Abstract
Oxidative stress is considered to be part of the pathogenic mechanism for community-acquired pneumonia (CAP) and is closely linked to inflammation. Attenuation of oxidative stress would be expected to reduce pulmonary damage. Antioxidants have been found to be effective in alleviating lung injury and protecting against damage of other organs.
The aim of the study was to compare the effect of adding N-acetylcysteine (NAC) to conventional treatment versus conventional treatment on oxidative stress, inflammatory factors, and radiological changes in CAP patients.
Eligible CAP patients at Weihai Municipal Hospital were stratified and randomly assigned to either NAC group or non-NAC group between August 2016 and March 2017. The NAC group received conventional treatment for pneumonia and NAC (1200 mg/d). Thenon-NAC group received conventional therapy. malondialdehyde (MDA), superoxide dismutase (SOD), total antioxidant capacity (TAOC), tumor necrosis factor-α (TNF-α), and computed tomography (CT) images were evaluated at baseline and after treatment. The primary endpoint indicators were the changes in oxidative stress parameters (MDA, TAOC, SOD) and TNF-α after treatment in the NAC group compared with those in the non-NAC group. The secondary endpoint indicator was any difference in CT scores after treatment in the NAC group compared with the non-NAC group.
Baseline levels of MDA, TAOC, SOD, and TNF-α were similar between the 2 groups before treatment. Plasma levels of MDA and TNF-α decreased more (P < .05 MDA:p 0.004, TNF-α:p <0.001) in the NAC group than the non-NAC group, and there was a reliable increase in TAOC content (p 0.005). There was no significant difference in increased plasma SOD activity between the groups (p 0.368), and the NAC group did not show a greater improvement from CT scores. No NAC-related adverse effects were observed.
Addition of NAC therapy for CAP patients reduced MDA and TNF-α and increased TAOC. Treatment with NAC may help to reduce oxidative and inflammatory damage in pneumonia patients.
Keywords: N-acetylcysteine, oxidative stress, pneumonia
1. Introduction
Pneumonia is an infection of the lung usually caused by bacteria and viruses. Alterations in oxidative metabolism are considered to be part of the pathogenic mechanism in the development and progression of community-acquired pneumonia (CAP). It was shown that infection with a respiratory syncytial virus induced significant down regulation of the antioxidant system in the airway in vivo, and this was likely to result in lung oxidative damage.[1] There is an increment increase in oxidative stress in CAP patients. Oxidative stress plays an important role in a host's innate immune response to foreign pathogens,[2] and increases the production of inflammation mediators in the respiratory system.
Oxidative stress is closely linked to inflammation. Increased production of interleukin (IL)-8 and tumor necrosis factor (TNF)-α, both attract inflammatory cells and increase oxidant production by these cells.[3] Lung cells release inflammatory mediators and cytokines, such as TNF-α, IL-1, and IL-8, in response to oxidative stress. TNF-α acts on mitochondria to generate reactive oxygen species (ROS), which take part in the activation of NF-kB and AP-1 (redox-sensitive transcription factors). Activation of NF-kB/AP-1 leads to the co-ordinate expression of antioxidant protective and proinflammatory genes.[4] Attenuation of oxidative stress would be expected to result in reduced pulmonary damage.
Antioxidants have been found to be effective in alleviating lung injury and protecting against damage of other organs, such as the heart, kidney, and liver in animal models of oxidative stress.[5] It was shown that vitamin C reduced proinflammatory and oxidative biomarkers in vivo of CAP patients.[6]
N-acetylcysteine (NAC), a thiol reducing agent, has mucolytic properties of degrading the disulfide bonds (S–S) to a sulfhydryl bond (–SH) in mucoprotein complexes that no longer cross-linking.[7] And it may also reduce the mucus elasticity and viscosity and facilitate the removal of pulmonary secretions.[7] Moreover, it prevents bacterial stimulation of mucin production and mucus hyper-secretion.[8] NAC exhibits direct as well as indirect antioxidant properties. Its direct effect is due to a free thiol group interacting with and scavenging ROS.[9] Its indirect antioxidant effect is related to its role as glutathione (GSH) precursor, resulting in increase of intracellular GSH concentration.
The administration of NAC in chronic obstructive pulmonary disease (COPD) and COPD exacerbations has shown benefit. High dose NAC ameliorated oxidative stress and inflammatory response in COPD exacerbation patients.[3] NAC pretreatment also significantly prevented TNF-a production in alveolar macrophages treated with ultrafine nickel particles.[10] High dose NAC therapy showed benefit in H1N1 influenza pneumonia patients.[11] Our present study aimed to compare the effect of NAC addition versus conventional treatment on oxidative stress, inflammatory factors, and radiological changes in CAP patients.
