Abstract
The catecholamine neurotransmitter dopamine (DA) exerts powerful modulatory control of physiology and behavior across phylogeny. Perturbations of DA signaling in humans are associated with multiple neurodegenerative and behavioral disorders, including Parkinson’s disease, attention-deficit/ hyperactivity disorder, addiction and schizophrenia. In the nematode C. elegans, DA signaling regulates mating behavior, learning, food seeking and locomotion. Previously, we demonstrated that loss of function mutations in the dat-1 gene that encodes the presynaptic DA transporter (DAT-1) results in a rapid cessation of movement when animals are placed in water, termed Swimming Induced Paralysis (Swip). Mutations in genes supporting DA biosynthesis, vesicular packaging and DA signaling suppresses Swip in dat-1 animals, consistent with paralysis as arising from excessive DA signaling at the extrasynaptic D2-type DA receptor DOP-3. Although animals grown on the vesicular monoamine transporter antagonist reserpine diminish Swip, the drug must be applied chronically, can impact the signaling of multiple biogenic amines, and has been reported to have penetrant, off-target actions. Here, we demonstrate that the antipsychotic drug azaperone potently and rapidly suppresses Swip behavior in either dat-1 mutants, as well as in wildtype animals treated with the DAT-1 antagonist nisoxetine, with genetic experiments consistent with DOP-3 antagonism as the mechanism of Swip suppression. Reversal of Swip in previously paralyzed dat-1 animals by azaperone application demonstrates an otherwise functionally-intact swimming circuit in these mutants. Finally, whereas azaperone suppresses DA-dependent Swip, the drug fails to attenuate the DA-independent paralysis induced by βPEA, aldicarb or genetic disruption of γ-aminobutyric acid (GABA) signaling. We discuss our findings with respect to the use of azaperone as a potent and selective tool in the identification and analysis of presynaptic mechanisms that regulate DA signaling.
Keywords: dopamine, azaperone, D2-type dopamine receptor, Swip, C. elegans
1. Introduction
The catecholamine neurotransmitter dopamine (DA) is a contributor to synaptic transmission across phylogeny, modulating a wide-spectrum of behaviors in both vertebrate and invertebrates. Diminished or excessive DA signaling is associated with multiple brain disorders, ranging from diseases associated with atypical movements such as dystonia, tardive dyskinesia and Parkinson’s disease to disorders that feature perturbations in cognition, impulse control and reward, including schizophrenia, Attention-Deficit Hyperactivity Disorder (ADHD), and addiction. Such associations have driven significant efforts over the past fifty years to dissect the molecular networks that control DA signaling. A key determinant of the temporal and spatial extent of DA action is the presynaptic DA transporter (DAT), a membrane protein that actively clears the neurotransmitter from the extracellular space following vesicular DA release. Our work, and that of colleagues in the field (Hansen et al. 2014; Jayanthi et al. 1998; Nass et al. 2002; Bowton et al. 2014; Mazei-Robison et al. 2008; Grunhage et al. 2000; Ng et al. 2014; Sakrikar et al. 2012), has identified multiple disease-associated mutations in the human gene encoding DAT (SLC6A3), reinforcing the clinical significance of efforts to better understand the mechanisms by which DAT expression, trafficking and function are regulated.
In an effort to extend studies of DAT and DAT regulatory genes to models with opportunities for both forward and reverse genetic approaches, we cloned the Caenorhabditis elegans DAT gene (dat-1) and established the transporter’s substrate and antagonist selectivity in transfected cells (Jayanthi et al. 1998). Our in vivo expression studies using transcriptional and translational fusion constructs revealed expression in the eight DA neurons present in the hermaphrodite (Nass et al. 2002) and localization of the transporter to DA synapses (McDonald et al. 2007), respectively, consistent with DAT-1 as the nematode ortholog of mammalian DAT. Subsequently, we defined an easily detectible motor phenotype that arises when loss-of-function dat-1 mutants are placed in water, termed Swip (Swimming-induced paralysis) (McDonald et al. 2007), and capitalized on this phenotype to implement a forward genetic screen for DA signaling regulators (Hardaway et al. 2012; Hardaway et al. 2015; Bermingham et al. 2017).
Although Swip is a readily discernable phenotype associated with DAT-1 dysfunction and excess DA signaling, multiple non-DAergic mechanisms control movement (Zheng et al. 1999; Liu et al. 2011; Liu, Chen, and Wang 2011; Richmond and Jorgensen 1999; Zhen and Samuel 2015) and as such, secondary tests must be implemented following a primary Swip screen to consider lines as harboring DA signaling linked gene mutations. In our past work (Hardaway et al. 2012), to corroborate the DAergic nature of induced mutations, we evaluated suppression of Swip following growth of lines on plates containing the vesicular monoamine transporter (VMAT, cat-2) inhibitor reserpine. Although useful, the chronic nature of reserpine exposure means that conclusions regarding mutation impact on ongoing DA signaling disruption are not evaluated. Moreover, CAT-1 can package other biogenic amines for release, including serotonin and octopamine, both of which are synthesized in the nematode (Avery and Horvitz 1990; Duerr et al. 1999). Finally, reserpine has been reported to have actions on other targets in the worm beyond CAT-1 (Saharia et al. 2012; Reckziegel et al. 2016).
Here, we demonstrate that the FDA-approved drug and D2 DA receptor antagonist azaperone is a potent and rapidly-acting modulator of Swip in animals genetically or pharmacologically rendered deficient in DAT-1 mediated DA uptake capacity, effects consistent with the drug’s ability to antagonize the D2-type DA receptor DOP-3. Azaperone not only precludes elaboration of Swip but also reverses Swip after paralysis has arisen, revealing an ongoing requirement for excess DA signaling in dat-1 mutants rather than a result of compensatory changes in DA signaling arising from lifelong DAT-1 deficiency. Finally, we demonstrate that azaperone fails to attenuate Swip induced through DA-independent pathways. Our findings reveal azaperone to be a powerful tool to validate DA and DOP-3 contributions to Swip and offers an effective approach to selecting lines from C. elegans mutagenesis screens that seek novel determinants of DA signaling.
2. Materials and methods
2.1. C. elegans strains
Worm strains were cultured on OP50 or NA22 bacterial lawns and maintained at 13°C – 20°C using standard methods (Brenner, 1974). C. elegans strains were obtained from the Caenorhabditis Genetics Center (University of Minnesota, Minneapolis, MN), or from Shohei Mitani of the National Bioresource Project at Tokyo Women’s Medical University. N2 Bristol was used as the wild-type strain. The dat-1(ok157) strain (a gift from J. Duerr and J. Rand, Oklahoma Medical Research Foundation, Oklahoma City, OK) was used as the DAT-1 loss of function allele. Strains LX703: dop-3(vs106) and LX702: dop-2(vs105) were also used, as they bear loss of function mutations in the D2-type DA receptors DOP-3 and DOP-2, respectively. These strains were crossed to dat-1(ok157) to construct double mutant lines BY600 and BY830, respectively. Strains BY1175: swip-10(tm5915) and BY990: swip-13(gk1234) carry null alleles of swip-10 and swip-13, respectively. For unc-49, we used the e407 which deletes all UNC-49B subunits.
2.2. Swip assays
Swip assays were performed as previously described (Hardaway et al. 2012; McDonald et al. 2007). To generate synchronous populations of L4 animals, gravid adult worms were lysed using hypochlorite treatment, and synchronized L1 animals were plated into OP50 plates. All manual assays were initiated by picking ten L4 hermaphrodites into a well of 100μL of distilled water plus or minus drug. The numbers of paralyzed versus swimming animals were scored after 10 min of incubation, except where noted otherwise. For each genotype and/or treatment, eight wells were scored, and every experiment was repeated on at least three separate days for an n = 24. For automated analyses, a single L4 animal was placed into a well of 20 μL of water with or without drug, and 10-min movies were captured from at least 30 worms per genotype/ treatment and their thrashing behavior was tracked and analyzed using custom-designed Worm Tracker and SwimR software (Hardaway et al. 2014) as described previously (Hardaway et al. 2014; Hardaway et al. 2015).
