Abstract
Previous research has linked sleep disturbance to anxiety. However, evidence for this relation has been inconsistent, largely limited to retrospective reports that do not account for daily variability, and silent on when the association is most pronounced. Thus, the present study utilized ecological momentary assessment (EMA) to examine the effects of daily deviations in total sleep time (TST) and person-average TST on anxiety and whether these effects varied as a function of time of day in a sample of unselected adults (N = 138). Results indicate that the amount of TST on a given night, relative to personal average TST, negatively predicted anxiety, and this relation was significant in the morning and afternoon, but not evening. In contrast, person-average TST was unrelated to average anxiety. Relations between TST and anxiety did not differ across objective (e.g., actigraphy) and subjective (e.g., sleep diary) measures. Furthermore, the pattern of results remained the same when controlling for previous day’s anxiety and were not bidirectional. These findings suggest that getting less sleep than is typical for the individual predicts subsequent anxiety, and this effect is particularly strong in the morning. Average sleep duration may be less important to the experience of anxiety than deviations from that average. These findings highlight the importance of EMA to examine how and when variability in sleep confers vulnerability for anxiety symptoms.
Recent attention has been given to the role of sleep disturbance in psychopathology, with accumulating evidence for the presence of sleep disturbance across the majority of disorders (Baglioni et al., 2017). In addition to general psychopathology, sleep disturbance has also been specifically linked to the experience of anxiety. Findings from prospective studies indicate sleep disturbance predicts symptoms of anxiety, including repetitive negative thinking (Cox, Cole, Kramer, & Olatunji, 2018) and general anxiety (Doane, Gress-Smith, & Breitenstein, 2015). Further, results from experimental studies indicate that both total sleep deprivation and partial sleep restriction result in increased anxiety (Babson, Trainor, Feldner, & Blumenthal, 2010; Reddy et al., 2017), suggesting a causal relation between acute sleep loss and elevated anxiety. Finally, sleep disturbance is evident in the majority of anxiety-related disorders (Cox & Olatunji, 2016) and predicts the development of an anxiety disorder (Batterham, Glozier, & Christensen, 2012).
Although some findings in the literature suggest a causal link between sleep disturbance and anxiety, evidence for this relation has not been wholly consistent. Indeed, in contrast to prospective evidence that sleep disturbance predicts anxiety, other studies have found a unidirectional relation between anxiety-related disorders and subsequent insomnia symptoms, though these findings are limited to adolescent samples (Alvaro, Roberts, Harris, & Bruni, 2017; Johnson, Roth, & Breslau, 2006). Similarly, although considerable research has found sleep-related deficits among those with anxiety disorders compared to controls, several studies have also found no such differences (see Cox & Olatunji, 2016 for a review). One possible source of these discrepancies relates to measurement. That is, the majority of extant research on sleep and anxiety has utilized retrospective measures that are vulnerable to recall bias (Shiffman, Stone, & Hufford, 2008). Further, previous research utilizing daily monitoring methods (i.e., sleep diaries, actigraphy) has generally aggregated these measures to yield average levels of sleep parameters. Such aggregation may mask the prospective impact of daily sleep variability on subsequent experiences of anxiety.
Many of these discrepant findings in the literature may be addressed with ecological momentary assessment (EMA) that enables researchers to sample sleep and anxiety as they occur in the participant’s natural environment, both reducing recall bias and enhancing ecological validity. Such EMA data can be analyzed with multilevel modeling, which allows for the examination of person-average, daily-average, and within-day variability of variables of interest. Indeed, recent findings utilizing these methods have yielded a more consistent link between sleep disturbance and anxiety-related outcomes. For example, decreased subjective daily sleep quality predicts next day stress and anxiety in unselected samples (Kalmbach, Arnedt, Swanson, Rapier, & Ciesla, 2017; Lee, Crain, McHale, Almeida, & Buxton, 2017). Similarly, decreased subjective daily sleep quality predicts next day worry among those with generalized anxiety disorder (GAD; Thielsch et al., 2015), and decreased subjective daily sleep quality and sleep efficiency predict next day posttraumatic stress disorder (PTSD) symptoms among those with PTSD (Short, Allan, & Schmidt, 2017). Although more recent research has employed EMA to demonstrate a link between daily fluctuations in sleep and subsequent anxiety, these findings are limited by lack of objective sleep measurement. Given previous evidence for discrepancies between objective and subjective sleep measures (Lauderdale, Knutson, Yan, Liu, & Rathouz, 2008), studies relying only on subjective sleep assessment may lack precision in characterizing the effect of sleep disturbance on anxiety.
An additional strength of EMA is the ability to examine time of day effects, allowing for more detailed characterization of how dynamic processes, such as anxiety, vary between morning, afternoon, and evening. Research on the diurnal course of anxiety is limited; however, extant findings indicate anxiety-related processes may peak at certain times of day. Among those with OCD, obsessions are most frequent in the afternoon (Nota, Gibb, & Coles, 2014). Similarly, among those with panic attacks, general anxiety and panic symptoms are highest in the afternoon; however, sense of threat is highest in the morning (Kenardy, Fried, Kraemer, & Taylor, 1992). To date, however, no study has tested whether the relation between daily sleep disturbance and anxiety may vary as a function of time of day. Evidence for time of day effects on this relation could highlight when therapeutic intervention might be most effective or implicate processes that peak at similar times as potential underlying mechanisms. Previous research suggests conflicting possibilities for the direction of a time of day effect. For example, a recovery model would suggest that anxiety would diminish over time following a stressor such as sleep loss (Verkuil, Brosschot, & Thayer, 2014), thus anxiety may be highest in the morning and lowest in the evening. In contrast, homeostatic sleep pressure increases with sustained wakefulness (Borbely, Daan, Wirz-Justice, & Deboer, 2016), and this accumulation process may interact with poor sleep to produce highest anxiety in the evening when sleep pressure is highest.
The aim of the present study was to address an important gap in the literature by utilizing both subjective and objective sleep measures and EMA to examine the link between daily sleep and anxiety in a sample of unselected adults. Total sleep time (TST) was chosen as the index of sleep, as this parameter has the most consistency with polysomnography when measured by actigraphy (Ancoli-Israel et al., 2003). For reasons given above, we hypothesized that decreased subjective and objective daily and average TST and increased average insomnia symptoms (i.e., difficulties with sleep initiation and/or maintenance) would predict increased anxiety. We then conducted exploratory analyses to examine whether time of day moderated the effect of TST on anxiety. Given previous evidence for bidirectional links between sleep and anxiety, we also tested an alternate model of bidirectional relations between daily TST and daily anxiety.
