Abstract
Rationale:
Pneumocardia and septic pulmonary embolism are uncommon complications of Klebsiella pneumoniae primary liver abscess (KPLA); however, they may lead to a poor clinical outcome.
Patient concerns:
A 67-year-old woman was admitted to our hospital with fever, chills, cough, and dyspnea for 4 days. She had a previous history of diabetes mellitus.
Diagnoses:
The chest computed tomography (CT) revealed multiple peripheral nodules in both lungs and wedge-shaped peripheral infiltrative lesions abutting the pleura, suggestive of septic pulmonary embolism. An abdominal CT on the following day showed a large liver abscess without gas formation and pneumocardia of the right ventricle.
Interventions:
After the antibiotic therapy of intravenous imipenem and drainage of the liver abscess, our patient made a complete recovery.
Outcomes:
The patient was discharged on the 25th hospital day after full recovery and was doing well on follow-up at 10 months.
Lessons:
KPLA is potentially fatal due to the associated serious metastatic complications. Attention must be paid not only to the primary focus of infection but also to infection of other organs. It is important to detect to diagnose the spread of infection accurately, in a timely manner, to improve the prognosis of this condition.
Keywords: Klebsiella pneumoniae, liver abscess, pneumocardia, septic pulmonary embolism
1. Introduction
Klebsiella pneumoniae primary liver abscess (KPLA) is an emerging disease[1] associated with septic complications. Pneumocardia[2] and septic pulmonary embolism (SPE)[3] are uncommon, but potentially fatal complications of KPLA. Pneumocardia has been reported only in emphysematous liver abscess,[2,4,5] and has not been previously reported with SPE. We report a unique case of pneumocardia associated with a nongas-forming liver abscess and SPE.
2. Case report
This case report was reviewed by the Institutional Review Board of Zhuji People's Hospital, the Affiliated Hospital, Wenzhou Medical University, Zhejiang, China. Informed consent was obtained from the patient for publication of this case report.
A 67-year-old woman was admitted to our hospital with a 4-day history of fever, chills, cough, and dyspnea. She had a previous history of diabetes mellitus. On physical examination, her temperature was 38.9°C and she was mildly icteric. She had bilateral rhonchi and crepitations on auscultation of the lungs. Her abdominal examination revealed no tenderness over the right hypochondrial region. Laboratory tests revealed a raised white blood cell count (10,400/mL) with 90.2% neutrophils, a hemoglobin level of 11.0 g/dL, and a platelet count of 81,000/μL. Her liver function tests revealed an aspartate aminotransferase level of 86 units/L, and alanine aminotransferase of 78 units/L; her bilirubin level was high (total bilirubin 49.3 μmol/L) and she had a high blood glucose (18.4 mmol/L). There was evidence of acute kidney injury with a blood urea nitrogen 24 mg/dL and creatinine of 1.7 mg/dL. A chest computed tomography (CT) scan (Fig. 1, panels A and B) was performed, which showed SPE presenting as multiple peripheral nodules in both lungs and wedge-shaped, peripheral, infiltrative lesions abutting the pleura. She had bilateral pleural effusions and a mass within the liver. Transthoracic echocardiography showed mild tricuspid regurgitation with no vegetations on the heart valves. She was commenced on intravenous imipenem; however, fever, chills, and dyspnea worsened. On day 2 of hospital admission, a contrast-enhanced abdominal CT (Fig. 1, panels C, D, and E) was carried out. The CT revealed a large liver abscess without evidence of gas formation; pneumocardia was observed with presence of air within the right ventricle. The patient was immediately placed in the supine Trendelenburg position, and 100% oxygen was administered. She was admitted to the telemetry unit for close observation. The liver abscess was drained by the bedside under ultrasonographic guidance with continued antibiotic treatment with intravenous imipenem. Her symptoms improved gradually; 48 hours later, transthoracic echocardiography did not reveal any evidence of air within the heart. The 2 initial blood cultures and pus culture from the liver abscess yielded K pneumoniae. The patient was discharged on the 25th hospital day after full recovery and was doing well on follow-up at 10 months.
Figure 1.
