Abstract
Background
Complex interactions between environmental and genetic factors influence the risk for developing alcohol use disorder (AUD) in humans. To date, studies of the impact of environment on AUD risk have primarily focused on psychological characteristics or on the effects of developmental exposure to ethanol. We recently observed that modifying levels of the long-chain ω-3 (LC ω-3) fatty acid, eicosapentaenoic acid (EPA), alters acute physiological responses to ethanol in C. elegans. Because mammals derive ω-3 fatty acids from their diet, here we asked if manipulating dietary levels of LC ω-3 fatty acids can affect ethanol-responsive behaviors in mice.
Methods
We used two well-characterized inbred mouse strains, C57BL/6J (B6) and DBA/2J (D2), that differ in their responses to ethanol. Age-matched young adult male mice were maintained on isocaloric diets that differed only by being enriched or depleted in LC ω-3 fatty acids. Animals were subsequently tested for acute ethanol sensitivity (locomotor activation and sedation), voluntary consumption, and metabolism. Fat deposition was also determined.
Results
We found that dietary levels of LC ω-3s altered ethanol sensitivity and consumption in a genotype-specific manner. Both B6 and D2 animals fed high LC ω-3 diets demonstrated lower ethanol-induced locomotor stimulation than those fed low LC ω-3 diets. Ethanol sedation and ethanol metabolism were greater in D2, but not B6 mice on the high LC ω-3 diet. Conversely, LC ω-3 dietary manipulation altered ethanol consumption in B6, but not in D2 mice. B6 mice on a high LC ω-3 diet consumed more ethanol in a 2-bottle choice intermittent access model than B6 mice on a low LC ω-3 diet.
Conclusions
Because ethanol sensitivity is predictive of risk for developing AUD in humans, our data indicate that dietary LC ω-3 levels should be evaluated for their impact on AUD risk in humans. Further, these studies indicate that genetic background can interact with fatty acids in the diet to significantly alter ethanol-responsive behaviors.
Keywords: ω-3 fatty acid, eicosapentaenoic acid, ethanol sensitivity, ethanol consumption, ethanol locomotor activation
Introduction
Both genetic and environmental factors strongly influence the risk of developing alcohol use disorder (AUD) (Prescott and Kendler 1999). Genetics contribute approximately 50% of the risk, and non-genetic factors contribute the rest (Prescott and Kendler 1999). While identification of the genetic variants that confer risk for developing AUD has received considerable effort both in human and animal model studies, very little is understood about specific non-genetic variables that can influence liability. Much of the work in that area has focused on psychological and sociological factors that predispose individuals to AUD, but discrete environmental factors that physiologically influence liability to AUD have not been identified.
A strong predictor of the risk for developing an AUD is an individual’s acute physiological response to ethanol, called the “level of response” (LR). LR is a complex phenotype that is measured in relatively naïve (non-tolerant) individuals. A low LR is associated with increased risk for developing an AUD (Heath et al. 1999, Schuckit et al. 2001, Schuckit et al. 2004). Much effort has been placed on identifying the molecular mechanisms underlying LR using animal models, and variation in the human homologs of genes that affect LR in models are often found to have association with AUD in humans (Grotewiel and Bettinger 2015).
Much less is known about the modulation of physiological LR by environmental factors. We previously showed that altering the levels of the long-chain ω-3 (LC ω-3) polyunsaturated fatty acid eicosapentaenoic acid (EPA) in the nematode Caenorhabditis elegans (C. elegans) could modify acute behavioral responses to ethanol (Raabe et al. 2014). Specifically, we found that animals that are deficient in EPA are unable to develop acute functional tolerance (AFT) to ethanol. In contrast, when we provided supplemental dietary EPA, we could enhance AFT (Raabe et al. 2014). In C. elegans, EPA is generated from arachidonic acid through the function of the FAT-1 enzyme, an ω-3 fatty acyl desaturase. Vertebrates do not have this enzyme function, so EPA in mammals is derived directly from diet (reviewed in (Superko et al. 2013). Different diets can strongly influence the amount of EPA in human brain tissue which can subsequently influence brain function (Dyall 2015). Significant dietary supplementation of the LC ω-3 fatty acids EPA and docosahexaenoic acid (DHA) in the form of fish oil is extremely common (Superko et al. 2013), yet the impact that these supplements may have on ethanol-responsive behaviors remains unknown.
Given the profound effect of EPA on acute behavioral responses to ethanol in C. elegans, we hypothesized that manipulating levels of dietary LC ω-3s in mammals may alter ethanol-responsive behaviors. Here, we demonstrate that C57BL/6J (B6) and DBA/2J (D2) mice fed high or low LC ω-3 diets have different behavioral responses to ethanol, and that the effects of these dietary fatty acid levels on different ethanol response phenotypes are modulated by genetic background.
Materials and Methods
Male B6 and D2 inbred mice from Jackson Laboratory (Bar Harbour, ME) were delivered at 5 weeks of age and individually housed in polycarbonate ventilated cages (28.5x17.5x12.5 cm) with corncob bedding on a 12- hour light-dark cycle (lights on at 7 am). Animals were maintained on the standard Virginia Commonwealth University rodent diet (Harlan/Teklad 7012) for one week to allow them to acclimate to the environment. After one week, the animals were switched to the test diets for the remainder of the experiment.
Specialty diets from Research Diets, Inc. (New Brunswick, NJ) were manufactured to be isocaloric and otherwise identical except for the oil that contributed the fat component. We designed the diets to either augment or deplete polyunsaturated ω-3 fatty acids, which we altered by varying the oils that provided the fat calories: we used either safflower oil or a mix of safflower and menhaden (fish) oils. The fatty acids manipulated were the LC ω-3 acids eicosapentaenoic acid (EPA, C20:5), and docosahexaenoic acid (DHA, C22:6), provided by menhaden oil, and ω-6 fatty acids provided by safflower oil. The high LC ω-3 fatty acid diet (D04092709R) was modified AIN-93G Rodent Diet with 1% w/w safflower oil and 9% w/w menhaden oil in ½″ pellet form presenting a fatty acid profile containing 14.3% EPA and 9.3% DHA and an ω-3/ω-6 ratio of 3.3. The low LC ω-3 fatty acid diet (D044092701P) was modified AIN-93G Rodent Diet with 10% w/w Safflower oil in ½″ pellet form presenting a fatty acid profile containing no detectable LC ω-3 EPA or DHA and an ω-3/ω-6 ratio of 0.002 (the small amount of ω-3 provided is in the form of linolenic acid). The standard grain-based diet used by VCU (Harlan/Teklad 7012) has a ω-3/ω-6 ratio of 0.1, where the ω-3 provided is linolenic acid. The typical North American diet, which is low in LC ω-3s, has a ω-3/ω-6 ratio of 0.06 to 0.1 (Kris-Etherton et al. 2000).
Please note that most standard rodent diets provide ω-3 fatty acids only in the form of the vegetable-based ω-3 fatty acid, linolenic acid (C18:ω3). Linolenic acid is considered a short-chain polyunsaturated fatty acid, which is not functionally equivalent to either of the long-chain ω-3 fatty acids, EPA (C20:ω3) or DHA (C22:ω3). Therefore, when making comparisons between diets for ω-3 fatty acids concentrations, linolenic acid concentrations should not be used as a substitute for EPA (C20:ω3) or DHA (C22:ω3).