For drug treatments, azaperone (Sigma-Aldrich, St. Louis, MO) was dissolved in DMSO to generate a 50 mM stock solution, which was used to generate dilutions in distilled water for Swip assays. The final concentration of DMSO in azaperone solutions of 50 μM, 250 μM, and 500 μM azaperone equals 0.1%, 0.25% and 0.5% respectively. Vehicle controls used the same DMSO concentrations in the absence of azaperone. For Swip reversal experiments (i.e. when azaperone was added to animals that had already paralyzed in water), azaperone stock solution was added to the liquid surface, or well’s edge, to reach the desired final concentration, without disturbing paralyzed worms. Nisoxetine hydrochloride (Sigma-Aldrich, St. Louis, MO) was used to induce Swip at 20 μM concentration as described in our prior studies (Bermingham et al. 2016) and prepared as a 100 μM stock solution in distilled water. Studies of the ability of azaperone to suppress nisoxetine-induced Swip were performed in solutions of both 20 μM nisoxetine and 250 μM azaperone. Aldicarb (Chem Service Inc., West Chester, PA) was prepared as a stock solution in distilled water at 25mM and used to induce Swip at a final concentration of 1 mM. β-phenylethylamine (βPEA) (a gift from Lucia Carvelli, Florida Atlantic University) was prepared as a stock solution in distilled water at 10 mM and used at a final concentration of 1 mM, as previously described (Safratowich et al. 2014). Treatments of worms with reserpine (Sigma-Aldrich, St. Louis, MO), dissolved in M9 buffer, were performed as previously described (McDonald et al. 2007), where synchronized L1 animals were grown on OP50 plates containing 0.6 mM reserpine. After ~48 hr of reserpine or vehicle incubation, L4 animals were used for Swip assays as described above.
2.3. Graphical and statistical methods
Swip assays scores were transferred to Microsoft Excel 2016 (Microsoft, Redmond, WA) to calculate averages and standard deviations. Data were analyzed statistically and graphed using either SwimR software (described above), or using Prism 6.0 (GraphPad, Inc., La Jolla, CA). Descriptions of all statistical tests with n and P values are noted in the figure legends.
3. Results
3.1. Acute azaperone treatment suppresses dat-1 SWIP phenotype
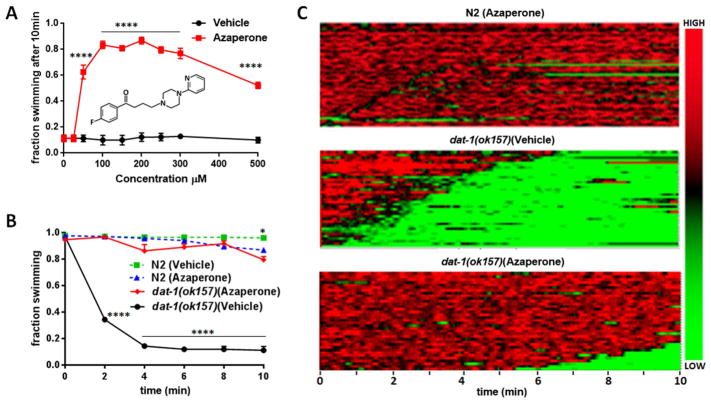
We previously demonstrated that animals lacking functional C. elegans DAT-1 proteins exhibit Swip minutes after being placed in water and that elevated DA signaling through the D2-type DA receptor DOP-3 was responsible for paralysis (McDonald et al. 2007). Previously, McCormick and colleagues reported that worms chronically exposed to the butyrophenone antipsychotic drug azaperone displayed significantly diminished phenotypes arising from tau overexpression, including movement defects, accumulation of tau aggregates, and GABA neuron degeneration (McCormick, Wheeler, et al. 2013). Animals rendered deficient in the D2-type DA receptors DOP-2 and DOP-3 also demonstrated tau phenotype suppression, suggesting that the action of azaperone in these studies was due to D2-type DA receptor antagonism. To examine whether acute exposure to azaperone can suppresses Swip, we examined the swimming behavior of dat-1(ok157) mutants in solutions of varying azaperone concentrations, and at different time points (Figure 1). Within 5–10 minutes, dat-1(ok157) mutants display ~90% Swip in the absence of drug (Figure 1A–B). Animals exposed to 50 μM azaperone displayed significantly reduced paralysis, with effects plateauing at 100–300 μM (Figure 1A). At concentrations higher than 500 μM, we observed diminished Swip reversal efficacy of azaperone, possibly a result of the higher DMSO concentrations (≥ 0.5%) used in these assays.
Figure 1. Azaperone exhibits dose and time-dependent capacity to suppress dat-1 Swip.
A) Concentration-response profile of azaperone to suppress Swip of dat-1(ok157) animals. Animals were transferred from plates to solutions of azaperone or vehicle at which time the extent of paralysis was assessed by eye. Each point reflects the analysis of 80 animals assayed on three different days, with total N ≥ 240. Data were analyzed by a two-way analysis of variance (ANOVA) with Bonferroni multiple comparison tests between azaperone and vehicle treatments at each concentration. A statistically significant effect of azaperone between vehicle and azaperone-treated dat-1(ok157) animals was apparent from 50 μM – 500 μM azaperone, with ****= P value < 0.0001. Inset shows azaperone chemical structure. B) Time-dependence of the swimming behavior of N2 and dat-1(ok157) animals treated either with azaperone (250μM) or with vehicle, recorded every 2 minutes. Data were evaluated using a two-way ANOVA with selected Bonferroni’s multiple comparisons tests, comparing genotypes at each dose of azaperone, with **** = P value < 0.0001. C) Automated heat maps produced from swimming videos to visualize thrashing frequencies of a population of animals over a 10 min assay window, with green color representing low frequency values (i.e. paralysis), and red representing high frequency values (i.e. swimming). The analysis depicts high swimming frequencies in N2 across the time of the assay which are similar to that of dat-1(ok157) animals treated with 250μM azaperone, where only a few animals demonstrate occasional or late paralysis (top and lower panels). In contrast, untreated dat-1(ok157) (i.e. vehicle; middle panel) exhibited a rapid, but individually variable onset of paralysis (n ≥ 40 for each panel). Bar on the right side of the heatmaps color codes HIGH and LOW frequencies of swimming.
To assess the rapidity of azaperone’s ability to suppress Swip, we tested dat-1(ok-157) as a function of time (Figure 1B). We scored dat-1 animals for Swip in 2 minute intervals over a 10 minute period, using a 250 μM azaperone concentration. As shown in Figure 1B, the effects of azaperone were rapid, with a significant difference from control incubations evident at 2 minutes. Indeed, at 2 min and all time points assayed thereafter azaperone-treated dat-1 animals swam to the same extent as azaperone-treated wildtype animals.
To provide a more quantitative assessment of the time dependence of azaperone on swimming behavior, we utilized a previously described, automated approach (Hardaway et al. 2014) to generate population heat maps of swimming frequency as a function of time (Figure 1C). Again, we observed that dat-1 mutants treated with azaperone (250 μM) demonstrated thrashing frequencies statistically indistinguishable from drug treated wildtype animals based on measures of the frequency of swimming, latency to paralyze and reversion probability (Figure 1C).
3.2. Comparison of azaperone actions on dat-1 mutants to reserpine and for other DA-dependent Swip mutants
As previously described, we assessed the DA dependency of Swip mutants in our initial forward genetic screen by evaluating restoration of swimming activity following multi-day growth of animals on the CAT-1 inhibitor reserpine (Hardaway et al. 2012). The chronic nature of the reserpine incubations and potential issues with neurotransmitter/target selectivity, led us to our efforts to explore azaperone as a substitute for reserpine. Indeed, as shown in Figure 2A, we found the extent of swimming restoration achieved with dat-1 mutants following acute azaperone incubations to be greater than that achieved with chronic treatment of animals with reserpine. The comparison provided here should not be considered as reporting relative quantitative effects of reserpine and azaperone, given the different nature of their applications, but rather to demonstrate that acute azaperone treatment can do as good, if not better job, in suppressing Swip, reducing opportunities for confounding plasticities that can emerge with chronic drug administration. To further validate an effective reversal of DA-dependent Swip, we tested azaperone for its effect on Swip arising in two mutants we recovered in our Swip screen and that we subsequently characterized as driving excess DA signaling through DOP-3 receptors, swip-10(tm5915) and swip-13(gk1234). swip-10 encodes an orthologue of mammalian MBLAC1, and when mutated generates hyperdopaminergia as a result of glutamate-dependent DA neuron hyperexcitability (Hardaway et al. 2015). swip-13 encodes the worm ortholog of the atypical MAP kinase ERK8, which we demonstrated to regulate DAT-mediated DA clearance through a Rho-dependent cell surface trafficking mechanism (Bermingham et al. 2017). Similar to dat-1 mutants, swip-10 and swip-13 paralysis was significantly rescued when incubated with 250 μM azaperone for 10 minutes (Figure 2B), indicating that azaperone can rescue SWIP caused by variety of mutations in the DA signaling pathway.
Figure 2. Comparison of suppression of Swip by azaperone in relation to reserpine and to genotype.
