Abstract
Extracellular vesicles (EVs), including exosomes, microvesicles, and others, have emerged as potential therapeutics for a variety of applications. Pre-clinical reports of EV efficacy in treatment of non-healing wounds, myocardial infarction, osteoarthritis, traumatic brain injury, spinal cord injury, and many other injuries and diseases demonstrate the versatility of this nascent therapeutic modality. EVs have also been demonstrated to be effective in humans, and clinical trials are underway to further explore their potential. However, for EVs to become a new class of clinical therapeutics, issues related to translation must be addressed. For example, approaches originally developed for cell biomanufacturing, such as hollow fiber bioreactor culture, have been adapted for EV production, but limited knowledge of how the cell culture microenvironment specifically impacts EVs restricts the possibility for rational design and optimization of EV production and potency. In this review, we discuss current knowledge of this issue and delineate potential focus areas for future research towards enabling translation and widespread application of EV-based therapeutics.
Keywords: Extracellular Vesicle, Exosome, Mesenchymal Stem Cell, Biomanufacturing, Microenvironment
1. Introduction
As natural carriers of bioactive cargo, extracellular vesicles (EVs) – especially microvesicles and exosomes – have emerged as potential therapeutics for a variety of applications. EVs have been shown to play roles in numerous physiological and pathological phenomena by imparting their bioactivity through various mechanisms, including signal transduction via protein or bioactive lipid ligand-mediated cell surface receptor activation (Bruno et al., 2009; Isakson et al., 2015), immune modulation by means of antigen presentation (Bobrie et al., 2011), and horizontal transfer of nucleic acids such as microRNAs (miRNAs) (Bovy et al., 2015; Valadi et al., 2007). Among many applications, regenerative or reparative effects of EVs have been observed in models of myocardial infarction (Lai et al., 2010), ischemia reperfusion injury (Chen et al., 2013), acute kidney injury (Bruno et al., 2012), skeletal muscle repair (Nakamura et al., 2015), and many others. EVs have also been utilized as biological drug carriers for delivery of therapeutic proteins (Sterzenbach et al., 2018), nucleic acids (Alvarez-Erviti et al., 2011, Lamichhane et al., 2016, 2015), and small molecules (Tian et al., 2014). Thus, interest in clinical translation of EV therapeutics is high.
However, significant barriers to translation of EV-based therapies remain. Noteworthy among these is the lack of a rationally designed scalable biomanufacturing process for EVs. Several methods have been reported to increase EV production, including cell stimulation in static culture by raising intracellular calcium (Constantinescu et al., 2010; Qu et al., n.d.), inducing hypoxia (Kucharzewska et al., 2013), and serum starvation (Aharon et al., 2008). Additionally, dynamic culture systems such as hollow fiber bioreactors have been used to increase EV yield (Watson et al., 2016). Immortalization of mesenchymal stem cells (MSCs) also enabled enhanced EV production capability (Chen et al., 2011). Yet, in all of these cases, there is a lack of fundamental understanding of how changes in cellular function imposed by the culture system or environment specifically affect EVs. This knowledge gap hinders rational design of EV biomanufacturing strategies.
In this review, we describe current cell culture methods used for EV production and summarize what is known about how the biophysical culture microenvironment impacts cellular responses and, hence, EV production. We further discuss how cell culture parameters might impact EV biogenesis, cargo, and therapeutic function. Increased knowledge of these topics could ultimately spur further study of the mechanisms linking cellular responses with EV function towards enabling rationally designed EV biomanufacturing.
2. Current culture systems for EV production
Cell culture configurations for controlled production of EVs can be grouped into two-dimensional (2D) and three-dimensional (3D) approaches. 2D systems include conventional tissue culture polystyrene flasks for growth of EV-producing cells, which is typically carried out in EV-free media or media previously depleted of EVs by various methods, most commonly ultracentrifugation (Gudbergsson et al., 2016). In general, EV collection is carried out from cells in a sub-confluent state after 24–48h of culture in collection media, however many papers do not report quantitative measures of cell confluence, seeding density, or collection time and frequency.
2.1. Media composition
It is important to note that, regardless of the culture system and source cells used, media composition plays a critical role in defining composition of generated EVs. The very use of EV-depleted media can impact cell growth (Eitan et al., 2015), function (Shelke et al., 2014), phenotype (Beninson and Fleshner, 2015), and differentiation potential (Aswad et al., 2016). Further, growth factors and other media additives can dramatically affect cell behavior in a wide variety of ways. Given the nearly innumerable possibilities, this review will not focus further on the effects of specific media components on EV generation. However, it is acknowledged that media selection -along with source cell selection, with the latter informing the former – will ultimately be a crucial component in the development of any next-generation EV biomanufacturing systems.
2.2. 3D culture systems
3D culture systems for EV production consist primarily of scaffold-based and scaffold-free approaches, as well as bioreactors. 3D scaffolds are able to capture physiological aspects that are missing in conventional 2D systems, such as 3D tissue architecture, extracellular matrix (ECM) composition, and heterotypic cell-cell interactions (Ader and Tanaka, 2014). The enhanced biomimicry provided by 3D environments may confer a more physiologically relevant phenotype to cells compared to 2D systems and, accordingly, can be leveraged for production of EVs with more natural features (e.g. cargo content, lipid and protein content, etc.). For example, kidney epithelial cells (KECs) cultured in 3D ECM-like scaffolds undergo cystogenesis and produce microvesicles, a phenomenon not observed in 2D cultures (Guo et al., 2008). EV secretion in this system occurred at the abluminal side of the cyst, indicating that the 3D environment does not directly foster EV secretion but rather supports KEC cystogenesis, which in turn results in a polarized vesicular production and trafficking. Additionally, scaffold composition can affect both quantity and quality of the EVs secreted, as shown by Griffith and associates (Jangamreddy et al., 2018). Poly(ethylene glycol) scaffolds functionalized with collagen-like peptides (PEG-CLP) were seeded with corneal epithelial cells (CECs) and tested in vitro and in vivo for the regeneration of cornea defects. Comparisons between conventional 2D cultures and 3D scaffolds made of recombinant collagen (RC) were carried out. Interestingly, CD9+ EVs localized differently in 2D (cell edges), in RC (cytoplasm) and in PEG-CLP (perinuclear region). Additionally, production of Rab7+ EVs was markedly higher in PEG-CLP scaffolds compared to RC, a finding corroborated in animal models, where PEG-CLP constructs stimulated cell ingrowth and the secretion of vast quantities of EVs compared to other scaffolds.
Overall, these results support the hypothesis that scaffold composition can be leveraged to direct EV production and cargo content. Yet, despite the above-mentioned advantages over 2D culture systems, 3D systems also present some limitations, namely the more difficult retrieval of EVs produced within large non-porous scaffolds (e.g. hydrogels). In 2D culture, EVs are easily collected from the medium, however 3D scaffolds may partially retain EVs, requiring further processing (e.g. enzymatic digestion of the construct) that may have detrimental effects on EV integrity and bioactivity. Difficulties with reproducibility and scalability of 3D constructs must also be considered and depend largely on the fabrication method used. Emergent technologies such as 3D printing may ultimately enable reliable 3D scaffold production for industrial-scale EV generation.
2.3. Bioreactors
Following from their use for generation of clinical scale quantities of therapeutic cells, bioreactors are also being employed for large-scale EV production. In particular, hollow fiber bioreactor technology has been utilized effectively (Kim et al., 2017; Nold et al., 2013; Watson et al., 2016). In this setup, cells are seeded into cylindrical hollow fibers through which media is circulated. These fibers are bundled up within a tubular shell, resulting in a high surface area available for cell seeding. Accordingly, these bioreactors can house 3 orders of magnitude (billions vs. millions) more cells than the largest cell culture flask (T175) (Watson et al., 2016), and continuous flow of media through these reactors allows for the collection of roughly 4-fold more EVs than from a traditional 2D flask (Figure 1). In fact, it has been estimated that to obtain the same amount of EVs in 20mL media from one day using a hollow fiber bioreactor, it would take 53 T175 cell culture flasks and 800mL of media (Watson et al., 2016). Other investigators produced EVs in a Biolevitator™, a commercially available bioreactor system where cells are cultured onto protein-coated magnetic particles within a vessel (Jarmalavičiūtė et al., 2015). Gas exchange and metabolite removal is provided by the continuous motion of the particles, which is directed by a magnet along the vertical axis, and by rotation of the culture vessel along the horizontal axis. Stem cells cultured in these conditions secreted exosomes that rescue human dopaminergic neurons from apoptosis, a phenomenon not observed with exosomes harvested in 2D cultures (Jarmalavičiūtė et al., 2015). Strikingly, microvesicles produced in the Biolevitator™ were ineffective and only exosomes had antiapoptotic effects on the dopaminergic neurons. While the overall yield in EV production was not indicated, this study clearly indicates that EVs produced by cells cultured in 3D systems under dynamic conditions are biologically active and have therapeutically relevant properties.
