Abstract
Background:
Robot-assisted partial nephrectomy (RPN) and focal therapy (FT) have both been successfully employed in the management of small renal masses. However, despite this being the era of minimally invasive surgery, few comparative studies exist on RPN and FT. The aim of our study is to review perioperative, renal functional and oncologic outcomes of FT and RPN in cT1 renal masses.
Methods:
Literature published in Medline, EMBASE, and Cochrane Library databases up to April 22, 2018, was systematically searched. We included literature comparing outcomes of FT (radiofrequency ablation, cryoablation, microwave ablation, and irreversible electroporation) and RPN. Studies that reported only on laparoscopic partial nephrectomy or open partial nephrectomy, and review articles, editorials, letters, or cost analyses were excluded. In total, data from 1166 patients were included.
Results:
From 858 total articles, 7 nonrandomized, observational studies were included. Compared with RPN, FT was associated with a significantly lower decrease of estimated glomerular filtration rate (weighted mean difference [WMD] −8.06 mL/min/1.73 m2; confidence interval [CI] −15.85 to −0.26; P = .04), and lower estimated blood loss (WMD −49.61 mL; CI −60.78 to −38.45; P < .001). However, patients who underwent FT had a significantly increased risk of local recurrence (risk ratio [RR] 9.89; CI 4.24–23.04; P < .001) and distant metastasis (RR 6.42; CI 1.70–24.33; P = .006). However, operative times, lengths of stay, and complication rates were revealed to be similar between FT and RPN.
Conclusion:
RPN has a substantial advantage in preventing cancer recurrence. However, in the era of minimally invasive surgery, FT has advantages in renal function preservation and less bleeding. Long-term follow-up for survival rates and comparative analysis of microwave ablation and irreversible electroporation are needed to extend FT for patients with significant morbidities and for those who need sufficient renal function preservation with minimal bleeding.
Keywords: ablation techniques, kidney, meta-analysis, nephrectomy, robotics
1. Introduction
For the management of small renal masses (SRMs), partial nephrectomy (PN) has been considered the standard treatment and active surveillance is an option in selected cases.[1,2] However, PN has been associated with a complication rate of approximately 20%.[3] Laparoscopic partial nephrectomy (LPN) is associated with a relatively longer learning curve and is regarded as a relatively complicated procedure. Recently, the introduction of a robotic system in renal surgery has overcome the difficulties faced in cases involving LPN. Robot-assisted partial nephrectomy (RPN) has the following advantages of meticulous dissection of the renal mass, short renorrhaphy time owing to articulating instruments, and improved perioperative outcomes compared with LPN.[4]
Additionally, focal therapy (FT) for SRMs, such as thermal (radiofrequency ablation [RFA], cryoablation [CA], microwave ablation [MWA]) ablation and nonthermal (irreversible electroporation [IRE]) ablation, have been introduced as an treatment option, which provides tumor control with less complications, improved renal function preservation, and shorter recovery time.[5] The 2017 American Urological Association (AUA) guidelines have recommended FT as an alternative to PN for renal masses <3 cm in size.[6] The European Association of Urology guidelines have recommended FT for patients with renal masses for whom surgery is contraindicated or in cases where severe comorbidities are present.[7] FT is performed via a laparoscopic or percutaneous approach, and these techniques have the benefit of renal parenchymal preservation without the need for surgical resection. Despite this being the era of minimally invasive surgery, few comparative studies exist on RPN and FT. Therefore, the objective of this study was to systematically review the perioperative, renal functional and oncologic outcomes of RPN and FT for cT1 renal masses.
2. Materials and methods
2.1. Search strategy
This study followed the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines.[8] This study involved the systematic review of literature published in the Medline, EMBASE, and Cochrane Central Register of Controlled Trials databases up to April 22, 2018. The search terms included (robotic OR robotic-assisted OR robot OR robot-assisted) AND (partial OR nephron sparing OR nephron-sparing) AND (cryoablation OR cryotherapy OR radiofrequency ablation OR RFA OR ablation OR irreversible electroporation OR IEP OR microwave ablation OR MWA). This search was not limited by language or year. This study was except for approval of ethics committee or institutional review board due to the study design of systematic review and meta-analysis.
2.2. Inclusion and exclusion criteria for study eligibility
We included the literature according to the following criteria. Comparative data were available on the treatment of cT1 renal masses via RPN and FT (CA, RFA, MWA, and IRE). Reports having information regarding at least 1 oncological outcome, renal functional outcome, or perioperative outcome. Data for continuous (standard deviation) or dichotomous variables must have been provided from the data source. We excluded the literature according to following criteria. Studies that reported only on LPN or open PN (OPN) review articles, editorials, letters, or purely cost analysis studies.
