Supplemental Digital Content is available in the text
Keywords: meta-analysis, ovarian cancer, platinum-based chemotherapy, polymorphism, XRCC1
Abstract
Objectives:
Although platinum-based chemotherapy is widely used for advanced ovarian cancer (OC), genetic polymorphisms can influence the chemotherapeutic response. This study investigated the association between XRCC1 polymorphisms Arg194Trp, Arg280His, and Arg399Gln, and overall survival (OS) in OC patients who received platinum-based chemotherapy.
Methods:
We systematically searched PubMed, Embase, the Cochrane library, Chinese National Knowledge Infrastructure, Wanfang, and Weipu databases for relevant studies from inception to October, 2017. OS was calculated using a random-effects model. Sensitivity, subgroup, and publication bias analyses were also performed.
Results:
Five studies involving 1159 OC patients were included. When compared with 194ArgArg, 194TrpTrp (hazard ratio [HR] 1.09, 95% confidence interval [CI] 0.71–1.69, P = .69) and 194TrpArg (HR 1.00, 95% CI 0.78–1.28, P = .98) carriers were not associated with OS. Similarly, compared with 280ArgArg carriers, neither 280HisHis (HR 1.39, 95% CI 0.82 to −2.34, P = .22) nor 280HisArg (HR 0.98, 95% CI 0.73 to −1.31, P = .90) affected OS. Furthermore, there were no significant differences in OS between 399GlnGln (HR 1.00, 95% CI 0.46–2.16, P > .99), 399GlnArg (HR 1.05, 95% CI 0.81–1.37, P = .70), and 399ArgArg. Finally, subgroup analysis suggested that 399GlnGln significantly decreased OS when the percentage of III or IV cases was >80.0% (HR 1.79, 95% CI 1.22–2.62, P = .003), while OS was increased when this percentage was <80.0% (HR 0.47, 95% CI 0.28–0.79, P = .004).
Conclusions:
This study indicated that XRCC1 Arg194Trp, Arg280His, and Arg399Gln did not affect OS after platinum-based chemotherapy in OC patients. However, disease status could affect the relationship between Arg399Gln and OS in these patients.
1. Introduction
Ovarian cancer (OC) is the eighth most common cancer and fifth leading cause of cancer death among women, with an estimated 3% of cancers and 6% of cancer deaths.[1] Currently, the standard treatment strategy for OC is a platinum (cisplatin or carboplatin) and a taxane-based chemotherapy (docetaxel or paclitaxel).[2,3] However, there is still a high incidence of relapse and death due to OC in patients receiving modern chemotherapy, which accounts for nearly 55% of cancer deaths after 5-year follow-up periods.[4,5] This difference in survival time might be related to inherited variants in genes, which, in turn, are correlated with the metabolism and resistance to these chemotherapy agents.[6]
Several polymorphisms have been illustrated to affect the activity of platinum agents, with the most common being DNA repair and glutathione S-transferase genes. X-ray repair cross complementing group 1 (XRCC1), a DNA repair gene, encodes a protein involved in the repair of single-strand breaks and in base excision repair of endogenous and exogenous oxidants. The common polymorphisms in XRCC1 are Arg194Trp, Arg280His, and Arg399Gln. Numerous studies have already illustrated the impact of XRCC1 on breast, colorectal, thyroid, non-Hodgkin lymphoma, lung, prostate, hematological, bladder, gastric, glioma, and hepatocellular carcinoma risk and prognosis.[7–16] However, the association between XRCC1 and overall survival (OS) in OC patients receiving platinum-based chemotherapy has not been determined. Several studies have reported the impact of XRCC1 polymorphisms Arg194Trp, Arg280His, and Arg399Gln on OS in OC receiving platinum-based chemotherapy. However, these results were not consistent,[17–21] with some studies indicating that these polymorphisms could improve OS, while others have reported differently. Thus, this study performed a meta-analysis summarizing the available data to investigate the impact of Arg399Gln, Arg194Trp, and Arg280His polymorphisms on the OS of OC patients receiving platinum-based chemotherapy.
