Abstract
The huge inter-individual differences in how people age have prompted researchers to examine whether people’s own perception of how old they are—their subjective age—could be a better predictor of relevant outcomes than their actual chronological age. Indeed, how old people feel does predict mortality hazards, and health-related measures such as walking speed may account for this association. In the present study, we extended this line of work by investigating whether subjective age also predicts walking speed and running speed in daily life or whether the predictive effects of subjective age for behavior manifest only within a controlled performance situation. We used data from 80 older participants (age range 62–82 years; M = 69.50, SD = 4.47) from the Berlin Aging Study II (BASE-II). Subjective age was assessed by self-report. Walking speed in the laboratory was measured with the Timed Up and Go test, and walking speed and running speed in real life were measured with an accelerometer. Results showed that compared to participants who felt older, those who felt younger than they actually were indeed walked faster in the laboratory, but they did not walk or run faster in real life. These patterns of results held when age, gender, education, BMI, comorbidity, depression, physical activity, and cognition were covaried. We discuss the role of stereotype threat in accounting for these results.
Keywords: Walking speed, Subjective age, Age stereotypes, Health, Running speed
Introduction
Old age is characterized by a high degree of variability between people (Baltes et al. 2006; Nelson and Dannefer 1992). This has raised the question of whether people’s own perception of how old they are—their subjective age—could be a better predictor of relevant outcomes than their chronological age. Indeed, how old people feel does predict mortality hazards (Kotter-Gruehn et al. 2009; Uotinen et al. 2005), risk of hospitalization (Stephan et al. 2016), and other health outcomes such as presence of chronic health problems and functional health (Spuling et al. 2013). Walking speed may operate as a mechanism linking subjective age and health outcomes or disability. Drawing from the seminal disablement process model’ (Verbrugge and Jette 1994), for example, walking speed as a valid and reliable indicator of functional status and health (Middleton et al. 2015) can be understood as an ‘impairment,’ lying in the causal chain between chronic diseases and disability. Thus, understanding links between subjective age—a psychological construct—and physical functioning like walking speed would present a potentially promising point of departure for psychological interventions aimed at successful aging and longevity and in a second step also at preserving quality of life (Gouveia et al. 2017). Recently, Stephan et al. (2015) reported that younger subjective age was associated with faster walking speed and less pronounced decline in walking speed over time. Similarly, Ihira et al. (2015) showed that feeling physically younger was associated with faster walking speed. Walking speed and changes in walking speed over time have been documented as important markers of functional health and predictors of mortality hazards (Cesari et al. 2005; Studenski et al. 2011). However, in the existing studies, walking speed was assessed in a laboratory test, in which participants walked a predetermined distance while they were being observed by an experimenter, and their walking speed was measured. Presumably, features of people’s daily activities should be even more strongly linked to health because people should be exposed to them on a routine basis. Thus, it is an important open question whether associations between subjective age and walking speed generalize beyond the controlled conditions of the laboratory and can also be found when walking speed in daily life is considered.
In the current study, we first attempted to replicate the findings from Stephan et al. (2015) by making use of a standard laboratory task of walking speed and examining its association with subjective age. We also tested whether associations between subjective age and walking speed hold when we controlled for the same relevant covariates used by Stephan et al. (2015)—age, gender, education, body mass index (BMI), disease burden, depression, physical activity, and cognition, with the exceptions of smoking because data on this variable were only available for half of our sample and race because this variable was not available in our data. One can safely assume that the BASE-II study sample was less ethnically diverse than the samples included in the paper by Stephan et al. (2015). Second, we attempted to extend earlier work by examining whether subjective age also predicts walking speed in daily life. The paradigm used earlier not only assesses the phenomenon under controlled conditions, but also within a social context with an experimenter present and observing the participants’ walking. The experimenter’s presence may lead to participants’ making age comparisons and experiencing stereotype threat in a physical performance situation in an aging study, thereby possibly amplifying the effects of subjective age (Swift et al. 2012). We considered associations of subjective age with real-life walking speed as measured with an actibelt accelerometry device at the zero-order level and after controlling for relevant covariates. Because walking in the laboratory test is carried out with the intention to cover a distance quickly, we examined two additional physical performance measures assessed in daily life with the actibelt, running speed, and the average speed within sequences of at least 100 steps.
