Abstract
Cystinosis is an autosomal recessive metabolic disease that belongs to the fa1mily of lysosomal storage disorders (LSDs). Initial symptoms of cystinosis correspond to the renal Fanconi syndrome. Patients then develop chronic kidney disease and multi-organ failure due to accumulation of cystine in all tissue compartments. LSDs are commonly characterized by a defective activity of lysosomal enzymes. Hematopoietic stem and progenitor cell (HSPC) transplantation is a treatment option for several LSDs based on the premise that their progeny will integrate in the affected tissues and secrete the functional enzyme, which will be recaptured by the surrounding deficient cells and restore physiological activity. However, in the case of cystinosis, the defective protein is a transmembrane lysosomal protein, cystinosin. Thus, cystinosin cannot be secreted, and yet, we showed that HSPC transplantation can rescue disease phenotype in the mouse model of cystinosis. In this review, we are describing a different mechanism by which HSPC-derived cells provide cystinosin to diseased cells within tissues, and how HSPC transplantation could be an effective one-time treatment to treat cystinosis but also other LSDs associated with a lysosomal transmembrane protein dysfunction.
Keywords: cystinosis, lysosomal storage disorders, hematopoietic stem and progenitor cells, gene therapy, lysosomal transfer, tunneling nanotubes
Introduction
Lysosomal storage disorders (LSDs) are a group of metabolic diseases characterized by a disruption of a lysosomal function leading to the storage of diverse macromolecules within lysosomes [1]. The progressive accumulation of incompletely degraded substrates in several tissues ultimately leads to multiple organ dysfunction and clinical complications that usually shorten the lifespan of affected children [1]. The majority of LSDs are due to defective activity of lysosomal hydrolases. For years, the only available treatment for LSDs was the enzyme replacement therapy (ERT) based on the discovery of Hasilik and colleagues that wildtype cells could secrete functional enzymes that are captured and directed to lysosomes of deficient cells through the mannose-6-phosphate receptor pathway [2]. Nine U.S. Food and Drug Administration (FDA)-approved ERT for the treatment of six LSDs (Mucopolysaccharidoses, MPS, I, II, and VI, Gaucher, Fabry, Pompe) are currently available and they are all administered by intravenous infusion, usually weekly or every other week, typically for the life of a patient [3]. Although ERT has improved patients’ life expectancy, the major limitation, beside a potential immune-response against the injected protein, is that administrated enzymes cannot cross the blood-brain barrier (BBB) which reduces treatment efficacy for LSDs with central nervous system (CNS) manifestations [4].
Among the ~ 50 known LSDs, 1/5 are caused by lysosomal membrane protein dysfunction [5]. Cystinosis, which belongs to this category, is an autosomal recessive metabolic disorder with an estimated incidence of 1/100,000 to 200,000 live births. The gene involved, CTNS gene, encodes for the 7 transmembrane H+-driven lysosomal cystine transporter, cystinosin [6–8]. Defects in CTNS results in accumulation of cystine, the oxidized dimer of the amino acid cysteine, within lysosomes resulting in intracellular crystal formation, cell death and eventually tissue damage. Because cystinosin is ubiquitously expressed, most of the organs are affected. However, depending on the type of mutation in the CTNS gene, there are different forms and severity of the disease [9, 10]. The most common and most severe form is infantile cystinosis (MIM 219800). Patients are normal at birth, but develop renal tubular Fanconi syndrome at 6–18 months of age, accompanied by failure to thrive, polyuria and polydipsia, dehydration, and hypophosphatemic rickets. Patients also develop chronic kidney disease that eventually leads to end stage renal failure requiring renal transplantation. They also present with photophobia and eventually later in life with retinal blindness, hypothyroidism, diabetes mellitus, muscle weakness and neurological defects [11]. There are two other forms of cystinosis that are rarer and less severe, juvenile cystinosis (MIM 219900) characterized by photophobia and glomerular and tubular alterations leading to proteinuria and eventually end-stage renal disease (ESRD) [12], and the ocular cystinosis (MIM 219750) defined by adult-onset of mild photophobia [13].
The current care for patients affected by cystinosis, beyond supportive therapy (dietary recommendations, indomethacin, angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, growth hormones, etc) [14], is the oral drug cysteamine (Mercapto-ethylamine), which allows lysosomal cystine clearance. Cysteamine has certainly improved the life expectancy of cystinotic patients and improved the disease outcome, especially if started before the age of 5 years [15]. However, this treatment delays disease progression, but is not a cure. Indeed, cysteamine fails to correct the Fanconi syndrome and only delays ESRD; dialysis and renal transplantation remain mandatory for most of the cystinotic patients [15–17]. Between supportive therapy and cysteamine, daily medication for a cystinotic patient can reach up to 60 pills. In addition, severe gastric side effects are often associated with cysteamine therapy. Therefore, there is a pressing need for a new therapy for cystinosis.
Hematopoietic Stem and Progenitor cell transplantation for non-hematopoietic diseases
In most cases, HSPC transplantation is used to treat blood-related diseases (e.g. blood or bone marrow malignancies, non-malignant blood disease, immunodeficiency disorders). However, HSPC transplantation can also treat non-hematopoietic disorders. Exploiting the property that HSPC can home to damage tissues, differentiate in hematopoietic cells and have a paracrine effect on neighboring cells, HSPC transplantation proved to be effective to treat LSDs that are due to defective lysosomal enzymes. Indeed, with a one-time intervention, bone marrow engrafted cells become a permanent source of healthy cells that can deliver functional lysosomal enzymes to neighbor diseased cells after integrating within tissues including the CNS [18]. Treatment of Hurler patients with HSPC transplantation has been the most gratifying. Indeed, bone marrow transplantation for severe MPS-I, performed before the age of two, prolongs survival and allows normal or near normal cognitive development and myocardial function [19]. Same results have been obtained for patients affected with Krabbe disease, a demyelinating disorder caused by a deficiency of galactosylceramidase if early transplantation is performed [20–22]. Nonetheless, for some LSDs (Sanfilippo or GM1 gangliosidosis), HSPC transplantation did not show clinical benefit [23, 24]. The reason for unsuccessful HSPC transplant is unclear. In the case of Sanfilippo, it is suggested that the donor-derived microglia cells secrete insufficient amounts of enzymes for cross-correction of the neuronal tissue [24].