Figure 1. Hollow fiber bioreactor and 3D culture impact on EV production and bioactivity.
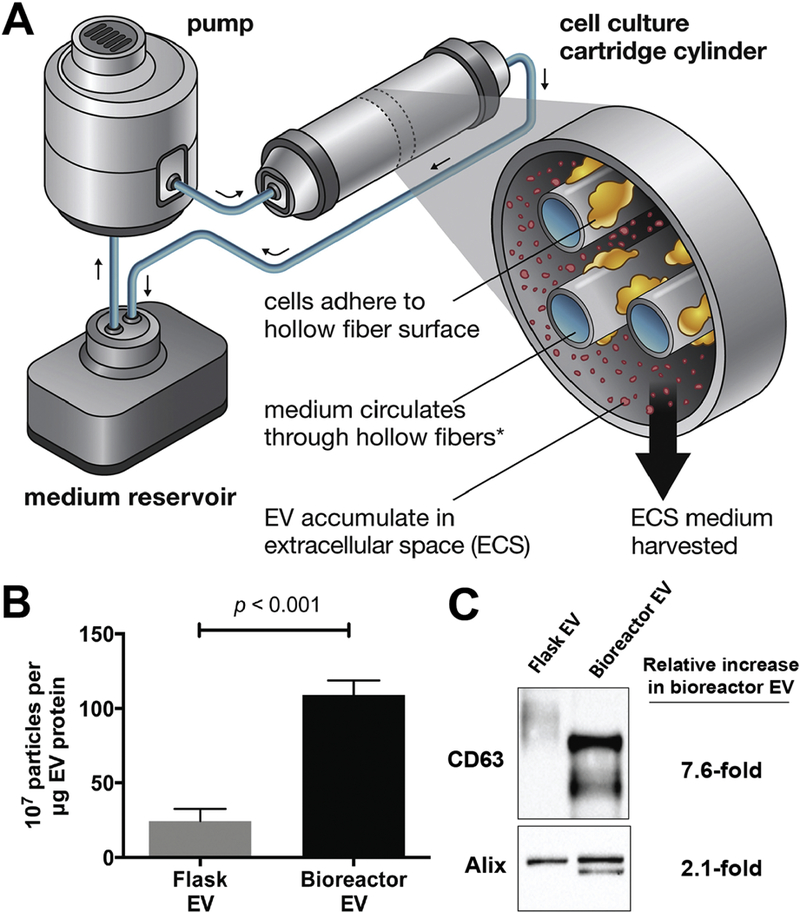
(A) Schematic of the hollow fiber bioreactor system. (B) HEK293 cells cultured in a hollow fiber bioreactor produced ~4-fold more EVs than cells cultured in conventional tissue culture flasks. Data presented as mean ± SEM and statistical significance was compared by ANOVA. (B) Western blot analysis showed 7.6-fold and 2.1-fold increase in EV-associated markers of EVs (20µg) from hollow-fiber bioreactor compared to tissue culture flask. Additional bands observed for CD63 and Alix in Bioreactor EVs is hypothesized to be due to differential glycosylation pattern and phosphorylation, respectively. Adapted via open access from Watson, D.C. et al. (2016).
Beyond increasing the total number of EVs produced, the utilization of continuous flow bioreactors necessarily imposes conditions that might impact the identity and function of EVs. Specifically, while EV production may benefit from the enhanced nutrient exchange enabled within a bioreactor system, the presence of flow-derived shear stress may also act as a mechanomodulator of EV secretion and uptake, with unknown consequences. Indicative of this phenomenon, Watson et. al. reported that EVs generated from hollow fiber bioreactor culture had a different size profile when compared to EVs from the same cells generated in 2D culture where both EV populations were collected using the same downstream process (Watson et al., 2016). Thus, future investigations should seek to assess the relevance of the mechanical perturbations brought on by flow on EV production and function, as has been done for cells in a variety of contexts (Santoro et al., 2015). Additionally, surface protein and intravesicular cargo content may be reduced with increased EV production in bioreactors on a per vesicle basis, possibly leading to reduced therapeutic potency via reduced cell uptake or other mechanisms. This phenomenon was observed for the rapid production of EV-like vesicles by cavitation. Using a nitrogen cavitation chamber, neutrophils suspended in media were able to produce 16-fold more vesicles compared to 2D culture (Gao et al., 2017). The vesicles produced by cavitation contained more integrin B2 (a plasma membrane marker of activated neutrophils) and less nucleic acids and proteins commonly found in exosomes, suggesting the force of the nitrogen gas against the cell membrane caused removal of parts of the plasma membrane and formation of vacant vesicles. While not completely analogous to bioreactor culture, this study reinforces the idea that increased production of EVs could have negative consequences on therapeutic potency and functionality if EV composition is not conserved.
3. Potential impact of cellular microenvironment on EVs
Tremendous progress has been made in EV production methods in only a short time, however there are still no rationally-designed approaches that have been specifically developed for EV generation. Currently, rational design of EV production is limited by a lack of fundamental knowledge of the relationships between specific production parameters and EV generation. While these parameters have scarcely been explicitly studied, it is clear that parental cell identity impacts EV cargo and function, suggesting that parameters that influence cells also affect EVs. One cell type for which environmental effects on cell function have been extensively characterized is mesenchymal stem/stromal cells (MSCs), which are responsive to culture substrate properties, extracellular matrix topography and rigidity, and various biochemical and mechanical cues (Figure 2). Below, we discuss effects of the cell culture environment on MSCs and how such effects might modify MSC EVs, which are of significant clinical interest for a variety of therapeutic applications (Merino-González et al., 2016).
Figure 2.
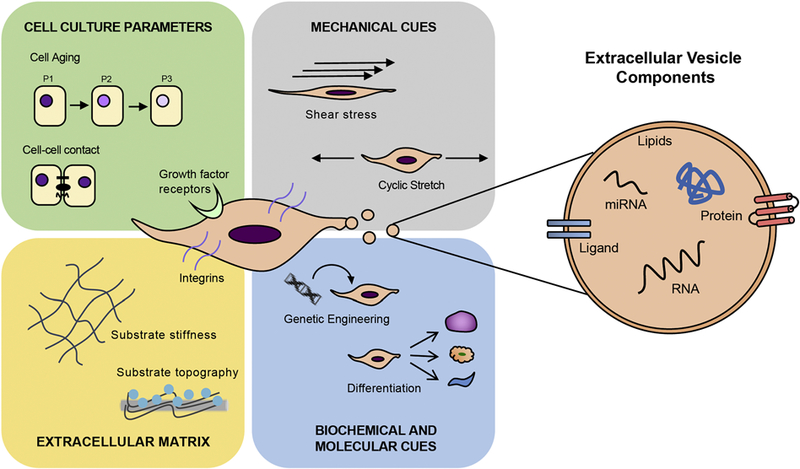
Schematic representations of factors influencing MSC-derived EV production and cargo composition.
3.1. Cell Culture Parameters:
3.1.1. Cell Density
EVs are typically harvested from cell cultures at 60–90% confluence (Gudbergsson et al., 2016), although confluence estimates may vary between individual researchers. Interestingly, previous studies have linked low seeding densities with rapid proliferation of MSCs as well as the highest percentage of multipotent cells compared to higher culture densities (Colter et al., 2000; Sekiya et al., 2002). Furthermore, cells experience contact inhibition at higher densities, which triggers confluent cells to enter quiescence (Lieberman MA, 1981). The variance in density has also been shown to impact growth kinetics (Colter et al., 2000). In one study, Ho et al. (2011) found that MSCs reached cellular senescence more rapidly in 100% confluent culture, and the doubling time was significantly prolonged (Ho J.H et al., 2011). They also showed that this process was independent of both telomere shortening and p53 activation. Instead, the group showed that the decrease in cell proliferation was due to the observed upregulation of cell cycle regulating protein, p16(INK4a), through Ras pathway (Ho J.H et al., 2011).
