Abstract
Rationale:
Programmed cell death-1 protein (PD-1) antibody is an immune-checkpoint inhibitor that triggers anti-tumor response by enhancing immune response. Although PD-1 antibody has been reported effective in some malignant tumor, it can also induce significant immune-related adverse events (irAEs) such as autoimmune diabetes.
Patient concerns:
A 67-year-old male patient with non-small cell lung cancer (NSCLS) presented with polydipsia, polyuria, weakness, and weight loss after use of anti-programmed cell death-1 antibody therapy. Hyperglycemia, high serum ketone, low bicarbonate and high anion gap were compatible with the criteria of diabetic ketoacidosis (DKA).
Diagnoses:
Autoimmune diabetes and diabetic ketoacidosis (DKA). The presence of low serum titers of c-peptide, high blood glucose together with diabetic ketoacidosis (DKA) that occurs shortly after the use of pembrolizumab strongly supported the diagnosis of anti-PD-1 induced autoimmune diabetes.
Interventions:
The patient stopped using pembrolizumab while continuous subcutaneous insulin infusion (CSII) was started at the same time. The insulin infusion was switched to multiple daily injection (MDI) after he was discharged from hospital.
Outcomes:
The patient is now a well-controlled insulin-dependent patient with palliative care of NSCLS.
Lessons:
Autoimmune diabetes induced by anti-programmed cell death-1 (PD-1)/programmed cell death 1 ligand 1 (PD-L1) therapy is a rare, but life threatening immune-related side effect. Physicians should closely monitor diabetes-related indexes of patients who have been undergoing the treatment of anti-PD-1/PD-L1 therapy.
Keywords: anti-programmed cell death-1 antibody, autoimmune diabetes, programmed cell death-1, pembrolizumab
1. Introduction
Pembrolizumab is a humanized IgG4 anti-programmed cell death-1 (PD-1) antibody serving as an immune-checkpoint inhibitor. It was approved by the food and drug administration (FDA) in 2015 as a breakthrough drug for the treatment of non-small cell lung cancer (NSCLS). PD-1 and its ligands (PD-L1 and PD-L2) are negative co-stimulatory molecules of T cell activation. Anti-PD-1 antibody send an inhibitory signal to T cell preventing it from recognizing and attacking tumor cells.[1] Rash, pruritus, thyroiditis, diarrhea, hepatitis, and pneumonitis are main immune-related side effects of anti-PD-1 therapy. Autoimmune diabetes induced by immunotherapy are rare, but life threatening immune-related adverse event (iRAE).[2] In this report, we describe the first case of autoimmune diabetes following the treatment of pembrolizumab therapy in Chinese population.
2. Case presentation
In 2016, a 67-year-old man, with no history of diabetes in his life or family, was diagnosed with NSCLS. He was first treated with navelbine for half a year with clinical deterioration and documented disease progression. He was then proposed for the treatment with 2 mg/kg of pembrolizumab every 3 weeks.
One week after the third infusion, he presented with complaints of polydipsia, polyuria, weakness, and weight loss. There was no polyphagia, visual blurring, palpitation, heat intolerance, upper respiratory inflammation, and any other syndrome. Laboratory tests done in the outpatient department revealed that his fasting blood glucose was 26.98 mmol/L and serum ketone was high at 5.1 mmol/L. As to physical examination, the patient's body temperature was 36.2 °C, blood pressure was 127/84 mmHg, pulse was 114 beats/min, and body mass index (BMI) was 25.1 kg/m2. The remaining findings of neurological system and other systems were unremarkable. He was admitted to the hospital and further laboratory investigations are showed in Table 1. Hyperglycemia, high serum ketone, low bicarbonate at 13.4 mmol/L, and anion gap high at 26 mmol/L were compatible with the criteria of diabetic ketoacidosis (DKA). Given the diagnosis of diabetes and DKA, no further pembrolizumab has been required to date and the patient was started on continuous subcutaneous insulin infusion (CSII). The insulin infusion was switched to multiple daily injection (MDI) after he was discharged from hospital.
