Abstract
Introduction:
To systematically compare immediate postoperative tracheal extubation (IPTE) with conventional tracheal extubation (CTE) and to determine whether IPTE can achieve an enhanced recovery for adult patients underwent liver transplantation (LT) without additional risks. We designed a systematic review and meta-analysis.
Methods:
The RCTs, cohorts, case–controls, or case series that explored outcomes of IPTE after LT for adults were involved in our study. The Newcastle–Ottawa scale was used to assess the risk of bias.
Results:
A total of 15 studies (n = 4144) were included, consisting of 10 studies (retrospective cohorts; n = 3387) for quantitative synthesis and 5 studies (1 prospective cohort, and 4 case series; n = 757) for qualitative synthesis. The pooled estimates suggested IPTE could reduce time to discharge from ICU stay (TDICU) (mean difference [MD] −2.12 days, 95% confidence interval [CI] −3.04 to −1.19 days), time to discharge from the hospital (TDH) (MD −6.43 days, 95% CI −9.53 to −3.33 days), re-intubation rate (RI) (odds ratio [OR] 0.29, 95% CI 0.22–0.39), morbidity rate (MR) (OR 0.15, 95% CI 0.08–0.30) and graft dysfunction rate (GD) (IPTE vs CTE: 0.3% vs 3.8%, P < .01), and had comparable ICU survival rate (ICUS) (OR 6.67 95% CI 1.34–33.35) when compared with CTE after LT.
Conclusions:
IPTE can achieve an enhanced recovery for adult patients underwent LT without additional re-intubation, morbidity, and mortality risks. However, further work needs to be done to establish the extent definitively through carefully designed and conducted RCTs.
Keywords: conventional tracheal extubation, enhanced recovery after surgery, immediate postoperative tracheal extubation, liver transplantation
Strengths and limitations of this study
This is, to our knowledge, the first systematic review and meta-analysis on immediate postoperative tracheal extubation (IPTE) for enhanced recovery after liver transplantation (LT), with the aim of promoting the clinical application of this practice.
The sources of heterogeneity were explored with two prior subgroup hypotheses: the anesthesia types (fast-track anesthesia and traditional anesthesia) and, and the time-intervalbased IPTE types (< 1-hour, < 4-hours and < 8-hours).
The qualitative analyses were conducted on the issues including indications, anesthesia, extubation criteria, complications, re-intubation causes and overall survival rates.
The potential limitations include all non-RCTs involved, the asymmetry of data sources due to a regional imbalance of technology development and the failure to quantitatively analyze readmission rate and costs due to the limited and different-baseline data.
1. Introduction
Liver transplantation (LT) is the most effective approach to treat decompensated liver cirrhosis so far.[1] Because of the particularity of this major surgery and the complexity of patients’ diseases, delayed postoperative tracheal extubation always exists. However, some studies have found this conventional tracheal extubation (CTE) may go against the postoperative rehabilitation of patients.[2–6] For example, prolonged mechanical ventilation increases the risk of pulmonary infection and intensifies the reduction of liver blood flow markedly in the context of high cardiac indices during LT and of compromised immune after LT, eventually leading to lung or liver failures,[7–9] increased risks of death, postponed intensive care unit (ICU) stay and addition of medical expenses.[10,11] To solve these problems, LT doctors pay attention to immediate postoperative tracheal extubation (IPTE), which was first introduced into LT as part of resource-utilization-emphasizing fast-tracking (nowadays, called enhanced recovery after surgery, ERAS[12]) 30 years ago.[13] In the last 10 years, a series of studies have demonstrated IPTE's feasibility and safety: 60% to 80% of LT patients could undergo IPTE in the operating room without an increased risk of subsequent reintubation.[5,14,15] Nevertheless, IPTE is still thought to be a traditional-contrary practice against the view of 48 hours-ventilation after major surgery; only a few large LT centers take a positive attitude to this challenging risky practice. Hence, our aims were to systematically compare IPTE with CTE and to determine whether IPTE can achieve an enhanced recovery for adult patients underwent LT without additional risks.
2. Methods
We have stated as required in the first paragraph of the methods section that this was a secondary study based on previously published results, thus no ethical approval and patient consent are required.
We followed the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) recommendations,[16] and the Meta-analysis of Observational Studies in Epidemiology (MOOSE) guidelines.[17]
2.1. Definition
In this study, IPTE refers to < 8 hours between extubation and end of surgery; successfully tracheal extubation refers to no need of reintubation within the first 48 hours after extubation.
2.2. Eligibility criteria
We considered all studies published in any language, designed with RCT, cohort, case-control, or case series, and comparing IPTE with CTE after LT for adults. Moreover, the following criteria were also required: including more than 5 patients; original complete publications with full-text accessible; no overlapping data between studies.
2.3. Literature search
We searched PubMed, Ovid Embase, Cochrane Central Register of Controlled Trials, and Web of Science from database inception up to June 2017 (and continuously updated to September 2017), any language, using MeSH as far as possible. We also identified potentially relevant studies by scanning reference lists of review articles and consultation with experts in the field. An information expert (DP) developed and conducted the search strategy (Appendix 1A).