Methods
Participants
The sample consisted of unselected undergraduate students and community adults (N = 151). Undergraduate students were recruited from psychology courses and were compensated with course credit. Community adults were recruited from flyers and ResearchMatch, a national health volunteer registry created by several academic institutions and supported by the U.S. National Institutes of Health as part of the Clinical Translational Science Award (CTSA) program, and were compensated with $25. Thirteen participants withdrew from the study for a final sample of 138 (75.5% female). Due to equipment availability, actigraphy data was collected on a subset of participants (n = 100)1. Three participants failed to complete sleep diaries. Due to a programming error, eight participants did not complete the Morningness-Eveningness Questionnaire. Thus, the final samples for the objective and subjective TST models included 98 and 130 participants, respectively.
The mean age of the sample was 22.48 (SD = 9.24), ranging from 18 to 64. The majority of the sample was college age (18–22; n = 117; 84.2%). The age distribution of the remainder of the sample was as follows: 23–29 (n = 8; 5.8%), 30–39 (n = 4; 2.9%), 40–49 (n = 3; 2.2%), 50–59 (n = 2; 1.4%), 60–64 (n = 3; 2.2%). The ethnicity composition was as follows: Caucasian (n = 80; 57.6%), African American (n = 17; 12.2%), Asian (n = 33; 23.9%), Hispanic/Latino (n = 7; 5.1%), Other (n = 1; 0.7%). 33% of the sample met criteria for a major form of psychopathology, 12% met criteria for a mood disorder, and 24% met criteria for an anxiety disorder as determined by the MINI International Neuropsychiatric Interview. 8% screened positive for clinical insomnia as determined by the Insomnia Severity Index.
Measures and Materials
Actigraphy.
Actigraphy is an objective sleep measure that estimates sleep and wake from motion (Ancoli-Israel et al., 2003). The present study utilized ActiGraph wGT3X-BT activity monitors (ActiGraph, Pensacola, FL). Previous research indicates that actigraphy is highly accurate when compared to polysomnography (Marino et al., 2013) and that the ActiGraph wGT3X-BT is reliable and valid for estimating sleep (Cellini, Buman, McDevitt, Ricker, & Mednick, 2013). Objective TST was calculated with the Sadeh algorithm (Sadeh, Sharkey, & Carskadon, 1994).
Consensus Sleep Diary (CSD; Carney et al., 2012).
The CSD is a 9-item sleep diary that asks participants about their last night of sleep. The CSD was developed by a panel of sleep experts to create a standard sleep diary for the assessment of daily sleep. Subjective TST is calculated as the difference between the time the participant began trying to sleep and the time of final awakening, minus sleep onset latency and wake after sleep onset.
Insomnia Severity Index (ISI; Bastien, Vallieres, & Morin, 2001).
The ISI is a 7-item selfreport measure of insomnia symptoms. Items include assessment of difficulties with sleep initiation and maintenance (e.g., “Difficulty falling asleep”) and subjective impairment (e.g., “How satisfied/dissatisfied are you with your current sleep pattern?”). Items are rated on a Likert scale from 1 (none) to 4 (very severe), and higher scores indicate increased insomnia symptoms. A score of 15 or higher indicates clinical insomnia (Bastien et al., 2001). The ISI demonstrated adequate internal consistency at time 1 (α = .86) in the present sample.
Mini International Neuropsychiatric Interview (MINI; Sheehan et al., 1998).
The MINI is a structured diagnostic interview that assesses 17 DSM disorders. The MINI was administered by bachelor- and master-level students trained and supervised by a licensed clinical psychologist.
Morningness-Eveningness Questionnaire (MEQ; Horne and Ostberg, 1976).
The MEQ is a 19-item self-report measure of chronotype and is thought to reflect the individual’s circadian rhythms. Items on the MEQ are rated on a Likert scale ranging from 1 to 6 with answer options varying by item content. Higher scores indicate increased morningness. The MEQ demonstrated adequate internal consistency (α = .84) in the present sample.
Momentary anxiety.
Momentary anxiety was measured with a single item (“How anxious do you feel right now?”) on a scale from 0 (“not anxious at all”) to 100 (“most anxious you could ever imagine feeling”). Momentary anxiety data were collected and managed using REDCap (Research Electronic Data Capture) hosted at Vanderbilt University (Harris et al. 2009). REDCap is a secure, web-based application designed to support data capture for research studies and is supported by UL1 TR000445 from NCATS/NIH.
Procedure
We collected data through a combination of laboratory and EMA methods over a 9-day period. On day 1, participants attended a laboratory session that included informed consent and administration of the MINI, MEQ, and ISI. We then gave participants the CSD, an actigraph, and instructions for the upcoming week. During days 2–8, we instructed participants to complete the CSD each morning. Participants also received a survey via email with the momentary anxiety item at 8am, 2pm, and 8pm and were instructed to complete each survey within 2 hours of receipt. On day 9, participants returned to the lab to return the CSD and actigraph and were debriefed. Data collection took place throughout the calendar year but did not include final exam periods or holidays. All data collection periods included one weekend. Day 1 took place on Monday, Tuesday, Wednesday, or Thursday, and day 9 took place on the following Tuesday, Wednesday, Thursday, or Friday.
We excluded from analysis all surveys that were completed outside of the specified 2hour period (5.6% of observations). We treated incomplete surveys as missing (15% of observations). These rates are similar to rates of missingness in previous EMA studies (e.g., Short et al., 2017).
Data Analytic Strategy
We fit three-level multilevel models with Mplus 7.4 (Muthen & Muthen, 1998–2015) to account for the three-level nested structure of the data: 3 timepoints (level 1 units) nested within 7 days (Level 2 units) nested within 151 people (Level 3 units). The level 1 predictor was time of day (centered at morning). Level-2 predictors were day-level TST (person-mean-centered so that deviations from zero represent deviations from the participant’s own average), day-level anxiety (i.e., person-mean-centered day-means of anxiety), and lagged day-level anxiety (i.e., lagged person-mean-centered day-means of anxiety). For example, a day-level TST value of −30 for day 1 would indicate that the participant slept 30 minutes less on day 1 than the participant’s average TST for the week. Level-3 predictors were person-average TST, person-average anxiety, ISI, MEQ, and age (all grand mean centered so that deviations from zero represent higher or lower values compared to the sample average). We included age as a covariate, given evidence for changes in sleep across the lifespan (Ohayon, Carskadon, Guilleminault, & Vitiello, 2004). We also included MEQ as a covariate to account for potential effects of individual circadian preferences. Two cross-level interaction terms served as additional predictors; these were daylevel TST × time-of-day (a level-2 by level-1 interaction) and person-average TST × time-of-day (a level-3 by level-1 interaction).