Methods
Participants and procedure
We used data from a subsample of older participants in the Berlin Aging Study II (BASE-II) for whom walking speed in the laboratory was measured with the Timed Up and Go (TUG) test and in real life with an accelerometer (n = 80, Mage = 69.50, 62–82 years; 43.80% women; see Table 1). BASE-II is a comprehensive multi-disciplinary study of more than 2,000 younger and older adults (Bertram et al. 2014; Gerstorf et al. 2016), of which only a subsample was asked to perform the walking speed measures.
Table 1.
Sample characteristics
| BASE-II subsample (n = 80) | ||
|---|---|---|
| M | SD | |
| Subjective age, relative score (− 0.07–0.37) | 0.11 | 0.08 |
| Subjective age, years raw score (40–75) | 61.63 | 7.00 |
| Laboratory walking speed, TUG, time in seconds (4.12–13.00) | 8.44 | 1.55 |
| Real-life walking speed, actibelt, m/s (0–1.78) | 1.17 | 0.32 |
| Real-life running speed, actibelt, m/s (0–2.54) | 1.30 | 0.55 |
| Speed within sequences of more than 100 Steps, actibelt, m/s (0–1.88 | 1.13 | 0.41 |
| Age, years (62–82) | 69.50 | 4.47 |
| Gender, % women | 37.70 | – |
| Education, years (8.50–18) | 14.29 | 3.02 |
| Depressive symptoms, CES-D (24.00–44.00) | 33.30 | 3.38 |
| Morbidity (0–3) | 0.53 | 0.91 |
| BMI, kg/m2 (17.69–36.26) | 26.57 | 3.75 |
| Average steps walked per day (.29–18127.10) | 7682.82 | 4340.74 |
| Cognitive composite z-score (-1.76–1.34) | 0.01 | 0.69 |
Cognitive composite z-score = Average of z-scored values from n-back accuracy, letter series accuracy, figure analogies sum correct, digit symbol accuracy, practical problems sum correct
TUG Timed Up and Go test, BMI body mass index
Participants completed psychosocial questionnaires at home, in-between two group-based cognitive testing sessions scheduled 1 week apart. The medical examination took place on two days at the campus of the Charité Universitätsmedizin Berlin and included a comprehensive medical history assessment performed by a physician and was supplemented by clinical examinations, additional blood laboratory assessments, and functional tests. At the medical examination, during which the TUG was performed, participants in the second half of BASE-II had the option to take home and wear the accelerometer over seven or more days whenever a device was available. Accelerometers were provided to as many participants as possible; however, there were not enough devices available to collect accelerometer data from everyone. Because accelerometers were provided to participants whenever they were available, participant selection for the accelerometer part of BASE-II happened randomly. Overall, accelerometry was only part of BASE-II for approximately the second half of all BASE-II participants as accelerometer devices were not available earlier. Participants did not receive additional compensation for wearing the accelerometer, but almost all who were asked agreed. Compared to the whole sample, participants in our subsample were older (t(1,904) = − 4.21, p < 0.001) and performed better on the Digit Symbol task (t(1,851) = 3.41, p = 0.001); they did not differ in terms of gender distribution, education, body mass index, comorbidity, and subjective age.