A) Extent of Swip suppression by acute treatment with azaperone or chronic treatment with reserpine. Pre-incubation of dat-1(ok157) animals on plates with reserpine for 48 hrs significantly, but partially suppresses Swip, whereas, acute treatment with azaperone (250μM) results in nearly complete suppression of Swip, exceeding the effects of reserpine. B) Azaperone at 250μM significantly suppress Swip of the hyperdopaminergic mutants swip-13(gk1234) and swip-10(tm5915), in a manner similar to its effect on dat-1(ok157). A small but significant effect of azaperone to induce paralysis was evident with N2 animals. Data in A and B were analyzed using a one-way ANOVA, with selected Bonferroni’s multiple comparisons tests, comparing each genotype’s azaperone treatment to vehicle treatment, using the corresponding concentration of DMSO. (* = P <.01, *** P < 0.001, **** P < 0.0001).
3.3. Azaperone suppresses pharmacologically-induced Swip
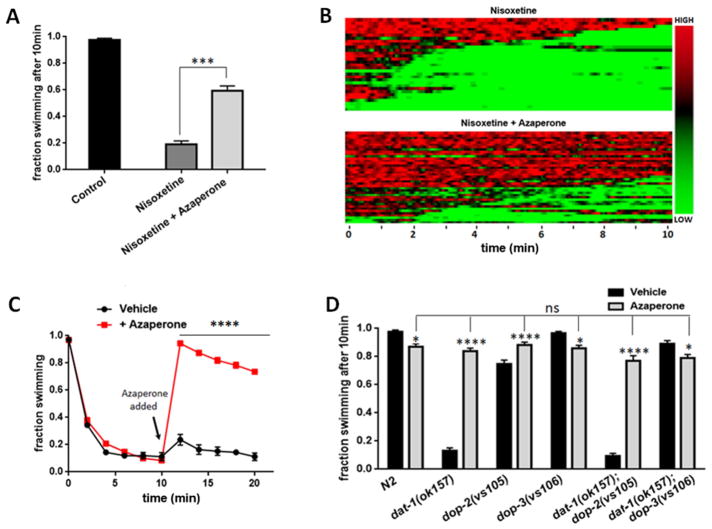
Our presumption that azaperone suppresses Swip because of blockade of DOP-3 disregards the possibility that the constitutive mutations used to induce hyperdopaminergia may induce compensatory, physiological adaptations, and it is one of these alterations targeted by azaperone. To diminish this concern, we tested the ability of azaperone to suppress Swip when paralysis is triggered acutely by pharmacological blockade of DAT-1. Previously, we showed that the mammalian norepinephrine transporter antagonist nisoxetine is a high-affinity antagonist of C. elegans DAT-1 (Jayanthi et al, 1998) and that acute application of the drug produces DA-dependent Swip in wildtype animals, effects lost in a dop-3 mutant (Bermingham et al. 2016). As previously demonstrated, transfer of wildtype worms into a solution containing 20 μM nisoxetine resulted in highly penetrant Swip within the 10 min time period of the swimming assay, as compared to animals tested in water alone (Figure 3A,B). Consistent with DOP-3 antagonism, nisoxetine-induce Swip was significantly attenuated when worms were added to a solution that contained both 20 μM nisoxetine and 250 μM azaperone. Although it seems likely that azaperone blocks nisoxetine-induced Swip by preventing activation of DOP-3 receptors, direct chemical inactivation of nisoxetine by azaperone, or an ability of azaperone to prevent import of nisoxetine, are acknowledged.
Figure 3. Azaperone demonstrates suppression and reversal of both genetically and pharmacologically-induced Swip, mediated by DOP-3.
A) Azaperone suppresses Swip induced by the DAT-1 inhibitor nisoxetine. N2 animals in basal conditions for 10 min (black bar). N2 acute treatment with 20μM nisoxetine for 10 min (dark grey bar). N2 animals treated with 20μM nisoxetine and 250μM azaperone. N ≥ 200 animals for each condition. Data were analyzed using a one-way ANOVA, with selected Bonferroni’s multiple comparisons tests. (***P value < 0.001) (light gray bar). B) Automated heat maps produced from swimming videos to visualize thrashing frequencies of a population of animals over a 10 min assay, using populations treated with 20μM nisoxetine (top panel), or 20μM nisoxetine and 250μM azaperone (lower panel). Green color representing low frequency movement (paralysis); red color indicates high frequency movement (swimming). Bar on the right side of the heatmaps color codes HIGH and LOW frequencies of swimming.
C) Azaperone treatment reverses Swip of paralyzed dat-1 animals. dat-1(ok157) animals were treated with either 250μM azaperone or vehicle after 10 minutes of incubation. n ≥ 40 for each condition. Data were analyzed using a two-way ANOVA with selected Bonferroni’s multiple comparisons tests comparing treated and untreated conditions at each time point, between 10 and 20 minutes. **** = P < 0.0001. D) Genetic evidence that azaperone suppresses dat-1(ok157) Swip by blocking the dopamine D2 type receptor DOP-3. Animals of different genotypes were treated with either 250μM azaperone or vehicle. N2 wildtype animals exhibit a small but significant paralysis induced by azaperone. dat-1(ok157); dop-2(vs105) double mutants exhibit suppression of Swip by azaperone comparable to that of dat-1(ok157) animals. dop-3(vs106) and dat-1(ok157); dop-3(vs106) animals lack suppression of Swip by azaperone. Data was analyzed using two-way ANOVA, with selected Bonferroni’s multiple comparisons tests, comparing the extent of swimming of each genotype in azaperone versus swimming in vehicle, as well as each genotype’s swimming behavior in azaperone relative to azaperone treated N2. (* = P < 0.1, *** = P < 0.001, **** = P < 0.0001, ns = non-significant, P > 0.05).
3.3. Azaperone treatment demonstrates rapid reversibility of Swip in dat-1 mutants
To date, restoration of normal swimming behavior following the onset of paralysis has not been demonstrated. Were azaperone to demonstrate such a property, mutant animals could be screened for both Swip and the DA-dependence of Swip in the same assay. Indeed, when we placed dat-1(ok157) mutants in water for 10 minutes to induce Swip, and then added azaperone (final concentration = 250 μM) to these solutions, swimming behavior was rapidly reinstated (Figure 3C), with > 90% of animals having resumed swimming after 2 minutes with the drug (Figure 3C). In contrast, paralyzed animals treated with the azaperone vehicle at the same time point as azaperone failed to recover from Swip across the subsequent 10 minutes recording period.
3.4. Genetic evidence for DOP-3 antagonism as the mechanism of Swip suppression by azaperone
The previous studies that examined the ability of azaperone to reduce phenotypes associated with tau overexpression (McCormick, Wheeler, et al. 2013) provided evidence that azaperone effects were mediated as a result of antagonism of both DOP-2 and DOP-3. As a dop-2 loss of function mutation does not attenuate the Swip exhibited by dat-1 mutants (Bermingham et al. 2016), we suspected that the actions of azaperone were likely to be solely mediated by DOP-3 antagonism. To test this hypothesis, we tested double mutants bearing both dat-1(ok157) and either dop-2(vs105) or dop-3(vs106) loss of function mutations (Figure 3D). As shown above, azaperone (250μM) effectively suppressed Swip of dat-1(ok157) animals, whereas the drug induced only a small degree of swimming inhibition on wildtype animals, also displayed by dop-2(vs105) and dop-3(vs106) mutants. Importantly, the ability of azaperone to reverse Swip was intact in dat-1; dop-2 double mutants, consistent with a lack of contribution of DOP-2 antagonism in the effects of azaperone. As expected, we found dat-1; dop-3 double mutants to swim at rates comparable to wildtype animals with no further swimming induction arising from azaperone treatments. We note a small amount of Swip generated by azaperone in N2, as well as in dop-3 mutants, at the concentration and duration of exposure used. As this effect is in the opposite direction as the suppression of Swip seen when dat-1 animals are exposed to azaperone, this suggests that the drug may antagonize a D2-type receptor that promotes Swip. We have previously presented evidence that DOP-2 receptors may act as presynaptic DA autoreceptors, providing for feedback inhibition of DA release (Bermingham et al. 2016). Azaperone antagonism of these receptors would be predicted to increase DA release and promote Swip. Future studies that restore DOP-2 expression to DA neurons selectively may help address this issue. Altogether, our findings provide genetic evidence that antagonism of DOP-3 is the most likely mechanism by which azaperone suppresses the Swip observed in dat-1 mutants.