All fitted three-level models predicting anxiety included a random intercept at level-2 (varying across days within person) and a random intercept at level-3 (varying across persons) because the day-level intraclass correlation (ICC) of .125 and the person-level ICC of .493 were both meaningfully large. We tested two three-level models to examine whether daily and person-average TST predicted anxiety and if the relation between (daily or person-average) TST and anxiety varied as a function of time of day. Model 1 utilized an objective measure of TST (e.g., actigraphy), and Model 2 utilized a subjective measure of TST (e.g., a sleep diary). In Models 1 and 2 predictor variables included time of day, day-level TST, person-average TST, day-level TST × time-of-day, person-average TST × time-of-day, ISI, age, and MEQ. Models 3 and 4 re-tested these models including lagged day-level anxiety as an additional covariate to control for the relative level of the previous day’s anxiety and a test of the effect of day-level anxiety on day level TST to examine bidirectional effects. There was no theoretical expectation of random slopes (of level 1 predictors’ slopes varying across level-2 or −3 units or level-2 predictors’ slopes varying across level-3 units). Nonetheless, a sensitivity analysis was undertaken allowing for random slopes of level-1 and level-2 predictors; it yielded nonsignificant random slope variances and did not alter main findings, so fixed-slope results are reported for Models 1–4.
Full information maximum likelihood (FIML) estimation was used, allowing persons with missing observations on the outcome to be retained under missing-at-random (MAR) assumptions. Furthermore, because creation of the lagged day-level anxiety variable induced covariate missingness-by-design (a lagged predictor is by definition missing on the first day) a generalization of procedures from Sterba (2014) was used at level-2 to retain these cases, again under MAR assumptions.
Results
Descriptive Results and Associations Between Study Variables
Average objective and subjective TST in the present sample were 385.66 minutes (SD = 59.04) and 433.69 minutes (SD = 60.38), respectively, and were consistent with average TST found in similar samples (Kalmbach et al., 2017; Slater et al., 2015). Objective and subjective TST were highly correlated, r = .74, p < .001, though subjective TST was significantly longer than objective TST, t(92) = −10.82, p < .001. Average level of insomnia symptoms was 6.87 (SD = 4.92), indicating a nonclinical average level of insomnia. Average daily anxiety was 14.32 (SD = 13.42), indicating mild anxiety consistent with daily anxiety ratings in a similar sample (Edmondson, Arndt, Alcantara, Chaplin, & Schwartz, 2015).
Conditional Main Effect of Day-level TST on Anxiety
Results from Model 1 (see Table 1) revealed a significant negative day-level conditional main effect of objective TST on anxiety. Likewise, results from Model 2 (see Table 1) revealed a significant negative day-level conditional main effect of subjective TST on anxiety.
Table 1.
Unstandardized coefficients for the hypothesized models predicting anxiety from day-level and person-average TST and insomnia symptoms.
| Model 1 Objective TST | |||||
|---|---|---|---|---|---|
| Fixed Effects | Outcome | Predictor | Est | SE | P |
| Level 1 (Observation level) | Anxiety | Time-of-day | .502 | 2.196 | .819 |
| Time-of-day x Day-level TST | .011 | .005 | .015 | ||
| Time-of-day x Person-avg TST | −.003 | .006 | .640 | ||
| Level 2 (Day level) | Day-level TST | −.024 | .007 | .000 | |
| Level 3 (Person level) | Intercept | 9.972 | 8.546 | .243 | |
| Person-avg TST | .012 | .022 | .575 | ||
| ISI | .877 | .260 | .001 | ||
| MEQ | −.152 | .138 | .273 | ||
| Age | −.225 | .113 | .047 | ||
| Variance components | |||||
| Level 1 | Residual variance | 114.121 | 4.933 | ||
| Level 2 | Intercept variance | 37.964 | 7.100 | ||
| Level 3 | Intercept variance | 126.814 | 20.140 | ||
| Model 2 Subjective TST | |||||
| Fixed Effects | Outcome | Predictor | Est | SE | P |
| Level 1 (Observation level) | Anxiety | Time-of-day | −2.559 | 2.334 | .273 |
| Time-of-day x Day-level TST | .010 | .004 | .016 | ||
| Time-of-day x Person-avg TST | .005 | .005 | .372 | ||
| Level 2 (Day level) | Day-level TST | −.026 | .006 | .000 | |
| Level 3 (Person level) | Intercept | 23.606 | 8.718 | .007 | |
| Person-avg TST | −.019 | .020 | .328 | ||
| ISI | .862 | .2401 | .000 | ||
| MEQ | −.147 | .124 | .234 | ||
| Age | −.262 | .122 | .032 | ||
| Variance components | |||||
| Level 1 | Residual variance | 135.267 | 5.170 | ||
| Level 2 | Intercept variance | 40.152 | 5.400 | ||
| Level 3 | Intercept variance | 146.138 | 20.169 | ||
Note. TST = Total sleep time; ISI = Insomnia Severity Index; MEQ = Morningness-Eveningness Questionnaire
Interactive Effect of Time of Day on the Relation between Day-level TST and Anxiety
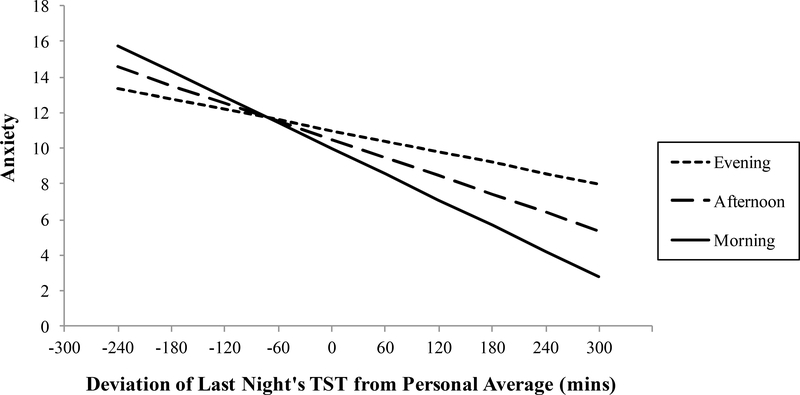
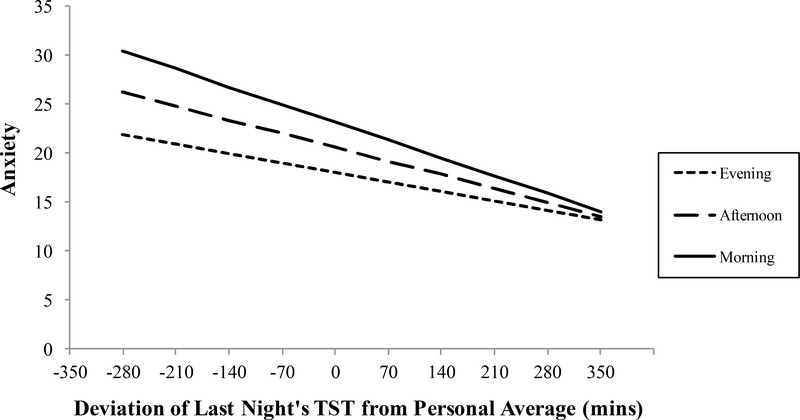
