Abstract
The prognostic significances of the germinal center B-cell-like (GCB) and non-germinal center B-cell-like (non-GCB) types of diffuse large B-cell lymphoma (DLBCL) have been reported to be different. We analyzed the effect of the cell of origin (COO) of bone marrow (BM) involvement in patients with DLBCL who were treated with rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) in a single institute.
The subtype of BM involvement was evaluated in 633 patients who were diagnosed with primary DLBCL and had been treated with R-CHOP. BM trephine biopsies were analyzed, and immunohistochemical staining of CD20, CD79a, and CD3 was performed. Additional staining of CD10, Bcl-6, and MUM1 was performed to determine the COO based on a previously reported algorithm.
BM involvement was present in 81 patients (12.8%). Among them, 30 patients (37.0%) had GCB-type BM involvement and 51 (63.0%) showed non-GCB-type involvement. Kaplan-Meier survival analysis showed that the non-GCB type had the worst progression-free survival (PFS) and overall survival (OS) (P <.001). In multivariate analysis controlled for the International Prognostic Index (IPI) score, non-GCB type was an independent predictor of PFS (P <.004) and OS (P =.042), whereas GCB type was not a prognostic factor independent of the IPI score.
Further prognostication based on the COO of BM involvement is a useful indicator of PFS, independent of IPI score. Accurate staging based on the COO should be included in the examination of BM in DLBCL.
Keywords: bone marrow involvement, diffuse large B-cell lymphoma, germinal center B-cell-like, non-germinal center B-cell-like
1. Introduction
Diffuse large B-cell lymphoma (DLBCL) is the most common type of non-Hodgkin lymphoma, accounting for 30% to 40% of new diagnoses.[1,2] It affects a broad age range of patients and shows a heterogeneous morphologic appearance, immunophenotype, and biological behavior. This heterogeneity led investigators to further subdivide DLBCL into different entities.[3] Based on cDNA microarray data, DLBCL can be divided into the prognostically significant subgroups of germinal center B-cell-like (GCB) DLBCL and non-germinal center B-cell-like (non-GCB) DLBCL.[4,5]
Immunohistochemical expression analysis is more cost-effective than cDNA microarray analysis, and Hans et al showed a close correlation between their proposed algorithm based on immunohistochemical staining and cDNA microarray analysis.[6] Many studies have used immunohistochemical expression of CD10, Bcl-6, and MUM1 to classify cases of DLBCL into GCB and non-GCB subtypes.[6–9] However, the survival data showed conflicting results; in some studies the GCB group showed better survival than the non-GCB group, whereas others showed no significant difference.
In addition to the pathological classification of DLBCL, the International Prognostic Index (IPI) based on the 5 clinical parameters of age, stage, performance status, serum lactate dehydrogenase (LDH) level, and number of extranodal sites is used to predict clinical outcome.[10] Bone marrow (BM) involvement at diagnosis of DLBCL is reported to be 10% to 30%,[11–15] and although BM involvement at diagnosis is related to poor prognosis, different morphologic types of DLBCL, such as concordant and discordant patterns, have different impacts on prognosis.[16]
Analysis of clinical outcomes according to the cell of origin (COO), such as GCB and non-GCB types, has yielded different results. Moreover, the impact of the COO has been reported to be different after the introduction of rituximab,[17,18] and the clinical impact of BM involvement based on the COO has not been analyzed in the context of recent clinical trials. The aim of this study was to assess the clinical significance of BM involvement in GCB and non-GCB types based on immunohistochemical expression profiles in patients treated with rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) after controlling for the IPI score.
2. Patients and methods
We included all patients in the electronic medical records of the Asan Medical Center who met the following criteria:
-
(i)
confirmed diagnosis of DLBCL on a pathology review;
-
(ii)
treated with rituximab immunotherapy and combination therapy (R-CHOP);
-
(iii)
underwent BM examination; and
-
(iv)
for BM-involved cases, BM slides were available for review and additional immunohistochemical staining.
