Highlights
-
•
We examine relationship of brain iron and cognition in childhood and adolescence.
-
•
Estimates of iron in basal ganglia and hippocampus from R2* relaxometry.
-
•
Age-related increase in non-heme iron content was observed in multiple brain areas.
-
•
Greater iron content was indicative of improved cognitive ability.
-
•
Non-heme iron appears to play a critical role in neural and cognitive development.
Keywords: Non-heme iron, Development, Cognition, R2* relaxometry
Abstract
Non-heme iron is a vital metabolic cofactor for many core processes of brain development including myelination, dendritogenesis, and neurotransmitter synthesis, and accumulates in the brain with age. However, little is known about development-related differences in brain iron and its association with emerging cognitive abilities during formative years. In this study, we estimated brain iron via R2* relaxometry in children ages 7–16 (N = 57; 38 females) and examined its relation to age-related differences in cognitive ability. As we hypothesized, age correlated positively with iron content in the hippocampus and across subregions of the basal ganglia. The magnitude of age differences in iron content differed between regions such that the largest effects were observed in basal ganglia subregions: globus pallidus, substantia nigra, caudate nucleus, and putamen, as compared to values obtained for the hippocampus and red nucleus. We did not observe sex or hemispheric differences in iron content. Notably, greater brain iron content was associated with both faster processing speed and higher general intelligence, and shared 21.4% of the age-related improvement in processing speed and 12.5% of the improvement in general intelligence. These results suggest that non-heme iron plays a central neurobiological role in the development of critical cognitive abilities during childhood.
1. Introduction
Iron is the most abundant metallic ion within the brain and serves a fundamental role in cellular processes integral to maintaining healthy brain function. While important to the whole body, the brain is especially sensitive to variation in iron availability (Hare et al., 2013), in part due to the high metabolic demands of the brain that are supported by the availability and action of iron. The brain accounts for roughly 20% of whole-body metabolic energy expenditure in adulthood, and even higher proportions, 30–50%, in early human development (Chugani, 1998; Kuzawa et al., 2014). Metabolic activity differentiates brain regions (Dallman, 1986) and this landscape changes with age (de Deungria et al., 2000). Due to its role in metabolic energy production, brain iron may play a critical role in maturation of the human nervous system (Larsen and Luna, 2015; Lozoff and Georgieff, 2006; Wang et al., 2012). Presumably to meet the demand of these processes, non-heme iron accumulates quickly across the first two decades of life and continues at a lesser rate across the lifespan (Aquino et al., 2009; Hallgren and Sourander, 1958; Li et al., 2014; Wang et al., 2012). The advent of non-invasive neuroimaging methods sensitive to brain iron content now accommodates in vivo study of non-heme iron in the developing brain. Here we aim to evaluate development-related differences in regional brain iron content and its relation to cognitive functions.
A distinction is made between heme and non-heme forms of biological iron. Heme iron is a component of hemoglobin that binds oxygen and is present in flowing or accumulated blood. Non-heme iron is stored within many cell types and participates in essential cellular and mitochondrial functions, including production of adenosine triphosphate (ATP) and DNA synthesis (Lozoff and Georgieff, 2006), and in the brain supports neurotransmission (Youdim and Yehuda, 2000) and myelination (Bartzokis, 2011; Connor and Menzies, 1996; Moos and Morgan, 2004; Todorich et al., 2009). Iron accumulation outside of binding complexes, and ensuing abundance of oxidative stress, disrupts mitochondria and cellular functions, which is a known putative mechanism of neurodegeneration in aging and related disease (Harman, 1956; Schenck and Zimmerman, 2004; Zecca et al., 2004; Raz and Daugherty, 2017). These observations have led some to suggest that non-heme iron content in neural tissue may serve as a useful proxy for brain health, and a biomarker of neurological deficits or disease (Schenck and Zimmerman, 2004). The presumed mechanism of neural insult is an aberration of fundamental cellular processes that are necessary for normal brain health and function. Whereas abundance of iron and related oxidative stress may drive neurodegeneration in adult aging, insufficient non-heme iron is expected to impair neural cognitive development. Despite the fundamental role of non-heme iron, the majority of in vivo non-heme brain iron studies have focused on neurodegeneration in later life (Daugherty and Raz, 2015), with lesser attention paid to brain iron in neural and cognitive development across the lifespan. In large part, what is known about the role of iron in neurological development comes from peripheral blood measures of ferritin in blood serum, which may not relate directly to non-heme iron concentrations in specific brain regions (Li et al., 2014; Rao and Georgieff, 2002).
The few investigations of non-heme iron in vivo and associations to performance in cognitive tasks during childhood suggest subcortical brain iron may serve an important role in the development of cognitive processes. Recent studies of typically developing children have shown that caudate iron is positively correlated with spatial intelligence (Carpenter et al., 2016) and working memory ability (Darki et al., 2016) in children, and decreased peripheral ferritin levels and thalamic iron have been observed in children with ADHD (Adisetiyo et al., 2014; Cortese et al., 2012). While these studies have garnered important insight into the trajectory of non-heme iron availability during childhood and its role in emergent cognitive abilities, much work remains to be done to elucidate the relationship of brain iron, brain maturation, and cognitive development in typically developing children and adolescents.
The regions of the basal ganglia have been the primary interest in studies of non-heme iron because they show robust and quantifiable levels of iron content that vary by individual, as seen in post-mortem (Hallgren and Sourander, 1958; Langkammer et al., 2010) and in vivo imaging studies (Aquino et al., 2009; Carpenter et al., 2016; Langkammer et al., 2010; Larsen and Luna, 2015; Li et al., 2014; Wang et al., 2012). Further, these regions are central to cognitive abilities that develop across childhood and adolescence, such as processing speed, cognitive-motor control, reward processing, and working memory function (Brown et al., 1997; Burgaleta et al., 2014; Carpenter et al., 2016; Darki et al., 2016; Khedr et al., 2008; MacDonald et al., 2014; Munoz and Humeres, 2012). These functions, as well as hippocampal-dependent relational memory function, contribute to general intelligence ability (Amat et al., 2008; Rhein et al., 2014; Sandman et al., 2014). Moreover, basal ganglia function appears to be especially vulnerable to deviation in iron availability, as implicated in iron deficient states (Lozoff, 2011; Lozoff et al., 2006; Lozoff and Georgieff, 2006; Sachdev, 1993). In particular, the striatal dopamine pathway is highly dependent on non-heme iron for neurotransmission (Adisetiyo et al., 2014; Erikson et al., 2000; Wiesinger et al., 2007), D2 receptor expression (Beard, 2003; Jellen et al., 2013), dopamine metabolism (Yehuda and Youdim, 1989; Youdim et al., 1989; Youdim and Green, 1978), and dopamine neuron excitability (Jellen et al., 2013). Interactive effects of non-heme iron abundance and dopaminergic system function are likely to contribute to maturation of dopamine-dependent cognitive processes. By comparison, less is known about hippocampal iron and cognitive development. Research in older adults suggests increased hippocampal iron relates to deficits in relational memory function (Rodrigue et al., 2013) and longitudinal decline in spatial navigation ability (Raz and Daugherty, 2017). Because the hippocampus is superbly sensitive to fluctuations in metabolic health even in adolescence (Yates et al., 2012), it is plausible that the availability and action of iron within this region may contribute to cognitive development.