Each diet was stored under argon gas at −80˚C to minimize oxidation. Regulated diet (~30g) was placed into the appropriate cage once per week. Mouse weight (g) and the remaining weight of the food (g) was recorded weekly to obtain food consumption and body weight measures. Leftover food was discarded.
Fatty acid extraction and quantification
EPA, DHA and the ω-6 fatty acid arachidonic acid (AA) were measured using a modification of a method previously described (Di Marzo et al. 2000). A seven-point calibration curve with a range of 100 to 10000 ng/mL, and a 0 ng/mL control were prepared in parallel with each batch of samples. 50 ng each of deuterated internal standards, EPA-d5, DHA-d5 and AA-d5 (Cayman Medical Company, Ann Arbor, Michigan) were added to each experimental sample (0.1g of tissue or 0.1 mL of fluid), calibration sample and 0 ng/mL control. 1 mL chloroform/methanol (2:1, v/v) was added to the samples. Tissue samples were then homogenized using an IKA-Labortechnik Ultra-Turrax T25® homogenizer (Wilmington, NC). 0.2 mL of 0.73% sodium chloride was added to each sample prior to mixing and centrifugation. The chloroform was collected and reserved. The aqueous phase was then extracted once more with chloroform/methanol, and the chloroform fractions for each sample were pooled and evaporated to dryness under nitrogen. These extracts were reconstituted with 100 μL mobile phase and placed in auto-sampler vials for HPLC/MS/MS analysis.
The HPLC/MS/MS analysis was performed on an Applied Biosystems 3200 Q trap with a turbo V source for TurbolonSpray or a Sciex 6500+ (Ontario, Canada) attached to a Shimadzu SCL HPLC system (Kyoto, Japan) controlled by Analyst 1.4.2 software. Chromatographic separation was performed on Discovery® HS C18 Column (15cm x 2.1mm, 3μm; Supelco: Bellefonte, PA).
Determining the Effects of Dietary Manipulation on ω-3 and ω-6 Concentrations
After one week of acclimation to the vivarium, mice were separated into diet treatment groups (n=20/genotype/diet) for a minimum of 9 weeks in order to stablize blood fatty acid levels. We measured blood concentrations of the LC ω-3 fatty acids DHA and EPA and the ω-6 fatty acid AA after the animals had been maintained on the experimental diets for nine weeks. Blood was collected from the submandibular vein (150 μL). Behavioral testing began after 9 weeks of experimental diet feeding. Mice remained on the assigned diets ad libitum throughout the experiments.
Ethanol-Induced Locomotor Activity
Mice were tested after 9 – 11 weeks on the experimental diets (n=20/diet/genotype). Mice were habituated to i.p. injections with normal saline (0.9% w/v) for two days and habituated to the locomotor activity boxes (Omnitech Electronics, Inc, Columbus, OH) for 5 minutes on day 1 and 15 minutes on day 2. On the test day, mice were habituated to the testing room for one hour and habituated to the locomotor activity box for a 15-minute session, injected with 1.5 g/kg ethanol, 2.0 g/kg ethanol (10% w/v i.p. in 0.9% saline), or 0.9% saline and immediately tested for locomotor behaviors in a 15-minute test session. For blood ethanol concentration (BEC) measurements, blood was collected by submandibular bleed immediately upon removal from the locomotor boxes, approximately 15 minutes after the ethanol injection.
Intermittent Ethanol Consumption
B6 mice began intermittent 2-bottle choice drinking in the home cage beginning in week 14 (day 101) of diet treatment for a total of 20 ethanol sessions and D2 mice began drinking in week 13 (day 94) of experimental diet treatment for a total of 19 ethanol sessions. Mice (n=20/diet) were given intermittent access to two bottles containing 15% v/v ethanol in tap water or tap water alone in 15mL conical tubes with rubber stoppers fitted with a ball bearing sipper tube. Ethanol bottles were placed on cages every other day on alternating sides at the beginning of the dark cycle. Water and diet treatment was present ad libitum. Cages without animals but with water and 15% v/v ethanol bottles were used to correct for evaporation and bottle leakage.
Loss of Righting Reflex (LORR)
B6 mice (n=11 high and 13 low LC ω-3 diet) were tested on day 136 and 150 of dietary treatment. D2 mice (n=18 high and n=18 low LC ω-3 diet) were tested on day 137 and 138 of dietary treatment. B6 and D2 mice were administered 3.8 g/kg ethanol (18% v/v in 0.9% saline, i.p.) to test the effects of a sedating dose of ethanol. We used the cylindrical apparatus for LORR establishment as described in (Ponomarev and Crabbe 2002). LORR is considered to be established after a mouse cannot right itself from the supine position after 5 seconds; this is tested with 2 consecutive turns of the cylindrical apparatus spaced 2–3 seconds apart. LORR onset was calculated as the time from ethanol administration to the time at the end of the 5 second period that the mouse remained in the supine position. Immediately after LORR onset, the mice were placed in the supine position in the V-cut of the feeding trough of empty feeding trays. Time to recovery of the righting reflex (LORR duration) was determined when the mouse righted to the prone position, placing all four paws on the feeding trough twice within a 30 second period. 150 μL blood was collected immediately after regaining the righting reflex. One B6 high ω-3 mouse was excluded due to probable misplaced ethanol injection.
Analysis of Ethanol Metabolism
An ethanol time course for 3.8 g/kg ethanol (18% v/v in 0.9% saline, i.p.) was conducted on days 166 and 170 of diet treatment for B6 mice and days 169 and 170 of diet treatment for D2 mice. After ethanol administration and the loss of righting reflex was established as described in the LORR procedure, blood samples (20 μL) were collected at 15, 30, 60 and 90 minute intervals.
BEC Determination
Blood was collected in heparinized tubes via retro-orbital or submandibular bleeds. Samples were stored at −80°C and then measured using head-space chromatography. The blood ethanol content was determined using a modified method routinely employed for the analysis of clinical and forensic samples for ethanol (Poklis et al. 2017). A seven-point calibration curve was prepared from 100 to 8000 mg/L ethanol. Analysis was performed using a Tekmar HT3 headspace autosampler attached to a Shimadzu 2014 Gas Chromatograph (GC) with a flame ionization detector (FID). The chromatographic separation was performed on a RTX-BAC1, 30 m x 0.32 mm id x 1.80 μM column (Restek Corp, Bellefonte, PA). The limit of detection for all volatiles was 50 mg/L with a determined linear range of 50 to 8000 mg/L for the assay.
Tissue Harvest
B6 (n=12 high LC ω-3 diet, n=13 low LC ω-3 diet) mice were sacrificed on day 208 of dietary treatment. D2 (n=20 high LC ω-3 diet; n=18 low LC ω-3 diet) mice were sacrificed on day 241 of dietary treatment. At the time of harvest, subcutaneous, gonadal, mesenteric, and intrascapular brown fat tissues were weighed and recorded. The heart, liver and microdissected brain regions were collected and stored at −80°C for future analysis. Body metrics including body weight (g), body density, and body length (cm) were recorded in order to determine the percentage of body-fat relative to mouse body mass. We determined body density by dividing dry body weight by the dry body weight minus wet/immersed body weight. We assumed that the residual volume of gas in the body of the mouse was small and was similar between subjects.