2. Materials and methods
2.1. Study design and subjects
The study was conducted at the Department of Respiratory, Weihai Municipal Hospital. All patients admitted to the hospital with community acquired pneumonia between August 2016 and March 2017 were consecutively enrolled. Pneumonia was defined as a new infiltrate in chest radiography together with symptoms and signs of a lower respiratory tract infection: fever, cough, and purulent sputum.[12]
The CAP diagnostic criteria were as follows: an acute pulmonary infiltrate evident on chest radiography and consistent with pneumonia; symptoms of a lower respiratory tract infection: fever, cough, and purulent sputum, with confirmatory findings of a clinical examination; acquisition of the infection outside a hospital, long-term care facility, or nursing home. The patients were included if they had the diagnostic criteria defined above, were diagnosed with bacterial community-acquired pneumonia according to American Thoracic Society/Infectious Diseases Society of America (ATS/IDSA) Guidelines[13,14] and if they were aged ≥18 years. Patients were excluded if they were of very advanced age (≥70 years old), had severe obesity, undertook heavy smoking, severe or multiple systemic diseases, severe pneumonia (pneumonia severity index [PSI] score IV–V), tuberculosis, fungus infection, primary viral pneumonia, or other diseases including: lung tumors, diffuse connective tissue diseases, sarcoidosis, pulmonary tuberculosis, parasitic infestations, bronchiectasis, pulmonary edema, immunosuppression or were receiving immunosuppressive therapy (daily dose of ≥20 mg prednisolone equivalent for >2 weeks), human immunodeficiency virus (HIV) infection, granulocytopenia (<1000 neutrophils/mm3), prior antimicrobial treatment before the hospital admission, or were using other antioxidant drugs. Diabetic patients with blood glucose levels >150 mg/dL were also not included in the study.[15] The study was approved by the institutional review board, and all participants provided written informed consent.
Stratified randomization was used to select the samples. The stratification was according to “comorbidities or not” and “smoking or not,” and the patients were then randomized in each stratification.
2.2. Grouping and intervention
Stratified patients were randomly assigned to either NAC group or non-NAC group. The NAC group received conventional treatment for pneumonia and NAC (1200 mg/d). The non-NAC group received conventional therapy alone. The drug, N-acetylcysteine (600 mg/tablet), was obtained from Hainan Zambon pharmaceutical company (Hainan, China), a subsidiary of Zambon Group, Italy. The conventional pneumonia medication included: antibiotics, expectorants, and antitussives. Patients in the NAC group received NAC at a dose of 600 mg twice daily by oral administration for 10 days and were monitored for incidence of NAC related side effects and drug interactions. There was no dose increase or decrease during the whole process. Routine treatment of pneumonia (antibiotics, expectorants, antitussives) was administered in both groups, and NAC intervention was specific to the NAC group.
2.3. Primary endpoint and secondary endpoint
The primary endpoint indicators were the changes in oxidative stress parameters (MDA, TAOC, SOD) and TNF-α after treatment in the NAC group compared with those in the non-NAC group. The secondary endpoint indicator was whether there was a difference in CT scores after treatment in the NAC group compared with the non-NAC group.
2.4. Data and sample collection
Baseline evaluation included: history taking, physical examination, routine laboratory tests, PSI score assessment, and chest computed tomography (CT) examination. The PSI score was used to evaluate CAP severity. Patients with PSI I–III (<91 points) were defined as low risk class, while patients were considered to be severe and have high risk if their PSI was Class IV or V (≥91 points or higher).[16] We collected venous blood samples from all patients on admission (Day 0) and after 7 days of treatment (Day 7).
2.5. Oxidative stress analysis
The blood samples were collected in lithium-heparin tubes and were centrifuged at 1500 × g for 10 minutes. Plasma was separated and stored at –80 °C until analysis. Index of oxidative injury (lipoperoxidative damage) was determined by malondialdehyde (MDA). Antioxidant capacity was estimated by superoxide dismutase (SOD) and total antioxidant capacity (TAOC). These parameters were measured by spectrophotometry. We used TNF-α as an inflammatory marker in CAP and detected it by ELISA. TNF-α reagent (Human TNF-α DKW12–1720) was purchased from Dakewe Bioengineering Co., Ltd., Shenzhen, China. MDA, TAOC, and SOD reagents were purchased from Nanjing Jiancheng Bioengineering Institute, Nanjing. The spectrophotometer (Model: V-1200B) was purchased from Shanghai Mapada Instruments Co., Ltd. Shanghai. The microplate Reader (Model: Labservk3) was purchased from ThermoFisher, Shanghai King Industry Co., Ltd, Shanghai.