R2* relaxometry is a non-invasive MRI technique that is sensitive to brain iron content (Daugherty and Raz, 2015; Langkammer et al., 2010; Vymazal et al., 1992). R2* relaxation time (1/T2*) is directly proportional to iron content in wet tissue (Langkammer et al., 2010), and greater specificity of the signal to iron is observed in subcortical gray matter as compared to white matter (Haacke et al., 2011). The current study employs R2* relaxometry to assess non-heme iron content in 57 children and adolescents in the hippocampus, basal ganglia (caudate nucleus, putamen, globus pallidus), red nucleus and substantia nigra. We examine the primary hypotheses that brain iron content increases across developmental age, and that greater brain iron content correlates with better cognitive performance.
2. Methods
2.1. Participants
This study reports on 57 children and adolescents (38 female), ages 7–16 years (M = 12.5, SD = 2.36) and of mixed race and ethnicity: 42% African American, 42% Caucasian, 7% Hispanic, and 9% biracial. Standardized intelligence scores (Kaufman Brief Intelligence Test, Second edition) of the whole sample indicated average intelligence (M = 100, SD = 17.2). Participants were recruited from Southeast Michigan through Wayne State University’s (WSU) website, Craigslist (Metro Detroit), and printed flyers. The Institutional Review Board of Wayne State University approved the study. Parental informed written consent and child/ adolescent assent were obtained prior to study participation. All participants spoke English as a first language. Participants were excluded if their parent/caregiver reported a history of head trauma, neurologic condition, learning disability, or contraindication for magnetic resonance imaging (MRI) for the child. All but one was right-handed. No subjects reported any pertinent clinical diagnoses, such as iron deficiency or anemia.
2.2. Cognitive measures
Unstandardized intelligence quotient (IQ) verbal and non-verbal scores, calculated from the Kaufman Brief Intelligence Test, Second Edition (Kaufman and Kaufman, 2004) were used as a measure of general cognitive function. The Woodcock-Johnson III Normative Update (W-J III NU) was administered to measure processing speed (Gs), including the Visual-Matching and Cross-Out subtests, while Digit Symbol was measured with the WISC III (Weschler, 1991; Woodcock et al., 2001). See Table 1 for a summary of scores by measure.
Table 1.
Summary of cognitive measures.
| Intelligent Quotient | Mean ± SD |
|---|---|
| Verbal | 64 ± 14 |
| Non-Verbal | 31 ± 5 |
| Composite | 197 ± 25 |
| Processing Speed | |
| Digit Symbol | 50 ± 15 |
| Cross Out | 19 ± 5 |
| Visual Matching | 40 ± 8 |
Average unstandardized WISC-III scores and WJ-NU processing speed scores by subtest.
2.3. Image acquisition and processing
MRI was performed on a Siemens Verio 3 T scanner equipped with a 12-channel headcoil. Regional iron content was estimated in vivo from an 11-echo multiple gradient-echo sequence: voxel size 0.5 × 0.5 × 2 mm; echo times (TE) = 5.68–31.38 ms with inter-echo interval of 2.57 ms; repetition time = 37 ms; flip angle = 15°; bandwidth = 465 Hz/pixel; field of view = 512 × 384. T2* data were processed using Signal Processing in NMR (SPIN software; MR Innovations, Inc., Detroit, MI, USA; http://www.mrinnovations.com/spin-lite; last accessed 9/14/2017), following our prior work (Daugherty et al., 2015). T2* maps were interpolated to a voxel size of 1 × 1 × 2 mm to improve signal-to-noise ratio and estimated by a maximum-likelihood fit function for TE-dependent exponential decay. On T2*-weighted images, regions with higher magnetic susceptibility, and thus higher iron content, appear darker. While T2* values do not translate directly to absolute iron concentration measured in wet tissue (Sehgal et al., 2005), susceptibility of subcortical gray matter is shown to be directly correlated with iron content (Langkammer et al., 2010; Schweser et al., 2011). R2* values were calculated from the inverse of the measured T2* (1/T2*), where higher values indicate greater iron content.
2.4. Regions of interest
We measured brain iron in six regions of interest (ROIs): caudate nucleus (Cd), globus pallidus (GP), putamen (Pt), hippocampus (Hc), red nucleus (RN), and substantia nigra (SN). Magnitude images acquired from the first echo of the multiple gradient-echo sequence were used to identify ROIs for each participant, following prior work (Daugherty et al., 2015). To sample representative, average T2* from each ROI, a standardized, circular mask of 24 pixels was placed in the center of each ROI, in each hemisphere and across three contiguous axial slices (adapted after (Rodrigue et al., 2013); see Fig. 1 for example mask placement. Differences in intensity value on T2* and corresponding magnitude images can bias visualization and segmentation of regional boundaries (Lorio et al., 2014). The masking procedure, as a representative average of the ROI, avoids this potential confound. In addition, the manual masking procedure avoided inclusion of partial voluming of adjacent white matter, cerebrospinal fluid and visualized vasculature in the measure of T2*. This process was implemented separately in left and right hemispheres and was thus sensitive to cross-hemispheric variation in neuroanatomy. The reliability of the mask placement procedure between two raters (J.H. and K.M.) was tested with an intra-class correlation coefficient (ICC(2)), assuming random rater error (Shrout and Fleiss, 1979). A sample of 10 participants was used to establish reliability for Cd, GP, Hc, RN, and SN, and a sample of n = 14 for Pt. ICC(2) was greater than 0.85 for left and right hemisphere T2* measures within each ROI and ICC(2) > 0.90 for bilateral averages.
Fig. 1.
Measurement of T2* signal in the basal ganglia and hippocampus Top: Magnitude images from the multiple gradient-echo sequence (axial orientation) were used as anatomical reference to bilaterally place circular masks within the center of six ROIs, across 3 contiguous slices (Colors: red = caudate nucleus (Cd), orange = globus pallidus (GP), yellow = putamen (Pt), fuchsia = hippocampus (Hc), green = red nucleus (RN), cyan= substantia nigra (SN)). Bottom: ROI masks were then transferred onto T2* maps to extract regional values, following correction for image noise. Darker regions correspond to increased R2* (1/T2*) and thus greater iron content.
2.5. Validation of iron estimates against post mortem quantified priors
A comparison was conducted to validate the assumption that extracted R2* values represent non-heme iron content. A prior study by Hallgren and Sourander (1958) reports post-mortem concentrations (per 100 g fresh weight) of non-heme iron from direct measurements of the Cd, GP, and Pt as a function of age. Using equations published in their study and the known participant ages at the time of scanning, we compared the post-mortem based estimates to the observed R2* relaxometry measures collected in the study.
