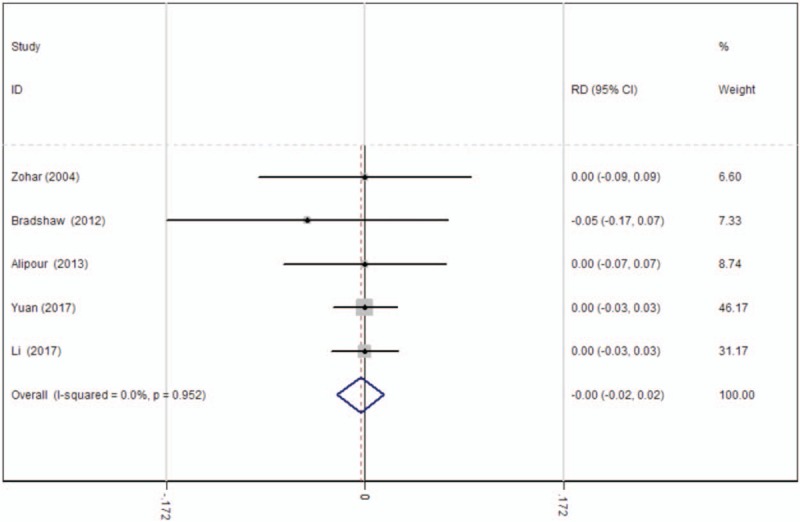
Forest plot diagram showing effect of oral tranexamic acid on the risk of pulmonary embolism. CI = confidence interval, RD = risk difference.
3.4.6. Publication bias and subgroup analysis
Publication bias was assessed for the main outcomes. A low risk of publication bias was found (Figs. 9–11); however, publication bias is an inherent weakness that exists in all meta-analyses.
Figure 9.
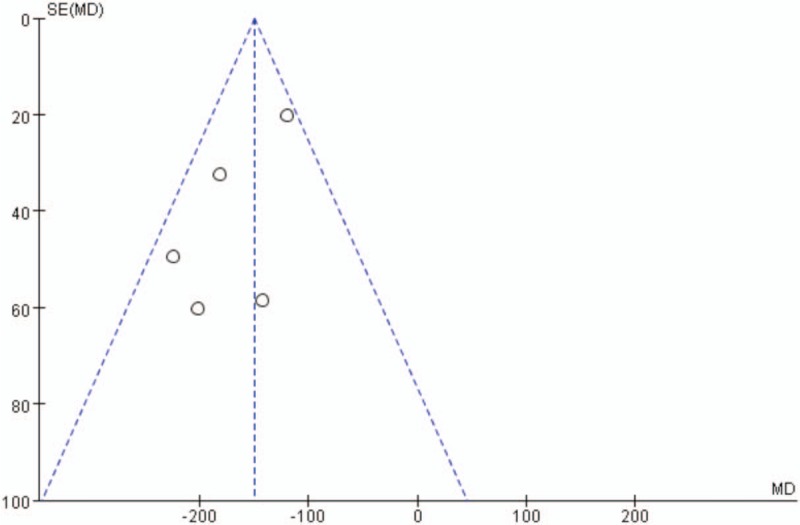
Funnel plot of total blood loss.
Figure 11.
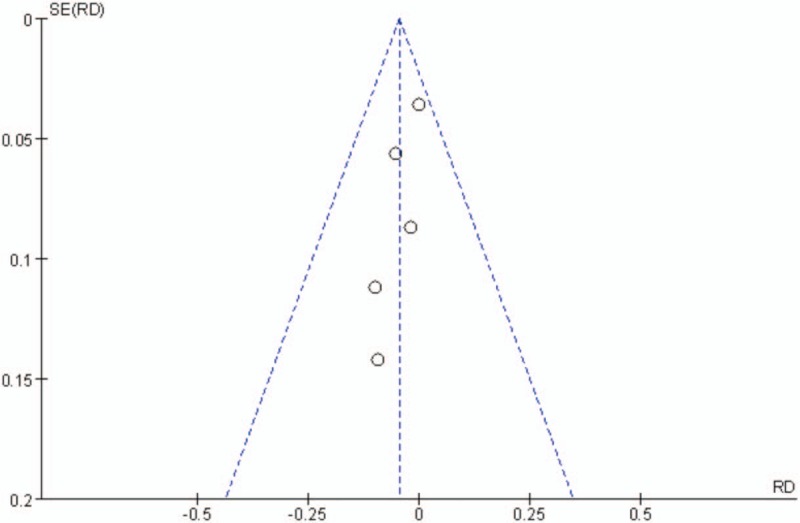
Funnel plot of transfusion rates.