2.3. Quality assessment of included studies
The quality of included studies in the meta-analysis was evaluated using the modified Newcastle–Ottawa scale (mNOS) for nonrandomized controlled trials.[9,10] The mNOS contains 3 elements: selection of subjects, comparability of the study groups, and assessment of outcome. A score of 0 to 8 was assigned to each study, and studies that achieved a score of ≥6 were considered to be high quality.
2.4. Data extraction and outcome measures
Two investigators (Young Eun Yoon and Dae Keun Kim) independently identified all literature that matched the inclusion criteria and also performed a cross-check. The data were extracted independently and any disagreement was resolved by a third investigator (Hyung Ho Lee).
The following study variables were collected: year of publication, study design, number of patients who underwent RPN or FT, subjects’ age, tumor size, RENAL nephrometry score or PADUA score, pathology, and follow-up period. Surgical outcomes included estimated blood loss (EBL), operative time, length of hospital stay, minor, major, and overall complications.
Postoperative complications were reported according to the Clavien–Dindo system, whereby a major complication was assigned a grade ≥3.[11] Functional outcomes included a change in estimated glomerular filtration rate (eGFR) according to the modification of diet in renal disease equation. Oncological outcomes included time to local recurrence and distant metastasis.
2.5. Statistical analysis
Continuous data were expressed as the mean difference with 95% confidence interval (CI); for dichotomous data an inverse variance was used, and data were expressed as odds ratio (OR) or risk ratio (RR) for binary variables with 95% CI. I2 values of 25%, 50%, and 75% correspond to low, medium, and high levels of heterogeneity, respectively. A fixed-effects model was used unless significantly high heterogeneity (I2 > 75%) existed between studies. A random-effects model was employed if heterogeneity existed. All analyses were conducted by using Review Manager, version 5.2 (The Cochrane Collaboration, Oxford, United Kingdom). All P values were 2-sided and considered statistically significant if P < .05.
3. Results
3.1. Patient and baseline characteristics
Figure 1 shows the PRISMA flowchart, depicting the identification of studies to their inclusion in the meta-analysis. We initially identified a total of 858 articles; after deleting duplications, we analyzed 708 abstracts. Subsequently, we excluded 690 articles upon abstract review. After a full-text review of 18 articles, 11 articles were excluded owing to different inclusion criteria (5), studies being deemed as review articles (3), and unavailable format (3). From the initial 858 articles, 7 nonrandomized, observational studies were included; these studies reported on a total of 1166 patients (RPN vs FT) up to April 22, 2018 (Table 1).
Figure 1.
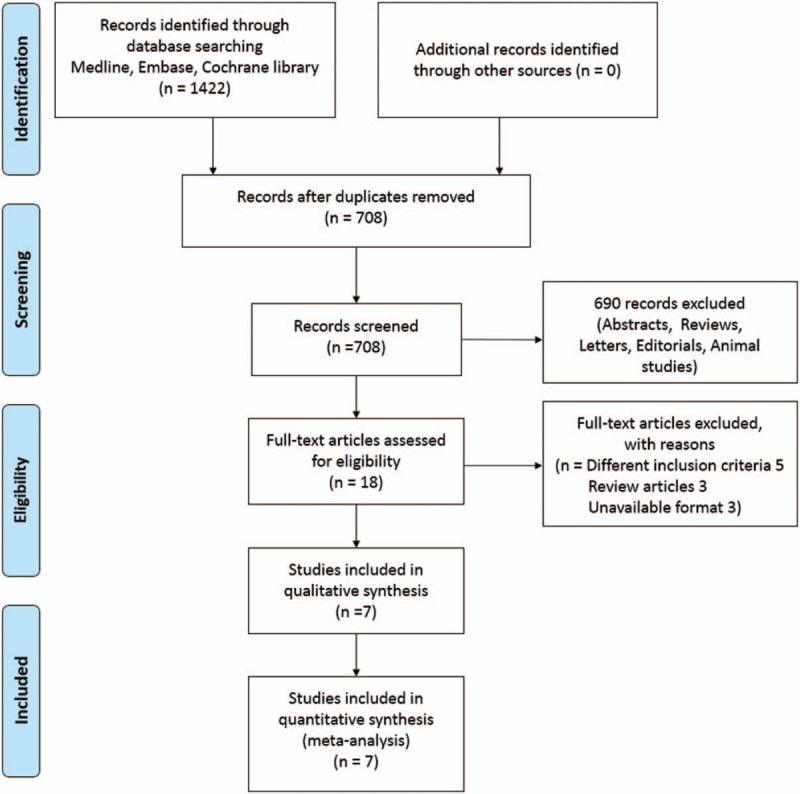
Flow diagram showing the selection of studies.
Table 1.
Characteristics of included studies.