Taken together, these studies suggest that cell culture parameters such as cell density can impact not only cell fate but also cause genetic changes, which may influence their secreted paracrine factors such as EVs. Recent work by our group using MSCs showed up to 100-fold decrease in EV production per cell at a higher density of 10,000 cells/cm2 compared to cells seeded at 100 cells/cm2; although no significant change in bioactivity of the EVs from low and high density cultures was observed as assessed in an endothelial cell gap closure assay (Patel et al., 2017) (Figure 3). In a different study by Llorente et al. (2013), dense prostate cancer cells, which have decreased cholesterol metabolism, secreted EVs enriched in cholesterol (Llorente A et al., 2013).
Figure 3. Impact of MSC passage and seeding density on EV production and vascular bioactivity.
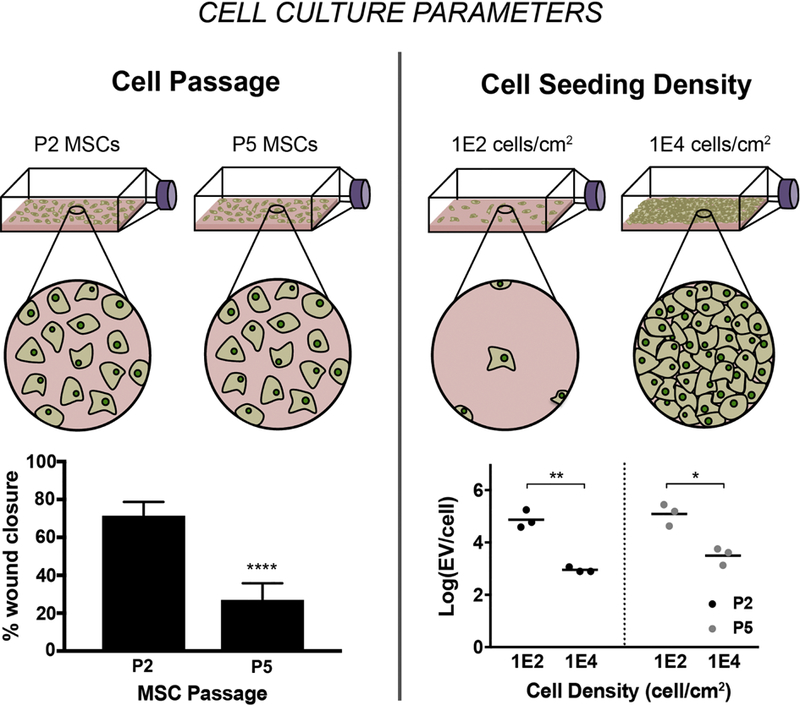
(A) EVs were isolated from MSCs at P2, P3, P4, and P5 were seeded at 1E2, 5E2, 1E3, 1E4 cells/cm2. For MSCs at all passages, increase in EV production is observed per cell with decreasing seeding densities. Statistical significance was calculated using two-way ANOVA with Tukey’s multiple comparison test (* p<0.05, ** p<0.01). Vascular bioactivity of EVs from different densities (B) and different passages (C) was analyzed using a gap closure assay with endothelial cells. (B) No significant difference in gap closure was observed for EVs from MSCs seeded at 1E2 and 1E4 cells/cm2. (C) Bioactivity of EVs significantly diminishes with increasing passage. Data are representative of three independent experiments with three replicates each (n = 3). Statistical significance was calculated using two-way ANOVA with Tukey’s multiple comparison test (* p<0.05, ** p<0.01, **** p<0.0001). Figure reprinted from Patel et al., 2017 via open access.
These studies further suggest that the production rate and composition of EVs are highly dependent upon their parent cells. While these results begin to establish the importance of cell culture parameters on EV production, better understanding of the molecular mechanisms of the observed phenomena is needed in order to inform rational design of therapeutic MSC EV production. For example, the observed decrease in EV production at higher densities may be due to the well-established phenomenon of contact inhibition. Alternatively, limited direct cell-cell contact in less confluent culture may increase demand for indirect cell-cell communication, such as that mediated by EVs, therefore leading to increased production of EVs per cell. It is likely that the impact of cell density on EV production as well as bioactivity is multifaceted. Thus, more work needs to be carried out to elucidate this mechanism towards establishing a scalable manufacturing pathway for therapeutic EVs.
3.1.2. Cell Aging/Passage
MSCs are found in bone marrow (BM) at a low frequency (Wexler et al., 2003), requiring high expansion in vitro to obtain relevant quantities for cell therapy as well as EV production. However, researchers have reported the association of long-term in vitro cell expansion with alterations in genes involved in the cell cycle, protein ubiquitination, and apoptosis (Izadpanah et al., 2008). Such changes can affect MSC differentiation potential; Liu et. al. report that aged MSCs demonstrate biased differentiation to adipocytes at the cost of osteoblasts (Liu et al., 2015). These changes can be attributed to loss of cell pluripotency as well as senescence during in vitro culture (Bonab et al., 2006; Izadpanah et al., 2008). The mechanisms of aging of MSCs include telomere attrition, DNA damage, and changes in microenvironmental factors with long-term MSC expansion, among others (Liu et al., 2015). This is corroborated in another study by Bonab et al., which showed that mean telomere length decreased by more than 15% in MSCs as early as the 9th passage. Furthermore, population doubling time decreased 5-fold by the 10th passage (Bonab et al., 2006).
These results suggest that MSCs enter senescence and begin losing their plasticity and genetic stability rapidly, which may be reflected in their EV cargo as demonstrated by our group in a recent study where we observed a significant decrease in endothelial gap closure bioactivity of MSC-derived EVs with increasing cell passage (Patel et al., 2017) (Figure 3). However, further investigations are warranted to establish a mechanistic link between the bioactivity phenomenon and the influence of long-term expansion of MSCs on their EVs. To begin understanding this connection, changes in EV cargo composition as well as molecular changes in cells treated with these EVs should be identified by applying unbiased approaches where possible (e.g. proteomics, transcriptomics). A detailed assessment of potential mechanism should follow via specific knockdown and overexpression experiments of identified molecular targets to corroborate their roles in EV-mediated bioactivity with increasing MSC passage.
3.2. Biochemical and molecular cues:
3.2.1. Cell Immortalization
To overcome the limited lifespan of MSCs and loss of MSC-derived EV bioactivity with increasing passage, Chen and colleagues investigated the effect of immortalizing MSCs on EV production and cardioprotective phenotype (Chen et al., 2011). The authors immortalized an embryonic stem cell (ESC)-derived MSC line (E1MYC16.3) and an umbilical cord-derived MSC line (CMSC3A1). Upon immortalization, both lines of MSCs demonstrated higher proliferative capacity and increased growth rate compared to their primary counterpart; however, cord-derived MSCs lost the ability to undergo adipogenic differentiation and had reduced adherence to tissue culture plastic, which is uncharacteristic of MSCs. Cord-derived MSCs also presented more disparity in gene regulation after MYC-immortalization than ESC-derived MSCs. The authors observed a general increase in EV production post-immortalization of ESC-derived MSCs, albeit for cord-derived MSCs, MYC-immortalization significantly reduced EV production. EVs from both cell lines were positive for exosomal markers CD9 and C81, but MYC protein was undetected. Evidently, EVs from both immortalized cell lines exhibited cardioprotective and therapeutic phenotype against myocardial ischemia/reperfusion injury. While this transformation reportedly produced EVs with preserved therapeutic potential, the process of cell immortalization required lentiviral transfection, which rendered a change in MSC phenotype to a non-MSC classification that could be reflected in the EV population over long-term in vitro expansion.
3.2.2. Cell Differentiation
Stem cell differentiation is a highly complex process requiring substantial changes in genetic regulation of cells, which are likely to be reflected in their EVs. In a recent study by Wang et al. (2018), the authors isolated EVs from conditioned media of MSCs at different stages of osteogenic differentiation and evaluated their potential to stimulate osteogenic differentiation of homotypic cells (Wang et al., 2018). Interestingly, their results suggest that homotypic cells treated with EVs from only late-stage differentiation showed increased ALP activity and extracellular matrix (ECM) mineralization, indicative of their osteogenic specification (Figure 4). Further, microarray-based miRNA analysis revealed variance in miRNA composition of EVs in a stage-dependent manner, with higher levels of miRNAs favoring osteogenic induction present in late-stage EVs compared to early-stage EVs. Other researchers have reported similar findings related to neuronal differentiation, where EVs derived from neuronal progenitor cells (PC12) at a late stage of differentiation induced neuronal differentiation of MSCs through miRNA transfer (Takeda and Xu, 2015). While the specific roles of miRNAs in EV function remain a subject of active investigation, these studies reinforce the concepts that parent cell phenotype dictates EV function and that molecular composition of parent cell is reflected in their EV population.