Figure 10.
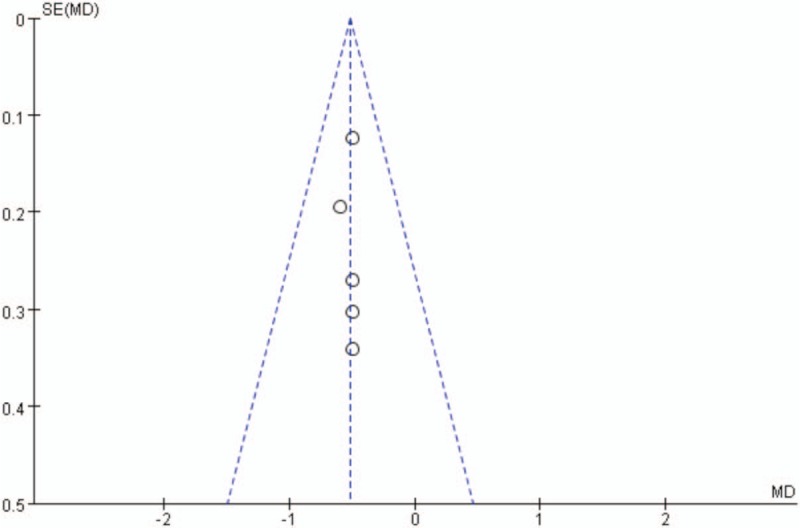
Funnel plot of hemoglobin decline.
Subgroup analysis is summarized in Table 2.
Table 2.
Outcome of subgroup analysis for main results.

4. Discussion
The objective of the meta-analysis is to assess the effectiveness and safety of oral antifibrinolytics for blood management in patients undergoing TKA. Pooled results indicated that oral TXA was associated with further decrease in total blood loss and transfusion rate when compared with the control. In addition, no difference in thromboembolic complication was found.
All outcomes in this meta-analysis were evaluated using the GRADE system. The overall scores of the included studies are presented in Table 3. Based on the concerns regarding the serious limitations and inconsistencies, we downgraded the evidence to low quality for the main outcomes. In addition, we also found significant heterogeneity for the total blood loss (I2 = 87.5%, P = .000), hemoglobin decline (I2 = 55.9%, P = .060) and transfusion requirement (I2 = 73.1%, P = .005). Differing from anesthesia methods, a dose of TXA and transfusion trigger would be a cause of heterogeneity. Thus, more RCTs are needed to confirm our conclusion. Overall, the evidence quality for the present meta-analysis is low which indicated that further research is likely to have a significant impact on the confidence in an estimate of effect and is also likely to change the estimate.
Table 3.
The GRADE evidence quality for main outcome.

The present meta-analysis indicated that oral TXA was able to effectively reduce total blood loss (WMD = −159.53, 95% CI: −256.95 to 62.11, P = .001; Fig. 4) and hemoglobin decline after TKA (WMD = −0.492, 95% CI: −0.696 to −0.288, P = .000; Fig. 5). Substantial evidence has demonstrated that the use of TXA was associated with excellent outcomes for patients undergoing TKA.[17,19] The oral form is cheap, easy to use and does not require specific equipment for administration. Since the oral form of TXA is available and is quick acting, is estimated that it has the comparative effect in blood management compared with the topical or intravenous form. Despite previous clinical research, the efficacy of the oral form of TXA in primary TKA was seldom reported. Therefore, no reliable conclusion has been reached regarding the effectiveness for blood sparing by oral routine of TXA. Meta-analysis is conducted as the main statistical method in our study. It could strengthen statistical power while also enlarging the sample size by pooling results of published studies that could provide stronger evidence. The present meta-analysis indicates that oral TXA can significantly reduce the calculated total blood loss and hemoglobin decline after TKA. Minimally invasive surgery, diathermy coagulation, the position of the knee, sealing of the intramedullary femoral canal, and the use of tourniquet were also effective method of reducing the requirement for blood transfusion.[20–23] Due to the limited number of the included studies, we did not perform a meta-analysis regarding these factors. Further investigation was still required.