Results from Model 1 also revealed a significant cross-level interaction between objective TST and time of day to predict anxiety. Simple slopes analyses for the cross-level interaction revealed that the amount of objective TST a person gets relative to their own personaverage has greatest negative impact on anxiety in the morning, β = −.024, z = −3.510, p < .01, followed by the afternoon, β = −.013, z = −2.593, p < .01, but no significant effect in the evening (see Table 1 and Figure 1). Similarly, results from Model 2 revealed a significant cross-level interaction between subjective TST and time of day to predict anxiety. Simple slopes analysis of the cross-level interaction revealed the same pattern: the amount of subjective TST a person gets relative to their own person-average has the strongest negative impact on anxiety in the morning, β = −.026, z = −4.446, p < .01, followed by afternoon, β = −.017, z = −3.942, p < .01, but no significant effect in the evening (see Table 1 and Figure 2).
Figure 1.
Simple regressions of the relationship between daily deviations in objective total sleep time (TST) predicting anxiety in the morning, afternoon, and evening. Slopes for morning and afternoon, but not evening, are significant (see text for details).
Figure 2.
Simple regressions of the relationship between daily deviations in subjective total sleep time (TST) predicting anxiety in the morning, afternoon, and evening. Slopes for morning and afternoon, but not evening, are significant (see text for details).
Effects of Person-Average TST on Anxiety
In contrast, results from Models 1 and 2 (see Table 1) revealed no significant conditional main effect of a person’s typical amount of objective or subjective TST on anxiety at level 3. That is, person-average TST for the week did not significantly predict person-average anxiety for the week. Likewise, there was no significant cross-level (level-3 × level-1) interaction between person-average TST and time of day to predict anxiety.
Effects of Insomnia Symptoms on Anxiety
Results from both Models 1 and 2 (see Table 1) revealed significant positive person-level effects of insomnia symptoms on anxiety. That is, increased insomnia symptoms predicted increased person-average anxiety.
Follow-up Analyses Controlling for Lagged Day-level Anxiety
Results from Models 3 and 4 revealed that, when controlling for lagged day-level anxiety, all conditional main effects and cross-level interactions involving day-level TST that were significant in Models 1 and 2 remained significant (regardless of whether TST was measured objectively or subjectively, see Table 2). Results from both models revealed that the person-level effect of insomnia symptoms on anxiety also remained significant when controlling for lagged day-level anxiety.
Table 2.
Unstandardized coefficients for the hypothesized models predicting anxiety from day-level and person-average TST and insomnia symptoms and day-level TST from day-level anxiety, controlling for lagged daily anxiety.
| Model 3 Objective TST | |||||
|---|---|---|---|---|---|
| Fixed Effects | Outcome | Predictor | Est | SE | p |
| Level 1 (Observation level) | Anxiety | Time-of-day | .504 | 2.197 | .818 |
| Time-of-day x Day-level TST | .011 | .005 | .015 | ||
| Time-of-day x Person-avg TST | −.003 | .006 | .639 | ||
| Level 2 (Day level) | Anxiety | Day-level TST | −.024 | .007 | <.001 |
| Lagged Day-level TST | .005 | .048 | .914 | ||
| TST | Day-level Anxiety | −.483 | .332 | .146 | |
| Level 3 (Person level) | Anxiety | Intercept | 9.969 | 8.550 | .244 |
| Person-avg TST | .012 | .022 | .575 | ||
| ISI | .876 | .260 | .001 | ||
| MEQ | −.152 | .138 | .273 | ||
| Age | −.225 | .113 | .047 | ||
| Variance components | |||||
| Level 1 | Residual variance | 114.173 | 4.938 | ||
| Level 2 | Intercept variance anxiety | 37.931 | 5.346 | ||
| Intercept variance TST | 5106.737 | 278.946 | |||
| Level 3 | Intercept variance | 126.907 | 20.168 | ||
| Model 4 Subjective TST | |||||
| Fixed Effects | Outcome | Predictor | Est | SE | p |
| Level 1 (Observation level) | Anxiety | Time-of-day | −2.556 | 2.334 | .274 |
| Time-of-day x Day-level TST | .010 | .004 | .015 | ||
| Time-of-day x Person-avg TST | .005 | .005 | .374 | ||
| Level 2 (Day level) | Anxiety | Day-level TST | −.027 | .006 | <.001 |
| Lagged day-level TST | −.038 | .040 | .347 | ||
| TST | Day-level TST | −.551 | .297 | .064 | |
| Level 3 (Person level) | Anxiety | Intercept | 23.550 | 8.702 | .007 |
| Person-avg TST | −.019 | .020 | .330 | ||
| ISI | .864 | .239 | .000 | ||
| MEQ | −.147 | .124 | .233 | ||
| Age | −.262 | .122 | .032 | ||
| Variance components | |||||
| Level 1 | Residual variance | 135.222 | 5.167 | ||
| Level 2 | Intercept variance anxiety | 40.235 | 5.418 | ||
| Intercept variance TST | 6305.421 | 300.106 | |||
| Level 3 | Intercept variance | 145.517 | 20.025 | ||
Note. TST = Total sleep time; ISI = Insomnia Severity Index; MEQ = Morningness-Eveningness Questionnaire
Follow-up Analyses Testing Bidirectional Effects
Results from Models 3 and 4 also indicated that there was no significant day-level conditional main effect of day-level anxiety on day-level objective or subjective TST (see Table 2). Results from both models revealed that all significant effects from Models 1 and 2 remained significant when controlling for lagged day-level anxiety and including the test of bidirectional effects.