Figure 4. Schematic describing that EVs from varying stages of differentiating MSCs induce osteogenic differentiation of MSCs differently.
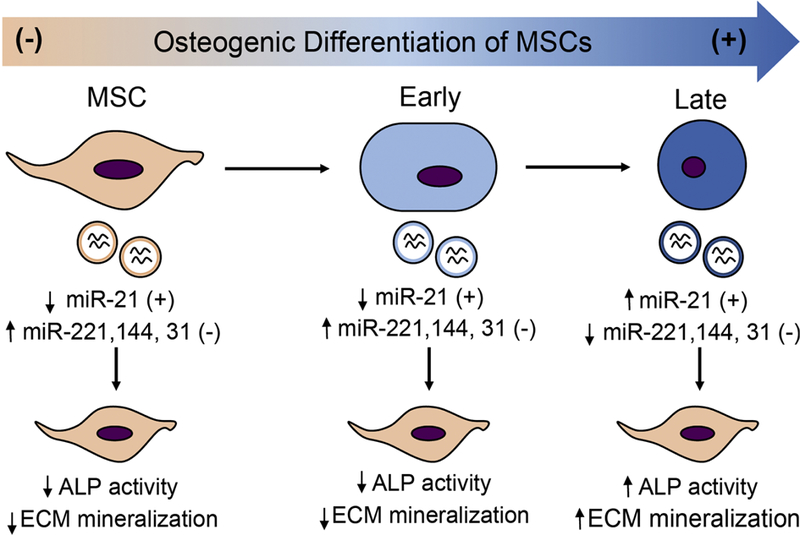
Osteogenic differentiation was induced in MSCs using osteogenic differentiation media. EVs were isolated from conditioned media collected at different stages after media change, namely, early and late stages. Exosomes from MSCs in growth media were used as control. Using an array-based method, expression of miRNA associated with osteogenic differentiation of MSCs was measured. ALP activity, ECM calcium, and phosphate was measured to evaluate osteogenic differentiation of MSCs after EV treatment. Adapted from data presented in Wang et al., (2018) via open access.
3.3. Extracellular Matrix:
3.3.1. Substrate Topography
Topography of the cell substrate or extracellular matrix has been shown to govern several aspects of MSC behavior, including migration, proliferation, and differentiation. Abagnale et al. (2015) systematically compared in vitro differentiation of MSCs on a variety of patterned polyimide surfaces (Abagnale et al., 2015). Differences in substrate grooves and ridges affected spreading and orientation of MSCs and guided them towards specific lineages. For example, 15 μm ridges increased adipogenic differentiation whereas 2 μm ridges enhanced osteogenic differentiation. Notably, nano-patterns with a periodicity of 650 nm increased differentiation towards both osteogenic and adipogenic lineages (Abagnale et al., 2015).
Other studies have focused on elucidating changes in gene expression profiles due to surface patterning. Dalby et al. (2008) compared the genetic profile of MSCs cultured on osteogenic nanoscale topographies to cells treated with dexamethasone, a corticosteroid routinely used to stimulate bone formation from MSCs. The topographies were shown to activate integrin-mediated actin cytoskeleton signaling and p38 mitogen-activated protein kinase (MAPK) signaling to manipulate MSC cytoskeleton towards osteogenic differentiation (Dalby et al., 2008, 2007). Lee et al. observed that etched microgrooves on titanium triggered differential expression of genes involved in cell adhesion, migration, and cytoskeletal reorganization in MSCs. They also showed that microgrooves resulted in increased type I collagen production, osteoblast differentiation, and activation of TGF-β signaling and MAPK signaling (Lee et al., 2012). In a different study, Kurpinski et al. (2016) used a micropatterned strip to align MSCs along the direction of uniaxial strain, which resulted in increased expression of calponin-1, a marker for smooth muscle cells (SMCs). However, perpendicular alignment of the cells caused no changes in gene expression of calponin-1 (Kurpinski et al., 2006). Thus, surface topography alone does not govern cellular differentiation but rather triggers a signaling pathway that elicits specific cell responses when synergizing with corresponding biochemical stimuli.
Despite numerous studies describing the effects of substrate topography on MSC fate, additional work needs be done to elucidate the resulting impact on MSC EVs. Tauro et al. (2013) showed that a single LIM1863 colon-cancer cell carcinoma organoid with basolateral and apical topography produced two distinct population of EVs. The basolateral and apical surfaces of the cells exocytose A33-and EpCAM-rich EVs, respectively, each with a different cargo composition (Tauro et al., 2013) (Figure 5). This study illustrates the impact of the mechanical environment on the production of bioactive EVs and thus the importance of its consideration when formulating a rational design for EV biomanufacturing.
Figure 5. ECM characteristics and mechanical stimulation of cells can dictate EV production, composition, and uptake profiles.
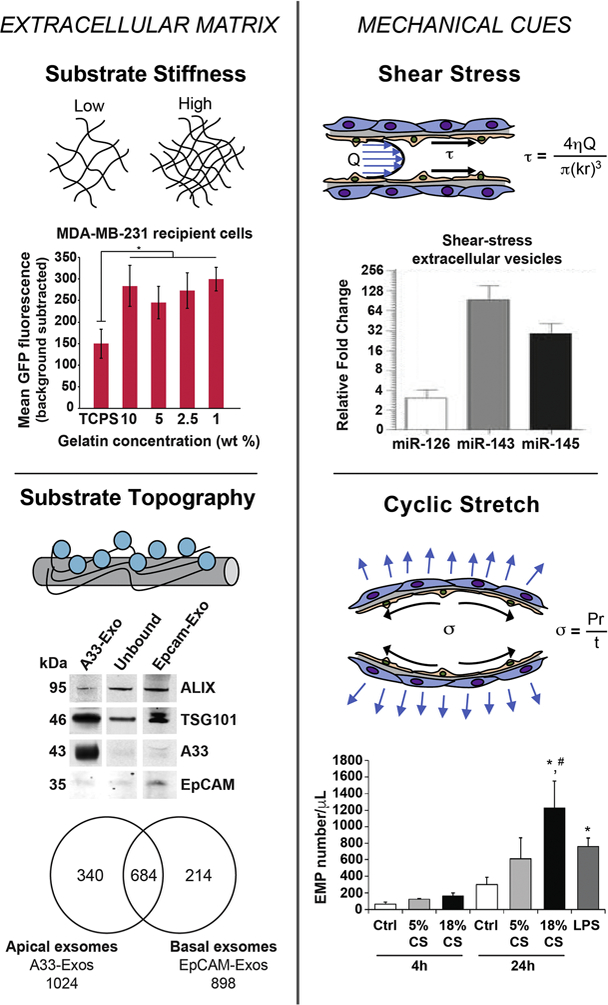
In the top left figure, breast cancer cell lines MCF-7 and MDA-MB-231 were seeded on gelatin substrate with varying stiffness. MDA-MB-231 cells seeded on gelatin matrix with different stiffness were treated with CD63-GFP-labled EVs. EV uptake was monitored following 2 hours of treatment through live-cell imaging. Seeding cell on softer gelatin matrices compared to tissue culture polystyrene (TCPS), resulted in significantly increased EV uptake for both cell lines. Adapted from Tauro et al., (2013) via open access. Bottom left figure shows western blot analysis of exosomes (10 µg) derived from LIM1863 cells from apical (EpCAM-Exos) or basolateral (A33-Exos) surfaces, which were enriched in EpCAM and A33, respectively. Both sources were also positive for exosomal markers TSG101 and Alix. Venn diagram shows differences in protein expression between EpCAM-Exos and A33-Exos. In total, 340 and 214 proteins were specifically found in A33-and EpCAM-Exos, respectively. In comparison, 684 proteins were present in both exosome sources. Adapted from Stranford et al., (2017) with permission. For the top right figure, EVs were isolated from HUVECs with or without exposure to shear-stress of 20 dynes/cm2 for 3 days. RNA analysis revealed that EVs derived from HUVECs under shear stress were highly enriched in miR-143/145, which are known to modulate SMC phenotype. Adapted from Hergenreider et al., (2012) with permission. For the bottom right figure, confluent lung-ECs were subjected to 5% CS or 18% CS for 4 and 24 hours or to LPS (1 µg/mL) for 24 hours. Lung-EC-derived microparticles (EMPs) (0.1–1 µm) were isolated from conditioned media and quantified by flow cytometry. Significant increase in EMP production is observed after 24 h exposure to 18% CS and LPS compared to the static control. Reprinted from Letsiou et al., (2015) with permission.