Blood sparing is not the only concern when evaluating the effectiveness of the oral form of TXA. TXA is a synthetic fibrinolytic inhibitor that competitively inhibits plasmin, plasminogen, and fibrin from combining. Theoretically, it would increase the prevalence of thromboembolic complications. The most common thrombotic events were DVT and PE which could induce severe results and even death after major orthopedic surgery. Published studies have demonstrated that no increased risk of DVT or PE was observed with the topical or intravenous administration of TXA. Similarly, all included articles in the present meta-analysis reflected no significant difference in the incidence rate of DVT or PE in the oral TXA groups which was in accordance with the previous studies. However, due to the small amount of included studies, more RCTs with longer follow-ups are required to confirm our conclusion. Surgical site infection remains a devastating complication that is a deep concern for many patients and surgeons alike. It may lead to periprosthetic joint infection which prolongs hospital stays, delays recoveries, and subsequent revision surgeries, leading to greater subsequent revision surgeries which are a financial burden. It has been reported that the infection rate of TKA is approximately 1% to 3%.[24] The present meta-analysis shows that there is no significant difference in the incidence of infection. More RCTs with long-term follow-ups are needed for further investigation.
Limitations existed in the current review that needed further attention, describing as follows: the limited number of studies and the small number of samples may have weaken our analysis; the analysis of some outcome measures such as total blood loss and hemoglobin decline were based on a high heterogeneity; follow-up duration was relatively short; only English studies were included, publication bias is unavoidable.
Implications for practice: Oral form of TXA should be considered for patients undergoing TKA. Although no significant difference in the incidence rate of DVT or PE were found, the risk of such complications should still be carefully considered. Implications for research: an adequately sized, well designed RCT for assessing TXA-related side-effects are required to shed more light on the efficiency of TXA given perioperatively to decrease blood loss during TKA.
5. Conclusion
The oral form of antifibrinolytics in TKA is able to significantly decrease blood loss, postoperative hemoglobin reduction, as well as transfusion requirements. No increased risk of postoperative complications was observed. Higher quality RCTs is necessary to confirm our finding.
Author contributions
Hua Li finishes the manuscript. Liqun Bai collects data. Yunhai Li and Zhiyuan Fang design the study. All authors read and approved the final manuscript.
Data curation: Liqun Bai, Yunhai Li, Zhiyuan Fang.
Writing – original draft: Hua Li.
Footnotes
Abbreviations: PE = pulmonary embolism, DVT = deep vein thrombosis, RCT = randomized controlled trial, RD = risk difference, TKA = total-knee arthroplasty, TXA = tranexamic acid, WMD = weighted mean difference.
The authors have no funding and conflicts of interest to disclose.
References
- [1].Horikawa A, Miyakoshi N, Shimada Y, et al. Comparison of clinical outcomes between total knee arthroplasty and unicompartmental knee arthroplasty for osteoarthritis of the knee: a retrospective analysis of preoperative and postoperative results. J Orthop Surg Res 2015;10:168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Hadzic A, Houle TT, Capdevila X, et al. Femoral nerve block for analgesia in patients having knee arthroplasty. Anesthesiology 2010;113:1014–5. [DOI] [PubMed] [Google Scholar]
- [3].Benoni G, Fredin H. Fibrinolytic inhibition with tranexamic acid reduces blood loss and blood transfusion after knee arthroplasty: a prospective, randomised, double-blind study of 86 patients. J Bone Joint Surg Br 1996;78:434–40. [PubMed] [Google Scholar]
- [4].Hiippala S, Strid L, Wennerstrand M, et al. Tranexamic acid (Cyklokapron) reduces perioperative blood loss associated with total knee arthroplasty. Br J Anaesth 1995;74:534–7. [DOI] [PubMed] [Google Scholar]