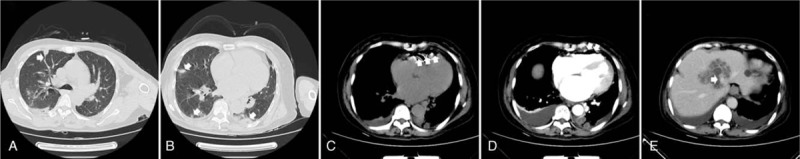
Chest CT showing a wedge-shaped peripheral lesion (arrow, panel A) with feeding vessel sign (arrow, panel A), peripheral nodules (arrow, panel B), and right-sided pleural effusion. Plain and contrast abdominal CT scans showed pneumocardia (arrow, panels C and D), and liver abscess (arrow, panel E). CT = computed tomography.
3. Discussion
Pyogenic liver abscess (PLA) is potentially fatal[1] with an incidence of about 1 in 45 to 100,000, with peak incidence in the fifth decade of life.[6] It carries a significant mortality rate of 6.0% to 19.6%.[7] PLA often manifests as bacteremia with subsequent metastatic complications, with a higher incidence in case of K pneumoniae infection.[8] In the past 2 decades, K pneumoniae has been reported to be the most common pathogen causing PLA, especially in the Asia Pacific region. KPLA is common in patients with diabetes mellitus, and often complicated by metastatic infections including brain abscess, endophthalmitis, infective endocarditis, SPE, osteomyelitis, prostatitis, and necrotizing fasciitis.[9] The incidence of metastatic infection ranges from 3.5% to 20%.[10]
Septic pulmonary embolism is an uncommon but serious metastatic complication of KPLA with a mortality of approximately 10% in the recent studies.[3] It is a nonthrombotic type of pulmonary embolism associated with septic thrombophlebitis and is composed of pathogens admixed with fibrin from the primary infected site. Embolization is characterized by obstruction of small pulmonary vessels, with secondary infection. There are relatively few reports of liver abscess associated with SPE.[11,12] KPLA can spread to the adjacent hepatic veins, inferior vena cava, and enter the pulmonary circulation, resulting in thrombophlebitis. Most SPE can be diagnosed based on CT findings, although the findings are not pathognomonic.[13] The main features on CT scan include bilateral, multiple nodules, a feeding-vessel sign, cavitations, and subpleural wedge-shaped peripheral lesions.[14] Symptoms are usually nonspecific; diagnostic delays may occur, leading to a poor prognosis.
Pneumocardia is a type of vascular air embolism (VAE). VAE is rare, but its true incidence is unknown, due to under-reporting and failure to diagnose in asymptomatic patients. The most common cause of VAE is iatrogenic, after procedures such as endoscopy, angiography, tissue biopsy, and surgical procedures.[15–17] Our patient did not undergo any procedure that may have resulted in VAE. Embolization occurs along a pressure gradient between the atmosphere and blood vessels into the circulatory system, resulting in arrhythmias, myocardial ischemia, ischemic strokes, and death.[18] The consequent physiological effects depend on the volume of air entrained, the rate of accumulation, and patient position at the time of embolization. However, under certain conditions, fatality may ensue from even a small volume of air entraining into the right heart. Hence, the occurrence of pneumocardia implies high mortality.[2,19] Rarely, pneumocardia may arise from a noniatrogenic reason, such as a gas-forming hepatic abscess, resulting from rupture of the abscess into the adjacent hepatic vein. The mechanism of gas formation in a liver abscess is not completely clear. However, diabetes mellitus is considered to be closely associated with emphysematous liver abscess, especially when K pneumoniae is the causative agent. Lee et al[20] hypothesized that contributive factors include in liver abscess caused by a gas-producing pathogen such as K pneumoniae, hyperglycemia with impaired local perfusion facilitating anaerobic metabolism, and failure of local circulation to remove the generated gas. To our knowledge, There are only 3 previous case reports of pneumocardia associated with gas-forming liver abscesses.