Cystinosis belongs to a different class of LSD as the protein involved is a transmembrane lysosomal protein. However, the idea of HSPC transplantation for the multisystemic disorder, cystinosis, emerged from an attempt to try to find an optimal vehicle to bring the functional protein to damaged tissues. Studies have been performed in the mouse model of cystinosis, the Ctns−/− mice [25, 26]. Ctns−/− mice accumulate cystine and cystine crystals in all tissues and develop similar symptoms to those observed in patients, proximal tubulopathy and ESRD by 15 months of age, ocular anomalies, bone and muscular defects, behavioral anomalies, and hypothyroidism [26–30]. We first hypothesized that mesenchymal stem cells (MSCs) would be the best candidate since they are multipotent stromal cells that can be mobilized from the bone marrow and differentiate into a variety of cell type of tissue cells including osteoblasts, chondrocytes, myocytes and adipocytes [31]. Moreover, several studies showed that, in the setting of renal injury, transplanted MSCs could generate mesangial and tubular epithelial cells [32] and restore renal structure and function [33, 34]. In our model, MSC transplantation only led to some short-term improvement in tissue cystine content and we observed by confocal microscopy that Green Florescent Protein (GFP)-expressing MSCs did not integrate efficiently within tissues [35]. In parallel, we also performed syngeneic whole bone marrow cell (BMC) transplantation in lethally irradiated Ctns−/− mice, which, surprisingly, led to the dramatic reduction of cystine content in all tissues tested [35]. Therefore, we also investigated the impact of purified HSPCs. Transplantation of Sca1+ HSPCs, Sca1 being the murine homolog marker for CD34 in humans, in lethally irradiated Ctns−/− mice, was as efficient as BMC transplantation leading to significant tissue cystine decrease [35].
HSPC transplantation leads to multi-organ rescue in the Ctns−/− mice
Kidney Preservation
Kidney is the primary tissue compartment impacted by cystinosis with the development of the renal Fanconi syndrome at 6–18 months of age [36]. In addition, the glomerular filtration rate progressively starts to deteriorate after 2 years of age which leads to ESRD at the end of the first decade [37]. In the appropriate genetic background, Ctns−/− mice develop kidney pathology, in particular a mild renal Fanconi syndrome, and eventually end-stage renal failure but late in life (~18 months of age) as opposed to cystinosis patients [29]. Characteristic histological anomalies of the disease could be observed such as swan neck deformities at the glomerulo-tubular junction, thick basal membrane and perivascular mononuclear infiltrates, and cystine crystals are present within interstitial and proximal tubular cells. HSPC transplantation in Ctns−/− mice prevented the progression of the kidney disease in cystinosis. Indeed, this treatment led to long-term preservation of the kidney function and structure including the Fanconi syndrome, despite lack of HSPC reprogramming into proximal tubular cells (PTCs) [38]. However, effective therapy depended on achieving at least 50% of donor-derived blood cells engraftment of Ctns-expressing HSPCs within the bone marrow. This means that, at least 50% of the exogenous HSPCs have to express a functional CTNS gene in the bone marrow for the treatment to be fully effective in the Ctns−/− mice; the cells will then become a permanent source of circulating blood cells expressing CTNS. We also tested the impact of HSPC transplantation in older mice, between 6 and 10 months of age, when the disease is already established, and observed normal kidney function if the blood engraftment was sufficient [38]. This would suggest that if injury is not too advanced, the remaining kidney tissue could be rescued or protected by stem cell therapy. However, we do not know if established kidney injury could be reversed. Of note, since the mice male are fertile, in contrast to men affected with cystinosis, impact of HSPC transplantation on fertility could not be evaluated.
Eye pathology
The second tissue impacted by cystinosis is the eye and the main ocular manifestation is crystal deposition within the cornea. Crystals can be observed by slit lamp examination as early as one year of age, increases with age, and gradually leads to photophobia, blepharospasm, keratopathy, and recurrent corneal erosion [39]. In older patients, filamentous keratopathy, band keratopathy, and peripheral corneal neovascularization are also observed [39–41]. The mouse model for cystinosis also develops ocular pathology similar to humans [28, 30]. Cornea is a challenging tissue for stem cell-mediated therapy because it is avascular rendering its access by HSPC-derived cells difficult. However, abundant HSPC-derived cells could be observed in the cornea at one year post-transplantation, but also in the retina, lens and ciliary margin [42]. Ctns expression in the eye was increased and cystine level decreased. Using in vivo confocal microscopy, we showed that, if engraftment of Ctns-expressing HSPCs was sufficient (more than 50%), cystine build up was almost completely prevented in the Ctns−/− corneas. We also demonstrated that we could restore normal intra-ocular pressure as well as normal corneal thickness and structure.
These results demonstrate the versatility of the HPSCs and their therapeutic potential for corneal disorders. However, the limitation resides in the risk of bone marrow transplantation when the pathology is strictly localized to the eye. Recent studies investigated the feasibility of intravitreal HSPC transplantation in different mouse models of retinal degeneration, macular oedema or ischemia showing phenotypical improvement [43–45].
Hypothyroidism
Accumulation of cystine crystals also leads to impairment of endocrine tissues; hypothyroidism being the most common endocrine involvement [46]. The team of Dr. Courtoy previously showed that Ctns−/− mice present with impaired thyroid hormone production resulting in subclinical hypothyroidism, thyrocyte hyperplasia/hypertrophy and accelerated cell turnover [27]. In collaboration with Dr. Courtoy’s lab, we showed that wild-type HSPC transplantation into Ctns−/− mice decreased thyroid cystine content, normalized thyroid function (TSH and T4), prevented thyrocyte hyperplasia and hypertrophy, and improved biosynthetic and lysosomal overloaded in Ctns−/− thyroid [47].
Overall, these data show that one single systemic injection of HPSC is sufficient to address the serious global effects of cystinosis for the life of the mice. However, cystinosin is a lysosomal transmembrane protein that cannot be secreted and the mechanism by which HSPC participate to the phenotypical rescue of the Ctns−/− mouse model was then unclear.
Mechanism of action of HSPC in the case of cystinosis
Fate of the transplanted HSPCs within tissues
Our first hypothesis to explain the drastic effect of BMC and HSPC transplantation to prevent the development of cystinosis were: (1) Wildtype HSPCs were integrating within the diseased tissue and differentiating into proficient tissue-specific cells or, (2) HSPCs were fusing with the deficient Ctns−/− tissue cells. In order to investigate the fate of HSPCs after transplantation, we generated a new system consisting of a DsRed+ Ctns−/− mouse model, constitutively expressing the red fluorescent protein DsRed, and HSPCs isolated from wildtype GFP-transgenic donor mice. This dual fluorescent model allowed us to unequivocally discern fusion events (yellow cells) from differentiation/transdifferentiation (green). We observed that the majority of the bone marrow-derived cells were strictly GFP+, excluding fusion as the main mechanism for tissue repair. In conjunction with lineage-specific antibody staining, we identified the GFP-expressing cells as macrophages within tissues [48]. Macrophages are among the most plastic of immune cells with a large variety of phenotypes and physiological functions [49, 50]. However, their impact for tissue repair in the context of a transmembrane protein was unknown.