All patients were staged according to the Ann Arbor system,[19] performance status was assigned according to the Eastern Cooperative Oncology Group (ECOG) Scale,[20] and the IPI score was calculated as previously described.[10] The IPI score was considered low when it was 0 to 2 and high when it was 3 to 5.
Two hematopathologists reviewed the BM trephine biopsies. BM involvement was confirmed by immunohistochemical analyses using monoclonal antibodies for CD20 (Novocastra, Newcastle, UK), CD3 (DAKO, Glostrup, Denmark), and CD79a (DAKO), following routine protocols for automated immunohistochemistry on the Ventana Benchmark XT (Ventana Medical Systems, Tucson, AZ). Additional staining of CD10 (Novocastra), Bcl-6 (Cell Marque, Rocklin, CA), and MUM1 (DAKO) were performed to classify the COO.
GCB and non-GCB types were assigned based on the algorithm proposed by Hans et al (Fig. 1).[6] Despite small variations, expression of CD10 and Bcl-6 has been shown to correlate with GCB DLBCL, and expression of MUM1 has been shown to correlate with non-GCB DLBCL[3] (Fig. 2).
Figure 1.
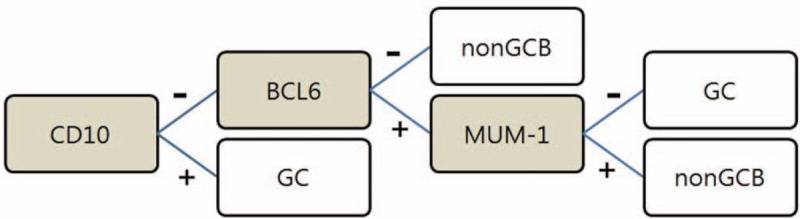
Algorithm proposed by Hans et al for classification of GC and non GCB diffuse large B cell lymphoma. GCB = germinal center B-cell-like, non-GCB = non-germinal center B-cell like.
Figure 2.
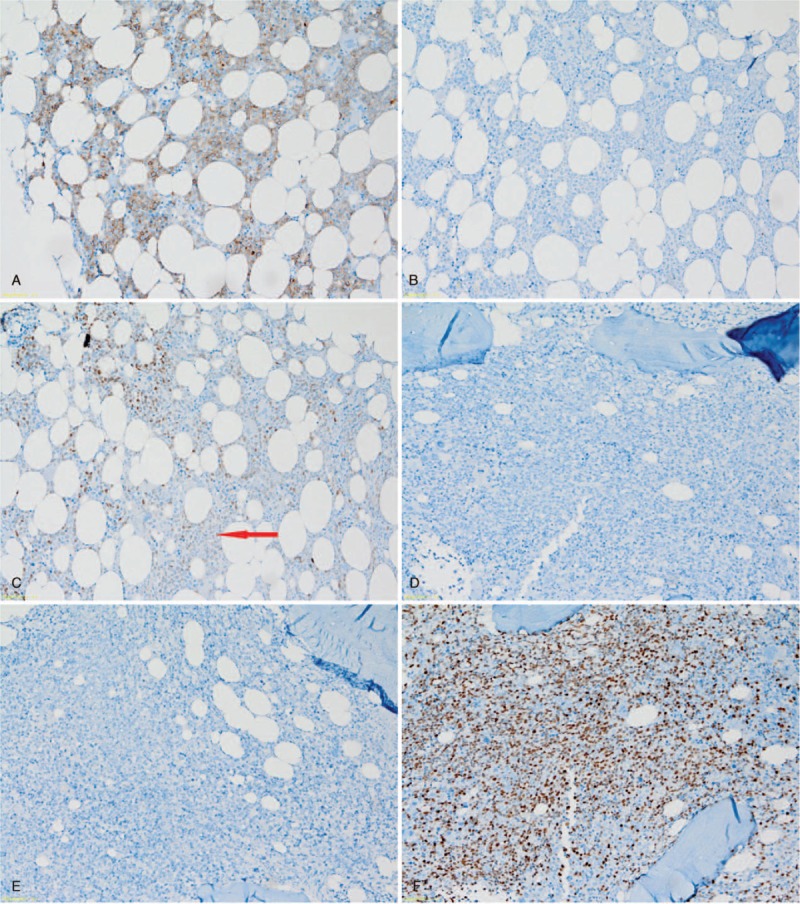
Representative immunohistochemical analysis of germinal center B-cell-like BM involvement with (A) CD10 positivity, (B) Bcl-6 negativity, and (C) MUM1 negativity; and non-germinal center B-cell-like BM involvement with (D) CD10 negativity, (E) Bcl-6 negativity and (F) MUM1 positivity; all magnification ×200. BM = bone marrow.