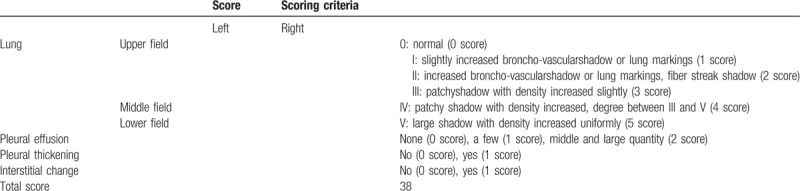
2.6. CT image assessment
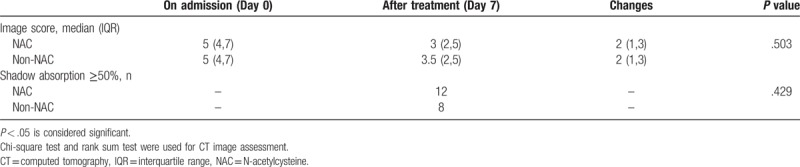
The patients had chest CT when they were admitted to hospital and after 10 days of treatment. We used semi-quantitative analysis to assess the pneumonia imaging absorption.[17–21] After 10 days treatment, patients in either group were estimated according to the chest CT shadow area absorption ≥50% or <50%. According to Table 1, (post-treatment score – pre-treatment score)/pre-treatment score × 100%. We also estimated pneumonic imaging changes with another method.[15,22–24] In this method, 3 sections of CT scans were analyzed: upper (above the carina), middle (below the carina and above the inferior pulmonary vein), and lower (below the inferior pulmonary vein). Two chest radiologists with >10 years’ experience as an attending physician reviewed the images independently initially, with a final finding reached by consensus when there was a discrepancy. They were blinded to the clinical information or clinical progress of the patients. According to Table 1, the differences in CT scores between the 2 groups before and after treatment were compared to evaluate the improvement in CT image after treatment.
Table 1.
CT image assessment.
2.7. Statistical analysis
The distribution of continuous data was assessed using the Kolmogorov–Smirnov test. Normally distributed data were presented as mean ± standard deviation and analyzed using the t test. Non-normally distributed data are presented as median (IQR) and analyzed using the Mann–Whitney U test. Categorical variables are presented as frequencies and were analyzed using the Fisher exact test. The difference in oxidative stress and inflammatory markers after treatment between the NAC group and the non-NAC group was assessed with analysis of covariance (ANCOVA) using the pretreatment indicators of the 2 groups as covariates. Analysis of imaging score changes was conducted by Wilcoxon rank sum test. SPSS 17.0 (IBM, Armonk, NY) was used for all analyses. Two-sided P-values <.05 were considered to be statistically significant.
3. Results
3.1. Baseline characteristics
Initially 86 patients were considered for inclusion in the study. Twenty-nine patients were excluded and so 37 cases were in the NAC group and 24 cases in the non-NAC group. Eighteen cases did not complete the study. So, 39 pneumonia patients completed the study. Of these, 21 subjects were included in the NAC group and 18 in the non-NAC group. The study flowchart which contains the details of the inclusions exclusions and reasons for non-completion is shown in Fig. 1.
Figure 1.
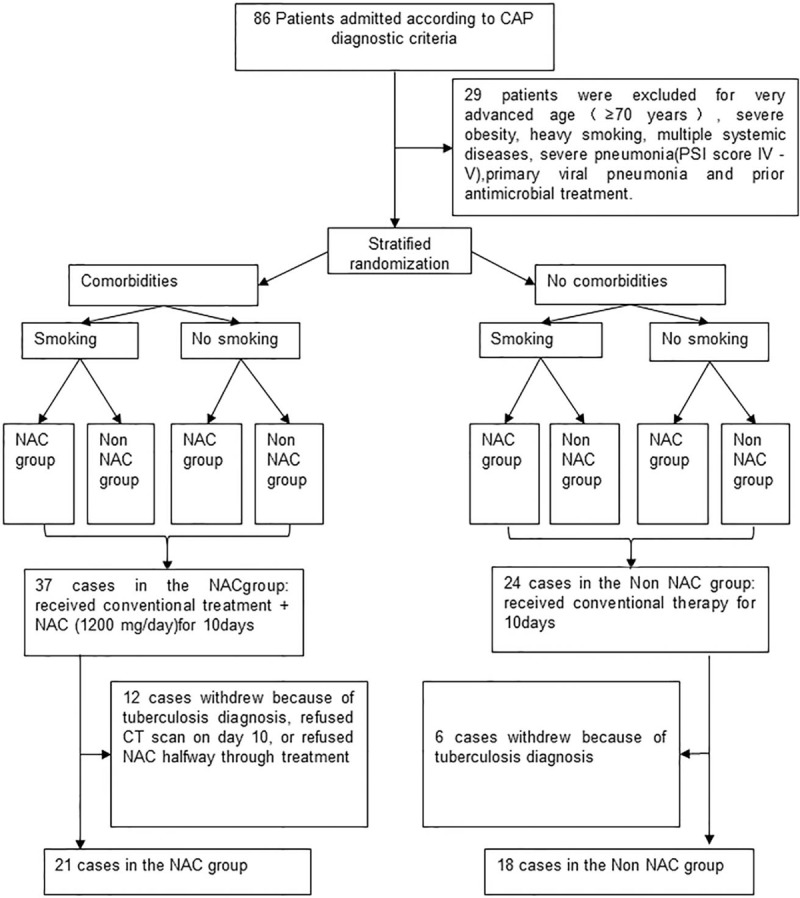
Flow chart showing the inclusion of patients in the study.