In vitro demonstration of lysosomal cross-correction
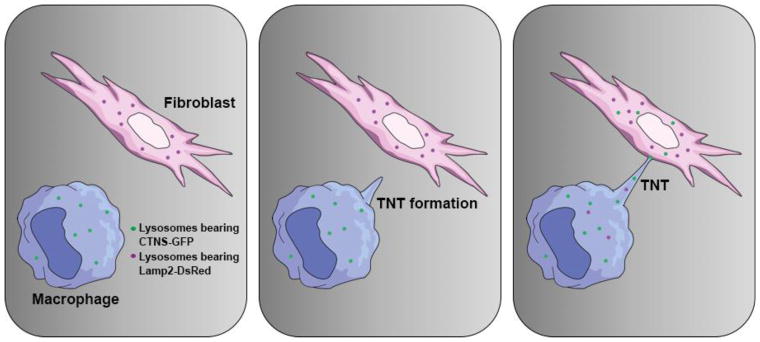
Cystinosin transfer from the HSPC-derived cells to the diseased cells was the most plausible explanation to account for the long-term tissue preservation in cystinosis. Cystinosin-containing microvesicles/exosomes shed by cystinosin-expressing cells have been shown as vehicles to decrease cystine in cystinotic cells [51, 52]. To address the mechanism in vitro, we established co-culture models using wildtype macrophages and DsRed+ Ctns/− fibroblasts. When wildtype macrophages were co-cultured with Ctns−/− fibroblasts, cystine levels decreased by ~75% in FACS-sorted fibroblasts whereas, when the two populations were physically separated using transwells porous to exclusively microvesicles, cystine levels decreased only by ~20%. These findings showed that direct cell:cell contact was necessary for an optimal cystinosin cross-correction as compared to microvesicle-mediated transfer. A large number of macrophages were extending long protrusions connecting to fibroblasts, also known as tunneling-nanotubes (TNTs) [48], which could be a route for cystinosin transfer. TNTs have been first described in vitro in 2004 by Rustom et al. [53] formed de novo between numerous cell types allowing complex connections between distant cells observed both in vitro [53, 54] and in vivo [55, 56]. They have been implicated in a wide variety of biological processes ranging from to bacterial and prion pathogenesis [57, 58] to calcium-mediated cellular communication [54, 59] but also lysosomal and mitochondrial trafficking [60, 61]. To verify the transfer of lysosomes via TNTs in our in vitro assays, we used macrophages transduced with self-inactivating lentivirus (SIN-LV) carrying the cDNA of the fusion protein cystinosin-GFP. Time-lapse confocal microscopy revealed that lysosomes (stained with LysoTracker) containing cystinosin-GFP migrated along the TNTs toward the fibroblasts [48]. Interestingly, we found that this transfer was bidirectional, cystinosin deficient lysosomes, marked with Lamp2-DsRed also travelled through TNTs to reach macrophages [48] (Figure 1). Lysosome fusion in both cell types probably occurs, releasing cystine in both cell types and providing a bidirectional correction, accounting for the efficient tissue cystine decrease.
Figure 1. Mechanism of in vitro lysosomal cross-correction via tunneling nanotubes.
Ctns−/− fibroblasts virally transduced to express the fusion protein Lamp2-DsRed are co-cultured with macrophages expressing Cystinosin-GFP. The macrophages extend tunneling-nanotubes (TNTs) toward the fibroblasts and establish an intercellular connection leading to a bidirectional exchange of lysosomes between both cell types. Fibroblasts are then rescued by the presence of healthy lysosomes carrying the functional cystinosin, while macrophages help discarding cystine-loaded lysosomes.
In vivo demonstration of lysosomal cross-correction within the Ctns−/− kidney
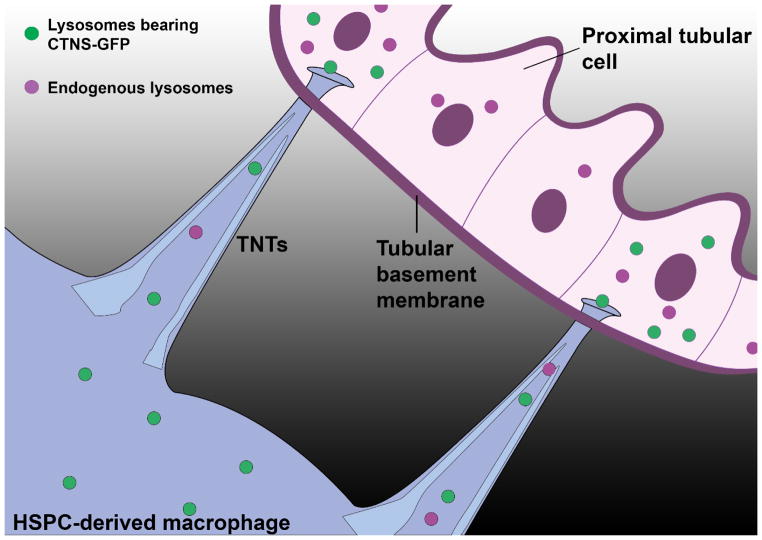
TNTs have been reported in vivo by others in the cornea [55] and lung [56], but not in a context of tissue repair. One of the primary and earliest sites affected in cystinosis is the proximal tubular cells (PTCs) responsible for the Fanconi syndrome. As mentioned earlier, we found that HSPC transplantation could prevent the kidney disease including the proximal tubulopathy [35, 38, 62]. And yet, PTCs are known to be protected from the interstitium by a thick tubular basement membrane (TBM) that strongly limits the passage of macromolecules [63]. By confocal microscopy, we observed that abundant GFP-expressing bone marrow-derived macrophages were indeed surrounding but never inside the proximal tubules in the Ctns−/− kidneys. However, long tubular protrusions were observed apposing on and even crossing the TBM [48]. To confirm that those nanotubes were able to deliver cystinosin-containing vesicles to PTCs, we transplanted Ctns−/− mice with DsRed+ HSPCs transduced with SIN-LV-CTNS-GFP, and observed GFP-positive lysosomes in the DsRed-expressing HSPC-derived macrophages surrounding the proximal tubules but also within the PTCs, providing a mechanism for the preservation of the proximal tubules in Ctns−/− mice [48] (Figure 2). To our knowledge, this is the first evidence of direct transfer of proteins/organelles from interstitial macrophages to epithelial cells via TNTs penetrating the TBM.
Figure 2. Mechanism of in vivo lysosomal cross-correction via tunneling nanotubes.
Transplanted Ctns−/− HSPCs ex-vivo transduced with SIN-LV carrying CTNS-GFP repopulate the bone marrow of Ctns−/− mice, migrate into the kidney where they differentiate into macrophages. Affected proximal tubular cells (PTCs) are protected from the extracellular environment by the tubular basement membrane (TBM). The rescue of PTCs requires that macrophages extend tunneling-nanotubes (TNTs) crossing the TBM to deliver functional cystinosin-bearing lysosomes and may be take away the endogenous cystine-loaded lysosomes (never shown in vivo) from the PTCs, accounting for the long-term recue of the proximal tubules in Ctns−/− treated by hematopoietic stem and progenitor cell (HSPC) transplantation.
