Abstract
Tumors are complex tissues composed of variable amounts of both non-cellular components (matrix proteins) and a multitude of stromal cell types, which are under an active cross-talk with tumor cells. Tumor-associated macrophages (TAMs) are the major leukocyte population among the tumor-infiltrating immune cells. Once they are infiltrated into tumor stroma they undergo a polarized activation, where the M1 and M2 phenotypes represent the two extreme of the polarization heterogeneity spectrum. It is known that TAMs acquire a specific phenotype (M2), oriented toward tumor growth, angiogenesis and immune-suppression. A growing body of evidences supports the presence of tuning mechanisms in order to skew or restraint the inflammatory response of TAMs and thus forces them to function as active tumor-promoting immune cells. The receptor of advanced glycation end-products (RAGE) is a member of the immunoglobulin protein family of cell surface molecules, being activated by several danger signals and thus signaling to promote the production of many pro-inflammatory molecules. Interestingly, this receptor is paradoxically expressed in both M1 and M2 macrophages phenotypes. This review addresses how RAGE signaling has been drifted away in M2 macrophages, and thus taking advantage of the abundance of RAGE ligands at tumor microenvironment, particularly HMGB1, to reinforce the supportive M2 macrophages strategy to support tumor growth.
Keywords: Receptor for advanced glycation end-products, Tumor microenvironment, Macrophage polarization, Alarmins, Tumor-associated macrophages
Introduction
It is estimated that almost 25% of all cancers are somehow associated with chronic infection and inflammation [1–4]. In addition, evidences derived from both epidemiological studies and basic research has demonstrated that organ-specific carcinogenesis is linked to a chronic and local inflammatory milieu, for instances, the H. pylori-induced gastric inflammation and the occurrence of gastric cancer and gastric mucosa-associated lymphoid tissue lymphoma have become in a classic example [5]. The presence of inflammatory elements at the microenvironment of neoplasic tissues is now well accepted, thus inflammation has been suggested to represent the 7th hallmark of cancer [6, 7].
Cancer and inflammation are mainly connected by two pathways [2, 8]. In the intrinsic pathway, both oncogenes and tumor suppressor genes drive the expression of inflammation-related programs, thus generating an inflammatory microenvironment. The extrinsic pathway is based on different conditions that cause non-resolving smoldering inflammation, and it is driven by inflammatory cells and a myriad of mediators produced by these cells. As masterpieces in the intersection of both pathways, different elements have been identified including transcription factors, such NF-kB, STAT3, cytokines, chemokines and cellular components, such as tumor-associated macrophages (TAMs) [9–15].
Among the most relevant cytokines in the context of cancer-related inflammation, it should be firstly mentioned TNF-α. It plays an important role in the triggering and maintenance of inflammation. Tumor-derived TNF-α has been consistently associated with tumor growth [16, 17], and its constitutive release has been associated with increased release of chemokines (CCL2, CXCL8, CXCL12), IL-6, VEGF and macrophage migration inhibitory factor (MIF-1). Thus, TNF-α is able to generate an autocrine tumor-promoting network [18]. IL-1 also promotes tumor growth and metastasis by inducing several pro-metastatic genes such as metalloproteinases, chemokines, growth factors and TGF-β [19]. Furthermore, patients with IL-1 producing tumors have generally bad prognosis [20]. IL-1 also stimulates the expression of endothelial adhesion molecules [21]. Strikingly, IL-1 can be also released from necrotizing tumor/stroma cells and may then function as an alarmin [22]. Whereas some tumors acquire the ability to down-regulate alarmins able to induce apoptosis or recruit antigen-presenting cells, other tumors hijack normal alarmin function to send out fraudulent SOS signals as a means to promote their survival [23].
IL-6 is another key growth-promoting and anti-apoptotic inflammatory cytokine [24, 25]. IL-6 protects normal and pre-malignant intestinal epithelial cells from apoptosis and promotes the proliferation of tumor-initiating cells by a mechanism involving STAT3 [26, 27].
Different studies carried out on gene-targeted mice have clearly demonstrated the key roles of chemokines in cancer biology, particularly as mediators of chronic inflammation, and thus supporting initiation and/or tumor progression in many solid tumors [28, 29]. There is a growing body of evidences supporting that CXCR4/CXCL12 is one of the most efficient pair of chemokine receptor/chemokine to enhance tumor growth, being associated with tumor progression in many tumor types, including gastric cancer [30], as well as in the homing of cancer cells to metastatic niches and in the recruitment of different cells types to the tumor microenvironment [31–33].
Tumor Microenvironment
Tumors are complex tissues composed of variable amounts of both non-cellular components (matrix proteins) and a multitude of stromal cell types, in addition to the ever-evolving neoplastic cells [34, 35]. It is important to highlight that in tumor microenvironment converge the persistent and abundance of a myriad of soluble signals in a hypoxic niche. In this context, tumor cells orchestrate microenvironment modifications by attracting or activating many of these non-tumoral cells. Thereby, there is a consensus that dynamics changes occurred in tumor microenvironment during the progression towards advanced tumor stages, thus determining the outcome of tumor growth, tumor dormancy, tumor invasion, metastasis and resistance to therapy [36].
This dynamics changes are mainly produced by the active cross-talk between tumor cells and tumor-infiltrating cells, which not only fail to mount an effective anti-tumour immune response, but also interact intimately to actively modified tumor development particularly by their actions towards promoting tumor progression, invasion, and metastasis. Of note, tumor-associated macrophages (TAMs) are the major leukocyte population among the tumor-infiltrating immune cells.
Tumor-Associated Macrophages (TAMs)
TAMs represent the major inflammatory component of the stroma of many tumors [37]. They are recruited early at tumor site where they promote tumor growth, switch to an angiogenic program, the resistance of tumor cells to apoptotic stimuli, tissue remodeling, invasion of tumor cells and the suppression of cytotoxic T-cell activities [38, 39].
Although the interactions between macrophages and tumor cells are incompletely defined, it is evident that the macrophage-dependent production of proteases, growth factors and cytokines regulates tumor seeding and the metastatic process [40]. The presence of immunocompetent cells, particularly T lymphocytes has been considered as a proof of an immunological antitumor response. Indeed the degree of T-lymphocytes infiltration has been consistently associated with more favorable clinical outcome [41]. In contrast, TAMs densities inversely correlate with patient prognosis in most solid tumors [42–44].
