Abstract
The systematic immune-inflammation index (SII) has been used to predict the prognosis of patients with various cancers. This study aimed to determine whether the preoperative SII was associated with postoperative survival among patients with operable colon cancer.
This retrospective study included 118 age- and sex-matched healthy subjects and 118 patients who underwent radical surgery for colon cancer between January 2011 and December 2013. The preoperative SII was calculated based on counts of neutrophils, lymphocytes, and platelets in the peripheral blood. Pearson correlation analysis was used to analyze the relationships between the SII and carcinoembryonic antigen (CEA) concentration, average length of stay (ALOS), and medical costs during hospitalization. The χ2 test or Fisher exact test was used to analyze the relationship between the preoperative SII and the postoperative survival rate.
The median SII value was 667.75 among patients with colon cancer, which was higher than the value among healthy subjects. A high SII (>667.75) was associated with a large tumor size and advanced TNM stage, although it was not associated with age, sex, tumor location, or pathological grade. Pearson correlation analysis revealed that the SII was positively correlated with serum CEA concentration, ALOS, and medical costs. Relative to a low SII, a high SII was significantly associated with a lower overall survival rate at 3 years and 5 years after surgery.
The present study's findings suggest that the preoperative SII is a useful prognostic index for patients with operative colon cancer.
Keywords: average length of stay, colon cancer, survival, systematic immune-inflammation index
1. Introduction
Colon cancer is one of the most common malignancies and poses a serious threat to human health.[1] The incidence of colon cancer has also risen in developing countries, including China. Clinicians generally use staging, pathological typing, and differentiation grading to predict the prognosis of this disease.[2,3] For example, TNM staging is commonly used to guide the choice of treatment and predict the postoperative life expectancy. However, patients with the same TNM stage often have a heterogeneous prognosis.[4] Moreover, despite the use of clinical staging and pathological grading systems, there is a lack of reliable prognostic systems for colon cancer that are based on simple blood indicators. Thus, it would be useful to identify other biomarkers to improve prognostication.
Recent studies have revealed a relationship between the host inflammatory response and tumor carcinogenesis, which suggests that the inflammatory response plays a role in the development, progression, and metastasis of cancer.[5,6] An ongoing systematic host reaction will also affect cancer progression,[7,8] and various inflammation-related indicators can be used to predict overall survival among patients with malignant tumors. For example, important inflammation markers include leukocytes, lymphocytes, neutrophils, platelets, and C-reactive protein. Recent studies have indicated that combinations of these systemic inflammatory parameters, including the neutrophil-lymphocyte ratio and the platelet-lymphocyte ratio, can predict prognosis for some malignant solid tumors.[9–11] Moreover, the systematic immune-inflammation index (SII) can predict prognosis among patients with liver cancer,[12] lung cancer,[13] gastric cancer,[14] and colorectal cancer.[15] Therefore, the present study aimed to evaluate whether the SII could predict survival among patients with colon cancer who underwent radical surgery, as well as the relationships between the preoperative SII and carcinoembryonic antigen (CEA) concentration, average length of stay (ALOS), and medical costs during hospitalization.
2. Patients and methods
This retrospective study included patients who underwent radical surgery for colon cancer between January 2011 and December 2013. The inclusion criteria were histologically confirmed colon cancer with complete clinical, laboratory, and follow-up data. The exclusion criteria were having undergone neoadjuvant therapy, intestinal perforation or obstruction, clinical evidence of infection, presence of hematological diseases, and the use of anti-inflammatory or immunosuppressive drugs. Based on these criteria, 118 patients (63 men and 55 women) with a median age of 60 years were included and 118 age- and sex-matched healthy subjects were also included.
Medical records were searched to obtain information regarding age, sex, clinicopathological features (tumor location, tumor size, histological type, and TNM stage), CEA concentration, ALOS, medical costs, and survival status at 1 year, 3 years, and 5 years after the operation. Preoperative blood samples had been used to obtain data regarding neutrophil, lymphocyte, and platelet counts. The SII was calculated as neutrophil × platelet/lymphocyte counts.[16]
2.1. Statistical analysis
Data were analyzed using SPSS software (version 18.0; SPSS Inc., Chicago, IL). The Mann–Whitney U test was used to compare the SII values between the patients and health subjects. Pearson correlation analysis was used to evaluate the relationships between the SII and CEA concentration, ALOS, and medical costs during hospitalization. The χ2 test or Fisher exact test were used to determine the relationship between the preoperative SII and the postoperative survival rate. Differences were considered statistically significant at P values of < .05.