Measures
Laboratory walking speed
The Timed Up and Go test (TUG; Podsiadlo and Richardson 1991) was used to measure participants’ walking speed in the laboratory. Participants were asked to rise from a chair 46 cm high with arm rest, walk three meters to a line on the floor, turn around, walk back to the chair, and sit down. The time in seconds that participants needed to complete the task was recorded. Although the TUG is not strictly only about walking speed, but requires additional abilities such as leg strength, balance, and coordination, it is highly correlated with pure measures of gait speed (between r = 0.745 and r = 0.816; Freter and Fruchter 2000) and seems to be comparable to pure measures of gait speed in predicting health outcomes (Viccaro et al. 2011). In completing the TUG, participants were asked to walk at their usual pace. Walking aids were permitted, but none of the participants in our subsample used them.
Real-life walking speed
We assessed walking speed in real life with the ‘actibelt,’ a belt worn around the waist with an accelerometer in its buckle using a 100 Hz sampling frequency. Earlier reports (Schimpl et al. 2011) have documented its utility and accuracy for measuring walking speed in healthy individuals (Motl et al. 2012). The device is unobtrusive and located close to a person’s center of mass. BASE-II participants wore the actibelt between 3 and 15 days (M = 9.01, SD = 2.01). We used walking speed (‘velocity.walking’) in meters/second averaged over the step intervals that had been classified as ‘walking’ according to the actibelt’s algorithm over at least two and at most 12 days. The actibelt’s algorithm distinguishes between walking and running; thus, walking speed and running speed are also calculated separately. According to information from the actibelt manual, accuracy of the walking speed measurement is high with errors amounting to less than .2 meters/second in 78% of the cases. One study has shown that the actibelt may overestimate walking speed in people with certain chronic diseases such as multiple sclerosis (Motl et al. 2012), but the participants in the BASE-II sample did not suffer from such conditions.
Real-life physical performance measures
To account for the assumption that the TUG includes a performance component and that walking within the TUG is carried out with the intention to cover a distance quickly, we examined two additional physical performance measures assessed in daily life with the actibelt; both of which were calculated by the actibelt’s algorithm. First, we considered running speed (‘velocity.running’) in meters/second averaged over the step intervals that had been classified as ‘running’ according to the actibelt’s algorithm over at least two and at most 12 days. Three participants did not run while wearing the actibelt; this was indicated by a value of ‘0’ on the running speed variable. Second, we considered the speed within step sequences of at least 100 steps (‘velocity.ratio100Av’) averaged over the step intervals that had been classified as ‘walking’ or ‘running’ according to the actibelt’s algorithm over at least two and at most 12 days.
Subjective age
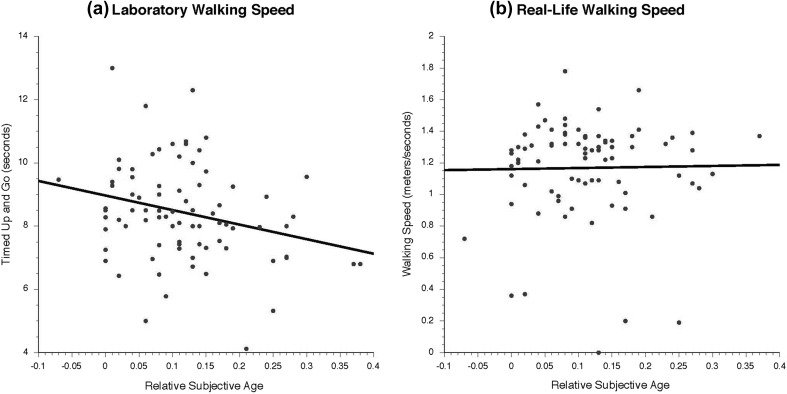
Participants indicated how old they felt in years. In line with previous research (Rubin and Berntsen 2006; Stephan et al. 2015), we calculated proportional discrepancy scores by subtracting participants’ subjective age from their chronological age and then dividing by chronological age. Positive scores indicate a younger subjective age. Proportional discrepancy scores three standard deviations above or below the mean were considered outliers (Stephan et al. 2015; Weiss and Lang 2012) and replaced with a score equivalent to the mean plus or minus three standard deviations, respectively; this was necessary for one participant only (Fig. 1).
