Abstract
Objectives
We determined differences in the prevalence of blood pressure (BP) phenotypes and the association of these phenotypes with left ventricular hypertrophy (LVH) for individuals meeting and not meeting various criteria used for defining a complete ambulatory BP monitoring (ABPM) recording.
Methods
We analyzed data for 1,141 participants from the Jackson Heart Study. Criteria evaluated included having ≥80% of planned readings with ≥1 reading per hour (Spanish ABPM Registry criteria), ≥70% of planned readings with a minimum of 20 daytime and 7 nighttime readings (2013 European Society of Hypertension [ESH] criteria), ≥14 daytime and ≥7 nighttime readings (2003 ESH criteria), ≥10 daytime and ≥5 nighttime readings (International Database of Ambulatory Blood Pressure in Relation to Cardiovascular Outcome [IDACO] criteria), and ≥14 daytime readings (United Kingdom National Institute of Health and Clinical Excellence [UK-NICE] criteria).
Results
Between 45.0% (Spanish ABPM Registry) and 91.8% (UK-NICE) of participants met different criteria for a complete ABPM recording. Across the various criteria evaluated, 55.5% to 57.8% of participants had nocturnal hypertension and 62.8% to 66.8% had non-dipping systolic blood pressure. Among participants with clinic-measured systolic/diastolic BP ≥140/90 mm Hg, 22.9% to 26.5% had white-coat hypertension. The prevalence of daytime, 24-hour, sustained and masked hypertension differed by ≤2% for participants meeting each criteria. The association of BP phenotypes with LVH was similar for participants meeting versus not meeting different criteria (each p-interaction>0.05).
Conclusion
Regardless of the criteria used for defining a complete ABPM recording, the prevalence of BP phenotypes and their association with LVH were similar.
Keywords: Ambulatory blood pressure monitoring, blood pressure, hypertension, criteria, left ventricular hypertrophy
INTRODUCTION
Ambulatory blood pressure monitoring (ABPM) provides a profile of an individual’s blood pressure (BP) during their normal daily activities [1]. Typically an individual wears a portable monitor for a 24-hour period during which BP is measured at pre-established time intervals of 15 to 30 minutes [2]. Because movement can result in reading errors, and the ABPM device may be removed and put back on, many people do not obtain 100% of planned BP readings. Different criteria have been used for defining a complete 24-hour ABPM recording. For an ABPM recording to be considered complete, the Spanish ABPM Registry required ≥80% of planned systolic BP (SBP) and diastolic BP (DBP) readings to be obtained with at least 1 reading per hour [3]. In contrast, other groups have required less stringent criteria. For example, the UK National Institute of Health and Clinical Excellence (UK-NICE) guideline requires ≥ 14 daytime readings for an ABPM recording to be considered complete [4]. Several other criteria have been used to define a complete ABPM recording in prior research studies or have been recommended by clinical practice guidelines (Table 1) [2,5,6].
Table 1.
Description of criteria used to consider a 24-hour ABPM recording complete.
| Criteria | ||||
|---|---|---|---|---|
| Origin | Percent of expected readings |
Number of daytime readings |
Number of nighttime readings |
Number of readings per hour |
| Spanish Ambulatory Blood Pressure Registry [3] | ≥ 80% | N/A | N/A | ≥ 1 |
| 2013 European Society of Hypertension (ESH) Position Paper [2] | ≥ 70% | ≥ 20 | ≥ 7 | N/A |
| 2003 ESH Recommendations [6] | N/A | ≥ 14 | ≥ 7 | N/A |
| The International Database of Ambulatory Blood Pressure in Relation to Cardiovascular Outcome (IDACO) [5] | N/A | ≥ 10 | ≥ 5 | N/A |
| UK National Institute for Health and Clinical Excellence (UK-NICE) Guidelines [4] | N/A | ≥ 14 | N/A | N/A |
For the present analyses, daytime was defined as 10:00 am to 8:00 pm and nighttime was defined as 12:00 am to 6:00 am.
N/A: Not applicable, origin did not require this criterion.
There are no evidence-based criteria for defining a complete 24-hour ABPM recording. Although the use of stringent criteria for defining a complete ABPM recording may provide more accurate estimates of the prevalence of various BP phenotypes (e.g., white coat hypertension) and of associations with outcomes, this practice will tend to exclude people with fewer ambulatory BP readings. This may lead to biased prevalence estimates and phenotype-outcome associations if systematic differences exist between individuals with fewer versus more ABPM readings. Therefore, the objective of this study was to determine whether the prevalence of BP phenotypes differs among people meeting some but not other published criteria used to define a complete 24-hour ABPM recording. We also evaluated whether the association of BP phenotypes with left ventricular hypertrophy (LVH) differs for people who meet one but not another set of criteria. Findings from this analysis may inform future research studies regarding whether less stringent criteria can be used for considering a 24-hour ABPM recording complete.
METHODS
Study Population
The Jackson Heart Study (JHS) enrolled a community-based cohort of 5,306 African Americans between 2000 and 2004. Details of the design and conduct of the JHS are published elsewhere [7,8]. Participants were recruited from urban and rural areas of 3 counties (Hinds, Madison, and Rankin) that comprise the Jackson, Mississippi metropolitan area. After an in-home interview and baseline examination, participants were invited to complete a 24-hour ABPM procedure, and a total of 1,146 participants underwent ABPM. For the current analysis, we excluded participants missing data on clinic SBP or DBP (n=5) for a sample size of 1,141 participants. For the analyses of the association between BP phenotypes and LVH, we further excluded 27 participants who did not have echocardiographic data. The protocol for the JHS was approved by the institutional review boards of the participating institutions, and all participants provided written informed consent. The current analysis of de-identified data was approved by the institutional review board at the University of Alabama at Birmingham.
Data Collection
Data for the current analyses were collected by questionnaires, a clinic examination and ABPM. Of relevance to the current analysis, data that were collected through the interview-administered questionnaires included age, sex, education, marital status, cigarette smoking, physical activity, history of cardiovascular disease (CVD), history of diabetes, and self-reported use of antihypertensive medication. Using a modified Baecke questionnaire, duration, frequency and intensity of physical activity were assessed and reported in four domains (active living, work, home life, and sports and exercise) [9,10]. Participants were considered to be taking antihypertensive medication if they self-reported use of medication to lower BP in the two weeks prior to their clinic examination. Measures obtained during the clinic examination included height, weight, and BP. Body mass index (BMI) was calculated as weight in kilograms divided by height in meters squared (kg/m2).