4. Azaperone fails to suppress DA-independent Swip
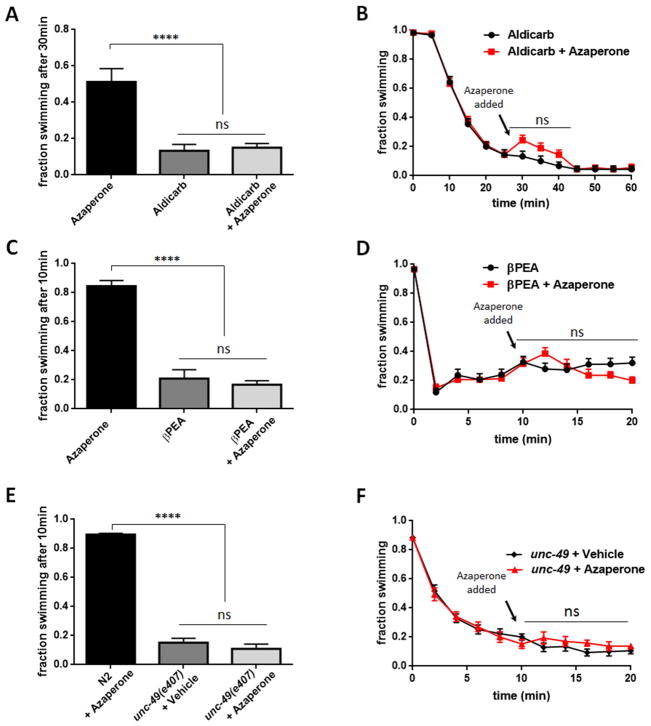
Although azaperone phenocopies the effects of a dop-3(vs106) mutation in suppressing DA dependent Swip, these effects could arise from a nonspecific stimulant effect targeting other pathways, with this effect simply unapparent in wild-type animals due to ceiling effects on swimming rates. To test this possibility, we analyzed the effect of azaperone on three DA-independent mechanisms that can trigger paralysis. β-Phenylethylamine (βPEA) is a physiologically active, endogenous trace amine that when applied exogenously can rapidly induce Swip in wildtype worms, effects that are DAT-1 independent, mediated by activation of the amine-gated chloride channel LGC-55 (Safratowich et al. 2014; Hossain, Wickramasekara, and Carvelli 2014). Consistent with azaperone as selective for DA-dependent Swip, we observed no effect of azaperone on βPEA-induced Swip when either animals were added to an azaperone (250μM) solution (Figure 4A) or when azaperone was added to reach the same concentration after βPEA-treated animals had already become paralyzed (Figure 4B).
Figure 4. Azaperone does not rescue DA-independent paralysis induced by treatments of N2 with βPEA or aldicarb, or by unc-49 mutant.
A) Azaperone (250μM) fails to suppress paralysis of N2 animals treated with aldicarb (1mM, 30 min) compared to aldicarb-treated N2. N ≥ 200. B) Azaperone fails to reverse paralysis of N2 animals treated with aldicarb. Both vehicle-incubated N2 and aldicarb-incubated N2 were treated with azaperone (250μM) or azaperone vehicle 30 min after initiation of swimming assay. N= 240 for both groups. C) Azaperone (250μM) fails to suppress paralysis of N2 animals treated with βPEA (1 mM, 10 min) compared to βPEA-treated N2. N for all groups = 240. D) Azaperone fails to reverse paralysis of N2 animals treated with βPEA. Both vehicle-incubated N2 and βPEA-incubated N2 were treated with azaperone (250μM) or azaperone vehicle 30 min after initiation of swimming assay. N= 160 for both groups. E) Azaperone (250μM) fails to suppress paralysis of unc-49(e407) animals compared to unc-49(e407) animals treated with vehicle. N for all groups = 240. F) Azaperone fails to reverse paralysis of unc-49(e407) animals. (unc-49(e407) animals were treated with azaperone (250μM) or vehicle 30 min after initiation of swimming assay. N= 160 for both groups. Data in A, C and E were analyzed using a one-way ANOVA, with selected Bonferroni’s multiple comparisons tests, comparing N2 with azaperone to other groups. Data in B, D, and F were analyzed by a two-way, repeated-measures ANOVA with selected Bonferroni’s comparisons between azaperone and control at each time point. (**** = P value < 0.0001, ns = nonsignificant, P > 0.05).
Aldicarb is an acetylcholinesterase (AChE) inhibitor that is widely used to study cholinergic synaptic transmission in C. elegans (Mahoney, Luo, and Nonet 2006; Oh and Kim 2017; Opperman and Chang 1991; Opperman et al. 2017; Mahoney et al. 2006). When applied exogenously, aldicarb causes acetylcholine (ACh) to accumulate at worm neuromuscular synapses, resulting in desensitization of postsynaptic nicotinic ACh receptors and muscle paralysis. The effects of aldicarb are typically assessed by a slowing or cessation of movement on plates. We found that nearly 90% of wildtype animals exposed to 1 mM aldicarb for 30 minutes in water exhibited Swip. In keeping with the DA dependence of azaperone action to suppress Swip, we observed no significant ability of azaperone to restore normal swimming behavior for aldicarb treated animals, whether animals were exposed to both solutions at the beginning of the swimming assay (Figure 4C) or when azaperone was added after aldicarb-induced paralysis had been established (Figure 4D).
As a final test of DA dependence for azaperone-induced Swip suppression, we tested the ability of the drug to restore swimming behavior of a mutant (unc-49(e407)) that impacts all B subunits of the C. elegans GABAA ionotropic receptor homolog UNC-49 (Bamber et al. 1999; Richmond and Jorgensen 1999). UNC-49 is expressed in body wall muscles and localizes at neuromuscular junctions to regulate normal locomotion (McIntire et al. 1993; McIntire, Jorgensen, and Horvitz 1993; Bamber et al. 1999). The e407 allele is functionally null for inhibitory GABA actions on body wall muscles (Richmond and Jorgensen 1999; Locke et al. 2009) and significantly reduces swimming activity in liquid media (Locke et al. 2009). Consistent with these data, approximately 90% of unc-49(e407) animals were paralyzed 10 minutes after transfer to water, an effect that failed to be suppressed by azaperone (250uM) incubation (Figure 4E). Moreover, azaperone failed to reinstate swimming behavior of paralyzed unc-49(e407) mutants (Figure 4F). We obtained a similar lack of effect of azaperone to rescue swimming when a less penetrant unc-49 allele (e382) was tested (data not shown). Along with the lack of effect of azaperone on aldicarb and βPEA-induced Swip, our findings that the antipsychotic lacks the ability to restore swimming behavior of unc49 mutants are consistent with a specificity of azaperone for suppression of DA-dependent Swip.
4. Discussion
The anatomical simplicity, transparency and short life cycle of the C. elegans make it a powerful tool for the study of neuronal development and function. In C. elegans, the neurotransmitter DA is produced by eight neurons in the hermaphrodite (McDonald et al. 2006) to regulate a wide range of behaviors, including locomotion, habituation to mechanical stimuli, mechanosensation, searching for food, motor and learning activates (Chase, Pepper, and Koelle 2004; Han et al. 2017; Hills, Brockie, and Maricq 2004; Sanyal et al. 2004; Sawin, Ranganathan, and Horvitz 2000; Vidal-Gadea et al. 2011). Use of the worm model to elucidate molecular networks that drive DA neuron development and signaling have capitalized on the ease by which the model can be operationalized for forward genetic screens (Doitsidou et al. 2013; Offenburger, Jongsma, and Gartner 2018; Chase, Pepper, and Koelle 2004; Hardaway et al. 2015; Bermingham et al. 2017). Previously, we capitalized on the model to screen for genes whose mutation results in Swip, phenocopying a lack of DA transporter (Bermingham et al. 2017; Hardaway et al. 2015; Hardaway et al. 2012). To support a role of our Swip mutants in modulating DAT or DA signaling, we grew animals on the CAT-1 inhibitor reserpine, and pursued lines demonstrating diminished Swip, presumably derived from an inability of reserpine-treated animals to package and release DA. A limit to the utility of reserpine is the role that CAT-1 plays in packaging serotonin and octopamine for release (Duerr et al. 1999). Additionally, the assumption that reserpine actions reflect solely an antagonism of CAT-1 can be questioned (Saharia et al. 2012; Reckziegel et al. 2016). Finally, the need for chronic incubations of animals with reserpine to affect DA depletion raises the possibility of compensatory changes in neuronal development that cloud interpretations of whether recovered mutations induce long-lasting compensatory changes that may or may not reflect a role in ongoing DA signaling.