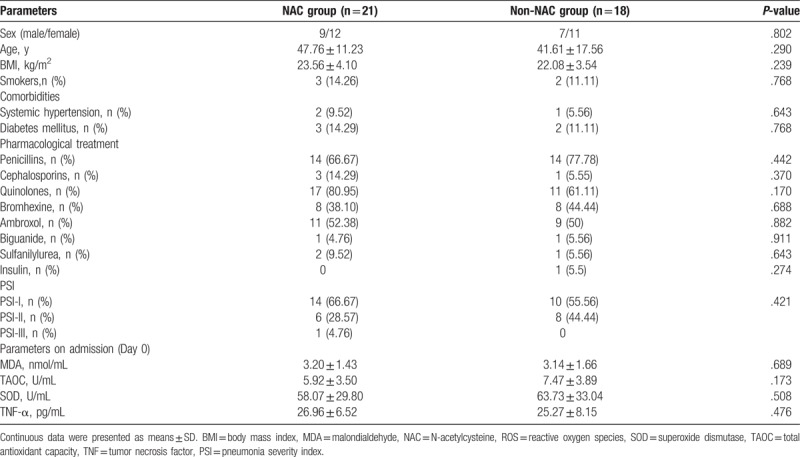
The baseline demographics are presented in Table 2. No significant difference was found between the NAC group (n = 21) and the non-NAC group (n = 18) in the parameters listed in Table 2.
Table 2.
Baseline characteristics in NAC and non-NAC group.
3.2. Comparison of oxidative stress and inflammation between the 2 groups
To avoid the influence of baseline values, we adopted covariance analysis to assess the difference of oxidative stress and inflammatory parameters after treatment between groups. After 7 days plasma levels of MDA and TNF-α decreased more (MDA:p 0.004, TNF-α:p <0.001) in the NAC group than the non-NAC group, and there was a reliable increase of TAOC content in the NAC group (p 0.005). An improvement was seen in plasma SOD activity after treatment, but there was no significant difference in the rise of plasma SOD activity between groups (p 0.368). The results are shown in Table 3 and Fig. 2. No side effects were reported with NAC administration.
Table 3.
Comparison of oxidative stress and inflammation between the NAC group and non-NACgroup.
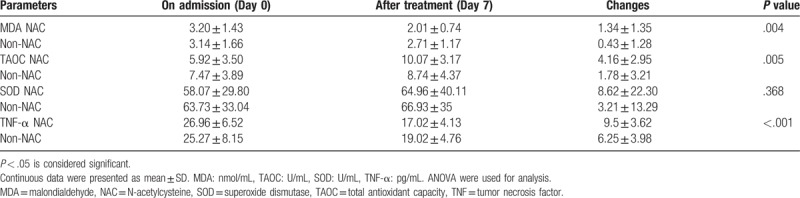
Figure 2.
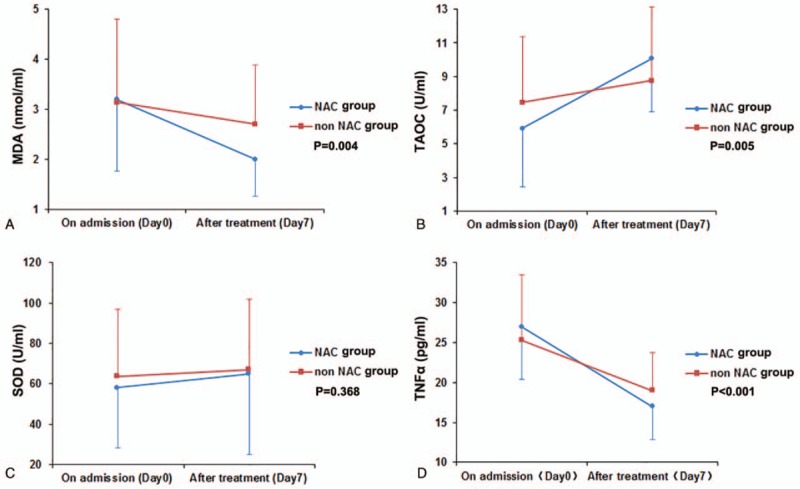
Comparison of oxidative stress and inflammation in the NAC and non-NAC groups before and after treatment. A: Comparison of malondialdehyde (MDA) in the NAC and non-NAC groups. B: Comparison of total antioxidant capacity (TAOC) in the NAC and non-NAC groups. C: Comparison of superoxide dismutase (SOD) in the NAC and non-NAC groups. D: Comparison of tumor necrosis factor alpha (TNF-α) in the NAC and non-NAC groups. NAC = N-acetylcysteine.
3.3. Comparison of CT changes between groups
The CT image changes after 10 days are shown in Table 4. There was no difference in the number of patients with imaging absorption ≥50% between the groups. From the CT score changes, the NAC group did not show a greater improvement in CT scans than the non-NAC group.
Table 4.
Comparison of CT image assessment between the NAC group and non-NAC group.
