Abstract
Background:
Perioperative inadvertent hypothermia in elderly urology patients undergoing transurethral resection of the prostate (TURP) is a well-known serious complication, as it increases the risk of myocardial ischemia, blood loss, and surgical wound infection. We conducted this prospective randomized controlled trial to evaluate the combined effect of a forced-air warming system and electric blanket in elderly TURP patients.
Methods:
Between January 2015 and October 2017, we recruited 443 elderly male patients undergoing elective TURP with subarachnoid blockade (SAB). These were randomly divided into 3 groups: group E (intraoperative warming using electric blankets set to 38°C; n = 128); group F (intraoperative warming using a forced-air warmer set to 38°C; n = 155) and group FE (intraoperative warming using a forced-air warmer plus electric blankets, both set to 38°C; n = 160). The primary outcome was shivering and their grades. Hemodynamic changes, esophageal temperature, recovery time, incidences of adverse effects, and patient and surgeon satisfaction were also recorded.
Results:
Baseline characteristics showed no significant differences when compared across the 3 groups (P >.05). Compared with groups E and F, both HR and mean arterial pressure (MAP) in group FE were significantly decreased from T6 to T10 (P <.05). Compared with groups E and F, esophageal temperature in group FE increased significantly from T5 to T10 (P <.05). Compared with group E, esophageal temperature in group F was significantly increased from T5 to T10 (P <.05). Compared with groups F and FE, post-anesthesia care unit (PACU) recovery time was longer in group E, while compared with group F, PACU recovery time was shorter in group FE (P <.05). Compared to patients in groups E and F, those in group FE had a significantly lower incidence of arrhythmia and shivering (P <.05). The number of patients with shivering grades 0 to 3 was higher in group E than in other groups, while the number of patients with shivering grade 2 was significantly higher in group F than in group FE (P <.05). Patient and surgeon satisfaction scores were higher in group FE than in groups E and F (P <.05).
Conclusions:
Use of a forced-air warming system combined with an electric blanket was an effective method with which to retain warmth among elderly TURP patients.
Keywords: elderly patients, electric blanket, forced-air warming system, perioperative inadvertent hypothermia, transurethral resection of the prostate
1. Introduction
Perioperative inadvertent hypothermia (PIH), in which core body temperature falls below 36°C, is a common event during surgery and may result from the anesthesia-induced impairment of thermoregulation, fluids used during surgery, and exposure to a cold operating room (OR) environment. Worryingly, PIH has been associated with an increased incidence of perioperative complications if not properly controlled.[1,2] Previous studies have confirmed that PIH may have several adverse effects on the pharmacokinetics of agents used during anesthesia on the myocardium, on surgical site infection rates, and the bloody clotting system, which can then lead to an extended hospital stay for patients.[3–5]
Transurethral resection of the prostate (TURP) is currently the most widely used surgical technique to treat benign prostatic hyperplasia (BPH).[6,7] Spinal anesthesia is commonly used during TURP; however, elderly patients under spinal anesthesia are vulnerable to hypothermia due to the specific characteristics of this patient group, such as reduced muscle content, higher body surface area/weight and reduced vasoconstriction in the skin.[8–10] General anesthesia is preferred when blockade is contraindicated, fails, or is refused by patients.[11] In addition, previous studies have found that the use of irrigation fluids at room temperature during TURP can lead to perioperative hypothermia. The use of isothermic irrigation fluid reduces this risk but does not eliminate it completely.[12,13] In light of these issues, forced-air warming is commonly used to warm patients intraoperatively, but may not achieve normothermia during a short procedure such as TURP.[14,15] The aim of this study was to investigate the effects of forced-air warming plus an electric blanket on perioperative hypothermia in elderly patients undergoing TURP under spinal anesthesia.
2. Methods
2.1. Patients
Ethical approval was obtained from the Institutional Review Board of Liaocheng People's Hospital, China and patients provided informed written consent for this prospective randomized controlled trial. The study was registered at chictr.org (ChiCTR-TRC-14004191). Between January 2015 and October 2017, we enrolled elderly male patients (aged >65 years, American Society of Anesthesiology [ASA] Grades I to II) scheduled for elective TURP under subarachnoid blockade (SAB). We excluded patients who were obese (body mass index [BMI] >30 kg/m2), required blood transfusion during surgery, had a history of ischemic heart disease, thyroid disease, cerebrovascular disease, Parkinson's disease or Raynaud's syndrome, those with severe diabetic autonomic neuropathy; those with a contraindication to regional anesthesia, those who had an infection of the urinary tract; and those with a core temperature above 38°C or below 36.5°C. Patients who were taking vasodilator/vasoconstrictor medications were also excluded from the study because such drugs can cause changes in thermoregulation. Our final study featured 443 elderly patients, who were divided into 3 groups according to the different rewarming methods used: group E (intraoperative warming using electric blankets set to 38°C; n = 128); group F (intraoperative warming using a forced-air warmer set to 38°C; n = 155) and group FE (intraoperative warming using a forced-air warmer and electric blankets, both set to 38°C; n = 160). Data were acquired from electronic charts and a DoCare Clinic electronic anesthesia recording system. Esophageal temperature was measured as an indicator of core body temperature.