3.3.2. Substrate Stiffness
MSC fate can be governed by the rigidity of the cell substrate (Engler et al., 2017). For example, MSCs plated on soft substrates (0.1–1 kPa) exhibited a neuronal phenotype, whereas on stiff substrates (25–40 kPa, physiological stiffness of collagenous bone), MSCs differentiated into an osteogenic lineage. On intermediate stiffness substrates (11–30 kPa) mimicking muscle substratum, MSCs displayed a myogenic phenotype. Further, the regulation of MSC specification by matrix stiffness was shown to involve non-muscle myosin (Engler et al., 2004). This observation was supported in a study by Zemel et al. (2010), who demonstrated that non-muscle myosin alignment was dependent on matrix rigidity, implying mechanical coupling between external environment and internal cytoskeletal organization (Zemel et al., 2010).
The influence of ECM stiffness on MSC specification has also been assessed in 3D culture systems. In a 3D alginate hydrogel with integrin-binding RGD peptides, the regulation of cell fate through stiffness coincided with observations in 2D cultures. MSCs committed to an osteogenic phenotype at intermediate (11–30 kPa) elasticity and adipogenic lineage in softer (2.5–5 kPa) hydrogels (Engler et al., 2017). However, 3D culture significantly altered cell morphology and proliferation rate compared to 2D culture. MSCs cultured in spheroids have been shown to be more spherical inside and elongated outside with an overall reduction of cytoskeletal molecules and the constituted ECM. Small, rounded MSCs are prone to differentiate into adipogenic lineage, whereas large, extended MSCs tend to differentiate into osteogenic lineages (Cesarz and Tamama, 2016). Thus, differences in dimensionality of MSC culture systems can alter cellular morphology, which is a key characteristic used to determine cellular phenotypes and fates of MSCs. Furthermore, the ECM formed by MSCs in 3D spheroid culture has a very low rigidity of less than 0.1 kPa, whereas the tissue culture plastic used in 2D culture has an elastic modulus in the GPa range. As described above, changes in substrate stiffness contribute to alterations of gene expression and cell phenotype (Cesarz and Tamama, 2016), which could dictate EV production and function.
Although the impact of matrix stiffness on EV production has yet to be sufficiently investigated, de Jong et al. (2012) showed that EVs have the ability to contribute to ECM stiffness. Specifically, EVs from hypoxic endothelial cells showed increased abundances of the ECM components fibronectin, collagen-4 and −12 subunits, and LOXL2 (de Jong et al., 2012). Similar trends could be possible for EVs obtained from cells cultured on substrates with varying stiffness. IN a different study, Stranford et al. (2017) recently conducted a systematic analysis of stiffness influencing EV uptake. Their findings showed a significant increase in EV uptake by breast cancer cell lines MDA-MB-231 and MCF-7 when seeded on softer gelatin matrix compared to stiff tissue culture polystyrene (Stranford et al., 2017) (Figure 5). Thus, the substrate stiffness that a cell experiences can affect both EV secretion and uptake, which could profoundly impact net EV production. To better understand the impact of stiffness on EV generation and cargo composition, a methodical evaluation needs to be carried out.
3.4. Mechanical Cues:
3.4.1. Fluid Shear Stress
Physiologically, MSCs present in the bone marrow and in circulation are exposed to shear stresses imposed by interstitial fluid and blood, respectively (Coughlin and Niebur, 2012). In culture, Castillo and Jacobs (2010) showed that shear stress in the range of 0.5 to 2.0 Pa increases MSC proliferation (Castillo and Jacobs, 2010). In another study by Rubin et al. (2007), similar shear stress decreased adipogenic differentiation in MSCs (Rubin et al., 2007), which was corroborated by Ruiz and Chen (2018) who showed that when MSCs seeded in a collagen matrix are subjected to stress, those experiencing low shear stress in the center of the construct differentiated into adipocytes, while those experiencing higher stresses on the surface of the construct differentiated into osteoblasts (Ruiz and Chen, 2008).
Although a number of studies have determined the influence of shear stress on MSCs, their impact on secretion of EVs is yet to be fully understood. As described earlier, Watson et al. (2016) showed that HEK293 cells cultured in a hollow fiber bioreactor with slow media circulation produced 40-fold more EVs to be used for drug delivery as compared to the conventional static cell culture (Figure 1B). Apart from the rate of EV production, cargo loading has also been shown to altered when source cells are exposed to shear stress. Hergenreider et al. (2012) showed that exposure of human umbilical vein endothelial cells (HUVECs) to shear stress of 20 dynes/cm2 for 72 hours increased miR-143/145 in HUVEC EVs by 30-fold compared to only 10-fold in the cells, suggesting targeted transcription of miRNAs specifically for EV-mediated release and eventual transfer to SMCs to regulate their function (Hergenreider et al., 2012) (Figure 5). Although researchers are beginning to investigate the impact of mechanical cues such as shear stress on EV production and cargo loading, further analysis with multiple shear stresses over different time periods need to be conducted to fully decipher the impact of shear stress on MSC EV production and bioactivity.
3.4.2. Cyclic Stretch
The effect of cyclic strain has been intensively researched in cardiovascular cells, especially cardiomyocytes and SMCs, as they are physiologically exposed to periodic stretch. However, the effect of cyclic stretch on MSCs has only recently been explored. Park et al. (2004) demonstrated that cyclic equiaxial strain in MSCs downregulates smooth muscle actin (SMA) and SM-22a, markers of SMC differentiation, whereas cyclic uniaxial strain transiently increases the expression of these markers intermittently. This transient expression correlated with cell reorganization in the perpendicular direction, relative to the direction of stretch. Thus, uniaxial strain may be used to guide MSC differentiation into SMCs (Park et al., 2004). However, more frequently, researchers have investigated the impact of cyclic stretch in combination with shear stress. For example, O’Cearbhaill et al. (2008) showed that under pulsatile pressure, radial distention of 5%, and a shear stress of 10 dyn/cm2 (1 Pa), MSCs exhibit similar mechanosensitive responses to those of endothelial cells (ECs) (O’Cearbhaill et al., 2008), reorienting along the direction of flow and displaying EC-like morphology. Interestingly, genetic profiling of these MSCs revealed greater expression of SMC-associated markers SMA and calponin (O’Cearbhaill et al., 2008). In another study, however, application of 5% circumferential stretch for 4 days following 2.5 dyn/cm2 shear stress for 1 day resulted in increased MSC expression of EC marker proteins vascular endothelial cadherin (VE-cad) and von Willebrand factor (vWF), with SMA and calponin being undetectable. These contradicting results demand further investigation focused on mechanisms by which mechanical forces impact MSC fate. Regardless, it is clear that these factors significantly impact MSC phenotype and have the ability to alter MSC EV characteristics and thus should be further evaluated.
Although the focus of this article is on understanding how microenvironmental factors affect EVs derived especially from MSCs, it widely applicable to EVs derived from any cell type. Several studies have shown therapeutic potential of EVs derived from different vascular cells including ECs and SMCs towards therapeutic vascularization. Significant efforts have been made to understand the physiological processes by which EVs mediate intercellular communication between ECs and SMCs (Hergenreider et al., 2012; van Balkom et al., 2013). Moreover, the impact of fluid shear stress and cyclic strain on ECs in mature blood vessels has been widely studied and has been summarized by Hsieh et al (2014), reiterating the importance of careful consideration of microenvironmental factors for generating therapeutic EVs from any given cell type. Interestingly, in a model of acute lung injury, Letsiou et al. (2015) showed that exposing endothelial cells to 18% cyclic stretch results in ~4-fold increase in EC-derived EVs compared to static cells, each with differing protein composition (Letsiou et al., 2015) (Figure 5).
4. Conclusions
In this review, we have outlined various factors that could impact EV biogenesis and function. The work done in this area so far supports the importance of careful consideration of the parameters discussed above on EV production. However, in-depth analyses identifying mechanistic links between these factors and EV phenotype still need to be conducted. Particularly, alterations in EV-associated lipids, proteins, and/or nucleic acid cargo and their downstream targets in the recipient cells should be assessed alongside the phenotypic changes imparted by EVs in recipient cells. A complete understanding of the multitude of ways in which these factors impact EV production and specific cargo components would inform rational design of a large scale biomanufacturing platform that could yield not only increased production, but also increased potency of therapeutic EVs. Although beyond the scope of this review, it is important to note that many factors in the downstream isolation and characterization processes of EVs can also significantly impact the outcome of EV production, as discussed in numerous reports (Gudbergsson et al., 2016; Lai et al., 2016; Tauro et al., 2012). Thus, continuous efforts toward establishing standardized protocols are paramount. Finally, a special focus on choosing appropriate cell lines and cell donors as well as establishing guidelines for consistent production of therapeutic EVs will also be necessary to overcome regulatory issues facing cell-based therapies. Cooperative efforts between researchers and the US Food and Drug Administration (FDA) will be crucial to ensure successful establishment of good manufacturing practice-grade production of therapeutic EVs.