[2,4,5] However, there are no previous reports of pneumocardia in nongas-forming liver abscesses or with SPE. It is unclear as to how pneumocardia may have occurred in the present case. We considered the possibility of infective endocarditis (IE) leading to pneumocardia in our patient. However, infective endocarditis due to gram-negative infections, including K pneumoniae, is rare, with an incidence of around 5% of all cases of endocarditis.[21] SPE is strongly associated with IE. Ye et al[22] systematically reviewed 168 cases of SPE due to various causes and found that echocardiography revealed vegetations in 48.6% of cases; 86.54% were located in the tricuspid valve. The presence of tricuspid vegetations supports the diagnosis of infective endocarditis. Although no vegetation was detected on transthoracic echocardiography (TTE) in our patient, the diagnosis of IE cannot be excluded in the presence of typical clinical features. TTE has a relatively low sensitivity for the detection of vegetations. In a multicenter review of echocardiographic examination in INTENSIVE care unit patients,[23] TTE as a first-line technique had a high specificity of 95% for the diagnosis and assessment of severity of IE, similar to that for transesophageal echocardiography (TEE). However, TTE had a sensitivity of only 46% in contrast to that for TEE, which has a 93% sensitivity. Furthermore, in 2016, Laiq et al[24] reported a case of IE, diagnosed based on the unusual finding of gas in the left ventricle on noncontrast CT. Our patient had evidence of air within the right ventricle, suggestive of IE involving the right heart. IE may have been the primary focus of infection with septic metastasis to other locations; however, but the initial manifestation of SPE without evidence of gas in the right ventricle on the initial chest CT supports the possibility of liver being the primary focus of infection. IE is a serious, potentially life-threatening illness, with a mortality of nearly 30% at the end of 1 year.[25] Early diagnosis is crucial in improving the prognosis.
4. Conclusions
Pneumocardia and SPE are metastatic complications of KPLA, resulting from bacteremia and septic thrombophlebitis. The most likely route of septic emboli may have been by invasion of the hepatic vein by the liver abscess; this may have led to septic thrombophlebitis, and spread to the inferior vena cava and the right heart, and through the pulmonary circulation into the lungs. Septic embolization to the lungs may manifest with relatively few, nonspecific symptoms; however, it may be potentially lethal. Hence, for patients with KPLA, prompt attention must be paid not only to the primary focus of infection but also to possible metastatic spread to other organs. Early diagnosis, combined with appropriate antibiotics and drainage, are important in successful treatment.
Author contributions
Conceptualization: Zhoujia Yao.
Data curation: Zhoujia Yao, Jun Zheng, Wenhong Wang.
Formal analysis: Zhoujia Yao, Youguang Si.
Funding acquisition: Zhoujia Yao.
Investigation: Zhoujia Yao.
Writing – original draft: Zhoujia Yao.
Writing – review & editing: Zhoujia Yao.
Footnotes
Abbreviations: CT = computed tomography, IE = infective endocarditis, KPLA = Klebsiella pneumoniae primary liver abscess, PLA = pyogenic liver abscess, SPE = septic pulmonary embolism, TEE = transesophageal echocardiography, TTE = transthoracic echocardiography, VAE = vascular air embolism.
Funding: Zhuji People's Hospital.
The authors have no conflicts of interest.
References
- [1].Sotto Mayor J, Robalo MM, Pacheco AP, et al. Pyogenic liver abscesses: uncommon presentation. BMJ Case Rep 2016. doi: 10.1136/bcr-2016-214841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Sakpal SV, Tichauer M, Chamberlain RS. Hepatic venous gas and pneumocardia. Dig Liver Dis 2009;41:915–6. [DOI] [PubMed] [Google Scholar]
- [3].Oh HG, Cha SI, Shin KM, et al. Risk factors for mortality in patients with septic pulmonary embolism. J Infect Chemother 2016;22:553–8. [DOI] [PubMed] [Google Scholar]
- [4].Keng LT, Fu YH, Lin YH. Gas-forming liver abscess with pneumocardia. J Emerg Med 2014;46:e185–186. doi: 10.1016/j.jemermed.2014.01.021. [DOI] [PubMed] [Google Scholar]