2.2. Anesthesia
Patients were not pre-medicated. The OR temperature was controlled at 22°C to 24°C and all operative procedures were performed by the same surgeon. On arrival in the OR, all patients were monitored with an automated system (Philips IntelliVue MP50, Philips Company) in accordance with the American Society of Anesthesiologists and were actively warmed from induction to the end of anesthesia. During surgery, supplemental oxygen (4 L/min) was delivered via a face mask. In both groups of patients, intravenous fluids were infused intraoperatively using an Animec warmer (Elltec Co. Ltd., Nagoya, Japan). Before performing spinal anesthesia, each patient received 10 mL/kg/h of lactated Ringer's solution over a period of 30 minutes. The infusion rate was then reduced to 5 mL/kg/h. The patient was administered with epidural anesthesia at the L3-4 interspace via a 25-G Quincke spinal needle. Motor blockade was evaluated by the Bromage motor scale. Sensory block level was assessed by the pinprick test. When systolic blood pressure fell to 80% below the baseline value, or to lower than 90 mmHg, we administered phenylephrine (40 μg) or ephedrine (6 mg) at 3-min intervals. Atropine (0.4 mg) was administered at 3-minute intervals when heart rate (HR) fell to 80% below the baseline value or to lower than 50 beats/min. All of the irrigation fluids (0.9% saline) were heated to 36°C during surgery. Patients were covered with 1 layer of surgical drapes over the chest, thigh, and calves during TURP. The warming setting was reduced if the verbal analogue score of general thermal comfort (VAS; scale 0–10, where 10 indicates extreme heat) exceeded 7, or by patient request. All patients with a core temperature <36.0°C received only lower-body forced-air warming set to 38°C in the post-anesthesia care unit (PACU).
2.3. Outcome measures
The primary outcome was shivering, which was graded using a scale validated by Crossley and Mahajan[16] in which 0 = no shivering, 1 = piloerection or peripheral vasoconstriction but no visible shivering, 2 = muscular activity in only 1 muscle group, 3 = muscular activity in more than 1 muscle group but not generalized, and 4 = shivering involving the whole body. We also recorded hemodynamic changes, esophageal temperature, recovery time, incidences of adverse effects, patients and surgeon satisfaction (on a 5-point scale where 5 = excellent, 4 = adequate, 3 = cannot say, 2 = inadequate, and 1 = poor).
2.4. Data collection
Perioperative hemodynamic data (MAP and HR) and esophageal temperature were obtained using a Phillips IntelVue monitor MP50 at the following time points: arrival in the OR (T0), just before the induction of anesthesia (T1), at the beginning of the operation (T2), 10 minutes (T3), 20 minutes (T4), 30 minutes (T5), 60 minutes (T6) after the onset of operation, at the end of operation (T7), and 10 minutes (T8), 20 minutes (T9), and 30 minutes (T10) after arriving at the PACU. We also recorded the length of the PACU stay based on Aldretes criteria.[17] The number of adverse effects, such as arrhythmia, hypotension, hypertension, agitation, hypoxemia, and shivering were also recorded at the end of the study.
2.5. Statistical analysis
Sample size was calculated on the basis of an expected 10% shorter in the incidence of patients experiencing shivering. For a study power of 80% (α = 0.05, β = 0.2), the required sample size per group was calculated to be 106 (PASS 11.0, NCSS Statistical Software, Kaysville, Utah). Assuming a dropout rate of 20%, the final sample size was determined to be 128 patients for each group.
The Kolmogorov–Smirnov test was used to assess the distribution of variables. Homogeneity of variance was determined using Levene test. Quantitative data were expressed as means and standard deviations, or medians and inter-quartile ranges (IQRs). Inter-group comparisons were performed using repeated-measures analysis of variance. Bonferroni's correction was used for post-hoc multiple comparisons. The non-parametric Wilcoxon–Mann–Whitney test was used for variables that were not normally distributed. Categorical data were expressed as frequencies and percentages and analyzed using chi-square tests or Fisher exact tests, when appropriate. Probability (P) values <.05 were considered to be statistically significant. All statistical analysis was performed with SPSS for Windows version 19.0 (SPSS Inc., Chicago, IL).
