Abstract
Background:
Complex regional pain syndromes (CRPS) is a disease that is poorly understood. It is a chronic pain syndrome characterized by sympathetic disruptions as well as CNS sensitization. Botulinum Toxin-A has been shown to have efficacy in Raynaud's as well as other neuropathic pain disorders. Perhaps BTX-A warrants experimentation in the treatment of CRPS.
Methods:
Patients with CRPS refractory to 2 or more regional sympathetic nerve blocks in 2007 were included in the study. Patient's were asked to rank their initial pain on a visual analog scale of 0 to 10 (10 being the worst pain). "Tenderness maps" were marked on patient's areas of most pain in 1 by 1 centimeter grids. Each box on the grid was injected with 10 IU of BTX-A after nerve blocks with 1% lidocaine. Treatment sessions occured on a monthly basis with VAS pain scores being re-assessed immediately before the new treatment. t Test, linear regression, and Cohen's D-test were used to analyze the correlation of the data.
Results:
Study sample was 20 patients. Etiology of CRPS was 6 amputations, 4 crush injuries, 4 penetrating injuries, and 2 lacerations. Average pain reduction on VAS scale achieved was 2.05 points. Average percentage pain reduction was 22.94%. Cohen's D Test also showed a meaningful difference with a score of 1.01. Linear regression R2 = 0.491. Maximum pain reduction, on average, was achieved by treatment 9.
Conclusion:
Despite the esoteric etiology of CRPS, BTX-A has a well-demonstrated mechanism of effect. BTX-A should be further explored as a treatment modality for CRPS.
Complex regional pain syndromes (CRPS) is characterized by pain, sympathetic dysregulation, and CNS sensitization. CRPS may evolve to disabling allodynia causing disuse of the affected limb, atrophy, and contractures. Incidence estimates are 5–26 cases per 100,000.1 Diagnostic criteria are pain disproportionate to inciting trauma, at least 1 positive symptom in 3 or more of the categories of dysfunction (sensory, vasomotor, sudomotor/edema, motor/trophic), at least 1 sign in 2 or more of the mentioned categories at the time of evaluation, and no alternative diagnosis.2 Theories of CRPS etiology include hypersensitive cutaneous and muscular adrenoceptors, genesis of postganglionic sympathetic fibers to dorsal root ganglia, and altered N-methyl-D-asparate (NMDA) receptor activity. Despite traditional treatments, patients often still experience significant pain and discomfort.3,4
Botulinum Toxin Type A (BTX-A, Botox) is shown to have long-term well-maintained effects in reducing pain in chronic muscular and neuropathic pain conditions refractory to treatment.2 BTX-A offers the possibility for a minimally invasive solution for these conditions. Botox has been shown to provide symptomatic relief in conditions involving vasomotor instability. In 2010, Neumeister5 demonstrated that perivascular injections of botulinum toxin in the hand had a profound symptomatic relieving effect in patients suffering from Raynaud’s Phenomenon. The vasomotor characteristics shared by Raynaud’s and CRPS raises the question that perhaps this correlation between the 2 can be extended to their response to botulinum toxin injections. The mechanism of action for Botox efficacy in these conditions remains poorly understood: NMDA receptor modulation, peripheral or central nervous system inhibition, reduced muscular spasm and hyperactivity, decreased adrenoception, or decreased sympathetic output. This study aims to investigate the efficacy of BTX-A for pain reduction in upper extremity CRPS refractory to regional sympathetic nerve blocks and to all the standard modalities of pain therapy.
METHODS
Patients covered by the Workman’s Compensation Commission and presenting to the Plastic Surgery Hand Clinics at the McGill University Health Center in 2007 with CRPS in the upper extremity with pain refractory to 2 or more treatments with regional sympathetic nerve blocks were included. Each patient was seen at the Montreal General Hospital Hand Clinic and deemed to be chronic suffers of CRPS with confirmation via triple phase bone scan. Patients were asked to quantify their pain using a visual analog version of the McGill Pain Scale, with 0 being absence of pain and 10 being the worst pain imaginable.6 Next, the patients’ hands were assessed for areas of tenderness. This revealed a “hypersensitivity map” on the patients’ upper extremity that was marked directly on the patient and drawn on preorganized upper extremity charts. A grid of 1 × 1 cm boxes was marked inside an area of tenderness and 10 IU of BTX-A was then injected inside each individual 1 × 1 cm box within this grid in the subcutaneous layer, essentially injecting BTX-A throughout the entirety of the area of hyperesthesia in 1 × 1 cm increments (Fig. 1, 2). Nerve blocks with lidocaine 1% with epinephrine were administered before BTX-A injections for injection tolerance. This process was repeated each month and the patients’ VAS pain score was obtained at the start of each session.