2.4. Study process
Two reviewers independently participated in the initial screening records based on the above-mentioned eligibility criteria, reviewing full-text articles, quality assessment, and collecting data from each eligible study using detailed instructions. The cross-check was performed to identify discrepancies. Any conflicts arising between the 2 reviewers were adjudicated by a third reviewer (JYY).
2.5. Risk of bias assessment
We used the Newcastle–Ottawa scale (NOS)[18] to assess the methodological quality of the studies included in this review. Two investigators independently assessed quality, and another resolved the discrepancies.
2.6. Data collection
We collected a total of 158 pieces (details seen in Appendix 1B) of information in 4 aspects: general characteristics (16 items), preoperative variables (53 items), intraoperative variables (57 items), and postoperative variables (32 items).
2.7. Data analysis
For quantitative synthesis, we assessed heterogeneity between studies using the χ2 test and I2 statistic. We pooled mean difference (MD) for continuous data using Inverse Variance methods and odds ratios (OR) for dichotomous data using Mantel–Haenszel methods, reporting pooled results along with their associated 95% confidence intervals (CI). The sources of heterogeneity were explored through 2 prior subgroup hypotheses: the anesthesia types (fast-track anesthesia and traditional anesthesia) and the time-interval-based IPTE types (< 1-hour, < 4-hours and < 8-hours). We undertook sensitivity analyses by using alternative effect measures (odds ratio ν relative risk; mean difference ν standardized mean difference). A P value of < .01 was considered significant.
3. Results
3.1. Search results
Our search yielded 683 potentially relevant reports. After screening titles and abstracts, we retrieved 138 reports for full-text screening and then excluded 123 ones for no useable data (n = 68), improper study design (n = 45), overlapping data (n = 1)[19] and conference abstracts (n = 9). Finally, a total of 15 studies, including 10 retrospective ones,[9,20–28] 1 prospective one[29] and 4 case series[2,30–32] were eligible for inclusion (Fig. 1). A total number of 4144 patients were recruited, including 3387 patients from 10 studies[9,20–28] for quantitative synthesis and 757 patients from 5 studies[2,29–32] for qualitative synthesis (Fig. 1).
Figure 1.
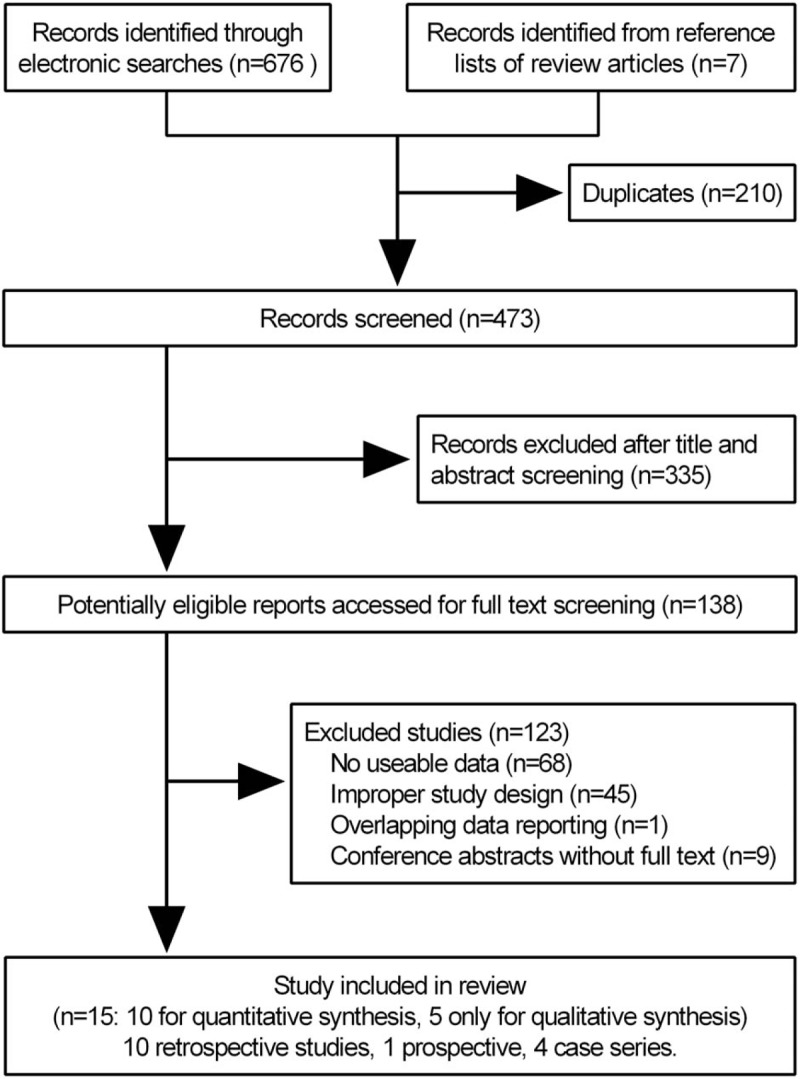
Flow chart of article selection.
