Abstract
Background:
The impact of magnesium sulfate on hemodynamic responses during laparoscopic cholecystectomy remains controversial. We conduct a systematic review and meta-analysis to explore the influence of magnesium sulfate on hemodynamic responses for laparoscopic cholecystectomy.
Methods:
We search PubMed, EMbase, Web of science, EBSCO, and Cochrane library databases through June 2018 for randomized controlled trials (RCTs) assessing the effect of magnesium sulfate on hemodynamic responses for laparoscopic cholecystectomy. Meta-analysis is performed using the random-effect model.
Results:
Four RCTs involving 208 patients are included in the meta-analysis. Overall, compared with control group in laparoscopic cholecystectomy, intravenous magnesium sulfate is associated with systolic blood pressure at 30 minutes [Std. MD = −1.34; 95% confidence interval (95% CI) = −1.86 to −0.82; P < .00001], diastolic blood pressure at 30 minutes (Std. MD = −1.40; 95% CI = −1.86 to −0.94; P < .00001), mean arterial pressure at 30 minutes (Std. MD = −1.19; 95% CI = −1.91 to −0.46; P = .001), systolic blood pressure at 10 minutes (Std. MD = −1.61; 95% CI = −2.08 to −1.13; P < .00001), diastolic blood pressure at 10 minutes (Std. MD = −1.54; 95% CI = −2.68 to −0.40; P = .008), heart rate at 30 minutes (Std. MD = −2.09; 95% CI = −2.87 to −1.32; P < .00001), but results in prolonged extubation time (Std. MD = 0.96; 95% CI = 0.18–1.74; P = .02).
Conclusion:
Magnesium sulfate can reduce blood pressure, but with the increase in extubation time for laparoscopic cholecystectomy.
Keywords: hemodynamic responses, laparoscopic cholecystectomy, magnesium sulfate, meta-analysis’ randomized controlled trials
1. Introduction
Laparoscopic cholecystectomy is known as one of the most common laparoscopic surgeries worldwide.[1–3] Carbon dioxide (CO2) is used for pneumoperitoneum, which may result in the adverse cardiovascular effects.[4–6] They mainly include an abrupt elevation of mean arterial pressure, systemic vascular resistance, and decreased cardiac output because of the release of both catecholamines and vasopressin.[7–9] In addition, the reverse Trendelenburg position in these surgeries can further decrease cardiac output.[10]
Many drugs have been developed for the attenuation of these vasopressor responses, and include opioids, beta blockers, magnesium, and α2 agonists.[11–13] Especially, magnesium is widely used to attenuate intubation-induced vasopressor response by blocking the release of catecholamines release from both adrenal medullae and from nerve terminals.[14] In addition, magnesium sulfate is reported to attenuate vasopressin-induced vasoconstriction by directly acting on blood vessels causing vasodilation.[10] With accumulating evidence, we perform a systematic review and meta-analysis of randomized controlled trials (RCTs) to investigate the efficacy of magnesium sulfate to control hemodynamic responses in laparoscopic cholecystectomy.
2. Materials and methods
Ethical approval and patient consent are not required because this is a systematic review and meta-analysis of previously published studies. The systematic review and meta-analysis are conducted and reported in adherence to PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses).[15]
2.1. Search strategy and study selection
Two investigators have independently searched the following databases (inception to June 2018): PubMed, EMbase, Web of science, EBSCO, and Cochrane library databases. The electronic search strategy is conducted using the following combination keywords
“magnesium sulfate” and “laparoscopic cholecystectomy.” We also check the reference lists of the screened full-text studies to identify other potentially eligible trials.
The inclusive selection criteria are as follows: population: patients undergoing laparoscopic cholecystectomy; intervention: magnesium sulfate; comparison: saline; study design: RCT; and studies published in English. Studies without hemodynamic data are excluded.
2.2. Data extraction and outcome measures
We have extracted the following information: author, number of patients, age, weight, surgical duration, etc. Data have been extracted independently by 2 investigators, and discrepancies are resolved by consensus. We also contact the corresponding author to obtain the data when necessary. No simplifications and assumptions are made. The primary outcomes are systolic blood pressure and diastolic blood pressure at 30 minutes after pneumoperitoneum. Secondary outcomes include mean arterial pressure at 30 minutes, systolic blood pressure and diastolic blood pressure at 10 minutes, heart rate at 30 minutes, and extubation time.