Statistics
We analyzed B6 and D2 data separately because we were interested in the relative changes due to dietary manipulation within each strain, and we did not compare data between strains. For fatty acid modulation, Student’s t-Tests were used to compare fatty acid levels between diets (high LC ω-3 vs. low LC ω-3). Two-way repeated measures (RM) ANOVAs were used for ethanol locomotor activation assays within each diet with ethanol dose (saline, 1.5 g/kg and 2.0 g/kg) as a factor and time (5 min, 10 min and 15 min) as the RM. For ethanol consumption and preference, two-way RM ANOVA were used between diet groups with a Tukey post-hoc test. Two-tailed, unpaired t-Tests were used to calculate significance for the loss of righting reflex assay. Two-way RM ANOVAs were used for ethanol pharmacokinetic studies. For all analyses, we used SigmaPlot (version 13) and significance was set at p < 0.05.
Results
Dietary manipulation strongly influenced ω-3 and ω-6 fatty acid profiles in B6 and D2 mice
Preliminary testing suggested that blood fatty acid concentrations stabilized after 8 weeks of dietary treatment, so we tested the fatty acid profile of animals after nine weeks on the experimental diets. We found that the dietary manipulation significantly altered EPA, DHA and AA concentrations relative to our initial measurements in both B6 and D2 mice. In B6 mice on a high LC ω-3 diet, EPA and DHA concentrations were significantly increased while AA concentrations were decreased as compared to B6 mice on a low LC ω-3 diet that significantly decreased EPA and DHA concentrations (Table 1; p<0.001 for all measures). Similarly, in D2 mice on high LC ω-3 diet, EPA and DHA concentrations were significantly higher and AA concentrations were significantly lower than D2 mice on a low LC ω-3 diet (p<0.001).
Table 1. ω-3 and ω-6 fatty acid profiles after 9 weeks of special diet.
Data are presented as mean (SEM).
| B6 | D2 | |||
|---|---|---|---|---|
| High ω-3 diet | Low ω-3 diet | High ω-3 diet | Low ω-3 diet | |
| EPA (ng/ml) | 2916.5 (176.1) | 0 (0)* | 4621.9 (344.0) | 0 (0) * |
| DHA (ng/ml) | 3962.5 (313.2) | 340.4 (11.4) * | 7849.8 (560.2) | 252.3 (50.8) * |
| AA (ng/ml) | 1011.5 (49.0) | 2581.5 (96.4) * | 1587.8 (125.0) | 2959.1 (210.86) * |
p<0.001 as compared to high ω-3 diet
Dietary LC ω-3s influenced ethanol-induced locomotor activation in B6 and D2 mice
We asked two questions about the effects of dietary LC ω-3 levels on locomotion. First, we asked if basal locomotion was different in animals maintained on the two diets by measuring locomotion for 15 minutes after an ip saline injection. We found that the diets did not alter the basal locomotor activity relative to each other in either the B6 or D2 strains (B6: F(1,35)=3.215, p=0.103, D2: F(1,35)=1.401, p=0.264).
Second, we examined ethanol-induced locomotor activation as an initial measure of LR to ethanol in animals in which LC ω-3 levels had been manipulated. Ethanol can induce acute locomotor activation in a genotype-dependent manner in mice; D2 animals demonstrate a more robust activation response than B6 animals (Dudek et al. 1991, Rose et al. 2013). We tested animals for 15 minutes after an injection of 0, 1.5 or 2.0 g/kg ethanol. We found that dietary manipulation influenced ethanol-induced locomotor activation in both strains (Figure 1).
Figure 1. Ethanol locomotor activation is lower in B6 and D2 mice fed a high ω-3 diet.
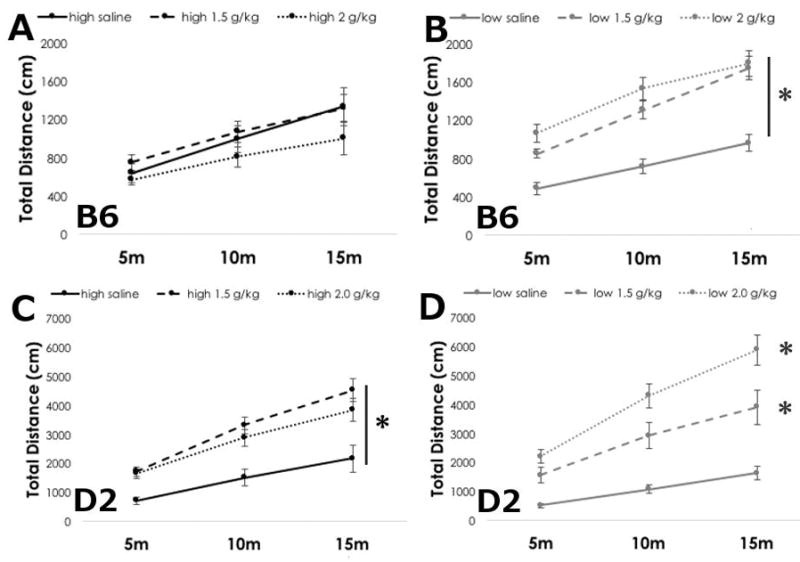
Mean +/− SEM cumulative locomotor activity of B6 mice on (A) high ω-3 diet or (B) low ω-3 diet after saline, or ethanol at 1.5 and 2.0 g/kg. Mean +/− SEM cumulative locomotor activity of D2 mice on (C) high ω-3 or (D) low ω-3 diet after saline, or ethanol at 1.5 or 2.0 g/kg. *p<0.05 vs. saline, main effect of diet by RM ANOVA.
Dietary LC ω-3 levels dramatically altered ethanol-induced locomotor activation responses in B6 mice. In agreement with previous reports that B6 animals do not demonstrate significant locomotor activation (Dudek et al. 1991, Rose et al. 2013), we found that B6 mice maintained on the high LC ω-3 diet were not activated in this assay (Figure 1A). Neither ethanol dose was significantly different than saline, and there was no main effect of dose on B6 mice (F(2,59)=1.367, p=0.280), or interaction between dose and time (F(4,59)=0.873, p=0.490). As expected, locomotor activity did increase over time (F(2,59)=47.130, p<0.001)
In marked contrast, we found that B6 animals on the low LC ω-3 diet expressed strong locomotor activation at both doses of ethanol (Figure 1B). Two-way RM ANOVA revealed significant main effects of dose (F(2,59)=19.989, p<0.001) and time (F(2,59)=108.926, p<0.001), as well as interactions between them (F(4,59)=3.473, p=0.018). Locomotor activity in saline treated mice was significantly lower than ethanol treated mice, but the effects of the two ethanol doses did not differ from each other.