4. Discussion
The pathophysiological mechanisms of CAP include microorganism invasion, airway damage, and activation of the immune defense systems. Polymorphonuclear neutrophils and macrophages kill these microorganisms by using ROS and lysosomal enzymes, including proteinases. The increase in ROS concentration and proteolytic enzymes may be reflected at the systemic level as an increment in the oxidative stress and airway remodeling biomarkers.[25] Oxidative stress takes part in host innate immune response to foreign pathogens, and increases the production of mediators of pulmonary inflammation.[15]
Numerous studies have shown that there was a higher oxidative stress in CAP patients compared with healthy volunteers.[26] The effects of oxidative stress in the airway as well as in other organs depend on ROS concentration and time of exposure. In general, higher levels of ROS produce damage in biomolecules (e.g., lipid peroxidation) and induce intracellular signaling pathways leading to cell death, mainly through apoptosis.[27] Secondly, the impairment of the antioxidant capacity in CAP could also enhance cell damage. CAP patients presented greater lipid peroxidation, and antioxidant status alterations correlated with clinical severity.[25]
In a way, weakening the oxidative stress may mitigate organ damage. In our study, there was a more significant decrease in plasma MDA level and a more considerable rise in plasma TAOC level after treatment in the NAC group, compared with those in the non-NAC group. These showed that NAC had increased the protective markers for oxidative stress. Besides, with NAC treatment, the TNF-α level decreased more in the NAC group than in the non-NAC group. It was reflected that NAC treatment could protect lung tissue by alleviating oxidative stress and diminishing inflammatory factors such as TNF-α.
N-acetylcysteine, a glutathione precursor, can replenish the total combined thiols (cysteine, cysteinylglycine, glutathione and homocysteine), interact with the electrophile groups of ROS, and then raise the total anti-oxidant capacity. NAC protects alveolar type II cells against injury induced by cigarette smoke in knockout mice lacking the nuclear factor erythroid 2-related factor-2 (Nrf2), which is a redox-sensitive transcription factor and is a key regulator of the antioxidant defense system.[28] NAC pretreatment effectively prevented thiobarbituric reactive substances accumulation, lung edema, and polymorphonuclear neutrophil (PMN) influx into the lungs induced by concentrated ambient thiobarbituric particles.[25] Previous studies have demonstrated the potential antioxidant, anti-inflammatory and mucolytic properties of NAC in COPD. Addition of NAC to the standard treatment of COPD exhibited beneficial effects in disease exacerbations, symptom improvement, and a decline in oxidative stress parameters.[29] High dose NAC improves clinical outcome of COPD exacerbation patients by ameliorating oxidative stress and inflammatory response thereby improving lung spirometry and pulmonary oxygenation.[3]
Besides, NAC meets the need by virtue of its anti-inflammatory action. Study indicates that IL-8, IL-6, and TNF-a could be strongly inhibited by NAC at the expression and release level in alveolar type II cells infected with influenza virus A and B and respiratory syncytial virus.[30] NAC inhibited the activation of NF-κB in alveolar macrophages induced by TNF-α,[31] and was an effective inhibitor of TNF-α/IL-1 β-stimulated intercellular adhesion molecule-1 (ICAM-1) and IL-8 release in endothelial and epithelial cells.[32]
Research suggests the effects of NAC differ in vivo and in vitro and are highly dose-dependent. In vitro anti-inflammatory effects were seen at high but not at low concentrations. NAC administered at high concentrations significantly inhibited the release of IL-1β, IL-8, and TNF-α induced by lipopolysaccharide (LPS) incubation in an ex vivo model of COPD exacerbation.[33] But, on the other hand, some long-term effectiveness is reported in several in vivo studies even at low doses. The lower doses of NAC given in vivo might require longer time in order to achieve sustained effects on the cellular thiols which lead to changes in the redox status.[34,35] This showed that high dose NAC (1200 mg/d) significantly decreased IL-8 levels after 10 days of treatment in patients with COPD exacerbations.[3] In our test, administration with NAC (1200 mg/d) for 7 days could significantly decreased TNF-α levels in CAP patients.
Most studies have shown NAC increased the SOD level or activity in lung injury. On the contrary, in our research, addition of NAC did not provide more improvement in SOD activity compared with conventional therapy, which was in accord with Forgiarini study.[36] Nagata research showed that NAC increased the protein and mRNA expression of MnSOD without altering the mRNA expression of other antioxidant enzymes, including GPx1 (glutathione peroxydase 1), CuZnSOD, and extracellular SOD (ecSOD).[37] Three isoforms of SOD have been found in mammalian cells: CuZnSOD, MnSOD, and ecSOD. We suppose that the effect of NAC on SOD might be determined by the kind of SOD, detecting time, and some other factors.
There was no difference in the improvement of pneumonia as assessed by CT between NAC group and non-NAC group. This might be because the observation period was not long enough. In addition, the pneumonia image absorption time could be influenced by the etiology, host factors, immune factors, and some other factors. So further investigation is needed to reveal any difference in clinical outcome between the 2 patient groups.
Different results might be found by alternative methods of administering NAC. Inhalation of NAC solution can directly act on the airway, so that it might come into effect rapidly, with high bioavailability. Yoshito research showed that ex vivo treatment of donor lungs with inhaled NAC reduced inflammatory response via its antioxidant activity in experimental porcine lung transplantation.[38] Study of NAC inhalation in ventilator-associated pneumonia showed that with prolonged mechanical ventilation, biofilm structure improved, biofilm culture positive rate and incidence of ventilator-associated pneumonia decreased. Tomioka pilot study[39] indicated that aerosolized NAC (352 mg/d) for idiopathic pulmonary fibrosis may delay disease progression.[40] In the literature we referred to when we planned this study in 2016, most studies had used NAC by oral administration in antioxidant study of patients with COPD and influenza.[3,8,9,11,29,41] Therefore oral administration was chosen in this study but on further consideration, using NAC solution by inhalation might be a better choice. The effect of inhalation of NAC solution on oxidative stress and inflammatory factors in respiratory diseases is an interesting subject for future study.