Demonstration of bone marrow cell rescue via TNTs in another kidney disease
This novel mechanism of transmembrane lysosomal protein correction, leading to long-term kidney preservation after HSPC transplantation in cystinosis, may be expanded for treatment of other inherited renal disorders. Thus, Dr. Devuyst’s group recently tested bone marrow transplantation in a mouse model of Dent disease [64]. Dent disease (MIM #300009) is a rare X-linked tubulopathy caused by mutations in the endosomal chloride-proton exchanger (ClC-5) resulting in defective receptor-mediated endocytosis, severe proximal tubule dysfunction, low molecular weight proteinuria, kidney stones, and renal failure [65]. They demonstrated that transplantation of wild-type bone marrow cells in Clcn5Y/− mice significantly improved proximal tubule dysfunction. Similar to our findings, they observed that kidney-engrafted cells were mononuclear phagocytes found in the interstitium, surrounding proximal tubules, and also extending TNTs. In vitro experiments showed that cell:cell contact was also mandatory to rescue defective endocytosis suggesting that not only lysosomes but also endosomes could be transferred to diseased cells via TNTs [64].
Mechanism of action in non-nephropathic tissues
We also studied the mechanism of HSPC-mediated tissue repair in the eye and thyroid. In the cornea, HSPCs also differentiated into macrophages that were also capable of generating TNTs and delivering cystinosin-bearing lysosomes to diseased adjacent corneal cells [42]. Similarly, HSPCs differentiated into macrophages/dendritic cells in the thyroid frequently apposed onto the follicular basement laminae and generating TNTs. However, in addition, some HSPC-derived cells were able to entirely cross the membrane laminae of thyrocytes and fully squeeze into the epithelial monolayer [47].
Form Bench to Bedside: Hematopoietic Stem Cell Gene Therapy using SIN-lentivirus vectors
Ex vivo HSPC gene therapy as a treatment option for genetic disorders
Because allogeneic HSPC transplantation in patients requires a compatible donor and still represents a procedure with high risk of morbidity and mortality, graft versus host disease being the major complication [66]. In contrast, autologous ex vivo gene-corrected HSPC transplantation represents a safer treatment option because it abrogates the risk of GVHD and immune rejection. However, it requires the use of virus vectors to introduce a normal copy of the gene, which could be a limitation to achieve high enough gene-corrected cell level and could integrate near cancer genes. SIN-LVs are now used for most of the ex vivo gene correction of the cells as they have a great ability to transduce human HSCs, and contain only one internal enhancer/promoter, which reduces the incidence of interactions with nearby cellular genes and thus significantly decreases the risk of oncogenic integration [67]. Clinical trials using SIN-LV to transduce human CD34+ HSPCs are being undertaken in the U.S. and Europe for genetic diseases especially immune deficiency disorders (e.g: X-linked severe combined immunodeficiency, adenosine deaminase deficiency and Wiskott-Aldrich syndrome) [68–70]. In the context of LSDs, an important example of successful HSPC gene therapy approach using SIN-LV is for Metachromatic leukodystrophy, due to the deficiency of the lysosomal enzyme, Arylsulfatase A (ARSA). Nine patients have been treated so far with no vector-related toxicity reported. The first 3 patients have been reported to have extensive and stable ARSA expression in the periphery and in the cerebrospinal fluid, with no manifestation of disease from 7–21 months after the predicted age of symptom onset [71]. Autologous CD34+ cells transduced with a SIN-LV strategy was also successful for the treatment of X-linked cerebral adrenoleukodystrophy (ALD) [72, 73]. A total of 17 boys have been treated in a phase II/III study and after 29.4 months’ follow-up; they all presented with significant gene-marking and ALD protein was physiologically expressed [74].
HSPC gene therapy for cystinosis
We developed an ex-vivo gene-modified HSPC strategy using a SIN-LV carrying CTNS cDNA, pCCL-CTNS, and tested this approach in the mouse model of cystinosis. Preclinical studies showed that transduced HSPCs kept their differentiative capabilities, populating all tissue compartments and allowing long-term transgene expression [62]. Cystine content was decreased in all tissues tested and kidney function was improved. We are currently finishing the pharmacological and toxicological studies and assembling the Investigational New Drug (IND) application for a phase I/II clinical trial to assess the safety and efficacy of autologous transplantation of CD34+ HSPCs ex vivo modified using pCCL-CTNS in patients affected with infantile cystinosis. If successful, i.e. if we can achieve significant reduction of cystine level and restore normal cellular functions in the majority of diseased cells, this treatment could be a lifelong therapy that may eliminate or reduce renal deterioration and the need for kidney transplantation, as well as, the long-term complications associated with cystinosis. However, it is important to note that this treatment will be the first ex vivo HSPC gene therapy for a lysosomal storage disease for which the protein involved is a transmembrane lysosomal protein so cautious optimism is warranted on the efficacy of such a strategy in patients with cystinosis.
Conclusion
Despite the fact that the protein involved in cystinosis is a transmembrane lysosomal protein, HSPC transplantation proved to be efficient to rescue the pathology through their differentiation into macrophages within tissues and the transfer of cystinosin-bearing lysosomes via TNTs to adjacent host cells. This study not only allowed us to reveal for the first time a new potential curative property of the HSPC-derived cells for cystinosis but also demonstrates that HSPCs can act as intelligent vehicles to deliver functional organelle-associated proteins to defective cells in the entire body. These findings open new perspectives to treat diseases for which HSPC transplantation was not considered as a treatment option. Indeed, as mentioned earlier, a new pre-clinical study for Dent disease showed that an endosomal transmembrane protein could be transferred to surrounded the proximal tubular cells through TNTs and prevent the proximal tubulupathy [64]. Most recently, because mitochondria can also be transferred via TNTs [56, 75–77], we also investigated if HSPC transplantation could help to prevent the mitochondrial disorder Friedreich’s ataxia, a neuromuscular degenerative disorder, for which there is no treatment. We showed that HSPCs were able to migrate efficiently to all the sites of injury, i.e. the brain, spinal cord and dorsal root ganglia (DRGs), but also to the heart and skeletal muscle, and differentiate into microglia/macrophages and deliver frataxin to neurons and myocytes [78]. Locomotor deficits and muscle weakness were prevented as well as degeneration of the large sensory neurons in DRGs, and mitochondrial dysfunction was improved in these tissues. Altogether, these findings highlight the potential of HSPC for tissue repair and may expend their use to treat a wider panel of hereditary disorders.
Acknowledgments
We thank Adrien Rocca for the design of the figures. This work was supported by the National Institute of Health (NIH) RO1-DK090058, the Cystinosis Research Foundation and the California Institute of Regenerative Medicine (CIRM, CLIN-09230).