D2 mice have been reported to exhibit a more robust ethanol-induced locomotor activation than B6 mice (Dudek et al. 1991, Rose et al. 2013), and we found that D2 mice demonstrated significant locomotor activation at both ethanol doses regardless of diet (Figure 1C&D). Interestingly, however, D2 mice on the high LC ω-3 diet had a less robust activation compared to mice on the low LC ω-3 diet. In D2 mice on the high LC ω-3 diet, we found significant main effects of dose (F(2,59)=6.943, p=0.006), and time (F(2,59)=125.433, p<0.001), and significant interactions between the two (F(4,59)=4.053, p<0.001). Ethanol-induced locomotor activity at 1.5 g/kg ethanol was not significantly different than 2.0 g/kg ethanol at any time point, indicating that, like in the B6 mice, dietary fatty acid levels had strong effects on the behavioral responses to ethanol. Locomotor activity at both ethanol doses were significantly greater than saline.
In D2 mice on a low LC ω-3 diet, we noted a dose- and time-dependent increase in locomotor activity (Figure 1D). Two-way RM ANOVA revealed significant main effects of dose (F(2,59)=18.046, p<0.001), and time (F(2,59)=148.959, p<0.001), and significant interactions between the two (F(4,59)= 14.174, p<0.001). Ethanol dose-dependently increased locomotor activity over saline-treated controls through the course of the assay.
Dietary LC ω-3 levels did not affect metabolism of 1.5 g/kg or 2 g/kg of ethanol
We determined BECs at 15 minutes after injection (Table 2). We found that BECs in B6 mice were not significantly different between diets (F(1,39)=1.124, p=0.264), but, as expected, were greater in the mice injected with 2.0 g/kg compared to the 1.5 g/kg dose (F(1,39)=26.925, p<0.001). No significant interactions between diet and dose were found (F(1,39)=0.0778, p=0.782).
Table 2. Blood ethanol content in B6 and D2 mice after ethanol-induced locomotor activation.
Data are presented as mean (SEM).
| B6 | D2 | |||
|---|---|---|---|---|
| High ω-3 diet | Low ω-3 diet | High ω-3 diet | Low ω-3 diet | |
| 1.5 g/kg ethanol | 171 (9.0) | 178 (6.5) | 228 (6.9) | 243 (14.3) |
| 2.0 g/kg ethanol | 215 (9.7) | 227 (10.2) | 276 (18.7) | 307 (16.0) |
We also found that BEC taken 15 min after the ethanol injection (Table 2) were not significantly different in D2 mice on the high or low LC ω-3 diets (F(1,39)=2.453, p=0.126). As expected, there was a significant difference in BEC between ethanol doses (F(1,39)=14.454, p<0.001) where the 1.5 g/kg ethanol dose gave lower BECs than the 2.0 g/kg dose. Ethanol dose and diet did not significantly interact (F(1,39)=0.297, p=0.589).
Dietary LC ω-3 levels influenced ethanol consumption in ethanol-preferring B6 mice, but did not change consumption in ethanol non-preferring D2 mice
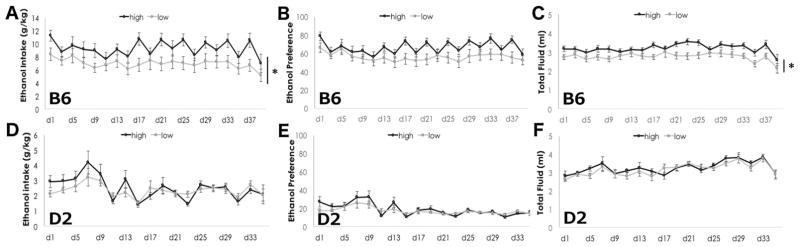
We measured ethanol consumption and preference using the intermittent access 2-bottle choice paradigm in which the animals were given the choice to consume 0 or 15% (v/v) ethanol every other day. Water was always available. We found that dietary LC ω-3 levels had a significant effect on ethanol consumption in B6 mice: B6 mice on the high LC ω-3 diet consumed more ethanol than B6 mice on the low LC ω-3 diet (Figure 2A). Two-way RM ANOVA revealed a main effect of diet (F(1,799)=4.156, p=0.049). On average, ethanol intake in B6 mice on a high LC ω-3 diet was 9.3 g/kg, while mice on a low LC ω-3 diet consumed 6.99 g/kg ethanol. We observed a main effect of time (F(1,19)=4.267, p<0.001), where the first day of ethanol intake was significantly greater than most other days (d9, d11, d15, d27, d35, and d39), but there were no significant interactions between diet and day (F(19,799)=1.325, p=0.159). Ethanol preference (Figure 2B) showed a trend towards significance for diet (F(1,797)=2.992, p=0.092) and there was a significant effect of day where day 1 preference was greater than most other days (d3, d7, d9, d11, d13, d15, d19, d23, d27, d35, d39). Total fluid consumed (Figure 2C) was also greater in B6 mice on the high LC ω-3 diet as compared to the low LC ω-3 diet (F(1,797)=5.848, p=0.020). Significant effects of day were also found for total fluid intake (F(19,797)=5.696, p<0.001) where fluid consumption on day 35 and day 39 was lower than all other days.
Figure 2. The level of dietary ω-3 fatty acids alters ethanol intake in B6, but not D2 mice.
Daily ethanol intake (A), preference (B) and total fluid intake (C) in B6 mice. Daily ethanol intake (D), preference (E), and total fluid intake (F) in D2 mice. Ethanol intake and total fluid were greater in B6 mice on a high ω-3 diet relative to B6 on a low ω-3 diet. Ethanol preference showed a trend towards increased preference in B6 mice on a high ω-3 diet relative to B6 on a low ω-3 diet. Ethanol intake, preference and total fluid intake were not altered by diet in D2 mice. Data are presented as mean +/− SEM.*p<0.05, main effect of diet by two-way RM ANOVA.
D2 mice consumed less ethanol than B6 mice, consuming only 2.4 g/kg ethanol in 24 hours using an intermittent 2-bottle choice assay (Figure 2D), and their ethanol intake was not significantly altered by diet (F(1,711)=0.697, p=0.409). Main effects of day were significant (F(17, 711)=5.065, p<0.001), where ethanol intake on days 7 and 9 were greater than most other days. Interaction between day and diet were not significant (F(17, 711)=0.977, p=0.459). Likewise, ethanol preference (Figure 2E) was not significantly different between diets (F(1,711)=1.38, p=0.247). We did note significant main effects of day (F(17, 711)=5.627, p<0.001) with days 7 and 9 ethanol preference greater than most other days. Interactions between diet and day were not significant (F(17,711)=0.926, p=0.543). Total fluid consumed (Figure 2F) was also not different in mice on the high LC ω-3 diet as compared to mice on the low LC ω-3 diet (F(1,711)=0.550, p=0.463). We observed significant effects of day for total fluid intake (F(17,711)=6.927, p<0.001) where fluid consumption on days 27, 29 and 33 were higher than most other days.