2.6. Analytic approach
First, to test for regional differences in iron content and differential age effects by region, we used a 2 (hemisphere) × 6 (ROI) repeated measures general linear model (GLM). Age (centered at the sample mean), age2 as a test of possible non-linear age differences, and sex were entered as covariates. If the age2 term was not significant beyond the linear effects of age alone, it was removed from the model, and all interactions between age and sex were tested and removed if not significant. In a second model, we tested for age differences in brain iron content and the relation to cognitive measures. Prior to modeling, the multiple cognitive measures were submitted to a principal components analysis (varimax rotation) to create composites representing processing speed and general intelligence (verbal and non-verbal, unstandardized IQ scores). Because the cognitive measures represent functions that are not specific to singular brain regions, we tested hypotheses with a composite of R2* that represented basal ganglia iron in general, calculated in a second PCA with a single component solution of all regions. This general component score was included as a predictor in univariate GLM analyses to test general brain iron content, age, and sex predicting differences in composite processing speed and general intelligence.
We observed strong positive correlations between age, composite of general iron content, and cognitive measures, which constitutes collinearity that will bias multivariate analyses that evaluate the effects of age and brain iron content on cognition within the same model. However, the presence of collinearity here is in line with our assumptions of a theoretical framework that includes age-related brain iron accumulation as part of typical cognitive development. In this regard, a commonality analysis is an exploratory step (Linderberg and Pötter, 1998) that quantifies the shared variance between age and iron content predicting cognitive outcomes. To a limited extent, we can consider this commonality representing the developmental process we aim to describe. Mediation analyses of developmental effects require assumptions of temporal precedence and causality, which a cross-sectional study design cannot suffice and the resulting estimates are indeterminate (Maxwell and Cole, 2007). A commonality analysis does not carry the same assumptions and offers a conservative account of the covariance, and as applied here identified the unique effect of age and the shared effect of age and brain iron content on cognitive ability. This was accomplished in a series of hierarchical linear regressions to identify the unique and shared variance components (Linderberg and Pötter, 1998). To provide further information of the effects of age and brain iron content predicting cognition, regression models were bootstrapped with bias-correction (5000 draws) to produce 95% confidence intervals (BS 95% CI), which can be interpreted as further evidence in support of the effect (α = 0.05) when not overlapping with zero. Because hypotheses pertaining to processing speed and general intelligence were tested separately, a Bonferroni correction for multiple comparisons was applied (α’ = 0.025) to the regression coefficients.
3. Results
3.1. Regional age differences in iron content
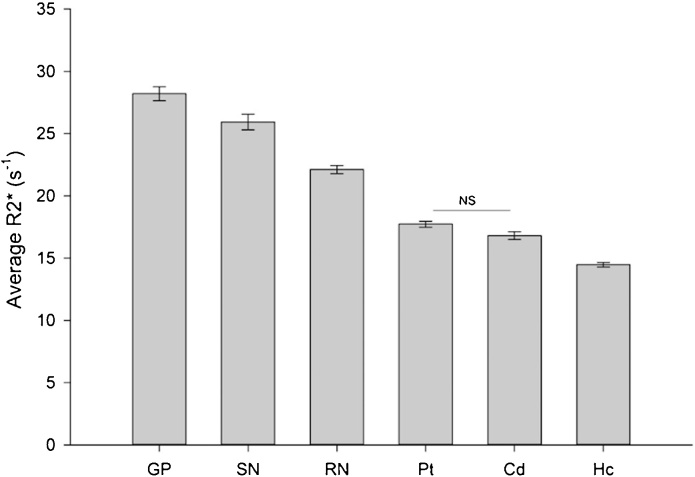
Observed R2* relaxometry values agreed with age-based post-mortem iron levels for Cd (r = 0.80, p < 0.001; BS 95% CI: 0.41/0.89), GP (r = 0.81, p < 0.001; BS 95% CI: 0.52/0.91), and Pt (r = 0.77, p < 0.001; BS 95% CI: 0.35/0.90) provided by Hallgren and Sourander (Hallgren & Sourander). Hemispheres were similar overall in iron content across regions, F(1,54) = 3.11, p = 0.08, as were age differences per hemisphere, F(1,54) = 1.33, p = 0.26, and all further analysis considered effects in bilateral average R2* per region. Regions differed in gross iron content, F(5,50) = 13.28, p < 0.001 (see Fig. 2). In rank order, R2* was greatest in the globus pallidus, substantia nigra, red nucleus, putamen, caudate, and least in the hippocampus (see Table 2).
Fig. 2.
Regional variation in iron content in youth.
All regions differed from each other (p’s < 0.05) with exception of Pt and Cd. Error bars represent standard error of the mean. Abbreviations: globus pallidus, GP; substantia nigra, SN; red nucleus, RN; putamen, Pt; caudate, Cd; hippocampus, Hc.
Table 2.
Measured R2* (1/T2*) by region of interest.
| Region of Interest | Left Hemisphere | Right Hemisphere | Bilateral Average |
|---|---|---|---|
| Globus Pallidus | 27.7 ± 4.4 | 29.1 ± 5.3 | 28.4 ± 4.3 |
| Substantia Nigra | 24.9 ± 6.1 | 27.8 ± 5.1 | 26.3 ± 4.7 |
| Red Nucleus | 22.0 ± 3.6 | 22.6 ± 2.7 | 22.3 ± 2.5 |
| Putamen | 17.8 ± 2.5 | 17.98 ± 2.7 | 17.9 ± 1.9 |
| Caudate Nucleus | 17.3 ± 3.1 | 16.6 ± 3.0 | 16.9 ± 2.5 |
| Hippocampus | 14.2 ± 1.8 | 14.8 ± 1.8 | 14.5 ± 1.5 |
Increased magnetic susceptibility corresponds with higher non-heme iron content. Values displayed here as M ± SD.
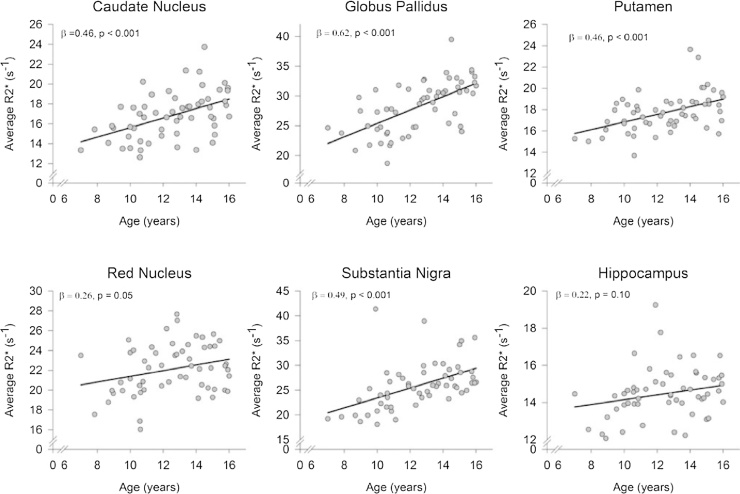
There was no evidence of non-linear age differences in iron content (F(1, 51) = 1.70, p = 0.20) nor differentiating between regions (age2 × region: F(5, 47) = 0.98, p = 0.44), and therefore the quadratic age term was removed from the model and only linear age differences were further considered. Sex was unrelated to individual differences in regional iron content (F(1, 53) = 0.44, p = 0.51) and did not interact with linear age effects (age × sex: F(1, 53) = 2.87, p = 0.10), and the interaction term was removed from the model. Age correlated positively with iron content across regions, F(1,54) = 48.44, p < 0.001, and the magnitude of linear age differences in iron content differed between regions (age × region): F(5,50) = 5.40, p < 0.001 (see Fig. 3). In rank order, age differences were largest in the globus pallidus (r = 0.62, p < 0.001), substantia nigra (r = 0.50, p < 0.001), caudate nucleus (r = 0.48, p < 0.001), and putamen (r = 0.47, p < 0.001), which were all of similar magnitude in this sample (all pair-wise Steiger Z* > 1.35, p = 0.09). Age differences were smaller, in the red nucleus (r = 0.27, p = 0.05) and in the hippocampus (r = 0.22, p = 0.10), the latter having only demonstrated a non-significant trend of age differences. Age differences in the red nucleus and hippocampus were smaller than in other regions examined (all pair-wise Steiger Z* < -1.62, p < 0.05; see Fig. 4).