Fig. 1.
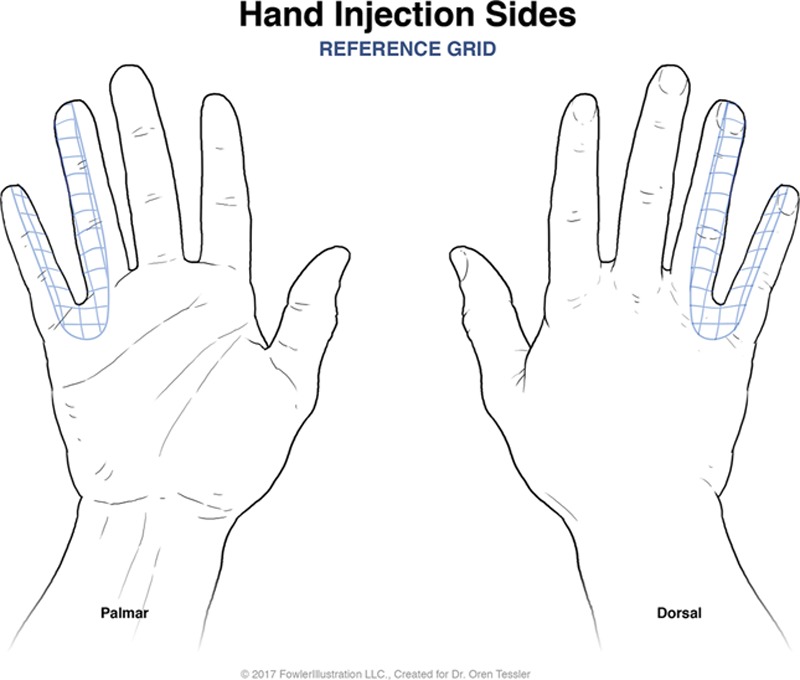
Schematic grid for BTX-A injections in the superimposed 1 × 1 cm grid system.
Fig. 2.
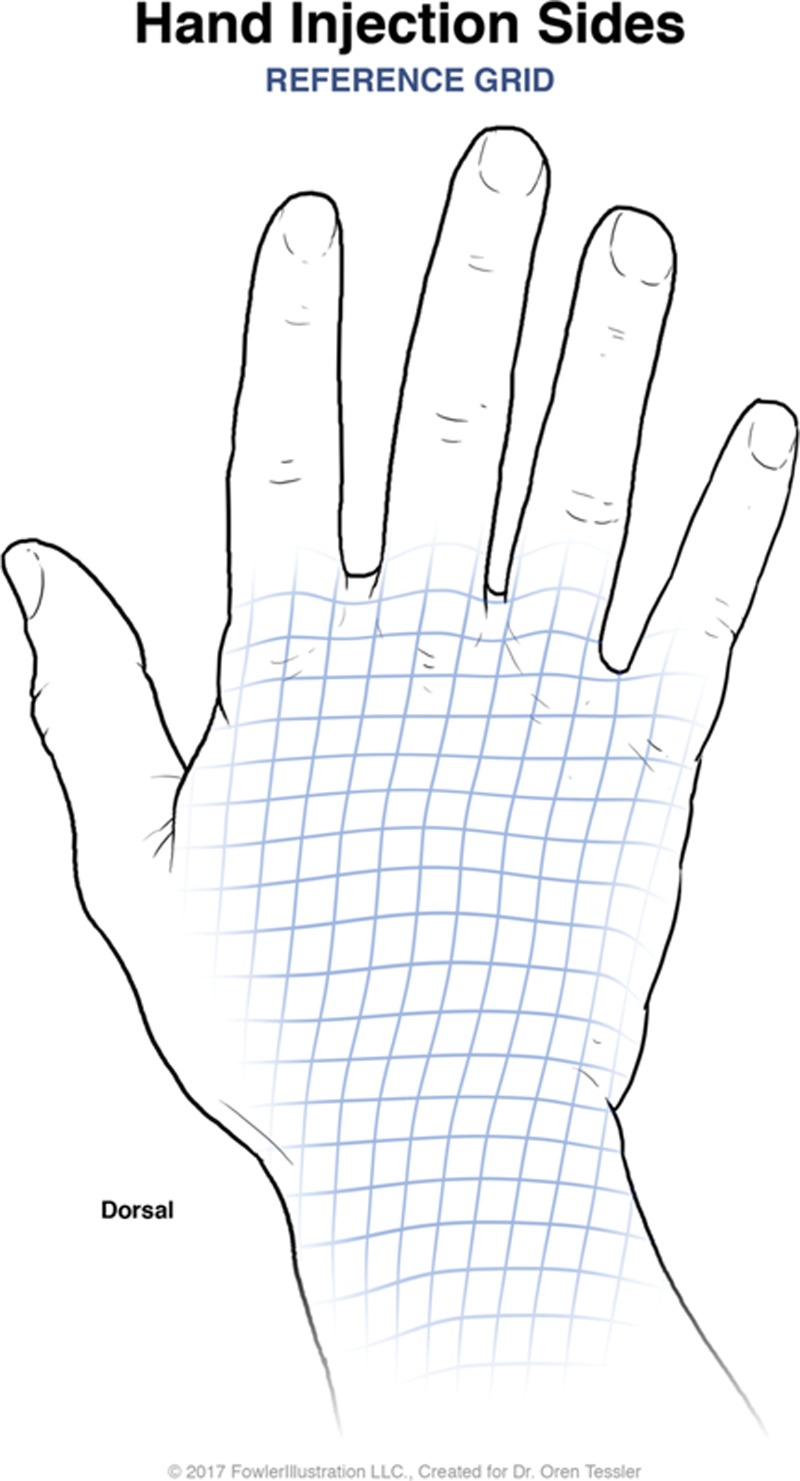
Second schematic for BTX-A injections in the superimposed 1 × 1 cm grid system.
Initial statistics on absolute pain reduction and percent reduction from initial scores were calculated (Fig. 3). The first measured outcome was average pain score reduction. Paired 1 tail t test was used to analyze change in pain score as a function of treatment session. Linear regression analysis was performed to explore if significant changes in pain score as a result of treatment session existed. Cohen’s D Test was used to verify meaningful difference between time points.
Fig. 3.
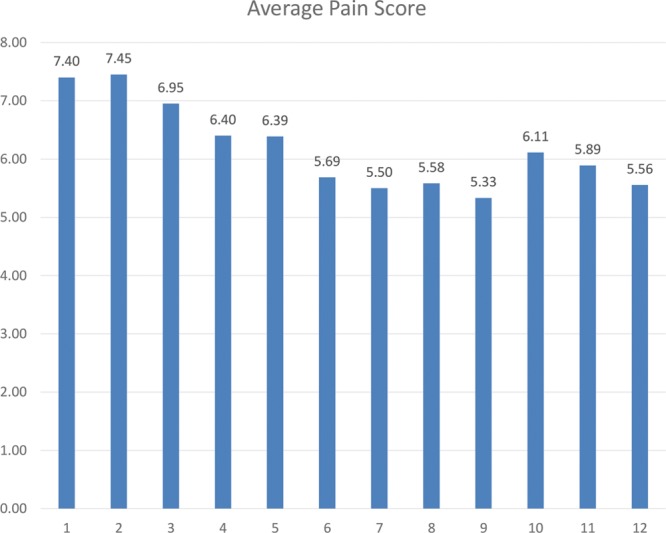
Absolute pain reduction from initial scores, and % of initial pain scores reduced.
RESULTS
Sample size was 20—12 men and 8 women—mean age was 50 (range, 33–64). Injuries leading to CRPS included 6 amputations (2 arm replantations, 4 finger