Sensitivity to a sedating dose of ethanol is dependent on dietary LC ω-3 levels in D2 mice, but not in B6 mice
We tested the sedative hypnotic effects of ethanol using the loss of righting reflex (LORR) assay. The sensitivity of B6 mice to a sedating/hypnotic dose was not significantly altered by LC ω-3 diet (Figure 3). Neither the latency to lose the righting reflex (Figure 3A, p=0.500) nor the duration of LORR was different between diets (Figure 3B, p=0.735). Mirroring the behavioral data, diet did not interact with BEC upon regaining the righting reflex (high LC ω-3 = 386 ± 22 mg/dL and low LC ω-3= 390 ± 16.4 mg/dL, p=0.907).
Figure 3. Sensitivity to a high dose of ethanol is greater in D2 mice on a high ω-3 diet.
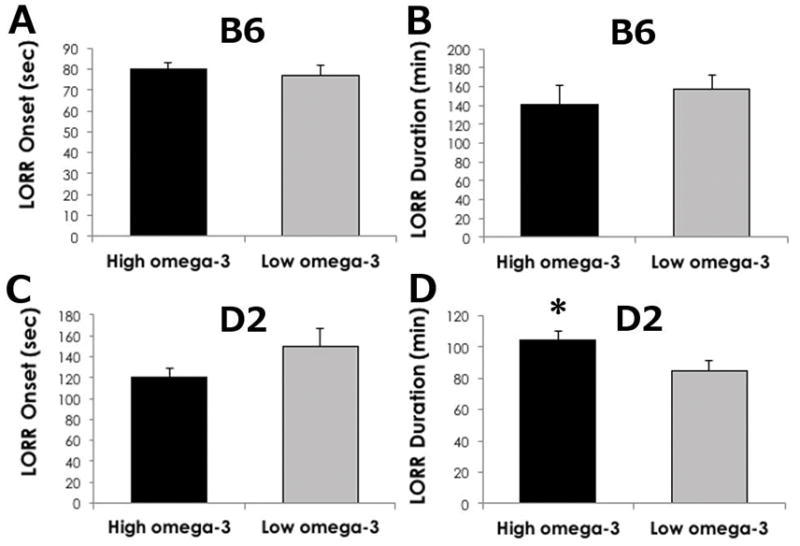
Latency to lose the righting reflex (in seconds) in (A) B6 and (C) D2 mice was not significantly altered by diet. The duration of sleep time (in minutes) was significantly greater in D2 mice (D) on high ω-3 diet relative to D2 mice on low ω-3 diet, but not in B6 mice (B). Data are reported as mean +/− SEM. *p<0.05 by t-Test in high vs. low ω-3 diet.
We found a very different result in D2 mice, in which dietary LC ω-3 levels significantly influenced sensitivity to a sedating/hypnotic dose of ethanol. The latency to lose the righting reflex was not altered by diet (Figure 3C, p=0.132). However, the duration of LORR was significantly greater in mice consuming the high LC ω-3 diet (Figure 3D, p=0.035), suggesting these mice are more sensitive to ethanol, or have a decreased development of acute functional tolerance to ethanol than mice on the low LC ω-3 diet. D2 mice fed the high LC ω-3 diet had lower BEC (366.1± 8.3 mg/dL) when they recovered the righting reflex compared to D2 mice fed a low LC ω-3 diet (404.9 ± 5.5 mg/dL, p=0.0004). These data indicate that the dietary manipulation of LC ω-3 fatty acids can strongly affect ethanol sensitivity.
Ethanol metabolism is influenced by dietary LC ω-3s in D2 mice, but not in B6 mice
We conducted a BEC time course using a high ethanol dose (3.8 g/kg, i.p.), and found that ethanol metabolism was not different between B6 mice on the high or low LC ω-3 diet (Figure 4A, F(1,110)=, p=0.703). As expected, BEC significantly decreased over time (F(3,110)=31.815, p<0.001), and we did not find significant interactions between diet and time (F(3,110)=0.115, p=0.951).
Figure 4. Ethanol metabolism is faster in D2 mice on high ω-3 diet.
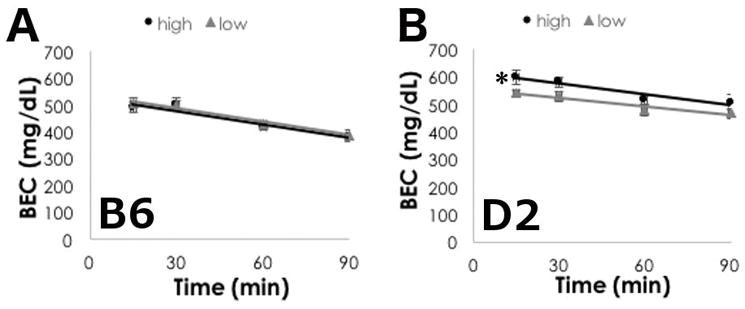
Mean +/− SEM of blood ethanol concentrations at 15, 30, 60 and 90 minutes after 3.8g/kg ethanol (i.p.) in (A) B6 and (B) D2 mice. Data are reported as mean +/− SEM. *p<0.05 for main effect of diet by two-way RM ANOVA.
We did, however, find that diet significantly altered ethanol metabolism in D2 mice. BECs were greater in D2 mice on the high LC ω-3 diet as compared to the low LC ω-3 diet (Figure 4B, F(1,75)=7.106, p=0.016), primarily driven by differences at the 15 and 30 minute time points. There was a significant effect of time (F(3,75)=8.077, p<0.001), but interactions between diet and time were not significant (F(3,75)=0.836, p=0.481).
Terminal body fat composition is different in D2 mice on high LC ω-3 diet
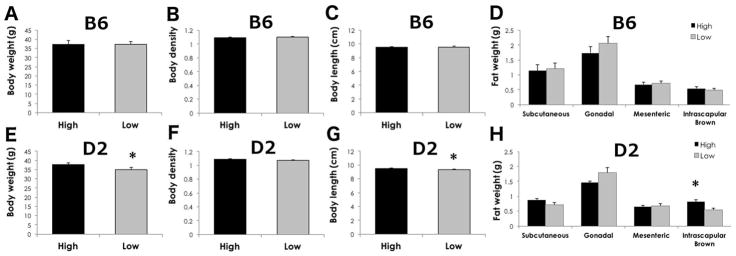
At the termination of the study, we collected wet and dry body weights to estimate differences in body density. We also collected four regions of fat pad deposition: subcutaneous, gonadal, mesenteric, and intrascapular brown fat to estimate relative differences in fat deposition in animals fed high and low LC ω-3 fatty acid diets. Given that the diets were matched to be isocaloric and contain similar amounts of total fat, we did not expect to see differences in body weight or fat composition. In B6 mice, none of the measures, body weight, body density, and all fat pad depositions, was significantly altered by diet (Figure 5, p>0.05). In D2 mice, however, body weight and body length (nose-anus) were slightly but significantly lower in mice on the low LC ω-3 diet (Figure 5, p=0.044 and p=0.047, respectively) relative to mice on the high LC ω-3 diet, suggesting that mice in the low LC ω-3 group were slightly smaller in body size. White fat pads and body density were not significantly different between D2 mice fed either diet (p>0.05). Interestingly, intrascapular brown fat was greater in D2 mice on the high LC ω-3 diet compared to mice on the low LC ω-3 diet (p=0.006).