Fig. 1.
Graphical illustration of associations between subjective age and walking speed for a sample of 80 BASE-II participants, using laboratory-based walking speed (left-hand panel A) and real-life walking speed (right-hand panel B). Participants who felt younger than they actually were indeed walked faster in the laboratory, but no associations were found in real life. The figure also highlights the tremendous amount of between-person heterogeneity in those associations. The black line represents the model-implied values
Covariates
Our models control for relevant individual difference characteristics. We based our selection of covariates on variables included in the paper by Stephan and colleagues (2015). First, we included socio-demographic measures: age, gender, and years of education. Second, depressive symptoms were assessed with the CES-D (Radloff 1977). Third, morbidity was assessed as part of the medical examination at the Charité University Hospital Berlin (Gerstorf et al. 2015). Diagnoses were obtained through participant reports, with select diagnoses (e.g., diabetes mellitus) being verified by additional (blood laboratory) tests. Diagnoses were used to compute a morbidity index largely based on the categories of the Charlson index, which is a weighted sum of moderate to severe, mostly chronic physical illnesses, including cardiovascular (e.g., congestive heart failure), cancer (e.g., lymphoma), and metabolic diseases (e.g., diabetes mellitus; Charlson et al. 1987; Meyer et al. 2016). Using the available data from the medical assessment, we matched the Charlson index as closely as possible. Fourth, cognition was measured with a unit-weighted composite of performance across five cognitive tests, an n-back task, a letter series accuracy task, a figure analogies task, a digit symbol task, and a practical problems task (Düzel et al. 2016; Mueller et al. 2016). In the n-back task, three one-digit numbers (ranging from 0 to 9) were presented sequentially in three cells situated horizontally, which were followed by the next sequence of three digits. This cycle was repeated 30 times. In each cycle, two-choice decisions on whether the current stimulus matches the stimulus shown three steps earlier in the sequence had to be made. Four practice trials including 30 runs were followed by six test trials with 30 runs. Participants made their decision via button-box presses with their left and right index fingers. The letter series accuracy task consisted of 22 items. Each item contained five letters followed by a question mark (e.g., c e g i k?). Items were displayed in the upper half of the screen, and five response alternatives were presented in the lower half. Items followed simple rules such as + 1, − 1, + 2, or + 2 + 1. Participants entered their response by touching one of the five response alternatives. The score was based on the total number of correct responses. Before the test phase, instructions and three practice items were given. The test phase was terminated when participants made three consecutive false responses, when they reached the maximum time limit (6 min), or after they had answered the last item of the test. Items were ordered by difficulty. In the figure analogies task, items followed the format ‘A is to B as C is to?’. One figure pair was presented in the upper left part of the screen and an incomplete figure was shown beside. Participants had to apply the same rule that was applied to the complete figure pair by choosing one of the five alternative responses, which were presented in the lower part. Participants entered their response by clicking with the mouse arrow one of the five alternatives. Before the test phase, instructions and three practice items were given. The test phase was terminated when participants made three consecutive false responses, when they reached the maximum time limit (10 min), or after they had answered the last item of the test. Items were ordered by difficulty. The digit symbol task consists of a code box with nine digit–symbol pairs, where each digit is paired with a corresponding symbol, and rows of double boxes, each with a digit in the top box and an empty lower box. Participants are asked to fill in as many corresponding symbols as possible in 90 s. The score indicates the number of correctly filled boxes, with penalty for wrong answers (score = correct–wrong). The practical problems task consists of 12 items depicting everyday problems such as the hours of a bus schedule, instructions for medication, a warranty for a technical appliance, a train map, as well as other forms and tables. For each item, the problems were presented in the upper part of the screen, and five alternative responses were shown in the lower part. Participants responded by clicking with the mouse arrow on one of the five alternatives. A single practice item was provided. The test phase was terminated when participants made three consecutive errors, when they reached the maximum time limit of 10 min, or after they had answered the last item of the test. Items were ordered by difficulty. Scores were converted to z-scores and averaged to yield a cognitive composite score; a higher score indicates better performance. Finally, we included the average number of steps walked each day as a measure of physical activity. Walking falls within the widely used definition of physical activity as ‘any bodily movement produced by skeletal muscles that results in energy expenditure’ by Caspersen et al. (1985). Similarly important, physical activity recommendations of the World Health Organization can be met by walking and the number of steps walked (Marshall et al. 2009), and steps walked have been shown to be a valid and cost-effective measure that is highly correlated with total physical activity (Sylvia et al. 2014).