Participants were asked to fast prior to their JHS examination. Venipuncture was conducted in the morning after participants were in a supine position for 20 minutes. Total and high-density lipoprotein (HDL) cholesterol were measured from blood samples taken during the clinic examination, using a Roche COBAS Fara analyzer in the central laboratory located at the University of Minnesota Department of Laboratory Medicine and Pathology. Estimated glomerular filtration rate (eGFR) was calculated using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation [11]. Reduced eGFR was defined as <60 mL/min per 1.73 m2 [12].
Clinic BP Readings
Clinic BP was measured in each participant’s right arm following a standardized protocol, using a Hawksley random zero sphygmomanometer and Littman stethoscope. The appropriate cuff size was determined by measuring each participant’s right arm circumference. Two BP readings, separated by a one-minute rest, were obtained and averaged to define clinic SBP and DBP. The random-zero sphygmomanometer has been shown to under-estimate BP [13]. Therefore, the random-zero BP measurements were calibrated to a semi-automated device (Omron-HEM-907, Omron Healthcare Inc., Lake Forest, IL) using robust regression as described previously [14].
Ambulatory BP Readings
ABPM was performed with a SpaceLabs 90207 device following the clinic examination. Readings were taken every 20 minutes during the 24-hour monitoring period. In a previous study, the differences in mean daytime and nighttime BP was small when using fixed-time periods compared with self-report or actigraphy to define the daytime and nighttime periods [15]. Therefore in order to omit asleep-awake transition periods during which BP changes markedly, fixed-time periods were used to define daytime (10 am to 8 pm) and nighttime (12 am to 6 am) periods [5,15,16]. Five sets of criteria were used to define a complete 24-hour ABPM recording (Table 1). These criteria were identified from previously published studies, position papers and guidelines, including the Spanish Ambulatory Blood Pressure Registry, the 2003 European Society of Hypertension (ESH) recommendations, the 2013 ESH recommendations, the International Database of Ambulatory Blood Pressure in Relation to Cardiovascular Outcome (IDACO), and the UK-NICE guidelines [2–6]. The criteria in the 2013 ESH recommendations were subsequently endorsed by the 2014 ESH guidelines for ABPM [17].
BP Phenotypes
Using the calibrated clinic BP measurements, clinic hypertension was defined as mean SBP ≥ 140 mmHg or DBP ≥ 90 mmHg. Daytime hypertension was defined as mean SBP ≥ 135 mmHg and/or DBP ≥ 85 mmHg based on all readings obtained between 10 am and 8 pm and nocturnal hypertension as mean SBP ≥ 120 mmHg and/or DBP ≥ 70 mmHg based on all readings obtained between midnight and 6 am [18]. Based on all available readings from the ABPM recording, 24-hour hypertension was defined as mean SBP ≥ 130 mmHg and/or DBP ≥ 80 mmHg. For SBP and DBP, separately, the white coat effect was defined as clinic BP minus daytime BP. We evaluated three BP phenotype domains: out-of-clinic hypertension (daytime, nocturnal, and 24-hour hypertension), a combination of clinic and out-of-clinic hypertension (sustained, white coat, and masked hypertension), and a diurnal BP pattern (non-dipping SBP; Supplemental Digital Content Table 1).
Echocardiography
Two-dimensional echocardiography was performed by certified technicians (Sonos 4500; Philips Medical Systems, Andover, MA) using standardized protocol [8]. LV dimensions, including interventricular septum thickness in diastole, LV internal dimension in diastole, and posterior wall thickness in diastole, were assessed according to the 2D method based on the 2015 American Society of Echocardiography (ASE) recommendations [19].
Echocardiographic Derived Variables
Left ventricular mass (LVM) was calculated using the 2015 ASE formula: 0.8 × (1.04 × {[interventricular septum thickness in diastole + LV internal dimension in diastole + posterior wall thickness in diastole]3 − LV internal dimension in diastole3)} + 0.6g [19]. LVM index (LVMI) was calculated as LV mass/body surface area [19]. LV hypertrophy (LVH) was defined as LVMI ≥ 96 g/m2 in women and LVMI ≥ 116 g/m2 in men [19].
Statistical Analysis
We calculated characteristics for participants meeting and not meeting each set of criteria for defining a complete 24-hour ABPM recording. Among participants meeting each set of criteria, we calculated the prevalence of BP phenotypes. The percentage of participants who met one set of criteria, but not each of the other criteria was calculated. The statistical significance of differences in the prevalence of each BP phenotype across criteria was determined using a 1,000 iteration bootstrap [20]. For the remaining analyses, we investigated pairwise sets of criteria where there was a >10% discordance in the proportion of participants classified as having a complete ABPM. Specifically, the analyses described below were conducted among participants who met the 2003 ESH, IDACO, and UK-NICE criteria, separately. The prevalence of each BP phenotype was calculated for participants meeting and not meeting the Spanish ABPM Registry, separately, and meeting and not meeting the 2013 ESH criteria, separately. Next, we calculated the unadjusted prevalence ratios (PR) for LVH associated with BP phenotypes among participants who met and did not meet the Spanish ABPM Registry and, separately, the 2013 ESH criteria using Poisson regression models with robust standard errors. The statistical significance of differences in the association between BP phenotypes and LVH for participants meeting versus not meeting the Spanish ABPM Registry and 2013 ESH criteria was evaluated in multivariable adjusted Poisson regression models using a multiplicative interaction term (e.g., indicator term for daytime hypertension * indicator term for meeting the Spanish ABPM Registry criteria). Also, multivariable-adjusted PRs were calculated to determine participant characteristics associated with meeting versus not meeting the Spanish ABPM Registry and 2013 ESH criteria. Multivariable adjustment models included age, sex, education, marital status, smoking status, physical activity, BMI, HDL and total cholesterol, reduced eGFR, history of CVD, history of diabetes, self-reported antihypertensive medication use, clinic SBP, clinic DBP, daytime SBP, daytime DBP, nighttime SBP, and nighttime DBP. To avoid co-linearity, we modeled clinic BP and daytime/nighttime BP in separate models. P-values < 0.05 were considered statistically significant. All analyses were conducted using SAS Version 9.3 (SAS Institute, Cary, NC).