Macrophages Plasticity
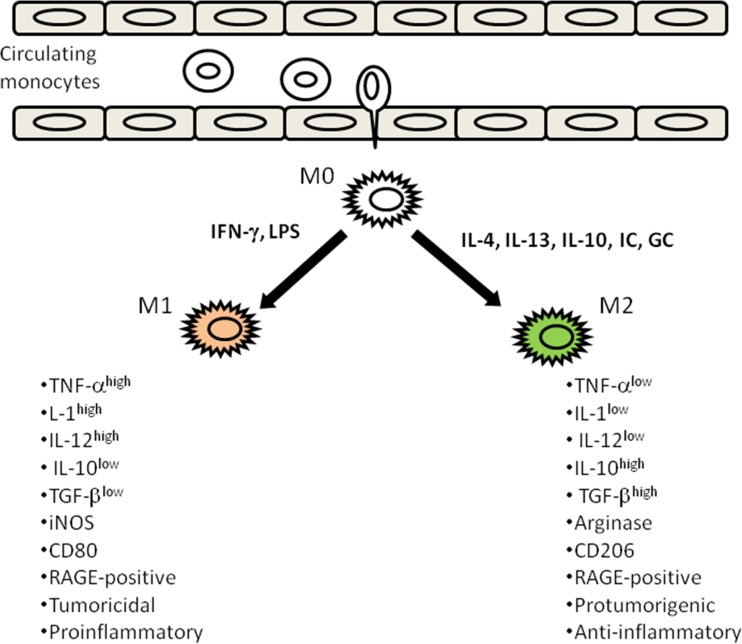
Macrophages are highly plastic cells, which undergo polarized activation. In this context, the “classically activated” or M1 phenotype (TNF-αhigh,IL-1high, IL-12high, IL-10low, TGFβlow) and the “alternatively activated” or M2 phenotype (TNF-αlow,IL-1low, IL-12low, IL-10high, TGFβhigh) represent two extreme phenotypes in this heterogeneity spectrum [45–47] (Fig. 1). Classical or M1 macrophages activation is produced in response to microbial products (LPS) or interferon-γ and display a classical expression pattern in regard to high IL-12 and IL-23 production and elicit antitumor and tissue destructive reactions. On the contrary, M2 cells respond to IL-4, IL-13, IL-10, immune complexes or glucocorticoids displaying a low IL-12, high IL-10 production pattern, poor antigen-presenting capacity and suppression of Th1 adaptative immunity, thus promoting tumor cell survival, proliferation and dissemination [48, 49].
Fig. 1.
Schematic representation of the two extreme phenotypes (M1 and M2) in the heterogeneity spectrum derived from macrophage polarization. M1 phenotype arises as a response to interferon-γ and LPS while M2 are generated in response to IL-4, IL-13, IL-10, immunocomplexes (IC), or glucocorticoids (GC). The classical M1 phenotype supports proinflammatry and tumoricidal responses. On the contrary M2 macrophages are supportive of anti-inflammatory and protumorigenic responses
TAMs as M2 Polarized Macrophages
It is known that TAMs acquire a specific phenotype (M2), oriented toward tumor growth, angiogenesis and immune-suppression. This M2 polarization is promoted by signals presented at the tumor microenvironment such as PGE2, TGF-β, IL-6, IL-10 which are produced by tumor cells and by TAMs themselves. The M2 phenotype of TAMs is associated with profound effects on tumor progression [50]. It is well accepted that NF-κB activation in cancer cells is mainly promoted by signals derived from the microenvironment itself, such as a cytokines/chemokines network, hypoxic conditions, and oxidative stress. NF-κB activation on cancer cells is known to contribute to their proliferation and survival [51]. In contrast, TAMs from advanced neoplasias display defective NF-κB activation. Accumulation of p50 homodimers, which act as repressor of NF-κB activation, has been reported in TAMs [52], thereby impairing the production of cytotoxic mediators such as nitric oxide (NO), cytokines, TNF-α, IL-1 and IL-12.
Intracellular signaling networks are tightly regulated in both M1 and M2 macrophage phenotypes. The cytokine signaling protein suppressor family (SOCS) represents a negative regulator for various cytokine-mediated signaling [53, 54]). This family is linked to macrophage polarization, SOCS3 member is essential to M1 polarized macrophages [55], whereas SOCS1 is up-regulated in M2 macrophages, being critical in sustaining the anti-inflammatory phenotype [56].
The Interferon Regulatory Factor (IRF) family also participates in signal transduction triggered by pattern recognition receptors [57]. Both IRF5 and IRF4, are key players in the commitment of macrophage polarization into M2 or M1 macrophages phenotypes, respectively [58, 59].
Additionally STAT3, a member of the STATs transcription factors, has been reported to be constitutively activated in tumor cells as well as in TAMs [60, 61], leading to the inhibition of proinflammatory mediators production.
The Receptor of Advanced Glycation End-Products (RAGE)
RAGE is a member of the immunoglobulin protein family of cell surface molecules, being activated by several danger signals, and thus functioning as a pattern-recognition receptor [62, 63]. In addition to the full-length receptor, RAGE undergoes extensive alternative splicing. However, endogenous soluble RAGE isoform may be generated by mechanisms different from alternative splicing, such as the participation of membrane associated-proteases, including the sheddase A disintegrin and metalloprotease-10 (ADAM-10) and the matrix metalloproteinase-9 (MMP-9) [64, 65]. Of note, soluble RAGE (sRAGE) may function as a decoy for ligands, and thus preventing the interaction with the membrane anchored full-length RAGE [66].
RAGE as a Multiligand Receptor
Advanced glycation end-products (AGEs) were the first identified RAGE ligands, particularly N-carboxymethyllysine-modified proteins [67, 68]. The presence of AGEs has been even detected in human cancer tissues [69].
Of particular importance, S100/calgranulins and high-mobility group box 1 (HMGB1) have been also identified as RAGE ligands. S100 proteins are responsible for different roles in cell cycle, but some members of the family, have relevant extracellular roles, particularly at sites of chronic inflammation, being able to active, via RAGE, different cell types, including macrophages [70]. HMGB1 belongs to the so-called damage-associated molecular pattern molecules or alarmins, which are released in response to infection or inflammatory stimuli, especially during tissue damage [71]. Both S100/calgranulins and HMGB1 are expressed and secreted not only by cancer cells but also by stroma-infiltrating cells [72, 73].
Noteworthy, the ligand HMGB1 may signal through RAGE and via TLRs (TLR2/TLR4). Activation of these receptors results in the activation of NF-kB, AP-1, thereby promoting inflammation [74]. Very recently, new data have highlighted the cross-talk between TLRs and RAGE, when both TIRAP and MyD88, two adaptor proteins for TLR-2 and TLR-4, also function for RAGE once it is phosphorylated by PKC-ζ upon binding of ligands, and thus partly share intracellular signaling pathways [75].
RAGE and Tumor Microenvironment
There is a growing body of evidences that clearly support that RAGE axis activation plays a central role in strengthening the inflammatory milieu at tumor microenvironment [76]. In this context, many open questions still remain to be answered particularly on the role of RAGE axis on different tumor infiltrating cell populations. In addition to the cancer cells themselves, tumors are also comprised of many RAGE-positive cell types, such as stromal cells. All these cellular components are under a very complex cross-talk mediated by a myriad of biological mediators. From the mechanistic point of view, a considerable amount of new pieces of knowledge has been added in the last years; however, much more remains to be understood regarding the role of RAGE in tumor biology, particularly in this complex cross-talk located at tumor microenvironment and where the net response favors tumor growth and progression. Although the functionality of RAGE in some tumor infiltrating cells has been described, the role of RAGE on tumor-associated macrophages have been recently started to be clarified.