3. Results
3.1. Baseline characteristics
The flow diagram depicting patient enrollment is shown in Figure 1. In total, 757 patients who underwent TURP between January 2015 and October 2017 were initially enrolled in the study. Of these, 314 patients were excluded for various reasons. The final sample size consisted of 443 patients. Of these, 36 patients were obese (BMI: >30 kg/m2), 23 required blood transfusion during surgery, 35 showed an ASA score which was higher than II, 156 had a history of ischemic heart disease, cerebrovascular disease, Parkinson's disease, Raynaud's syndrome or severe diabetic autonomic neuropathy, 22 patients underwent general anesthesia, 8 patients had an infection of the urinary tract or core temperature above 38°C or below 36.5°C, 23 patients needed vasodilator or vasoconstrictor medications during surgery, and 11 patients were excluded after surgery owing to an incomplete set of clinical data.
Figure 1.
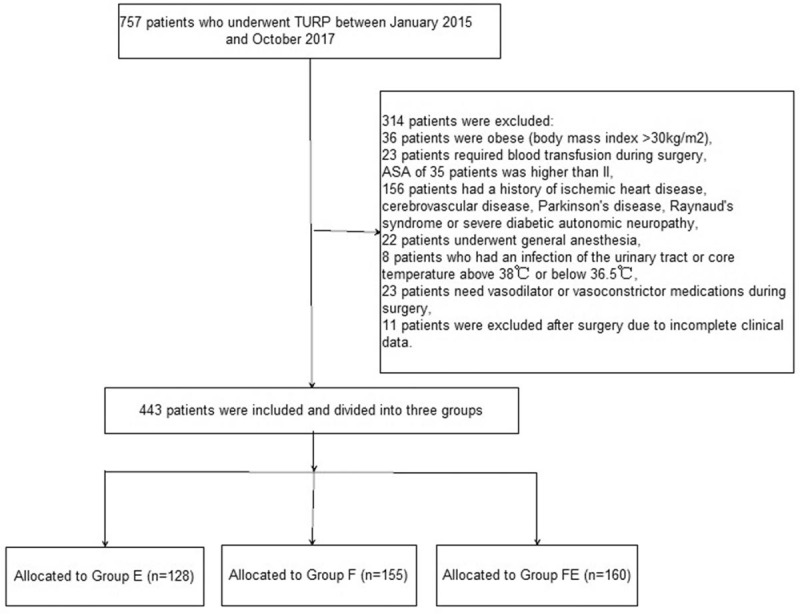
Flow diagram depicting patient enrolment.
There were no significant differences among the 3 groups with respect to age, BMI, ASA grade, OR temperature, median level of sensory block, total irrigation fluid, total infused fluid, prostate weight, International Prostate Symptom Score (IPSS), maximal urinary flow rate, residual urine, serum prostatic specific antigen (PSA) level, median level of sensory block, and the duration of anesthesia and operation (Table 1).
Table 1.
Patients’ characteristics and Intraoperative data.

3.2. Perioperative hemodynamic data
Baseline HR and MAP were not significantly different when compared across the 3 groups (P >.05, Fig. 2). Compared with groups E and F, HR in group FE was significantly lower from T6 to T10 (P <.05, Fig. 2). Compared with groups E and F, MAP in group FE was also significantly lower from T6 to T10 (P <.05, Fig. 2). Compared with groups E and F, esophageal temperature in group FE was significantly higher from T5 to T10 (P <.05, Fig. 3). Compared with group E, esophageal temperature in group F was also significantly higher from T5 to T10 (P <.05, Fig. 3). Compared with groups F and FE, the recovery time of PACU was significantly longer in group E, while compared with group F, the recovery time of PACU was significantly shorter in group FE (P <.05, Table 1). The satisfaction scores of both patients and surgeon were significantly higher in group FE than the other 2 groups (P <.05, Table 2).
Figure 2.