3.2. Characteristics of studies
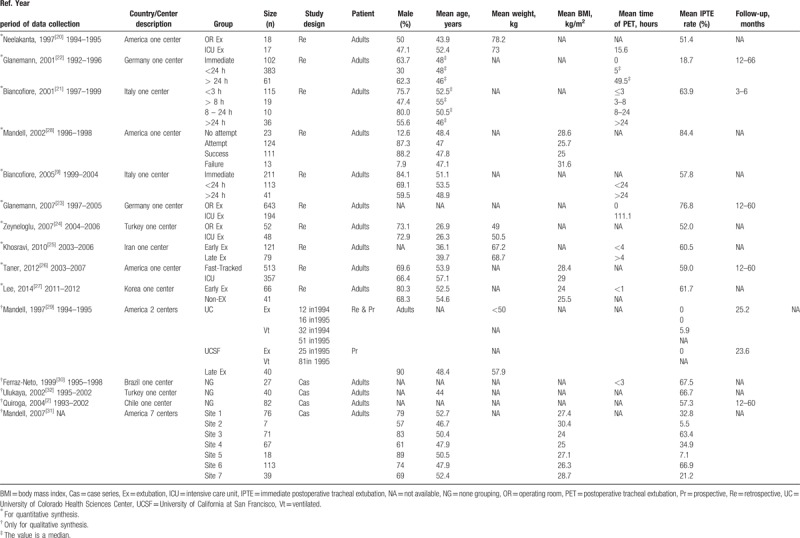
Of the 15 included studies, 13 (86%) were unicentric, 1 (7%) was bicentric and 1 (7%) was multicentric (7 involved centers); because 3 centers[9,21,31] were duplicated, there are actually only 19 involved centers. Of these 19 involved LT centers, 7 (37%) were in North America, 6 (32%) were in Europe, 4 (21%) were in Asia and 2 (10%) were in South America. The enrolled sample sizes ranged from 27 to 870, with a mean age range of 26 to 57 years, mean weight range of 49.0 to 78.2 kg, mean body mass index (BMI) range of 24.0 to 31.6 kg/m2, mean postoperative ventilation time range of 0–111.1 hours, mean IPTE rate range of 5.5% to 84.4% and follow-up range of 3 to 66 months (Table 1).
Table 1.
Characteristics of studies included for meta-analysis and systematic review.
3.3. Baseline features comparison for IPTE and CTE after LT
No significant differences were found between IPTE and CTE regarding recipient age (MD −1.66 years, 95% CI −3.45 to 0.13 years) (Fig. 2A), Child C status % (OR 0.75, 95% CI 0.39–1.45) (Fig. 2B), recipient BMI (MD −1.30 kg/m2, 95% CI –2.41 to −0.18 kg/m2) (Fig. 2C), model for end-stage liver disease (MELD) scores (MD −2.46, 95% CI −4.45 to −0.47) (Fig. 2D), preoperative creatinine (MD −14.58 μmol/L, 95% CI −30.9 to −1.73 μmol/L) (Fig. 2E) and cold ischemic time of graft (MD 4.18 minutes, 95% CI −17.43 to −25.78 minutes) (Fig. 2F). Still, there were significant differences between the 2 approaches regarding the amount of packed red cell (PCR) transfused (MD −4.30 U, 95% CI −6.24 to −2.37 U) (Fig. 2G) and the duration of surgery (MD −32.02 minutes, 95% CI −47.73 to −16.31 minutes) (Fig. 2H). The subgroup analysis indicated a correlation between anesthesia types and IPTE. The fast-track anesthesia favored IPTE through less amount of PCR transfused and less duration of surgery (Fig. 2G and H). Likewise, the subgroup analysis by time-interval-based IPTE types revealed an inclination for the short time-interval IPTE (<1-hour) by less amount of PCR transfused and less duration of surgery (Figs. D and E in Appendix 2).
Figure 2.