Footnotes
Competing Interests: S.C. is a Scientific Board member and member of the Board of Trustees of the Cystinosis Research Foundation. S.C. is a cofounder, shareholder and a member of both the scientific board and board of directors of GenStem Therapeutics Inc. The terms of this arrangement have been reviewed and approved by the University of California San Diego in accordance with its conflict of interest policies.
References
- 1.Pastores GM, Barnett NL. Current and emerging therapies for the lysosomal storage disorders. Expert Opin Emerg Drugs. 2005;10:891–902. doi: 10.1517/14728214.10.4.891. [DOI] [PubMed] [Google Scholar]
- 2.Hasilik A, Klein U, Waheed A, Strecker G, von Figura K. Phosphorylated oligosaccharides in lysosomal enzymes: identification of alpha-N-acetylglucosamine(1)phospho(6)mannose diester groups. Proc Natl Acad Sci U S A. 1980;77:7074–7078. doi: 10.1073/pnas.77.12.7074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ratko TA, Marbella A, Godfrey S, Aronson N. Enzyme-Replacement Therapies for Lysosomal Storage Diseases. Rockville, MD: Agency for Healthcare Research and Quality (US); 2013. Report No.: 12(13)-EHC154-EF. [PubMed] [Google Scholar]
- 4.Enns GM, Huhn SL. Central nervous system therapy for lysosomal storage disorders. Neurosurg Focus. 2008;24:E12. doi: 10.3171/FOC/2008/24/3-4/E11. [DOI] [PubMed] [Google Scholar]
- 5.Ruivo R, Anne C, Sagne C, Gasnier B. Molecular and cellular basis of lysosomal transmembrane protein dysfunction. Biochim Biophys Acta. 2009;1793:636–649. doi: 10.1016/j.bbamcr.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 6.Cherqui S, Kalatzis V, Trugnan G, Antignac C. The targeting of cystinosin to the lysosomal membrane requires a tyrosine-based signal and a novel sorting motif. J Biol Chem. 2001;276:13314–13321. doi: 10.1074/jbc.M010562200. [DOI] [PubMed] [Google Scholar]
- 7.Kalatzis V, Cherqui S, Antignac C, Gasnier B. Cystinosin, the protein defective in cystinosis, is a H(+)-driven lysosomal cystine transporter. EMBO J. 2001;20:5940–5949. doi: 10.1093/emboj/20.21.5940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Town M, Jean G, Cherqui S, Attard M, Forestier L, Whitmore SA, Callen DF, Gribouval O, Broyer M, Bates GP, van’t Hoff W, Antignac C. A novel gene encoding an integral membrane protein is mutated in nephropathic cystinosis. Nat Genet. 1998;18:319–324. doi: 10.1038/ng0498-319. [DOI] [PubMed] [Google Scholar]
- 9.Anikster Y, Shotelersuk V, Gahl WA. CTNS mutations in patients with cystinosis. Hum Mutat. 1999;14:454–458. doi: 10.1002/(SICI)1098-1004(199912)14:6<454::AID-HUMU2>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 10.Attard M, Jean G, Forestier L, Cherqui S, van’t Hoff W, Broyer M, Antignac C, Town M. Severity of phenotype in cystinosis varies with mutations in the CTNS gene: predicted effect on the model of cystinosin. Hum Mol Genet. 1999;8:2507–2514. doi: 10.1093/hmg/8.13.2507. [DOI] [PubMed] [Google Scholar]
- 11.Gahl WA, Thoene JG, Schneider JA. Cystinosis. New Engl J Med. 2002;347:111–121. doi: 10.1056/NEJMra020552. [DOI] [PubMed] [Google Scholar]
- 12.Goldman H, Scriver CR, Aaron K, Delvin E, Canlas Z. Adolescent cystinosis: comparisons with infantile and adult forms. Pediatrics. 1971;47:979–988. [PubMed] [Google Scholar]
- 13.Cogan DG, Kuwabara T, Kinoshita J, Sheehan L, Merola L. Cystinosis in an adult. J Am Med Assoc. 1957;164:394–396. doi: 10.1001/jama.1957.02980040034009. [DOI] [PubMed] [Google Scholar]
- 14.Emma F, Nesterova G, Langman C, Labbe A, Cherqui S, Goodyer P, Janssen MC, Greco M, Topaloglu R, Elenberg E, Dohil R, Trauner D, Antignac C, Cochat P, Kaskel F, Servais A, Wuhl E, Niaudet P, Van’t Hoff W, Gahl W, Levtchenko E. Nephropathic cystinosis: an international consensus document. Nephrol Dial Transplant. 2014;29(Suppl 4):iv87–94. doi: 10.1093/ndt/gfu090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brodin-Sartorius A, Tete MJ, Niaudet P, Antignac C, Guest G, Ottolenghi C, Charbit M, Moyse D, Legendre C, Lesavre P, Cochat P, Servais A. Cysteamine therapy delays the progression of nephropathic cystinosis in late adolescents and adults. Kidney Int. 2012;81:179–189. doi: 10.1038/ki.2011.277. [DOI] [PubMed] [Google Scholar]
- 16.Cherqui S. Cysteamine therapy: a treatment for cystinosis, not a cure. Kidney Int. 2012;81:127–129. doi: 10.1038/ki.2011.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gahl WA, Balog JZ, Kleta R. Nephropathic cystinosis in adults: natural history and effects of oral cysteamine therapy. Ann Intern Med. 2007;147:242–250. doi: 10.7326/0003-4819-147-4-200708210-00006. [DOI] [PubMed] [Google Scholar]
- 18.Biffi A. Hematopoietic Stem Cell Gene Therapy for Storage Disease: Current and New Indications. Mol Ther. 2017;25:1155–1162. doi: 10.1016/j.ymthe.2017.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Krivit W. Allogeneic stem cell transplantation for the treatment of lysosomal and peroxisomal metabolic diseases. Springer Semin Immunopathol. 2004;26:119–132. doi: 10.1007/s00281-004-0166-2. [DOI] [PubMed] [Google Scholar]
- 20.Caniglia M, Rana I, Pinto RM, Fariello G, Caruso R, Angioni A, Dionisi Vici C, Sabetta G, De Rossi G. Allogeneic bone marrow transplantation for infantile globoid-cell leukodystrophy (Krabbe’s disease) Pediatr Transplant. 2002;6:427–431. doi: 10.1034/j.1399-3046.2002.02026.x. [DOI] [PubMed] [Google Scholar]
- 21.Escolar ML, Poe MD, Provenzale JM, Richards KC, Allison J, Wood S, Wenger DA, Pietryga D, Wall D, Champagne M, Morse R, Krivit W, Kurtzberg J. Transplantation of umbilical-cord blood in babies with infantile Krabbe’s disease. New Engl J Med. 2005;352:2069–2081. doi: 10.1056/NEJMoa042604. [DOI] [PubMed] [Google Scholar]