Figure 5. D2 mice on high ω-3 diet have more intrascapular brown fat.
Mean +/− SEM of body weight (A, E), body density (B, F), body length (C, G) and fat pads (D, H) in B6 (A–D) and D2 mice. Body weight, body length and intrascapular brown fat were lower in D2 mice on low ω-3 diet (E–H). *p<0.05 by t-test in high vs. low ω-3 diet.
Discussion
There is much interest in identifying factors that predispose individuals to developing AUD. While genetic variation plays an important role, very little is understood about non-genetic factors that alter the molecular effects of alcohol, and thereby influence the propensity to abuse alcohol. In this study, we investigated the impact of manipulating the levels of dietary long-chain polyunsaturated ω-3 fatty acids on acute ethanol sensitivity and ethanol consumption in two genetically divergent mouse strains with different ethanol reponse phenotypes. We found that altering only the levels of LC ω-3 fatty acids and ω-6 fatty acids in otherwise identical diets could cause significant alterations in several behavioral responses to ethanol. Furthermore, using the C57/BL6 and DBA/2J strains of mice, we found that diet could interact with the genetic background to significantly alter different phenotypic measures of acute ethanol sensitivity and, importantly, voluntary ethanol consumption (Table 3).
Table 3.
Summary of results of behavioral assays. Data are reported for mice on the high ω-3 diet compared to mice on the low ω-3 diet.
| Assay | B6 mice | D2 mice |
|---|---|---|
| Ethanol-induced locomotor activation | High < Low | High < Low |
| BEC at low ethanol dose | no difference | no difference |
| LORR onset | no difference | no difference |
| LORR duration | no difference | High > Low |
| BEC at RORR | no difference | High < Low |
| Ethanol consumption | High > Low | no difference |
| Ethanol preference | High > Low (trend) | no difference |
| Total fluid intake | High > Low | no difference |
| Ethanol pharmacokinetics at 3.8 g/kg | no difference | High > Low |
BEC = blood ethanol content, LORR = loss of righting reflex, RORR = regaining of righting reflex
Altering dietary LC ω-3 levels had quite different effects on different ethanol response phenotypes in the two strains of mice. Low dose ethanol can cause locomotor activation in a manner that is modulated by genotype (Dudek et al. 1991, Rose et al. 2013). We found that in both strains, animals fed the high LC ω-3 diet had less ethanol-induced locomotor activation than animals fed the low LC ω-3 diet. As has been previously reported, we observed that D2 mice displayed greater ethanol-induced locomotor activity than B6 mice (Dudek et al. 1991). Dietary LC ω-3s modified this response; at low locomotor-activating doses of ethanol, D2 mice on the diet depleted in LC ω-3 fatty acids displayed a dose-dependent increase in locomotor activity after acute ethanol. This is similar to the reported behavior of D2 mice on standard laboratory chow (Rose et al. 2013). In D2 mice on the high LC ω-3 diet, however, while we did observe ethanol-induced locomotor activation, the mice did not display a dose-dependent increase in locomotor activity. We interpret this blunting to mean that the maximal activating response was achieved at low-to-moderate ethanol doses in D2 animals fed the high LC ω-3 diet, or, alternatively, that those animals are more sensitive to the sedating effects of ethanol at lower doses, masking any additional activating effects. In either case, these data suggest that high levels of LC ω-3 fatty acids decrease sensitivity to the acute locomotor-activing effects of ethanol in D2 mice.
Dietary LC ω-3 levels also tuned the acute ethanol sensitivity of B6 mice. Interestingly, in B6 mice, which do not always display robust ethanol-induced locomotor activation (Melon and Boehm 2011, Rose et al. 2013), we found that animals on a diet depeleted in LC ω-3 fatty acids demonstrated convincing ethanol-induced locomotor activation, but not in a dose-dependent manner. In contrast, B6 mice on a high LC ω-3 diet demonstrated no ethanol-induced locomotor activation. These data suggest that depleting LC ω-3 fatty acids decreased the animals’ sensitivity to the depressing effects of ethanol, revealing the locomotor activating effects at the 1.5 g/kg dose. These data are consistent with the results of a recent study in which fish oil supplements were reported to attenuate cocaine locomotor sensitivity in rats on a high-fat diet (Serafine et al. 2016). These differences in responses at low, locomotor-activating ethanol doses between animals on the experimental diets are not easily explained by simple differences in ethanol metabolism at this early time point. When measured immediately after the locomotor assay, we could not detect a difference in blood ethanol levels between animals on the different diet treatments.
We tested the effects of dietary LC ω-3 levels on the response to a high, sedating dose of ethanol in the loss of righting reflex assay, and found that here, too, there were differences in the effects of dietary manipulation of fatty acid levels in the two strains of mice. We found that while dietary manipulation had no effect on the LORR duration in B6 mice, D2 mice fed high LC ω-3 diet took significantly longer to recover the righting reflex than D2 mice on the low LC ω-3 diet, and the blood ethanol concentration upon waking was lower in the high LC ω-3-fed mice. These observations, combined with the locomotor activation data, suggest that the high LC ω-3 diet enhanced sensitivity to ethanol (i.e. ethanol sedation) in the D2 strain.
Intriguingly, when we examined a timecourse of ethanol metabolism after a 3.8 g/kg injection, we found that the diets also influenced blood ethanol concentrations in D2 mice. Animals fed the high LC ω-3 diet had slightly greater BECs early (at 15 and 30 minutes) during the ethanol time course, but were similar at the latter times compared to animals fed the low LC ω-3 diet. This suggests that there was a more rapid rate of ethanol elimination in D2 mice on high LC ω-3 diet. We were particularly struck by this result, because in human genetics studies, a commonly replicated genetic predictor of susceptibility to develop AUD is allelic variation in genes that regulate ethanol metabolism (Edenberg and Foroud 2014). Our data point to the possibility that dietary LC ω-3 fatty acid levels may influence ethanol metabolism, and thereby are good candidates for influencing alcohol dependence liability.
We found that animals on the two diets appeared to grow equally well, because their sizes, body weights, and overall body fat at the end of the experiment did not differ between dietary treatments. To determine if there was a more subtle difference in fat deposits, we examined the fat pads in the animals. In almost all cases, there was no difference in the weight of fat pads between animals on the two diets. We did find, however, that D2 animals maintained on the high LC ω-3 diet had more intrascapular brown fat. Because brown fat is involved in metabolism, this may point to a possible explanation for the faster rate of ethanol metabolism in these mice. Future experiments will be needed to determine how LC ω-3 fatty acid content and fat content/deposition play a role in ethanol sensitivity and metabolism.
Ultimately, our goal was to ask if modulating levels of dietary LC ω-3 fatty acids could modify voluntary ethanol consumption in mammals. We found that B6 mice drank more ethanol and demonstrated a trend for higher ethanol preference if they were maintained on a diet high in LC ω-3 compared to B6 mice on a diet depleted of LC ω-3s. Total fluid intake was also slightly increased in B6 mice on a diet high in LC ω-3. Importantly, this increased total fluid consumption is driven by increased drinking from the ethanol bottle by B6 mice on a high LC ω-3 diet since since there was a significant increase in the volume of ethanol consumed (p=0.024), but not a significant difference in the volume of water consumed (p=0.425). D2 mice tend not to drink ethanol due to its taste (McCool and Chappell 2014), and, indeed, we found that D2 mice did not drink large volumes of ethanol in the 2-bottle choice test and diet did not modify ethanol intake or preferences.