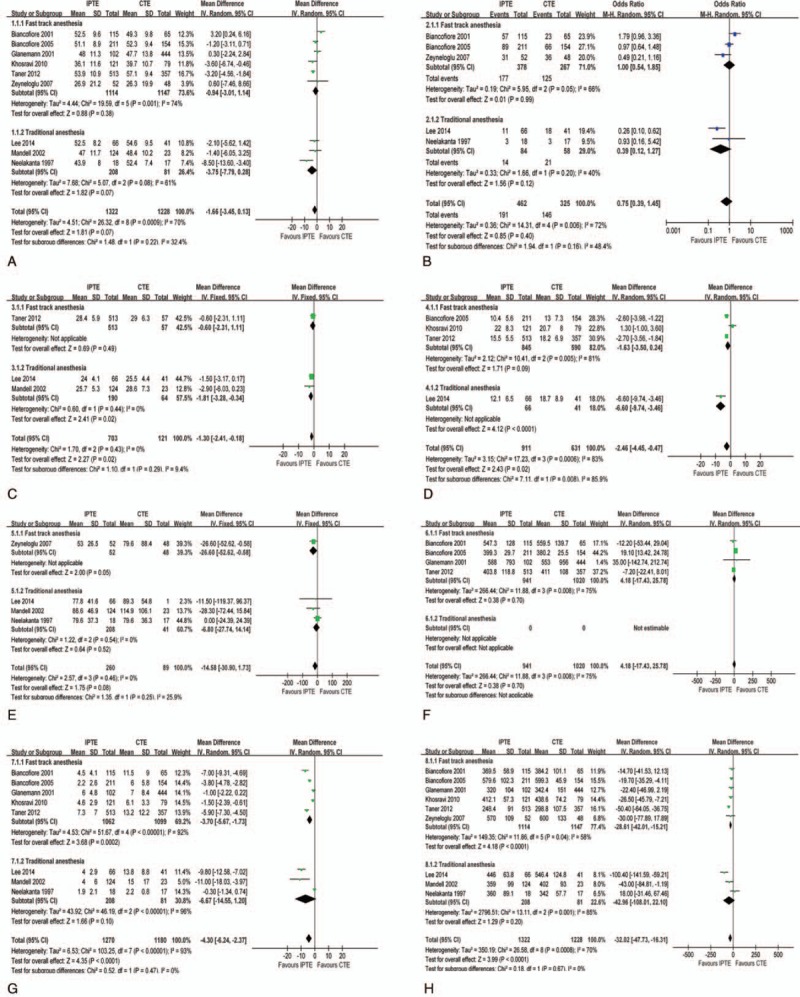
Baseline features comparison for IPTE and CTE after LT. (A). Recipient age; (B). Recipient BMI; (C). Child C status %; (D). MELD score; (E). Preoperative creatinine; (F) Cold ischemic time of graft; (G) amount of PCR transfused (H) Duration of surgery.
No obvious heterogeneity was found regarding the left baseline items (Figs. A–F in Fig. 2 and Figs. A–C in Appendix 2). The sensitivity analysis also did not show any significant change in the pooled effects (Figs. F–M in Appendix 2). In addition, regarding Child C status (%), one study[27] adopted the data form of Child B+C %, however, it did not change the pooled effect as removed from the main analysis (Fig. N in Appendix 2).
3.4. Outcome comparison between IPTE and CTE after LT
Of the 10 studies for quantitative analysis, 7 reported time to discharge from ICU stay (TDICU), with a mean range of 0.9 to 5.7 days in IPTE group and 1.5 to 11.2 days in CTE group; 6 reported time to discharge from the hospital (TDH), with a mean range of 9.1–29.6 days in IPTE group and 19 to 31 days in CTE group; 8 reported re-intubation rate (RI), with a mean range of 0% to 11.7% in IPTE group and 0% to 35.6% in CTE group; 3 reported morbidity rate (MR), with a mean range of 0% to 10.6% in IPTE group and 11.8% to 43.9% in CTE group; 4 reported ICU survival rate (ICUS), with a mean range of 98.5% to 100% in IPTE group and 73.8% to 98.3% in CTE group.
IPTE had the significant advantages over CTE in reducing TDICU (MD −2.12 days, 95% CI −3.04 to −1.19 days; Fig. 3A), TDH (MD −6.43 days, 95% CI −9.53 to −3.33 days; Fig. 3B), RI (odds ratio [OR] 0.29, 95% CI 0.22–0.39; Fig. 3C) and MR (OR 0.15, 95% CI 0.08–0.30; Fig. 3D) through main analysis. The results from 2 kinds of subgroup analyses were identical to those of the main analysis (Fig. A–D in Fig. 3, and Figs. O–Q in Appendix 2). Nor was there a difference by the sensitivity analysis using alternative effect measures (Figs. R–V in Appendix 2), indicating the robustness of main results.
Figure 3.
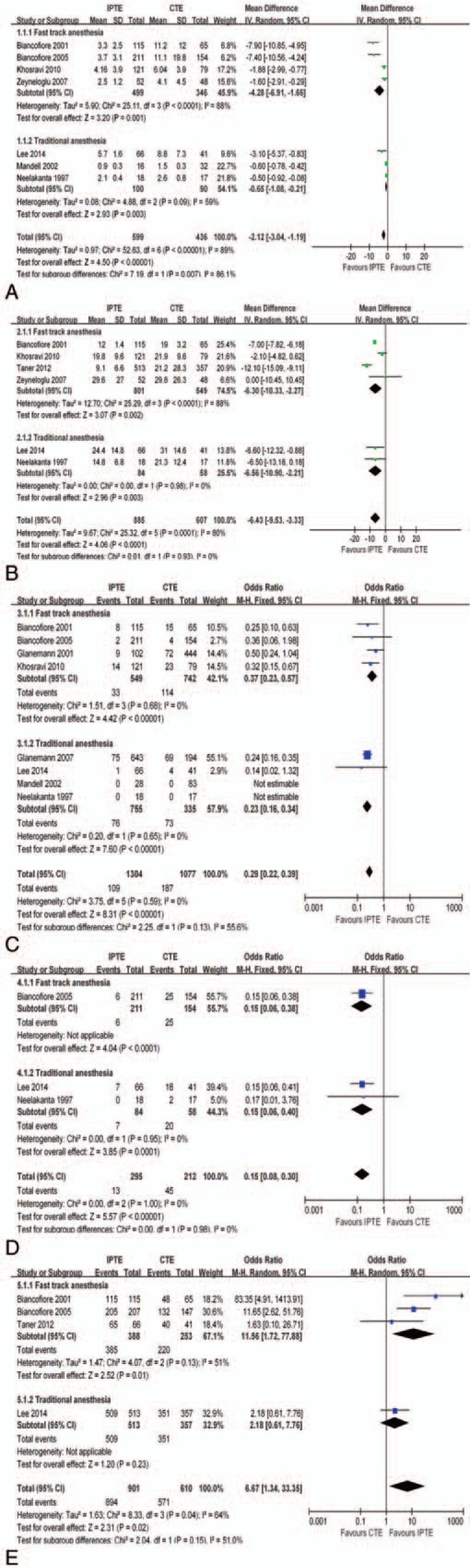
Outcome comparison between IPTE and CTE after LT. (A). Times to discharge from ICU stay (TDICU); (B). Times to discharge from the hospital (TDH); (C). Re-intubation rate (RI); (D). Morbidity rate (MR); (E). ICU survival rate (ICUS).