RAGE and Macrophage Functionality
We and others have demonstrated that RAGE engagement activates redox-sensitive transcription factors such as NF-κB, and subsequently induce the production of pro-inflammatory molecules such as IL-1, IL-6 and TNF-alpha, NO and superoxide [77–84]. Very interestingly, sRAGE was shown to bind and induce monocytes survival and differentiation into macrophages, which paralleled by increases in the expression of mannose receptors and in CCR5 chemokine receptor [85]. Mannose receptor engagement by tumoral mucins modulates cytokine production by TAM toward an immune-suppressive profile [86].Furthermore, the CCR5 ligand CCL5, is known to increase the presence of tumor-associated macrophages (TAM) and inhibiting potential anti-tumor T cell activities, at least in breast cancer [87].
CCL2 is one of the highly represented chemokines in a wide range of tumor, as well as one of the important determinants of human tumor macrophages content [88]. RAGE over-expression has been linked to the induction of CCL2 expression [89].
One of the earlier events downstream RAGE engagements is the activation of reactive oxygen species (ROS)-generating enzymes such as NADPH oxidase (NOX), thereby contributing to the activation of redox signaling pathways. TAMs in thyroid carcinoma displayed strong immunostaining for NOX2, the catalytic subunit of NAPH oxidase [90]. Although ROS appear to be related more to “classic” M1 macrophages phenotype, is important to highlight the contribution of ROS to an immunosuppressive environment [91], as well as to invadopodia and podosome formation which facilitates tumor invasive behavior [92].
Macrophages are key elements of innate immunity and host defense, and where pattern recognition receptors are able to sense “danger signals” resulting from tissue damage and necrosis. In the innate immune system, many receptor systems are capable of adapting their responsiveness to marked and sustained increases in the concentration of extracellular ligand(s) (as occurs in tumor microenvironment) and use the steady-state levels to generate appropriate negative feedback mechanisms that effectively shut down signal transduction [93].
In this sense, the nature of the “tuning machinery” displayed by M2 macrophages might involve not only the recruitment of negative feedback pathways affecting RAGE/TLRs downstream signaling but also other gene expression regulation mechanisms, such as chromatin modification and microRNAs, thereby promoting the skewed profile ascribed to M2 macrophages. Strikingly, it has been suggested that acute inflammatory state in macrophages is transient and unstable and evolves to a tolerant state associated with features of the “alternative” macrophage activation [94].
Restraining or Skewing Inflammatory Response in TAMs
A growing body of evidences supports the presence of tuning mechanisms in order to skew or restraint the inflammatory response of TAMs and thus forces them to function as active tumor-promoting immune cells. Some major mechanisms have described, particularly those based on the acquisition of a tolerant state, the induction of epigenetic changes as well the marked changes in the miRNAs profile.
Tolerance
As mentioned above, TAMs displayed a defective NF-κB signaling, resembling the classical LPS tolerance [52]. Furthermore, recent data derived from microarrays and advanced bioinformatics analysis determined that gene expression pattern in endotoxin tolerance is very similar to that found in M2 macrophages [95].
Taking in mind that RAGE is now considered as a member of pattern recognition receptor family, it is noteworthy that macrophage tolerance can be also induced by stimulation through TLRs, thus representing a general regulatory strategy to control inflammation triggered by TLRs signaling [96]. In the classical endotoxin tolerance, TLR signaling is suppressed, either by TLR4 desensitization or by induction of molecules able to inhibit TLR signaling such as IRAK-M, SHIP, SOCS1 and A20 [97]. TAMs produce low amounts of proinflammatory cytokines, a condition that may resemble some aspects of tolerance, which may then contribute to the immunosuppressive tumor microenvironment. Strikingly, recent reports have highlighted the relevance of chromatin modifications leading to a tolerant phenotype [98].
Epigenetics
Inflammation involves a very complex regulatory network. In addition to transcription factors families, chromatin structure modification has been shown to be a key regulator of many inflammatory genes. Chromatin remodeling via histone modification is one of the key epigenetic mechanisms that plays a pivotal role in the maintenance of both active and suppressed states of gene expression depending of the site of methylation [99, 100]. Of note, epigenetic regulation has been recently reported in alternative phenotype of murine macrophages, particularly a decreased in H3K27 (histone H3 at lysine-27) methylation at the promoter of M2 marker genes and a paralleled increased in the demethylase Jumonji domain containing-3 (Jmjd3) expression [101]. Furthermore, transcription-prone histone modification at the IL-10 promoter has been described in TLR signaling in TAMs, thereby enhancing IL-10-mediated immunosupression [102]. Noteworthy, RAGE activation by S100B is able to induce the expression of thioredoxin-interacting protein (TXNIP), the endogenous inhibitor of ROS-scavenging protein thioredoxin (TRX). Additionally, TXNIP over-expression abolished H3K9 tri-methylation, a marker for gene inactivation, and increased H3K9 acetylation, an indicator of gene induction, at proximal Cox2 promoter [103].
MicroRNAs
In the context of innate immunity and particularly on macrophages gene expression profile changes during the course of an inflammatory reaction, microRNAs have emerged as important regulators [104]. Of note, miR-155 also regulates inflammatory cytokine production in TAMs by targeting the enhancer binding protein C/EBPβ [105]. Conversely, miR-155 has been reported to target IL-13Rα1, which is known to trigger an M2 phenotype [106]. Noteworthy, IL-10, a crucial anti-inflammatory cytokine, inhibits miR-155 transcription by an STAT-3 dependent mechanism [107]. RF5 is a member of the interferon regulatory factor family, which is crucial in the up-regulation of the M1 phenotype markers and inhibits transcriptional activation of IL-10, a marker of M2 phenotype [108]. Strikingly, miR-146a is known to dampen down inflammatory response, by targeting genes such as IRF-5 and other signaling molecules down-stream MyD88, such as TRAF-6 and IRAK1 [109]. Of note, MyD88 blocking largely abrogated the RAGE-mediated intracellular signaling [75]. miR146a over-expression also results in a decrease of proinflammatory signals such as CXCL8 [110], IL-6 [111] and TNF-α [112]. Furthermore, miR21 controls inflammation by down-regulation the translation of the pro-inflammatory tumor suppressor programmed cell death 4(PDCD4), an inhibitor of IL-10 production [113].
Skewing RAGE Signaling. An Emerging Mechanism
We have recently reported that RAGE is equally expressed in both M1 and M2 macrophages polarized phenotypes and that RAGE activation by the alarmin HMGB1, highly abundant at tumor microenvironment, promotes protumoral activities of M2 macrophages based on their abilities to enhance tumor cell invasion and promoting angiogenesis. All these effects were abrogated by RAGE-targeting knockdown [114].