Table 1.
Laboratory data of the patient.
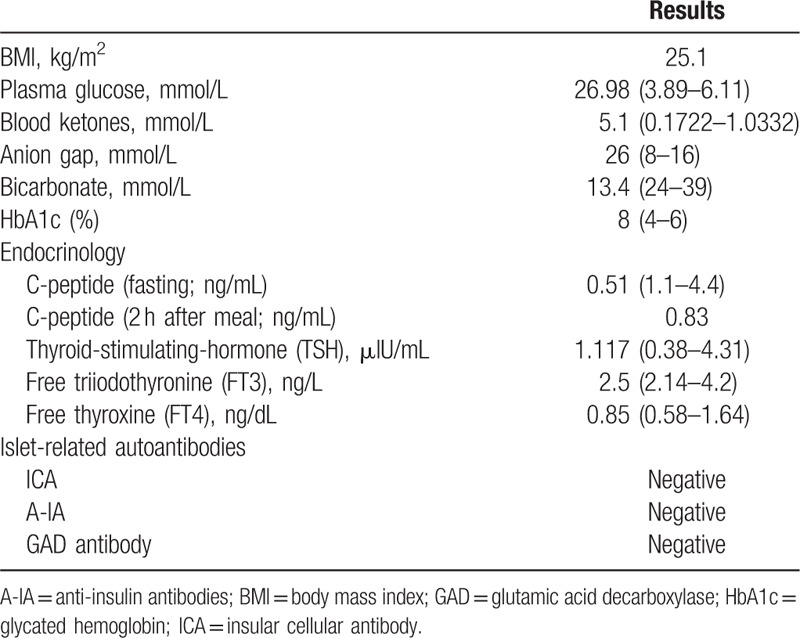
After case discussion and literature review, the patient was diagnosed with anti-PD-1 induced autoimmune diabetes. To date, the man is a well-controlled insulin-dependent patient with palliative care of NSCLS. Meanwhile, at the time of discharge and 3 months after discharge, the C-peptide level remained 0 ng/mL.
3. Discussion
In this report, we describe a case of anti-PD-1 therapy-induced autoimmune diabetes in Chinese population, which characterized with a rapid onset of DKA. The use of pembrolizumab was considered as the cause of autoimmune diabetes, for there were no other potential factors such as personal or family history of autoimmune diseases, viral infection, pancreatic metastasis or use of drugs that induce diabetes, and only pembrolizumab could be identified.
Furthermore, blockading the PD-1/PD-L1 pathway in non-obese diabetic (NOD) mice may precipitate the onset and progression of autoimmune diabetes.[3] Anti-PD-1/PD-L1 drugs might have the same effect. Besides, Fujisawa et al[4] demonstrated that autoimmune diabetes patients have a significant reduction in PD-1 expression in CD4 (+) T cells compared with healthy controls. Another study suggested that decreased PD-1 expression on T cell derived from T1D patients can cause abnormal activation of T cell and then destroy the β cells.[5] So the reduction of PD-1 on T cell may play an important role in the progressing of anti-PD-1 therapy-induced diabetes.
Although the mechanism underlying anti-PD-1/PD-L1 antibody induced autoimmune diabetes is not well understood, 42 cases have been reported that patients presented diabetes were secondary to PD-1 or PD-L1 inhibitors. Twelve out of 42 were treated with pembrolizumab.[6–8] The levels of C-peptide was low in most patients (30 of 32 tested patients).[8] The low or undetectable C-peptide indicated the rapid onset of diabetes with rapid β-cell destruction. Similarly, in our patient, there was a low C-peptide level at the onset of autoimmune diabetes.