Acknowledgements:
This work was supported by the American Heart Association (16PRE30770016 to DBP), the MSCRF Postdoctoral Fellowship Program (#4301172 to MS), the National Institutes of Health (HL112905 and HL141611 to SMJ), the National Science Foundation (CBET 1750542 to SMJ), the National Institute of Biomedical Imaging and Bioengineering / National Institutes of Health (NIBIB/NIH) Center for Engineering Complex Tissues (P41 EB023833), and the University of Maryland.
Abbreviations:
- EV
extracellular vesicle
- miRNA
microRNA
- 2D
two-dimensional
- 3D
three-dimensional
- ECM
extracellular matrix
- KEC
kidney epithelial cell
- CEC
corneal epithelial cell
- RC
recombinant collagen
- MSC
mesenchymal stem cells
- ESC
embryonic stem cell
- E1MYC16.3
MYC-immortalized ESC-derived MSC line
- CMSC3A1
MYC-immortalized umbilical cord-derived MSC line
- PC12
neuronal progenitor cells
- MAPK
p38 mitogen activated protein kinase
- SMC
smooth muscle cells
- HUVEC
human umbilical vein endothelial cells
- SMA
smooth muscle actin
- EC
endothelial cell
- VE-cad
vascular endothelial cadherin
- vWF
von-Willebrand factor
- FDA
US Food and Drug Administration.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- Abagnale G, Steger M, Nguyen VH, Hersch N, Sechi A, Joussen S, Denecke B, Merkel R, Hoffmann B, Dreser A, Schnakenberg U, Gillner A, Wagner W, 2015. Surface topography enhances differentiation of mesenchymal stem cells towards osteogenic and adipogenic lineages. Biomaterials 61, 316–326. https://doi.org/http://doi.org/10.1016/j.biomaterials.2015.05.030 [DOI] [PubMed] [Google Scholar]
- Ader M, Tanaka EM, 2014. Modeling human development in 3D culture. Curr. Opin. Cell Biol 31, 23–28. https://doi.org/https://doi.org/10.1016/j.ceb.2014.06.013 [DOI] [PubMed] [Google Scholar]
- Aharon A, Tamari T, Brenner B, 2008. Monocyte-derived microparticles and exosomes induce procoagulant and apoptotic effects on endothelial cells. Thromb. Haemost 100, 878–885. https://doi.org/10.1160/TH07-11-0691 [DOI] [PubMed] [Google Scholar]
- Alvarez-Erviti L, Seow Y, Yin H, Betts C, Lakhal S, Wood MJA, 2011. Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat Biotech 29, 341–345. [DOI] [PubMed] [Google Scholar]
- Aswad H, Jalabert A, Rome S, 2016. Depleting extracellular vesicles from fetal bovine serum alters proliferation and differentiation of skeletal muscle cells in vitro. BMC Biotechnol 16, 32 https://doi.org/10.1186/s12896-016-0262-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beninson LA, Fleshner M, 2015. Exosomes in fetal bovine serum dampen primary macrophage IL-1β response to lipopolysaccharide (LPS) challenge. Immunol. Lett 163, 187–192. https://doi.org/https://doi.org/10.1016/j.imlet.2014.10.019 [DOI] [PubMed] [Google Scholar]
- Bobrie A, Colombo M, Raposo G, Théry C, 2011. Exosome Secretion: Molecular Mechanisms and Roles in Immune Responses. Traffic 12, 1659–1668. https://doi.org/10.1111/j.1600-0854.2011.01225.x [DOI] [PubMed] [Google Scholar]
- Bonab MM, Alimoghaddam K, Talebian F, Ghaffari SH, Ghavamzadeh A, Nikbin B, 2006. Aging of mesenchymal stem cell in vitro. BMC Cell Biol 7 https://doi.org/10.1186/1471-2121-7-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bovy N, Blomme B, Frères P, Dederen S, Nivelles O, Lion M, Carnet O, Martial JA, Noël A, Thiry M, Jérusalem G, Josse C, Bours V, Tabruyn SP, Struman I, 2015. Endothelial exosomes contribute to the antitumor response during breast cancer neoadjuvant chemotherapy via microRNA transfer. Oncotarget 6, 10253–66. https://doi.org/10.18632/oncotarget.3520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruno S, Grange C, Collino F, Deregibus MC, Cantaluppi V, Biancone L, Tetta C, Camussi G, 2012. Microvesicles derived from mesenchymal stem cells enhance survival in a lethal model of acute kidney injury. PLoS One 7, e33115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruno S, Grange C, Deregibus MC, Calogero RA, Saviozzi S, Collino F, Morando L, Busca A, Falda M, Bussolati B, Tetta C, Camussi G, 2009. Mesenchymal Stem Cell-Derived Microvesicles Protect Against Acute Tubular Injury. J. Am. Soc. Nephrol 20, 1053–1067. https://doi.org/10.1681/ASN.2008070798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castillo AB, Jacobs CR, 2010. Mesenchymal Stem Cell Mechanobiology. Curr. Osteoporos. Rep 8, 98–104. https://doi.org/10.1007/s11914-010-0015-2 [DOI] [PubMed] [Google Scholar]
- Cesarz Z, Tamama K, 2016. Spheroid Culture of Mesenchymal Stem Cells. Stem Cells Int 2016, 9176357 https://doi.org/10.1155/2016/9176357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Wang Y, Pan Y, Zhang L, Shen C, Qin G, Ashraf M, Weintraub N, Ma G, Tang Y, 2013. Cardiac progenitor-derived exosomes protect ischemic myocardium from acute ischemia/reperfusion injury. Biochem. Biophys. Res. Commun 431, 566–571. https://doi.org/10.1016/j.bbrc.2013.01.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen TS, Arslan F, Yin Y, Tan SS, Lai RC, Boon A, Choo H, Padmanabhan J, Lee CN, Pv De Kleijn D, Lim SK, 2011. Enabling a robust scalable manufacturing process for therapeutic exosomes through oncogenic immortalization of human ESC-derived MSCs. J. Transl. Med 9, 47 https://doi.org/10.1186/1479-5876-9-47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colter DC, Class R, DiGirolamo CM, Prockop DJ, 2000. Rapid Expansion of Recycling Stem Cells in Cultures of Plastic-Adherent Cells from Human Bone Marrow Rapid expansion of recyclil plastic-adherent cells from. Source Proc. Natl. Acad. Sci United States Am 97, 3213–3218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantinescu P, Wang B, Kovacevic K, Jalilian I, Bosman GJCGM, Wiley JS, Sluyter R, 2010. P2X7 receptor activation induces cell death and microparticle release in murine erythroleukemia cells. Biochim. Biophys. Acta -Biomembr 1798, 1797–1804. https://doi.org/10.1016/j.bbamem.2010.06.002 [DOI] [PubMed] [Google Scholar]
- Coughlin TR, Niebur GL, 2012. Fluid shear stress in trabecular bone marrow due to low-magnitude high-frequency vibration. J. Biomech 45, 2222–2229. https://doi.org/10.1016/j.jbiomech.2012.06.020 [DOI] [PubMed] [Google Scholar]
- Dalby MJ, Andar A, Nag A, Affrossman S, Tare R, McFarlane S, Oreffo ROC, 2008. Genomic expression of mesenchymal stem cells to altered nanoscale topographies. J. R Soc. Interface 5, 1055 LP-1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalby MJ, Gadegaard N, Tare R, Andar A, Riehle MO, Herzyk P, Wilkinson CDW, Oreffo ROC, 2007. The control of human mesenchymal cell differentiation using nanoscale symmetry and disorder. Nat Mater 6, 997–1003. [DOI] [PubMed] [Google Scholar]