Here, we demonstrate that the butyrophenone antipsychotic azaperone both antagonizes and reverses DA-dependent Swip upon acute application, and present evidence to implicate the D2-type DA receptor DOP-3 in azaperone actions. DA receptors are targets for many drugs that treat neuropsychiatric disorders (Creese, Burt, and Snyder 1976; Girault and Greengard 2004), where many clinically effective antipsychotics block D2-type DA receptors (Snyder et al. 1970; Creese, Burt, and Snyder 1976; Seeman et al. 1976; McCormick, Wilson, et al. 2013; McCormick, Wheeler, et al. 2013). An action of azaperone in C. elegans first emerged from a chemical screen for drugs that reduce tau accumulation and neurotoxicity, using a chronic treatment regimen initiated at embryonic stages in the cuticle-compromised bus-8 strain, employed to facilitate azaperone exposure (McCormick, Wheeler, et al. 2013). We demonstrate that azaperone acts rapidly on animals with a normal cuticle to antagonize DA signaling, an important consideration as cuticle-compromised animals are not typically the backgrounds preferred for genetic screens and these animals may also behave poorly in behavioral tests or be hypersensitive to exogenous chemical agents. Our dose response analyses demonstrate that azaperone can act at relatively low concentrations (50 μM) with a maximal azaperone effects on dat-1 SWIP reached at 100 – 300 μM, similar to the range previously observed in studies of tau toxicity using chronic treatments (McCormick, Wheeler, et al. 2013). We wish to note that, in the absence of a rapidly acting and specific DOP-3 agonist, it is difficult to rule out other targets that could support the ability of azaperone to suppress Swip. Logical candidates would be CAT-2 and CAT-1, proteins responsible for DA synthesis and packaging. We were unable to find published evidence of azaperone, or butyrophenone antipsychotic drugs in general as capable of antagonizing these proteins in either worm or man. We also believe that the rapid actions of azaperone are unlikely to reflect blockade of CAT-2 or CAT-1, which would likely require a greater period of time than direct post-synaptic receptor blockage to result in loss of DA stores and DA signaling.
The acetylcholinesterase inhibitor (AChE), aldicarb is an example of a cuticle-penetrating and fast-acting drug whose use has propelled screens for regulators of synaptic transmission as well as mechanistic dissections of genes proposed to act in cholinergic circuits (Mahoney, Luo, and Nonet 2006; Bany, Dong, and Koelle 2003; Oh and Kim 2017; Burns et al. 2017; Miller et al. 2000; Opperman and Chang 1991; Opperman et al. 2017; Mullen et al. 2007). Aldicarb blocks the enzyme AChE, leading to continuous accumulation of extracellular acetylcholine (ACh), which eventually results in motor paralysis. By analogy, we recently reported nisoxetine as a DAT-1 antagonist that acts within minutes to block DA clearance and increase DA availability, leading to Swip (Bermingham et al. 2016). Just as enhancement or suppression of aldicarb-induced paralysis has been a tool to identify pharmacological agents or genetic mutations that regulate ACh signaling (Jorgensen et al. 1995; Nonet et al. 1997; McCulloch et al. 2017; Wabnig et al. 2015), we believe that nisoxetine and azaperone can facilitate elucidation of molecular networks needed to achieve normal DA signaling.
As shown above, azaperone suppression of SWIP is dose- and time-dependent (see Figure 1A,B). As shown by McCormick and colleagues (McCormick et al., 2013) with respect to actions to suppress tau aggregates and neural degeneration, we observed an inverted U-shaped dose response for azaperone with respect to Swip rescue, where higher doses (Figure 1A), and longer incubation times (Figure 3C) demonstrate reduced activity. High concentrations of DMSO can induce immobility in C. elegans (Dagenhardt et al. 2017) and our own studies with wildtype animals reveals that incubations of mutants with high concentrations of azaperone must be compared against parallel diluted vehicle. Of course, it is also possible, if not likely, that targets besides DOP-3 begin to drive Swip at higher concentrations or longer times of incubation.
In our prior forward genetic studies, we used relatively high concentrations (600 μM) of reserpine and grew animals from initial lysis to late L4s prior to Swip studies, which we speculated could induce developmental and behavioral changes that might lead to false conclusions as to mutation specificity as DA-related. This hypothesis is bolstered by findings that chronic reserpine incubations can impact worm survival and stress responses (Saharia et al. 2012; Saharia et al. 2016; Srivastava et al. 2008). Recently, Reckziegel et al. (2016) studied reserpine-induced toxicity in worms, revealing many developmental defects and behavioral modifications including changes in egg laying, defecation cycles and locomotion rate on food. Additionally, reserpine was reported to cause neurodegeneration of dopaminergic CEP neurons and decreases the survival in cat-1 and dat-1 loss-of-function mutant worms (Reckziegel et al. 2016). A concern for dopamine neuron toxicity arises from findings that chronic reserpine treatment in rodents induces oxidative stress, possibly a result of elevations of highly oxidizable DA in the cytosol (Thomas, Francescutti-Verbeem, and Kuhn 2009; Teixeira et al. 2008). Thus, the acute exposure to azaperone should preclude effects on behavior and development when implementing a pharmacological approach to complement the use of gene disruptions.
With respect to the use of azaperone as a secondary test in genetic studies that score for excess DA signaling via Swip, we demonstrated that the drug not only reverses paralysis achieved by loss of function alleles in dat-1, but also suppresses Swip of two recently published DA-dependent Swip mutants, swip-10 (Hardaway et al. 2015) and swip-13(Bermingham et al. 2017). Swip-10 mutants elevate DA neuron excitability and DA release whereas swip-13 mutants result in reduced cell surface DAT-1, in both cases causing excess DA signaling and DOP-3 mediated Swip. Rescue studies indicate that the critical site of action of SWIP-10 is in glia that lie in proximity to DA neurons, whereas SWIP-13 acts presynaptically to modulate DAT trafficking. The ability of azaperone to rescue these two functionally and spatially different mutations, as well as its inability to limit paralysis triggered by changes in cholinergic and GABAergic signaling, underscores the utility of azaperone to limit forays arising from genetic screens or pharmacological treatments to those linked to DA signaling pathways.
Since azaperone could preclude the initiation of Swip by dat-1(157) mutants, we wondered whether the drug could reverse paralysis of mutant animals after they had paralyzed. In theory, constitutive loss of DAT-1 availability could have induced changes in circuitry during development that leads to poor thrashing behavior. Similarly, reversal of Swip in a dat-1(ok157); dop-3(vs106) double mutant might only reflect a role for DOP-3 in worm development, and that at the time of assay, DA signaling through the receptor is irrelevant. However, our studies demonstrating rapid reversal of dat-1(ok157) Swip by acute azaperone application demonstrate that this is not the case (Figure 3C), and indicates that use of azaperone to mobilize paralyzed animals can be a powerful option for the selection of animals from a Swip screen as ones arising from hyperdopaminergia.
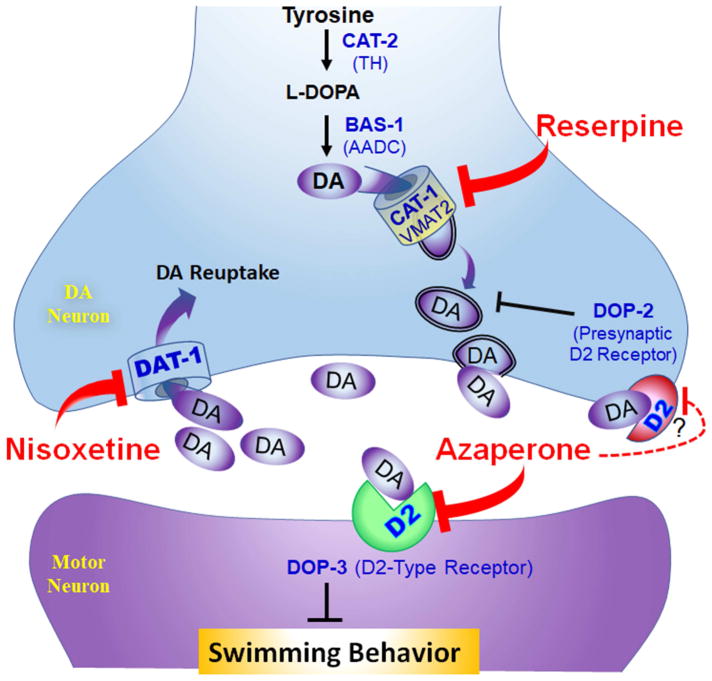
Our genetic analysis of azaperone effects on dat-1 Swip implicate targeting by the drug of the D2-type DA receptor DOP-3 (Figure 3D). The C. elegans genome encodes two D2-like DA receptors, DOP-2 and DOP-3 (Chase, Pepper, and Koelle 2004). As previously shown (McDonald et al. 2007; Bermingham et al. 2016), we show here that mutation of dop-3, but not dop-2, is sufficient to rescue dat-1 Swip, with levels or swimming activity similar to that established by azaperone. Moreover, we observed no additivity in swimming behavior treating dat-1(ok157); dop-3(vs106) double mutants with azaperone. Given the possibility of ceiling effects with the latter experiments, we feel a stronger argument for DOP-3 specificity is apparent in studies where we demonstrated full Swip suppression by azaperone –treated dat-1(ok157); dop-2(vs105) double mutants. DOP-3 receptors act as extrasynaptic DA receptors on cholinergic motor neurons where they are believed to inhibit motor neuron excitability and/or ACh release (Chase et al. 2004). Consistent with this view, DOP-3 re-expression in motor neurons is sufficient to restore Swip in dat-1;dop-3 double mutants (Allen et al. 2011). Our prior studies (Bermingham et al. 2016) with nisoxetine indicate that DOP-2 acts as a presynaptic autoreceptor to limit DA release, and hence its blockade by azaperone would be predicted to augment, not attenuate dat-1(ok157) Swip. We summarize the most mechanism for azaperone actions based on our pharmacological and genetic studies, along with the actions of reserpine and nisoxetine, in Fig 5. Interestingly, studies of azaperone’s action on tau accumulation and toxicity azaperone (McCormick, Wheeler, et al. 2013) are consistent with an additivity of DOP-2 an DOP-3 signaling antagonism. Whether DOP-3 and DOP-2 act together through cell autonomous mechanisms in the latter studies is as yet unclear, but regardless the mechanism of action for tau effects, Swip appears to derive from distinct effects.