RESULTS
Participant Characteristics
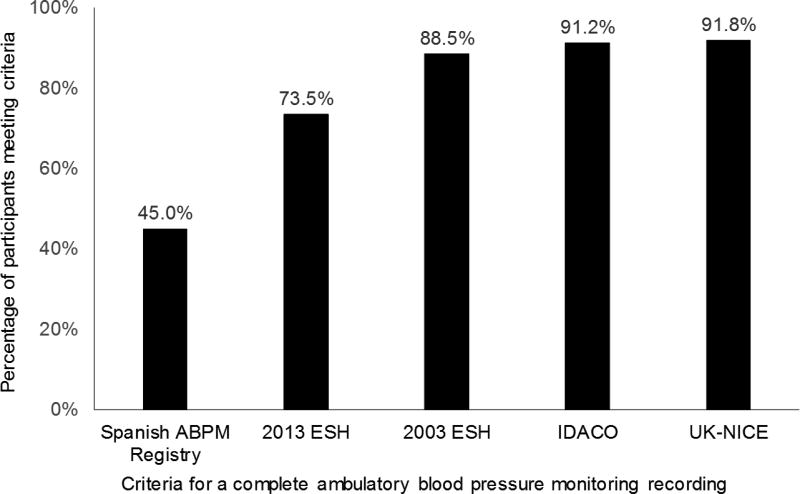
Of the 1,141 participants included in this analysis, the mean age was 59 years and 32% were male. The proportion of participants with a complete ABPM ranged from 45.0% for the Spanish ABPM Registry criteria to 91.8% for the UK-NICE criteria (Figure 1). Participants not meeting versus meeting each set of criteria were more likely to be current smokers, except the Spanish ABPM Registry criteria, and have a higher BMI (Table 2). Compared to participants meeting the 2013 ESH criteria, those not meeting the criteria were more likely to have less than a high school education, participate in moderate or vigorous physical activity, and have lower HDL-cholesterol. Participants who met versus did not meet the Spanish ABPM Registry criteria had a higher total cholesterol and were more likely to have a reduced eGFR. Mean 24-hour SBP and DBP was lower among participants meeting versus not meeting the IDACO criteria while mean DBP was lower among participants meeting versus not meeting the 2003 ESH and UK-NICE criteria for a complete ABPM recording. The mean white coat effect for SBP and DBP was larger but negative among participants meeting versus not meeting the IDACO criteria.
Figure 1.
Percentage of participants meeting each set of criteria for having a complete ambulatory blood pressure monitoring recording.
See Table 1 for the criteria for a complete ambulatory blood pressure monitoring recording ABPM - ambulatory blood pressure monitoring; ESH – European Society of Hypertension; IDACO - International Database of Ambulatory Blood Pressure in Relation to Cardiovascular Outcome; UK-NICE - UK National Institute of Health and Clinical Excellence
Table 2.
Characteristics of Jackson Heart Study participants who did and did not meet each set of criteria for considering a 24-hour ABPM recording complete.
| Met criteria for a complete ABPM recording | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Spanish ABPM Registry |
2013 ESH | 2003 ESH | IDACO | UK-NICE | ||||||
| Yes (n=514) |
No (n=627) |
Yes (n=839) |
No (n=302) |
Yes (n=1,010) |
No (n=131) |
Yes (n=1,041) |
No (n=100) |
Yes (n=1,047) |
No (n=94) |
|
| Age, years | 59.1 (10.6) | 59.1 (11.6) | 58.9 (10.8) | 59.5 (11.9) | 59.9 (11.0) | 57.9 (12.2) | 59.2 (10.9) | 57.6 (13.2) | 59.2 (11.1) | 57.9 (11.7) |
| Male, % | 32.9 | 30.9 | 32.7 | 29.5 | 32.5 | 26.7 | 32.1 | 29.0 | 32.4 | 25.5 |
| < High School Education, % | 20.9 | 18.9 | 18.2* | 24.3* | 19.0 | 26.0 | 19.3 | 25.0 | 19.0* | 28.7* |
| Married, % | 57.0 | 51.9 | 55.8 | 49.7 | 54.9 | 48.9 | 55.2* | 44.0* | 54.6 | 50.0 |
| Current Smoker, % | 9.4 | 12.1 | 9.5* | 14.7* | 9.9* | 18.6* | 10.1* | 19.4* | 10.1* | 19.4* |
| Any Moderate or Vigorous Physical Activity, % | 51.9 | 52.0 | 46.0* | 54.2* | 52.2 | 48.9 | 51.7 | 53.0 | 52.3 | 46.8 |
| BMI (kg/m2) | 30.0 (5.9)* | 32.4 (6.7)* | 30.7 (6.4)* | 32.9 (6.3)* | 31.1 (6.5)* | 33.1 (6.4)* | 31.1 (6.4)* | 33.3 (6.4)* | 31.2 (6.5)* | 33.1 (6.4)* |
| HDL-Cholesterol (mg/dL) | 54.7 (15.1) | 53.1 (15.0) | 54.4 (15.2)* | 52.6 (14.7)* | 54.1 (15.2) | 52.3 (13.8) | 54.0 (15.1) | 52.7 (15.1) | 54.1 (15.1) | 51.4 (14.0) |
| Total Cholesterol (mg/dL) | 204.1 (41.1)* | 199.1 (38.5)* | 201.9 (40.1) | 200.1 (38.8) | 201.9 (40.2) | 197.3 (35.6) | 201.6 (40.0) | 199.2 (36.7) | 202.0 (40.4) | 194.7 (30.5) |
| eGFR <60 ml/min/1.73 m2, % | 8.3* | 4.9* | 6.9 | 5.1 | 6.3 | 7.1 | 6.3 | 7.2 | 6.4 | 6.5 |
| History of CVD, % | 11.1 | 11.2 | 10.5 | 12.9 | 10.7 | 14.5 | 10.6 | 17.0 | 10.6 | 17.0 |
| History of Diabetes, % | 25.6 | 24.2 | 24.6 | 25.7 | 24.5 | 27.7 | 24.3 | 30.3 | 24.3 | 31.2 |
| Antihypertensive Medication Use, % | 56.3 | 57.6 | 56.7 | 58.1 | 56.8 | 58.9 | 56.6 | 61.6 | 56.7 | 60.2 |
| Clinic BP | ||||||||||
| SBP, mmHg | 127.3 (16.6) | 127.9 (14.9) | 127.8 (16.2) | 127.3 (14.1) | 127.7 (16.0) | 127.4 (13.2) | 127.7 (15.9) | 127.7 (13.7) | 127.7 (15.9) | 127.6 (13.0) |
| DBP, mmHg | 74.0 (8.4) | 74.8 (8.5) | 74.4 (8.6) | 74.3 (8.1) | 74.4 (8.5) | 74.2 (8.1) | 74.4 (8.5) | 74.1 (8.5) | 74.4 (8.5) | 74.4 (8.3) |
| Daytime BP | ||||||||||
| SBP, mmHg | 130.1 (13.7) | 129.1 (13.7) | 129.9 (13.7) | 128.6 (13.8) | 129.6 (13.6) | 129.3 (14.7) | 129.5 (13.5) | 130.5 (15.4) | 129.5 (13.5) | 129.5 (14.9) |
| DBP, mmHg | 78.0 (9.3) | 78.0 (9.5) | 78.0 (9.3) | 77.8 (9.8) | 77.9 (9.3) | 78.5 (10.4) | 77.9 (9.3) | 79.0 (10.8) | 77.8 (9.3) | 79.5 (10.7) |