- de Jong OG, Verhaar MC, Chen Y, Vader P, Gremmels H, Posthuma G, Schiffelers RM, Gucek M, van Balkom BWM, 2012. Cellular stress conditions are reflected in the protein and RNA content of endothelial cell-derived exosomes. J. Extracell. Vesicles 1, 10.3402/jev.v1i0.18396. https://doi.org/10.3402/jev.v1i0.18396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eitan E, Zhang S, Witwer KW, Mattson MP, 2015. Extracellular vesicle–depleted fetal bovine and human sera have reduced capacity to support cell growth. J. Extracell. Vesicles 4, 10.3402/jev.v4.26373. https://doi.org/10.3402/jev.v4.26373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engler AJ, Griffin MA, Sen S, Bönnemann CG, Sweeney HL, Discher DE, 2004. Myotubes differentiate optimally on substrates with tissue-like stiffness: pathological implications for soft or stiff microenvironments. J. Cell Biol 166, 877–887. https://doi.org/10.1083/jcb.200405004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engler AJ, Sen S, Sweeney HL, Discher DE, 2017. Matrix Elasticity Directs Stem Cell Lineage Specification. Cell 126, 677–689. https://doi.org/10.1016/j.cell.2006.06.044 [DOI] [PubMed] [Google Scholar]
- Gao J, Wang S, Wang Z, 2017. High yield, scalable and remotely drug-loaded neutrophil-derived extracellular vesicles (EVs) for anti-inflammation therapy. Biomaterials 135, 62– 73. https://doi.org/10.1016/j.biomaterials.2017.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gudbergsson JM, Johnsen KB, Skov MN, Duroux M, 2016. Systematic review of factors influencing extracellular vesicle yield from cell cultures. Cytotechnology 68, 579–592. https://doi.org/10.1007/s10616-015-9913-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Q, Xia B, Moshiach S, Xu C, Jiang Y, Chen Y, Sun Y, Lahti JM, Zhang XA, 2008. The microenvironmental determinants for kidney epithelial cyst morphogenesis. Eur. J. Cell Biol 87, 251–266. https://doi.org/10.1016/j.ejcb.2007.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hergenreider E, Heydt S, Treguer K, Boettger T, Horrevoets AJG, Zeiher AM, Scheffer MP, Frangakis AS, Yin X, Mayr M, Braun T, Urbich C, Boon RA, Dimmeler S, 2012. Atheroprotective communication between endothelial cells and smooth muscle cells through miRNAs. Nat Cell Biol 14, 249–256. [DOI] [PubMed] [Google Scholar]
- Ho JH, Ma W-H, Chen Y-F, Tseng T-C, Chen M-H, & L. OK, 2011. Cell contact accelerates replicative senescence of human mesenchymal stem cells independent of telomere shortening and p53 activation: Roles of ras and oxidative stress. Cell Transplant 20, 1209–1220. [DOI] [PubMed] [Google Scholar]
- Hsieh H-J, Liu C-A, Huang B, Tseng AHH, Wang DL, 2014. Shear-induced endothelial mechanotransduction: the interplay between reactive oxygen species (ROS) and nitric oxide (NO) and the pathophysiological implications. J. Biomed. Sci 21, 3 https://doi.org/10.1186/1423-0127-21-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isakson M, de Blacam C, Whelan D, McArdle A, Clover AJP, 2015. Mesenchymal Stem Cells and Cutaneous Wound Healing: Current Evidence and Future Potential. Stem Cells Int 2015, 831095 https://doi.org/10.1155/2015/831095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izadpanah R, Kaushal D, Kriedt C, Tsien F, Patel B, Dufour J, Bunnell BA, 2008. Long-term In vitro Expansion Alters the Biology of Adult Mesenchymal Stem Cells. Cancer Res 68, 4229–38. https://doi.org/10.1158/0008-5472.CAN-07-5272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jangamreddy JR, Haagdorens MKC, Mirazul Islam M, Lewis P, Samanta A, Fagerholm P, Liszka A, Ljunggren MK, Buznyk O, Alarcon EI, Zakaria N, Meek KM, Griffith M, 2018. Short peptide analogs as alternatives to collagen in pro-regenerative corneal implants. Acta Biomater https://doi.org/https://doi.org/10.1016/j.actbio.2018.01.011 [DOI] [PMC free article] [PubMed]
- Jarmalavičiūtė A, Tunaitis V, Pivoraitė U, Venalis A, Pivoriūnas A, 2015. Exosomes from dental pulp stem cells rescue human dopaminergic neurons from 6-hydroxy-dopamine– induced apoptosis. Cytotherapy 17, 932–939. https://doi.org/https://doi.org/10.1016/j.jcyt.2014.07.013 [DOI] [PubMed] [Google Scholar]
- Kim MH, Wu WH, Choi JH, Kim JH, Hong S-H, Jun JH, Ko Y, Lee JH, 2017. Conditioned medium from the three-dimensional culture of human umbilical cord perivascular cells accelerate the migration and proliferation of human keratinocyte and fibroblast. J. Biomater. Sci. Polym. Ed 1–15. https://doi.org/10.1080/09205063.2017.1340045 [DOI] [PubMed]
- Kucharzewska P, Christianson HC, Welch JE, Svensson KJ, Fredlund E, Ringnér M, Mörgelin M, Bourseau-Guilmain E, Bengzon J, Belting M, 2013. Exosomes reflect the hypoxic status of glioma cells and mediate hypoxia-dependent activation of vascular cells during tumor development. Proc. Natl. Acad. Sci. U. S. A 110, 7312–7. https://doi.org/10.1073/pnas.1220998110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurpinski K, Chu J, Hashi C, Li S, 2006. Anisotropic mechanosensing by mesenchymal stem cells. Proc. Natl. Acad. Sci. U. S. A 103, 16095–16100. https://doi.org/10.1073/pnas.0604182103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai RC, Arslan F, Lee MM, Sze NSK, Choo A, Chen TS, Salto-Tellez M, Timmers L, Lee CN, El Oakley RM, Pasterkamp G, de Kleijn DPV, Lim SK, 2010. Exosome secreted by MSC reduces myocardial ischemia/reperfusion injury. Stem Cell Res 4, 214–222. https://doi.org/http://dx.doi.org/10.1016/j.scr.2009.12.003 [DOI] [PubMed] [Google Scholar]
- Lai RC, Yeo RWY, Padmanabhan J, Choo A, de Kleijn DPV, Lim SK, 2016. Isolation and Characterization of Exosome from Human Embryonic Stem Cell-Derived C-Myc-Immortalized Mesenchymal Stem Cells, in: Gnecchi M (Ed.), Mesenchymal Stem Cells: Methods and Protocols Springer New York, New York, NY, pp. 477–494. https://doi.org/10.1007/978-1-4939-3584-0_29 [DOI] [PubMed] [Google Scholar]
- Lamichhane TN, Jeyaram A, Patel DB, Parajuli B, Livingston NK, Arumugasaamy N, Schardt JS, Jay SM, 2016. Oncogene Knockdown via Active Loading of Small RNAs into Extracellular Vesicles by Sonication. Cell. Mol. Bioeng 9, 315–324. https://doi.org/10.1007/s12195-016-0457-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamichhane TN, Raiker RS, Jay SM, 2015. Exogenous DNA Loading into Extracellular Vesicles via Electroporation is Size-Dependent and Enables Limited Gene Delivery. Mol. Pharm 12, 3650–7. https://doi.org/10.1021/acs.molpharmaceut.5b00364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MH, Kang JH, Lee SW, 2012. The significance of differential expression of genes and proteins in human primary cells caused by microgrooved biomaterial substrata. Biomaterials 33, 3216–3234. https://doi.org/10.1016/j.biomaterials.2012.01.034 [DOI] [PubMed] [Google Scholar]
- Letsiou E, Sammani S, Zhang W, Zhou T, Quijada H, Moreno-Vinasco L, Dudek SM, Garcia JGN, 2015. Pathologic Mechanical Stress and Endotoxin Exposure Increases Lung Endothelial Microparticle Shedding. Am. J. Respir. Cell Mol. Biol 52, 193–204. https://doi.org/10.1165/rcmb.2013-0347OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman MA GL, 1981. Density-dependent regulation of cell growth: an example of a cell-cell recognition phenomenon. J Membr Biol 63, 1–11. [DOI] [PubMed] [Google Scholar]
- Liu H, Xia X, Li B, 2015. Minireview Mesenchymal stem cell aging : Mechanisms and influences on skeletal and non-skeletal tissues 1099–1106. https://doi.org/10.1177/1535370215591828 [DOI] [PMC free article] [PubMed]
- Llorente A1, Skotland T, Sylvänne T, Kauhanen D, Róg T, Orłowski A, Vattulainen I, Ekroos K,SK, 2013. Molecular lipidomics of exosomes released by PC-3 prostate cancer cells. Biochim. Biophys. Acta 183, 1302–1309. [DOI] [PubMed] [Google Scholar]