2. Methods
2.1. Data sources, search strategy, and selection criteria
This review was performed according to the Meta-analysis of Observational Studies in Epidemiology (MOOSE) guidelines issued in 2000.[22] Two authors systematically searched electronic databases to select all studies relating to the impact of XRCC1 polymorphisms Arg194Trp, Arg280His, and Arg399Gln on OS in OC receiving platinum-based chemotherapy. The electronic databases PubMed, Embase, the Cochrane library, Chinese National Knowledge Infrastructure, Wanfang, and Weipu were searched with the following core search terms: (ovarian or ovary) and (neoplasms or cancer or neoplasm or carcinoma or tumor) and (platinum or cisplatin or carboplatin or nedaplatin or oxaliplatin) and (“X-ray repair complementing defective repair in Chinese hamster cells 1” or “X-ray repair cross complementing 1” or XRCC1). We conducted manual search for the reference lists of retrieved studies or relevant review to identify additional published studies. To identify unpublished literature, databases of abstracts were searched and contact with authors was also used.
Studies were considered eligible if they fulfilled all of the following criteria: participants: all included patients were diagnosed with OC; intervention: all included patients received platinum-based chemotherapy; control: all studies included comparisons of different categories of the polymorphic genotypes XRCC1 Arg194Trp, Arg280His, or Arg399Gln; outcomes: all studies reported the OS in patients with different genotypes. The study selection process was performed by 2 authors independently; and a third author determined the final criteria for any inconsistencies.
2.2. Data collection and quality assessment
Two authors were responsible for the extraction of data from eligible studies using a standardized data extraction table. Disagreements were resolved by group discussion or a third author, if consensus could not be reached. First author's name, publication year, country, sample size, mean age, percentage of each category of disease stage, tumor grade, tumor type, and OS for each category of genotypes were collected. Bias of individual studies was examined by 2 authors independently, according to the Newcastle–Ottawa Scale (NOS) score, which is quite comprehensive when evaluating the quality of observational studies in meta-analysis.[23] The bias of selection (4 items), comparability (1 item), and outcome (3 items) were assessed during this process.
2.3. Data synthesis and analysis
To determine the impact of XRCC1 polymorphisms Arg194Trp, Arg280His, and Arg399Gln on OS in OC patients who received platinum-based chemotherapy, hazard ratios (HRs) with its 95% confidence intervals (CIs) were calculated. A random-effects model was performed to address underlying variation among the included studies.[24,25] Heterogeneity in the pooled analyses was determined by statistical analyses, using the Q statistic for homogeneity and the I2 statistic. P value of <.10 or I2 > 50% was regarded as significant heterogeneity.[26,27] Sensitivity analyses were performed to evaluate the influence of single studies on overall analysis.[28] Subgroup analyses, including mean age (≥50.0 vs <50.0 years), disease stage (percentage of III and IV ≥80.0% vs <80.0%), and study quality (NOS ≥7 vs <7), were conducted. Publication biases were estimated by using funnel plots, and Egger and Begg tests.[29,30] Two-sided P value less than .05 was regarded as statistically significant. All statistical analyses were performed using the STATA software (version 10.0; Stata Corporation, College Station, TX).
2.4. Ethical approval
As this article is a meta-analysis of the previous work of literature, in which informed consent has already been obtained by the previous clinical researcher, hence approval of the ethics committee was not required.
3. Results
3.1. Search results
Figure 1 presents the whole process of study selection. There were 601 potentially relevant articles identified after the systematic search of electronic databases and manual searches. After reviewing titles or abstracts, 562 of them were excluded due to duplicate and irrelevant titles, leaving 39 articles for further full text review. Twenty-five studies were discarded at the stage of full text review. Among the remaining 14 citations, 5 studies were finally identified and included for analysis[17–21]; the rest of them were excluded for the following reasons: incomparable data, nonrelevant genotypes, and focus on other outcomes.
Figure 1.