- [5].Tichauer M, Sakpal SV, Chamberlain RS, et al. Right ventricular pneumocardia secondary to hepatic abscesses. Case Rep Gastroenterol 2010;4:498–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Pinto E, Sousa M, Costa A. Pyogenic liver abscesses: from the interventional radiologist point of view. Rev Clin Hosp Prof Dr Fernando Fonseca 2013;1:27–33. [Google Scholar]
- [7].Chung YFA. Pyogenic liver abscess: predicting failure to improve outcome. Neth J Med 2008;66:183–4. [PubMed] [Google Scholar]
- [8].Leederman ER, Crum NF. Pyogenic liver abscess with a focus on Klebsiella pneumoniae as a primary pathogen: an emerging disease with unique clinical characteristics. Am J Gastroenterol 2005;100:322–31. [DOI] [PubMed] [Google Scholar]
- [9].Keller JJ, Tsai MC, Lin CC, et al. Risk of infections subsequent to pyogenic liver abscess: a nationwide population-based study. Clin Microbiol Infect 2013;19:717–22. [DOI] [PubMed] [Google Scholar]
- [10].Lee SS, Chen YS, Tsai HC, et al. Predictors of septic metastatic infection and mortality among patients with Klebsiella pneumoniae liver abscess. Clin Infect Dis 2008;47:642–50. [DOI] [PubMed] [Google Scholar]
- [11].Kamano Y, Ohashi H, Kikuchi T, et al. Liver abscess and Aeromonas bacteremia with septic pulmonary embolism. Intern Med 2003;42:1047–9. [DOI] [PubMed] [Google Scholar]
- [12].Plemmons RM, Dooley DP, Longfield RN. Septic thrombophlebitis of the portal vein (pylephlebitis): diagnosis and management in the modern era. Clin Infect Dis 1995;21:1114–20. [DOI] [PubMed] [Google Scholar]
- [13].Deng-Wei Chou, Shu-Ling Wu, Kuo-Mou Chung, et al. Septic pulmonary embolism caused by a Klebsiella pneumoniae liver abscess: clinical characteristics, imaging findings, and clinical courses. Clinics (Sao Paulo) 2015;70:400–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Kuhlman JE, Fishman EK, Teigen C. Pulmonary septic emboli: diagnosis with CT. Radiology 1990;174:211–3. [DOI] [PubMed] [Google Scholar]
- [15].Freund MC, Petersen J, Goder KC, et al. Systemic air embolism during percutaneous core needle biopsy of the lung: frequency and risk factors. BMC Pulm Med 2012;12:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Bessereau J, Genotelle N, Chabbaut C, et al. Long-term outcome of iatrogenic gas embolism. Intensive Care Med 2010;36:1180–7. [DOI] [PubMed] [Google Scholar]
- [17].Vesely TM. Air embolism during insertion of central venous catheters. J Vasc Interv Radiol 2001;12:1291–5. [DOI] [PubMed] [Google Scholar]
- [18].Mirski MA, Lele AV, Lunei Fitzsimmons, et al. Diagnosis and treatment of vascular air embolism. Anesthesiology 2007;106:164–77. [DOI] [PubMed] [Google Scholar]
- [19].Haggerty KA, George TJ, Arnaoutakis GJ, et al. Portal venous air resulting in pneumocardia: a rare presentation of intestinal ischemia. Ann Thorac Surg 2012;93:e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Lee CH, Leu HS, Wu TS, et al. Risk factors for spontaneous rupture of liver abscess caused by Klebsiella pneumonia. Diagn Microbiol Infect Dis 2005;52:79–84. [DOI] [PubMed] [Google Scholar]
- [21].Rivero A, Gomez E, Alland D, et al. K2 serotype Klebsiella pneumoniae causing a liver abscess associated with infective endocarditis. J Clin Microbiol 2010;48:639–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Rui Y, Zhao L, Wang C, et al. Clinical characteristics of septic pulmonary embolism in adults: a systematic review. Respir Med 2014;108:1–8. [DOI] [PubMed] [Google Scholar]
- [23].Keynan Y, Singal R, Kumar K, et al. Infective endocarditis in the intensive care unit. Crit Care Clin 2013;29:923–51. [DOI] [PubMed] [Google Scholar]
- [24].Laiq Z, Yarmohammadi H, Nabeel Y, et al. Presence of gas in the left ventricle due to infective endocarditis. J Cardiovasc Comput Tomogr 2016;10:335–6. [DOI] [PubMed] [Google Scholar]
- [25].Cahill TJ, Baddour LM, Habib G, et al. Challenges in infective endocarditis. J Am Coll Cardiol 2017;69:325–44. [DOI] [PubMed] [Google Scholar]