| Nighttime BP | ||||||||||
| SBP, mmHg | 120.7 (16.4) | 121.8 (15.6) | 121.3 (16.0) | 120.9 (16.0) | 121.1 (15.8) | 123.5 (19.4) | 121.0 (15.8) | 128.2 (20.9) | 121.3 (15.9) | 119.6 (17.3) |
| DBP, mmHg | 68.5 (9.6) | 68.7 (10.9) | 68.8 (9.7) | 67.7 (12.2) | 68.5 (9.7) | 70.6 (17.7) | 68.4 (9.8) | 76.2 (22.1) | 68.6 (9.9) | 68.7 (17.6) |
| 24-Hour BP | ||||||||||
| SBP, mmHg | 126.8 (14.0) | 126.6 (14.0) | 126.9 (13.9) | 126.1 (14.3) | 126.5 (13.8) | 128.2 (15.3) | 126.3 (13.8)* | 130.2 (15.6)* | 126.6 (13.8) | 128.0 (15.7) |
| DBP, mmHg | 74.5 (8.8) | 74.4 (9.5) | 74.7 (8.8) | 74.1 (10.1) | 74.2 (8.9)* | 76.3 (11.1)* | 74.1 (8.9)* | 78.0 (11.4)* | 74.3 (8.9)* | 76.6 (11.8)* |
| White Coat Effect | ||||||||||
| SBP, mmHg | −2.8 (15.3) | −1.2 (14.2) | −2.1 (14.9) | −1.3 (14.2) | −1.9 (14.8) | −1.9 (14.1) | −4.8 (10.4)* | −2.9 (14.4)* | −1.9 (14.8) | −2.0 (13.6) |
| DBP, mmHg | −4.0 (10.0) | −3.2 (9.8) | −3.6 (9.9) | −3.4 (9.8) | −3.5 (9.8) | −4.3 (10.0) | −3.4 (9.8)* | −1.8 (14.8)* | −3.4 (9.8) | −5.1 (10.1) |
| Left Ventricular Hypertrophy, % | 11.7 | 14.6 | 13.2 | 13.5 | 12.8 | 16.9 | 12.9 | 17.2 | 13.0 | 16.0 |
Numbers reported in table are mean (standard deviation) or percent.
ABPM: ambulatory blood pressure monitoring; ESH: European Society of Hypertension; IDACO: The International Database of Ambulatory Blood Pressure in Relation to Cardiovascular Outcome; NICE: UK National Institute for Health and Clinical Excellence; BMI: body mass index; HDL: high density lipoprotein; eGFR: estimated glomerular filtration rate; BP: blood pressure; SBP: systolic blood pressure; DBP: diastolic blood pressure.
White coat effect was calculated as the difference between clinic BP and daytime BP (clinic BP minus daytime BP).
p<0.05 when comparing those who do versus do not meet specified criteria.
Meeting Versus Not Meeting Different Criteria for a complete ABPM recording
All participants who met the Spanish ABPM Registry criteria also met the 2003 ESH, IDACO, and UK-NICE criteria, and only 1.2% did not meet the 2013 ESH criteria (Table 3). Among participants who met the 2013 ESH criteria, 39.5% did not meet the Spanish ABPM Registry criteria and all met the 2003 ESH, IDACO, and UK-NICE criteria. Approximately 50% of participants meeting the 2003 ESH, IDACO, and UK-NICE criteria did not meet the Spanish ABPM Registry criteria, and between 15% and 20% of participants meeting the 2003 ESH, IDACO, and UK-NICE criteria did not meet the 2013 ESH criteria.
Table 3.
Percentage of participants meeting a set of criteria for considering a 24-hour ABPM recording complete who did not meet an alternative set of criteria.
| Criteria Met | ||||||
|---|---|---|---|---|---|---|
| Spanish ABPM Registry (n = 514) |
2013 ESH (n = 839) |
2003 ESH (n = 1,010) |
IDACO (n = 1,041) |
UK-NICE (n = 1,047) |
||
| Criteria Not Met | Spanish ABPM Registry | - | 39.5% | 49.1% | 50.6% | 50.9% |
| 2013 ESH | 1.2% | - | 16.9% | 19.4% | 19.9% | |
| 2003 ESH | 0.0% | 0.0% | - | 3.0% | 3.5% | |
| IDACO | 0.0% | 0.0% | 0.0% | - | 3.1% | |
| UK-NICE | 0.0% | 0.0% | 0.0% | 2.5% | - | |
ABPM: ambulatory blood pressure monitoring; ESH: European Society of Hypertension; IDACO: The International Database of Ambulatory Blood Pressure in Relation to Cardiovascular Outcome; UK-NICE: UK National Institute for Health and Clinical Excellence.
Prevalence of BP Phenotypes by Five Different Criteria for Defining a Complete Recording
The prevalence of daytime, nocturnal, 24-hour, sustained and masked hypertension were within 2% among participants who met each set of criteria for a complete 24-hour ABPM recording (Table 4). The prevalence of white coat hypertension was within 4% among participants who met each set of criteria for a complete 24-hour ABPM recording. The prevalence of white coat hypertension was higher while the prevalence of a non-dipping BP pattern was lower among participants who met the Spanish ABPM Registry criteria, compared to those who met the other sets of criteria. Consistent with this finding, among participants who met the 2003 ESH, IDACO, and UK-NICE criteria, a lower prevalence of non-dipping SBP was present for those meeting versus not meeting the Spanish ABPM Registry criteria (Supplemental Digital Content Table 2). The prevalence of all other BP phenotypes were not statistically significantly different between those meeting versus not meeting the Spanish ABPM Registry or the 2013 ESH criteria.
Table 4.