Ethical approval for this study was obtained from the institutional review board of Asan Medical Center, which is officially accredited by the Forum for Ethical Review Committees in Asia and the Western Pacific.
To compare clinical characteristics between the groups, an independent t-test was used for continuous variables and the χ2 test and Fisher exact test were used for categorical variables. Progression-free survival (PFS) was calculated as the time from the date of first pathological diagnosis to the date of relapse, disease progression, or death from any cause. Overall survival (OS) was calculated from the date of diagnosis until death from any cause. The Kaplan-Meier method was used to assess PFS and OS and the log-rank test was used for comparison between the groups. The Cox proportional hazard model was used to perform multivariate analysis to assess the independent effect of prognostic variables on outcome. Data were analyzed using SAS software (SAS 9.3, SAS Institute Inc., Cary, NC) and SPSS software (SPSS version 18.0 for Windows; SPSS Inc., Chicago, IL).
3. Results
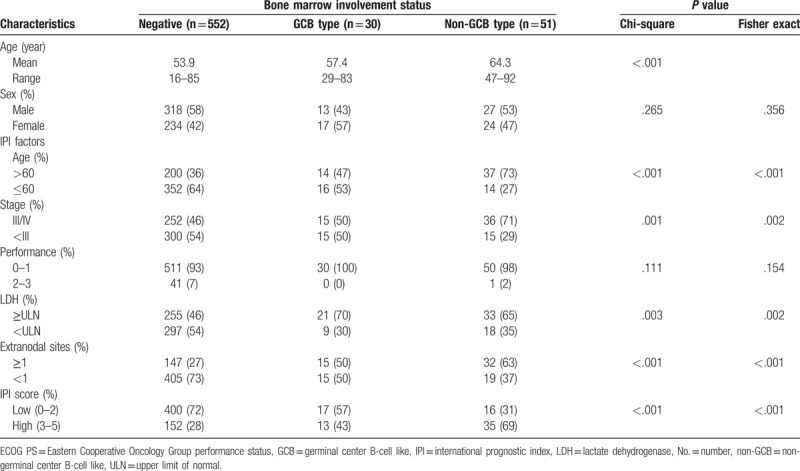
We identified 633 patients who met the inclusion criteria. Of these, 81 patients (12.8%) presented with BM involvement at the time of diagnosis: 30 (37.0%) had GCB-type BM involvement and 51 (63.0%) showed non-GCB-type involvement. The baseline clinical characteristics were analyzed according to no involvement, GCB-type involvement, or non-GCB-type involvement, and are listed in Table 1. The mean age was significantly different among the 3 groups (P <.001). All IPI factors showed a statistically significant difference among the groups (P <.003), except for ECOG performance status. As a result, the percentage of patients with a high IPI score was significantly different in the non-GCB (69%), GCB (43%), and no involvement (28%) groups (P <.001).
Table 1.
Patient and clinical characteristics according to status of bone marrow involvement.
The 3-year PFS and OS were significantly different among the groups (PFS: 78.8% vs 67.3% vs 43.3%, P <.001 and OS: 74.1% vs 54.9% vs 42.3%, P <.001 for no involvement vs GCB-type vs non-GCB-type; Fig. 3). However, the difference in PFS and OS between the no involvement group and the GCB-type group was not statistically significant (P =.50 and P =.14, respectively).