Physical activity assessments
Participants were given a short one-on-one tutorial on how to use the accelerometer along with supplementary information to take home. During the instruction phase, the accelerometer was switched on by a trained experimenter, and participants were advised not to switch it off until the last day of their measurement, usually seven days later. Participants were asked to remove the accelerometer only while showering, taking a bath, or swimming.
Statistical analyses
To test our hypothesis that subjective age predicts walking speed in the laboratory and in real life, we used stepwise regression modeling. Predictors were mean-centered and entered into the model in the following order: First, we included subjective age, our main predictor of interest. Following Stephan and colleagues (2015), we ran a second model, adding demographic characteristics, physical health indicators, depressive symptoms, physical activity level, and cognitive performance as covariates.
Results
Laboratory walking speed was unrelated to real-life walking speed (r = 0.13, p = 0.27) and physical performance measures, namely walking speed within sequences of more than 100 steps (r = 0.01, p = 0.95), and running speed (r = − 0.17, p = 0.13). Correlations between all measures are reported in Table 2. Results of regression analyses examining associations of subjective age with different types of walking speed and physical performance are reported in Table 3, both with and without adjusting for key covariates.
Table 2.
Correlations between measures
| (2) | (3) | (4) | (5) | (6) | (7) | (8) | (9) | (10) | (11) | (12) | (13) | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (1) Laboratory-based walking speed | 0.13 | − 0.17 | 0.01 | − 0.25* | 0.21 | − 0.05 | − 0.30* | 0.20 | − 0.10 | 0.12 | − 0.27* | − 0.10 |
| (2) Real-life walking speed | – | 0.66* | 0.81* | 0.01 | 0.07 | 0.06 | − 0.20 | 0.02 | 0.02 | − 0.05 | − 0.03 | 0.56* |
| (3) Real-life running speed | – | – | 0.82* | − 0.04 | − 0.19 | 0.15 | − 0.04 | − 0.26* | 0.26* | − 0.21 | 0.15 | 0.71* |
| (4) Real-life walking speed in sequences of 100 + steps | – | – | – | − 0.05 | 0.004 | 0.11 | − 0.15 | − 0.02 | 0.17 | − 0.06 | 0.11 | 0.69* |
| (5) Subjective age, relative score | – | – | – | – | 0.02 | − 0.10 | 0.08 | − 0.02 | − 0.04 | 0.03 | − 0.17 | − 0.06 |
| (6) Age | – | – | – | – | – | − 0.29* | 0.06 | 0.07 | − 0.35* | 0.26* | − 0.12 | − 0.12 |
| (7) Gender | – | – | – | – | – | – | − 0.14 | − 0.21 | 0.21 | − 0.14 | − 0.05 | 0.24* |
| (8) Education | – | – | – | – | – | – | – | − 0.16 | 0.04 | 0.07 | 0.29* | − 0.01 |
| (9) BMI | – | – | – | – | – | – | – | – | − 0.12 | 0.17 | − 0.12 | − 0.25 |
| (10) Depressive symptoms | – | – | – | – | – | – | – | – | – | − 0.24* | 0.08 | − 0.22 |
| (11) Morbidity | – | – | – | – | – | – | – | – | – | – | 0.02 | − 0.07 |
| (12) Cognition | – | – | – | – | – | – | – | – | – | – | – | 0.21 |
| (13) Steps walked per day | – | – | – | – | – | – | – | – | – | – | – | 1 |
*Indicates p ≤ 0.05
Table 3.