Prevalence of ambulatory blood pressure phenotypes among participants meeting each set of criteria for considering a 24-hour ABPM recording complete.
| Criteria Met | |||||
|---|---|---|---|---|---|
| Spanish ABPM Registry (n=514) |
2013 ESH (n=839) |
2003 ESH (n=1,010) |
IDACO (n=1,041) |
UK-NICE (n=1,047) |
|
| Daytime Hypertension | 38.1% | 38.6% | 38.4% | 38.3% | 38.5% |
| Nocturnal Hypertension | 55.5% | 57.8% | 57.1% | 56.7% | 57.3% |
| 24-Hour Hypertension | 41.6% | 43.6% | 42.5% | 42.3% | 42.7% |
| Sustained Hypertension | 14.0% | 15.4% | 15.2% | 14.9% | 15.1% |
| White Coat Hypertension | 26.5% | 23.2% | 23.1% | 23.3% | 22.9% |
| Masked Hypertension | 34.1% | 30.7% | 30.5% | 30.5% | 30.6% |
| Non-Dipping Pattern | 62.8% | 66.3% | 66.5% | 66.4% | 66.8% |
ABPM: ambulatory blood pressure monitoring; ESH: European Society of Hypertension; IDACO: The International Database of Ambulatory Blood Pressure in Relation to Cardiovascular Outcome; UK-NICE: UK National Institute for Health and Clinical Excellence.
Differences in the prevalence of phenotypes across criteria were not statistically significant, with the exception of non-dipping pattern. The prevalence of non-dipping pattern differed when comparing ESH2013, ESH 2003, IDACO and UK-NICE with Spanish ABP Registry.
LVH
Among participants meeting the 2003 ESH, IDACO, and UK-NICE criteria, having versus not having daytime, nocturnal, 24-hour, and masked hypertension were each associated with a higher prevalence ratio of LVH for those meeting and not meeting the Spanish ABPM Registry criteria and 2013 ESH criteria although some of the 95% confidence intervals were wide (Table 5). Sustained hypertension was associated with LVH among those meeting the Spanish ABPM Registry criteria and the 2013 ESH criteria but the associations for those not meeting these criteria were weaker and not statistically significant. White coat hypertension and non-dipping BP were not associated with LVH for participants meeting or not meeting the Spanish ABPM Registry criteria and 2013 ESH criteria. The associations of each BP phenotype with LVH were not statistically significantly different for participants meeting versus not meeting the Spanish ABPM Registry criteria and the 2013 ESH criteria (all p-values for interaction>0.05).
Table 5.
Prevalence ratios for left ventricular hypertrophy associated with ambulatory blood pressure phenotypes among participants meeting and not meeting the Spanish Ambulatory Blood Pressure Registry criteria (left panel) and the 2013 ESH criteria (right panel), restricted to those who met the 2003 ESH (top panel), IDACO (middle panel), and UK-NICE (bottom panel) criteria.
| Among participants meeting 2003 ESH criteria | ||||||
| Spanish ABPM Registry criteria met | 2013 ESH criteria met | |||||
| Yes (N=497) | No (N=487) | Yes (N=818) | No (N=166) | |||
| Prevalence Ratio (95% CI) | p-value† | Prevalence Ratio (95% CI) | p-value† | |||
| Daytime Hypertension | 3.35 (1.93–5.79) | 2.00 (1.24–3.22) | 0.304 | 2.60 (1.76–3.83) | 2.10 (0.83–5.31) | 0.426 |
| Nocturnal Hypertension | 2.31 (1.29–4.17) | 2.26 (1.29–3.97) | 0.771 | 2.09 (1.36–3.22) | 4.22 (1.22–14.58) | 0.495 |
| 24-Hour Hypertension | 2.90 (1.68–5.02) | 1.97 (1.21–3.20) | 0.498 | 2.38 (1.60–3.53) | 2.15 (0.85–5.45) | 0.655 |
| Sustained Hypertension | 2.29 (1.29–4.07) | 1.59 (0.91–2.78) | 0.381 | 2.00 (1.31–3.06) | 1.24 (0.36–4.30) | 0.276 |
| White Coat Hypertension | 1.34 (0.49–3.71) | 0.35 (0.05–2.51) | 0.192 | 0.97 (0.40–2.38) | NR | NR |
| Masked Hypertension | 1.76 (1.04–2.98) | 1.55 (0.95–2.53) | 0.880 | 1.56 (1.026–2.31) | 2.14 (0.83–5.53) | 0.610 |
| Non-Dipping Pattern | 1.22 (0.70–2.11) | 1.17 (0.68–2.00) | 0.973 | 1.21 (0.80–1.83) | 1.22 (0.43–3.42) | 0.953 |
| Among participants meeting IDACO criteria | ||||||
| Spanish ABPM Registry criteria met | 2013 ESH criteria met | |||||
| Yes (N=497) | No (N=518) | Yes (N=818) | No (N=197) | |||
| Prevalence Ratio (95% CI) | p-value† | Prevalence Ratio (95% CI) | p-value† | |||
| Daytime Hypertension | 3.35 (1.93–5.79) | 1.93 (1.22–3.06) | 0.255 | 2.60 (1.76–3.83) | 1.85 (0.82–4.20) | 0.313 |
| Nocturnal Hypertension | 2.31 (1.29–4.17) | 2.07 (1.22–3.48) | 0.923 | 2.09 (1.36–3.22) | 2.59 (1.02–6.56) | 0.811 |
| 24-Hour Hypertension | 2.90 (1.68–5.02) | 1.90 (1.19–3.02) | 0.434 | 2.38 (1.60–3.53) | 1.89 (0.84–4.29) | 0.519 |
| Sustained Hypertension | 2.29 (1.29–4.07) | 1.51 (0.87–2.64) | 0.310 | 2.00 (1.31–3.06) | 1.03 (0.31–3.47) | 0.159 |
| White Coat Hypertension | 1.34 (0.49–3.71) | 0.33 (0.05–2.37) | 0.175 | 0.97 (0.40–2.38) | NR | NR |
| Masked Hypertension | 1.76 (1.04–2.98) | 1.55 (0.97–2.50) | 0.900 | 1.56 (1.06–2.31) | 2.05 (0.89–4.74) | 0.582 |
| Non-Dipping Pattern | 1.22 (0.70–2.11) | 1.22 (0.73–2.07) | 0.909 | 1.21 (0.80–1.83) | 1.40 (0.55–3.54) | 0.829 |
| Among participants meeting UK-NICE criteria | ||||||
| Spanish ABPM Registry criteria met | 2013 ESH criteria met | |||||
| Yes (N=497) | No (N=523) | Yes (N=818) | No (N=202) | |||
| Prevalence Ratio (95% CI) | p-value† | Prevalence Ratio (95% CI) | p-value† | |||
| Daytime Hypertension | 3.35 (1.93–5.79) | 1.80 (1.14–2.84) | 0.182 | 2.60 (1.76–3.83) | 1.53 (0.70–3.35) | 0.159 |
| Nocturnal Hypertension | 2.31 (1.29–4.17) | 2.28 (1.30–3.98) | 0.791 | 2.09 (1.36–3.22) | 4.27 (1.24–14.64) | 0.535 |
| 24-Hour Hypertension | 2.90 (1.68–5.02) | 1.93 (1.21–3.06) | 0.482 | 2.38 (1.60–3.53) | 2.02 (0.92–4.46) | 0.669 |
| Sustained Hypertension | 2.28 (1.29–4.07) | 1.53 (0.89–2.63) | 0.334 | 2.00 (1.31–3.06) | 1.18 (0.41–3.45) | 0.223 |
| White Coat Hypertension | 1.34 (0.49–3.71) | 0.32 (0.05–2.32) | 0.169 | 0.97 (0.40–2.38) | NR | NR |
| Masked Hypertension | 1.76 (1.04–2.98) | 1.55 (0.97–2.48) | 0.917 | 1.56 (1.06–2.31) | 0.49 (0.22–1.10) | 0.541 |
| Non-Dipping Pattern | 1.22 (0.70–2.11) | 1.17 (0.68–1.99) | 0.966 | 1.21 (0.80–1.83) | 1.23 (0.44–3.42) | 0.957 |
ABP: ambulatory blood pressure; ESH: European Society of Hypertension; IDACO: The International Database of Ambulatory Blood Pressure in Relation to Cardiovascular Outcome; UK-NICE: UK National Institute for Health and Clinical Excellence; CI: Confidence Interval.