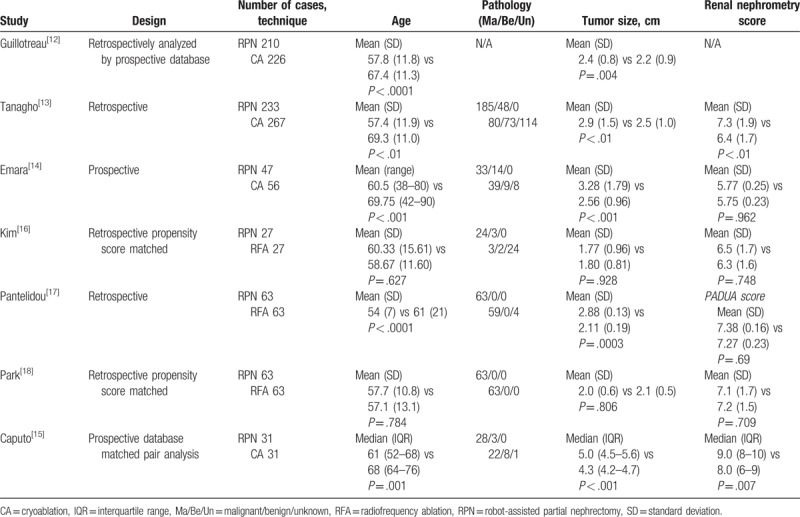
A total of 674 and 492 (CA 339, RFA 153) patients underwent RPN and FT, respectively. Four studies compared the outcomes between RPN and CA,[12–15] and 3 studies compared the outcomes between RPN and RFA.[16–18] From 4 studies comparing RPN and CA, 2 studies involved laparoscopic CA,[12,14] and 2 studies involved both laparoscopic or percutaneous CA.[13,15] All RFA studies involved percutaneous RFA. There was only a single arm of studies on the effectiveness of MWA or IEP, and there was no study comparing MWA or IEP with RPN. The cohort sizes ranged from 54 to 436 patients. Study participants were from the United States (3), United Kingdom (2), and South Korea (2). Mean tumor sizes were under 4 cm except for Caputo et al.[15] Renal complexity score was analyzed based on the RENAL Nephrometry score, except for Pantelidou et al,[17] which involved the PADUA system.[3] No difference in renal mass complexity was observed except in 2 studies.[13,15]
3.2. Perioperative outcomes
Data on EBL were reported in 2 studies. Compared with RPN, patients who underwent FT had lower EBL (weighted mean difference [WMD] −49.61 mL; CI −60.78 to −38.45; P < .001). A fixed-effects model was used on EBL rate owing to the low degree of heterogeneity (I2 = 0%). Operative time (WMD −59.91; CI −225.04 to −105.21; P = .48) and lengths of stay (WMD −2.9; CI −7.54 to −1.75; P = .22) were similar between RPN and FT (Fig. 2). A random-effects model was used because of a high heterogeneity rate (I2 = 99%).
Figure 2.
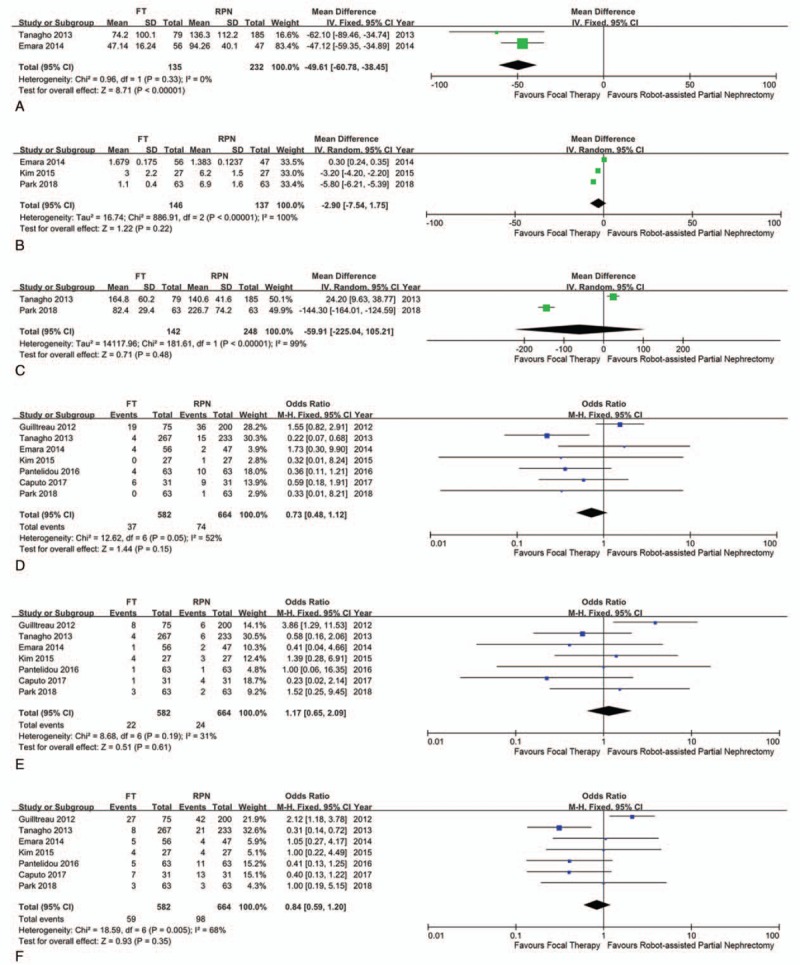
Forest plot and meta-analysis of perioperative and postoperative outcomes: (A) estimated blood loss; (B) length of stay; (C) operative time; (D) minor complication; (E) major complication; and (F) overall complication.