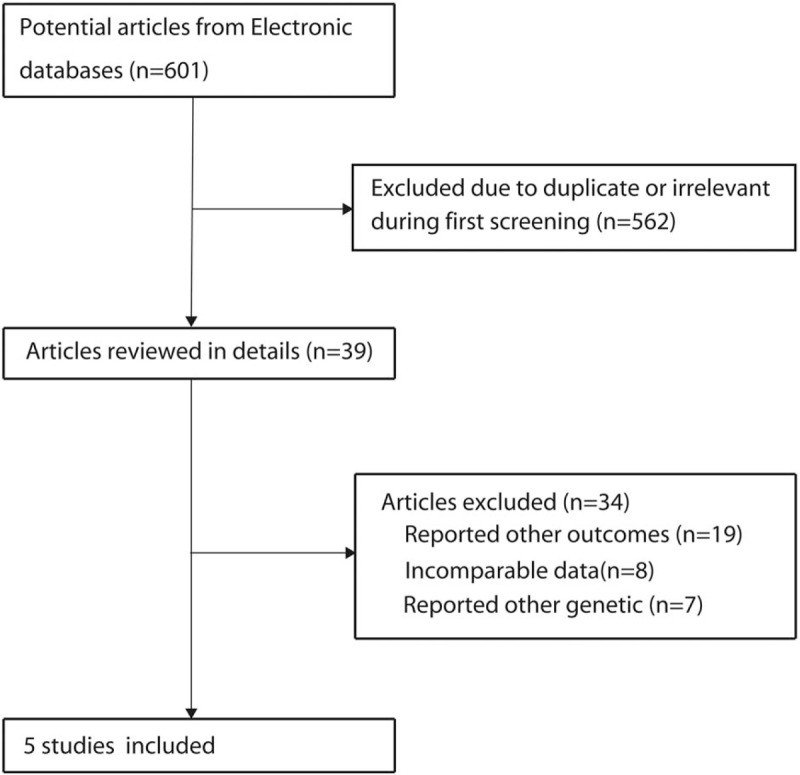
Flow diagram of the literature search and trials selection process.
3.2. Study characteristics
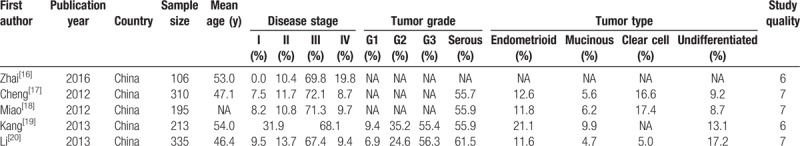
The characteristics of all included studies are listed in Table 1. Five studies reporting 1159 OC patients who received platinum-based chemotherapy were included in this study. These articles were all published in English, and their year of publication ranged from 2012 to 2016. The sample size ranged from 106 to 335 patients and the mean age ranged from 46.4 to 54.0 years. Further, Table 1 provides information on the disease status, tumor grade, and tumor type. Study quality was assessed using the NOS score; 3 studies had a score of 7, while the remaining 2 studies had a score of 6.
Table 1.
Baseline characteristics of the studies included in the systematic review and meta-analysis.
3.3. Arg194Trp
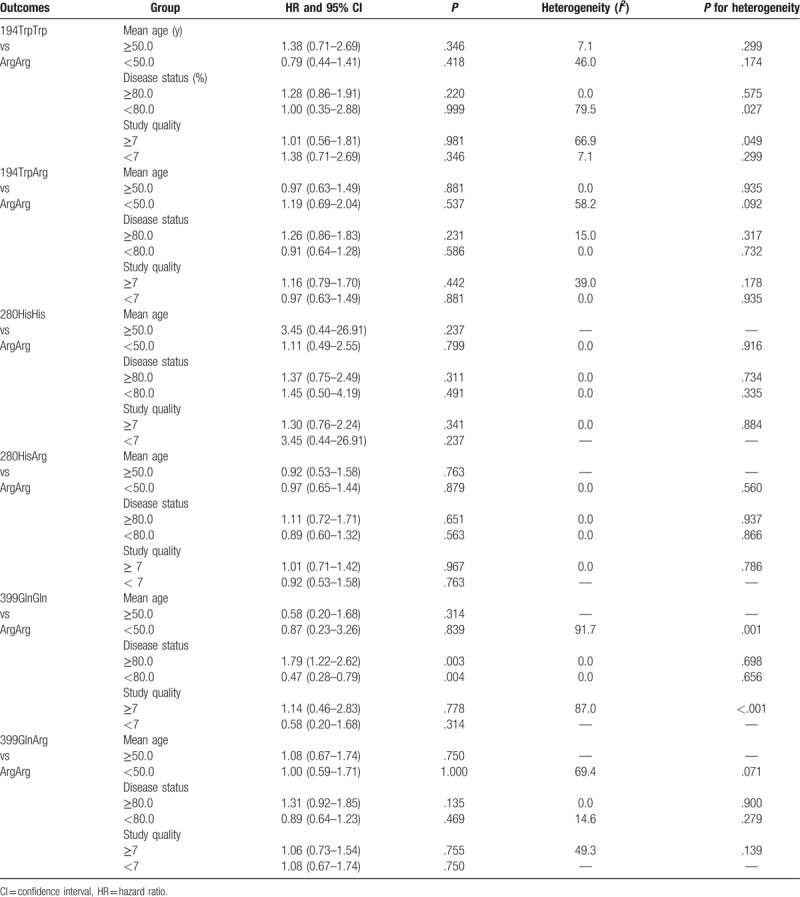