This study has some limitations. The major one being the small sample size. This insufficiency could reduce the test power, limit the ability of our study to generalize, and prevent adjustment for confounding effects. While in some similar studies, the sample number is not large either. De Backer et al[41] studied 12 patients to study the effect of high-dose N-acetylcysteine on airway geometry, inflammation, and oxidative stress in COPD patients. Yuanyuan Chen's research showed Vitamin C mitigated oxidative stress and proinflammatory mediator in severe CAP, with 15 patients in each group.[6] Trefler used small sample in a pilot study to show oxidative stress in immunocompetent patients with severe community-acquired pneumonia.[26] For all that, it is suggested that the study with large sample if possible with patients from multiple centers may have stronger test efficiency, and provide a more convincing conclusion.
Another limitation is that the study did not record details of the etiologic agent. Patients in our study were diagnosed with bacterial community-acquired pneumonia according to ATS/IDSA Guidelines. For the pathological features, treatment and disease course of tuberculosis and fungus infection are different from those of bacterial pneumonia, patients considered to have tuberculosis or fungus infection were not included. Primary viral pneumonia was defined in patients presenting during the acute phase of influenza virus illness with acute respiratory disease and unequivocal alveolar opacification involving ≥2 lobes with negative respiratory and blood bacterial cultures. There might be some differences in oxidative stress status between bacterial and viral pneumonia.[26] Primary viral pneumonia cases were not admitted to the study either.
It has been reported that different microorganisms may elicit different cytokine activation patterns in CAP. The lowest inflammatory expression was found in unknown cause and the highest was found in Legionella pneumophila, Streptococcus pneumoniae, and Enterobacteriaceae.Atypical bacteria exhibit an inflammatory pattern closer to that of viruses.[42] In our study, although viral pneumonia, tuberculosis, and fungus infection were not included, we did not make further stratification in patients selected on the basis of pathogen, which might likely reveal more details in changes of oxidative stress and inflammatory cytokines, and this is also likely to lead to bias.
The study showed no difference in the improvement of CT between NAC group and non-NAC group. One reason for this might be the observation period was not long enough. This is also one of the limitations.
We included patients consecutively in this study. For various reasons, some patients who were selected at the beginning were excluded or signed out halfway. Some factors such as age, sex, body mass index, smoking habits, comorbidities, drugs, and pathogen, may have impact on the oxidative stress and affect the equilibrium between groups. So, the patients who had very advanced age (≥70 years old), severe obesity, heavy smoking, or other sever systemic diseases were not admitted into the study groups. The levels of oxidative stress and inflammatory cytokines in severe pneumonia differ from those in non-severe pneumonia. Thus, we chose patients with PSI score I–III for allocation into the study groups. There might be difficulties in collecting patients who have pneumonia alone without other comorbidities within a certain time period. Thus, there could be potential bias, and this is one of the reasons for the small sample size. Although we have taken some measures, for instance, stratified randomization and some exclusion methods mentioned above, selection bias still existed.
In our study, we did not make stratification on the basis of pathogens, and the confounding effect that pathogens may have had on the 2 groups was not adjusted for. Therefore, this is also likely to be one cause for bias.
In addition, we did not make this a double-blind study. There might be interference from observers’ subjective factors. This is another likely reason for bias.
Therefore, we consider that a further study using a large sample over a longer research period in pneumonia patients with different etiologies and severity may reveal more details of the effect of antioxidant treatment.
5. Conclusion
In summary, we demonstrated that CAP exhibited significant increase of oxidative stress. Addition of NAC therapy to the CAP patients reduced MDA and TNF-α and increase TAOC more than standard therapy alone. Thus, our study suggests that NAC inhibited oxidative stress and reduced the inflammatory factors in pneumonia. Treatment with antioxidants NAC might reduce oxidative and inflammatory damage in pneumonia patients.
Author contributions
Investigation: Qianwen Zhang, Yuanrong Ju, Yan Ma, Tao Wang.
Methodology: Qianwen Zhang, Yuanrong Ju, Yan Ma, Tao Wang.
Resources: Qianwen Zhang.
Validation: Qianwen Zhang.
Visualization: Qianwen Zhang.
Writing – original draft: Qianwen Zhang, Yuanrong Ju, Yan Ma, Tao Wang.
Writing – review & editing: Qianwen Zhang, Yuanrong Ju, Yan Ma, Tao Wang.
Footnotes
Abbreviations: CAP = community-acquired pneumonia, COPD = chronic obstructive pulmonary disease, CT = computed tomography, IL = interleukin, MDA = malondialdehyde, NAC = N-acetylcysteine, ROS = reactive oxygen species, SOD = superoxide dismutase, TAOC = total antioxidant capacity, TNF = tumor necrosis factor.