- 22.Wright MD, Poe MD, DeRenzo A, Haldal S, Escolar ML. Developmental outcomes of cord blood transplantation for Krabbe disease: A 15-year study. Neurology. 2017;89:1365–1372. doi: 10.1212/WNL.0000000000004418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shield JP, Stone J, Steward CG. Bone marrow transplantation correcting beta-galactosidase activity does not influence neurological outcome in juvenile GM1-gangliosidosis. J Inherit Metab Dis. 2005;28:797–798. doi: 10.1007/s10545-005-0089-7. [DOI] [PubMed] [Google Scholar]
- 24.Welling L, Marchal JP, van Hasselt P, van der Ploeg AT, Wijburg FA, Boelens JJ. Early Umbilical Cord Blood-Derived Stem Cell Transplantation Does Not Prevent Neurological Deterioration in Mucopolysaccharidosis Type III. JIMD Rep. 2015;18:63–68. doi: 10.1007/8904_2014_350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cherqui S, Kalatzis V, Forestier L, Poras I, Antignac C. Identification and characterisation of the murine homologue of the gene responsible for cystinosis, Ctns. BMC Genomics. 2000;1:2. doi: 10.1186/1471-2164-1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cherqui S, Sevin C, Hamard G, Kalatzis V, Sich M, Pequignot MO, Gogat K, Abitbol M, Broyer M, Gubler MC, Antignac C. Intralysosomal cystine accumulation in mice lacking cystinosin, the protein defective in cystinosis. Mol Cell Biol. 2002;22:7622–7632. doi: 10.1128/MCB.22.21.7622-7632.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gaide Chevronnay HP, Janssens V, Van Der Smissen P, Liao XH, Abid Y, Nevo N, Antignac C, Refetoff S, Cherqui S, Pierreux CE, Courtoy PJ. A mouse model suggests two mechanisms for thyroid alterations in infantile cystinosis: decreased thyroglobulin synthesis due to endoplasmic reticulum stress/unfolded protein response and impaired lysosomal processing. Endocrinology. 2015;156:2349–2364. doi: 10.1210/en.2014-1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kalatzis V, Serratrice N, Hippert C, Payet O, Arndt C, Cazevieille C, Maurice T, Hamel C, Malecaze F, Antignac C, Muller A, Kremer EJ. The ocular anomalies in a cystinosis animal model mimic disease pathogenesis. Pediatr Res. 2007;62:156–162. doi: 10.1203/PDR.0b013e31809fda89. [DOI] [PubMed] [Google Scholar]
- 29.Nevo N, Chol M, Bailleux A, Kalatzis V, Morisset L, Devuyst O, Gubler MC, Antignac C. Renal phenotype of the cystinosis mouse model is dependent upon genetic background. Nephrol Dial Transplant. 2010;25:1059–1066. doi: 10.1093/ndt/gfp553. [DOI] [PubMed] [Google Scholar]
- 30.Simpson J, Nien CJ, Flynn K, Jester B, Cherqui S, Jester J. Quantitative in vivo and ex vivo confocal microscopy analysis of corneal cystine crystals in the Ctns knockout mouse. Mol Vis. 2011;17:2212–2220. [PMC free article] [PubMed] [Google Scholar]
- 31.Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 32.Yokoo T, Sakurai K, Ohashi T, Kawamura T. Stem cell gene therapy for chronic renal failure. Curr Gene Ther. 2003;3:387–394. doi: 10.2174/1566523034578221. [DOI] [PubMed] [Google Scholar]
- 33.Herrera MB, Bussolati B, Bruno S, Fonsato V, Romanazzi GM, Camussi G. Mesenchymal stem cells contribute to the renal repair of acute tubular epithelial injury. Int J Mol Med. 2004;14:1035–1041. [PubMed] [Google Scholar]
- 34.Morigi M, Imberti B, Zoja C, Corna D, Tomasoni S, Abbate M, Rottoli D, Angioletti S, Benigni A, Perico N, Alison M, Remuzzi G. Mesenchymal stem cells are renotropic, helping to repair the kidney and improve function in acute renal failure. J Am Soc Nephrol. 2004;15:1794–1804. doi: 10.1097/01.asn.0000128974.07460.34. [DOI] [PubMed] [Google Scholar]
- 35.Syres K, Harrison F, Tadlock M, Jester JV, Simpson J, Roy S, Salomon DR, Cherqui S. Successful treatment of the murine model of cystinosis using bone marrow cell transplantation. Blood. 2009;114:2542–2552. doi: 10.1182/blood-2009-03-213934. [DOI] [PubMed] [Google Scholar]
- 36.Brodehl J, Hagge W, Gellissen K. Changes in kidney function in cystinosis. I. Inulin, PAH and electrolyte clearance in various stages of the disease. Ann Paediatr. 1965;205:131–154. [PubMed] [Google Scholar]
- 37.Markello TC, Bernardini IM, Gahl WA. Improved renal function in children with cystinosis treated with cysteamine. New Engl J Med. 1993;328:1157–1162. doi: 10.1056/NEJM199304223281604. [DOI] [PubMed] [Google Scholar]
- 38.Yeagy BA, Harrison F, Gubler MC, Koziol JA, Salomon DR, Cherqui S. Kidney preservation by bone marrow cell transplantation in hereditary nephropathy. Kidney Int. 2011;79:1198–1206. doi: 10.1038/ki.2010.537. [DOI] [PubMed] [Google Scholar]
- 39.Gahl WA, Kuehl EM, Iwata F, Lindblad A, Kaiser-Kupfer MI. Corneal crystals in nephropathic cystinosis: natural history and treatment with cysteamine eyedrops. Mol Genet Metab. 2000;71:100–120. doi: 10.1006/mgme.2000.3062. [DOI] [PubMed] [Google Scholar]
- 40.Kaiser-Kupfer MI, Caruso RC, Minkler DS, Gahl WA. Long-term ocular manifestations in nephropathic cystinosis. Arch Ophthalmol. 1986;104:706–711. doi: 10.1001/archopht.1986.01050170096030. [DOI] [PubMed] [Google Scholar]
- 41.Tsilou ET, Rubin BI, Reed GF, Iwata F, Gahl W, Kaiser-Kupfer MI. Age-related prevalence of anterior segment complications in patients with infantile nephropathic cystinosis. Cornea. 2002;21:173–176. doi: 10.1097/00003226-200203000-00009. [DOI] [PubMed] [Google Scholar]