Regression results examining the association between subjective age and different types of walking speed and physical performance (laboratory walking speed, real-life walking speed, real-life running speed, real-life walking speed in sequences of 100 + steps) with and without covariates
| Predictors | Laboratory-based walking speed | Real-life walking speed | Real-life running speed | Real-life walking speed in sequences of 100 + steps | ||||
|---|---|---|---|---|---|---|---|---|
| Model 1 | Model 2 | Model 1 | Model 2 | Model 1 | Model 2 | Model 1 | Model 2 | |
| Subjective age | − 0.25* | − 0.28* | 0.01 | 0.06 | − 0.04 | − 0.02 | − 0.05 | 0.02 |
| Age | – | 0.14 | – | 0.13 | – | − 0.03 | – | 0.13 |
| Gender | – | − 0.05 | – | − 0.08 | – | − 0.06 | – | − 0.04 |
| Education | – | − 0.12 | – | − 0.17 | – | − 0.02 | – | − 0.12 |
| BMI | – | 0.12 | – | 0.12 | – | − 0.04 | – | 0.16 |
| Morbidity | – | 0.06 | – | − 0.05 | – | − 0.08 | – | − 0.03 |
| Depressive symptoms | – | − 0.001 | – | − 0.04 | – | 0.1 | – | 0.09 |
| Steps walked per day | – | − 0.03 | – | 0.65* | – | 0.68* | – | 0.73* |
| Cognition | – | − 0.21* | – | − 0.06 | – | − 0.02 | – | 0.01 |
| Total R2 | 0.062 | 0.229 | <0.001 | 0.416 | 0.001 | 0.522 | 0.002 | 0.539 |
| F | 5.16 | 2.11 | 0.01 | 5.06 | 0.11 | 7.78 | 0.18 | 8.30 |
| (df1, df2) | (1, 78) | (9, 64) | (1, 78) | (9, 64) | (1, 78) | (9, 64) | (1, 78) | (9, 64) |
*Indicates p ≤ 0.05. Coefficients reported are standardized regression coefficients
Subjective age and laboratory walking speed
We first considered the predictive effects of subjective age for walking speed in the laboratory. Indeed, a younger subjective age was related to a faster walking speed on the TUG (b = –0.25, t(78) = − 2.27, p = 0.026). To describe more clearly what this means, we re-calculated the analysis using the difference between actual and subjective age in years: Feeling 8 years younger was associated with an average reduction of approximately half a second in TUG performance (effect size d = − 0.46). When we entered all covariates into the regression model, the association between subjective age and walking speed remained significant (b = − 0.28, t(64) = − 2.45, p = 0.017). In this second model that included subjective age and the covariates, none of the covariates were associated with laboratory walking speed. Given the variability in the number of days that participants wore the actibelt, we conducted follow-up analyses, in which we controlled for the number of days on which participants wore the device; the pattern of results remained the same.
Subjective age and real-life walking speed
The middle panel of Table 3 reports associations of subjective age with walking speed measured in real life. Subjective age did not predict walking speed in real life (e.g., Model 2: b = 0.06, t(64) = 0.63, p > 0.10). In a conjoint model, a greater number of steps walked per day (b = 0.65, t(64) = 5.77, p < 0.001) was associated with faster walking speed (i.e., more meters per second). In zero-order models, number of steps walked was also associated with walking speed (b = 0.56, t(78) = 6.16, p < 0.001). Again, a follow-up analysis in which we controlled for the number of days that participants wore the actibelt yielded the same pattern of results.