Discussion
The findings of the present study suggest that decreased TST relative to personal average predicts subsequent anxiety. Otherwise stated, when individuals get less sleep than they usually do on a given night, anxiety increases on the following day. These findings are consistent with previous EMA research linking daily subjective sleep disturbance to decreased positive affect, increased negative affect, and increased anxiety (Kalmbach et al., 2017; McCrae et al., 2008) and extend previous findings by revealing a similar relation with anxiety using both subjective and objective sleep measurement. Likewise, these findings are consistent with research linking acute sleep loss to increased anxiety (Babson et al., 2010) and replicate this relation on a daily level. Together, these findings suggest that getting less sleep than typical for the individual increases subsequent levels of anxiety. Interestingly, this result is inconsistent with a recent study indicating no relation between daily subjective TST and subsequent PTSD symptoms in a PTSD sample (Short et al., 2017). However, the Short et al. study utilized grand mean centering of TST, such that in their study the effect of TST was an “uninterpretable blend” (Cronbach, 1976) of the purely level-3 effect of person-average TST (nonsignificant in the current study) and the purely level-2 effect of day-level deviations from person average TST (significant in the current study). Hence, Short et al.’s results may indeed be consistent with ours if they had disaggregated the level-specific effects of TST on anxiety. Alternatively, meta-analytic findings indicate that TST is not disrupted in PTSD (Kobayashi, Boarts, & Delahanty, 2007); thus TST may have differential predictive value for anxiety as a function of the normative vs. clinical nature of the sample.
The present study also found that time of day moderated the relation between day-level TST and anxiety, such that the relation between the amount of last night’s TST (relative to a person’s average TST) and the amount of subsequent anxiety (relative to a person’s average anxiety) was significant in the morning and afternoon, but not significant in the evening. Importantly, the inclusion of MEQ scores in the model reduces the likelihood of a confounding effect of individual differences in circadian activity preferences. This finding is consistent with a recent study linking decreased TST with increased morning anxiety among adolescents with GAD (Mullin et al., 2017). One possibility is that variability in TST has a proximal effect on affective experience that dissipates over the course of the day. This interpretation is consistent with a recovery model, such that a response (i.e., elevated anxiety) to a stressor (i.e., shorter sleep duration than typical) diminishes over time (Hellhammer & Schubert, 2012). Additionally, sleep deprivation is associated with increased serotonergic activity (Elmenhorst, Kroll, Matusch, & Bauer, 2012), which may contribute to decreased evening anxiety. Alternatively, this effect may reflect variations in underlying circadian processes impacted by sleep. For example, recent findings indicate that nights characterized by less than typical objective TST are associated with lower levels of waking cortisol the following day (Van Lenten & Doane, 2016), representing an alteration from the normative circadian cortisol pattern, in which cortisol levels are highest in the morning (Van Cauter, Polonksy, & Scheen, 1997). Given links between anxiety and low waking cortisol levels and flatter diurnal cortisol rhythm (Doane et al., 2013), diminished waking cortisol may be one mechanism by which less than typical TST impacts subsequent anxiety.
Notably, person-average subjective and objective TST were unrelated to person-average anxiety in the present study. This finding is consistent with previous EMA studies finding no relation between person-average TST and anxiety (Kalmbach et al., 2017). Extant research suggests that there may be individual differences in sleep need, such that the duration of TST required for adaptive functioning may vary between individuals based on factors such as gender, age, and sleepiness (see Blunden & Galland, 2014 for a review). Given the within-person findings, it may be the case that the relation between TST and anxiety is not driven by the typical numbers of hours of sleep one gets, but rather deviations from one’s individual norm. This finding highlights the importance of utilizing EMA to reveal nuances in the relations between complex processes like sleep and anxiety that unfold on a day to day level. One implication of the present findings is that individuals with comorbid anxiety and sleep disturbance may benefit most from intervention in the morning when their anxiety is highest. Alternatively, delineation of whether an individual’s current sleep profile represents a deviation from their norm may inform decisions on whether or not to intervene on sleep.
A limitation of the present study is that one week may be too limited a sampling period to detect a relation between person-average TST and person-average anxiety in an unselected sample. That is, chronic deviations from individual TST average may accumulate over time, perhaps necessitating a longer window across which to compute a reliable person-average. This accumulation of insufficient TST may confer vulnerability for increased anxiety. This interpretation is supported by the finding that insomnia symptoms were associated with increased person-average anxiety in the present study. Though insomnia symptoms are not necessarily indicative of chronically reduced TST, this finding suggests that chronic sleep disturbance is linked to increased anxiety over the course of a week. This finding is consistent with considerable extant research implicating insomnia symptoms as a prospective predictor of anxiety (Cox et al., 2018) and its disorders (Neckelmann, Mykletun, & Dahl, 2007). Future research examining sleep and anxiety over longer periods of time is necessary to determine whether deviations in individual TST may accumulate over time and increase the likelihood of experiencing elevated anxiety.
Importantly, the present study found that daily anxiety did not predict daily TST, suggesting that the relationship between TST and anxiety is not bidirectional. Previous research examining daily variability in affective function and sleep has found evidence for a detrimental impact of daily stress and negative affect on sleep parameters (Lee et al., 2017), including TST (Kalmbach, Pillai, Roth, & Drake, 2014). In contrast, one recent study found no relationship between daily anxious arousal and subsequent subjective TST (Kalmbach et al., 2017), consistent with the present findings. Thus, there may be complex relations between sleep and affective function, such that mood-related processes may impact sleep, while sleep may impact anxiety-related processes. This interpretation is consistent with a recent study finding that sleep disturbance predicts increased anxiety during the transition between high school and college, while depression symptoms predict increased sleep disturbance (Doane et al., 2015). Together with the finding that the relationship between daily TST and anxiety remained robust after the inclusion of lagged daily anxiety as a covariate, this finding suggests that the relationship between TST and anxiety is not better explained by an upstream impact of anxiety on TST. That is, night’s characterized by less sleep than is typical for the individual are followed by days characterized by increased anxiety, and this relationship is not due to previous day’s anxiety or an effect of previous anxiety on subsequent TST. Though preliminary, these findings suggest that initial disturbances in TST may precede subsequent TST-anxiety relations and provide further support to the rejection of sleep disturbances as epiphenomenon of symptoms of psychopathology.