All included studies reported overall complications as well as major and minor complications according to the Clavien–Dindo classification system. In terms of overall complications and minor complications, FT had lower complication rates compared with RPN. The overall complication result was revealed of OR 0.73 (CI 0.36–1.49; P = .39), and the minor complication was revealed of OR 0.62 (CI 0.29–1.31; P = .21). However, both did not reach statistical significance and had high heterogeneity (overall complication I2 = 52%, minor complication I2 = 68%). A fixed-effects model was used for major complication analysis owing to a medium degree of heterogeneity (I2 = 31%); there was a higher rate of major complications for RPN but with no significant difference (OR 1.17; CI 0.65–2.09; P = .61).
3.3. Functional outcomes
The data reporting renal functional outcomes were heterogeneous among the included studies, such as eGFR percentage preservation, eGFR difference, and creatinine-level change. All 7 studies reported renal functional outcomes (Table 2). From these studies, the mean eGFR difference and standard deviation could be extracted from the 3 studies. A forest plot analysis revealed a heterogeneous result (I2 = 89%) and the follow-up period among included studies varied (1–35.8 months). Despite heterogeneous parameters, FT was associated with a significantly low decrease of eGFR (WMD −8.06 mL/min/1.73 m2; CI −15.85 to −0.26; P = .04) compared with RPN (Fig. 3).
Table 2.
Renal functional outcomes of included literature.
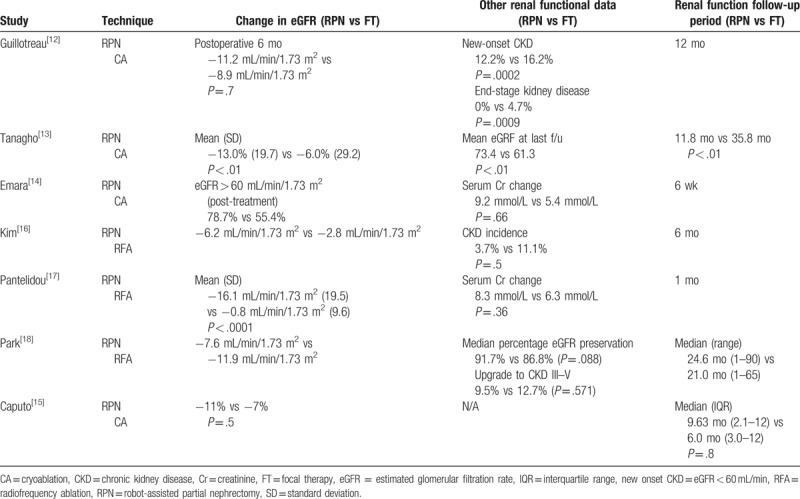
Figure 3.

Forest plot and meta-analysis of renal functional outcome of estimated glomerular filtration rate change.
3.4. Oncological outcomes
The mean or median follow-up period of oncological outcomes of RPN was 4.8 to 24.6 months, and FT was 16.7 to 47.5 months. Seven studies reported on local recurrence rate and 3 studies reported on distant metastasis rate. These studies were included for meta-analysis. A fixed-effects model was used on both local recurrence and metastasis rate owing to a low degree of heterogeneity (I2 = 0%). Patients who underwent FT had a significantly increased risk of local recurrence (RR 9.89; CI 4.24–23.04; P < .001) and significantly increased risk of distant metastasis (RR 6.42; CI 1.70–24.33, P = .006) compared with patients who underwent RPN (Fig. 4). However, 2 propensity score matched studies with similar basic characteristics revealed no difference in local recurrence between RPN and FT (Table 3).[16,18]
Figure 4.
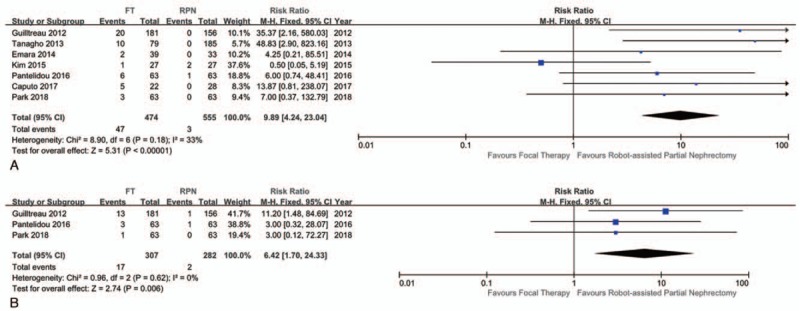
Forest plot and meta-analysis of oncological outcomes: (A) local recurrence and (B) distant metastasis.