- 42.Rocca CJ, Kreymerman A, Ur SN, Frizzi KE, Naphade S, Lau A, Tran T, Calcutt NA, Goldberg JL, Cherqui S. Treatment of Inherited Eye Defects by Systemic Hematopoietic Stem Cell Transplantation. Invest Ophthalmol Vis Sci. 2015;56:7214–7223. doi: 10.1167/iovs.15-17107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Moisseiev E, Smit-McBride Z, Oltjen S, Zhang P, Zawadzki RJ, Motta M, Murphy CJ, Cary W, Annett G, Nolta JA, Park SS. Intravitreal Administration of Human Bone Marrow CD34+ Stem Cells in a Murine Model of Retinal Degeneration. Invest Ophthalmol Vis Sci. 2016;57:4125–4135. doi: 10.1167/iovs.16-19252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Park SS, Caballero S, Bauer G, Shibata B, Roth A, Fitzgerald PG, Forward KI, Zhou P, McGee J, Telander DG, Grant MB, Nolta JA. Long-term effects of intravitreal injection of GMP-grade bone-marrow-derived CD34+ cells in NOD-SCID mice with acute ischemia-reperfusion injury. Invest Ophthalmol Vis Sci. 2012;53:986–994. doi: 10.1167/iovs.11-8833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Siqueira RC, Messias A, Gurgel VP, Simoes BP, Scott IU, Jorge R. Improvement of ischaemic macular oedema after intravitreal injection of autologous bone marrow-derived haematopoietic stem cells. Acta Ophthalmol. 2015;93:e174–176. doi: 10.1111/aos.12473. [DOI] [PubMed] [Google Scholar]
- 46.Chan AM, Lynch MJ, Bailey JD, Ezrin C, Fraser D. Hypothyroidism in cystinosis. A clinical, endocrinologic and histologic study involving sixteen patients with cystinosis. Am J Med. 1970;48:678–692. doi: 10.1016/s0002-9343(70)80002-x. [DOI] [PubMed] [Google Scholar]
- 47.Gaide Chevronnay HP, Janssens V, Van Der Smissen P, Rocca CJ, Liao XH, Refetoff S, Pierreux CE, Cherqui S, Courtoy PJ. Hematopoietic Stem Cells Transplantation Can Normalize Thyroid Function in a Cystinosis Mouse Model. Endocrinology. 2016;157:1363–1371. doi: 10.1210/en.2015-1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Naphade S, Sharma J, Gaide Chevronnay HP, Shook MA, Yeagy BA, Rocca CJ, Ur SN, Lau AJ, Courtoy PJ, Cherqui S. Brief reports: Lysosomal cross-correction by hematopoietic stem cell-derived macrophages via tunneling nanotubes. Stem cells. 2015;33:301–309. doi: 10.1002/stem.1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mantovani A, Biswas SK, Galdiero MR, Sica A, Locati M. Macrophage plasticity and polarization in tissue repair and remodelling. J Pathol. 2013;229:176–185. doi: 10.1002/path.4133. [DOI] [PubMed] [Google Scholar]
- 50.Martinez FO, Gordon S. The M1 and M2 paradigm of macrophage activation: time for reassessment. F1000Prime. 2014 doi: 10.12703/P6-13. (E Collection) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Iglesias DM, El-Kares R, Taranta A, Bellomo F, Emma F, Besouw M, Levtchenko E, Toelen J, van den Heuvel L, Chu L, Zhao J, Young YK, Eliopoulos N, Goodyer P. Stem cell microvesicles transfer cystinosin to human cystinotic cells and reduce cystine accumulation in vitro. PloS One. 2012;7:e42840. doi: 10.1371/journal.pone.0042840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Thoene J, Goss T, Witcher M, Mullet J, N’Kuli F, Van Der Smissen P, Courtoy P, Hahn SH. In vitro correction of disorders of lysosomal transport by microvesicles derived from baculovirus-infected Spodoptera cells. Mol Genet Metab. 2013;109:77–85. doi: 10.1016/j.ymgme.2013.01.014. [DOI] [PubMed] [Google Scholar]
- 53.Rustom A, Saffrich R, Markovic I, Walther P, Gerdes HH. Nanotubular highways for intercellular organelle transport. Science. 2004;303:1007–1010. doi: 10.1126/science.1093133. [DOI] [PubMed] [Google Scholar]
- 54.Watkins SC, Salter RD. Functional connectivity between immune cells mediated by tunneling nanotubules. Immunity. 2005;23:309–318. doi: 10.1016/j.immuni.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 55.Chinnery HR, Pearlman E, McMenamin PG. Cutting edge: Membrane nanotubes in vivo: a feature of MHC class II+ cells in the mouse cornea. J Immunol. 2008;180:5779–5783. doi: 10.4049/jimmunol.180.9.5779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Islam MN, Das SR, Emin MT, Wei M, Sun L, Westphalen K, Rowlands DJ, Quadri SK, Bhattacharya S, Bhattacharya J. Mitochondrial transfer from bone-marrow-derived stromal cells to pulmonary alveoli protects against acute lung injury. Nat Med. 2012;18:759–765. doi: 10.1038/nm.2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Onfelt B, Nedvetzki S, Benninger RK, Purbhoo MA, Sowinski S, Hume AN, Seabra MC, Neil MA, French PM, Davis DM. Structurally distinct membrane nanotubes between human macrophages support long-distance vesicular traffic or surfing of bacteria. J Immunol. 2006;177:8476–8483. doi: 10.4049/jimmunol.177.12.8476. [DOI] [PubMed] [Google Scholar]
- 58.Gousset K, Schiff E, Langevin C, Marijanovic Z, Caputo A, Browman DT, Chenouard N, de Chaumont F, Martino A, Enninga J, Olivo-Marin JC, Mannel D, Zurzolo C. Prions hijack tunnelling nanotubes for intercellular spread. Nat Cell Biol. 2009;11:328–336. doi: 10.1038/ncb1841. [DOI] [PubMed] [Google Scholar]
- 59.Smith IF, Shuai J, Parker I. Active generation and propagation of Ca2+ signals within tunneling membrane nanotubes. Biophys J. 2011;100:L37–39. doi: 10.1016/j.bpj.2011.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yasuda K, Khandare A, Burianovskyy L, Maruyama S, Zhang F, Nasjletti A, Goligorsky MS. Tunneling nanotubes mediate rescue of prematurely senescent endothelial cells by endothelial progenitors: exchange of lysosomal pool. Aging (Albany NY) 2011;3:597–608. doi: 10.18632/aging.100341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wang X, Gerdes HH. Transfer of mitochondria via tunneling nanotubes rescues apoptotic PC12 cells. Cell Death Differ. 2015;22:1181–1191. doi: 10.1038/cdd.2014.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Harrison F, Yeagy BA, Rocca CJ, Kohn DB, Salomon DR, Cherqui S. Hematopoietic stem cell gene therapy for the multisystemic lysosomal storage disorder cystinosis. Mol Ther. 2013;21:433–444. doi: 10.1038/mt.2012.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Abrahamson DR, Leardkamolkarn V. Development of kidney tubular basement membranes. Kidney Int. 1991;39:382–393. doi: 10.1038/ki.1991.50. [DOI] [PubMed] [Google Scholar]