Figure 3.
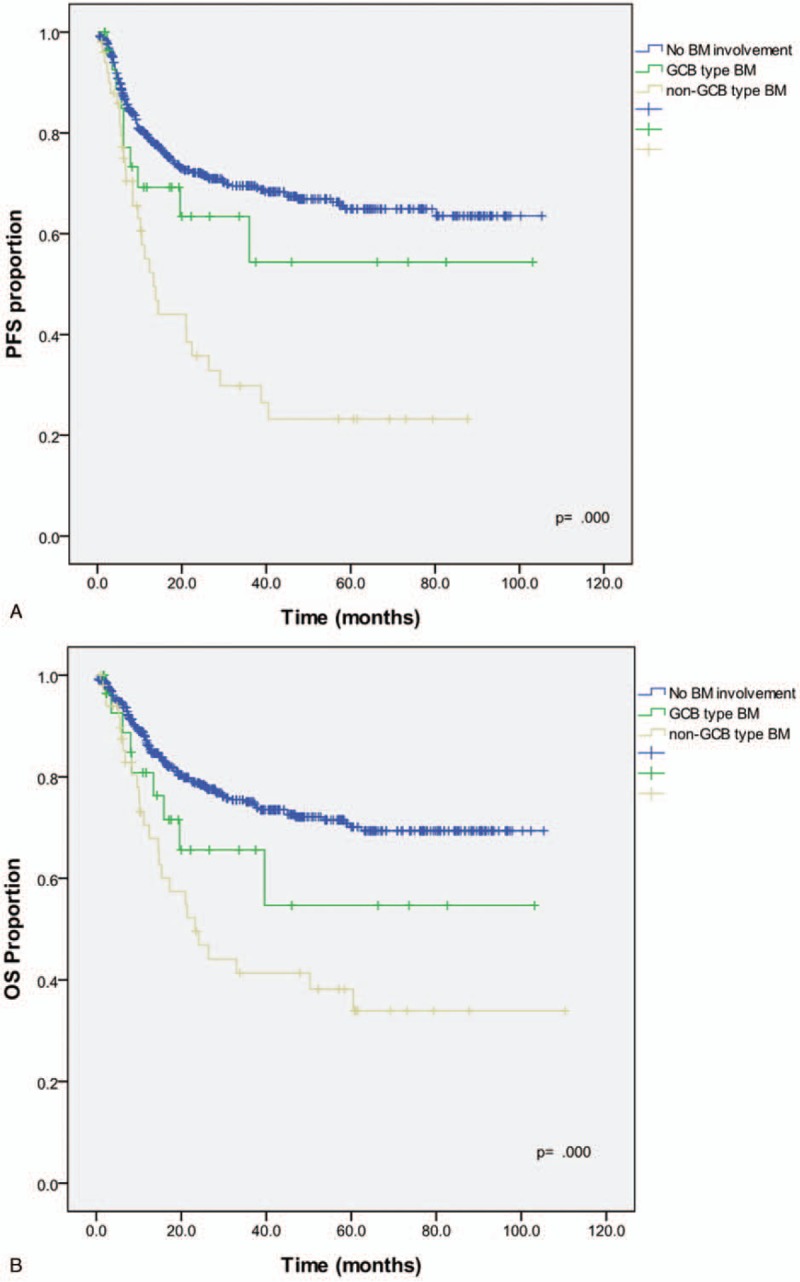
Kaplan–Meier survival curves of (A) PFS and (B) OS for the GCB, non-GCB, and no bone marrow involvement groups. PFS = progression-free survival, OS = overall survival, GCP = germinal center B-cell-like type, non-GCB = non-germinal center B-cell-like type.