NR: Not reported because there were no participants with white coat hypertension defined by 24-hour ambulatory blood pressure who also had LVH in this group and therefore a prevalence ratio could not be calculated.
p-values reported in this table are testing the statistical significance of differences in the prevalence ratios between those who did and did not meet the specified criteria for a complete ABPM recording.
Factors Associated with Not Meeting More Stringent ABPM Criteria
Among participants who met the 2003 ESH, IDACO and UK-NICE criteria, older age and having a higher BMI were associated with a higher multivariable-adjusted prevalence ratio for not meeting the Spanish ABPM Registry criteria and 2013 ESH criteria (Supplemental Digital Content Table 3). Reduced eGFR was associated with a lower multivariable-adjusted prevalence ratio for not meeting these criteria. None of the other factors studied were associated with not meeting versus meeting the Spanish ABPM Registry criteria or 2013 ESH criteria.
DISCUSSION
In this study, almost all participants who met the Spanish ABPM Registry criteria for a complete 24-hour ABPM recording also met the 2013 ESH, 2003 ESH, IDACO and UK-NICE criteria. As expected, a substantial proportion of participants who satisfied the less stringent 2003 ESH, IDACO and UK-NICE criteria did not meet the Spanish ABPM Registry criteria or the 2013 ESH criteria. Despite these differences, the prevalence of BP phenotypes was similar for participants meeting each set of criteria. Furthermore, among participants who met the 2003 ESH, IDACO and UK-NICE criteria for a complete 24-hour ABPM recording, the association between BP phenotypes and LVH were similar for those meeting, and not meeting the Spanish ABPM Registry and 2013 ESH criteria. These data suggest that using less stringent criteria including 2003 ESH, IDACO, and UK-NICE to define a complete ABPM should produce unbiased results in research studies.
There are no firm data on which to base recommendations for defining a complete ABPM recording [2]. Given the lack of data it is not surprising that the criteria for a complete ABPM recording have not been harmonized across position statements and clinical practice guidelines. Requiring a higher number of readings to define a complete ABPM recording can result in more stable BP estimates and should minimize the effect of any spikes or troughs of single BP readings. However, using less stringent criteria for a complete ABPM will increase the number of people with complete recordings and presumably increase the generalizability of results.
There are few data evaluating the impact of using different criteria for defining a complete ABPM recording. The percentage of participants excluded from published analyses due to not having a complete ABPM recording has often not been reported. In prior analyses of the Coronary Artery Risk Development in Young Adults (CARDIA) study and the JHS, 11.1% and 8.9% of participants, respectively, have been excluded due to not meeting the IDACO criteria [21,22]. The current study demonstrated that a substantially higher percentage of participants would be excluded from analyses if the Spanish ABPM Registry or 2013 ESH criteria were used to define a complete ABPM recording.
Although the overall prevalence of BP phenotypes was similar for participants meeting each set of criteria evaluated in the current study, there were sub-groups of participants who were less likely to meet the more stringent ABPM criteria. Participants who were older and had a higher BMI were less likely to meet the criteria for the Spanish ABPM Registry and 2013 ESH guidelines. Older individuals may be less likely to have a valid ABPM recording due to a higher prevalence of arrhythmias [23,24]. Challenges of conducting ABPM in obese individuals are well recognized and include issues of the cuff bladder size and conical-shaped arms [2]. ABPM is particularly important in these populations because older adults have a high prevalence of white coat hypertension and obese adults have a high prevalence of masked hypertension [25,26]. Having reduced eGFR was associated with a lower likelihood of not having a complete ABPM recording as defined by the Spanish Registry or ESH 2013 criteria.
The goal of the current analysis was to inform future studies on the criteria to be used to define a complete ABPM recording and the effect of this choice on the population prevalence of phenotypes and their associations with outcomes. It remains unclear how many readings are required to accurately diagnose an individual with daytime and nocturnal hypertension, or other phenotypes, on ABPM. The number of clinic BP readings needed to diagnose hypertension has been evaluated in previous studies. For example, in a study of US Veterans followed for 18 months, the probability that an individual with a single SBP reading ≥ 140 mmHg actually has clinic-measured SBP ≥ 140 mmHg is less than 70% [27]. This probability increases to more than 92% for an individual with clinic-measured SBP ≥ 140 mmHg based on the average of 5 SBP readings taken over 5 visits. Although applying less stringent criteria may be acceptable for research studies and generating population estimates, more stringent criteria (e.g., ≥80% of planned BP readings) may be desirable to diagnose hypertension or guide the titration of treatment for individual patients. This should be evaluated in future studies.
The current study highlights the importance of training staff to conduct ABPM. Some individuals may experience discomfort while undergoing ABPM [28]. However in a study of 1,010 adults, approximately half with and half without hypertension, 46.4% reported a willingness to tolerate mild discomfort and an additional 37.0% would tolerate moderate or extreme discomfort to obtain an accurate estimate of their BP [29]. Based on our experience, having staff carefully explain the ABPM procedure, what individuals should expect during the monitoring period, and approaches to prevent failed readings (e.g., to stop moving before the inflation of the BP cuff, not talking when having their BP measured) can result in successful recordings for the vast majority of individuals. Obtaining 100% of planned BP readings should be the goal of ABPM and would prevent the need to decide among criteria for defining a complete recording.