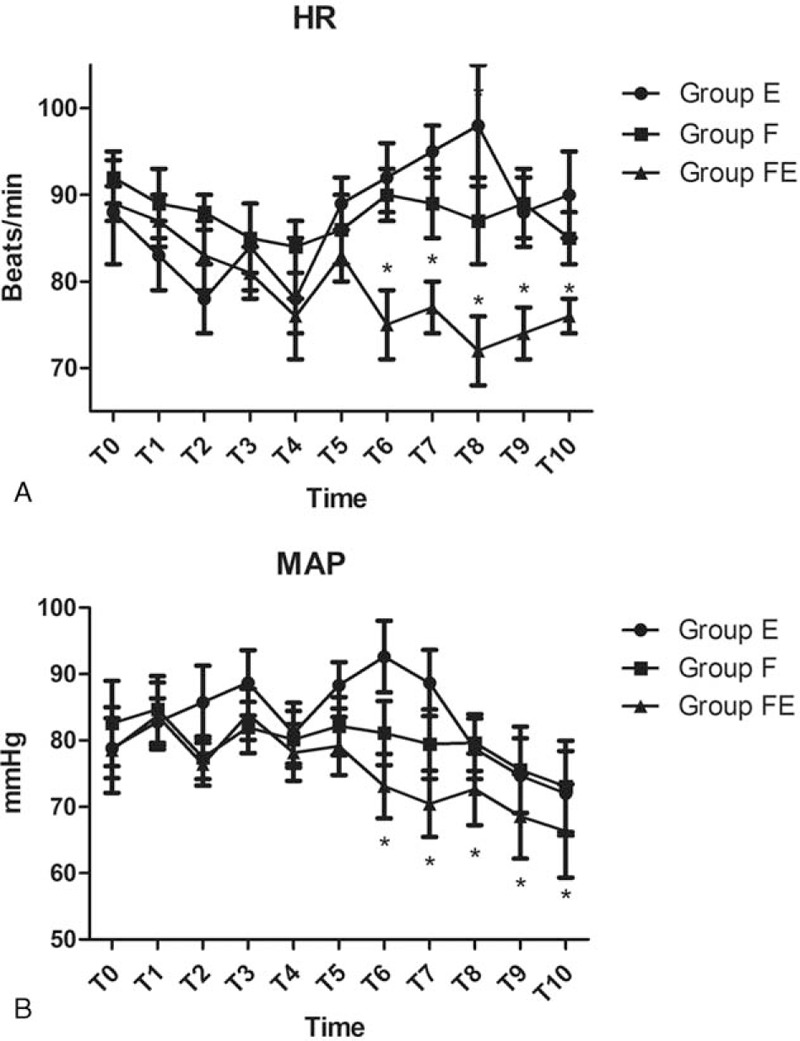
Hemodynamic data for the 3 groups of patients at various timepoints: arrival in the operating room (T0), just before the induction of anesthesia (T1), at the beginning of the operation (T2), 10 minutes (T3), 20 minutes (T4), 30 minutes (T5), 60 minutes after the onset of operation (T6), at the end of operation (T7), and 10 minutes (T8), 20 minutes (T9), and 30 minutes (T10) after arriving in the PACU. ∗P <.05 versus Group E.
Figure 3.
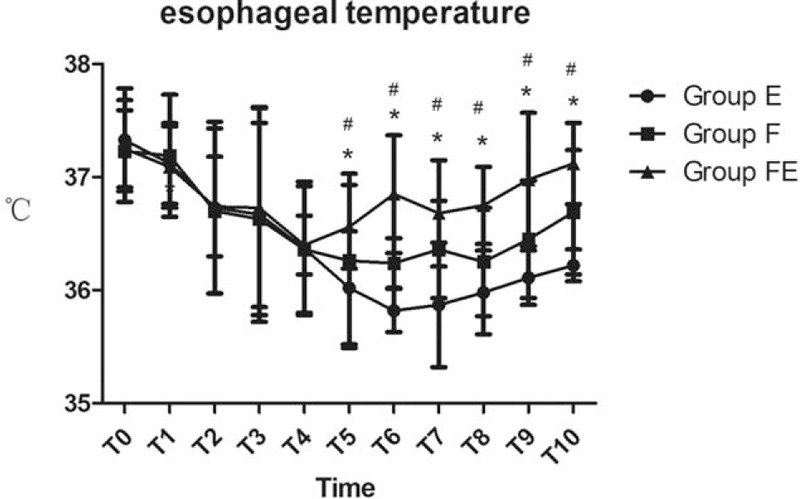
Esophageal temperature data for the 3 groups of patients at various timepoints: arrival in the operating room (T0), just before anesthesia induction (T1), at the beginning of the operation (T2), 10 minutes (T3), 20 minutes (T4), 30 minutes (T5), 60 minutes after the onset of operation (T6), at the end of operation (T7), and 10 minutes (T8), 20 minutes (T9), and 30 minutes (T10) after arriving in the PACU. ∗P <.05 versus Group E, #P <.05 versus Group F.
Table 2.
Satisfaction scores of patients and surgeons.