It is interesting to consider how these sensitivity phenotypes correspond to the original observations in C. elegans, where EPA levels acted like a rheostat to modify acute functional tolerance (AFT), which is reasonably similar to the murine LORR assay. In worms, more EPA enhanced AFT (decreased the sedation time), and loss of EPA inhibited AFT (increased sedation time) (Raabe et al. 2014). In contrast, here we show that in the D2 strain of mice, higher levels of LC ω-3s increased sedation time. One intriguing possibility is that the DHA in these studies, which was not tested in C. elegans, had important and opposite effects on ethanol sensitivity than did EPA. However, while we cannot yet reconcile these disperate results, it is not without precedent for the direction of effect of drugs in different organisms to be different. For example, in humans, opioids are strong behavioral depressants, whereas in rodents, opioids cause robust locomotor activation (Katz 1982).
Long-chain ω-3 fatty acids are derived from the diet in mammals, and there are significant differences in LC ω-3 levels in humans (reviewed in (Superko et al. 2013)). Many people routinely use fish oil supplementation to increase EPA and DHA levels, and this can have a significant effect on plasma LC ω-3 levels (Superko et al. 2013). Increasing evidence points to important roles for LC ω-3 fatty acids in neurobiological disorders such as bipolar disorder (reviewed in (Saunders et al. 2016)) and alcoholism.
This study clearly demonstrates that dietary supplementation of LC ω-3 fatty acids can both modify acute behavioral responses to ethanol and can influence ethanol drinking in mice. These data add to a growing appreciation that LC ω-3 fatty acids play important roles in neurobiological conditions across phyla. For example, in rats, addition of the LC ω-3 fatty acid DHA reduced alcohol consumption in a stress-reactive genetic animal model of bipolar disorder and co-morbid alcoholism, and in alcohol-preferring P rats (Le-Niculescu et al. 2011). In humans, dietary supplementation of LC ω-3 fatty acids was shown to decrease the anxiety and cortisol levels in abstinent alcoholics in a residential treatment facility (Barbadoro et al. 2013).
However, the complex relationship between LC ω-3 fatty acid levels and ethanol consumption is not yet fully understood. There are some suggestions that LC ω-3 levels are themselves modulated in response to ethanol consumption. Increased levels of DHA were found in the umbilical cord blood of infants exposed to ethanol during early gestation (Denkins et al. 2000), and dysregulation of LC ω-3 levels was correlated with alcoholic liver injury in very heavy drinking males seeking treatment (Vatsalya et al. 2016). In other studies, relationships between LC ω-3 levels and alcohol drinking are observed, but whether LC ω-3 levels influence alcohol drinking, or alcohol drinking modulates LC ω-3 levels is less clear. In healthy subjects in the human IMMIDIET study, higher alcohol consumption was associated with higher blood levels of both EPA and DHA in women, and with higher blood levels of EPA in men (di Giuseppe et al. 2009). In postmortem samples of human orbitofrontal cortex, higher levels of DHA were correlated with a greater severity of alcohol abuse (McNamara et al. 2008).
A relationship between diet and ethanol response phenotypes has been observed previously. Recently, Marshall et al. (2015) directly tested the possibility that differences in standard laboratory diets may play a role in intra-laboratory variability in ethanol response phenotypes, particularly in measures of voluntary consumption. While the important components of the diets were not identified, this study clearly demonstrated that diet alone can modify both ethanol consumption and acute LR phenotypes in B6 mice.
Our data demonstrate that the levels of dietary LC ω-3 fatty acids alter mechanisms in B6 and D2 mice to modify ethanol sensitivity and, in B6 mice, to modulate ethanol drinking. In humans, such changes in ethanol responses may have a meaningful impact on the risk for developing alcohol dependence (Heath et al. 1999, Schuckit et al. 2001, Schuckit et al. 2004). Importantly, we found that there was a significant impact of genetic background on the effects of dietary manipulation of LC ω-3 levels. While ethanol response phenotypes of both B6 and D2 mice were modified by dietary LC ω-3 levels, the individual phenotypes affected were somewhat different in the two different genetic backgrounds. This raises the intriguing possibility that the influence of the interaction between environmental and genetic factors on the liability to develop AUD may be explained in part by dietary factors such as LC ω-3 fatty acid levels. Further, these data strongly suggest that genetic variation in lipid metabolism machinery may be important in predisposing people to AUD, and is worthy of further study.
Acknowledgments
This project was supported by National Institutes of Health P50 AA022537 and P30 DA033934. We thank Dr. Michael Grotewiel, Dr. Michael F. Miles, and Dr. Tina Herfel for their thoughtful advice and comments on these studies.
Support: VCU-ARC: P50AA022537 and P30DA033934.
Footnotes
The authors declare no conflicts of interest.