Another notable finding in the present study is the negative relationship between age and person-average anxiety, such that younger participants reported increased anxiety. This finding is consistent with previous research indicating that anxiety disorders typically onset in late adolescence (Kessler et al., 2005) and that the transition to college may be a sensitive period for increased anxiety, as well as sleep problems (Doane et al., 2015). Similarly, anxiety may decrease with age, as extant research suggests that anxiety disorder severity diminishes across adulthood (Ramsawh, Raffa, Edelen, Rende, & Keller, 2009), and older adults exhibit lower anxious responding to threat compared to younger adults (Teachman & Gordon, 2009). Thus, age may buffer the impact of sleep on anxiety. This finding highlights the importance of examining sleep and anxiety in a more age diverse sample to better characterize changes in sleep and anxiety across the lifespan.
The present findings indicate that nights characterized by less sleep than is typical for the individual predict increased anxiety the next day, even after controlling for previous levels of anxiety. Further, this effect is strongest in the morning and diminishes over the course of the day. Although average TST over the week was not linked to average anxiety in the present study, increased insomnia symptoms were associated with increased average anxiety over the week. These results are consistent with cognitive and cognitive-behavioral models of insomnia, which suggest that insomnia is maintained and perpetuated by worry and anxiety about insufficient sleep (Harvey 2005; Morin, 2004). Such a feedback loop between sleep and anxiety may distinguish those with chronic symptoms that reach clinical significance from those who are resilient to transient fluctuations in sleep and anxiety. These findings may have implications for the treatment of anxiety-related disorders. That is, treatments targeting deviations from normal sleep and general sleep disturbance may enhance anxiety interventions, particularly given that sleep problems often persist after treatment of anxiety-related disorders (Belleville, Guay, & Marchand, 2011). Indeed, previous research indicates that cognitive behavior therapy for insomnia (CBTI) improves anxiety symptoms (Belleville, Cousineau, Levrier, & St-Pierre-Delorme, 2011), and one recent study found that the effect of CBTI on mood and anxiety symptoms was mediated by decreased insomnia symptoms (de Bruin, Bogels, Oort, & Meijer, 2017).
An important strength of the present study is the measurement of both objective and subjective TST. Given that objective and subjective sleep estimates often differ (Lauderdale et al., 2008), the concordance observed between methods in the prediction of anxiety is notable. One explanation for such concordance is the ability to parse between- and within-person effects; that is, examining only the relations between average levels of processes that vary within and between days may miss relations of day to day variability. Given recent evidence for daily variability in sleep predicting daily variability in anxiety and related outcomes in unselected and clinical samples (Kalmbach et al., 2017; Short et al., 2017), EMA may be an important tool for future research examining the effects of sleep on symptoms of affective disorders.
Despite the strengths of the present approach, it is important to consider these findings within the context of the study limitations. First, this study utilized an unselected sample, and these findings may not generalize to anxiety-disordered populations. Similarly, the relative age and racial homogeneity of the present sample may limit generalizability to more diverse populations. Second, anxiety was assessed with a single-item. Additional research utilizing a multimethod anxiety assessment is necessary to characterize fully the impact of daily variability in sleep on daily variability in anxiety. Third, although temporal precedence of sleep assessment and the inclusion of lagged anxiety as a covariate strengthened the case for TST as a predictor of subsequent anxiety, a strong causal argument cannot be made without experimental manipulation of sleep. Future research examining the effect of a sleep restriction paradigm that is tailored to individual TST averages is necessary to fully examine the effect of less than average TST on subsequent anxiety. Fourth, the present study examined time of day effects on the relationship between TST and anxiety. An additional related question for future research regards the role of individual circadian phase, which may have a differential impact on the present effect. Fifth, the present design leaves unanswered the question of mechanism. That is, how might getting less than individual average TST contribute to increased anxiety the following day? Future research using EMA studies of daily sleep and anxiety may benefit from examining possible mediating processes, such as alterations in diurnal cortisol or deficits in cognitive function.
Decreased TST relative to personal average predicts next day anxiety
This effect is strongest in the morning
Average TST over a week is unrelated to average anxiety
Results are consistent across sleep measurement methods
Results are consistent when controlling for previous day’s anxiety
Acknowledgements
The authors would like to thank Jennifer Guenther, Eliza Kramer, Olivia Lee, Alex McIntyre, and Powell Newbern for assistance with data collection and Sarah Jessup for comments on an earlier draft of this manuscript.
Funding
This work was supported by a National Institute of Mental Health 1F31MH113271–01A1 and a Graduate Student Summer Research Award from Vanderbilt University awarded to the first author.
Footnotes
Conflict of Interest
The authors declare no conflict of interest.