2.3. Quality assessment in individual studies
Methodological quality of the included studies is independently evaluated using the modified Jadad scale.[16] There are 3 items for Jadad scale: randomization (0–2 points), blinding (0–2 points), dropouts and withdrawals (0–1 points). The score of Jadad Scale varies from 0 to 5 points. An article with Jadad score ≤2 is considered to be of low quality. If the Jadad score ≥3, the study is thought to be of high quality.[17]
2.4. Statistical analysis
We estimate the standard mean difference (Std. MD) with 95% confidence interval (CI) for continuous outcomes (systolic blood pressure and diastolic blood pressure at 30 minutes after pneumoperitoneum, mean arterial pressure at 30 minutes, systolic blood pressure and diastolic blood pressure at 10 min, heart rate at 30 minutes, and extubation time). A random-effects model is used regardless of heterogeneity. Heterogeneity is reported using the I2 statistic, and I2 > 50% indicates significant heterogeneity.[18] Whenever significant heterogeneity is present, we search for potential sources of heterogeneity via omitting 1 study in turn for the meta-analysis or performing subgroup analysis. Publication bias is not evaluated because of the limited number (<10) of included studies. All statistical analyses are performed using Review Manager Version 5.3 (The Cochrane Collaboration, Software Update, Oxford, UK).
3. Results
3.1. Literature search, study characteristics, and quality assessment
A detailed flowchart of the search and selection results is shown in Fig. 1. Four hundred fifty-five potentially relevant articles are identified initially. Finally, 4 RCTs that meet our inclusion criteria are included in the meta-analysis.
Figure 1.
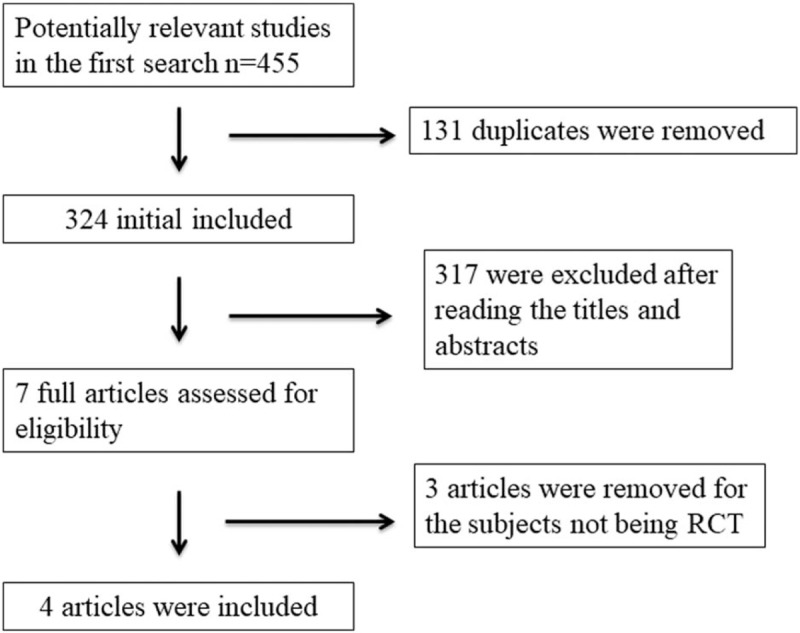
Flow diagram of study searching and selection process.
The baseline characteristics of the 4 eligible RCTs in the meta-analysis are summarized in Table 1. The 4 studies are published between 2013 and 2017, and sample sizes range from 32 to 60 with a total of 208. All included RCTs report injection magnesium sulfate 50 mg/kg before pneumoperitoneum versus matched saline.
Table 1.
Characteristics of included studies.