Regarding ICUS, the P-value of .02 derived from the main analysis did not satisfy our previous set value (P < .01) for the significance of the difference. However, subgroup analysis showed IPTE had a higher ICUS than CTE did when fast-track anesthesia was adopted (OR 11.56 95% CI 1.72–77.88; Fig. 3E).
3.5. Indications, anesthesia, extubation criteria, complications, re-intubation causes, and overall survival rates
For IPTE, there was a total of 954 patients diagnosed with postnecrotic cirrhosis and 302 patients with liver malignancy (Appendix 3A). Hepatitis C virus-related cirrhosis was the most common diagnosis, which accounted for 21.9% of all indications in the IPTE group. The constituent of indications was generally similar in both groups but still had a little difference. Compared with CTE group, IPTE group had a little larger proportion of patients with the multi-virus-related cirrhosis and hepatic cellular cancer (HCC), yet had a smaller proportion of patients with acute liver failure and re-liver-transplantation (Fig. 4A and Appendix 3A).
Figure 4.
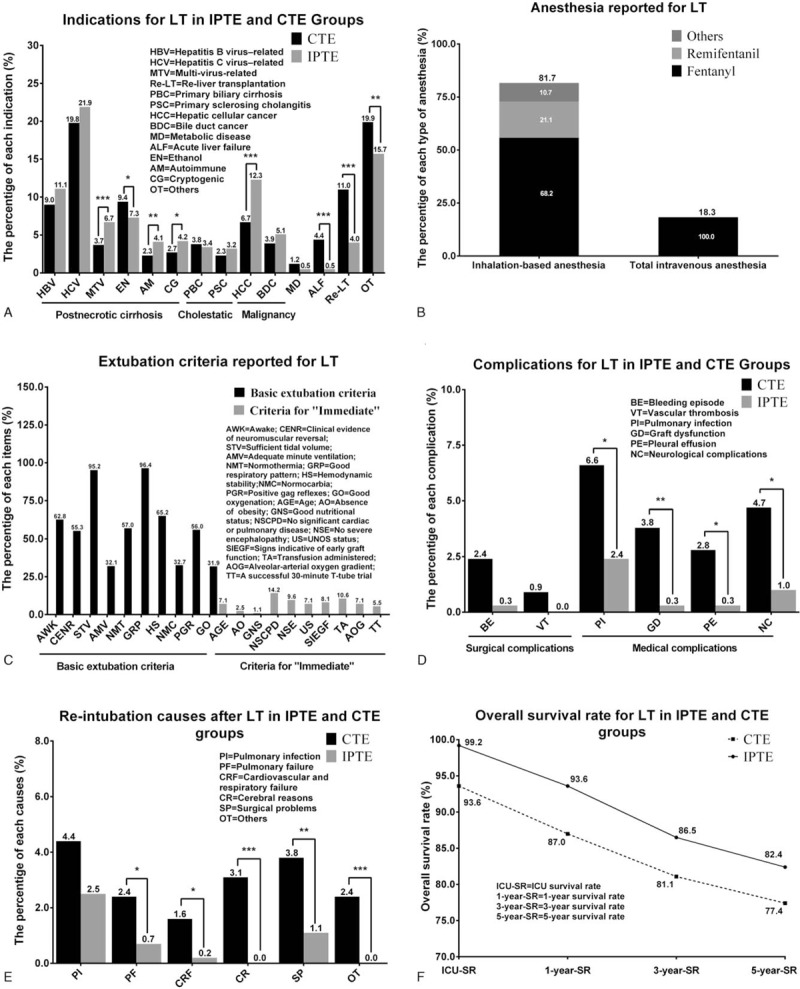
Indications, anesthesia, extubation criteria, complications, re-intubation causes and overall survival rate. (A). Indications for LT in IPTE and CTE groups; (B). Anesthesia reported for LT; (C). Extubation criteria reported for LT; (D). Complications for LT in IPTE and CTE groups; (E) Re-intubation causes after LT in IPTE and CTE groups; (F) Overall survival rate for LT in IPTE and CTE groups.