Subjective age and real-life performance measures
The right-hand panel of Table 3 reports associations of subjective age with real-life performance measures. Again, results revealed that subjective age did not predict running speed (e.g., Model 2: b = − 0.02, t(64) = − 0.02, p > 0.10), but steps walked per day did (b = 0.68, t(64) = 7.17, p < 0.001). Similarly, subjective age did not predict speed within sequences of more than 100 steps (e.g., Model 2: b = 0.02, t(64) = − 0.23, p > 0.10). In Model 2 that included subjective age and all covariates, number of steps walked was associated with speed within sequences of more than 100 steps (b = 0.73, t(64) = 7.83, p < 0.001). Controlling for the number of days on which participants wore the actibelt in a follow-up analysis yielded the same pattern of results.
Discussion
The objective of our study was to examine whether and how subjective age is associated with two types of walking speed, as measured in the laboratory and in real life and with other real-life physical performance measures, while accounting for socio-demographic characteristics, depressed affect, morbidity, physical activity level, and cognitive performance. Our study was particularly well suited to examining these questions because walking speed was assessed in two different contexts. In line with Stephan et al. (2015), we found that younger subjective age was related to faster walking speed measured in the laboratory. This effect appears to be quite robust because it holds when socio-demographic factors, health, depressive symptoms, physical activity level, and cognitive performance were taken into consideration, even in our relatively small sample.
Surprisingly, subjective age was not associated with real-life walking speed. A possible explanation could be that associations between subjective age and walking speed are only activated in a performance situation. Having to complete a timed walking test in front of an experimenter could lead participants to experience age-related stereotype threat (Swift et al. 2012) and thus result in slower walking speed the older participants feel. However, in daily life, thoughts related to one’s age may not be constantly activated and thus not influence real-life walking speed. This interpretation remains to be tested in future research, for example, by having participants complete a laboratory-based walking test without direct observation by an experimenter, by reducing the impact of stereotype threat in such a performance situation, or by priming participants with the concept of age as they go about their daily lives. This study suggests that the link between subjective age and walking speed or physical performance emerges mostly when greater effort is required, and is amplified when effort and difficulty increase or when there is a performance goal. The TUG is a complex task, which may lead to a stronger activation of subjective age compared to routine tasks in daily life.
It is also possible that laboratory speed and real-life walking speed are qualitatively different, a notion supported by these measures’ not being correlated. In this study, we were limited to using the aggregated accelerometer measures provided by the company that manufactures the actibelt; raw data were not available. By also examining the association between subjective age and accelerometer measures that may also represent performance—namely running speed and speed within longer step sequences—we attempted to minimize the qualitative differences between TUG and accelerometer measure. However, some qualitative differences may have persisted. Not only is walking speed in real life measured over a longer time period than in the TUG, and previous studies have demonstrated that walking speed is slower when measured over longer compared to shorter durations or distances (Simonsick et al. 2001). Associations between walking speed measured over longer compared to shorter durations with outcome measures such as cognitive performance are also weaker (Pasma et al. 2014). Furthermore, whereas the accelerometer measure in real life may provide a relatively pure measure of walking speed, the TUG conducted in the laboratory also involves coordination and balance skills (getting up from and sitting down on a chair, walking around an obstacle). It remains to be tested whether these skills are differentially associated with subjective age. A measure that presumably allows immediate comparability between laboratory and real life would be walking speed in a situation, in which people are striving to ‘perform well’ and in which this ‘performance’ includes both speed and coordinative skills. For example, hurrying to catch a bus on the other side of the road could represent such a situation; it requires speed as well as several coordinative skills.
Taken as a whole, the current study suggests that the link between subjective age and walking speed or mobility performance emerges mostly when greater effort is required, and is amplified when effort and difficulty increase or when there is a performance goal. The TUG is a complex task that may lead to a stronger activation of subjective age compared to activities in daily life. This interpretation remains to be tested in future research because it could shed light on the circumstances under which subjective age affects outcomes relevant to successful aging.