amputations), 4 crush injuries, 4 penetrating injuries with foreign bodies, and 2 complex lacerations. Patients received an average of 8.85 treatment sessions (range, 4–12). All patients received 10 IU of Botox per square centimeter, with a maximum dose of 100 units in 1 session. Thus, if the hypersensitivity area was greater than 10 cm2, we divided the 100 units by the area to determine the number of units injected into each square centimeter. One hundred IU Botox was reconstituted with 2 mL normal saline and injected with a 30-gauge needle.
Average pain reduction on the VAS was 2.05 points, with average pain reduction percentage of 22.94%. t Test showed there was significant improvement in pain scores in patients receiving BTX-A (P < 0.01). Cohen’s D Test showed a meaningful difference with a score of 1.01. Linear regression R2 = 0.491 (Fig. 4). Average maximum pain relief was achieved by session 9.
Fig. 4.
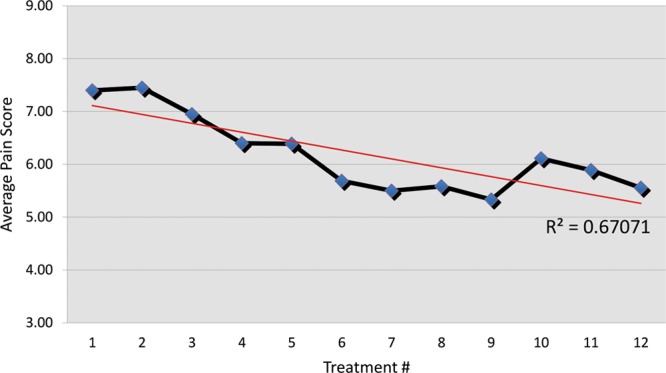
Linear regression demonstrating pain scores as treatment episodes progress.