Figure 5. Schematic representation of DA neurotransmission in C. elegans involved in Swip and sites of proposed action of reserpine, nisoxetine and azaperone.
Shown is a DA presynaptic terminal where DA is synthesized from the amino acid tyrosine by sequential actions of CAT-2, the C. elegans ortholog of the mammalian tyrosine hydroxylase (TH), and BAS-1, the C. elegans ortholog of aromatic amino acid decarboxylase (AADC), and then imported into synaptic vesicles by CAT-1, the C. elegans ortholog of the mammalian vesicular monoamine transporter, type 2 (VMAT2). DA release is believed to be activated upon placing worms in water to facilitate a change of motor behavior from crawling to swimming, with excess DA release or inadequate DA clearance producing Swip. Following vesicular release, DA acts on either extrasynaptic D2 type DA receptors (DOP-3) or presynaptic D2-type DA autoreceptors (DOP-2), and is cleared from extracellular spaces by DAT-1, the C. elegans ortholog of the mammalian DA transporter (DAT). Motor neuron DOP-3 activation by DA inhibits motor neurons leading to Swip. Presynaptic DOP-2 activation by DA inhibits DA release. Reserpine blocks CAT-1, preventing loading of DA into synaptic vesicles, and relieves DA-dependent Swip. Nisoxetine blocks DAT-1 and results in Swip due to excess DA action on DOP-3 receptors. Azaperone acts to antagonize both DOP-2 and DOP-3. Azaperone blockade of presynaptic DOP-2 relieves DA release inhibition, inducing Swip. Azaperone blockade of motor neuron DOP-3 blocks Swip induced by loss of function mutations in DAT-1 or by nisoxetine inhibition of DAT-1. The more significant arrow illustrating DOP-3 antagonism by azaperone versus DOP-2 is meant to illustrate that with respect to Swip blockade, antagonism of DOP-3 is dominant at the concentrations and times of application of azaperone used.
5. Conclusions
In the current study, we demonstrated that the antipsychotic drug azaperone suppresses Swip in C. elegans, when this phenotype derives from excessive DA signaling. Our data shows that low concentrations of azaperone suppress both genetically and pharmacologically-induced Swip, as revealed through suppression of the paralytic effects of a loss of function dat-1(ok157) mutation and by suppression of paralysis triggered by acute incubations with the DAT-1 antagonist nisoxetine. Our genetic analysis supports the antagonism by azaperone of DA D2-type receptor DOP-3, consistent with previous observations in C. elegans and human cells. Unlike the CAT-1 inhibitor reserpine, azaperone exert its effect immediately following acute application, thereby avoiding potentially undesirable developmental effects that arias from prolonged exposure to drug and the accumulation of cytosolic DA. Furthermore, azaperone suppression of worm paralysis appears specific for DA-dependent paralysis, as the drug did not restore movement to animals treated with βPEA or aldicarb, nor does it act to reverse paralysis arising in unc-49 mutants. Altogether, our work reveals azaperone to have the actions that warrant its use in genetic screens for genes that contribute to DA signaling as well as in studies that dissect DOP-3 signaling mechanisms.
Highlights.
The antipsychotic azaperone suppresses swimming-induced paralysis (Swip) in C. elegans
Azaperone effects on Swip are specific to paralysis driven by excess DA signaling
Azaperone suppression of Swip involves blockade of D2-type DOP-3 DA receptors
Azaperone restores swimming behavior to animals paralyzed by excess DOP-3 stimulation
Ongoing DA receptor signaling drives Swip in dat-1 animals, not circuit compensations
Acknowledgments
The authors thank Chelsea Gibson, Andrew Giles, and Lucia Carvelli for helpful discussions during the completion of this work, and Peter Rodriguez, Sean Mellish, Matthew Gross, Catherine Nettesheim, Erika Castriz, Samara Vilca, and Rania Katamish for general technical support. The work was supported by NIH award MH095044 to R.D.B.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Allen AT, Maher KN, Wani KA, Betts KE, Chase DL. Coexpressed D1- and D2-like dopamine receptors antagonistically modulate acetylcholine release in Caenorhabditis elegans. Genetics. 2011;188:579–90. doi: 10.1534/genetics.111.128512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avery L, Horvitz HR. Effects of starvation and neuroactive drugs on feeding in Caenorhabditis elegans. J Exp Zool. 1990;253:263–70. doi: 10.1002/jez.1402530305. [DOI] [PubMed] [Google Scholar]
- Bamber BA, Beg AA, Twyman RE, Jorgensen EM. The Caenorhabditis elegans unc-49 locus encodes multiple subunits of a heteromultimeric GABA receptor. J Neurosci. 1999;19:5348–59. doi: 10.1523/JNEUROSCI.19-13-05348.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bany IA, Dong MQ, Koelle MR. Genetic and cellular basis for acetylcholine inhibition of Caenorhabditis elegans egg-laying behavior. J Neurosci. 2003;23:8060–9. doi: 10.1523/JNEUROSCI.23-22-08060.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bermingham DP, Hardaway JA, Refai O, Marks CR, Snider SL, Sturgeon SM, Spencer WC, Colbran RJ, Miller DM, 3rd, Blakely RD. The atypical MAP kinase SWIP-13/ERK8 regulates dopamine transporters through a Rho-dependent mechanism. J Neurosci. 2017;37:9288–304. doi: 10.1523/JNEUROSCI.1582-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bermingham DP, Hardaway JA, Snarrenberg CL, Robinson SB, Folkes OM, Salimando GJ, Jinnah H, Blakely RD. Acute blockade of the Caenorhabditis elegans dopamine transporter DAT-1 by the mammalian norepinephrine transporter inhibitor nisoxetine reveals the influence of genetic modifications of dopamine signaling in vivo. Neurochem Int. 2016;98:122–8. doi: 10.1016/j.neuint.2016.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowton E, Saunders C, Reddy IA, Campbell NG, Hamilton PJ, Henry LK, Coon H, Sakrikar D, Veenstra-VanderWeele JM, Blakely RD, Sutcliffe J, Matthies HJ, Erreger K, Galli A. SLC6A3 coding variant Ala559Val found in two autism probands alters dopamine transporter function and trafficking. Transl Psychiatry. 2014;4:e464. doi: 10.1038/tp.2014.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns AR, Bagg R, Yeo M, Luciani GM, Schertzberg M, Fraser AG, Roy PJ. The novel nematicide wact-86 interacts with aldicarb to kill nematodes. PLoS Negl Trop Dis. 2017;11:e0005502. doi: 10.1371/journal.pntd.0005502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chase DL, Pepper JS, Koelle MR. Mechanism of extrasynaptic dopamine signaling in Caenorhabditis elegans. Nat Neurosci. 2004;7:1096–103. doi: 10.1038/nn1316. [DOI] [PubMed] [Google Scholar]
- Creese I, Burt DR, Snyder SH. Dopamine receptor binding predicts clinical and pharmacological potencies of antischizophrenic drugs. Science. 1976;192:481–3. doi: 10.1126/science.3854. [DOI] [PubMed] [Google Scholar]