3. Results
3.1. SII in patients with colon cancer and healthy subjects
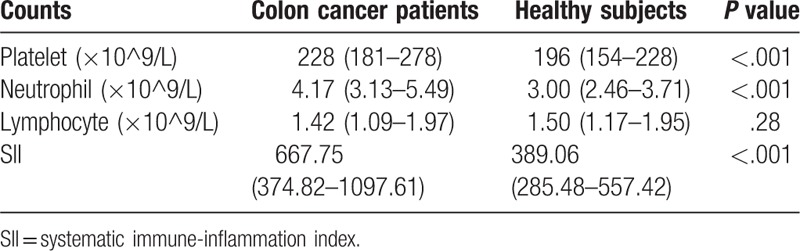
The present study included 118 patients with colon cancer and 118 healthy subjects. There were no significant differences in age and sex between the 2 groups. Relative to the healthy subjects, the patients with colon cancer had significantly higher counts of platelets and neutrophils in their peripheral blood, although no significant difference was detected in the lymphocyte counts. The median SII value was 667.75, and colon cancer patients had a clearly higher SII value than the healthy subjects (Table 1).
Table 1.
The value of SII in patients with colon cancer and healthy subjects.
3.2. Relationships between SII and clinical parameters
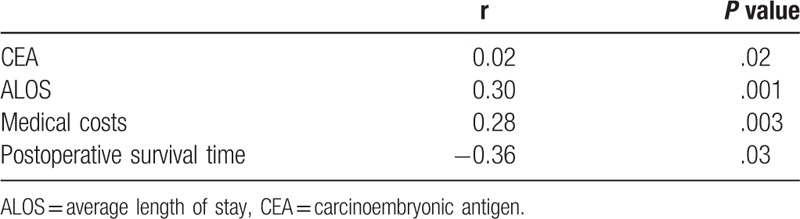
The clinical and pathological characteristics of the patients with colon cancer are shown in Table 2. The patients were subsequently categorized as having high or low SII values, based on the median value, which revealed that a high SII was significantly associated with larger tumor size (P = .03) and advanced TNM stage (P = .04), but not with sex (P = .10), tumor location (P = .64), or pathological grade (P = .05). The Pearson correlation analysis revealed that the SII was positively correlated with serum CEA concentration (P = .02), ALOS (P = .001), and medical costs (P = .003), but negatively correlated with postoperative survival time (P = .03) (Table 3).
Table 2.
Association of SII with clinicopathologic characteristics in patients with colon cancer.
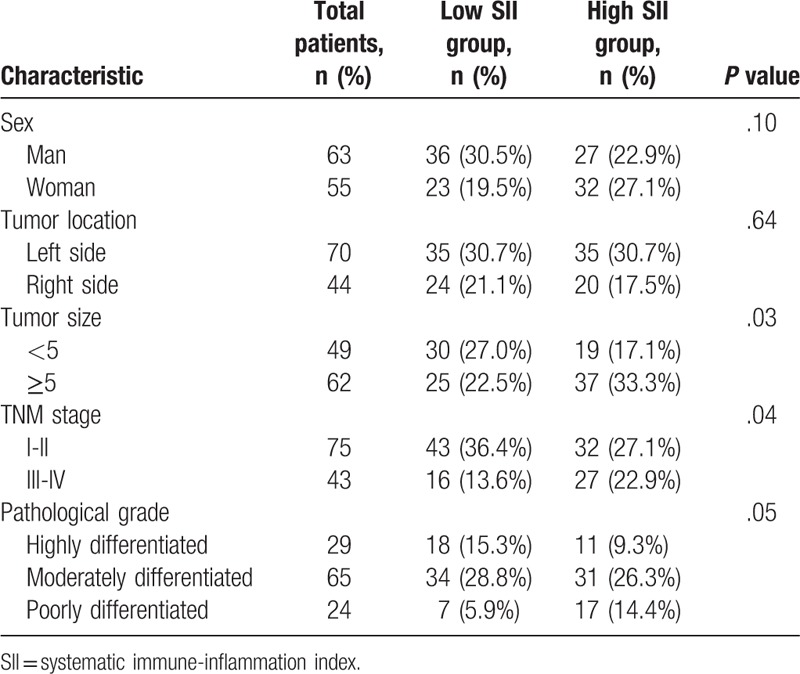
Table 3.
Association of SII with CEA, ALOS, medical costs and postoperative survival time.
3.3. Association of SII with the postoperative survival rate
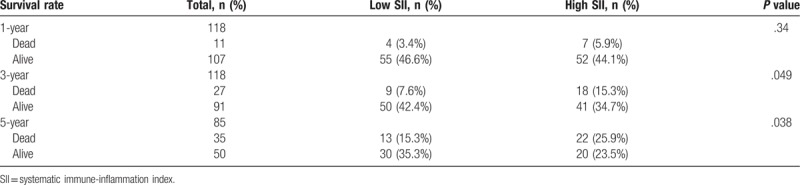
We further analyzed the relationship between the preoperative SII and the postoperative survival rate (Table 4). All patients completed >3 years of follow-up, although only 5-year follow-up data were only available for 85 patients, including 35 patients who had died (41.2%) and 50 patients who remained alive (58.8%). There was no significant difference in overall survival after 1 year between the high and low SII groups. However, the high SII group had significantly lower rates of overall survival after 3 years (34.7% [41/118] vs 42.4% [50/118]; P = .049) and after 5 years (23.5% [20/85] vs 35.3% [30/85]; P = .038).