The summary results showed that 194TrpTrp (HR 1.09, 95% CI 0.71–1.69, P = .69; Fig. 2A) and 194TrpArg (HR 1.00, 95% CI 0.78–1.28, P = .99; Fig. 2B) carriers were not associated with OS improvement in OC patients treated with platinum-based chemotherapy as compared with 194ArgArg. Significant heterogeneity was observed among 194TrpTrp versus 194ArgArg (I2 = 50.0%, P = .092) studies, while no evidence of heterogeneity was found among 194TrpArg versus 194ArgArg (I2 = 0.0%, P = .93) studies. The findings of sensitivity and subgroup analyses were consistent with overall analysis (Table S1, S2, and Table 2). Finally, no evidence of publication bias was observed (Figs. S1 and S2).
Figure 2.
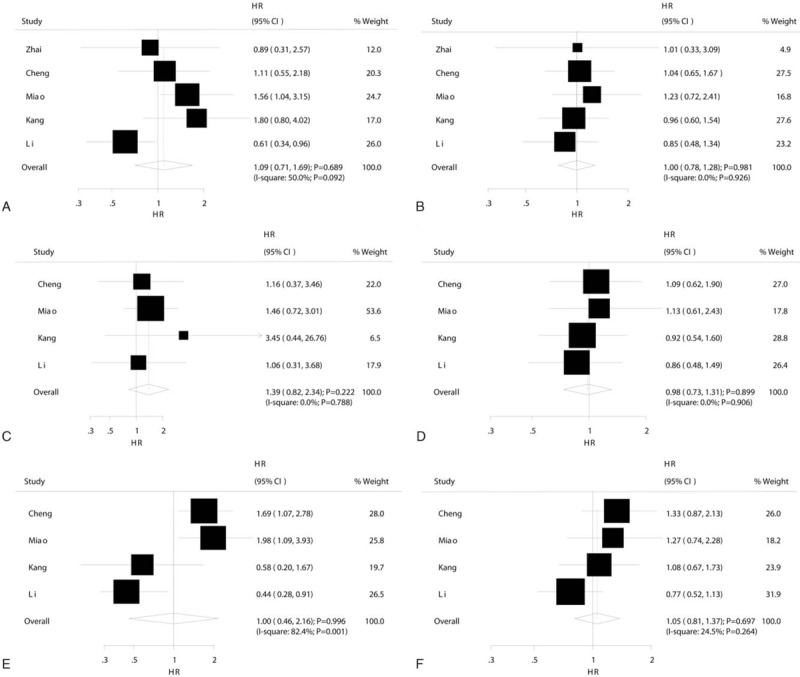
Forest plots of XRCC1 mutant type homozygote and heterozygote versus wild type homozygote on overall survival in ovarian cancer patients who received platinum-based chemotherapy. (A) 194TrpTrp versus 194ArgArg. (B) 194TrpArg versus 194ArgArg. (C) 280HisHis versus 280ArgArg. (D) 280HisArg versus 280ArgArg. (E) 399GlnGln versus 399ArgArg. (F) 399GlnArg versus 399ArgArg.