- [5].Veien M, Sorensen JV, Madsen F, et al. Tranexamic acid given intraoperatively reduces blood loss after total knee replacement: a randomized, controlled study. Acta Anaesthesiol Scand 2002;46:1206–11. [DOI] [PubMed] [Google Scholar]
- [6].Iturbe T, Cornudella R, Serrablo A, et al. Adverse events associated with autologous and allogenic blood transfusion. J Bone Joint Surg Am 2000;82-A:1514–5. [DOI] [PubMed] [Google Scholar]
- [7].Faught C, Wells P, Fergusson D, et al. Adverse effects of methods for minimizing perioperative allogeneic transfusion: a critical review of the literature. Transfus Med Rev 1998;12:206–25. [DOI] [PubMed] [Google Scholar]
- [8].Benoni G, Lethagen S, Fredin H. The effect of tranexamic acid on local and plasma fibrinolysis during total knee arthroplasty. Thromb Res 1997;85:195–206. [DOI] [PubMed] [Google Scholar]
- [9].Panchmatia JR, Chegini S, Lobban C, et al. The routine use of tranexamic acid in hip and knee replacements. Bull NYU Hosp Jt Dis 2012;70:246–9. [PubMed] [Google Scholar]
- [10].Martin JG, Cassatt KB, Kincaid-Cinnamon KA, et al. Topical administration of tranexamic acid in primary total hip and total knee arthroplasty. J Arthroplasty 2014;29:889–94. [DOI] [PubMed] [Google Scholar]
- [11].Melvin JS, Stryker LS, Sierra RJ. Tranexamic acid in hip and knee arthroplasty. J Am Acad Orthop Surg 2015;23:732–40. [DOI] [PubMed] [Google Scholar]
- [12].Alshryda S, Mason J, Vaghela M, et al. Topical (intra-articular) tranexamic acid reduces blood loss and transfusion rates following total knee replacement: a randomized controlled trial (TRANX-K). J Bone Joint Surg Am 2013;95:1961–8. [DOI] [PubMed] [Google Scholar]
- [13].Jaszczyk M, Kozerawski D, Kolodziej L, et al. Effect of single preoperative dose of tranexamic acid on blood loss and transfusion in hip arthroplasty. Ortop Traumatol Rehabil 2015;17:265–73. [DOI] [PubMed] [Google Scholar]
- [14].Zohar E, Ellis M, Ifrach N, et al. The postoperative blood-sparing efficacy of oral versus intravenous tranexamic acid after total knee replacement. Anesth Anal 2004;99:1679–83. [DOI] [PubMed] [Google Scholar]
- [15].Lee QJ, Ching WY, Wong YC. Blood sparing efficacy of oral tranexamic acid in primary total knee arthroplasty: a randomized controlled trial. Knee Surg Relat Res 2017;29:57–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Alipour M, Tabari M, Keramati M, et al. Effectiveness of oral tranexamic acid administration on blood loss after knee artroplasty: a randomized clinical trial. Transfus Apher Sci 2013;49:574–7. [DOI] [PubMed] [Google Scholar]
- [17].Yuan X, Li B, Wang Q, et al. Comparison of 3 routes of administration of tranexamic acid on primary unilateral total knee arthroplasty: a prospective, randomized, controlled study. J Arthroplasty 2017;32:2738–43. [DOI] [PubMed] [Google Scholar]
- [18].Bradshaw, Anthony R, Monoghan, et al. Oral tranexamic acid reduces blood loss in total knee replacement arthroplasty. Curr Orthop Pract 2012;23:209–12. [Google Scholar]
- [19].Liu W, Yang C, Huang X, et al. Tranexamic acid reduces occult blood loss, blood transfusion, and improves recovery of knee function after total knee arthroplasty: a comparative study. J Knee Surg 2018;31:239–46. [DOI] [PubMed] [Google Scholar]
- [20].Vandenbussche E, Duranthon LD, Couturier M, et al. The effect of tourniquet use in total knee arthroplasty. Int Orthop 2002;26:306–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Ko PS, Tio MK, Tang YK, et al. Sealing the intramedullary femoral canal with autologous bone plug in total knee arthroplasty. J Arthroplasty 2003;18:6–9. [DOI] [PubMed] [Google Scholar]
- [22].Ong SM, Taylor GJ. Can knee position save blood following total knee replacement? Knee 2003;10:81–5. [DOI] [PubMed] [Google Scholar]
- [23].Tria AJ, Jr, Coon TM. Minimal incision total knee arthroplasty: early experience. Clin Orthop Relat Res 2003;185–90. [DOI] [PubMed] [Google Scholar]
- [24].Soriano A, Bori G, Garcia-Ramiro S, et al. Timing of antibiotic prophylaxis for primary total knee arthroplasty performed during ischemia. Clin Infect Dis 2008;46:1009–14. [DOI] [PubMed] [Google Scholar]