- Dagenhardt J, Trinh A, Sumner H, Scott J, Aamodt E, Dwyer DS. Insulin signaling deficiency produces immobility in Caenorhabditis elegans that models diminished motivation states in man and responds to antidepressants. Mol Neuropsychiatry. 2017;3:97–107. doi: 10.1159/000478049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doitsidou M, Flames N, Topalidou I, Abe N, Felton T, Remesal L, Popovitchenko T, Mann R, Chalfie M, Hobert O. A combinatorial regulatory signature controls terminal differentiation of the dopaminergic nervous system in C. elegans. Genes Dev. 2013;27:1391–405. doi: 10.1101/gad.217224.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duerr JS, Frisby DL, Gaskin J, Duke A, Asermely K, Huddleston D, Eiden LE, Rand JB. The cat-1 gene of Caenorhabditis elegans encodes a vesicular monoamine transporter required for specific monoamine-dependent behaviors. J Neurosci. 1999;19:72–84. doi: 10.1523/JNEUROSCI.19-01-00072.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girault JA, Greengard P. The neurobiology of dopamine signaling. Arch Neurol. 2004;61:641–4. doi: 10.1001/archneur.61.5.641. [DOI] [PubMed] [Google Scholar]
- Grunhage F, Schulze TG, Muller DJ, Lanczik M, Franzek E, Albus M, Borrmann-Hassenbach M, Knapp M, Cichon S, Maier W, Rietschel M, Propping P, Nothen MM. Systematic screening for DNA sequence variation in the coding region of the human dopamine transporter gene (DAT1) Mol Psychiatry. 2000;5:275–82. doi: 10.1038/sj.mp.4000711. [DOI] [PubMed] [Google Scholar]
- Han B, Dong Y, Zhang L, Liu Y, Rabinowitch I, Bai J. Dopamine signaling tunes spatial pattern selectivity in C. elegans. Elife. 2017:6. doi: 10.7554/eLife.22896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen FH, Skjorringe T, Yasmeen S, Arends NV, Sahai MA, Erreger K, Andreassen TF, Holy M, Hamilton PJ, Neergheen V, Karlsborg M, Newman AH, Pope S, Heales SJ, Friberg L, Law I, Pinborg LH, Sitte HH, Loland C, Shi L, Weinstein H, Galli A, Hjermind LE, Moller LB, Gether U. Missense dopamine transporter mutations associate with adult parkinsonism and ADHD. J Clin Invest. 2014;124:3107–20. doi: 10.1172/JCI73778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardaway JA, Hardie SL, Whitaker SM, Baas SR, Zhang B, Bermingham DP, Lichtenstein AJ, Blakely RD. Forward genetic analysis to identify determinants of dopamine signaling in Caenorhabditis elegans using swimming-induced paralysis. G3 (Bethesda) 2012;2:961–75. doi: 10.1534/g3.112.003533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardaway JA, Sturgeon SM, Snarrenberg CL, Li Z, Xu XZ, Bermingham DP, Odiase P, Spencer WC, Miller DM, 3rd, Carvelli L, Hardie SL, Blakely RD. Glial expression of the caenorhabditis elegans gene swip-10 supports glutamate dependent control of extrasynaptic dopamine signaling. J Neurosci. 2015;35:9409–23. doi: 10.1523/JNEUROSCI.0800-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardaway JA, Wang J, Fleming PA, Fleming KA, Whitaker SM, Nackenoff A, Snarrenberg CL, Hardie SL, Zhang B, Blakely RD. An open-source analytical platform for analysis of C. elegans swimming-induced paralysis. J Neurosci Methods. 2014;232:58–62. doi: 10.1016/j.jneumeth.2014.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hills T, Brockie PJ, Maricq AV. Dopamine and glutamate control area-restricted search behavior in Caenorhabditis elegans. J Neurosci. 2004;24:1217–25. doi: 10.1523/JNEUROSCI.1569-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hossain M, Wickramasekara RN, Carvelli L. beta-Phenylethylamine requires the dopamine transporter to increase extracellular dopamine in Caenorhabditis elegans dopaminergic neurons. Neurochem Int. 2014;73:27–31. doi: 10.1016/j.neuint.2013.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayanthi LD, Apparsundaram S, Malone MD, Ward E, Miller DM, Eppler M, Blakely RD. The Caenorhabditis elegans gene T23G5.5 encodes an antidepressant- and cocaine-sensitive dopamine transporter. Mol Pharmacol. 1998;54:601–9. [PubMed] [Google Scholar]
- Jorgensen EM, Hartwieg E, Schuske K, Nonet ML, Jin Y, Horvitz HR. Defective recycling of synaptic vesicles in synaptotagmin mutants of Caenorhabditis elegans. Nature. 1995;378:196–9. doi: 10.1038/378196a0. [DOI] [PubMed] [Google Scholar]
- Liu P, Chen B, Wang ZW. Gap junctions synchronize action potentials and Ca2+ transients in Caenorhabditis elegans body wall muscle. J Biol Chem. 2011;286:44285–93. doi: 10.1074/jbc.M111.292078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu P, Ge Q, Chen B, Salkoff L, Kotlikoff MI, Wang ZW. Genetic dissection of ion currents underlying all-or-none action potentials in C. elegans body-wall muscle cells. J Physiol. 2011;589:101–17. doi: 10.1113/jphysiol.2010.200683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locke CJ, Kautu BB, Berry KP, Lee SK, Caldwell KA, Caldwell GA. Pharmacogenetic analysis reveals a post-developmental role for Rac GTPases in Caenorhabditis elegans GABAergic neurotransmission. Genetics. 2009;183:1357–72. doi: 10.1534/genetics.109.106880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahoney TR, Liu Q, Itoh T, Luo S, Hadwiger G, Vincent R, Wang ZW, Fukuda M, Nonet ML. Regulation of synaptic transmission by RAB-3 and RAB-27 in Caenorhabditis elegans. Mol Biol Cell. 2006;17:2617–25. doi: 10.1091/mbc.E05-12-1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahoney TR, Luo S, Nonet ML. Analysis of synaptic transmission in Caenorhabditis elegans using an aldicarb-sensitivity assay. Nat Protoc. 2006;1:1772–7. doi: 10.1038/nprot.2006.281. [DOI] [PubMed] [Google Scholar]
- Mazei-Robison MS, Bowton E, Holy M, Schmudermaier M, Freissmuth M, Sitte HH, Galli A, Blakely RD. Anomalous dopamine release associated with a human dopamine transporter coding variant. J Neurosci. 2008;28:7040–6. doi: 10.1523/JNEUROSCI.0473-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick AV, Wheeler JM, Guthrie CR, Liachko NF, Kraemer BC. Dopamine D2 receptor antagonism suppresses tau aggregation and neurotoxicity. Biol Psychiatry. 2013;73:464–71. doi: 10.1016/j.biopsych.2012.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick PN, V, Wilson S, Wilson AA, Remington GJ. Acutely administered antipsychotic drugs are highly selective for dopamine D2 over D3 receptors. Pharmacol Res. 2013;70:66–71. doi: 10.1016/j.phrs.2013.01.002. [DOI] [PubMed] [Google Scholar]
- McCulloch KA, Qi YB, Takayanagi-Kiya S, Jin Y, Cherra SJ., 3rd Novel mutations in synaptic transmission genes suppress neuronal hyperexcitation in caenorhabditis elegans. G3 (Bethesda) 2017;7:2055–63. doi: 10.1534/g3.117.042598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald PW, Hardie SL, Jessen TN, Carvelli L, Matthies DS, Blakely RD. Vigorous motor activity in Caenorhabditis elegans requires efficient clearance of dopamine mediated by synaptic localization of the dopamine transporter DAT-1. J Neurosci. 2007;27:14216–27. doi: 10.1523/JNEUROSCI.2992-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald PW, Jessen T, Field JR, Blakely RD. Dopamine signaling architecture in Caenorhabditis elegans. Cell Mol Neurobiol. 2006;26:593–618. doi: 10.1007/s10571-006-9003-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntire SL, Jorgensen E, Horvitz HR. Genes required for GABA function in Caenorhabditis elegans. Nature. 1993;364:334–7. doi: 10.1038/364334a0. [DOI] [PubMed] [Google Scholar]
- McIntire SL, Jorgensen E, Kaplan J, Horvitz HR. The GABAergic nervous system of Caenorhabditis elegans. Nature. 1993;364:337–41. doi: 10.1038/364337a0. [DOI] [PubMed] [Google Scholar]