3.4. Arg280His
Overall, 280HisHis (HR 1.39, 95% CI 0.82–2.34, P = .22; Fig. 2C) and 280HisArg (HR 0.98, 95% CI 0.73–1.31, P = .90; Fig. 2D) carriers were not associated with OS improvement in OC patients treated with platinum-based chemotherapy as compared with 280ArgArg. There were no evidence of heterogeneity among the included studies on 280HisHis versus 280ArgArg (I2 = 0.0%, P = .79) and 280HisArg versus 280ArgArg (I2 = 0.0%, P = .91). After sensitivity analysis and sequential exclusion of each study, it was found that the conclusion was not affected by the exclusion of any specific study (Tables S3 and S4). In addition, the results of subgroup analyses were consistent with overall analysis, as presented in Table 2. Finally, no significant publication bias was observed (Figs. S3 and S4).
Table 2.
Subgroup analyses according to the mean age, disease status, and study quality.
3.5. Arg399Gln
The summary results showed that 399GlnGln (HR 1.00, 95% CI 0.46–2.16, P > .99; Fig. 2E) and 399GlnArg (HR 1.05, 95% CI 0.81–1.37, P = .70; Fig. 2F) carriers had no impact on OS in OC patients who received platinum-based chemotherapy as compared with 399ArgArg. Further, substantial heterogeneity was observed among the included studies on 399GlnGln versus 399ArgArg (I2 = 82.4%, P = .001), while insignificant heterogeneity was detected among those on 399GlnArg versus 399ArgArg (I2 = 24.5%, P = .26). Sensitivity analysis was conducted, and after each study was sequentially excluded from the pooled analysis; the conclusion was not affected by the exclusion of any specific study (Tables S5 and S6). Subgroup analysis indicated that 399GlnGln negatively affects OS, as compared with 399ArgArg, when the percentage of III and IV cases is ≥80.0% (HR 1.79, 95% CI 1.22–2.62, P = .003), while it contributed a beneficial impact on OS than 399ArgArg if the percentage of III and IV <80.0% (HR 0.47, 95% CI 0.28–0.79, P = .004) (Table 2). Finally, there was no significant publication bias for 399GlnGln versus 399ArgArg and 399GlnArg versus 399ArgArg (Figs. S5 and S6).
4. Discussion
To the best of our knowledge, this is the first meta-analysis to investigate the role of XRCC1 polymorphisms Arg194Trp, Arg280His, and Arg399Gln, on OS in OC patients who received platinum-based chemotherapy. This comprehensive quantitative study included 1159 OC patients from 5 studies with a broad range of patient characteristics. The summary results from this study indicated that Arg194Trp, Arg280His, and Arg399Gln did not affect OS in patients who received platinum-based chemotherapy. However, 399GlnGln carriers provided conflicting conclusions for patients with different disease status as compared with 399ArgArg.
The Arg194Trp and Arg399Gln genetic polymorphisms are the most extensively studied single-nucleotide polymorphisms of XRCC1 gene. These polymorphisms, which lead to the encoded amino acid changes that might affect the normal function of XRCC1 protein, might alter the efficiency of DNA repair and result in carcinogenesis development.[10] But, in our study, we did not find a significant association between these 2 genes and OC, which showed good agreement with 2 previous studies conducted in Russia and Korea.[31,32] Only in further stratified analysis by disease status, 399GlnGln was shown to be predictive in OS when different percentage of patients with stage III and IV were analyzed. Also, a study of 103 patients with stage III and IV nonsmall cell lung cancer also found XRCC1 Arg399Gln was associated with a decrease in OS after platinum treatment.[33] So, it indicates that the use of XRCC1 Arg399Gln polymorphisms should be investigated in specific disease stage patients with a larger sample size in the future to ensure a more accurate and robust conclusion. XRCC1 Arg280His is also an amino acid variant, and the codon 280 is located outside the known functional domains of XRCC1, unlike Arg194Trp and Arg399Gln .[34] Functional studies using lymphocytes suggested that the 280His polymorphism diminishes genomic stability.[35] But, in our study, no association was found between XRCC1 Arg280His and OC prognosis.