The authors have no conflicts of interest to disclose.
References
- [1].Hosakote YM, Jantzi PD, Esham DL, et al. Viral-mediated inhibition of antioxidant enzymes contributes to the pathogenesis of severe respiratory syncytial virus bronchiolitis. Am J Respir Crit Care Med 2011;183:1550–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Kratzer E, Tian Y, Sarich N, et al. Oxidative stress contributes to lung injury and barrier dysfunction via microtubule destabilization. Am J Respir Cell Mol Biol 2012;47:688–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Mahmoud Abd El Hafiz A, Mohammed El Wakeel L, Mohammed El Hady H, et al. High dose N-acetyl cysteine improves inflammatory response and outcome in patients with COPD exacerbations. Egypt J Chest Dis Tubercul 2013;62:51–7. [Google Scholar]
- [4].Rahman I, MacNee W. Oxidative stress and regulation of glutathione in lung inflammation. Eur Respir J 2000;16:534–54. [DOI] [PubMed] [Google Scholar]
- [5].Andrades M, Ritter C, de Oliveira MR, et al. Antioxidant treatment reverses organ failure in rat model of sepsis: role of antioxidant enzymes imbalance, neutrophil infiltration, and oxidative stress. J Surg Res 2011;167:e307–13. [DOI] [PubMed] [Google Scholar]
- [6].Chen Y, Luo G, Yuan J, et al. Vitamin C mitigates oxidative stress and tumor necrosis factor-alpha in severe community-acquired pneumonia and LPS-induced macrophages. Mediators Inflamm 2014;2014:426740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Santus P, Corsico A, Solidoro P, et al. Oxidative stress and respiratory system: pharmacological and clinical reappraisal of N-acetylcysteine. COPD 2014;11:705–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Sadowska AM, Verbraecken J, Darquennes K, et al. Role of N-acetylcysteine in the management of COPD. Int J Chron Obstruct Pulmon Dis 2006;1:425–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Bachh A, Shah N, Bhargava R, et al. Effect of oral N-acetylcysteine in COPD - a randomised controlled trial. JK-Practitioner 2007;14:12–6. [Google Scholar]
- [10].Dick CA, Brown DM, Donaldson K, et al. The role of free radicals in the toxic and inflammatory effects of four different ultrafine particle types. Inhal Toxicol 2003;15:39–52. [DOI] [PubMed] [Google Scholar]
- [11].Lai KY, Ng WY, Osburga Chan PK, et al. High-dose N-acetylcysteine therapy for novel H1N1 influenza pneumonia. Ann Intern Med 2010;152:687–8. [DOI] [PubMed] [Google Scholar]
- [12].Lisboa T, Blot S, Waterer GW, et al. Radiologic progression of pulmonary infiltrates predicts a worse prognosis in severe community-acquired pneumonia than bacteremia. Chest 2009;135:165–72. [DOI] [PubMed] [Google Scholar]
- [13].Qu JM, Cao B. Chinese Medical Association for Respiratory Diseases Guidelines for Diagnosis and Treatment of Adult Community-Acquired Pneumonia in China (2016 Edition. 39), 4. 2016;241–242. [DOI] [PubMed] [Google Scholar]
- [14].Mandell LA, Wunderink RG, Anzueto A, et al. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis 2007;44suppl:S27–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Akkaya A, Öztürk Ö. Total antioxidant capacity and C-reactive protein levels in patients with community-acquired pneumonia. Turk J Medicalences 2008;38:537–44. [Google Scholar]
- [16].Fernandez-Botran R, Uriarte SM, Arnold FW, et al. Contrasting inflammatory responses in severe and non-severe community-acquired pneumonia. Inflammation 2014;37:1158–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Yin RY. Preliminary compilation and application of the scale for assessing the absorption of pneumonia in chest radiographs. Chin J Respir Crit Care 2012;11:2. [Google Scholar]
- [18].Feng F, Jiang Y, Yuan M, et al. Association of radiologic findings with mortality in patients with avian influenza H7N9 pneumonia. PLoS One 2014;9:e93885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Kirtland SH, Winterbauer RH. Slowly resolving, chronic, and recurrent pneumonia. Clin Chest Med 1991;12:303–18. [PubMed] [Google Scholar]
- [20].Li M, Liu J, Tan R. Risk factors for slowly resolving pneumonia in the intensive care unit. J Microbiol Immunol Infect 2016;49:654–62. [DOI] [PubMed] [Google Scholar]
- [21].Rome L, Murali G, Lippmann M. Nonresolving pneumonia and mimics of pneumonia. Med Clin North Am 2001;85:1511–30. xi. [DOI] [PubMed] [Google Scholar]
- [22].Yin RY, Li M, Xu HR, et al. Preliminary compilation and application of pneumonia chest radiograph absorption evaluation scale. Chin J Respir Crit Care Med 2012;11:185–6. [Google Scholar]