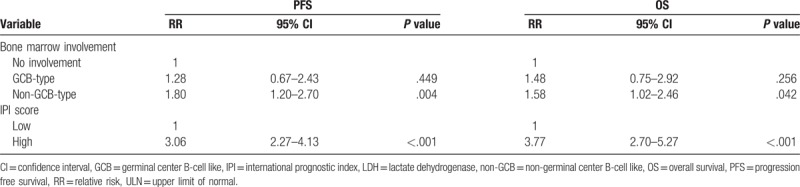
Multivariate analysis to control for the effect of the IPI score revealed that non-GCB-type BM involvement had a negative effect on PFS, independent of the IPI score (relative risk [RR], 1.8; 95% confidence interval [CI], 1.2 to 2.7; P =.004) and on OS, independent of the IPI score, with borderline statistical significance (RR, 1.58; 95% CI, 1.0–2.5; = .042) (Table 2).
Table 2.
Multivariate Cox regression model of bone marrow involvement, IPI score, and individual IPI factors for PFS and OS.
4. Discussion
BM examination at DLBCL diagnosis is useful for evaluating disease stage and is included in the IPI, a widely used indicator of clinical prognosis.[10] However, BM involvement can be further stratified based on 3 immunohistochemical markers and used as an indicator of prognosis independent of the IPI score. The algorithm suggested by Hans et al[6] based on CD10, Bcl-6, and MUM1 staining has been evaluated by many groups, with highly controversial results.[21–23] Moreover, the responses of the GCB and non-GCB DLBCL groups to treatment had been reported to be different according to the therapeutic regimens administered.[18,24] In our study, we included only patients treated with R-CHOP and evaluated the COO of DLBCL in BM specimens.
The Hans method has 70% to 80% concordance with the gene expression profiling (GEP) classification, while the remaining 20% to 30% of cases are discordant.[6,21] However, immunohistochemical staining is a widely used method in laboratory settings and is highly cost-effective compared to GEP. Based on our study, classification of BM-involved DLBCL using 3 markers provides a clinically useful prognostic indicator. There were significant differences in the clinical features of no, GCB-type, and non-GCB-type BM involvement, including age, stage, LDH level, and presence of extranodal involvement. The IPI score was significantly higher in the non-GCB group than in the no involvement and GCB groups.
The Kaplan-Meier method for survival analysis showed a statistically significant difference in PFS and OS among the no involvement, GCB, and non-GCB groups. Univariate analysis revealed that the non-GCB group had the worst outcome, whereas the difference between the no involvement group and the GCB group was statistically insignificant for both PFS and OS.
The IPI was originally defined and published in 1993 based on mathematical modeling of candidate prognostic factors in 2031 patients with de novo diffuse aggressive lymphoma.[10] In this study, a high IPI score was associated with non-GCB BM involvement, and multivariate analysis was subsequently carried out to investigate whether the COO classification is a prognostic factor independent of the IPI score. Non-GCB BM involvement was found to be a negative prognostic factor for PFS and OS independent of the IPI score. IPI factors had a greater influence on OS; when individual IPI factors were evaluated, LDH level and extranodal sites influenced PFS, and all 5 factors influenced OS. This might be due to the fact that the IPI was designed based on OS rather than PFS.[10]
Many issues have been raised regarding the presence of non-GCB DLBCL in elderly patients and whether certain pathological features represent a natural process of aging B cells.[25] The resistance of non-GCB DLBCL to chemotherapy, and the resultant poor prognosis is thought to be due to constitutive activation of NF-κB and its antiapoptotic target genes.[5,26] The level of neuron-specific enolase is higher in non-GCB DLBCL than in GCB DLBCL,[27] and although the reason for this is not clear, it further defines the biological difference between the 2 subtypes. In the present study, non-GCB BM involvement had poorer PFS than that of GCB or no involvement in R-CHOP-treated patients within a single institute.
In conclusion, subdividing BM-involved DLBCL based on the Hans’ classification method had clinical significance in our study. Addition of 3 immunohistochemical markers is relatively simple in a laboratory setting, and determining the histological subtype is of clinical value as a prognostic indicator independent of the IPI score.
Author contributions
Conceptualization: Hyun-Sook Chi.
Investigation: Hyoeun Shim.