3.3. Adverse events
The main adverse events are presented in Table 3. Compared to patients in groups E and F, those in group FE had a significantly lower incidence of arrhythmia and shivering (P <.05, Table 3). The number of patients with a shivering grade of 0 to 3 was significantly higher in group E than in the other groups, while the number of patients with a shivering grade of 2 was statistically higher in group F than in group FE (P <.05, Table 4). In contrast, there were no significant differences among the 3 groups with respect to the incidence of hypoxemia, hypotension, agitation, and hypertension.
Table 3.
Comparison of the main adverse events among groups.

Table 4.
Comparison of the shivering grade among groups.

4. Discussion
In this study, we found that the combination of an electric blanket and the use of a forced-air warming system set to 38°C was an effective warmth-retaining method for elderly urology patients undergoing TURP. This process also reduced the incidence of arrhythmia and shivering.
Patients undergoing TURP are usually elderly patients with systemic diseases, most-commonly with conditions affecting the respiratory and circulatory systems.[18] As a consequence, anesthetic techniques used during such techniques must consider the co-morbidities of this patient population in order to reduce the incidence of postoperative complications. Spinal anesthesia is acknowledged to be the most common technique for reducing impact upon critically ill patients and providing better surgical conditions. However, this technique is also associated with technical difficulties, takes longer to reach adequate levels of analgesia, can lead to blockade failure; patients can also experience post-dural puncture lumbago and back pain.[19–21] The existing literature predominantly suggests that sensory level should not be greater than T9 in order to observe patient symptoms for potential early TURP syndrome such as versical perforation.[22,23] However, some of the previous reports show that spinal anesthesia can significantly impair thermoregulation by altering afferent thermal inputs, inhibiting vasomotor and shivering responses, redistributing body heat, and predisposing patients to perioperative hypothermia. As a result, the threshold for shivering and vasoconstriction may decrease by 0.5°C to 0.9°C during spinal anesthesia.[24,25]
Because the thermal retardation capability of elderly patients is significantly reduced, they are at greatest risk of hypothermia during TURP, which can lead to serious complications such as blood loss and the infection of surgical wounds.[26] Thus, it is important to monitor and control body temperature and prevent hypothermia in elderly patients undergoing TURP. In a previous study, Torossian et al reported that 28% of patients were actively heated during regional anesthesia in Europe as compared to 43% during general anesthesia, and that body temperature was monitored in 6% of patients undergoing regional anesthesia as compared to 25% during general anesthesia.[27] Hypothermia may also lead to increased levels of circulating catecholamines, resulting in tachycardia, hypertension, and systemic vasoconstriction. The consequential increase in plasma norepinephrine concentrations can enhance cardiac irritability and promote the development of ventricular arrhythmias.[28] Furthermore, previous studies have reported that hypothermia can trigger alterations in electrocardiographic parameters, particularly the prolongation of PR, QRS, and QT intervals. Assessment of the QT interval is therefore clinically important because prolongation of repolarization is often associated with poor cardiac conditions.[29] Ten years ago, Frank et al conducted a randomized, controlled study in 300 high-risk cardiac patients and found that perioperative myocardial ischemia and ventricular tachycardia were more prevalent in hypothermic patients.[30] One previous study also reported that perioperative hypothermia can increase left ventricular afterload, indicating increased myocardial work and oxygen demand, which could likely result in myocardial ischemia and be associated with post-operative instability and prolonged recovery.[31] Consistent with this data, we found that the incidence of arrhythmia in our study was lower in group F2.
The amount of irrigation fluid used during TURP should be optimized to provide a clear and good view of the operative field, be isotonic relative to the plasma, be non-toxic and non-hemolytic in case of absorption; it should also be poorly ionized, rapidly excreted and cheap, as large volumes are required for TURP. The rate of intravasation of irrigating fluids during TURP depends partly on temperature as the density and fluidity of fluids vary with temperature. One previous study reported that when the temperature of the irrigation solution was increased from 17°C to 37°C, the mean absorption rate was predicted to increase by approximately 54% and that the theoretical “safe” duration of surgery reduced by 65%.[7] As a result, it is recognized that elderly patients undergoing TURP should not be excessively hydrated, partly because irrigation fluid might be absorbed through prostatic venous channels, and partly because elderly patients may not be able to tolerate such volume overload. Consistent with these studies, we found that the hemodynamics of patients in group FE was more stable and that recovery time was in this group of patients.