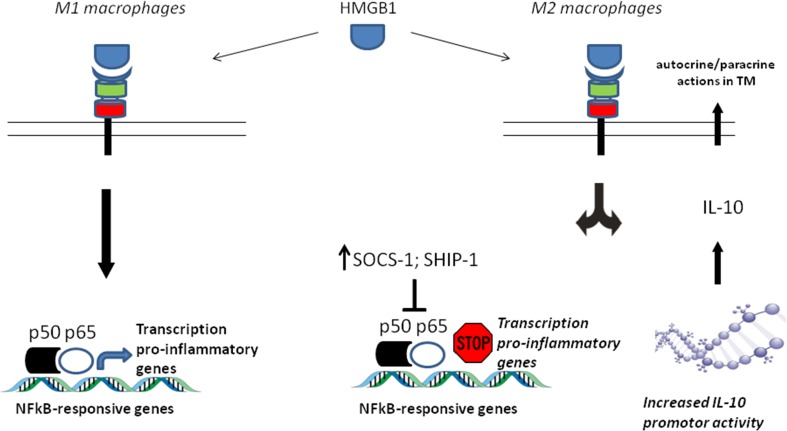
Additionally, RAGE activation by HMGB1 in M2 macrophages did not produce NFkB activation supporting how cell signaling triggering by RAGE activation in these cells has been drifted away from its classical NFkB activation-dependent proinflamatory response, by a mechanism that involved the induction of both the suppressor of cytokine signaling 1 (SOCS1) and the Src homology-2 domain-containing inositol 5-phosphatase 1 (SHIP-1), which are negative regulators of NFkB activation [114].
Another important finding supporting the fact that RAGE activation-mediated proinflammatory signaling has been skewed in M2 macrophages was that RAGE activation by HMGB1 in this phenotype induced a transcription-prone histone modification at the IL-10 promoter, and this epigenetic imprinting correlates with increments of HMGB1-induce IL-10 production, as reported independently by two groups [115, 116] (Fig. 2).
Fig. 2.
Skewing RAGE signaling seems to be an emerging mechanism to reinforce the anti-inflammatory and protumorigenic actions of M2 macrophages. Although during polarization active transcriptomic reprogramming occurs on of macrophages, RAGE still remained expressed and functional on M2 macrophages but its canonical proinflammatory signaling has been drifted away, either by increasing expression of NFkB activation inhibitors such as SOCS1 and SHIP1 or by transcription-prone histone modification at the IL-10 promoter, thus taking advantage of the abundance of its ligands on tumor microenvironment, particularly HMGB1, to reinforce a supportive tumor growth strategy
In summary, new data has emerged demonstrating how complex are the cellular cross-talk in tumor stroma which may even skew the canonical responses that we can expect for individual cell types. This is particularly interesting based on the fact that many tumor stroma cells undergo a process of phenotypic polarization, and most of them also express RAGE.
Finally, during the last years, TAMs have been recognize as an active and bidirectional modulator of immune response, and therefore a particular interest has raised to be considered as target for the treatment of tumors. It is known that TAMs are able to even modulate the efficiency of chemotherapy, radiation therapy, and immunotherapy in different stages of various tumors [117]. Of note, reeducation of TAMs towards an anti-tumor cell population is now a promising approach for the treatment of tumors. However, this goal will be only reached when we fully understand all cellular and molecular mechanisms underlying the switch from tumor-suppressing to tumor-promoting phenotypes.
Conclusion
Once macrophages infiltrate tumors they undergo a polarized activation, where the M1 and M2 phenotypes represent the two extreme of the polarization heterogeneity spectrum. At present, there is a consensus about the presence of tuning mechanisms in order to skew or restraint the inflammatory response of TAMs and thus forces them to function as active tumor-promoting immune cells. The activation of the receptor of advanced glycation end-products by HMGB1, a the highly abundant alarmin at tumor microenvironment, has emerged as a new mechanism which is able to drifted away the canonical proinflamatory signaling observed in classically activated macrophages and thus strengthening their supportive tumor growth behaviour.
Acknowledgments
This work was supported by grant 1130337 from Programa Fondecyt, Comisión Nacional de Ciencia y Teconología, Chile.
References
- 1.Blakwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;237:539–545. doi: 10.1016/S0140-6736(00)04046-0. [DOI] [PubMed] [Google Scholar]
- 2.Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454:436–444. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- 3.Rakoff-Nahoum S, Medzhitov R. Toll-like receptors and cancer. Nat Rev Cancer. 2009;9:57–63. doi: 10.1038/nrc2541. [DOI] [PubMed] [Google Scholar]
- 4.H zur Hausen (2006) Infections causing human cancer. Wiley-VCH. 531 p
- 5.McNamara D, El-Omar E. Helicobacter pylori infection and the pathogenesis of gastric cancer: a paradigm for host-bacterial interactions. Dig Liver Dis. 2008;40:504–509. doi: 10.1016/j.dld.2008.02.031. [DOI] [PubMed] [Google Scholar]
- 6.Mantovani A. Inflaming matastasis. Nature. 2009;457:36–37. doi: 10.1038/457036b. [DOI] [PubMed] [Google Scholar]
- 7.Colotta F, Allavena P, Sica A, et al. Cancer-related inflammation, the seventh hallmark of cancer: links to genetic instability. Carcinogenesis. 2009;7:1073–1081. doi: 10.1093/carcin/bgp127. [DOI] [PubMed] [Google Scholar]
- 8.Balkwill F, Mantovani A. Cancer-related inflammation: common themes and therapeutic opportunities. Semin Cancer Biol. 2012;22:33–40. doi: 10.1016/j.semcancer.2011.12.005. [DOI] [PubMed] [Google Scholar]
- 9.Mantovani A, Garlanda C, Allavena P. Molecular pathways and targets in cancer-related inflammation. Ann Med. 2010;42:161–170. doi: 10.3109/07853890903405753. [DOI] [PubMed] [Google Scholar]
- 10.Sethi G, Sung B, Aggarwal BB. Nuclear factor-kappaB activation: from bench to bedside. Exp Biol Med. 2008;233:21–31. doi: 10.3181/0707-MR-196. [DOI] [PubMed] [Google Scholar]
- 11.Kundu JK, Surh YJ. Inflammation: gearing the journey to cancer. Mutat Res. 2008;659:15–30. doi: 10.1016/j.mrrev.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 12.Garlanda C, Riva F, Polentarutti N, et al. Intestinal inflammation in mice deficient in Tir8, an inhibitory member of the IL-1 receptor family. Proc Natl Acad Sci U S A. 2004;101:3522–3526. doi: 10.1073/pnas.0308680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yu H, Pardoll D, Jove R. STATs in cancer inflammation and immunity: a leading role for STAT3. Nat Rev Cancer. 2009;9:798–809. doi: 10.1038/nrc2734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kortylewski M, Yu H. Role of Stat3 in suppressing anti-tumor immunity. Curr Opin Immunol. 2008;20:228–233. doi: 10.1016/j.coi.2008.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kortylewski M, Xin H, Kujawski M, et al. Regulation of the IL-23 and IL-12 balance by Stat3 signaling in the tumor microenvironment. Cancer Cell. 2009;15:114–123. doi: 10.1016/j.ccr.2008.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Balkwill F. Tumour necrosis factor and cancer. Nat Rev Cancer. 2009;9:361–371. doi: 10.1038/nrc2628. [DOI] [PubMed] [Google Scholar]