- Merino-González C, Zuñiga FA, Escudero C, Ormazabal V, Reyes C, Nova-Lamperti E, Salomón C, Aguayo C, 2016. Mesenchymal stem cell-derived extracellular vesicles promote angiogenesis: Potencial clinical application. Front. Physiol 7, 1–9. https://doi.org/10.3389/fphys.2016.00024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura Y, Miyaki S, Ishitobi H, Matsuyama S, Nakasa T, Kamei N, Akimoto T, Higashi Y, Ochi M, 2015. Mesenchymal-stem-cell-derived exosomes accelerate skeletal muscle regeneration. FEBS Lett 589, 1257–1265. https://doi.org/10.1016/j.febslet.2015.03.031 [DOI] [PubMed] [Google Scholar]
- Nold P, Brendel C, Neubauer A, Bein G, Hackstein H, 2013. Good manufacturing practice-compliant animal-free expansion of human bone marrow derived mesenchymal stroma cells in a closed hollow-fiber-based bioreactor. Biochem. Biophys. Res. Commun 430, 325–330. https://doi.org/10.1016/j.bbrc.2012.11.001 [DOI] [PubMed] [Google Scholar]
- O’Cearbhaill ED, Punchard MA, Murphy M, Barry FP, McHugh PE, Barron V, 2008. Response of mesenchymal stem cells to the biomechanical environment of the endothelium on a flexible tubular silicone substrate. Biomaterials 29, 1610–1619. https://doi.org/10.1016/j.biomaterials.2007.11.042 [DOI] [PubMed] [Google Scholar]
- Park JS, Chu JSF, Cheng C, Chen F, Chen D, Li S, 2004. Differential effects of equiaxial and uniaxial strain on mesenchymal stem cells. Biotechnol. Bioeng 88, 359–368. https://doi.org/10.1002/bit.20250 [DOI] [PubMed] [Google Scholar]
- Patel DB, Gray KM, Santharam Y, Lamichhane TN, Stroka KM, Jay SM, 2017. Impact of Cell Culture Parameters on Production and Vascularization Bioactivity of Mesenchymal Stem Cell‐Derived Extracellular Vesicles. Bioeng. Transl. Med [DOI] [PMC free article] [PubMed]
- Qu Y, Ramachandra L, Mohr S, Franchi L, Harding CV, Nunez G, Dubyak GR, n.d. P2X7 Receptor-Stimulated Secretion of MHC Class II-Containing Exosomes Requires the ASC/NLRP3 Inflammasome but Is Independent of Caspase-1 1 https://doi.org/10.4049/jimmunol.0802968 [DOI] [PMC free article] [PubMed]
- Rubin CT, Capilla E, Luu YK, Busa B, Crawford H, Nolan DJ, Mittal V, Rosen CJ, Pessin JE, Judex S, 2007. Adipogenesis is inhibited by brief, daily exposure to high-frequency, extremely low-magnitude mechanical signals. Proc. Natl. Acad. Sci. U. S. A 104, 17879–17884. https://doi.org/10.1073/pnas.0708467104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz SA, Chen CS, 2008. Emergence of patterned stem cell differentiation within multicellular structures. Stem Cells 26, 2921–2927. https://doi.org/10.1634/stemcells.2008-0432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santoro M, Lamhamedi-Cherradi S-E, Menegaz BA, Ludwig JA, Mikos AG, 2015. Flow perfusion effects on three-dimensional culture and drug sensitivity of Ewing sarcoma. Proc. Natl. Acad. Sci. U. S. A 112, 10304–10309. https://doi.org/10.1073/pnas.1506684112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekiya I, Larson BL, Smith JR, Pochampally R, Cui J, Prockop DJ, 2002. Expansion of Human Adult Stem Cells from Bone Marrow Stroma: Conditions that Maximize the Yields of Early Progenitors and Evaluate Their Quality. Stem Cells 20, 530–541. https://doi.org/10.1634/stemcells.20-6-530 [DOI] [PubMed] [Google Scholar]
- Shelke GV, Lässer C, Gho YS, Lötvall J, 2014. Importance of exosome depletion protocols to eliminate functional and RNA-containing extracellular vesicles from fetal bovine serum. J. Extracell. Vesicles 3, 10.3402/jev.v3.24783. https://doi.org/10.3402/jev.v3.24783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterzenbach U, Putz U, Low L-H, Silke J, Tan S-S, Howitt J, 2018. Engineered Exosomes as Vehicles for Biologically Active Proteins. Mol. Ther 25, 1269–1278. https://doi.org/10.1016/j.ymthe.2017.03.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stranford DM, Hung ME, Gargus ES, Shah RN, Leonard JN, 2017. A Systematic Evaluation of Factors Affecting Extracellular Vesicle Uptake by Breast Cancer Cells. Tissue Eng Part A 23, ten.tea.2017.0158. https://doi.org/10.1089/ten.tea.2017.0158 [DOI] [PMC free article] [PubMed]
- Takeda YS, Xu Q, 2015. Neuronal differentiation of human mesenchymal stem cells using exosomes derived from differentiating neuronal cells. PLoS One 10 https://doi.org/10.1371/journal.pone.0135111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tauro BJ, Greening DW, Mathias RA, Ji H, Mathivanan S, Scott AM, Simpson RJ, 2012. Comparison of ultracentrifugation, density gradient separation, and immunoaffinity capture methods for isolating human colon cancer cell line LIM1863-derived exosomes Methods 56, 293–304. https://doi.org/http://doi.org/10.1016/j.ymeth.2012.01.002 [DOI] [PubMed] [Google Scholar]
- Tauro BJ, Greening DW, Mathias RA, Mathivanan S, Ji H, Simpson RJ, 2013. Two Distinct Populations of Exosomes Are Released from LIM1863 Colon Carcinoma Cell-derived Organoids. Mol. Cell. Proteomics 12, 587–598. https://doi.org/10.1074/mcp.M112.021303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian Y, Li S, Song J, Ji T, Zhu M, Anderson GJ, Wei J, Nie G, 2014. A doxorubicin delivery platform using engineered natural membrane vesicle exosomes for targeted tumor therapy. Biomaterials 35, 2383–2390. https://doi.org/https://doi.org/10.1016/j.biomaterials.2013.11.083 [DOI] [PubMed] [Google Scholar]
- Valadi H, Ekström K, Bossios A, Sjöstrand M, Lee JJ, Lötvall JO, 2007. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat. Cell Biol 9, 654–659. https://doi.org/10.1038/ncb1596 [DOI] [PubMed] [Google Scholar]
- van Balkom BWM, de Jong OG, Smits M, Brummelman J, den Ouden K, de Bree PM, van Eijndhoven MAJ, Pegtel DM, Stoorvogel W, Würdinger T, Verhaar MC, 2013. Endothelial cells require miR-214 to secrete exosomes that suppress senescence and induce angiogenesis in human and mouse endothelial cells. Blood 121, 3997 LP-4006. [DOI] [PubMed] [Google Scholar]
- Wang X, Omar O, Vazirisani F, Thomsen P, Ekstro K, 2018. Mesenchymal stem cell-derived exosomes have altered microRNA profiles and induce osteogenic differentiation depending on the stage of differentiation 4–6. [DOI] [PMC free article] [PubMed]
- Watson DC, Bayik D, Srivatsan A, Bergamaschi C, Valentin A, Niu G, Bear J, Monninger M, Sun M, Morales-Kastresana A, Jones JC, Felber BK, Chen X, Gursel I, Pavlakis GN, 2016. Efficient production and enhanced tumor delivery of engineered extracellular vesicles. Biomaterials 105, 195–205. https://doi.org/10.1016/j.biomaterials.2016.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wexler SA, Donaldson C, Denning-Kendall P, Rice C, Bradley B, Hows JM, 2003. Adult bone marrow is a rich source of human mesenchymal “stem” cells but umbilical cord and mobilized adult blood are not. Br. J. Haematol 121, 368–374. https://doi.org/10.1046/j.1365-2141.2003.04284.x [DOI] [PubMed] [Google Scholar]
- Zemel A, Rehfeldt F, Brown AEX, Discher DE, Safran SA, 2010. Optimal matrix rigidity for stress fiber polarization in stem cells. Nat. Phys 6, 468–473. https://doi.org/10.1038/nphys1613 [DOI] [PMC free article] [PubMed] [Google Scholar]