There are several strengths of the current study. The JHS is a large community-based sample that measured ABPM and clinic BP following standardized protocols. Additionally, a large number of covariates were collected, allowing the assessment of factors associated with meeting versus not meeting criteria for a complete ABPM recording. Despite these strengths, the findings from this study should be interpreted in the context of known and potential limitations. Clinic BP was measured using a Hawksley random zero sphygmomanometer, which has been suggested to underestimate SBP and DBP [30]. The JHS enrolled only African Americans and the findings may not be generalizable to other race/ethnic groups. ABPM was performed in a selected sub-group of JHS participants. Upon comparing demographic and clinical characteristics of JHS participants who did and did not volunteer to complete ABPM, differences were present [22]. Also, 24-hr ABPM was only performed once, which may result in misclassification of BP phenotypes. There have not been enough CVD events among the subset of JHS participants who underwent ABPM to study whether the association between BP phenotypes and CVD events differs for individuals meeting one but not another set of criteria for a complete ABPM. However, we were able to study LVH, a marker of hypertension-related organ damage and a well-established risk factor for CVD.
In conclusion, the prevalence of BP phenotypes was similar for participants meeting several different published criteria for defining a complete ABPM recording. Also, among participants meeting the less stringent criteria (i.e., 2003 ESH, IDACO and UK-NICE criteria), the associations between BP phenotypes and LVH were similar for participants who did and did not meet the more stringent criteria (i.e., the Spanish ABPM Registry and 2013 ESH criteria). Excluding participants by applying criteria that are more stringent will result in reduced statistical power while not affecting the prevalence of BP phenotypes or phenotype-outcome associations. While achieving 100% of planned readings should be the goal for all individuals undergoing ABPM, the current analyses suggests less strict criteria can be used to define a complete recording in research studies.
Supplementary Material
Supplemental Digital Content Table 1. Definitions of blood pressure phenotypes.
Supplemental Digital Content Table 2. Prevalence of blood pressure phenotypes for participants not meeting and meeting the Spanish Ambulatory Blood Pressure Registry (top panel) and 2013 ESH criteria (bottom panel) criteria, among those who met the 2003 ESH, IDACO, and UK-NICE criteria.
Supplemental Digital Content Table 3. Participant characteristics associated with not meeting versus meeting the Spanish Ambulatory Blood Pressure Registry (left panel) and 2013 ESH criteria (right panel), among those who met the 2003 ESH, IDACO, and UK-NICE criteria.
Acknowledgments
The Jackson Heart Study is supported by contracts HHSN268201300046C, HHSN268201300047C, HHSN268201300048C, HHSN268201300049C, HHSN268201300050C from the National Heart, Lung, and Blood Institute and the National Institute on Minority Health and Health Disparities. This work was also supported by the National Institutes of Health (HL047540, HL117323, HL117323-02S2, K24-HL125704, 2T32HL007854-21) from the National Heart, Lung, and Blood Institute, Bethesda, MD. The authors thank the participants and data collection staff of the Jackson Heart Study. The views expressed in this manuscript are those of the authors and do not necessarily represent the views of the National Heart, Lung, and Blood Institute; the National Institutes of Health; or the U.S. Department of Health and Human Services. Dr. Booth received support from F31 HL129701 from the National Heart, Lung, and Blood Institute. Drs. Muntner, Schwartz, Shimbo and Thomas received support from 15SFRN2390002 from the American Heart Association.
Dr. Daichi Shimbo is a consultant for Abbott Vascular and Novartis Pharmaceuticals Corporation. Drs. Paul Muntner and Matthew Loop receive grant support from Amgen Inc.
Footnotes
Conflicts of Interest: For the remaining authors none were declared.
DISCLOSURES
Drs. Samantha G. Bromfield, John N. Booth III, Joseph E. Schwartz, Samantha Seals, S. Justin Thomas, Yuan-I Min, and Gbenga Ogedegbe have no disclosures.
References
- 1.Shimbo D, Abdalla M, Falzon L, Townsend RR, Muntner P. Role of Ambulatory and Home Blood Pressure Monitoring in Clinical Practice: A Narrative Review. Ann Intern Med. 2015;163(9):691–700. doi: 10.7326/M15-1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.O'Brien E, Parati G, Stergiou G, Asmar R, Beilin L, Bilo G, et al. European Society of Hypertension position paper on ambulatory blood pressure monitoring. J Hypertens. 2013;31(9):1731–1768. doi: 10.1097/HJH.0b013e328363e964. [DOI] [PubMed] [Google Scholar]
- 3.Gorostidi M, Sarafidis PA, de la Sierra A, Segura J, de la Cruz JJ, Banegas JR, et al. Differences between office and 24-hour blood pressure control in hypertensive patients with CKD: A 5,693-patient cross-sectional analysis from Spain. Am J Kidney Dis. 2013;62(2):285–294. doi: 10.1053/j.ajkd.2013.03.025. [DOI] [PubMed] [Google Scholar]
- 4.McManus RJ, Caulfield M, Williams B National Institute for Health Clinical Excellence. NICE hypertension guideline 2011: evidence based evolution. BMJ. 2012;344:e181. doi: 10.1136/bmj.e181. [DOI] [PubMed] [Google Scholar]
- 5.Thijs L, Hansen TW, Kikuya M, Bjorklund-Bodegard K, Li Y, Dolan E, et al. The International Database of Ambulatory Blood Pressure in relation to Cardiovascular Outcome (IDACO): protocol and research perspectives. Blood Press Monit. 2007;12(4):255–262. doi: 10.1097/mbp.0b013e3280f813bc. [DOI] [PubMed] [Google Scholar]
- 6.O'Brien E, Asmar R, Beilin L, Imai Y, Mallion JM, Mancia G, et al. European Society of Hypertension recommendations for conventional, ambulatory and home blood pressure measurement. J Hypertens. 2003;21(5):821–848. doi: 10.1097/00004872-200305000-00001. [DOI] [PubMed] [Google Scholar]
- 7.Taylor HA, Jr, Wilson JG, Jones DW, Sarpong DF, Srinivasan A, Garrison RJ, et al. Toward resolution of cardiovascular health disparities in African Americans: design and methods of the Jackson Heart Study. Ethn Dis. 2005;15(4 Suppl 6) S6-4-17. [PubMed] [Google Scholar]