Table 4.
Association of SII with survival rate at 1-, 3- and 5-year after operation.
4. Discussion
The present study revealed that the SII, which is an immuno-inflammatory index based on peripheral neutrophil, platelet, and lymphocyte counts, could predict prognosis among patients with operable colon cancer. Furthermore, patients with colon cancer had higher SII values than the healthy subjects, and a high SII value was associated with longer hospital stays and higher medical costs. Moreover, the SII was negatively correlated with postoperative survival time, and patients with high SII values had lower overall survival rates at 3 years and 5 years, relative to patients with low SII values.
The interaction between inflammation and cancer has been widely studied, and the SII effectively reflects the relationship between the inflammatory response and immune status. For example, a high SII value reflects changes in the cancer microenvironment that favor the development, progression, and metastasis of cancer. Neutrophils participate in the different stages of carcinogenesis, including enhancing tumor cell proliferation, migration and invasion, and tumor immunosuppression,[17,18] and can also secrete inflammatory mediators that promote tumor progression.[19,20] Platelets also secrete several growth factors and angiogenesis regulators that promote tumor growth.[21] Furthermore, lymphocytes are a type of immune cells that can clear tumor cells through cellular and humoral immune mechanisms.[22] Thus, the systemic inflammatory response plays an important role in tumor formation. The present study's findings indicate that patients with colon cancer had relatively high neutrophil and platelet counts (vs healthy subjects), which highlights the correlation between inflammation and tumor progression.
Present research has also revealed a link between inflammation-based indicators and clinicopathological features. Thus, the SII can be used as a simple, easily accessible, and inexpensive index of tumor size and CEA concentration. However, ours are the first findings to indicate that the preoperative SII was positively correlated with hospitalization duration and related medical costs, which may help physicians create a postoperative rehabilitation plan for patients with colon cancer.
The SII is considered an important predictor for various cancers. For example, Hu et al[12] suggested that the preoperative SII might be associated with circulating tumor cells and might predict prognosis among patients with hepatocellular carcinoma. Tomita et al[13] also reported that an elevated SII was negatively correlated with overall survival among patients with non-small cell lung cancer. In addition, the SII can predict prognosis among patients receiving first-line bevacizumab chemotherapy for metastatic colorectal cancer,[23] and Chen et al[15] have reported that the SII was able to predict overall survival among patients with colorectal cancer. In this context, the patient's prognosis is influenced by both the tumor's clinicopathological features and the host's inflammatory response, which is likely why the preoperative SII value has such good prognostic ability.
The present study revealed that the SII had good prognostic value among patients undergoing radical surgery for colon cancer. Furthermore, patients with a high SII value were likely to require a prolonged hospital stay and incur greater medical expenses. However, the present study also has 2 important limitations. First, this was a retrospective single-center study with a relatively small sample size. Second, we only included patients who underwent radical surgery and did not consider patients with inoperable advanced colon cancer. Therefore, a large-scale prospective study is needed to verify the preliminary results from the present study.
Acknowledgments
We would like to thank Editage (www.editage.com) for English language editing.
Author contributions
Data curation: Zhong-Hong Wang, Meng-Hui Zhang, Tian-Heng Ma, Xiao-Fei Chen.
Formal analysis: Xiao-Zhong Yang.
Investigation: Zhong-Hong Wang, Shang-Nong Wu.
Project administration: Xiao-Zhong Yang.
Resources: Xiao-Fei Chen.
Software: Tian-Heng Ma, Shang-Nong Wu.
Writing – original draft: Ming-Yue Tao.
Writing – review & editing: Hong-Gang Wang.
Hong-Gang Wang orcid: 0000-0003-4761-0407.
Footnotes
Abbreviations: ALOS = average length of stay, CEA = carcinoembryonic antigen, SII = systematic immune-inflammation index.
M-YT and Z-HW contributed equally to this work.
This study was reviewed and approved by The Affiliated Huaian No.1 People's Hospital of Nanjing Medical University Institutional Review Board. The patient provided written informed consents before participation in this study.
The authors have no conflicts of interest to disclose.