- [23].Majewska E, Kasielski M, Luczynski R, et al. Elevated exhalation of hydrogen peroxide and thiobarbituric acid reactive substances in patients with community acquired pneumonia. Respir Med 2004;98:669–76. [DOI] [PubMed] [Google Scholar]
- [24].Weatherly MR, Palmer CG, Peters ME, et al. Wisconsin cystic fibrosis chest radiograph scoring system. Pediatrics 1993;91:488–95. [PubMed] [Google Scholar]
- [25].Castillo RL, Carrasco RA, Alvarez PI, et al. Relationship between severity of adult community-acquired pneumonia and impairment of the antioxidant defense system. Biol Res 2013;46:207–13. [DOI] [PubMed] [Google Scholar]
- [26].Trefler S, Rodriguez A, Martin-Loeches I, et al. Oxidative stress in immunocompetent patients with severe community-acquired pneumonia. A pilot study. Med Intensiva 2014;38:73–82. [DOI] [PubMed] [Google Scholar]
- [27].Valko M, Leibfritz D, Moncol J, et al. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol 2007;39:44–84. [DOI] [PubMed] [Google Scholar]
- [28].Messier EM, Day BJ, Bahmed K, et al. N-acetylcysteine protects murine alveolar type II cells from cigarette smoke injury in a nuclear erythroid 2-related factor-2-independent manner. Am J Respir Cell Mol Biol 2013;48:559–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Kale SB, Patil AB, Kale A. Effects of administration of oral n-acetylcysteine on oxidative stress in chronic obstructive pulmonary disease patients in rural population. Int J Basic Clin Pharmacol 2016;5:775–81. [Google Scholar]
- [30].Mata M, Morcillo E, Gimeno C, et al. (NAC) inhibit mucin synthesis and pro-inflammatory mediators in alveolar type II epithelial cells infected with influenza virus A and B and with respiratory syncytial virus (RSV). Biochem Pharmacol 2011;82:548–55. [DOI] [PubMed] [Google Scholar]
- [31].Li YQ, Zhang ZX, Xu YJ, et al. N-Acetyl-L-cysteine and pyrrolidine dithiocarbamate inhibited nuclear factor-kappaB activation in alveolar macrophages by different mechanisms. Acta Pharmacol Sin 2006;27:339–46. [DOI] [PubMed] [Google Scholar]
- [32].Radomska-Lesniewska DM, Sadowska AM, Van Overveld FJ, et al. Influence of N-acetylcysteine on ICAM-1 expression and IL-8 release from endothelial and epithelial cells. J Physiol Pharmacol 2006;57suppl:325–34. [PubMed] [Google Scholar]
- [33].Cazzola M, Calzetta L, Facciolo F, et al. Pharmacological investigation on the anti-oxidant and anti-inflammatory activity of N-acetylcysteine in an ex vivo model of COPD exacerbation. Respir Res 2017;18:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Sadowska AM, Manuel YKB, De Backer WA. Antioxidant and anti-inflammatory efficacy of NAC in the treatment of COPD: discordant in vitro and in vivo dose-effects: a review. Pulm Pharmacol Ther 2007;20:9–22. [DOI] [PubMed] [Google Scholar]
- [35].Sadowska AM, Manuel-y-Keenoy B, Vertongen T, et al. Effect of N-acetylcysteine on neutrophil activation markers in healthy volunteers: in vivo and in vitro study. Pharmacol Res 2006;53:216–25. [DOI] [PubMed] [Google Scholar]
- [36].Forgiarini LF, Forgiarini LA, Jr, da Rosa DP, et al. N-acetylcysteine administration confers lung protection in different phases of lung ischaemia-reperfusion injury. Interact Cardiovasc Thorac Surg 2014;19:894–9. [DOI] [PubMed] [Google Scholar]
- [37].Nagata K, Iwasaki Y, Yamada T, et al. Overexpression of manganese superoxide dismutase by N-acetylcysteine in hyperoxic lung injury. Respir Med 2007;101:800–7. [DOI] [PubMed] [Google Scholar]
- [38].Yamada Y, Iskender I, Arni S, et al. Ex vivo treatment with inhaled N-acetylcysteine in porcine lung transplantation. J Surg Res 2017;218:341–7. [DOI] [PubMed] [Google Scholar]
- [39].Qu D, Ren XX, Guo LY. Effect of N-acetylcysteine inhalation on ventilator-associated pneumonia caused by biofilm in endotracheal tubes. ZhonghuaErKeZa Zhi 2016;54:278–82. [DOI] [PubMed] [Google Scholar]
- [40].Tomioka H, Kuwata Y, Imanaka K, et al. A pilot study of aerosolized N-acetylcysteine for idiopathic pulmonary fibrosis. Respirology 2005;10:449–55. [DOI] [PubMed] [Google Scholar]
- [41].De Backer J, Vos W, Van Holsbeke C, et al. Effect of high-dose N-acetylcysteine on airway geometry, inflammation, and oxidative stress in COPD patients. Int J Chron Obstruct Pulmon Dis 2013;8:569–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Menendez R, Sahuquillo-Arce JM, Reyes S, et al. Cytokine activation patterns and biomarkers are influenced by microorganisms in community-acquired pneumonia. Chest 2012;141:1537–45. [DOI] [PMC free article] [PubMed] [Google Scholar]