The measurement of central blood temperature with pulmonary artery catheters currently represents the gold standard for measuring body core temperature.[32] However, it is inconvenient or uncomfortable to monitor core temperature using these alternative methods in conscious patients or those who are lightly sedated during regional anesthesia.[33] For this reason, we adopted nasopharyngeal temperature for our present study. Shivering is commonly associated with spinal and epidural anesthesia, particularly in elderly patients undergoing TURP. Shivering during surgery may increase the risk of injury to the urethra, bladder, and rectum. However, the precise mechanisms underlying shivering process are still not clear.[34] One previous study reported that preoperative warming for 20 minutes was unable to maintain a core body temperature of >36°C during TURP, and that the incidence of shivering was higher.[35] These previous findings were inconsistent with those obtained in our present study. Furthermore, in our present study, both the incidence and grade of shivering were lower in the group who experienced intraoperative warming using a forced-air warmer and electric blankets set to 38°C. This was probably due to the fact that all of the irrigation fluids were heated to 36°C during surgery in our study. In recent times, a variety of drugs have been used to treat or prevent postoperative shivering, including pethidine, tramadol, clonidine, physostigmine, clonidine, ketamine, and MgSO4.[36] Despite premedication with these drugs, it is still important to adopt specific measures to maintain normothermia.
One previous study reported a mean nadir of 35.6°C 3 hours after the onset of anesthesia, and that mean temperatures returned to normal 7 hours after the onset of anesthesia.[26] These authors also found that the drop in body temperature was correlated with time but not with prostate size, patient size, or age.[26] However, in most of our patients, the mean nadir in our study occurred at T6 (60 minutes after the onset of surgery) and mean body core temperature returned to normal 2 hours after the onset of surgery. These observations are consistent with another study which reported that core temperature usually decreases markedly in the first hour of anesthesia, mostly due to the redistribution of body heat from the core to the periphery, rather than heat loss. The difference may be due to the different methods used to retain warmth and the different volumes of irrigation fluids used during surgery. Another study reported that the temperature of elderly patients may continue to drop in the PACU because of the inability of this patient group to vasoconstrict or shiver below the level of the subarachnoid block they received.[37] However, in our study, the esophageal temperature of elderly patients did not continue to fall in the PACU, most probably because of the fact we used different methods to rewarm our patients (lower-body forced-air warming setting at 38°C, as opposed to the upper-body warming blanket used in the previous study[37]). A new radiant warming device (Suntouch, Fisher and Paykel, Auckland, New Zealand) has been recently proposed as an alternative to forced-air warming. Researchers have shown that the forced-air warming device produced a higher core temperature than the Suntouch for the first 60 minutes after the induction of anesthesia. However, it may be difficult for surgeons to obtain the Suntouch due to its high-cost.[26] Currently, there are many strategies which can be used to reduce thermal loss in surgical patients, including cotton blankets, surgical drapes, plastic sheeting, forced-air warming, electric, and circulating-water mattresses placed underneath the patient, circulating-water garment systems wrapped around the trunk and extremities, warm water and pulsating negative pressure on 1 arm, as well as flexible adherent hydrogel matrixes.[35–37]
There are several limitations associated with this study. First, this was a single-center randomized controlled study; a large multi-center prospective trial is now needed to verify the conclusion of this study. Second, we did not consider the difference between smoking and drinking across the 3 groups of patients. As Araújo et al observed, most patients with TURP syndrome are smokers; of these, those that also drink alcohol were associated with higher morbidity rates.[38] Third, a previous study found that the incidence of postoperative shivering was higher in elderly females than males.[39] Thus, further studies are now needed to investigate the effect of this scenario in female patients. Fourth, we did not measure skin temperatures at the time of applying forced air warming. Consequently, it was not possible to estimate the total heat body content. Finally, we did not measure the plasma concentrations of norepinephrine.
In summary, our study has shown that the combined use of a forced-air warmer and an electric blanket set to 38°C was an effective method for elderly patients to retain warmth while undergoing TURP. In addition, this practice also reduced the incidence of arrhythmia and shivering.
Author contributions
Yan Xiao, Rui Zhang and Xueli Chen conceived and designed the trial. Yan Xiao and Rui Zhang collected the data. Xueli Chen analyzed the data while Yan Xiao and Xueli Chen wrote the manuscript.
Data curation: Rui Zhang.
Formal analysis: Xueli Chen.
Methodology: Xueli Chen.
Software: Rui Zhang.
Writing – original draft: Yan Xiao.
Writing – review & editing: Yan Xiao.
Footnotes
Abbreviations: ASA = American Society of Anesthesiology, BMI = body mass index, HR = heart rate, MAP = mean arterial pressure, OR = operating room, PACU = post-anesthesia care unit, PIH = perioperative inadvertent hypothermia, TURP = transurethral resection of the prostate.