- 17.Charles KA, Kulbe H, Soper R, et al. The tumor-promoting actions of TNF-alpha involve TNFR1 and IL-17 in ovarian cancer in mice and humans. J Clin Invest. 2009;119:3011–3023. doi: 10.1172/JCI39065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kulbe H, Thompson R, Wilson JL, et al. The inflammatory cytokine tumor necrosis factor-alpha generates an autocrine tumor-promoting network in epithelial ovarian cancer cells. Cancer Res. 2007;67:585–592. doi: 10.1158/0008-5472.CAN-06-2941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Naldini A, Filippi I, Miglietta D, et al. Interleukin-1beta regulates the migratory potential of MDAMB231 breast cancer cells through the hypoxia-inducible factor-1alpha. Eur J Cancer. 2010;46:3400–3408. doi: 10.1016/j.ejca.2010.07.044. [DOI] [PubMed] [Google Scholar]
- 20.Dinarello CA. The paradox of pro-inflammatory cytokines in cancer. Cancer Metastasis Rev. 2006;25:307–313. doi: 10.1007/s10555-006-9000-8. [DOI] [PubMed] [Google Scholar]
- 21.Arguello F, Baggs RB, Graves BT, et al. Effect of IL-1 on experimental bone/bone-marrow metastases. Int J Cancer. 1992;52:802–807. doi: 10.1002/ijc.2910520522. [DOI] [PubMed] [Google Scholar]
- 22.Chen CJ, Kono H, Golenbock D, et al. Identification of a key pathway required for the sterile inflammatory response triggered by dying cells. Nat Med. 2007;13:851–856. doi: 10.1038/nm1603. [DOI] [PubMed] [Google Scholar]
- 23.Coffelt SB, Scandurro AB. Tumors sound the alarmin(s) Cancer Res. 2008;68:6482–6485. doi: 10.1158/0008-5472.CAN-08-0044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lin WW, Karin M. A cytokine-mediated link between innate immunity, inflammation, and cancer. J Clin Invest. 2007;117:1175–1183. doi: 10.1172/JCI31537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Naugler WE, Karin M. The wolf in sheep’s clothing: the role of interleukin-6 in immunity, inflammation and cancer. Trends Mol Med. 2008;14:109–119. doi: 10.1016/j.molmed.2007.12.007. [DOI] [PubMed] [Google Scholar]
- 26.Bollrath J, Phesse TJ, von Burstin VA, et al. gp130-mediated Stat3 activation in enterocytes regulates cell survival and cell-cycle progression during colitis-associated tumorigenesis. Cancer Cell. 2009;15:91–102. doi: 10.1016/j.ccr.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 27.Grivennikov S, Karin E, Terzic J, et al. IL-6 and Stat3 are required for survival of intestinal epithelial cells and development of colitis-associated cancer. Cancer Cell. 2009;15:103–113. doi: 10.1016/j.ccr.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Allavena P, Germano G, Marchesi F, et al. Chemokines in cancer related inflammation. Exp Cell Res. 2011;17:664–673. doi: 10.1016/j.yexcr.2010.11.013. [DOI] [PubMed] [Google Scholar]
- 29.Lazennec G, Richmond A. Chemokines and chemokine receptors: new insights into cancer-related inflammation. Trends Mol Med. 2010;16:133–144. doi: 10.1016/j.molmed.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen G, Chen SM, Wang X, et al. Inhibition of chemokine (CXC motif) ligand 12/chemokine (CXC motif) receptor 4 axis (CXCL12/CXCR4)-mediated cell migration by targeting mammalian target of rapamycin (mTOR) pathway in human gastric carcinoma cells. J Biol Chem. 2012;287:12132–12141. doi: 10.1074/jbc.M111.302299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sun X, Cheng G, Hao M, et al. CXCL12 / CXCR4 / CXCR7 chemokine axis and cancer progression. Cancer Metastasis Rev. 2010;29:709–722. doi: 10.1007/s10555-010-9256-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Boldajipour B, Mahabaleshwar H, Kardash E, et al. Control of chemokine-guided cell migration by ligand sequestration. Cell. 2008;132:463–473. doi: 10.1016/j.cell.2007.12.034. [DOI] [PubMed] [Google Scholar]
- 33.Balkwill FR. The chemockine system and cancer. J Pathol. 2012;226:148–157. doi: 10.1002/path.3029. [DOI] [PubMed] [Google Scholar]
- 34.Whiteside TL. The tumor microenvironment and its role in promoting tumor growth. Oncogene. 2008;27:5904–5912. doi: 10.1038/onc.2008.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mbeunkui F, Johann DJ., Jr Cancer and the tumor microenvironment: a review of an essential relationship. Cancer Chemother Pharmacol. 2009;63:571–582. doi: 10.1007/s00280-008-0881-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sautès-Fridman C, Cherfils-Vicini J, Damotte D, et al. Tumor microenvironment is multifaceted. Cancer Metastasis Rev. 2011;30:13–25. doi: 10.1007/s10555-011-9279-y. [DOI] [PubMed] [Google Scholar]
- 37.Solinas G, Germano G, Mantovani A, et al. Tumor -associated macrophages (TAM) as major players of the cancer-related inflammation. J Leukoc Biol. 2009;86:1065–1073. doi: 10.1189/jlb.0609385. [DOI] [PubMed] [Google Scholar]
- 38.Solinas G, Marchesi F, Garlanda C, et al. Inflammation-mediated promotion of invasion and metastasis. Cancer Metastasis Rev. 2010;29:243–248. doi: 10.1007/s10555-010-9227-2. [DOI] [PubMed] [Google Scholar]
- 39.Sica A. Role of tumor-associated macrophages in cancer-related inflammation. Exp Oncol. 2010;32:153–158. [PubMed] [Google Scholar]
- 40.Galon J, Costes A, Sanchez-Cabo F, et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313:1960–1964. doi: 10.1126/science.1129139. [DOI] [PubMed] [Google Scholar]
- 41.Taylor RC, Patel A, Panageas KS, et al. Tumor-infiltrating lymphocytes predict sentinel lymph node positivity in patients with cutaneous melanoma. J Clin Oncol. 2007;25:869–875. doi: 10.1200/JCO.2006.08.9755. [DOI] [PubMed] [Google Scholar]