The result of our study is inconsistent with the conclusion of previous meta-analysis on predictive value of XRCC1 polymorphisms in patients with various cancers, such as lung cancer, gastric, and colorectal cancer.[36,37] Genetic polymorphisms of drug target genes, genes involving in detoxification pathways and DNA repair pathways may influence the anticancer therapeutic efficacy of platinum drugs and reveal platinum sensitivity in patients.[10] Therefore, other genetic variations may also be significantly associated with outcomes, and combined analysis of various genes for prognosis of platinum-based chemotherapy should be considered. Furthermore, the sample size and ethnic factor may be another reason. In our study, the data on other ethnicities were not available, like Russian[31] or Korean,[32] and only 5 Chinese studies were included. Therefore, the most important reason for negative findings by our meta-analyses is insufficient study power to detect effects. Moreover, the relationship between ethnic factor and XRCC1 polymorphisms was not illustrated, which should be considered in future studies.
The limitations of our study were as follows: the adjusted factors of the extracted data on survival time were different among the included studies, which might have affected the progression of the disease and death; substantial heterogeneity among the studies on 194TrpTrp versus 194ArgArg and OS in patients who received platinum-based chemotherapy was not fully addressed by subgroup analysis; subgroup analyses based on other baseline characteristics of patients were not conducted because they were not available in the included studies; the overall results among allele or mutation versus wild types were not calculated because the results were not available in an individual study; publication bias was an inevitable problem because the current study was based on published studies; and pooled data and individual data were not available, which prevented detailed analysis.
5. Conclusions
Overall, genetic polymorphisms in XRCC1 gene were not likely to be associated with OS in OC patients with platinum-based chemotherapy in Chinese. However, the relationship between XRCC1 polymorphisms and OS should be investigated more carefully in well-designed pharmacogenetics and functional studies with large sample sizes in diverse ethnic populations in the future.
Author contributions
Conceptualization: Zhuo Zhang.
Data curation: Zhuo Zhang, Guanyan Mu, Shuqing Chen, Kun Hu.
Investigation: Zhuo Zhang, Qiufen Xie.
Methodology: Zhuo Zhang, Guanyan Mu, Shuang Zhou.
Project administration: Qian Xiang, Yimin Cui.
Software: Shuang Zhou.
Supervision: Yimin Cui.
Visualization: Shuqing Chen, Shuang Zhou.
Writing – original draft: Zhuo Zhang.
Writing – review & editing: Zhuo Zhang, Qian Xiang, Yimin Cui.
Supplementary Material
Footnotes
Abbreviations: CI = confidence interval, HR = hazard ratio, MOOSE = Meta-analysis of Observational Studies in Epidemiology, NOS = Newcastle–Ottawa Scale, OC = ovarian cancer, OS = overall survival, XRCC1 = x-ray repair cross-complementing protein 1.
Funding: This study was supported by grants from the National Key Technologies R&D Program (No. 2016YFC0904900), National Natural Science Foundation of China (No. 81573504 and No. 81673509), Beijing Natural Science Foundation (No. 7171012), and National Science and Technology Major Projects for “Major New Drugs Innovation and Development” (No. 2017ZX09304028 and No. 2017ZX09101001).
The authors have no potential conflict of interest to declare.
Supplemental Digital Content is available for this article.