There was no difference on other sleep measures between participants who received an actigraph and those who did not.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alvaro PK, Roberts RM, Harris JK, & Bruni O (2017). The direction of the relationship between symptoms of insomnia and psychiatric disorders in adolescents. Journal of Affective Disorders, 207, 167–174. http://dx.doi.org/10.1016/j.jad.2016.08.032 [DOI] [PubMed] [Google Scholar]
- Ancoli-Israel S, Cole R, Alessi C, Chambers M, Moorcroft W, & Pollak CP (2003). The role of actigraphy in the study of sleep and circadian rhythms. SLEEP, 26, 342–392. [DOI] [PubMed] [Google Scholar]
- Babson KA, Trainor CD, Feldner MT, & Blumenthal H (2010). A test of the effects of acute sleep deprivation on general and specific self-reported anxiety and depressive symptoms: An experimental extension. Journal of Behavior Therapy and Experimental Psychiatry, 41, 297–303. http://dx.doi.org/10.1016/j.jbtep.2010.02.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baglioni C, Nanovska S, Regen W, Spiegelhalder K, Feige B, Nissen C, … Riemann D. (2017). Sleep and mental disorders: A meta-analysis of polysomnographic research. Psychological Bulletin, 142, 969–990. http://dx.doi.org/10.1037/bul0000053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastien CH, Vallieres A, & Morin CM (2001). Validation of the Insomnia Severity Index as an outcome measure for insomnia research. Sleep Medicine, 2, 297–307. http://dx.doi.org/10.1016/s1389-9457(00)00065-4 [DOI] [PubMed] [Google Scholar]
- Batterham PJ, Glozier N, & Christensen H (2012). Sleep disturbance, personality and the onset of depression and anxiety: Prospective cohort study. Australian & New Zealand Journal of Psychiatry, 46, 1089–1098. http://dx.doi.org/10.1177/0004867412457997 [DOI] [PubMed] [Google Scholar]
- Belleville G, Cousineau H, Levrier K, & St-Pierre-Delorme ME (2011). Meta-analytic review of the impact of cognitive-behavior therapy for insomnia on concomitant anxiety. Clinical Psychology Review, 31, 638–652. http://dx.doi.org/10.1016/j.cpr.2011.02.004 [DOI] [PubMed] [Google Scholar]
- Belleville G, Guay S, & Marchand A (2011). Persistence of sleep disturbances following cognitive-behavior therapy for posttraumatic stress disorder. Journal of Psychosomatic Research, 70, 318–327. http://dx.doi.org/10.1016/j.jpsychores.2010.09.022 [DOI] [PubMed] [Google Scholar]
- Blunden S & Galland B (2014). The complexities of defining optimal sleep: Empirical and theoretical considerations with a special emphasis on children. Sleep Medicine Reviews, 18, 371–378. http://dx.doi.org/10.1016/j.smrv.2014.01.002 [DOI] [PubMed] [Google Scholar]
- Borbely AA, Daan S, Wirz-Justice A, & Deboer T (2016). The two-process model of sleep regulation: A reappraisal. Journal of Sleep Research, 25, 131–143. http://dx.doi.org/10.1111/jsr.12371 [DOI] [PubMed] [Google Scholar]
- Carney CE, Buysse DJ, Ancoli-Israel S, Edinger JD, Krystal AD, Lichstein K, & Morin CM (2012). The Consensus Sleep Diary: Standardizing prospective sleep self-monitoring. SLEEP, 35, 287–302. http://dx.doi.org/10.5665/sleep.1642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cellini N, Buman MP, McDevitt EA, Ricker AA, & Mednick SC (2013). Direct comparison of two actigraphy devices with polysomnographically recorded naps in healthy young adults. Chronobiology International, 30, 691–698. http://dx.doi.org/10.3109/07420528.2013.782312 [DOI] [PubMed] [Google Scholar]
- Cox RC, Cole DA, Kramer EL, & Olatunji BO (2018). Prospective associations between sleep disturbance and repetitive negative thinking: The mediating roles of focusing and shifting attentional control. Behavior Therapy, 49, 21–31. https://doi.org/10.1016/j.beth.2017.08.007 [DOI] [PubMed] [Google Scholar]
- Cox RC & Olatunji BO (2016). A systematic review of sleep disturbance in anxiety and related disorders. Journal of Anxiety Disorders, 37, 104–129. http://dx.doi.org/10.1016/j.janxdis.2015.12.001 [DOI] [PubMed] [Google Scholar]
- Cronbach LJ (1976). Research on classrooms and schools: Formulation of questions, design, and analysis. Stanford, CA: Stanford University Evaluation Consortium. [Google Scholar]
- de Bruin EJ, Bogels SM, Oort FJ, & Meijer AM (in press). Improvements of adolescent psychopathology after insomnia treatment: Results from a randomized controlled trial over 1 year. The Journal of Child Psychology and Psychiatry. http://dx.doi.org/10.1111/jcpp.12834 [DOI] [PubMed] [Google Scholar]
- Doane LD, Gress-Smith JL, & Breitenstein RS (2015). Multi-method assessments of sleep over the transition to college and the associations with depression and anxiety symptoms. Journal of Youth and Adolescence, 44, 389–404. https://doi.org/10.1007/s10964-014-0150-7 [DOI] [PubMed] [Google Scholar]
- Edmondson D, Arndt J, Alcantara C, Chaplin W, & Schwartz JE (2015). Self-esteem and the acute effect of anxiety on ambulatory blood pressure. Psychosomatic Medicine, 77, 833–841. https://doi.org/10.1097/PSY.0000000000000219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmenhorst D, Kroll T, Matusch A, & Bauer A (2012). Sleep deprivation increases cerebral serotonin 2A receptor binding in humans. SLEEP, 35, 1615–1623. http://dx.doi.org/10.5665/sleep.2230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, & Conde JG (2009). Research electronic data capture (REDCap): A metadata-driven methodology and workflow process for providing translational research informatics support. Journal of Biomedical Informatics, 42, 377–381. https://doi.org/10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey AG (2005). A cognitive theory and therapy for chronic insomnia. Journal of Cognitive Psychotherapy, 19, 41–59. http://dx.doi.org/10.1891/jcop.19.1.41.66332 [Google Scholar]
- Hellhammer J & Schubert M (2012). The physiological response to Trier Social Stress Test relates to subjective measures of stress during but not before or after the test. Psychoneuroendrocrinology, 37, 119–124. https://doi.org/10.1016/j.psyneuen.2011.05.012 [DOI] [PubMed] [Google Scholar]
- Horne JA & Ostberg O (1976). A self-assessment questionnaire to determine morningnesseveningness in human circadian rhythms. International Journal of Chronobiology, 4, 97–110. [PubMed] [Google Scholar]
- Johnson EO, Roth T, & Breslau N (2006). The association of insomnia with anxiety disorders and depression: Exploration of the direction of risk. Journal of Psychiatric Research, 40, 700–708. https://doi.org/10.1016/j.jpsychires.2006.07.008 [DOI] [PubMed] [Google Scholar]