- 8.Carpenter MA, Crow R, Steffes M, Rock W, Heilbraun J, Evans G, et al. Laboratory, reading center, and coordinating center data management methods in the Jackson Heart Study. Am J Med Sci. 2004;328(3):131–144. doi: 10.1097/00000441-200409000-00001. [DOI] [PubMed] [Google Scholar]
- 9.Baecke JA, Burema J, Frijters JE. A short questionnaire for the measurement of habitual physical activity in epidemiological studies. Am J Clin Nutr. 1982;36(5):936–942. doi: 10.1093/ajcn/36.5.936. [DOI] [PubMed] [Google Scholar]
- 10.Dubbert PM, Carithers T, Ainsworth BE, Taylor HA, Jr, Wilson G, Wyatt SB. Physical activity assessment methods in the Jackson Heart Study. Ethn Dis. 2005;15(4 Suppl 6) S6-56-61. [PubMed] [Google Scholar]
- 11.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, 3rd, Feldman HI, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.KDIGO CKD Work Group. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl. 2013;3:1–150. doi: 10.1038/ki.2013.243. [DOI] [PubMed] [Google Scholar]
- 13.Mackie A, Whincup P, McKinnon M. Does the Hawksley random zero sphygmomanometer underestimate blood pressure, and by how much? J Hum Hypertens. 1995;9(5):337–343. [PubMed] [Google Scholar]
- 14.Abdalla M, Booth JN, 3rd, Seals SR, Spruill TM, Viera AJ, Diaz KM, et al. Masked Hypertension and Incident Clinic Hypertension Among Blacks in the Jackson Heart Study. Hypertens. 2016;68(1):220–226. doi: 10.1161/HYPERTENSIONAHA.115.06904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Booth JN, Muntner P, Abdalla M, et al. Differences in night-time and daytime ambulatory blood pressure when diurnal periods are defined by self-report, fixed-times, and actigraphy: Improving the Detection of Hypertension study. J. Hypertens. 2016;34(2):235–243. doi: 10.1097/HJH.0000000000000791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fagard R, Brguljan J, Thijs L, Staessen J. Prediction of the actual awake and asleep blood pressures by various methods of 24 h pressure analysis. J. Hypertens. 1996;14(5):557–563. doi: 10.1097/00004872-199605000-00003. [DOI] [PubMed] [Google Scholar]
- 17.Parati G, Stergiou G, O'Brien E, Asmar R, Beilin L, Bilo G, et al. European Society of Hypertension practice guidelines for ambulatory blood pressure monitoring. J Hypertens. 2014;32(7):1281–1366. doi: 10.1097/HJH.0000000000000221. [DOI] [PubMed] [Google Scholar]
- 18.Mancia G, Fagard R, Narkiewicz K, Redon J, Zanchetti A, Bohm M, et al. 2013 ESH/ESC Guidelines for the management of arterial hypertension: the Task Force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC) J Hypertens. 2013;31(7):1281–1357. doi: 10.1097/01.hjh.0000431740.32696.cc. [DOI] [PubMed] [Google Scholar]
- 19.Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28(1):1–39. e14. doi: 10.1016/j.echo.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 20.Carpenter J, Bithell J. Bootstrap confidence intervals: when, which, what? A practical guide for medical statisticians. Stat Med 100. 19(1):1141–1164. doi: 10.1002/(sici)1097-0258(20000515)19:9<1141::aid-sim479>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 21.Muntner P, Lewis CE, Diaz KM, Carson AP, Kim Y, Calhoun D, et al. Racial differences in abnormal ambulatory blood pressure monitoring measures: Results from the Coronary Artery Risk Development in Young Adults (CARDIA) study. Am J Hypertens. 2015;28(5):640–648. doi: 10.1093/ajh/hpu193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Diaz KM, Veerabhadrappa P, Brown MD, Whited MC, Dubbert PM, Hickson DA. Prevalence, Determinants, and Clinical Significance of Masked Hypertension in a Population-Based Sample of African Americans: The Jackson Heart Study. Am J Hypertens. 2015;28(7):900–908. doi: 10.1093/ajh/hpu241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Heeringa J, van der Kuip DA, Hofman A, Kors JA, van Herpen G, Stricker BH, et al. Prevalence, incidence and lifetime risk of atrial fibrillation: the Rotterdam study. Eur Heart J. 2006;27(8):949–953. doi: 10.1093/eurheartj/ehi825. [DOI] [PubMed] [Google Scholar]
- 24.Go AS, Hylek EM, Phillips KA, Chang Y, Henault LE, Selby JV, et al. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA. 2001;285(18):2370–2375. doi: 10.1001/jama.285.18.2370. [DOI] [PubMed] [Google Scholar]
- 25.Tanner RM, Shimbo D, Seals SR, Reynolds K, Bowling CB, Ogedegbe G, et al. White-Coat Effect Among Older Adults: Data From the Jackson Heart Study. J Clin Hypertens. 2016;18(2):139–145. doi: 10.1111/jch.12644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Asayama K, Sato A, Ohkubo T, Mimura A, Hayashi K, Kikuya M, et al. The association between masked hypertension and waist circumference as an obesity-related anthropometric index for metabolic syndrome: the Ohasama study. Hypertens Res. 2009;32(6):438–443. doi: 10.1038/hr.2009.37. [DOI] [PubMed] [Google Scholar]
- 27.Powers BJ, Olsen MK, Smith VA, Woolson RF, Bosworth HB, Oddone EZ. Measuring blood pressure for decision making and quality reporting: where and how many measures? Ann Intern Med. 2011;154(12):781–788. doi: 10.7326/0003-4819-154-12-201106210-00005. W-289-790. [DOI] [PubMed] [Google Scholar]
- 28.Viera AJ, Lingley K, Hinderliter AL. Tolerability of the Oscar 2 ambulatory blood pressure monitor among research participants: a cross-sectional repeated measures study. BMC Med Res Methodol. 2011;11:59. doi: 10.1186/1471-2288-11-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Viera AJ, Tuttle L, Zeng J. Dollars and Discomfort: What Will People Be Willing to Give for Better Blood Pressure Assessment? J Clin Hypertens. 2016;18(5):422–423. doi: 10.1111/jch.12680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.O'Brien E, Mee F, Atkins N, O'Malley K. Inaccuracy of the Hawksley random zero sphygmomanometer. Lancet. 1990;336:1465–1468. doi: 10.1016/0140-6736(90)93177-q. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Digital Content Table 1. Definitions of blood pressure phenotypes.
Supplemental Digital Content Table 2. Prevalence of blood pressure phenotypes for participants not meeting and meeting the Spanish Ambulatory Blood Pressure Registry (top panel) and 2013 ESH criteria (bottom panel) criteria, among those who met the 2003 ESH, IDACO, and UK-NICE criteria.
Supplemental Digital Content Table 3. Participant characteristics associated with not meeting versus meeting the Spanish Ambulatory Blood Pressure Registry (left panel) and 2013 ESH criteria (right panel), among those who met the 2003 ESH, IDACO, and UK-NICE criteria.