References
- [1].Jemal A, Siegel R, Xu J, et al. Cancer statistics, 2010. CA Cancer J Clin 2010;60:277–300. [DOI] [PubMed] [Google Scholar]
- [2].Ozols RF, Bundy BN, Greer BE, et al. Phase III trial of carboplatin and paclitaxel compared with cisplatin and paclitaxel in patients with optimally resected stage III ovarian cancer: a Gynecologic Oncology Group study. J Clin Oncol 2003;21:3194–200. [DOI] [PubMed] [Google Scholar]
- [3].du Bois A, Luck HJ, Meier W, et al. A randomized clinical trial of cisplatin/paclitaxel versus carboplatin/paclitaxel as first-line treatment of ovarian cancer. J Natl Cancer Inst 2003;95:1320–9. [DOI] [PubMed] [Google Scholar]
- [4].Hoskins WJ, Bundy BN, Thigpen JT, et al. The influence of cytoreductive surgery on recurrence-free interval and survival in small-volume stage III epithelial ovarian cancer: a Gynecologic Oncology Group study. Gynecol Oncol 1992;47:159–66. [DOI] [PubMed] [Google Scholar]
- [5].McGuire V, Jesser CA, Whittemore AS. Survival among U.S. women with invasive epithelial ovarian cancer. Gynecol Oncol 2002;84:399–403. [DOI] [PubMed] [Google Scholar]
- [6].Holohan C, Van Schaeybroeck S, Longley DB, et al. Cancer drug resistance: an evolving paradigm. Nat Rev Cancer 2013;13:714–26. [DOI] [PubMed] [Google Scholar]
- [7].Chen XP, Wen HF, Zhang F, et al. Assessment of the Link between XRCC1 Arg399Gln Polymorphism and Breast Cancer: a Meta-Analysis in a Single Ethnic Group. Clin Lab 2017;63:725–31. [DOI] [PubMed] [Google Scholar]
- [8].Wang L, Qian J, Ying C, et al. X-ray cross-complementing groups 1 rs1799782 C > T polymorphisms and colorectal cancer susceptibility: a meta-analysis based on Chinese Han population. J Cancer Res Ther 2016;12suppl:C264–c267. [DOI] [PubMed] [Google Scholar]
- [9].Li Y, Bai O, Cui J, et al. Genetic polymorphisms in the DNA repair gene, XRCC1 associate with non-Hodgkin lymphoma susceptibility: a systematic review and meta-analysis. Eur J Med Genet 2016;59:91–103. [DOI] [PubMed] [Google Scholar]
- [10].Yuan Z, Li J, Hu R, et al. Predictive assessment in pharmacogenetics of XRCC1 gene on clinical outcomes of advanced lung cancer patients treated with platinum-based chemotherapy. Sci Rep 2015;5:16482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Chen Y, Li T, Li J, et al. X-ray repair cross-complementing group 1 (XRCC1) Arg399Gln polymorphism significantly associated with prostate cancer. Int J Biol Markers 2015;30:e12–21. [DOI] [PubMed] [Google Scholar]
- [12].Du L, Liu Y, Xue P, et al. The Arg399Gln polymorphism in the XRCC1 gene is associated with increased risk of hematological malignancies. Tumour Biol 2015;36:4545–54. [DOI] [PubMed] [Google Scholar]
- [13].Dong LM, Zhang XY, Teng H, et al. Meta-analysis demonstrates no association between XRCC1 Arg399Gln polymorphism and bladder cancer risk. Genet Mol Res 2014;13:9976–85. [DOI] [PubMed] [Google Scholar]
- [14].Zhao DY, Cheng L, Yu J, et al. XRCC1 genetic polymorphism Arg339Gln, Arg194Trp, Arg280His and gastric cancer risk: an evidence based decision. Cancer Biomark 2014;14:449–56. [DOI] [PubMed] [Google Scholar]
- [15].Xu C, Chen P, Liu W, et al. Association between the XRCC1 Arg194Trp polymorphism and glioma risk: an updated meta-analysis. Asian Pac J Cancer Prev 2014;15:7419–24. [DOI] [PubMed] [Google Scholar]
- [16].Zhang XL, Lu Y, Yang S, et al. An updated meta-analysis between the association of XRCC1 Arg399Gln polymorphism and hepatocellular carcinoma risk. Asian Pac J Cancer Prev 2014;15:3273–8. [DOI] [PubMed] [Google Scholar]
- [17].Zhai XH, Huang J, Wu FX, et al. Impact of XRCC1, GSTP1, and GSTM1 polymorphisms on the survival of ovarian carcinoma patients treated with chemotherapy. Oncol Res Treat 2016;39:440–6. [DOI] [PubMed] [Google Scholar]