So far, to the best of our knowledge, this is the first case report of autoimmune diabetes following the treatment of anti-PD-1 therapy in China. Many PD-1 inhibitor has officially hit the Chinese mainland this year, with thousands of patients starting to use these drugs. Autoimmune diabetes is a rare but serious side-effect of anti-PD-1/PD-L1 therapy. The US FDA has updated with a warning for the development of autoimmune diabetes following the use of pembrolizumab. Patients undergoing the treatment of anti-PD-1/PD-L1 therapy should be closely monitored about diabetes related indexes including blood glucose and glycated hemoglobin (HbA1c) before and during the treatment. Patients with sudden onset of DKA should be alert to the occurrence of PD-1 related diabetes. Further studies are required to identify the pathogenesis of anti-PD-1/PD-L1 induced autoimmune diabetes.
Acknowledgments
The authors would like to thank all staff involved in the care of the patient presented in this case report.
Author contributions
Conceptualization: Ji Hu.
Investigation: Sicheng Li, Yi Zhang, Zhichun Sun.
Project administration: Chen Fang.
Supervision: Ji Hu.
Validation: Ji Hu.
Writing – original draft: Sicheng Li, Zhichun Sun.
Writing – review & editing: Yi Zhang, Chen Fang.
Footnotes
Abbreviations: BMI = body mass index, DKA = diabetic ketoacidosis, HbA1c = glycated hemoglobin (a1C), NK = nature killer, NOD = non-obese diabetic, NSCLS = non-small cell lung cancer, PD-1 = programmed cell death-1, PD-L1 = programmed cell death 1 ligand 1, PD-L2 = programmed cell death 1 ligand 2.
SL and YZ have contributed equally to this work.
Ethics approval and consent to participate: All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2008.
Consent to publish: Written informed consent was obtained from the patient for publication of this case report and any accompanying images. A copy of the consent is available for review by the editor of this journal.
Availability of data and materials: The datasets presented in the current report are available from the corresponding author on reasonable request.
Funding: This study was funded by grants from the National Natural Science Foundation of China (grant number 81600607 to CF and 81471041 to JH) and Postgraduate Research & Practice Innovation Program of Jiangsu Province (grant number SJCX18_0854 to SL). This work was also supported by the Open Research Project of Shanghai Key Laboratory of Diabetes Mellitus (SHKLD-KF-1604) and Ministry of Science and Technology of China under Award Number 2016YFC1305202.
The authors declare that they have no conflicts of interest.
References
- [1].Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med 2012;366:2443–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Food and Drug Administration. Pembrolizumab prescribing information. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/125514s012lbl.pdf Accessed August 18, 2018. [Google Scholar]
- [3].Ansari MJ, Salama AD, Chitnis T, et al. The programmed death-1 (PD-1) pathway regulates autoimmune diabetes in nonobese diabetic (NOD) mice. J Exp Med 2003;1:63–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Fujisawa R, Haseda F, Tsutsumi C, et al. Low programmed cell death-1 (PD-1) expression in peripheral CD4(+) T cells in Japanese patients with autoimmune type 1 diabetes. Clin Exp Immunol 2015;180:452–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Granados HM, Draghi A, II, Tsurutani N, et al. Programmed cell death-1, PD-1, is dysregulated in T cells from children with new onset type 1 diabetes. PLoS One 2017;12:e0183887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Gauci ML, Laly P, Vidal-Trecan T, et al. Autoimmune diabetes induced by PD-1 inhibitor-retrospective analysis and pathogenesis: a case report and literature review. Cancer Immunol Immunother 2017;66:1399–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Thoreau B, Gouaillier-Vulcain F, Machet L, et al. Acute lower limb ischaemia and diabetes in a patient treated with anti-PD1 monoclonal antibody for metastatic melanoma. Acta Derm Venereol 2017;97:408–9. [DOI] [PubMed] [Google Scholar]
- [8].Clotman K, Janssens K, Specenier P, et al. Programmed cell death-1 (PD-1) inhibitor induced type 1 diabetes mellitus: mini-review. J Clin Endocrinol Metab 2018;doi: 10.1210/jc.2018-00728. [DOI] [PubMed] [Google Scholar]