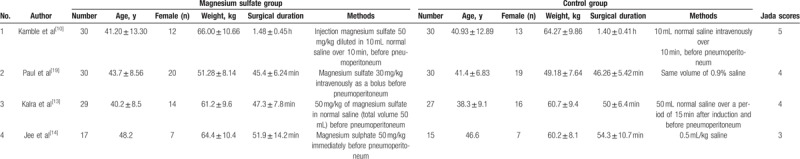
Among the 4 studies included here, 2 studies report systolic blood pressure and diastolic blood pressure at 30 minutes after pneumoperitoneum,[10,14] 2 studies report mean arterial pressure at 30 minutes,[10,19] 2 studies report systolic blood pressure and diastolic blood pressure at 10 minutes,[10,14] 2 studies report heart rate at 30 minutes,[10,19] and 3 studies report extubation time.[10,13,14] Jadad scores of the 4 included studies vary from 3 to 5, and all 4 studies are considered to be high-quality ones according to quality assessment.
3.2. Primary outcomes: systolic blood pressure and diastolic blood pressure at 30 minutes
These outcome data are analyzed with the random-effects model, and the pooled estimate of the 2 included RCTs suggested that compared with control group during laparoscopic cholecystectomy, magnesium sulfate can significantly decrease the systolic blood pressure at 30 minutes [Std. MD = −1.34; 95% confidence interval (95% CI) = −1.86 to −0.82; P < .00001], with low heterogeneity among the studies (I2 = 21%, heterogeneity P = .26) (Fig. 2). Consistently, magnesium sulfate results in substantially reduced diastolic blood pressure at 30 minutes (Std. MD = −1.40; 95% CI = −1.86 to −0.94; P < .00001), with no heterogeneity among the studies (I2 = 0%, heterogeneity P = .57) (Fig. 3).
Figure 2.
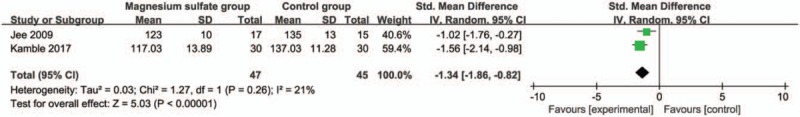
Forest plot for the meta-analysis of systolic blood pressure at 30 min (mm Hg).
Figure 3.
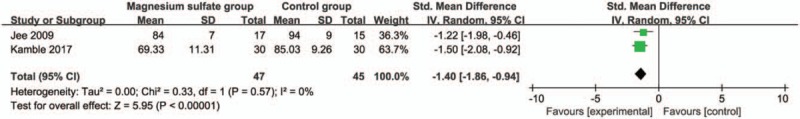
Forest plot for the meta-analysis of diastolic blood pressure at 30 min (mm Hg).
3.3. Sensitivity analysis
Low or even no heterogeneity is observed among the included studies for the primary outcomes. Thus, we do not perform sensitivity analysis by omitting 1 study in each turn to detect the source of heterogeneity.
3.4. Secondary outcomes
Compared with control group in laparoscopic cholecystectomy, magnesium sulfate is associated with significantly reduced mean arterial pressure at 30 minutes (Std. MD = −1.19; 95% CI = −1.91 to −0.46; P = .001; Fig. 4), systolic blood pressure at 10 minutes (Std. MD = −1.61; 95% CI = −2.08 to −1.13; P < .00001; Fig. 5), diastolic blood pressure at 10 minutes (Std. MD = −1.54; 95% CI = −2.68 to −0.40; P = .008; Fig. 6), heart rate at 30 minutes (Std. MD = −2.09; 95% CI = −2.87 to −1.32; P < .00001; Fig. 7), but leads to the increase in extubation time (Std. MD = 0.96; 95% CI = 0.18–1.74; P = .02; Fig. 8).
Figure 4.
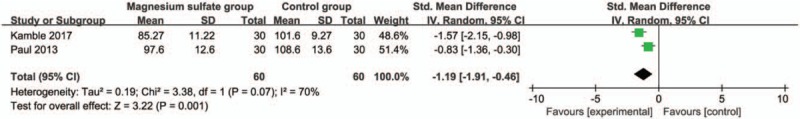
Forest plot for the meta-analysis of mean arterial pressure at 30 min (mm Hg).
Figure 5.
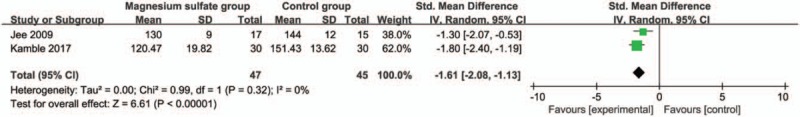
Forest plot for the meta-analysis of systolic blood pressure at 10 min (mm Hg).