- 42.Qian B, Deng Y, Im JH, et al. A distinct macrophage population mediates metastatic breast cancer cell extravasation, establishment and growth. PLoS One. 2009;4:e6562. doi: 10.1371/journal.pone.0006562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen J, Yao Y, Gong C, et al. CCL18 from tumor-associated macrophages promotes breast cancer metastasis via PITPNM3. Cancer Cell. 2011;19:541–555. doi: 10.1016/j.ccr.2011.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kurahara H, Shinchi H, Mataki Y, et al. Significance of M2-polarized tumor-associated macrophage in pancreatic cancer. J Surg Res. 2011;167:e211–e219. doi: 10.1016/j.jss.2009.05.026. [DOI] [PubMed] [Google Scholar]
- 45.Das A, Sinha M, Datta S, et al. Monocyte and macrophage plasticity in tissue repair and regeneration. Am J Pathol. 2015;185:2596–2606. doi: 10.1016/j.ajpath.2015.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Locati M, Mantovani A, Sica A. Macrophage activation and polarization as an adaptive component of innate immunity. Adv Immunol. 2013;120:163–184. doi: 10.1016/B978-0-12-417028-5.00006-5. [DOI] [PubMed] [Google Scholar]
- 47.Giorgio S. Macrophages: plastic solutions to environmental heterogeneity. Inflamm Res. 2013;62:835–843. doi: 10.1007/s00011-013-0647-7. [DOI] [PubMed] [Google Scholar]
- 48.Gordon S, Martinez F. Alternative activation of macrophages: mechanism and functions. Immunity. 2010;32:593–604. doi: 10.1016/j.immuni.2010.05.007. [DOI] [PubMed] [Google Scholar]
- 49.Jetten N, Verbruggen S. Anti-inflammatory M2, but not pro-inflammatory M1 macrophages promote angiogenesis in vivo. Angiogenesis. 2014;1:109–118. doi: 10.1007/s10456-013-9381-6. [DOI] [PubMed] [Google Scholar]
- 50.Mantovani A, Sozzani S, Locati M, et al. Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol. 2002;23:549–555. doi: 10.1016/s1471-4906(02)02302-5. [DOI] [PubMed] [Google Scholar]
- 51.Sica A, Bronte V. Altered macrophage differentiation and immune dysfunction in tumor development. J Clin Invest. 2007;117:1155–1166. doi: 10.1172/JCI31422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Porta C, Rimoldi M, Raes G, et al. Tolerance and M2 (alternative) macrophage polarization are related processes orchestrated by p50 nuclear factor kappaB. Proc Natl Acad Sci U S A. 2009;106:14978–14983. doi: 10.1073/pnas.0809784106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fujimoto M, Naka T. Regulation of cytokine signaling by SOCS family molecules. Trends Immunol. 2003;24:659–666. doi: 10.1016/j.it.2003.10.008. [DOI] [PubMed] [Google Scholar]
- 54.Yoshimura A, Naka T, Kubo M. SOCS proteins, cytokine signalling and immune regulation. Nat Rev Immunol. 2007;7:454–465. doi: 10.1038/nri2093. [DOI] [PubMed] [Google Scholar]
- 55.Liu Y, Stewart KN, Bishop E, et al. Unique expression of suppressor of cytokine signaling 3 is essential for classical macrophage activation in rodents in vitro and in vivo. J Immunol. 2008;180:6270–6278. doi: 10.4049/jimmunol.180.9.6270. [DOI] [PubMed] [Google Scholar]
- 56.Whyte CS, Bishop ET, Rückerl D, et al. Suppressor of cytokine signaling (SOCS)1 is a key determinant of differential macrophage activation and function. J Leukoc Biol. 2011;90:845–854. doi: 10.1189/jlb.1110644. [DOI] [PubMed] [Google Scholar]
- 57.Ozato K, Tailor P, Kubota T. The interferon regulatory factor family in host defense: mechanism of action. J Biol Chem. 2007;282:20065–20069. doi: 10.1074/jbc.R700003200. [DOI] [PubMed] [Google Scholar]
- 58.Krausgruber T, Blazek K, Smallie T, et al. IRF5 promotes inflammatory macrophage polarization and TH1-TH17 responses. Nat Immunol. 2011;12:231–238. doi: 10.1038/ni.1990. [DOI] [PubMed] [Google Scholar]
- 59.Satoh T, Takeuchi O, Vandenbon A, et al. The Jmjd3-Irf4 axis regulates M2 macrophage polarization and host responses against helminth infection. Nat Immunol. 2010;11:936–944. doi: 10.1038/ni.1920. [DOI] [PubMed] [Google Scholar]
- 60.Capece D, Fischietti M, Verzella D, et al. The inflammatory microenvironment in hepatocellular carcinoma: a pivotal role for tumor-associated macrophages. Biomed Res Int. 2013;2013:187204. doi: 10.1155/2013/187204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yuan F, Fu X, Shi H, et al. Induction of murine macrophage M2 polarization by cigarette smoke extract via the JAK2/STAT3 pathway. PLoS One. 2014;8(9):e107063. doi: 10.1371/journal.pone.0107063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.González I, Romero J, Rodríguez BL, et al. The immunobiology of the receptor of advanced glycation end-products: trends and challenges. Immunobiology. 2013;218:790–797. doi: 10.1016/j.imbio.2012.09.005. [DOI] [PubMed] [Google Scholar]
- 63.Rojas A, Pérez-Castro R, González I, et al. The emerging role of the receptor for advanced glycation end products on innate immunity. Int Rev Immunol. 2014;33:67–80. doi: 10.3109/08830185.2013.849702. [DOI] [PubMed] [Google Scholar]
- 64.Raucci A, Cugusi S, Antonelli A, et al. A soluble form of the receptor for advanced glycation endproducts (RAGE) is produced by proteolytic cleavage of the membrane-bound form by the sheddase a disintegrin and metalloprotease 10 (ADAM10) FASEB J. 2008;22:3716–3727. doi: 10.1096/fj.08-109033. [DOI] [PubMed] [Google Scholar]
- 65.Zhang L, Bukulin M, Kojro E, et al. Receptor for advanced glycation end products is subjected to protein ectodomain shedding by metalloproteinases. J Biol Chem. 2008;283:35507–35516. doi: 10.1074/jbc.M806948200. [DOI] [PubMed] [Google Scholar]
- 66.Santilli F, Vazzana N, Bucciarelli LG, et al. Soluble forms of RAGE in human diseases: clinical and therapeutical implications. Curr Med Chem. 2009;16:940–952. doi: 10.2174/092986709787581888. [DOI] [PubMed] [Google Scholar]
- 67.Kislinger T, Fu C, Huber B, et al. N(epsilon)-(carboxymethyl)lysine adducts of proteins are ligands for receptor for advanced glycation end products that activate cell signaling pathways and modulate gene expression. J Biol Chem. 1999;274:31740–31749. doi: 10.1074/jbc.274.44.31740. [DOI] [PubMed] [Google Scholar]