Regarding anesthesia, the fast-track anesthesia is a balanced anesthetic regimen that aims at early-extubation. This anesthetic approach usually adopted inhalation-based anesthetic techniques, prominently featured with the increased use (21.1%) of remifentanil in recent years and taking up the majority with a proportion of 81.7% when compared with total intravenous anesthesia (Fig. 4B and Appendix 3B).
With respect to extubation criteria, a good respiratory pattern, a sufficient tidal volume, and an awake state were the top 3 basic items, which accounted for 96.4%, 95.2%, and 62.8%, respectively (Fig. 4C and Appendix 3C). In criteria for “immediate,” the top 3 items were no significant cardiac or pulmonary disease, no large blood transfusion and no severe encephalopathy, which accounted for 14.2%, 10.6%, and 9.6%, respectively (Fig. 4C and Appendix 3C).
Regarding complications, the differences were from the graft dysfunction rate (GD), which was 0.3% in the IPTE group, while 3.8% in the CTE group (P < .01) (Fig. 4D and Appendix 3D).
About the re-intubation cause, pulmonary infection was the most common one, accounted for 2.5% in the IPTE group and 4.4% in the CTE group. In addition, the CTE group had more re-intubation cases due to encephalopathy and surgical problems (P < .01) (Fig. 4E and Appendix 3E).
The overall survival rates for LT were shown in Fig. 4F and Appendix 3F. The ICU, 1-, 3- and 5-year OS, were pooled to be 99.2%, 93.6%, 86.5%, and 82.4%, respectively, favoring IPTE.
3.6. Evidence from the studies for qualitative synthesis
Of the 5 studies[2,29–32] for qualitative synthesis, one[29] was a prospective cohort and 4[2,30–32] were case series. The prospective cohort,[29] a 2-center study, indicated IPTE of selected LT patients to be safe with the reintubation rate of 8% (2 out of 25) and to be cost-effective with the average saving of $2709 in Colorado, 1995.
Of the 4 case series,[2,30–32] one[31] was a 7-institutions-involved study. Their results showed a few pulmonary or surgically related adverse events occurred in 7.7% of 391 patients after IPTE. The remaining 3 studies[2,30,32] confirmed the safety and cost-effectiveness of IPTE.
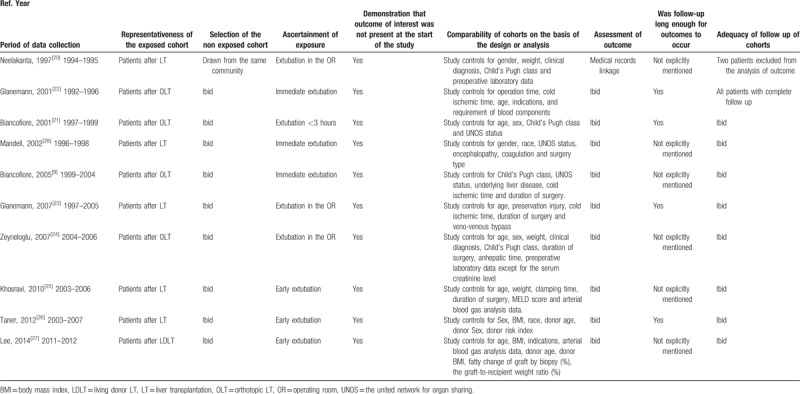
3.7. Quality of included studies and risk of bias
All the studies used patients’ medical records for their analyses (Table 2). The IPTE exposures were inconsistent across those studies, 7[9,20–23,25,27] studies described the detailed time interval, but 3[24,26,28] studies failed to give the detailed description. We sparingly pigeonholed the 3 ones as “< 8 hours ν control,” with the risk of adjustment questionable. Moreover, most cohort studies failed to report the level of quality of follow-up. Because of these limitations, the risk of bias associated with eligible data was low to moderate.
Table 2.
Risk of bias of cohort studies included for quantitative synthesis.
4. Discussion
In this systematic review and analysis of the ten retrospective studies (low to moderate risk of bias involving 1965 cases of IPTE among 3387 patients), one prospective study (moderate risk of bias involving 53 cases of IPTE among 217 patients) and 4 case series (moderate risk of bias involving 540 IPTE among 540 patients) we found the evidence to suggest IPTE has its advantages over CTE, regarding of reducing TDICU, TDH, RI, MR, GD, and of a similar ICUS with CTE. Potential risk factors should be considered when considering the actual situation. Regarding of TDH, not a few researchers gave up the analysis of the relationship between extubation and TDH, due to confounding factors such as episodes of patient's rejection, payment issues, out of service for hospital discharge on weekends and holidays and so on. Another issue is the possibility of the bias that comes from the regional imbalance of data sources. By 2013, LT was performed in over 80 countries worldwide,[33] however, 86.6% (3588/4144) of our data came from only 4 countries in Europe and North America as a result of the technical imbalance.