Figure 6.
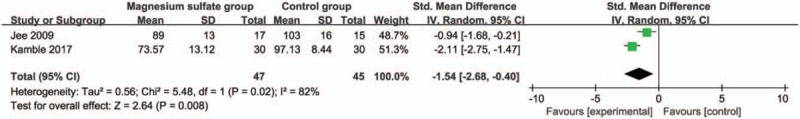
Forest plot for the meta-analysis of diastolic blood pressure at 10 min (mm Hg).
Figure 7.
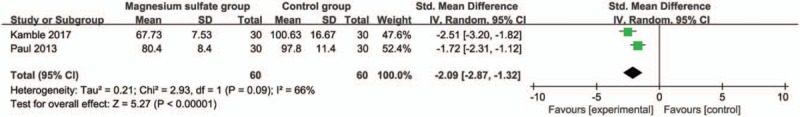
Forest plot for the meta-analysis of heart rate at 30 min.
Figure 8.
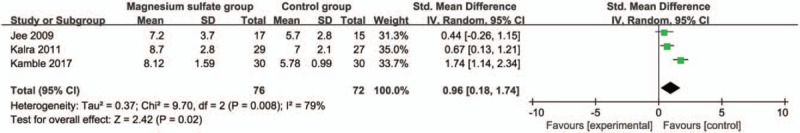
Forest plot for the meta-analysis of extubation time (min).
4. Discussion
Elevated intra-abdominal pressure induced by pneumoperitoneum and CO2 can produce some adverse effects on the cardiovascular system.[20,21] Plasma level of catecholamines and vasopressin is found to increase immediately after pneumoperitoneum.[22,23] Increased catecholamine level further activates the renin-angiotensin aldosterone system (RAAS) and some characteristic hemodynamic alterations, including decreased cardiac output, elevated arterial pressure, and increased systemic/pulmonary vascular resistance.[24,25] Vasopressin also results in elevation of arterial pressure and systemic vascular resistance.[8]
Magnesium has a vasodilator action, thus contributing to the reduction of blood pressure. Magnesium sulfate has been reported to attenuate the adverse hemodynamic response of endotracheal intubation in one previous study.[26] One RCT reveals effectively blunted hemodynamic responses to pneumoperitoneum and lower arterial pressure and heart rate after using magnesium sulfate for laparoscopic cholecystectomy than those in control intervention.[19]
Magnesium sulfate at a dose of 50 mg/kg over 2 to 3 minutes before pneumoperitoneum is reported to effectively attenuate the hemodynamic responses, without severe hypotension or bradycardia through reducing the plasma catecholamines and vasopressin levels.[14] These effects of magnesium can act at serum concentrations of 2 to 4 mmol/L, and a dose of 50 mg/kg has been shown to achieve these levels.[14,27,28] Our meta-analysis suggests that intravenous magnesium sulfate at a dose of 50 mg/kg is associated with significantly reduced blood pressure at 10 and 30 minutes after pneumoperitoneum, as well as heart rate at 30 minutes during laparoscopic cholecystectomy.
Magnesium is effective to attenuate vasopressin-stimulated vasoconstriction and adverse hemodynamic response of endotracheal intubation.[29] In addition, magnesium sulfate in a dose of 30 mg/kg bolus before induction and 10 mg/kg/h continuous infusion has the ability to decrease anesthetic requirement.[30] Prolonged time to verbal response observed occurs because of central nervous system depressant action. Magnesium sulfate is able to produce general anesthesia and enhance the activity of local anesthetic drugs.[31]
Magnesium sulfate can potentiate the neuromuscular blockade of nondepolarizing relaxants and this is evident one with a prolonged time to extubation.[10,32] Consistently, the increase in extubation time is observed after the use of magnesium sulfate in our meta-analysis. Regarding the incidence of adverse effects, one included RCT involves 30 patients in each magnesium sulfate group and control group. The results reveal that only single patient suffers from hypotension in magnesium sulfate group, and 12 patients have hypertension just in control group. No bradycardia is found in 2 groups.[19]
This meta-analysis has several potential limitations that should be taken into account. Firstly, our analysis is based on only 4 RCTs and all of them have a relatively small sample size (n < 100). More RCTs with a large sample size should be conducted to confirm this issue. Next, it is not available to perform the meta-analysis of some important adverse events such as hypotension and hypertension based on current included RCTs. Finally, some unpublished and missing data may lead bias to the pooled effect.