- 68.Cho SJ, Roman G, Yeboah F, et al. The road to advanced glycation end products: a mechanistic perspective. Curr Med Chem. 2007;14:1653–1671. doi: 10.2174/092986707780830989. [DOI] [PubMed] [Google Scholar]
- 69.van Heijst JW, Niessen HW, Hoekman K, et al. Advanced glycation end products in human cancer tissues: detection of N-epsilon-(carboxymethyl)lysine and argpyrimidine. Ann N Y Acad Sci. 2005;1043:725–733. doi: 10.1196/annals.1333.084. [DOI] [PubMed] [Google Scholar]
- 70.Marenholz I, Heizmann CW, Fritz G. S100 proteins in mouse and man: from evolution to function and pathology (including an update of the nomenclature) Biochem Biophys Res Commun. 2004;322:1111–1122. doi: 10.1016/j.bbrc.2004.07.096. [DOI] [PubMed] [Google Scholar]
- 71.Lotze MT, Tracey KJ. High mobility group box-1 protein (HMGB1): nuclear weapon in damage-associated molecular pattern the immune arsenal. Nat Rev Immunol. 2005;5:331–342. doi: 10.1038/nri1594. [DOI] [PubMed] [Google Scholar]
- 72.Salama I, Malone PS, Mihaimeed F, et al. A review of the S100 proteins in cancer. Eur J Surg Oncol. 2008;34:357–364. doi: 10.1016/j.ejso.2007.04.009. [DOI] [PubMed] [Google Scholar]
- 73.Campana L, Bosurgi L, Rovere-Querini P. HMGB1: a two-headed signal regulating tumor progression and immunity. Curr Opin Immunol. 2008;20:518–523. doi: 10.1016/j.coi.2008.04.012. [DOI] [PubMed] [Google Scholar]
- 74.van Beijnum JR, Buurman WA, Griffioen AW. Convergence and amplification of toll-like receptor (TLR) and receptor for advanced glycation end products (RAGE) signaling pathways via high mobility group B1 (HMGB1) Angiogenesis. 2008;11:91–99. doi: 10.1007/s10456-008-9093-5. [DOI] [PubMed] [Google Scholar]
- 75.Sakaguchi M, Murata H, Yamamoto K, et al. TIRAP, an adaptor protein for TLR2/4, transduces a signal from RAGE phosphorylated upon ligand binding. PLoS One. 2011;6:e23132. doi: 10.1371/journal.pone.0023132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rojas A, Figueroa H, Morales E. Fueling inflammation at tumor microenvironment: the role of multiligand/RAGE axis. Carcinogenesis. 2010;31:334–341. doi: 10.1093/carcin/bgp322. [DOI] [PubMed] [Google Scholar]
- 77.Vlassara H, Brownlee M, Manogue KR, et al. Cachectin/TNF and IL-1 induced by glucose-modified proteins: role in normal tissue remodeling. Science. 1988;240:1546–1548. doi: 10.1126/science.3259727. [DOI] [PubMed] [Google Scholar]
- 78.Rojas A, Caveda L, Romay C, et al. Effect of advanced glycosylation end products on the induction of nitric oxide synthase in murine macrophages. Biochem Biophys Res Commun. 1996;225:358–362. doi: 10.1006/bbrc.1996.1180. [DOI] [PubMed] [Google Scholar]
- 79.Wautier MP, Chappey O, Corda S, et al. Activation of NADPH oxidase by AGE links oxidant stress to altered gene expression via RAGE. Am J Physiol Endocrinol Metab. 2001;280:E685–E694. doi: 10.1152/ajpendo.2001.280.5.E685. [DOI] [PubMed] [Google Scholar]
- 80.Wu CH, Huang CM, Lin CH, et al. Advanced glycosylation end products induce NF-kappaB dependent iNOS expression in RAW 264.7 cells. Mol Cell Endocrinol. 2002;194:9–17. doi: 10.1016/s0303-7207(02)00212-5. [DOI] [PubMed] [Google Scholar]
- 81.Kokkola R, Andersson A, Mullins G, et al. RAGE is the major receptor for the proinflammatory activity of HMGB1 in rodent macrophages. Scand J Immunol. 2005;61:1–9. doi: 10.1111/j.0300-9475.2005.01534.x. [DOI] [PubMed] [Google Scholar]
- 82.Rashid G, Korzets Z, Bernheim J. Advanced glycation end products stimulate tumor necrosis factor-alpha and interleukin-1 beta secretion by peritoneal macrophages in patients on continuous ambulatory peritoneal dialysis. Isr Med Assoc J. 2006;8:36–39. [PubMed] [Google Scholar]
- 83.Hama S, Takeichi O, Saito I, et al. Involvement of inducible nitric oxide synthase and receptor for advanced glycation end products in periapical granulomas. J Endod. 2007;33:137–141. doi: 10.1016/j.joen.2006.11.011. [DOI] [PubMed] [Google Scholar]
- 84.Berbaum K, Shanmugam K, Stuchbury G, et al. Induction of novel cytokines and chemokines by advanced glycation end-products determined with a cytometric bead array. Cytokine. 2008;41:198–203. doi: 10.1016/j.cyto.2007.11.012. [DOI] [PubMed] [Google Scholar]
- 85.Wang Y, Wang H, Piper MG, et al. sRAGE induces human monocyte survival and differentiation. J Immunol. 2010;185:1822–1835. doi: 10.4049/jimmunol.0903398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Allavena P, Chieppa M, Bianchi G, et al. Engagement of the mannose receptor by tumoral mucins activates an immune suppressive phenotype in human tumor-associated macrophages. Clin Dev Immunol. 2010;54:71–79. doi: 10.1155/2010/547179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Soria G, Ben-Baruch A. The inflammatory chemokines CCL2 and CCL5 in breast cancer. Cancer Lett. 2008;267:271–285. doi: 10.1016/j.canlet.2008.03.018. [DOI] [PubMed] [Google Scholar]
- 88.Zhang J, Patel L, Pienta KJ. Targeting chemokine (C-C motif) ligand 2 (CCL2) as an example of translation of cancer molecular biology to the clinic. Prog Mol Biol Transl Sci. 2010;95:31–53. doi: 10.1016/B978-0-12-385071-3.00003-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hayakawa E, Yoshimoto T, Sekizawa N, et al. Overexpression of receptor for advanced glycation end-products induces monocyte chemoattractant protein-1 expression in rat vascular smooth muscle cell line. J Atheroscler Thromb. 2012;19:13–22. doi: 10.5551/jat.9472. [DOI] [PubMed] [Google Scholar]
- 90.Caillou B, Talbot M, Weyemi U, et al. Tumor-associated macrophages (TAMs) form an interconnected cellular supportive network in anaplastic thyroid carcinoma. PLoS One. 2011;6:e22567. doi: 10.1371/journal.pone.0022567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Corzo CA, Cotter MJ, Cheng P, et al. Mechanism regulating reactive oxygen species in tumor-induced myeloid-derived suppressor cells. J Immunol. 2009;182:5693–5701. doi: 10.4049/jimmunol.0900092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Yamaguchi H, Pixley F, Condeelis J. Invadopodia and podosomes in tumor invasion. Eur J Cell Biol. 2006;85:213–218. doi: 10.1016/j.ejcb.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 93.Domínguez PM, Ardavín C. Differentiation and function of mouse monocyte-derived dendritic cells in steady state and inflammation. Immunol Rev. 2010;234:90–104. doi: 10.1111/j.0105-2896.2009.00876.x. [DOI] [PubMed] [Google Scholar]