To date, our study provides the first systematic review and meta-analysis of outcome comparison between IPTE and CTE after LT. We found and identified 5 reviews[4,5,8,15,34] which overviewed IPTE after LT. The 5 reviews reached complete agreement on IPTE's advantages of reducing TDICU, TDH, and costs. With respect to cost saving, we failed to do the quantitative synthesis because of the different baseline of the data. According to the existing literature, we learned that ICU care fee accounts for more than 25% of the total cost of LT.[10] The reducing of TDICU theoretically could reduce the high cost of ICU services[4,34] and facilitate the management of available resources.[4]
Minimizing the incidence of complications and maximizing the survival rates should be the prerequisite for IPTE.[15] Our results showed reduced RI, MR, and comparable ICUS, which were coordinated with the most reviews.[4,15,34] We also found the pulmonary complication was the most common cause for re re-intubation but could be resolved in most cases by means of increasing ambient oxygen concentration.[34] Moreover, we found the graft dysfunction rate was significantly lower in the IPTE group than that in the CTE group, which was not revealed in the previous reviews.
Interestingly, a successful IPTE is more likely to happen to patients with liver malignancy, which may be ignored by most researchers. On this point, our result confirmed Taner's[26] view that patients with higher MELD scores assigned by sickest-first prioritization–based allocation system due to the diagnosis of hepatic cellular cancer or cholangiocarcinoma might be ideal candidates for IPTE after LT.
5. Conclusion
In summary, the available evidence suggests that compared with CTE, IPTE can reduce TDICU, TDH, RI, MR, GD and have a similar ICUS. Our conclusion would be of assistance in the promotion of clinical application of IPTE. However, further carefully designed and conducted RCTs are warranted to definitively establish the extent, if any, of increased risk.
Author contributions
Conceptualization: Jianbo Li, Chengdi Wang, Lunan Yan, Jiayin Yang.
Data curation: Jianbo Li, Chengdi Wang.
Formal analysis: Yuting Jiang, Jiulin Song, Longhao Zhang, Nan Chen, Rui Zhang, Lan Yang, Qin Yao, Li Jiang.
Methodology: Jian Yang.
Writing – original draft: Yang Yang.
Writing – review & editing: Tao Zhu, Weimin Li, Lunan Yan.
Footnotes
Abbreviations: BMI = body mass index, CI = confidence intervals, CTE = conventional tracheal extubation, GD = graft dysfunction rate, GD = graft dysfunction rate, HCC = hepatic cellular cancer, ICU = postponed intensive care unit, ICUS = ICU survival rate, IPTE = immediate postoperative tracheal extubation, LT = liver transplantation, MD = mean difference, MELD = model for end-stage liver disease, MOOSE = Meta-analysis of Observational Studies in Epidemiology, MR = morbidity rate, NOS = Newcastle–Ottawa scale, OR = odds ratio, PRISMA = Preferred Reporting Items for Systematic Reviews and Meta-analyses, RI = re-intubation rate, TDH = time to discharge from the hospital, TDICU = time to discharge from ICU stay.
JL, CW and YJ contributed equally to this work.
Funding Supported by a grant from the National Science and Technology Major Project of China (Grant number 81470037 to Prof. JY).
The authors have no conflicts of interest to disclose.
References
- [1].Starzl TE. The long reach of liver transplantation. Nat Med 2012;18:1489–92. [DOI] [PubMed] [Google Scholar]
- [2].Quiroga M, Rodriguez MG, Montalvan C, et al. Trends in mechanical ventilation and immediate extubation after liver transplantation in a single center in Chile. Transplant Proc 2004;36:1683–4. [DOI] [PubMed] [Google Scholar]
- [3].Faenza S, Ravaglia MS, Cimatti M, et al. Analysis of the causal factors of prolonged mechanical ventilation after orthotopic liver transplant. Transplant Proc 2006;38:1131–4. [DOI] [PubMed] [Google Scholar]
- [4].Wu J, Rastogi V, Zheng SS. Clinical practice of early extubation after liver transplantation. Hepatobil Pancr Dis Int 2012;11:577–85. [DOI] [PubMed] [Google Scholar]
- [5].Aniskevich S, Pai SL. Fast track anesthesia for liver transplantation: review of the current practice. World J Hepatol 2015;7:2303–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Yuan H, Tuttle-Newhall JE, Chawa V, et al. Prognostic impact of mechanical ventilation after liver transplantation: a national database study. Am J Surg 2014;208:582–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Luo XR, Zeng GB, Liu SR, et al. Impact of adaptive positive end expiratory pressure and mechanical ventilation on hemodynamics and oxygen kinetics in post-liver transplantation patients. Zhongguo Wei Zhong Bing Ji Jiu Yi Xue 2007;19:404–7. [PubMed] [Google Scholar]
- [8].Plevak DJ, Torsher LC. Fast tracking in liver transplantation. Liver Transplant Surg 1997;3:447–8. [DOI] [PubMed] [Google Scholar]