Table 3.
Oncological outcomes of included literature.
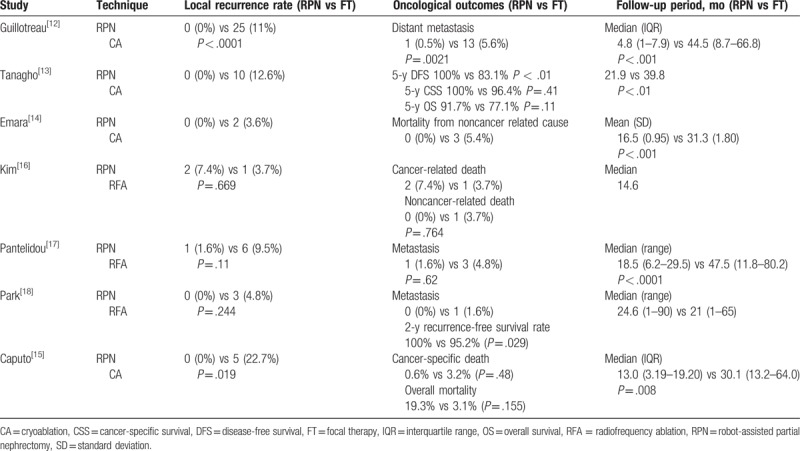
In terms of cancer-specific survival (CSS) rate and overall survival (OS) rate, Tanagho et al have reported a 5-year CSS rate of 100% versus 96.4% (RPN vs FT); P = .41, and an OS rate of 91.7% versus 77.1% (RPN vs FT); P = .11.[13] Kim et al have reported cancer-related death to be 7.4% versus 3.7% (RPN vs FT); P = .764,[16] and Caputo et al have reported cancer-specific death to be 0.6% versus 3.2%; P = .48, and overall mortality to be 3.1% versus 19.3% (RPN vs FT); P = .155.[15]
3.5. Quality assessment by mNOS
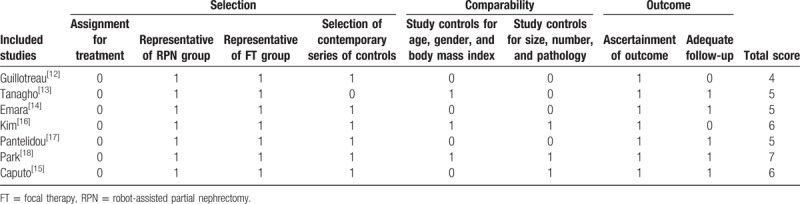
Table 4 presents the quality of each article, as assessed using the mNOS. The median quality score was 5 (range 4–7) out of a total possible score of 8. Three high-quality studies were identified and scored more than 5 stars on mNOS.[15,16,18]
Table 4.
Quality of analyzed studies and risk of bias according to the modified Newcastle–Ottawa scale.
4. Discussion
Surgical treatment for SRMs has developed over the years with the increase of minimally invasive procedures.[19] During the treatment of SRMs, cancer control, renal function preservation, and patient morbidity should be balanced.[20] Therefore, optimal treatment option selection is crucial and depends on patient characteristics, predicted oncological and functional outcomes, and risk of treatment. Recently, RPN and FT have gained popularity in the treatment of SRMs; however, few studies have compared the outcomes between RPN and FT.
Finding the optimal indication for FT in cases involving SRMs is challenging owing to the lack of literature comparing RPN with FT. To our knowledge, this study provides the first systematic review and meta-analysis comparing RPN and FT for SRMs. We have investigated 7 nonrandomized, observational studies comparing RPN and FT because RCTs are lacking. The primary endpoint was oncological outcome (local recurrence and distant metastasis of tumor) and the follow-up period was relatively short; therefore, meta-analysis of CSS and OS were inappropriate. The secondary endpoints were renal functional outcome and perioperative outcomes.