The authors declare that this research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- [1].John M, Crook D, Dasari K, et al. Comparison of resistive heating and forced-air warming to prevent inadvertentperioperative hypothermia. Br J Anaesth 2016;116:249–54. [DOI] [PubMed] [Google Scholar]
- [2].Allen TK, Habib AS. Inadvertent perioperative hypothermia induced by spinal anesthesia for cesarean delivery might be more significant than wethink: are we doing enough to warm our parturients. Anesth Analg 2018;126:7–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Grigore AM, Murray CF, Ramakrishna H, et al. A core review of temperature regimens and neuro protection during cardiopulmonary bypass: does rewarming rate matter. Anesth Analg 2009;109:1741–51. [DOI] [PubMed] [Google Scholar]
- [4].Shaw CA, Steelman VM, DeBerg J, et al. Effectiveness of active and passive warming for the prevention of inadvertent hypothermia in patients receiving neuraxial anesthesia: a systematic review and meta-analysis of randomized controlled trials. J Clin Anesth 2017;38:93–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Campbell G, Alderson P, Smith AF, et al. Warming of intravenous and irrigation fluids for preventing inadvertent perioperative hypothermia. Cochrane Database Syst Rev 2015;4:CD009891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Bayir H, Yildiz I, Erdem F, et al. Effect of perioperative inadvertent hypothermia on the ECG parameters in patients undergoingtransu rethral resection. Eur Rev Med Pharmacol Sci 2016;20:1445–9. [PubMed] [Google Scholar]
- [7].de Freitas Fonseca M, Andrade CM, Jr, de Mello MJ, et al. Effect of temperature on fluidity of irrigation fluids. Br J Anaesth 2011;106:51–6. [DOI] [PubMed] [Google Scholar]
- [8].Barry MJ, Roehrborn CG. Benign prostatic hyperplasia. BMJ 2001;323:1042–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Thangasamy IA, Chalasani V, Bachmann A, et al. Photoselective vaporisation of the prostate using 80-W and 120-W laser versus transurethral resection of the prostate for benign prostatic hyperplasia: a systematic review with meta-analysis from 2002 to 2012. Eur Urol 2012;62:315–23. [DOI] [PubMed] [Google Scholar]
- [10].Kim SY, Cho JE, Hong JY, et al. Comparison of intrathecal fentanyl and sufentanil in low-dose dilute bupivacaine spinal anaesthesia for transurethral prostatectomy. Br J Anaesth 2009;103:750–4. [DOI] [PubMed] [Google Scholar]
- [11].Žura M, Kozmar A, Šakić K, et al. Effect of spinal and general anesthesia on serum concentration of pro-inflammatory and anti-inflammatory cytokines. Immunobiology 2012;217:622–7. [DOI] [PubMed] [Google Scholar]
- [12].Okeke LI. Effect of warm intravenous and irrigating fluids on body temperature during transurethral resection of the prostate gland. BMC Urol 2007;7:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Pit MJ, Tegelaar RJ, Venema PL. Isothermic irrigation during transurethral resection of the prostate: effects on peri-operative hypothermia, blood loss, resection time and patient satisfaction. Br J Urol 1996;78:99–103. [DOI] [PubMed] [Google Scholar]
- [14].Egan C, Bernstein E, Reddy D, Ali M, Paul J, Yang D, Sessler DI. [DOI] [PubMed] [Google Scholar]
- [15].Vassilieff N, Rosencher N, Sessler DI, et al. Shivering threshold during spinal anesthesia is reduced in elderly patients. Anesthesiology 1995;83:1162–6. [DOI] [PubMed] [Google Scholar]
- [16].Crossley AW, Mahajan RP. The intensity of postoperative shivering is unrelated to axillary temperature. Anaesthesia 1994;49:205–7. [DOI] [PubMed] [Google Scholar]
- [17].Ead H. From Aldrete to PADSS: Reviewing discharge criteria after ambulatory surgery. J Perianesth Nurs 2006;21:259–67. [DOI] [PubMed] [Google Scholar]
- [18].Hartley B. Older patient perioperative care as experienced via transurethral resection of the prostate (TURP). J Perioper Pract 2014;24:135–40. [DOI] [PubMed] [Google Scholar]
- [19].Chen TY, Tseng CC, Wang LK, et al. The clinical use of small-dose tetracaine spinal anesthesia for transurethral prostatectomy. Anesth Analg 2001;92:1020–3. [DOI] [PubMed] [Google Scholar]