- Miller KG, Emerson MD, McManus JR, Rand JB. RIC-8 (Synembryn): a novel conserved protein that is required for G(q)alpha signaling in the C. elegans nervous system. Neuron. 2000;27:289–99. doi: 10.1016/s0896-6273(00)00037-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullen GP, Mathews EA, Vu MH, Hunter JW, Frisby DL, Duke A, Grundahl K, Osborne JD, Crowell JA, Rand JB. Choline transport and de novo choline synthesis support acetylcholine biosynthesis in Caenorhabditis elegans cholinergic neurons. Genetics. 2007;177:195–204. doi: 10.1534/genetics.107.074120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nass R, Hall DH, Miller DM, 3rd, Blakely RD. Neurotoxin-induced degeneration of dopamine neurons in Caenorhabditis elegans. Proc Natl Acad Sci U S A. 2002;99:3264–9. doi: 10.1073/pnas.042497999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng J, Zhen J, Meyer E, Erreger K, Li Y, Kakar N, Ahmad J, Thiele H, Kubisch C, Rider NL, Morton DH, Strauss KA, Puffenberger EG, D’Agnano D, Anikster Y, Carducci C, Hyland K, Rotstein M, Leuzzi V, Borck G, Reith ME, Kurian MA. Dopamine transporter deficiency syndrome: phenotypic spectrum from infancy to adulthood. Brain. 2014;137:1107–19. doi: 10.1093/brain/awu022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nonet ML, Staunton JE, Kilgard MP, Fergestad T, Hartwieg E, Horvitz HR, Jorgensen EM, Meyer BJ. Caenorhabditis elegans rab-3 mutant synapses exhibit impaired function and are partially depleted of vesicles. J Neurosci. 1997;17:8061–73. doi: 10.1523/JNEUROSCI.17-21-08061.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Offenburger SL, Jongsma E, Gartner A. Mutations in Caenorhabditis elegans neuroligin-like glit-1, the apoptosis pathway and the calcium chaperone crt-1 increase dopaminergic neurodegeneration after 6-OHDA treatment. PLoS Genet. 2018;14:e1007106. doi: 10.1371/journal.pgen.1007106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh KH, Kim H. Aldicarb-induced paralysis assay to determine defects in synaptic transmission in caenorhabditis elegans. Bio Protoc. 2017:7. doi: 10.21769/BioProtoc.2400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opperman CH, Chang S. Effects of aldicarb and fenamiphos on acetycholinesterase and motility of Caenorhabditis elegans. J Nematol. 1991;23:20–7. [PMC free article] [PubMed] [Google Scholar]
- Opperman KJ, Mulcahy B, Giles AC, Risley MG, Birnbaum RL, Tulgren ED, Dawson-Scully K, Zhen M, Grill B. The HECT family ubiquitin ligase EEL-1 regulates neuronal function and development. Cell Rep. 2017;19:822–35. doi: 10.1016/j.celrep.2017.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reckziegel P, Chen P, Caito S, Gubert P, Soares FA, Fachinetto R, Aschner M. Extracellular dopamine and alterations on dopamine transporter are related to reserpine toxicity in Caenorhabditis elegans. Arch Toxicol. 2016;90:633–45. doi: 10.1007/s00204-015-1451-7. [DOI] [PubMed] [Google Scholar]
- Richmond JE, Jorgensen EM. One GABA and two acetylcholine receptors function at the C. elegans neuromuscular junction. Nat Neurosci. 1999;2:791–7. doi: 10.1038/12160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safratowich BD, Hossain M, Bianchi L, Carvelli L. Amphetamine potentiates the effects of beta-phenylethylamine through activation of an amine-gated chloride channel. J Neurosci. 2014;34:4686–91. doi: 10.1523/JNEUROSCI.3100-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saharia K, Arya U, Kumar R, Sahu R, Das CK, Gupta K, Dwivedi H, Subramaniam JR. Reserpine modulates neurotransmitter release to extend lifespan and alleviate age-dependent Abeta proteotoxicity in Caenorhabditis elegans. Exp Gerontol. 2012;47:188–97. doi: 10.1016/j.exger.2011.12.006. [DOI] [PubMed] [Google Scholar]
- Saharia K, Kumar R, Gupta K, Mishra S, Subramaniam JR. Reserpine requires the D2-type receptor, dop-3, and the exoribonuclease, eri-1, to extend the lifespan in C. elegans. J Biosci. 2016;41:689–95. doi: 10.1007/s12038-016-9652-7. [DOI] [PubMed] [Google Scholar]
- Sakrikar D, Mazei-Robison MS, Mergy MA, Richtand NW, Han Q, Hamilton PJ, Bowton E, Galli A, Veenstra-Vanderweele J, Gill M, Blakely RD. Attention deficit/hyperactivity disorder-derived coding variation in the dopamine transporter disrupts microdomain targeting and trafficking regulation. J Neurosci. 2012;32:5385–97. doi: 10.1523/JNEUROSCI.6033-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanyal S, Wintle RF, Kindt KS, Nuttley WM, Arvan R, Fitzmaurice P, Bigras E, Merz DC, Hebert TE, van der Kooy D, Schafer WR, Culotti JG, Van Tol HH. Dopamine modulates the plasticity of mechanosensory responses in Caenorhabditis elegans. EMBO J. 2004;23:473–82. doi: 10.1038/sj.emboj.7600057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawin ER, Ranganathan R, Horvitz HR. C. elegans locomotory rate is modulated by the environment through a dopaminergic pathway and by experience through a serotonergic pathway. Neuron. 2000;26:619–31. doi: 10.1016/s0896-6273(00)81199-x. [DOI] [PubMed] [Google Scholar]
- Seeman P, Lee T, Chau-Wong M, Wong K. Antipsychotic drug doses and neuroleptic/dopamine receptors. Nature. 1976;261:717–9. doi: 10.1038/261717a0. [DOI] [PubMed] [Google Scholar]
- Snyder SH, Taylor KM, Coyle JT, Meyerhoff JL. The role of brain dopamine in behavioral regulation and the actions of psychotropic drugs. Am J Psychiatry. 1970;127:199–207. doi: 10.1176/ajp.127.2.199. [DOI] [PubMed] [Google Scholar]
- Srivastava D, Arya U, SoundaraRajan T, Dwivedi H, Kumar S, Subramaniam JR. Reserpine can confer stress tolerance and lifespan extension in the nematode C. elegans. Biogerontology. 2008;9:309–16. doi: 10.1007/s10522-008-9139-5. [DOI] [PubMed] [Google Scholar]
- Teixeira AM, Trevizol F, Colpo G, Garcia SC, Charao M, Pereira RP, Fachinetto R, Rocha JB, Burger ME. Influence of chronic exercise on reserpine-induced oxidative stress in rats: behavioral and antioxidant evaluations. Pharmacol Biochem Behav. 2008;88:465–72. doi: 10.1016/j.pbb.2007.10.004. [DOI] [PubMed] [Google Scholar]
- Thomas DM, Francescutti-Verbeem DM, Kuhn DM. Increases in cytoplasmic dopamine compromise the normal resistance of the nucleus accumbens to methamphetamine neurotoxicity. J Neurochem. 2009;109:1745–55. doi: 10.1111/j.1471-4159.2009.06094.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidal-Gadea A, Topper S, Young L, Crisp A, Kressin L, Elbel E, Maples T, Brauner M, Erbguth K, Axelrod A, Gottschalk A, Siegel D, Pierce-Shimomura JT. Caenorhabditis elegans selects distinct crawling and swimming gaits via dopamine and serotonin. Proc Natl Acad Sci U S A. 2011;108:17504–9. doi: 10.1073/pnas.1108673108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wabnig S, Liewald JF, Yu SC, Gottschalk A. High-throughput all-optical analysis of synaptic transmission and synaptic vesicle recycling in Caenorhabditis elegans. PLoS One. 2015;10:e0135584. doi: 10.1371/journal.pone.0135584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhen M, Samuel AD. C. elegans locomotion: small circuits, complex functions. Curr Opin Neurobiol. 2015;33:117–26. doi: 10.1016/j.conb.2015.03.009. [DOI] [PubMed] [Google Scholar]
- Zheng Y, Brockie PJ, Mellem JE, Madsen DM, Maricq AV. Neuronal control of locomotion in C. elegans is modified by a dominant mutation in the GLR-1 ionotropic glutamate receptor. Neuron. 1999;24:347–61. doi: 10.1016/s0896-6273(00)80849-1. [DOI] [PubMed] [Google Scholar]