Fig. 3.
Regional differences in the age-R2* iron content measurements correlation strength. *Indicates standardized regression coefficients that differed, p < 0.05, from each other, or from zero. Abbreviations: globus pallidus, GP; substantia nigra, SN; red nucleus, RN; putamen, Pt; caudate, Cd; hippocampus, Hc.
Fig. 4.
Age-related increases in regional iron content as measured by R2* relaxometry.
Iron content was significantly positively related to age in the caudate, globus pallidus, putamen, and substantia nigra. Age differences were smaller in the red nucleus and hippocampus.
3.2. Cognitive correlates to brain iron content
To test the functional relevance of age-related differences in brain iron content, we examined associations between measures of brain iron and both processing speed and general intelligence (verbal and non-verbal IQ unstandardized scores). PCA was used to derive a composite score representing general brain iron content, processing speed and general intelligence. The PCA of cognitive scores produced a two-component solution: processing speed measures loaded highly onto a single component (>0.87), and verbal and non-verbal IQ scores (both = 0.89) on a separate component. Iron content in the Cd (r = 0.29, p = 0.03), Pt (r = 0.34, p < 0.01), GP (r = 0.27, p = 0.04), and SN (r = 0.42, p < 0.01) correlated with general intelligence scores, whereas only Cd (r = 0.50, p < 0.001) and Pt (r = 0.35, p < 0.01) iron was correlated with processing speed, and Hc (both p > 0.69) and RN (both p > 0.33) iron were unrelated to cognitive performance. Because we did not have specific hypotheses per region, we created a composite of general iron content across all ROIs (see Table 3 for loadings) for further hypothesis testing.
Table 3.
Summary of Principal Component Analyses (PCA) of R2* signal, IQ, and processing speed.
| Component | Eigenvalue | % Variance |
|---|---|---|
| Global R2* signal | 2.51 | 41.8 |
| Intelligence Quotient (IQ) | 1.61 | 80.5 |
| Processing Speed (Gs) | 2.35 | 78.5 |
| Global R2* signal | Loading | |
| Caudate nucleus | 0.81 | |
| Globus pallidus | 0.75 | |
| Putamen | 0.69 | |
| Hippocampus | 0.59 | |
| Red nucleus | 0.58 | |
| Substantia nigra | 0.37 | |
| Intelligence Quotient (IQ) | ||
| Verbal | 0.89 | |
| Non-verbal | 0.89 | |
| Processing Speed (Gs) | ||
| Digit Symbol | 0.9 | |
| Cross-Out | 0.89 | |
| Visual Matching | 0.87 | |
All regions exhibited high loadings onto their respective component. Global R2* signal, IQ, and Gs, were used to assess the impact of global iron content on cognitive ability in a commonality analysis.
Greater general brain iron content predicted faster processing speed (F(1,55) = 5.96, p = 0.02, α’ = 0.025; BS 95% CI: 0.10/0.53) and better general intelligence (F(1,55) = 7.92, p = 0.01, α’ = 0.025; BS 95% CI: 0.09/0.71). Older age was also associated with faster processing speed (F(1,55) = 35.59, p < 0.001, α’ = 0.025; BS 95% CI: 0.19/0.34) and higher general intelligence (F(1, 55) = 23.38, p < 0.001, α’ = 0.025; BS 95% CI: 0.14/0.32; see Fig. 5). Commonality analysis identified that greater iron content shared 21.4% of the age-related improvement in processing speed and 12.5% of the improvement in general intelligence. Sex was included as a covariate in all models but was unrelated to either processing speed or general intelligence (all p > 0.06). Taken together, older developmental age was associated with greater brain iron content and this accounts for 21.4% of age-related improvement in processing speed, and 12.5% of age-related improvement in general intelligence.
Fig. 5.
Correlations between age and general brain iron content with processing speed and general intelligence.
Age positively correlated with faster processing speed (A) and higher general intelligence (B), and greater iron content was positively correlated with higher cognitive ability (processing speed (C) and general intelligence (D)).
4. Discussion
We investigated childhood age differences in brain iron content and its relation to cognitive ability. R2* relaxometry was used to non-invasively estimate non-heme iron in vivo within the basal ganglia and hippocampus of 57 typically developing children and adolescents, ages 7–16 years old. Iron content varied significantly between brain regions and iron content in all regions, except the hippocampus, was positively correlated with age. The magnitude of age differences varied between regions, with larger effects in regions of the basal ganglia, as compared to the hippocampus. Older age and greater composite iron content were each associated with faster processing speed and higher general intelligence.
Relative regional differences of R2* observed in this sample were similar to those observed in other studies of child and adult brains across the lifespan, where the globus pallidus has consistently shown the highest R2* signal (Ghadery et al., 2015; Haacke et al., 2010; Langkammer et al., 2010; Li et al., 2014) and highest concentration per mg/kg fresh weight, followed by the substantia nigra, red nucleus, putamen, and caudate nucleus (Darki et al., 2016; Hallgren and Sourander, 1958; Langkammer et al., 2010, 2012; Persson et al., 2015). The abundance of iron in the basal ganglia has been suggested to support dopamine neurotransmission that is a fundamental property of these regions (Adisetiyo et al., 2014; Erikson et al., 2000; Wiesinger et al., 2007). The striatum and its numerous connections to the cortex undergo protracted development into adolescence, which may in part be dependent upon the availability and action of regional iron content (Carpenter et al., 2016; Darki et al., 2016; Larsen and Luna, 2015).
This is consistent with the notion that non-heme iron is a vital metabolic cofactor for core cellular mechanisms and neurobiological processes necessary for proper brain development, including myelination and neurotransmission (Beard, 2003; Georgieff, 2011; Lozoff and Georgieff, 2006); Lozoff and Georgieff, 2006). Brain metabolism and volume continue to increase from birth until adulthood (Kuzawa et al., 2014; Lenroot and Giedd, 2006; Narvacan et al., 2017; Wierenga et al., 2014), and so the demand for non-heme iron is expected to also increase with age. Post-mortem investigations (Hallgren and Sourander, 1958), as well as MRI studies in adults (Aquino et al., 2009; Li et al., 2014) and children (Carpenter et al., 2016; Larsen and Luna, 2015), support a positive correlation between age and iron content and differences in age-related magnitude by region (Daugherty and Raz, 2013). In this sample of normally-developing children and adolescents, age-related increases were significant across the majority of areas examined, but smaller by comparison in the red nucleus, and non-significant in the hippocampus. A possible explanation for this is that the red nucleus and hippocampus acquire non-heme iron at a lesser rate across development. An alternative is that developmental change in these regions peak at different time points in development respectively, as a result of change in regional volume (Narvacan et al., 2017) or metabolic demand (de Deungria et al., 2000).