References
- [1].Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. Ca Cancer J Clin 2015;65:87–108. [DOI] [PubMed] [Google Scholar]
- [2].Xiong Y, You W, Hou M, et al. Nomogram integrating genomics with clinicopathological features improves prognosis prediction for colorectal cancer. Mol Cancer Res 2018;16:1373–84. [DOI] [PubMed] [Google Scholar]
- [3].Liu J, Zeng W, Huang C, et al. Predictive and prognostic implications of mutation profiling and microsatellite instability status in patients with metastatic colorectal carcinoma. Gastroenterol Res Pract 2018;2018:1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Fan XJ, Wan XB, Fu XH, et al. Phosphorylated p38, a negative prognostic biomarker, complements TNM staging prognostication in colorectal cancer. Tumour Biol 2014;35:10487–95. [DOI] [PubMed] [Google Scholar]
- [5].Valdés-Rives SA, González-Arenas A. Autotaxin-lysophosphatidic acid: from inflammation to cancer development. Mediators Inflamm 2017;2017:9173090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Hosseini F, Mahdian-Shakib A, Jadidi-Niaragh F, et al. Anti-inflammatory and anti-tumor effects of (-l-guluronic acid (G2013) on cancer-relatedinflammation in a murine breast cancer model. Biomed Pharmacother 2018;98:793–800. [DOI] [PubMed] [Google Scholar]
- [7].Diakos CI, Charles KA, Mcmillan DC, et al. Cancer-related inflammation and treatment effectiveness. Lancet Oncol 2014;15:e493–503. [DOI] [PubMed] [Google Scholar]
- [8].Crusz SM, Balkwill FR. Inflammation and cancer: advances and new agents. Nat Rev Clin Oncol 2015;12:584–96. [DOI] [PubMed] [Google Scholar]
- [9].Li Y, Jia H, Yu W, et al. Nomograms for predicting prognostic value of inflammatory biomarkers in colorectal cancer patients after radical resection. Int J Cancer 2016;139:220–31. [DOI] [PubMed] [Google Scholar]
- [10].Zou Z, Liu H, Ning N, et al. Clinical significance of pre-operative neutrophil lymphocyte ratio and platelet lymphocyte ratio as prognostic factors for patients with colorectal cancer. Oncol Lett 2016;11:2241–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Hong T, Shen D, Chen X, et al. A novel systematic inflammation related index is prognostic in curatively resected non-metastatic colorectal cancer. Am J Surg 2018;216:450–7. [DOI] [PubMed] [Google Scholar]
- [12].Hu B, Yang XR, Xu Y, et al. Systemic immune-inflammation index predicts prognosis of patients after curative resection for hepatocellular carcinoma. Clin Cancer Res 2014;20:6212–22. [DOI] [PubMed] [Google Scholar]
- [13].Tomita M, Ayabe T, Maeda R, et al. Systemic immune-inflammation index predicts survival of patients after curative resection for non-small cell lung cancer. In Vivo 2018;32:663–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Wang K, Diao F, Ye Z, et al. Prognostic value of systemic immune-inflammation index in patients with gastric cancer. Chin J Cancer 2017;36:75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Chen JH, Zhai ET, Yuan YJ, et al. Systemic immune-inflammation index for predicting prognosis of colorectal cancer. World J Gastroenterol 2017;23:6261–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Chiaruttini G, Mele S, Opzoomer J, et al. B cells and the humoral response in melanoma: The overlooked players of the tumor microenvironment. Oncoimmunology 2017;6:e1294296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Swierczak A, Mouchemore KA, Hamilton JA, et al. Neutrophils: important contributors to tumor progression and metastasis. Cancer Metastasis Rev 2015;34:735–51. [DOI] [PubMed] [Google Scholar]
- [18].Mayer C, Darb-Esfahani S, Meyer AS, et al. Neutrophil granulocytes in ovarian cancer-induction of epithelial-to-mesenchymal-transition and tumor cell migration. J Cancer 2016;7:546–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Moses K, Brandau S. Human neutrophils: their role in cancer and relation to myeloid-derived suppressor cells. Seminars in Immunology 2016;28:187–96. [DOI] [PubMed] [Google Scholar]
- [20].Felix K, Gaida MM. Neutrophil-derived proteases in the microenvironment of pancreatic cancer-active players in tumor progression. Int J Biol Sci 2016;12:302–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Li N. Platelets in cancer metastasis: to help the “villain” to do evil. International Journal of Cancer 2016;138:2078–87. [DOI] [PubMed] [Google Scholar]
- [22].Karn T, Pusztai L, Rody A, et al. The influence of host factors on the prognosis of breast cancer: stroma and immune cell components as cancer biomarkers. Curr Cancer Drug Targets 2015;15:652–64. [DOI] [PubMed] [Google Scholar]
- [23].Passardi A, Scarpi E, Cavanna L, et al. Inflammatory indexes as predictors of prognosis and bevacizumab efficacy in patients with metastatic colorectal cancer. Oncotarget 2016;7:33210–9. [DOI] [PMC free article] [PubMed] [Google Scholar]