- [9].Biancofiore G, Bindi ML, Romanelli AM, et al. Fast track in liver transplantation: 5 years’ experience. Eur J Anaesthesiol 2005;22:584–90. [DOI] [PubMed] [Google Scholar]
- [10].Showstack J, Katz PP, Lake JR, et al. Resource utilization in liver transplantation: effects of patient characteristics and clinical practice. NIDDK Liver Transplantation Database Group. JAMA 1999;281:1381–6. [DOI] [PubMed] [Google Scholar]
- [11].Taylor MC, Greig PD, Detsky AS, et al. Factors associated with the high cost of liver transplantation in adults. Can J Surg 2002;45:425–34. [PMC free article] [PubMed] [Google Scholar]
- [12].Steenhagen E. Enhanced recovery after surgery: it's time to change practice!. Nutr Clin Pract 2016;31:18–29. [DOI] [PubMed] [Google Scholar]
- [13].Rossaint R, Slama K, Jaeger M, et al. Fluid restriction and early extubation for successful liver transplantation. Transplant Proc 1990;22:1533–4. [PubMed] [Google Scholar]
- [14].Blaszczyk B, Wronska B, Klukowski M, et al. Factors affecting breathing capacity and early tracheal extubation after liver transplantation: analysis of 506 cases. Transplant Proc 2016;48:1692–6. [DOI] [PubMed] [Google Scholar]
- [15].Glanemann M, Busch T, Neuhaus P, et al. Fast tracking in liver transplantation. Immediate postoperative tracheal extubation: feasibility and clinical impact. Swiss Med Wkly 2007;137:187–91. [DOI] [PubMed] [Google Scholar]
- [16].Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 2009;339:b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 2000;283:2008–12. [DOI] [PubMed] [Google Scholar]
- [18].Stang A. Critical evaluation of the Newcastle–Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 2010;25:603–5. [DOI] [PubMed] [Google Scholar]
- [19].Glanemann M, Langrehr JM, Muller AR, et al. Incidence and risk factors of prolonged mechanical ventilation and causes of reintubation after liver transplantation. Transplant Proc 1998;30:1874–5. [DOI] [PubMed] [Google Scholar]
- [20].Neelakanta G, Sopher M, Chan S, et al. Early tracheal extubation after liver transplantation. J Cardiothorac Vasc Anesth 1997;11:165–7. [DOI] [PubMed] [Google Scholar]
- [21].Biancofiore G, Romanelli AM, Bindi ML, et al. Very early tracheal extubation without predetermined criteria in a liver transplant recipient population. Liver Transpl 2001;7:777–82. [DOI] [PubMed] [Google Scholar]
- [22].Glanemann M, Langrehr J, Kaisers U, et al. Postoperative tracheal extubation after orthotopic liver transplantation. Acta Anaesthesiol Scand 2001;45:333–9. [DOI] [PubMed] [Google Scholar]
- [23].Glanemann M, Hoffmeister R, Neumann U, et al. Fast tracking in liver transplantation: which patient benefits from this approach? Transplant Proc 2007;39:535–6. [DOI] [PubMed] [Google Scholar]
- [24].Zeyneloglu P, Pirat A, Guner M, et al. Predictors of immediate tracheal extubation in the operating room after liver transplantation. Transplant Proc 2007;39:1187–9. [DOI] [PubMed] [Google Scholar]
- [25].Khosravi MB, Lahsaei M, Ghafaripour S, et al. Factors affecting early and late extubation in liver transplant patients. Iran Red Crescent Me 2010;12:172–5. [Google Scholar]
- [26].Taner CB, Willingham DL, Bulatao IG, et al. Is a mandatory intensive care unit stay needed after liver transplantation? Feasibility of fast-tracking to the surgical ward after liver transplantation. Liver Transpl 2012;18:361–9. [DOI] [PubMed] [Google Scholar]
- [27].Lee S, Sa GJ, Kim SY, et al. Intraoperative predictors of early tracheal extubation after living-donor liver transplantation. Kor J Anesthesiol 2014;67:103–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Mandell MS, Lezotte D, Kam I, et al. Reduced use of intensive care after liver transplantation: patient attributes that determine early transfer to surgical wards. Liver Transpl 2002;8:682–7. [DOI] [PubMed] [Google Scholar]
- [29].Mandell MS, Lockrem J, Kelley SD. Immediate tracheal extubation after liver transplantation: experience of two transplant centers. Anesth Analg 1997;84:249–53. [DOI] [PubMed] [Google Scholar]
- [30].Ferraz-Neto BH, Silva ED, Afonso RC, et al. Early extubation in liver transplantation. Transplant Proc 1999;31:3067–8. [DOI] [PubMed] [Google Scholar]
- [31].Mandell MS, Stoner TJ, Barnett R, et al. A multicenter evaluation of safety of early extubation in liver transplant recipients. Liver Transpl 2007;13:1557–63. [DOI] [PubMed] [Google Scholar]
- [32].Ulukaya S, Ayanoglu HO, Acar L, et al. Immediate tracheal extubation of the liver transplant recipients in the operating room. Transplant Proc 2002;34:3334–5. [DOI] [PubMed] [Google Scholar]
- [33].Zarrinpar A, Busuttil RW. Liver transplantation: past, present and future. Nat Rev Gastroenterol Hepatol 2013;10:434–40. [DOI] [PubMed] [Google Scholar]
- [34].Mandell MS, Campsen J, Zimmerman M, et al. The clinical value of early extubation. Curr Opin Organ Transplant 2009;14:297–302. [DOI] [PubMed] [Google Scholar]