The basal ganglia and hippocampus support a number of cognitive processes, including higher-order cognitive functions such as general intelligence (Amat et al., 2008; Rhein et al., 2014). Indeed, greater iron content (collectively in all regions) shared 21.4% of the age-related differences in processing speed and 12.5% of variability in general intelligence. Older children and adolescents with greater iron content performed better on tests of these cognitive abilities. Striatal network connectivity and efficiency of dopamine signaling correlate with these cognitive functions (Dunnett, 2005), and the accumulation of iron content in these regions during adolescence appears to follow the protracted development of the system (Larsen and Luna, 2015; Rosenberg and Lewis, 1995; Teicher et al., 1995). Therefore, disruption of non-heme iron homeostasis within subcortical nuclei may confer differences across a large array of cognitive abilities, given the role of the basal ganglia and hippocampus in widely distributed neural cognitive networks (Brown et al., 1997; Grahn et al., 2009; Middleton and Strick, 1994, 2000; Moretti et al., 2017).
Our findings, and others (Carpenter et al., 2016; Darki et al., 2016; Larsen and Luna, 2015), suggest that non-heme iron and its regulatory mechanisms play a biologically supportive role during early life cognitive development. We found here that greater iron content supported better cognitive ability, which is the inverse to evidence from cognitive aging studies that describe higher iron content correlated with worse cognitive ability (Daugherty et al., 2015; Penke et al., 2012). Excessive iron deposition is also often seen in neurodevelopmental disorders and degradation of cognitive processes (Daugherty et al., 2015). Better understanding of the role of non-heme iron in supporting energy-expensive neurological processes, such as cognition, is important for building a comprehensive model of neurological health across the lifespan, with an emphasis on sensitive periods of development.
The reported evidence should be considered with the following limitations. First, the study is cross-sectional and the commonality analysis was explorative, therefore we cannot evaluate developmental change nor offer conclusions about mediation of age on developing cognition via brain iron (Lindenberger et al., 2011). Longitudinal study is necessary to assess age-related changes in iron content and to investigate the indirect relationship between age and cognitive development via changes in iron content. With these considerations in mind, we characterized the degree of shared variance between age, brain iron content and cognitive ability as a description of individual differences during this developmental period. Second, the sample size is modest. To address possible power concerns, we reduced the number of comparisons by focusing on theoretically-driven regions of interest and provided bootstrapped bias-corrected 95% confidence intervals of effects. Third, the cognitive measures were general in nature and did not allow for precise investigation of more specific cognitive correlates related to regions of interest—e.g., relational memory and hippocampal iron. Related to this, we used an ROI-based approach with 24-pixel masks across three contiguous slices to measure average R2* signal as a representative estimate of iron content in the basal ganglia and hippocampus. Therefore, the measurements reported here are a limited assessment of iron content within those regions. The representation of iron within a region appears to not be uniform (Aquino et al., 2009; Li et al., 2011), however it is unclear if the non-uniform presentation of iron aligns with anatomical or functional divisions within a structure. Future studies that include higher-resolution imaging may accommodate study of iron within different portions of a single brain region—e.g., caudate head and body, dorsal and ventral putamen, and the subfields of the hippocampus.
Fourth, we report estimates of iron content from R2* relaxometry, which is a well-validated method, but is biased by the presence of myelinated fibers and other sources of field inhomogeneity (Daugherty and Raz, 2015). This is a consideration when interpreting the results presented here, as several of the subcortical nuclei examined contain myelinated fibers. Nonetheless, the large iron concentrations observed in the basal ganglia have been confirmed with post-mortem histology and validated against R2* estimates of iron content (Haacke et al., 2010; Langkammer et al., 2010). The high agreement between post-mortem measures of iron and R2* estimates in the basal ganglia suggests that this bias is negligible within those regions, although estimates of post-mortem hippocampal iron was not available for comparison. The hippocampus is expected to have relatively lesser iron content, and therefore the R2* signal may be more sensitive to the presence of myelinated fibers and this may be an alternate explanation of the small age differences we observed in that region. Estimates of iron from other neuroimaging methods, such as quantitative susceptibility mapping, are less vulnerable to bias from the presence of myelin (Daugherty and Raz, 2015) and may be more suitable for the study of hippocampal iron, and potentially whole brain analysis.
Fifth, there remains a substantial degree of individual differences in cognition and iron content that are unaccounted for in the reported models. Additional factors should be considered, such as the contribution of individual polymorphisms in genes that encode the transport and storage of iron in the body (Blasco et al., 2017; Jones and Jellen, 2017; Kepinska et al., 2015; Rouault, 2016), and the influence of external factors, such as diet (Hagemeier et al., 2015; Pino et al., 2017). Finally, extant studies describing the relationship of iron and R2* are primarily of adults and the clinical relevance of iron accumulation during adolescence in the context of healthy development remains unknown. Further research is needed to validate the sensitivity and specificity of MRI-based estimates of iron in the developing brain.
5. Conclusion
These findings provide new information as to the nature of iron accumulation across childhood, feasibility of R2* relaxometry studies, and the critical role of non-heme iron in cognitive development. The knowledge that non-heme iron shortages may inflict deficits on cognition further motivates the need for in vivo studies of iron homeostasis during childhood that may inform future studies of interventions aimed to promote healthy development.
Conflict of interest
None.
Acknowledgements
This project was supported by awards to M.E.T. from the National Institutes of Health, MH110793 and ES026022, and by a NARSAD Young Investigator Award. This research was also supported, in part, by National Institutes of Health award AG011230 and by the Beckman Institute Postdoctoral Fellowship (University of Illinois at Urbana-Champaign) award to A.M.D, with funding provided by the Arnold and Mabel Beckman Foundation. The authors thank Andrea Bedway, Hilary Marusak, Lauren Grove, and Pavan Jella for their assistance in data acquisition, management and/or analyses. The authors also thank participant families who generously shared their time.