- [18].Cheng CX, Xue M, Li K, et al. Predictive value of XRCC1 and XRCC3 gene polymorphisms for risk of ovarian cancer death after chemotherapy. Asian Pac J Cancer Prev 2012;13:2541–5. [DOI] [PubMed] [Google Scholar]
- [19].Miao J, Zhang X, Tang QL, et al. Prediction value of XRCC 1 gene polymorphism on the survival of ovarian cancer treated by adjuvant chemotherapy. Asian Pac J Cancer Prev 2012;13:5007–10. [DOI] [PubMed] [Google Scholar]
- [20].Kang S, Sun HY, Zhou RM, et al. DNA repair gene associated with clinical outcome of epithelial ovarian cancer treated with platinum-based chemotherapy. Asian Pac J Cancer Prev 2013;14:941–6. [DOI] [PubMed] [Google Scholar]
- [21].Li K, Li W. Association between polymorphisms of XRCC1 and ADPRT genes and ovarian cancer survival with platinum-based chemotherapy in Chinese population. Mol Cell Biochem 2013;372:27–33. [DOI] [PubMed] [Google Scholar]
- [22].Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 2000;283:2008–12. [DOI] [PubMed] [Google Scholar]
- [23].Wells GA, Shea BJ, O’Connell D, et al. The Newcastle–Ottawa Scale (NOS) for assessing the quality of non-randomized studies in meta-analysis. Appl Eng Agriculture 2014;18:727–34. [Google Scholar]
- [24].DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986;7:177–88. [DOI] [PubMed] [Google Scholar]
- [25].Ades AE, Lu G, Higgins JP. The interpretation of random-effects meta-analysis in decision models. Med Decis Making 2005;25:646–54. [DOI] [PubMed] [Google Scholar]
- [26].Deeks JJ, Higgins JP, Altman DG. Analysing Data and Undertaking Meta-Analyses. In: Higgins JP, Green S, Cochrane Handbook for Systematic Reviews of Interventions. John Wiley & Sons, Ltd; 2008. Available from: https://onlinelibrary.wiley.com/doi/10.1002/9780470712184.ch9 Accessed October 23, 2018. [Google Scholar]
- [27].Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003;327:557–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Tobias A. Assessing the influence of a single study in meta-analysis. Stata Techn Bull 1999;8:7526–9. [Google Scholar]
- [29].Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994;50:1088–101. [PubMed] [Google Scholar]
- [31].Khrunin AV, Moisseev A, Gorbunova V, et al. Genetic polymorphisms and the efficacy and toxicity of cisplatin-based chemotherapy in ovarian cancer patients. Pharmacogenomics J 2010;10:54–61. [DOI] [PubMed] [Google Scholar]
- [32].Kim HS, Kim MK, Chung HH, et al. Genetic polymorphisms affecting clinical outcomes in epithelial ovarian cancer patients treated with taxanes and platinum compounds: a Korean population-based study. Gynecol Oncol 2009;113:264–9. [DOI] [PubMed] [Google Scholar]
- [33].Gurubhagavatula S, Liu G, Park S, et al. XPD and XRCC1 genetic polymorphisms are prognostic factors in advanced non-small-cell lung cancer patients treated with platinum chemotherapy. J Clin Oncol 2004;22:2594–601. [DOI] [PubMed] [Google Scholar]
- [34].Shen MR, Jones IM, Mohrenweiser H. Nonconservative amino acid substitution variants exist at polymorphic frequency in DNA repair genes in healthy humans. Cancer Res 1998;58:604–8. [PubMed] [Google Scholar]
- [35].Kiuru A, Lindholm C, Heilimo I, et al. Influence of DNA repair gene polymorphisms on the yield of chromosomal aberrations. Environ Mol Mutagen 2005;46:198–205. [DOI] [PubMed] [Google Scholar]
- [36].Li DJ, Xiao D. Association between the XRCC1 polymorphisms and clinical outcomes of advanced NSCLC treated with platinum-based chemotherapy: a meta-analysis based on the PRISMA statement. BMC Cancer 2017;17:501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Wu H, Xu C, Chen G, et al. X-ray repair cross-complementing 1 polymorphism and prognosis of platinum-based chemotherapy in gastric and colorectal cancer: a meta-analysis. J Gastroenterol Hepatol 2014;29:926–33. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