This meta-analysis highlighted that RPN had an advantage in cancer control compared with FT. Patients who underwent FT had an RR of 9.89 for local recurrence and an RR of 6.42 for distant metastasis compared with patients who underwent RPN. These results reveal that extirpative surgical treatment, such as RPN, has superiority in terms of cancer control in comparison to FT. However, the overall follow-up period for oncologic outcomes was longer in the FT group, which could result in a bias regarding the relatively high recurrence rates, especially metastasis. Metastatic progression could be strongly influenced by the different follow-up periods between RPN and FT. The majority of included studies had a significantly longer follow-up period in the FT group, which could account for the higher metastasis rate. Additionally, considering “secondary efficacy,” which is the determined oncological outcome after second ablation, risk of recurrence could be decreased. In another study, secondary ablation after first FT seemed to be effective in cancer control and metastasis was not higher compared with PN.[21] However, second ablation was not considered in the included literature. Interestingly, the matched studies for which basic characteristics were similar revealed a similar local recurrence rate between RPN and FT.[16,18]
Regarding the limitation of oncological outcomes, we could not perform a meta-analysis of CSS or OS due to the relatively short follow-up period and lack of information. However, several studies that have investigated CSS and OS revealed no difference between RPN and FT.[13,15,16]
Conversely, FT was associated with significantly better renal function preservation and low EBL. FT was associated with a low decrease of eGFR (WMD −8.06 mL/min/1.73 m2) compared with RPN, and additionally FT had a lower EBL of WMD −49.61 mL.
Analysis of complications was confined to postoperative complication graded by Clavien–Dindo classification.[11] Interestingly, complication rate was similar between the RPN and FT groups. In other studies, OPN and LPN had high complication rates compared with FT.[10,22] Previous literature has included a combination of surgical modalities, such as OPN and LPN or, LPN ± small series of RPN; however, this study only compared FT to RPN. Although there was technical development on the management of SRMs by RPN, major complication rate was still higher than FT with no statistical significance. However, both overall complication rate and minor complication rate were lower in the FT group and also it did not reach statistical significance.
Previously, FT has been reported to be the option of treatment for SRMs with less complications and improved renal function preservation when compared with radical nephrectomy.[23] Based on the guidelines of the 2017 AUA, FT has been considered to be an alternative therapy to PN for SRMs <3 cm.[6] Recently, there is evidence supporting the treatment of larger renal masses such as cT1b renal tumor.[24]
The source of energy of FT is thermal (CA, RFA, and MWA) or nonthermal (IRE). RFA has the advantage of cauterizing blood vessels, which is protective against hemorrhage, and CA has the advantage of monitoring the ablation zone with CT or ultrasonography in real time. However, both RFA and CA have the disadvantages of the “heat-sink” effect, whereby blood flow carries energy from the FT zone and decreases the effect of energy. MWA has the advantage of rapid heating by microwave band and has less “heat-sink” effect. IRE is the most recent FT technique that has no “heat-sink” effect and minimal damage to adjacent tissue; however, so far, no long-term data have been reported.[5]
There were several systematic reviews comparing CA with PN (OPN and LPN) or CA with LPN. Klatte et al have reported the comparative outcome of laparoscopic CA and PN (OPN and LPN).[22] CA resulted in a higher risk of local tumor progression. However, CA had a lower risk of perioperative complications. Since then, the same group reported on the comparative outcome of laparoscopic CA and (LPN and RPN), which concluded with similar results.[10]
No literature has compared WMA or IRE with surgical treatment. Only single arm studies of WMA or IRE have been published. Klapperich et al have investigated the efficacy and complication after percutaneous MWA for cT1a renal cell carcinoma.[25] The authors concluded that after percutaneous MWA, there was no change in renal function, 3% procedure-related complication, and 3-year local progression-free survival, CSS, OS were 88%, 100%, and 91%, respectively. IRE is a nonthermal ablation technique that leads to apoptosis of the ablation area by creating a disturbance in the cell membrane homeostasis.[26] The first clinical human study of IRE on renal mass was published in 2011.[27] From a total of 10 cases of IRE in renal tumors, 5 cases showed complete response and the other 5 cases had progressive disease. Several complications were evident, such as partial ureteral obstruction, hematuria, adrenal injury, and transient decrease of renal function. On the most recent large IRE study, a total of 41 patients with small low complexity tumors were investigated.[28] Using the NanoKnife System with a 22-month follow-up period, no complication was observed. The initial treatment success rate was 93%, and the 2-year local recurrence-free survival rate was 87%.
This meta-analysis has several limitations that should be noted. First, no randomized controlled trial (RCT) study was included, 5 studies were analyzed, retrospectively, and the other 2 studies were analyzed prospectively, which could have introduced selection bias. Second, a few studies had a small sample size and a suboptimal follow-up period in this meta-analysis. Third, there was marked heterogeneity for several variables and the definition of local recurrence in each included article was not clear. This may also lead to high heterogeneity result. Fourth, sensitivity analysis and publication bias could not be assessed due to the small number (<10 cases) of included studies. Fifth, due to relatively newer technologies, no comparative study of MWA and IRE was compared with surgical treatment. However, we believe that this article is meaningful because, to the best of our knowledge, this study is the first meta-analysis to establish the comparison between RPN and FT in cT1 renal tumor.