References
- Adisetiyo V., Jensen J.H., Tabesh A., Deardorff R.L., Fieremans E., Di Martino A. Multimodal MR imaging of brain iron in attention deficit hyperactivity disorder: a noninvasive biomarker that responds to psychostimulant treatment? Radiology. 2014;272(2):524–532. doi: 10.1148/radiol.14140047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amat J.A., Bansal R., Whiteman R., Haggerty R., Royal J., Peterson B.S. Correlates of intellectual ability with morphology of the hippocampus and amygdala in healthy adults. Brain Cogn. 2008;66(2):105–114. doi: 10.1016/j.bandc.2007.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aquino D., Bizzi A., Grisoli M., Garavaglia B., Bruzzone M.G., Nardocci N. Age-related iron deposition in the basal ganglia: quantitative analysis in healthy subjects. Radiology. 2009;252(1):165–172. doi: 10.1148/radiol.2522081399. [DOI] [PubMed] [Google Scholar]
- Bartzokis G. Alzheimer’s disease as homeostatic responses to age-related myelin breakdown. Neurobiol. Aging. 2011;32(8):1341–1371. doi: 10.1016/j.neurobiolaging.2009.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beard J. Iron deficiency alters brain development and functioning. J. Nutr. 2003;133(5 Suppl 1):1468S–1472S. doi: 10.1093/jn/133.5.1468S. [DOI] [PubMed] [Google Scholar]
- Blasco G., Moreno-Navarrete J.M., Rivero M., Perez-Brocal V., Garre-Olmo J., Puig J. The gut metagenome changes in parallel to waist circumference, brain iron deposition, and cognitive function. J. Clin. Endocrinol. Metab. 2017;102(8):2962–2973. doi: 10.1210/jc.2017-00133. [DOI] [PubMed] [Google Scholar]
- Brown L.L., Schneider J.S., Lidsky T.I. Sensory and cognitive functions of the basal ganglia. Curr. Opin. Neurobiol. 1997;7(2):157–163. doi: 10.1016/s0959-4388(97)80003-7. [DOI] [PubMed] [Google Scholar]
- Burgaleta M., MacDonald P.A., Martinez K., Roman F.J., Alvarez-Linera J., Ramos Gonzalez A. Subcortical regional morphology correlates with fluid and spatial intelligence. Hum. Brain Mapp. 2014;35(5):1957–1968. doi: 10.1002/hbm.22305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter K.L., Li W., Wei H., Wu B., Xiao X., Liu C. Magnetic susceptibility of brain iron is associated with childhood spatial IQ. Neuroimage. 2016;132:167–174. doi: 10.1016/j.neuroimage.2016.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chugani H.T. A critical period of brain development: studies of cerebral glucose utilization with PET. Prev. Med. 1998;27(2):184–188. doi: 10.1006/pmed.1998.0274. [DOI] [PubMed] [Google Scholar]
- Connor J.R., Menzies S.L. Relationship of iron to oligodendrocytes and myelination. Glia. 1996;17(2):83–93. doi: 10.1002/(SICI)1098-1136(199606)17:2<83::AID-GLIA1>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Cortese S., Angriman M., Lecendreux M., Konofal E. Iron and attention deficit/hyperactivity disorder: what is the empirical evidence so far? A systematic review of the literature. Expert Rev. Neurother. 2012;12(10):1227–1240. doi: 10.1586/ern.12.116. [DOI] [PubMed] [Google Scholar]
- Dallman P.R. Biochemical basis for the manifestations of iron deficiency. Annu. Rev. Nutr. 1986;6:13–40. doi: 10.1146/annurev.nu.06.070186.000305. [DOI] [PubMed] [Google Scholar]
- Darki F., Nemmi F., Moller A., Sitnikov R., Klingberg T. Quantitative susceptibility mapping of striatum in children and adults, and its association with working memory performance. Neuroimage. 2016;136:208–214. doi: 10.1016/j.neuroimage.2016.04.065. [DOI] [PubMed] [Google Scholar]
- Daugherty A., Haacke E.M., Raz N. Striatal iron content predicts its shrinkage and changes in verbal working memory after two years in healthy adults. J. Neurosci. 2015;35(17):6731–6743. doi: 10.1523/JNEUROSCI.4717-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daugherty A., Raz N. Age-related differences in iron content of subcortical nuclei observed in vivo: a meta-analysis. Neuroimage. 2013;70:113–121. doi: 10.1016/j.neuroimage.2012.12.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daugherty A., Raz N. Appraising the role of iron in brain aging and cognition: promises and limitations of MRI methods. Neuropsychol. Rev. 2015;25(3):272–287. doi: 10.1007/s11065-015-9292-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Deungria M., Rao R., Wobken J.D., Luciana M., Nelson C.A., Georgieff M.K. Perinatal iron deficiency decreases cytochrome c oxidase (CytOx) activity in selected regions of neonatal rat brain. Pediatr. Res. 2000;48(2):169–176. doi: 10.1203/00006450-200008000-00009. [DOI] [PubMed] [Google Scholar]
- Dunnett S.B. 1st ed. Elsevier; Amsterdam ; Boston: 2005. Dopamine. [Google Scholar]
- Erikson K.M., Jones B.C., Beard J.L. Iron deficiency alters dopamine transporter functioning in rat striatum. J. Nutr. 2000;130(11):2831–2837. doi: 10.1093/jn/130.11.2831. [DOI] [PubMed] [Google Scholar]
- Georgieff M.K. Long-term brain and behavioral consequences of early iron deficiency. Nutr. Rev. 2011;69(Suppl 1):S43–S48. doi: 10.1111/j.1753-4887.2011.00432.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghadery C., Pirpamer L., Hofer E., Langkammer C., Petrovic K., Loitfelder M. R2* mapping for brain iron: associations with cognition in normal aging. Neurobiol. Aging. 2015;36(2):925–932. doi: 10.1016/j.neurobiolaging.2014.09.013. [DOI] [PubMed] [Google Scholar]
- Grahn J.A., Parkinson J.A., Owen A.M. The role of the basal ganglia in learning and memory: neuropsychological studies. Behav. Brain Res. 2009;199(1):53–60. doi: 10.1016/j.bbr.2008.11.020. [DOI] [PubMed] [Google Scholar]
- Haacke E.M., Miao Y., Liu M., Habib C.A., Katkuri Y., Liu T. Correlation of putative iron content as represented by changes in R2* and phase with age in deep gray matter of healthy adults. J. Magn. Reson. Imaging. 2010;32(3):561–576. doi: 10.1002/jmri.22293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haacke E.M., Reichenbach J., Ebrary I. 1 ed. Wiley-Blackwell; Hoboken, N.J: 2011. Susceptibility Weighted Imaging in MRI: Basic Concepts and Clinical Applications. [Google Scholar]
- Hagemeier J., Tong O., Dwyer M.G., Schweser F., Ramanathan M., Zivadinov R. Effects of diet on brain iron levels among healthy individuals: an MRI pilot study. Neurobiol. Aging. 2015;36(4):1678–1685. doi: 10.1016/j.neurobiolaging.2015.01.010. [DOI] [PubMed] [Google Scholar]
- Hallgren B., Sourander P. The effect of age on the non-haemin iron in the human brain. J. Neurochem. 1958;3(1):41–51. doi: 10.1111/j.1471-4159.1958.tb12607.x. [DOI] [PubMed] [Google Scholar]