- [20].Vaghadia H, Neilson G, Lennox PH. Selective spinal anesthesia for outpatient transurethral prostatectomy (TURP): randomized controlled comparison of chloroprocaine with lidocaine. Acta Anaesthesiol Scand 2012;56:217–23. [DOI] [PubMed] [Google Scholar]
- [21].St Pierre M, Breuer G, Strembski D, et al. Does an electronic cognitive aid have an effect on the management of severe gynaecological TURP syndrome? A prospective, randomised simulation study. BMC Anesthesiol 2017;17:72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Boukatta B, Sbai H, Messaoudi F, et al. Transurethral resection of prostate syndrome: report of a case. Pan Afr Med J 2013;14:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Shah T, Flisberg P. Early recognition of the two cases of TURP syndrome in patients receiving spinal anaesthesia. Anaesth Intensive Care 2006;34:520–1. [PubMed] [Google Scholar]
- [24].du Toit L, van Dyk D, Hofmeyr R, et al. Core temperature monitoring in obstetric spinal anesthesia using an ingestible telemetric sensor. Anesth Analg 2018;126:190–5. [DOI] [PubMed] [Google Scholar]
- [25].Usta B, Gozdemir M, Demircioglu RI, Muslu B, Sert H, Yaldiz A. [Google Scholar]
- [26].Torrie JJ, Yip P, Robinson E. Comparison of forced-air warming and radiant heating during transurethral prostatic resection under spinal anaesthesia. Anaesth Intensive Care 2005;33:733–8. [DOI] [PubMed] [Google Scholar]
- [27].Torossian A, Van Gerven E, Geertsen K, et al. Active perioperative patient warming using a self-warming blanket (BARRIER EasyWarm) is superior to passive thermal insulation: a multinational, multicenter, randomized trial. J Clin Anesth 2016;34:547–54. [DOI] [PubMed] [Google Scholar]
- [28].Wold RM, Kondratiev T, Tveita T. Myocardial calcium overload during graded hypothermia and after rewarming in an in vivo rat model. Acta Physiol (Oxf) 2013;207:460–9. [DOI] [PubMed] [Google Scholar]
- [29].Chhabra L, Devadoss R, Liti B, et al. Electrocardiographicchanges inhypothermia: a review. Ther Hypothermia Temp Manag 2013;3:54–62. [DOI] [PubMed] [Google Scholar]
- [30].Frank SM, Fleisher LA, Breslow MJ, et al. Perioperative maintenance of normothermia reduces the incidence of morbid cardiac events. A randomized clinical trial. JAMA 1997;277:1127–34. [PubMed] [Google Scholar]
- [31].Kelly FE, Nolan JP. The effects of mild induced hypothermia on the myocardium: a systematic review. Anaesthesia 2010;65:505–15. [DOI] [PubMed] [Google Scholar]
- [32].Crawford SW, Hickman RO, Ulz L, et al. Use of the Hickman-Crawfordcritical care catheter in marrow transplant recipients: apulmonary artery catheter-adaptable central venous access. Crit Care Med 1994;22:347–52. [DOI] [PubMed] [Google Scholar]
- [33].Niven DJ, Gaudet JE, Laupland KB, et al. Accuracy of peripheral thermometers for estimating temperature: a systematic review and meta-analysis. Ann Intern Med 2015;163:768–77. [DOI] [PubMed] [Google Scholar]
- [34].Alfonsi P. Postanaesthetic shivering: epidemiology, pathophysiology, and approaches to prevention and management. Drugs 2001;61:2193–205. [DOI] [PubMed] [Google Scholar]
- [35].Madrid E, Urrútia G, Roqué i Figuls M, et al. Active body surface warming systems for preventing complications caused by inadvertent perioperative hypothermia in adults. Cochrane Database Syst Rev 2016;4:CD009016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Kranke P, Eberhart LH, Roewer N, et al. Pharmacological treatment of postoperative shivering: a quantitative systematic review of randomized controlled trials. Anesth Analg 2002;94:453–60. [DOI] [PubMed] [Google Scholar]
- [37].Eichenberger AS, Haller G, Cheseaux N, et al. A clinical pathway in a post-anaesthesia care unit to reduce length of stay, mortality and unplanned intensive care unit admission. Eur J Anaesthesiol 2011;28:859–66. [DOI] [PubMed] [Google Scholar]
- [38].Araújo LM, Klamt JG, Garcia LV. Anesthesia for transurethral resection of the prostate: comparison between two periods in a university hospital. Rev Bras Anestesiol 2005;55:197–206. [DOI] [PubMed] [Google Scholar]
- [39].Frank SM, Fleisher LA, Olson KF, et al. Multivariate determinants of early postoperative oxygen consumption in elderly patients. Effects of shivering, body temperature, and gender. Anesthesiology 1995;83:241–9. [DOI] [PubMed] [Google Scholar]