Despite the several limitations of this meta-analysis, this study represents a detailed overview between RPN and FT. So far, it is clear that RPN achieves a superior oncological outcome and comparable perioperative outcomes. FT could be performed in selected patients in whom there is a high risk of renal failure, high comorbidity, and who require minimal bleeding, such as multiple renal masses or single kidney. Furthermore, renal function was usually measured by eGFR which was calculated by serum creatinine level; however, further study with more exact renal function assessment could be performed by emerging urinary markers such as Neutrophil Gelatinase-associated Lipocalin or Kidney Injury Molecule-1.[29] Also, long-term follow-up, large volume, prospective, multicenter RCT studies including MWA and IRE are needed for confirmation of our findings.
5. Conclusions
This study results indicate that RPN has a substantial advantage in oncologic control, such as local recurrence and distant metastasis. However, in the era of minimally invasive surgery, FT has the advantage in renal function preservation and is associated with less bleeding. Long-term follow-up for survival rates and comparative analyses of MWA, and IRE are needed to extend FT for patients with significant morbidities and for those who need sufficient renal function preservation with minimal bleeding
Author contributions
Young Eun Yoon: Methodology and writing of the article; Hyung Ho Lee: data curation, formal analysis, and investigation; Dae Keun Kim: investigation, methodology, conceptualization, writing original draft, writing review and editing, and under supervision; Sung Yul Park: methodology, supervision, and validation; Hong Sang Moon: conceptualization, investigation, and supervision; Seung Ryeol Lee: data curation and formal analysis. Young Kwon Hong: investigation, methodology, and validation; Dong Soo Park: investigation; methodology, and validation.
Conceptualization: Ki Hong Kim, Hong Sang Moon, Dae Keun Kim.
Data curation: Hyung Ho Lee, Seung Ryeol Lee.
Formal analysis: Hyung Ho Lee, Seung Ryeol Lee.
Investigation: Hyung Ho Lee, Ki Hong Kim, Hong Sang Moon, Young Kwon Hong, Dong Soo Park.
Methodology: Young Eun Yoon, Ki Hong Kim, Sung Yul Park, Young Kwon Hong, Dong Soo Park.
Supervision: Sung Yul Park, Hong Sang Moon.
Validation: Sung Yul Park, Young Kwon Hong, Dong Soo Park.
Writing – original draft: Young Eun Yoon, Dae Keun Kim.
Writing – review & editing: Dae Keun Kim.
Dae Keun Kim orcid: 0000-0003-3237-6304.
Footnotes
Abbreviations: AUA = American Urological Association, CA = cryoablation, CI = confidence interval, CSS = cancer-specific survival, EBL = estimated blood loss, eGFR = estimated glomerular filtration rate, FT = focal therapy, IRE = irreversible electroporation, LPN = laparoscopic partial nephrectomy, mNOS = modified Newcastle–Ottawa scale, MWA = microwave ablation, OPN = open partial nephrectomy, OR = odds ratio, OS = overall survival, PN = partial nephrectomy, RFA = radiofrequency ablation, RPN = robot-assisted partial nephrectomy, RR = risk ratio, SRMs = small renal masses, WMD = weighted mean difference.
This research was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (NRF-2017R1C1B5018097). This research was supported by Basic Science Research Program through the National Research Foundation of Korea(NRF) funded by the Ministry of Education (NRF-2018R1D1A1B07051225).
The authors have no conflicts of interest to disclose.
References
- [1].Campbell SC, Novick AC, Belldegrun A, et al. Guideline for management of the clinical T1 renal mass. J Urol 2009;182:1271–9. [DOI] [PubMed] [Google Scholar]
- [2].Park SW, Lee SS, Lee DH, et al. Growth kinetics of small renal mass: initial analysis of active surveillance registry. Investig Clin Urol 2017;58:429–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Ficarra V, Novara G, Secco S, et al. Preoperative aspects and dimensions used for an anatomical (PADUA) classification of renal tumours in patients who are candidates for nephron-sparing surgery. Eur Urol 2009;56:786–93. [DOI] [PubMed] [Google Scholar]
- [4].Choi JE, You JH, Kim DK, et al. Comparison of perioperative outcomes between robotic and laparoscopic partial nephrectomy: a systematic review and meta-analysis. Eur Urol 2015;67:891–901. [DOI] [PubMed] [Google Scholar]
- [5].Johnson BA, Cadeddu JA. Current opinion in urology 2017: focal therapy of small renal lesions. Curr Opin Urol 2018;28:166–71. [DOI] [PubMed] [Google Scholar]
- [6].Campbell S, Uzzo RG, Allaf ME, et al. Renal mass and localized renal cancer: AUA guideline. J Urol 2017;198:520–9. [DOI] [PubMed] [Google Scholar]