DISCUSSION
CRPS is characterized by persistent pain disproportionate to the injury consisting of sensory, vasomotor, sudomotor, and motor dysfunction.2,4,7 Degood et al.8 showed 69% of CRPS patients were unemployed as a result of their condition, greater than the rate of unemployment from chronic headaches (37%) and chronic back pain (58%).2 Nerve blocks and pharmaceuticals have been investigated as treatments, but are insufficient and not a permanent cure.9
This study demonstrated BTX-A injections to areas of maximum tenderness had significant improvements in self-assessed pain scores. Pain reducing effect of BTX-A seems to surpass its expected effect duration of 6–7 months evidenced by continually low pain scores with subsequent follow-up.10,11 Due to the novel experimental design and the treatment of CRPS being in its infancy, the amount of botulinum toxin injected (maximum 100 units) was arbitrary. A randomized trial gauging the dose-effect of botulinum toxin on CRPS could be of interest. The maximum duration of botulinum toxin effects is documented at several months; however, subsequent injections were deliberately scheduled in 1 month intervals for patient compliance, adherence, loss to follow-up prevention.
CONCLUSIONS
Despite the poorly understood mechanism of how BTX-A effects CRPS, this study proved BTX-A effective in reducing self-assessed pain in CRPS patients that were refractory to traditional treatments.
Footnotes
Published online 16 October 2018.
Disclosure: The authors have no financial interest to declare in relation to the content of this article. The Article Processing Charge was paid for by the senior author, Oren E.S. Tessler.
REFERENCES
- 1.O’Connel N, Wand B, McAuley J, et al. Interventions for treating pain and disability in adults with complex regional pain syndrome- an overview of systematic reviews (Review). Cochrane Database Syst Rev. 2013;4:1. doi: 10.1002/14651858.CD009416. pub2. www.cochranelibrary.com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Borchers AT, Gershwin ME. Complex regional pain syndrome: a comprehensive and critical review. Autoimmun Rev. 2014;13:242. [DOI] [PubMed] [Google Scholar]
- 3.Galer BS, Jensen M. Neglect-like symptoms in complex regional pain syndrome: results of a self-administered survey. J Pain Symptom Manage. 1999;18:213. [DOI] [PubMed] [Google Scholar]
- 4.de Mos M, de Bruijn AG, Huygen FJ, et al. The incidence of complex regional pain syndrome: a population-based study. Pain. 2007;129:12. [DOI] [PubMed] [Google Scholar]
- 5.Neumeister MW. Botulinum toxin type A in the treatment of Raynaud’s phenomenon. J Hand Surg Am. 2010;35:2085. [DOI] [PubMed] [Google Scholar]
- 6.Jensen MP, Chen C, Brugger AM. Interpretation of visual analog scale ratings and change scores: a reanalysis of two clinical trials of postoperative pain. J Pain. 2003;4:407. doi: 10.1016/S1526-5900(03)00716-8. [DOI] [PubMed] [Google Scholar]
- 7.Beerthuizen A, Stronks DL, Van’t Spijker A, et al. Demographic and medical parameters in the development of complex regional pain syndrome type 1 (CRPS1): prospective study on 596 patients with a fracture. Pain. 2012;153:1187. [DOI] [PubMed] [Google Scholar]
- 8.Degood DE, Cundiff GW, Adams LE, et al. A psychosocial and behavioral comparison of reflex sympathetic dystrophy, low back pain, and headache patients. 1993;54:317. [DOI] [PubMed] [Google Scholar]
- 9.Sarangi PP, Ward AJ, Smith EJ, et al. Algodystrophy and osteoporosis after tibial fractures. J Bone Joint Surg Br. 1993;75:450. [DOI] [PubMed] [Google Scholar]
- 10.Ward AB, Molenaers G, Colosimo C, et al. Clinical value of botulinum toxin in neurological indications. Eur J Neurol. 2006;13:20. [DOI] [PubMed] [Google Scholar]
- 11.Brashear A, Gordon MF, Elovic E, et al. Intramuscular injection of botulinum toxin for the treatment of wrist and finger. N Engl J Med. 2002;6:382. [DOI] [PubMed] [Google Scholar]