- Kalmbach DA, Pillai V, Roth T, & Drake CL (2014). The interplay between daily affect and sleep: A 2-week study of young women. Journal of Sleep Research, 23, 636–645. https://doi.org/10.1111/jsr.12190 [DOI] [PubMed] [Google Scholar]
- Kalmbach DA, Arnedt JT, Swanson LM, Rapier JL, & Ciesla JA (2017). Reciprocal dynamics between self-rated sleep and symptoms of depression and anxiety in young adult women: A 14-day diary study. Sleep Medicine, 33, 6–12. http://dx.doi.org/10.1016/j.sleep.2016.03.014 [DOI] [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, & Walters EE (2005). Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Archives of General Psychiatry, 62, 593–603. http://dx.doi.org/10.1001/archpsyc.62.6.593 [DOI] [PubMed] [Google Scholar]
- Kobayashi I, Boarts JM, & Delahanty DL (2007). Polysomnographically measured sleep abnormalities in PTSD: A meta-analytic review. Psychophysiology, 44, 660–669. http://dx.doi.org/10.1111/j.1469-8986.2007.537.x [DOI] [PubMed] [Google Scholar]
- Lauderdale DS, Knutson KL, Yan LL, Liu K, & Rathouz PJ (2008). Sleep duration: how well do self-reports reflect objective measures? The CARDIA Sleep Study. Epidemiology, 19, 838–845. http://dx.doi.org/10.1097/EDE.0b013e318187a7b0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Crain TL, McHale SM, Almeida DM, & Buxton OM (2017). Daily antecedents and consequences of nightly sleep. Journal of Sleep Research, 26, 498–509. http://dx.doi.org/10.1111/jsr.12488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCrae CS, McNamara JPH, Rowe MA, Dzierzewski JM, Dirk J, Marsiske M, & Craggs JG (2008). Sleep and affect in older adults: Using multilevel modeling to examine daily associations. Journal of Sleep Research, 17, 42–53. http://dx.doi.org/10.1111/j.1365-2869.2008.00621.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morin CM (2004). Cognitive-behavioral approaches to the treatment of insomnia. Journal of Clinical Psychiatry, 65, 33–40. [PubMed] [Google Scholar]
- Mullin BC, Pyle L, Haraden D, Riederer J, Brim N, Kaplan D, & Novins D (2017). A preliminary multimethod comparison of sleep among adolescents with and without generalized anxiety disorder. Journal of Clinical Child & Adolescent Psychology, 46, 198–210. https://doi.org/10.1080/15374416.2016.1220312 [DOI] [PubMed] [Google Scholar]
- Muthén LK and Muthén BO (1998–2012). Mplus User’s Guide. Seventh Edition. Los Angeles, CA: Muthén & Muthén. [Google Scholar]
- Neckelmann D, Mykletun A, & Dahl AA (2007). Chronic insomnia as a risk factor for developing anxiety and depression. SLEEP, 30, 873–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nota JA, Gibb BE, & Coles ME (2014). Obsessions and time of day: A self-monitoring study in individuals with obsessive-compulsive disorder. Journal of Cognitive Psychotherapy: An International Quarterly, 28, 134–144. http://dx.doi.org/10.1891/0889-8391.28.2.134 [DOI] [PubMed] [Google Scholar]
- Ohayon MM, Carskadon MA, Guilleminault C, & Vitiello MV (2004). Meta-analysis of quantitative sleep parameters from childhood to old age in healthy individuals: Developing normative sleep values across the human lifespan. SLEEP, 27, 1255–1273. [DOI] [PubMed] [Google Scholar]
- Ramsawh HJ, Raffa SD, Edelen MO, Rende R, & Keller MB (2009). Anxiety in middle adulthood: effects of age and time on the 14-year course of panic disorder, social phobia and generalized anxiety disorder. Psychological Medicine, 39, 615–624. https://doi.org/10.1017/S0033291708003954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy R, Palmer CA, Jackson C, Farris SG, & Alfano CA (2017). Impact of sleep restriction versus idealized sleep on emotional experience, reactivity and regulation in healthy adolescents. Journal of Sleep Research, 26, 516–525. https://doi.org/10.1111/jsr.12484 [DOI] [PubMed] [Google Scholar]
- Sadeh A, Sharkey KM, & Carskadon MA (1994). Activity-based sleep-wake identification: An empirical test of methodological issues. SLEEP, 17, 201–207. [DOI] [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavas J, Weiller E, . . . Dunbar GC. (1998). The Mini-International Interview (M. I. N. I.): The development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. Journal of Clinical Psychiatry, 59, 22–33. [PubMed] [Google Scholar]
- Shiffman S, Stone AA, & Hufford MR (2008). Ecological momentary assessment. Annual Review of Clinical Psychology, 4, 1–32. https://doi.org/10.1146/annurev.clinpsy.3.022806.091415 [DOI] [PubMed] [Google Scholar]
- Short NA, Allan NP, & Schmidt NB (2017). Sleep disturbance as a predictor of affective functioning and symptom severity among individuals with PTSD: An ecological momentary assessment study. Behaviour Research and Therapy, 97, 146–153. http://dx.doi.org/10.1016/j.brat.2017.07.014 [DOI] [PubMed] [Google Scholar]
- Slater JA, Botsis T, Walsh J, King S, Straker LM, & Eastwood PR (2015). Assessing sleep using hip and wrist actigraphy. Sleep and Biological Rhythms, 13, 172–180. http://dx.doi.org/10.1111/sbr.12103 [Google Scholar]
- Sterba SK (2014). Handling missing covariates in conditional mixture models under missing at random assumptions. Multivariate Behavioral Research, 49, 614–632. http://dx.doi.org/10.1080/00273171.2014.950719 [DOI] [PubMed] [Google Scholar]
- Teachman BA & Gordon T (2009). Age differences in anxious responding: Older and calmer, unless the trigger is physical. Psychology and Aging, 24, 703–714. http://dx.doi.org/10.1037/a0016813 [DOI] [PubMed] [Google Scholar]
- Thielsch C, Ehring T,. Nestler S, Wolters J, Kopei I, Rist F, … Andor T (2015). Metacognitions, worry and sleep in everyday life: Studying bidirectional pathways using Ecological Momentary Assessment in GAD patients. Journal of Anxiety Disorders, 33, 53–61. http://dx.doi.org/10.1016/j.janxdis.2015.04.007 [DOI] [PubMed] [Google Scholar]
- Van Cauter E, Polonksy KS, & Scheen AJ (1997). Roles of circadian rhythmicity and sleep in human glucose regulation. Endocrine Reviews, 18, 716–738. [DOI] [PubMed] [Google Scholar]
- Van Lenten SA & Doane LD (2016). Examining multiple sleep behaviors and diurnal salivary cortisol and alpha-amylase: Within- and between-person associations. Psychoneuroendocrinology, 68, 100–110. http://dx.doi.org/10.1016/j.psyneuen.2016.02.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verkuil B, Brosschot JF, & Thayer JF (2014). Cardiac reactivity to and recovery from acute stress: Temporal associations with implicit anxiety. International Journal of Psychophysiology, 92, 85–91. http://dx.doi.org/10.1016/j.ijpsycho.2014.03.002 [DOI] [PubMed] [Google Scholar]