Methodology: Hyun-Sook Chi.
Project administration: Hyun-Sook Chi.
Resources: Jooryung Huh, Cheolwon Suh.
Supervision: Seongsoo Jang, Chan-Jeoung Park, Hyun-Sook Chi, Hyoeun Shim.
Writing – original draft: Hyoeun Shim.
Writing – review & editing: Min-Chul Cho, Yousun Chung, Hyoeun Shim.
Footnotes
Abbreviations: BM = bone marrow, COO = cell of origin, DLBCL = diffuse large B-cell lymphoma, ECOG = Eastern Cooperative Oncology Group, GCB = germinal center B-cell-like, GEP = gene expression profiling, IPI = International Prognostic Index, LDH = lactate dehydrogenase, non-GCB = non-germinal center B-cell like, OS = overall survival, PFS = progression free survival, R-CHOP = rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone.
The authors have no conflicts of interest to disclose.
References
- [1].The Non-Hodkin's Lymphoma Classification Project A clinical evaluation of the international lymphoma study group classification of non-hodgkin's lymphoma. Blood 1997;89:3909–18. [PubMed] [Google Scholar]
- [2].Coiffier B. Diffuse large cell lymphoma. Curr Opin Oncol 2001;13:325–34. [DOI] [PubMed] [Google Scholar]
- [3].Hunt KE, Reichard KK. Diffuse large B-cell lymphoma. Arch Pathol Lab Med 2008;132:118–24. [DOI] [PubMed] [Google Scholar]
- [4].Alizadeh AA, Eisen MB, Davis RE, et al. Distinct types of diffuse large B-cell lymphoma identified by gene expression profiling. Nature 2000;403:503–11. [DOI] [PubMed] [Google Scholar]
- [5].Rosenwald A, Wright G, Chan WC, et al. The use of molecular profiling to predict survival after chemotherapy for diffuse large-B-cell lymphoma. N Engl J Med 2002;346:1937–47. [DOI] [PubMed] [Google Scholar]
- [6].Hans CP, Weisenburger DD, Greiner TC, et al. Confirmation of the molecular classification of diffuse large B-cell lymphoma by immunohistochemistry using a tissue microarray. Blood 2004;103:275–82. [DOI] [PubMed] [Google Scholar]
- [7].Barrans SL, Carter I, Owen RG, et al. Germinal center phenotype and bcl-2 expression combined with the International Prognostic Index improves patient risk stratification in diffuse large B-cell lymphoma. Blood 2002;99:1136–43. [DOI] [PubMed] [Google Scholar]
- [8].Colomo L, Lopez-Guillermo A, Perales M, et al. Clinical impact of the differentiation profile assessed by immunophenotyping in patients with diffuse large B-cell lymphoma. Blood 2003;101:78–84. [DOI] [PubMed] [Google Scholar]
- [9].Chang CC, McClintock S, Cleveland RP, et al. Immunohistochemical expression patterns of germinal center and activation B-cell markers correlate with prognosis in diffuse large B-cell lymphoma. Am J Surg Pathol 2004;28:464–70. [DOI] [PubMed] [Google Scholar]
- [10].The International Non-Hodgkin's Lymphoma Prognostic Factors Project A predictive model for aggressive non-Hodgkin's lymphoma. N Engl J Med 1993;329:987–94. [DOI] [PubMed] [Google Scholar]
- [11].Campbell J, Seymour JF, Matthews J, et al. The prognostic impact of bone marrow involvement in patients with diffuse large cell lymphoma varies according to the degree of infiltration and presence of discordant marrow involvement. Eur J Haematol 2006;76:473–80. [DOI] [PubMed] [Google Scholar]
- [12].Chung R, Lai R, Wei P, et al. Concordant but not discordant bone marrow involvement in diffuse large B-cell lymphoma predicts a poor clinical outcome independent of the International Prognostic Index. Blood 2007;110:1278–82. [DOI] [PubMed] [Google Scholar]
- [13].Hodges GF, Lenhardt TM, Cotelingam JD. Bone marrow involvement in large-cell lymphoma. Prognostic implications of discordant disease. Am J Clin Pathol 1994;101:305–11. [DOI] [PubMed] [Google Scholar]