- Hare D., Ayton S., Bush A., Lei P. A delicate balance: iron metabolism and diseases of the brain. Front. Aging Neurosci. 2013;5:34. doi: 10.3389/fnagi.2013.00034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harman D. Aging: a theory based on free radical and radiation chemistry. J. Gerontol. 1956;11(3):298–300. doi: 10.1093/geronj/11.3.298. [DOI] [PubMed] [Google Scholar]
- Jellen L.C., Lu L., Wang X., Unger E.L., Earley C.J., Allen R.P. Iron deficiency alters expression of dopamine-related genes in the ventral midbrain in mice. Neuroscience. 2013;252:13–23. doi: 10.1016/j.neuroscience.2013.07.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones B.C., Jellen L.C. Systems genetics analysis of iron and its regulation in brain and periphery. Methods Mol. Biol. 2017;1488:467–480. doi: 10.1007/978-1-4939-6427-7_22. [DOI] [PubMed] [Google Scholar]
- Kaufman A., Kaufman N. 2nd ed. American Guidance Services; Circle, MN: 2004. K-BIT: Kaufman Brief Intelligence Test. [Google Scholar]
- Kepinska M., Szyller J., Milnerowicz H. The influence of oxidative stress induced by iron on telomere length. Environ. Toxicol. Pharmacol. 2015;40(3):931–935. doi: 10.1016/j.etap.2015.10.002. [DOI] [PubMed] [Google Scholar]
- Khedr E., Hamed S.A., Elbeih E., El-Shereef H., Ahmad Y., Ahmed S. Iron states and cognitive abilities in young adults: neuropsychological and neurophysiological assessment. Eur. Arch. Psychiatry Clin. Neurosci. 2008;258(8):489–496. doi: 10.1007/s00406-008-0822-y. [DOI] [PubMed] [Google Scholar]
- Kuzawa C.W., Chugani H.T., Grossman L.I., Lipovich L., Muzik O., Hof P.R. Metabolic costs and evolutionary implications of human brain development. Proc. Natl. Acad. Sci. U. S. A. 2014;111(36):13010–13015. doi: 10.1073/pnas.1323099111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langkammer C., Krebs N., Goessler W., Scheurer E., Ebner F., Yen K. Quantitative MR imaging of brain iron: a postmortem validation study. Radiology. 2010;257(2):455–462. doi: 10.1148/radiol.10100495. [DOI] [PubMed] [Google Scholar]
- Langkammer C., Schweser F., Krebs N., Deistung A., Goessler W., Scheurer E. Quantitative susceptibility mapping (QSM) as a means to measure brain iron? A post mortem validation study. Neuroimage. 2012;62(3):1593–1599. doi: 10.1016/j.neuroimage.2012.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen B., Luna B. In vivo evidence of neurophysiological maturation of the human adolescent striatum. Dev. Cogn. Neurosci. 2015;12:74–85. doi: 10.1016/j.dcn.2014.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenroot R.K., Giedd J.N. Brain development in children and adolescents: insights from anatomical magnetic resonance imaging. Neurosci. Biobehav. Rev. 2006;30(6):718–729. doi: 10.1016/j.neubiorev.2006.06.001. [DOI] [PubMed] [Google Scholar]
- Li W., Wu B., Batrachenko A., Bancroft-Wu V., Morey R.A., Shashi V. Differential developmental trajectories of magnetic susceptibility in human brain gray and white matter over the lifespan. Hum. Brain Mapp. 2014;35(6):2698–2713. doi: 10.1002/hbm.22360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W., Wu B., Liu C. Quantitative susceptibility mapping of human brain reflects spatial variation in tissue composition. Neuroimage. 2011;55(4):1645–1656. doi: 10.1016/j.neuroimage.2010.11.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindenberger U., von Oertzen T., Ghisletta P., Hertzog C. Cross-sectional age variance extraction: what's change got to do with it? Psychol. Aging. 2011;26(1):34–47. doi: 10.1037/a0020525. [DOI] [PubMed] [Google Scholar]
- Linderberg U., Pötter U. The complex nature of unique and shared effects in hierarchical linear regression: implications for developmental psychology. Psychol. Methods. 1998;3(2):218–230. [Google Scholar]
- Lorio S., Lutti A., Kherif F., Ruef A., Dukart J., Chowdhury R. Disentangling in vivo the effects of iron content and atrophy on the ageing human brain. Neuroimage. 2014;103:280–289. doi: 10.1016/j.neuroimage.2014.09.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozoff B. Early iron deficiency has brain and behavior effects consistent with dopaminergic dysfunction. J. Nutr. 2011;141(4):740S–746S. doi: 10.3945/jn.110.131169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozoff B., Beard J., Connor J., Barbara F., Georgieff M., Schallert T. Long-lasting neural and behavioral effects of iron deficiency in infancy. Nutr. Rev. 2006;64(5 Pt 2):S34–S43. doi: 10.1301/nr.2006.may.S34-S43. discussion S72-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozoff B., Georgieff M.K. Iron deficiency and brain development. Semin. Pediatr. Neurol. 2006;13(3):158–165. doi: 10.1016/j.spen.2006.08.004. [DOI] [PubMed] [Google Scholar]
- MacDonald A.A., Seergobin K.N., Tamjeedi R., Owen A.M., Provost J.S., Monchi O. Examining dorsal striatum in cognitive effort using Parkinson’s disease and fMRI. Ann. Clin. Transl. Neurol. 2014;1(6):390–400. doi: 10.1002/acn3.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxwell S.E., Cole D.A. Bias in cross-sectional analyses of longitudinal mediation. Psychol. Methods. 2007;12(1):23–44. doi: 10.1037/1082-989X.12.1.23. [DOI] [PubMed] [Google Scholar]
- Middleton F.A., Strick P.L. Anatomical evidence for cerebellar and basal ganglia involvement in higher cognitive function. Science. 1994;266(5184):458–461. doi: 10.1126/science.7939688. [DOI] [PubMed] [Google Scholar]
- Middleton F.A., Strick P.L. Basal ganglia output and cognition: evidence from anatomical, behavioral, and clinical studies. Brain Cogn. 2000;42(2):183–200. doi: 10.1006/brcg.1999.1099. [DOI] [PubMed] [Google Scholar]
- Moos T., Morgan E.H. The metabolism of neuronal iron and its pathogenic role in neurological disease: review. Ann. N. Y. Acad. Sci. 2004;1012:14–26. doi: 10.1196/annals.1306.002. [DOI] [PubMed] [Google Scholar]
- Moretti R., Caruso P., Crisman E., Gazzin S. Basal ganglia: their role in complex cognitive procedures in experimental models and in clinical practice. Neurol. India. 2017;65(4):814–825. doi: 10.4103/neuroindia.NI_850_16. [DOI] [PubMed] [Google Scholar]
- Munoz P., Humeres A. Iron deficiency on neuronal function. Biometals. 2012;25(4):825–835. doi: 10.1007/s10534-012-9550-x. [DOI] [PubMed] [Google Scholar]
- Narvacan K., Treit S., Camicioli R., Martin W., Beaulieu C. Evolution of deep gray matter volume across the human lifespan. Hum. Brain Mapp. 2017;38(8):3771–3790. doi: 10.1002/hbm.23604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penke L., Valdes Hernandez M.C., Maniega S.M., Gow A.J., Murray C., Starr J.M. Brain iron deposits are associated with general cognitive ability and cognitive aging. Neurobiol. Aging. 2012;33(3):510–517. doi: 10.1016/j.neurobiolaging.2010.04.032. e512. [DOI] [PubMed] [Google Scholar]