5. Conclusion
Magnesium sulfate is effective to attenuate the hemodynamic responses during laparoscopic cholecystectomy, but results in the prolonged extubation time.
Author contributions
Data curation: Juyi Zhang.
Funding acquisition: Juan Yang.
Investigation: Juyi Zhang.
Methodology: Juyi Zhang, Yubin Wang.
Software: Hao Xu.
Supervision: Juan Yang.
Writing – original draft: Yubin Wang.
Writing – review & editing: Hao Xu.
Footnotes
Abbreviations: CI = confidence interval, RAAS = renin angiotensin aldosterone system, RCTs = randomized controlled trials, SMD = standard mean difference.
The authors declare no conflict of interest. Research involving human participants and/or animals is not applicable.
References
- [1].Kao LS, Ball CG, Chaudhury PK. for Members of the Evidence Based Reviews in Surgery G Evidence-based reviews in surgery: early cholecystectomy for cholecystitis. Ann Surg 2018;[Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- [2].Ozkardes AB, Tokac M, Dumlu EG, et al. Early versus delayed laparoscopic cholecystectomy for acute cholecystitis: a prospective, randomized study. Int Surg 2014;99:56–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].von Strauss Und Torney M, Aghlmandi S, Zeindler J, et al. High-resolution standardization reduces delay due to workflow disruptions in laparoscopic cholecystectomy. Surg Endosc 2018;[Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- [4].Sarkany P, Lengyel S, Nemes R, et al. Non-invasive pulse wave analysis for monitoring the cardiovascular effects of CO2 pneumoperitoneum during laparoscopic cholecystectomy: a prospective case-series study. BMC Anesthesiol 2014;14:98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Chen YQ, Xie YY, Wang B, et al. Effect of stellate ganglion block on hemodynamics and stress responses during CO2-pneumoperitoneum in elderly patients. J Clin Anesth 2017;37:149–53. [DOI] [PubMed] [Google Scholar]
- [6].Nijssen MA, Schreinemakers JM, Meyer Z, et al. Complications after laparoscopic cholecystectomy: a video evaluation study of whether the critical view of safety was reached. World J Surg 2015;39:1798–803. [DOI] [PubMed] [Google Scholar]
- [7].Wahba RW, Beique F, Kleiman SJ. Cardiopulmonary function and laparoscopic cholecystectomy. Can J Anaesth 1995;42:51–63. [DOI] [PubMed] [Google Scholar]
- [8].Myre K, Rostrup M, Buanes T, et al. Plasma catecholamines and haemodynamic changes during pneumoperitoneum. Acta Anaesthesiol Scand 1998;42:343–7. [DOI] [PubMed] [Google Scholar]
- [9].Walder AD, Aitkenhead AR. Role of vasopressin in the haemodynamic response to laparoscopic cholecystectomy. Br J Anaesth 1997;78:264–6. [DOI] [PubMed] [Google Scholar]
- [10].Kamble SP, Bevinaguddaiah Y, Nagaraja DC, et al. Effect of magnesium sulfate and clonidine in attenuating hemodynamic response to pneumoperitoneum in laparoscopic cholecystectomy. Anesth Essays Res 2017;11:67–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Lentschener C, Axler O, Fernandez H, et al. Haemodynamic changes and vasopressin release are not consistently associated with carbon dioxide pneumoperitoneum in humans. Acta Anaesthesiol Scand 2001;45:527–35. [DOI] [PubMed] [Google Scholar]
- [12].Koivusalo AM, Scheinin M, Tikkanen I, et al. Effects of esmolol on haemodynamic response to CO2 pneumoperitoneum for laparoscopic surgery. Acta Anaesthesiol Scand 1998;42:510–7. [DOI] [PubMed] [Google Scholar]
- [13].Kalra NK, Verma A, Agarwal A, et al. Comparative study of intravenously administered clonidine and magnesium sulfate on hemodynamic responses during laparoscopic cholecystectomy. J Anaesthesiol Clin Pharmacol 2011;27:344–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Jee D, Lee D, Yun S, et al. Magnesium sulphate attenuates arterial pressure increase during laparoscopic cholecystectomy. Br J Anaesth 2009;103:484–9. [DOI] [PubMed] [Google Scholar]