- [14].Robertson LE, Redman JR, Butler JJ, et al. Discordant bone marrow involvement in diffuse large-cell lymphoma: a distinct clinical-pathologic entity associated with a continuous risk of relapse. J Clin Oncol 1991;9:236–42. [DOI] [PubMed] [Google Scholar]
- [15].Yan Y, Chan WC, Weisenburger DD, et al. Clinical and prognostic significance of bone marrow involvement in patients with diffuse aggressive B-cell lymphoma. J Clin Oncol 1995;13:1336–42. [DOI] [PubMed] [Google Scholar]
- [16].Sehn LH, Scott DW, Chhanabhai M, et al. Impact of concordant and discordant bone marrow involvement on outcome in diffuse large B-cell lymphoma treated with R-CHOP. J Clin Oncol 2011;29:1452–7. [DOI] [PubMed] [Google Scholar]
- [17].Fang C, Xu W, Li JY. A systematic review and meta-analysis of rituximab-based immunochemotherapy for subtypes of diffuse large B cell lymphoma. Ann Hematol 2010;89:1107–13. [DOI] [PubMed] [Google Scholar]
- [18].Morton LM, Cerhan JR, Hartge P, et al. Immunostaining to identify molecular subtypes of diffuse large B-cell lymphoma in a population-based epidemiologic study in the pre-rituximab era. Int J Mol Epidemiol Genet 2011;2:245–52. [PMC free article] [PubMed] [Google Scholar]
- [19].Carbone PP, Kaplan HS, Musshoff K, et al. Report of the committee on Hodgkin's disease staging classification. Cancer Res 1971;31:1860–1. [PubMed] [Google Scholar]
- [20].Oken MM, Creech RH, Tormey DC, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol 1982;5:649–55. [PubMed] [Google Scholar]
- [21].Veelken H, Vik Dannheim S, Schulte Moenting J, et al. Immunophenotype as prognostic factor for diffuse large B-cell lymphoma in patients undergoing clinical risk-adapted therapy. Ann Oncol 2007;18:931–9. [DOI] [PubMed] [Google Scholar]
- [22].Dupuis J, Gaulard P, Hemery F, et al. Respective prognostic values of germinal center phenotype and early (18)fluorodeoxyglucose-positron emission tomography scanning in previously untreated patients with diffuse large B-cell lymphoma. Haematologica 2007;92:778–83. [DOI] [PubMed] [Google Scholar]
- [23].Ott G, Ziepert M, Klapper W, et al. Immunoblastic morphology but not the immunohistochemical GCB/nonGCB classifier predicts outcome in diffuse large B-cell lymphoma in the RICOVER-60 trial of the DSHNHL. Blood 2010;116:4916–25. [DOI] [PubMed] [Google Scholar]
- [24].Wilson W, Jung SH, Porcu P, et al. A cancer and leukemia group B multi-center study of DA-EPOCH-rituximab in untreated diffuse large B-cell lymphoma with analysis of outcome by molecular subtype. Haematologica 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Thunberg U, Enblad G, Berglund M. Classification of diffuse large B-cell lymphoma by immunohistochemistry demonstrates that elderly patients are more common in the non-GC subgroup and younger patients in the GC subgroup. Haematologica 2012;97:e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Davis RE, Brown KD, Siebenlist U, et al. Constitutive nuclear factor kappaB activity is required for survival of activated B cell-like diffuse large B cell lymphoma cells. J Exp Med 2001;194:1861–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Wang L, Liu P, Chen X, et al. Serum neuron-specific enolase is correlated with clinical outcome of patients with non-germinal center B cell-like subtype of diffuse large B-cell lymphoma treated with rituximab-based immunochemotherapy. Med Oncol 2012;29:2153–8. [DOI] [PubMed] [Google Scholar]