- 64.Gabriel SS, Belge H, Gassama A, Debaix H, Luciani A, Fehr T, Devuyst O. Bone marrow transplantation improves proximal tubule dysfunction in a mouse model of Dent disease. Kidney Int. 2017;91:842–855. doi: 10.1016/j.kint.2016.11.016. [DOI] [PubMed] [Google Scholar]
- 65.Devuyst O, Thakker RV. Dent’s disease. Orphanet J Rare Dis. 2010;5:28. doi: 10.1186/1750-1172-5-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pallera AM, Schwartzberg LS. Managing the toxicity of hematopoietic stem cell transplant. J Support Oncol. 2004;2:223–237. 241, 246–227. discussion 237–228. [PubMed] [Google Scholar]
- 67.Montini E, Cesana D, Schmidt M, Sanvito F, Ponzoni M, Bartholomae C, Sergi Sergi L, Benedicenti F, Ambrosi A, Di Serio C, Doglioni C, von Kalle C, Naldini L. Hematopoietic stem cell gene transfer in a tumor-prone mouse model uncovers low genotoxicity of lentiviral vector integration. Nat Biotechnol. 2006;24:687–696. doi: 10.1038/nbt1216. [DOI] [PubMed] [Google Scholar]
- 68.De Ravin SS, Wu X, Moir S, Anaya-O’Brien S, Kwatemaa N, Littel P, Theobald N, Choi U, Su L, Marquesen M, Hilligoss D, Lee J, Buckner CM, Zarember KA, O’Connor G, McVicar D, Kuhns D, Throm RE, Zhou S, Notarangelo LD, Hanson IC, Cowan MJ, Kang E, Hadigan C, Meagher M, Gray JT, Sorrentino BP, Malech HL. Lentiviral hematopoietic stem cell gene therapy for X-linked severe combined immunodeficiency. Sci Transl Med. 2016;8:335ra357. doi: 10.1126/scitranslmed.aad8856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ferrua F, Aiuti A. Twenty-Five Years of Gene Therapy for ADA-SCID: From Bubble Babies to an Approved Drug. Hum Gene Ther. 2017;28:972–981. doi: 10.1089/hum.2017.175. [DOI] [PubMed] [Google Scholar]
- 70.Morris EC, Fox T, Chakraverty R, Tendeiro R, Snell K, Rivat C, Grace S, Gilmour K, Workman S, Buckland K, Butler K, Chee R, Salama AD, Ibrahim H, Hara H, Duret C, Mavilio F, Male F, Bushman FD, Galy A, Burns SO, Gaspar HB, Thrasher AJ. Gene therapy for Wiskott-Aldrich syndrome in a severely affected adult. Blood. 2017;130:1327–1335. doi: 10.1182/blood-2017-04-777136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Biffi A, Montini E, Lorioli L, Cesani M, Fumagalli F, Plati T, Baldoli C, Martino S, Calabria A, Canale S, Benedicenti F, Vallanti G, Biasco L, Leo S, Kabbara N, Zanetti G, Rizzo WB, Mehta NA, Cicalese MP, Casiraghi M, Boelens JJ, Del Carro U, Dow DJ, Schmidt M, Assanelli A, Neduva V, Di Serio C, Stupka E, Gardner J, von Kalle C, Bordignon C, Ciceri F, Rovelli A, Roncarolo MG, Aiuti A, Sessa M, Naldini L. Lentiviral hematopoietic stem cell gene therapy benefits metachromatic leukodystrophy. Science. 2013;341:1233158. doi: 10.1126/science.1233158. [DOI] [PubMed] [Google Scholar]
- 72.Cartier N, Hacein-Bey-Abina S, Bartholomae CC, Bougneres P, Schmidt M, Kalle CV, Fischer A, Cavazzana-Calvo M, Aubourg P. Lentiviral hematopoietic cell gene therapy for X-linked adrenoleukodystrophy. Methods Enzymol. 2012;507:187–198. doi: 10.1016/B978-0-12-386509-0.00010-7. [DOI] [PubMed] [Google Scholar]
- 73.Cartier N, Hacein-Bey-Abina S, Bartholomae CC, Veres G, Schmidt M, Kutschera I, Vidaud M, Abel U, Dal-Cortivo L, Caccavelli L, Mahlaoui N, Kiermer V, Mittelstaedt D, Bellesme C, Lahlou N, Lefrere F, Blanche S, Audit M, Payen E, Leboulch P, l’Homme B, Bougneres P, Von Kalle C, Fischer A, Cavazzana-Calvo M, Aubourg P. Hematopoietic stem cell gene therapy with a lentiviral vector in X-linked adrenoleukodystrophy. Science. 2009;326:818–823. doi: 10.1126/science.1171242. [DOI] [PubMed] [Google Scholar]
- 74.Eichler F, Duncan C, Musolino PL, Orchard PJ, De Oliveira S, Thrasher AJ, Armant M, Dansereau C, Lund TC, Miller WP, Raymond GV, Sankar R, Shah AJ, Sevin C, Gaspar HB, Gissen P, Amartino H, Bratkovic D, Smith NJC, Paker AM, Shamir E, O’Meara T, Davidson D, Aubourg P, Williams DA. Hematopoietic Stem-Cell Gene Therapy for Cerebral Adrenoleukodystrophy. N Engl J Med. 2017;377:1630–1638. doi: 10.1056/NEJMoa1700554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Plotnikov EY, Khryapenkova TG, Galkina SI, Sukhikh GT, Zorov DB. Cytoplasm and organelle transfer between mesenchymal multipotent stromal cells and renal tubular cells in co-culture. Exp Cell Res. 2010;316:2447–2455. doi: 10.1016/j.yexcr.2010.06.009. [DOI] [PubMed] [Google Scholar]
- 76.Plotnikov EY, Khryapenkova TG, Vasileva AK, Marey MV, Galkina SI, Isaev NK, Sheval EV, Polyakov VY, Sukhikh GT, Zorov DB. Cell-to-cell cross-talk between mesenchymal stem cells and cardiomyocytes in co-culture. J Cell Mol Med. 2008;12:1622–1631. doi: 10.1111/j.1582-4934.2007.00205.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Spees JL, Olson SD, Whitney MJ, Prockop DJ. Mitochondrial transfer between cells can rescue aerobic respiration. Proc Natl Acad Sci U S A. 2006;103:1283–1288. doi: 10.1073/pnas.0510511103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Rocca CJ, Goodman SM, Dulin JN, Haquang JH, Gertsman I, Blondelle J, Smith JLM, Heyser CJ, Cherqui S. Transplantation of wild-type mouse hematopoietic stem and progenitor cells ameliorates deficits in a mouse model of Friedreich’s ataxia. Sci Transl Med. 2017 doi: 10.1126/scitranslmed.aaj2347. [DOI] [PMC free article] [PubMed] [Google Scholar]