- Persson N., Wu J., Zhang Q., Liu T., Shen J., Bao R. Age and sex related differences in subcortical brain iron concentrations among healthy adults. Neuroimage. 2015;122:385–398. doi: 10.1016/j.neuroimage.2015.07.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pino J.M.V., da Luz M.H.M., Antunes H.K.M., Giampa S.Q.C., Martins V.R., Lee K.S. Iron-restricted diet affects brain ferritin levels, dopamine metabolism and cellular prion protein in a region-specific manner. Front. Mol. Neurosci. 2017;10:145. doi: 10.3389/fnmol.2017.00145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao R., Georgieff M.K. Perinatal aspects of iron metabolism. Acta Paediatr. Suppl. 2002;91(438):124–129. doi: 10.1111/j.1651-2227.2002.tb02917.x. [DOI] [PubMed] [Google Scholar]
- Raz N., Daugherty A.M. Pathways to brain aging and their modifiers: Free-radical-induced energetic and neural decline in senescence (FRIENDS) model - a mini-review. Gerontology. 2017 doi: 10.1159/000479508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhein C., Muhle C., Richter-Schmidinger T., Alexopoulos P., Doerfler A., Kornhuber J. Neuroanatomical correlates of intelligence in healthy young adults: the role of basal ganglia volume. PLoS One. 2014;9(4) doi: 10.1371/journal.pone.0093623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigue K.M., Daugherty A.M., Haacke E.M., Raz N. The role of hippocampal iron concentration and hippocampal volume in age-related differences in memory. Cereb. Cortex. 2013;23(7):1533–1541. doi: 10.1093/cercor/bhs139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg D.R., Lewis D.A. Postnatal maturation of the dopaminergic innervation of monkey prefrontal and motor cortices: a tyrosine hydroxylase immunohistochemical analysis. J. Comp. Neurol. 1995;358(3):383–400. doi: 10.1002/cne.903580306. [DOI] [PubMed] [Google Scholar]
- Rouault T.A. Mitochondrial iron overload: causes and consequences. Curr. Opin. Genet. Dev. 2016;38:31–37. doi: 10.1016/j.gde.2016.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachdev P. The neuropsychiatry of brain iron. J. Neuropsychiatry Clin. Neurosci. 1993;5(1):18–29. doi: 10.1176/jnp.5.1.18. [DOI] [PubMed] [Google Scholar]
- Sandman C.A., Head K., Muftuler L.T., Su L., Buss C., Davis E.P. Shape of the basal ganglia in preadolescent children is associated with cognitive performance. Neuroimage. 2014;99:93–102. doi: 10.1016/j.neuroimage.2014.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenck J.F., Zimmerman E.A. High-field magnetic resonance imaging of brain iron: birth of a biomarker? NMR Biomed. 2004;17(7):433–445. doi: 10.1002/nbm.922. [DOI] [PubMed] [Google Scholar]
- Schweser F., Deistung A., Lehr B.W., Reichenbach J.R. Quantitative imaging of intrinsic magnetic tissue properties using MRI signal phase: an approach to in vivo brain iron metabolism? Neuroimage. 2011;54(4):2789–2807. doi: 10.1016/j.neuroimage.2010.10.070. [DOI] [PubMed] [Google Scholar]
- Sehgal V., Delproposto Z., Haacke E.M., Tong K.A., Wycliffe N., Kido D.K. Clinical applications of neuroimaging with susceptibility-weighted imaging. J. Magn. Reson. Imaging. 2005;22(4):439–450. doi: 10.1002/jmri.20404. [DOI] [PubMed] [Google Scholar]
- Shrout P.E., Fleiss J.L. Intraclass correlations: uses in assessing rater reliability. Psychol. Bull. 1979;86(2):420–428. doi: 10.1037//0033-2909.86.2.420. [DOI] [PubMed] [Google Scholar]
- Teicher M.H., Andersen S.L., Hostetter J.C., Jr. Evidence for dopamine receptor pruning between adolescence and adulthood in striatum but not nucleus accumbens. Brain Res. Dev. Brain Res. 1995;89(2):167–172. doi: 10.1016/0165-3806(95)00109-q. [DOI] [PubMed] [Google Scholar]
- Todorich B., Pasquini J.M., Garcia C.I., Paez P.M., Connor J.R. Oligodendrocytes and myelination: the role of iron. Glia. 2009;57(5):467–478. doi: 10.1002/glia.20784. [DOI] [PubMed] [Google Scholar]
- Vymazal J., Brooks R.A., Zak O., McRill C., Shen C., Di Chiro G. T1 and T2 of ferritin at different field strengths: effect on MRI. Magn. Reson. Med. 1992;27(2):368–374. doi: 10.1002/mrm.1910270218. [DOI] [PubMed] [Google Scholar]
- Wang J., Shaffer M.L., Eslinger P.J., Sun X., Weitekamp C.W., Patel M.M. Maturational and aging effects on human brain apparent transverse relaxation. PLoS One. 2012;7(2) doi: 10.1371/journal.pone.0031907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weschler D. 3rd ed. Psychological Corporation; San Antonio, TX: 1991. Wechsler Intelligence Scale for Children. [Google Scholar]
- Wierenga L., Langen M., Ambrosino S., van Dijk S., Oranje B., Durston S. Typical development of basal ganglia, hippocampus, amygdala and cerebellum from age 7 to 24. Neuroimage. 2014;96:67–72. doi: 10.1016/j.neuroimage.2014.03.072. [DOI] [PubMed] [Google Scholar]
- Wiesinger J.A., Buwen J.P., Cifelli C.J., Unger E.L., Jones B.C., Beard J.L. Down-regulation of dopamine transporter by iron chelation in vitro is mediated by altered trafficking, not synthesis. J. Neurochem. 2007;100(1):167–179. doi: 10.1111/j.1471-4159.2006.04175.x. [DOI] [PubMed] [Google Scholar]
- Woodcock R., McGrew K., Mather N. Riverside Publishing; Rolling Meadows, IL: 2001. Woodcock-Johnson III Test of Achievement. [Google Scholar]
- Yates K.F., Sweat V., Yau P.L., Turchiano M.M., Convit A. Impact of metabolic syndrome on cognition and brain: a selected review of the literature. Arterioscler. Thromb. Vasc. Biol. 2012;32(9):2060–2067. doi: 10.1161/ATVBAHA.112.252759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yehuda S., Youdim M.B. Brain iron: a lesson from animal models. Am. J. Clin. Nutr. 1989;50(3 Suppl):618–625. doi: 10.1093/ajcn/50.3.618. discussion 625-619. [DOI] [PubMed] [Google Scholar]
- Youdim M.B., Ben-Shachar D., Yehuda S. Putative biological mechanisms of the effect of iron deficiency on brain biochemistry and behavior. Am. J. Clin. Nutr. 1989;50(3 Suppl):607–615. doi: 10.1093/ajcn/50.3.607. discussion 615-607. [DOI] [PubMed] [Google Scholar]
- Youdim M.B., Green A.R. Iron deficiency and neurotransmitter synthesis and function. Proc. Nutr. Soc. 1978;37(2):173–179. doi: 10.1079/pns19780022. [DOI] [PubMed] [Google Scholar]
- Youdim M.B., Yehuda S. The neurochemical basis of cognitive deficits induced by brain iron deficiency: involvement of dopamine-opiate system. Cell Mol. Biol. (Noisy-Le-Grand) 2000;46(3):491–500. [PubMed] [Google Scholar]
- Zecca L., Youdim M.B., Riederer P., Connor J.R., Crichton R.R. Iron, brain ageing and neurodegenerative disorders. Nat. Rev. Neurosci. 2004;5(11):863–873. doi: 10.1038/nrn1537. [DOI] [PubMed] [Google Scholar]