References
- Barbadoro P, Annino I, Ponzio E, Romanelli RM, D’Errico MM, Prospero E, Minelli A. Fish oil supplementation reduces cortisol basal levels and perceived stress: a randomized, placebo-controlled trial in abstinent alcoholics. Mol Nutr Food Res. 2013;57:1110–4. doi: 10.1002/mnfr.201200676. [DOI] [PubMed] [Google Scholar]
- Denkins YM, Woods J, Whitty JE, Hannigan JH, Martier SS, Sokol RJ, Salem N., Jr Effects of gestational alcohol exposure on the fatty acid composition of umbilical cord serum in humans. Am J Clin Nutr. 2000;71:300S–6S. doi: 10.1093/ajcn/71.1.300s. [DOI] [PubMed] [Google Scholar]
- di Giuseppe R, de Lorgeril M, Salen P, Laporte F, Di Castelnuovo A, Krogh V, Siani A, Arnout J, Cappuccio FP, van Dongen M, Donati MB, de Gaetano G, Iacoviello L European Collaborative Group of the IP. Alcohol consumption and n-3 polyunsaturated fatty acids in healthy men and women from 3 European populations. Am J Clin Nutr. 2009;89:354–62. doi: 10.3945/ajcn.2008.26661. [DOI] [PubMed] [Google Scholar]
- Di Marzo V, Breivogel CS, Tao Q, Bridgen DT, Razdan RK, Zimmer AM, Zimmer A, Martin BR. Levels, metabolism, and pharmacological activity of anandamide in CB(1) cannabinoid receptor knockout mice: evidence for non-CB(1), non-CB(2) receptor-mediated actions of anandamide in mouse brain. J Neurochem. 2000;75:2434–44. doi: 10.1046/j.1471-4159.2000.0752434.x. [DOI] [PubMed] [Google Scholar]
- Dudek BC, Phillips TJ, Hahn ME. Genetic analyses of the biphasic nature of the alcohol dose-response curve. Alcohol Clin Exp Res. 1991;15:262–9. doi: 10.1111/j.1530-0277.1991.tb01867.x. [DOI] [PubMed] [Google Scholar]
- Dyall SC. Long-chain omega-3 fatty acids and the brain: a review of the independent and shared effects of EPA, DPA and DHA. Front Aging Neurosci. 2015;7:52. doi: 10.3389/fnagi.2015.00052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edenberg HJ, Foroud T. Genetics of alcoholism. Handb Clin Neurol. 2014;125:561–71. doi: 10.1016/B978-0-444-62619-6.00032-X. [DOI] [PubMed] [Google Scholar]
- Grotewiel M, Bettinger JC. Drosophila and Caenorhabditis elegans as Discovery Platforms for Genes Involved in Human Alcohol Use Disorder. Alcohol Clin Exp Res. 2015;39:1292–311. doi: 10.1111/acer.12785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heath AC, Madden PA, Bucholz KK, Dinwiddie SH, Slutske WS, Bierut LJ, Rohrbaugh JW, Statham DJ, Dunne MP, Whitfield JB, Martin NG. Genetic differences in alcohol sensitivity and the inheritance of alcoholism risk. Psychol Med. 1999;29:1069–81. doi: 10.1017/s0033291799008909. [DOI] [PubMed] [Google Scholar]
- Katz RJ. Morphine- and endorphin-induced behavioral activation in the mouse: implications for mania and some recent pharmacogenetic studies. Ann N Y Acad Sci. 1982;398:291–301. doi: 10.1111/j.1749-6632.1982.tb39501.x. [DOI] [PubMed] [Google Scholar]
- Kris-Etherton PM, Taylor DS, Yu-Poth S, Huth P, Moriarty K, Fishell V, Hargrove RL, Zhao G, Etherton TD. Polyunsaturated fatty acids in the food chain in the United States. Am J Clin Nutr. 2000;71:179S–88S. doi: 10.1093/ajcn/71.1.179S. [DOI] [PubMed] [Google Scholar]
- Le-Niculescu H, Case NJ, Hulvershorn L, Patel SD, Bowker D, Gupta J, Bell R, Edenberg HJ, Tsuang MT, Kuczenski R, Geyer MA, Rodd ZA, Niculescu AB. Convergent functional genomic studies of omega-3 fatty acids in stress reactivity, bipolar disorder and alcoholism. Transl Psychiatry. 2011;1:e4. doi: 10.1038/tp.2011.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall SA, Rinker JA, Harrison LK, Fletcher CA, Herfel TM, Thiele TE. Assessment of the Effects of 6 Standard Rodent Diets on Binge-Like and Voluntary Ethanol Consumption in Male C57BL/6J Mice. Alcohol Clin Exp Res. 2015;39:1406–16. doi: 10.1111/acer.12773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCool BA, Chappell AM. Persistent enhancement of ethanol drinking following a monosodium glutamate-substitution procedure in C57BL6/J and DBA/2J mice. Alcohol. 2014;48:55–61. doi: 10.1016/j.alcohol.2013.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNamara RK, Jandacek R, Rider T, Tso P, Stanford KE, Hahn CG, Richtand NM. Deficits in docosahexaenoic acid and associated elevations in the metabolism of arachidonic acid and saturated fatty acids in the postmortem orbitofrontal cortex of patients with bipolar disorder. Psychiatry Res. 2008;160:285–99. doi: 10.1016/j.psychres.2007.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melon LC, Boehm SL., 2nd Role of genotype in the development of locomotor sensitization to alcohol in adult and adolescent mice: comparison of the DBA/2J and C57BL/6J inbred mouse strains. Alcohol Clin Exp Res. 2011;35:1351–60. doi: 10.1111/j.1530-0277.2011.01471.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poklis JL, Wolf CE, 2nd, Peace MR. Ethanol concentration in 56 refillable electronic cigarettes liquid formulations determined by headspace gas chromatography with flame ionization detector (HS-GC-FID) Drug Test Anal. 2017;9:1637–40. doi: 10.1002/dta.2193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponomarev I, Crabbe JC. A novel method to assess initial sensitivity and acute functional tolerance to hypnotic effects of ethanol. J Pharmacol Exp Ther. 2002;302:257–63. doi: 10.1124/jpet.302.1.257. [DOI] [PubMed] [Google Scholar]
- Prescott CA, Kendler KS. Genetic and environmental contributions to alcohol abuse and dependence in a population-based sample of male twins. Am J Psychiatry. 1999;156:34–40. doi: 10.1176/ajp.156.1.34. [DOI] [PubMed] [Google Scholar]
- Raabe RC, Mathies LD, Davies AG, Bettinger JC. The omega-3 fatty acid eicosapentaenoic acid is required for normal alcohol response behaviors in C. elegans. PLoS One. 2014;9:e105999. doi: 10.1371/journal.pone.0105999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose JH, Calipari ES, Mathews TA, Jones SR. Greater ethanol-induced locomotor activation in DBA/2J versus C57BL/6J mice is not predicted by presynaptic striatal dopamine dynamics. PLoS One. 2013;8:e83852. doi: 10.1371/journal.pone.0083852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders EF, Ramsden CE, Sherazy MS, Gelenberg AJ, Davis JM, Rapoport SI. Omega-3 and Omega-6 Polyunsaturated Fatty Acids in Bipolar Disorder: A Review of Biomarker and Treatment Studies. J Clin Psychiatry. 2016;77:e1301–e8. doi: 10.4088/JCP.15r09925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuckit MA, Edenberg HJ, Kalmijn J, Flury L, Smith TL, Reich T, Bierut L, Goate A, Foroud T. A genome-wide search for genes that relate to a low level of response to alcohol. Alcohol Clin Exp Res. 2001;25:323–9. [PubMed] [Google Scholar]
- Schuckit MA, Smith TL, Anderson KG, Brown SA. Testing the level of response to alcohol: social information processing model of alcoholism risk--a 20-year prospective study. Alcohol Clin Exp Res. 2004;28:1881–9. doi: 10.1097/01.alc.0000148111.43332.a5. [DOI] [PubMed] [Google Scholar]
- Serafine KM, Labay C, France CP. Dietary supplementation with fish oil prevents high fat diet-induced enhancement of sensitivity to the locomotor stimulating effects of cocaine in adolescent female rats. Drug Alcohol Depend. 2016;165:45–52. doi: 10.1016/j.drugalcdep.2016.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Superko HR, Superko SM, Nasir K, Agatston A, Garrett BC. Omega-3 fatty acid blood levels: clinical significance and controversy. Circulation. 2013;128:2154–61. doi: 10.1161/CIRCULATIONAHA.113.002731. [DOI] [PubMed] [Google Scholar]
- Vatsalya V, Song M, Schwandt ML, Cave MC, Barve SS, George DT, Ramchandani VA, McClain CJ. Effects of Sex, Drinking History, and Omega-3 and Omega-6 Fatty Acids Dysregulation on the Onset of Liver Injury in Very Heavy Drinking Alcohol-Dependent Patients. Alcohol Clin Exp Res. 2016;40:2085–93. doi: 10.1111/acer.13197. [DOI] [PMC free article] [PubMed] [Google Scholar]