- [7].Ljungberg B, Cowan NC, Hanbury DC, et al. EAU guidelines on renal cell carcinoma: the 2010 update. Eur Urol 2010;58:398–406. [DOI] [PubMed] [Google Scholar]
- [8].Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol 2009;62:e1–34. [DOI] [PubMed] [Google Scholar]
- [9].Stang A. Critical evaluation of the Newcastle–Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 2010;25:603–5. [DOI] [PubMed] [Google Scholar]
- [10].Klatte T, Shariat SF, Remzi M. Systematic review and meta-analysis of perioperative and oncologic outcomes of laparoscopic cryoablation versus laparoscopic partial nephrectomy for the treatment of small renal tumors. J Urol 2014;191:1209–17. [DOI] [PubMed] [Google Scholar]
- [11].Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 2004;240:205–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Guillotreau J, Haber GP, Autorino R, et al. Robotic partial nephrectomy versus laparoscopic cryoablation for the small renal mass. Eur Urol 2012;61:899–904. [DOI] [PubMed] [Google Scholar]
- [13].Tanagho YS, Bhayani SB, Kim EH, et al. Renal cryoablation versus robot-assisted partial nephrectomy: Washington University long-term experience. J Endourol 2013;27:1477–86. [DOI] [PubMed] [Google Scholar]
- [14].Emara AM, Kommu SS, Hindley RG, et al. Robot-assisted partial nephrectomy vs laparoscopic cryoablation for the small renal mass: redefining the minimally invasive “gold standard”. BJU Int 2014;113:92–9. [DOI] [PubMed] [Google Scholar]
- [15].Caputo PA, Zargar H, Ramirez D, et al. Cryoablation versus partial nephrectomy for clinical T1b renal tumors: a matched group comparative analysis. Eur Urol 2017;71:111–7. [DOI] [PubMed] [Google Scholar]
- [16].Kim SH, Lee ES, Kim HH, et al. A propensity-matched comparison of perioperative complications and of chronic kidney disease between robot-assisted laparoscopic partial nephrectomy and radiofrequency ablative therapy. Asian J Surg 2015;38:126–33. [DOI] [PubMed] [Google Scholar]
- [17].Pantelidou M, Challacombe B, McGrath A, et al. Percutaneous radiofrequency ablation versus robotic-assisted partial nephrectomy for the treatment of small renal cell carcinoma. Cardiovasc Interv Radiol 2016;39:1595–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Park BK, Gong IH, Kang MY, et al. RFA versus robotic partial nephrectomy for T1a renal cell carcinoma: a propensity score-matched comparison of mid-term outcome. Eur Radiol 2018;28:2979–85. [DOI] [PubMed] [Google Scholar]
- [19].Ljungberg B, Bensalah K, Canfield S, et al. EAU guidelines on renal cell carcinoma: 2014 update. Eur Urol 2015;67:913–24. [DOI] [PubMed] [Google Scholar]
- [20].Heuer R, Gill IS, Guazzoni G, et al. A critical analysis of the actual role of minimally invasive surgery and active surveillance for kidney cancer. Eur Urol 2010;57:223–32. [DOI] [PubMed] [Google Scholar]
- [21].Aron M, Kamoi K, Remer E, et al. Laparoscopic renal cryoablation: 8-year, single surgeon outcomes. J Urol 2010;183:889–95. [DOI] [PubMed] [Google Scholar]
- [22].Klatte T, Grubmuller B, Waldert M, et al. Laparoscopic cryoablation versus partial nephrectomy for the treatment of small renal masses: systematic review and cumulative analysis of observational studies. Eur Urol 2011;60:435–43. [DOI] [PubMed] [Google Scholar]
- [23].Lucas SM, Stern JM, Adibi M, et al. Renal function outcomes in patients treated for renal masses smaller than 4 cm by ablative and extirpative techniques. J Urol 2008;179:75–9. [DOI] [PubMed] [Google Scholar]
- [24].Schulman AA, Tay KJ, Polascik TJ. Expanding thermal ablation to the “intermediate-sized” renal mass: clinical utility in T1b tumors. Transl Androl Urol 2017;6:127–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Klapperich ME, Abel EJ, Ziemlewicz TJ, et al. Effect of tumor complexity and technique on efficacy and complications after percutaneous microwave ablation of stage T1a renal cell carcinoma: a single-center, retrospective study. Radiology 2017;284:272–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Narayanan G, Doshi MH. Irreversible electroporation (IRE) in renal tumors. Curr Urol Rep 2016;17:15. [DOI] [PubMed] [Google Scholar]
- [27].Thomson KR, Cheung W, Ellis SJ, et al. Investigation of the safety of irreversible electroporation in humans. J Vasc Interv Radiol 2011;22:611–21. [DOI] [PubMed] [Google Scholar]
- [28].Canvasser NE, Sorokin I, Lay AH, et al. Irreversible electroporation of small renal masses: suboptimal oncologic efficacy in an early series. World J Urol 2017;35:1549–55. [DOI] [PubMed] [Google Scholar]
- [29].Lucarelli G, Mancini V, Galleggiante V, et al. Emerging urinary markers of renal injury in obstructive nephropathy. Biomed Res Int 2014;2014:303298. [DOI] [PMC free article] [PubMed] [Google Scholar]