- 94.Ivashkiv LB. Inflammatory signaling in macrophages: transitions from acute to tolerant and alternative activation states. Eur J Immunol. 2011;41:2477–2481. doi: 10.1002/eji.201141783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Pena OM, Pistolic J, Raj D, et al. Endotoxin tolerance represents a distinctive state of alternative polarization (M2) in human mononuclear cells. J Immunol. 2011;186:7243–7254. doi: 10.4049/jimmunol.1001952. [DOI] [PubMed] [Google Scholar]
- 96.West MA, Heagy W. Endotoxin tolerance: a review. Crit Care Med. 2002;30:S64–S73. [PubMed] [Google Scholar]
- 97.Foster SL, Medzhitov R. Gene-specific control of the TLR-induced inflammatory response. Clin Immunol. 2009;130:7–15. doi: 10.1016/j.clim.2008.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Chen J, Ivashkiv LB. IFN-γ abrogates endotoxin tolerance by facilitating toll-like receptor-induced chromatin remodeling. Proc Natl Acad Sci U S A. 2010;107:19438–19443. doi: 10.1073/pnas.1007816107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kouzarides T. Chromatin modifications and their functions. Cell. 2007;128:693–705. doi: 10.1016/j.cell.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 100.Mohtat D, Susztak K. Fine tuning gene expression: the epigenome. Semin Nephrol. 2010;30:468–476. doi: 10.1016/j.semnephrol.2010.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ishii M, Wen H, Corsa CA, et al. Epigenetic regulation of the alternatively activated macrophage phenotype. Blood. 2009;114:3244–3254. doi: 10.1182/blood-2009-04-217620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Banerjee S, Halder K, Bose A, et al. TLR signaling-mediated differential histone modification at IL-10 and IL-12 promoter region leads to functional impairments in tumor-associated macrophages. Carcinogenesis. 2011;32:1789–1797. doi: 10.1093/carcin/bgr208. [DOI] [PubMed] [Google Scholar]
- 103.Perrone L, Devi TS, Hosoya K, et al. Thioredoxin interacting protein (TXNIP) induces inflammation through chromatin modification in retinal capillary endothelial cells under diabetic conditions. J Cell Physiol. 2009;221:262–272. doi: 10.1002/jcp.21852. [DOI] [PubMed] [Google Scholar]
- 104.Alam MM, O'Neill LA. MicroRNAs and the resolution phase of inflammation in macrophages. Eur J Immunol. 2011;41:2482–2485. doi: 10.1002/eji.201141740. [DOI] [PubMed] [Google Scholar]
- 105.He M, Xu Z, Ding T, et al. MicroRNA-155 regulates inflammatory cytokine production in tumor-associated macrophages via targeting C/EBPbeta. Cell Mol Immunol. 2009;6:343–352. doi: 10.1038/cmi.2009.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Martinez-Nunez RT, Louafi F, Sanchez-Elsner T. The interleukin 13 (IL-13) pathway in human macrophages is modulated by microRNA-155 via direct targeting of interleukin 13 receptor alpha1 (IL13Ralpha1) J Biol Chem. 2011;286:1786–1794. doi: 10.1074/jbc.M110.169367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.McCoy CE, Sheedy FJ, Qualls JE, et al. IL-10 inhibits miR-155 induction by toll-like receptors. J Biol Chem. 2010;285:20492–20498. doi: 10.1074/jbc.M110.102111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Krausgruber T, Blazek K, Smallie T, et al. RF5 promotes inflammatory macrophage polarization and TH1-TH17 responses. Nat Immunol. 2011;12:231–238. doi: 10.1038/ni.1990. [DOI] [PubMed] [Google Scholar]
- 109.Quinn SR, O'Neill LA. A trio of microRNAs that control toll-like receptor signalling. Int Immunol. 2011;23:421–425. doi: 10.1093/intimm/dxr034. [DOI] [PubMed] [Google Scholar]
- 110.Perry MM, Moschos SA, Williams AE, et al. Rapid changes in microRNA-146a expression negatively regulate the IL-1beta-induced inflammatory response in human lung alveolar epithelial cells. J Immunol. 2008;15(180):5689–5698. doi: 10.4049/jimmunol.180.8.5689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Bhaumik D, Scott GK, Schokrpur S, et al. MicroRNAs miR-146a/b negatively modulate the senescence-associated inflammatory mediators IL-6 and IL-8. Aging. 2009;1:402–411. doi: 10.18632/aging.100042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Nahid MA, Pauley KM, Satoh M, et al. miR-146a is critical for endotoxin-induced tolerance: Implication in innate immunity. J Biol Chem. 2009;284:34590–34599. doi: 10.1074/jbc.M109.056317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Sheedy FJ, Palsson-McDermott E, Hennessy EJ, et al. Negative regulation of TLR4 via targeting of the proinflammatory tumor suppressor PDCD4 by the microRNA miR-21. Nat Immunol. 2010;11:141–147. doi: 10.1038/ni.1828. [DOI] [PubMed] [Google Scholar]
- 114.Rojas A, Delgado-López F, Perez-Castro R, et al. HMGB1 enhances the protumoral activities of M2 macrophages by a RAGE-dependent mechanism. Tumour Biol. 2016;37(3):3321–3329. doi: 10.1007/s13277-015-3940-y. [DOI] [PubMed] [Google Scholar]
- 115.Huber R, Meier B, Otsuka A, et al. Tumour hypoxia promotes melanoma growth and metastasis via High Mobility Group Box-1 and M2-like macrophages. Sci Report. 2016;18(6):29914. doi: 10.1038/srep29914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Rojas A, Araya P, Romero J, et al (2016) HMGB1-mediated RAGE activation mechanism in M2 macrophages. In: Proceedings of the 107th Annual Meeting of the American Association for Cancer Research; 2016 Apr 16-20; New Orleans, LA. Philadelphia (PA): AACR; Cancer Res. 76 (14 Suppl):Abstract nr 725
- 117.De Palma M, Lewis CE. Macrophage regulation of tumor responses to anticancer therapies. Cancer Cell. 2013;23:277–286. doi: 10.1016/j.ccr.2013.02.013. [DOI] [PubMed] [Google Scholar]