- [15].Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol 2009;62:1006–12. [DOI] [PubMed] [Google Scholar]
- [16].Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials 1996;17:1–2. [DOI] [PubMed] [Google Scholar]
- [17].Kjaergard LL, Villumsen J, Gluud C. Reported methodologic quality and discrepancies between large and small randomized trials in meta-analyses. Ann Intern Med 2001;135:982–9. [DOI] [PubMed] [Google Scholar]
- [18].Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med 2002;21:1539–58. [DOI] [PubMed] [Google Scholar]
- [19].Paul S, Biswas P, Bhattacharjee DP, et al. Effects of magnesium sulfate on hemodynamic response to carbon dioxide pneumoperitoneum in patients undergoing laparoscopic cholecystectomy. Anesth Essays Res 2013;7:228–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Gurusamy KS, Vaughan J, Davidson BR. Low pressure versus standard pressure pneumoperitoneum in laparoscopic cholecystectomy. Cochrane Database Syst Rev 2014;CD006930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Amin B, Zhang C, Yan W, et al. Effects of pneumoperitoneum of laparoscopic cholecystectomy on the coagulation system of patients: a prospective observational study. Chin Med J (Engl) 2014;127:2599–604. [PubMed] [Google Scholar]
- [22].Le V, Kurnutala L, SchianodiCola J, et al. Premedication with intravenous ibuprofen improves recovery characteristics and stress response in adults undergoing laparoscopic cholecystectomy: a randomized controlled trial. Pain Med 2016;[Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- [23].Calvo-Soto P, Martinez-Contreras A, Hernandez BT, et al. Spinal-general anaesthesia decreases neuroendocrine stress response in laparoscopic cholecystectomy. J Int Med Res 2012;40:657–65. [DOI] [PubMed] [Google Scholar]
- [24].Richardson JD, Trinkle JK. Hemodynamic and respiratory alterations with increased intra-abdominal pressure. J Surg Res 1976;20:401–4. [DOI] [PubMed] [Google Scholar]
- [25].Shoar S, Naderan M, Ebrahimpour H, et al. A prospective double-blinded randomized controlled trial comparing systemic stress response in laparoascopic cholecystectomy between low-pressure and standard-pressure pneumoperitoneum. Int J Surg 2016;28:28–33. [DOI] [PubMed] [Google Scholar]
- [26].James MF, Beer RE, Esser JD. Intravenous magnesium sulfate inhibits catecholamine release associated with tracheal intubation. Anesth Analg 1989;68:772–6. [PubMed] [Google Scholar]
- [27].James MF, Cork RC, Dennett JE. Cardiovascular effects of magnesium sulphate in the baboon. Magnesium 1987;6:314–24. [PubMed] [Google Scholar]
- [28].Pritchard JA, Pritchard SA. Standardized treatment of 154 consecutive cases of eclampsia. Am J Obstet Gynecol 1975;123:543–52. [DOI] [PubMed] [Google Scholar]
- [29].Laurant P, Touyz RM, Schiffrin EL. Effect of magnesium on vascular tone and reactivity in pressurized mesenteric resistance arteries from spontaneously hypertensive rats. Can J Physiol Pharmacol 1997; 75:293–300. [PubMed] [Google Scholar]
- [30].Telci L, Esen F, Akcora D, et al. Evaluation of effects of magnesium sulphate in reducing intraoperative anaesthetic requirements. Br J Anaesth 2002;89:594–8. [DOI] [PubMed] [Google Scholar]
- [31].Peck CH, Meltzer SJ. Anesthesia in human beings by intravenous injection of magnesium sulphate. J Am Med Assoc 1916;LXVII:1131–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Fuchs-Buder T, Wilder-Smith OH, Borgeat A, et al. Interaction of magnesium sulphate with vecuronium-induced neuromuscular block. Br J Anaesth 1995;74:405–9. [DOI] [PubMed] [Google Scholar]