We acknowledge several limitations of our study. First, we only had cross-sectional information and cannot draw inferences regarding temporal ordering or directionality of associations (Lindenberger et al. 2011). Longitudinal data would allow examination of multidirectional dynamics between walking speed, subjective age, and the covariates. Second, inherent in applying convenience sampling are selection effects at both population and sample levels. The question remains, for example, whether our findings generalize to population segments with more severe health limitations. Third, our sample was relatively small, potentially resulting in limited statistical power. Fourth, we relied on number of steps as a measure of overall physical activity. Although the two are highly correlated, not all activities are encompassed by number of steps; activity intensity is also not reflected (Sylvia et al. 2014). Furthermore, the meaning of number of steps may differ by a person’s functional status; for example, a frail person may take smaller steps, thus, resulting in a higher number of steps that do not necessarily correspond to covering a longer distance or a higher activity intensity.
Our study confirms prior findings that suggest that how people perceive their own age—as measured by their subjective age—has consequences for their performance in controlled situations. We did not find an association between subjective age and characteristics of behavior (walking speed) across situations in real life. In order to better understand how perceptions about own age affect important real-life outcomes, it will be crucial to identify situations that trigger them.
Conclusion
The main objective of our study was to examine associations of subjective age with laboratory and real-life walking speed and other real-life physical performance indicators, over and above relevant covariates including demographic characteristics, physical health, depressive symptoms, physical activity level, and cognitive functioning. Walking speed in the laboratory was predicted by subjective age, whereas walking speed and running speed in real life were not. Our findings provide additional insight into associations of subjective age and walking speed. Mechanism-oriented designs are needed in future studies to better understand and empirically test possible underlying pathways.
Acknowledgements
This manuscript reports data from the Berlin Aging Study II (BASE-II). The BASE-II research project (Co-PIs are Lars Bertram, Denis Gerstorf, Ulman Lindenberger, Graham Pawelec, Elisabeth Steinhagen-Thiessen, and Gert G. Wagner) is supported by the German Federal Ministry of Education and Research (Bundesministerium für Bildung und Forschung, BMBF) under grant numbers #16SV5536 K, #16SV5537, #16SV5538, and #16SV5837, and #01UW0808). Another source of funding is the Max Planck Institute for Human Development, Berlin, Germany. Additional contributions (e.g., equipment, logistics, personnel) are made from each of the other participating sites. Further details about the study can be obtained at https://www.base2.mpg.de/en. We thank Martin Daumer from the Sylvia Lawry Centre for Multiple Sclerosis Research (SLCMSR), e.V., Munich, Germany for his input regarding the use of the actibelt accelerometer. The SLCMSR co-developed the device. N. N. was supported by a Horizon 2020 Marie Sklodowska-Curie Individual Fellowship (grant number H2020-MSCA-IF-2014 661555).
References
- Baltes, PB, Lindenberger, U, Staudinger, UM (2006). Lifespan theory in developmental psychology. In R. M. Lerner (Ed.), Handbook of child psychology Vol. 1: Theoretical models of human development (6th ed., pp. 569–664). New York, NY: Wiley. doi: 10.1002/9780470147658.chpsy0111
- Bertram L, Böckenhoff A, Demuth I, Düzel S, Eckardt R, Li S-C, Steinhagen-Thiessen E. Cohort profile: the Berlin aging study II (BASE-II) Int J Epidemiol. 2014;43:703–712. doi: 10.1093/ije/dyt018. [DOI] [PubMed] [Google Scholar]
- Caspersen CJ, Powell KE, Christenson GM. Physical activity, exercise, and physical fitness: definitions and distinctions for health-related research. Public Health Rep. 1985;100:126–131. [PMC free article] [PubMed] [Google Scholar]
- Cesari M, Kritchevsky SB, Penninx BW, Nicklas BJ, Simonsick EM, Newman AB, Visser M. Prognostic value of usual gait speed in well-functioning older people—results from the health, aging and body composition study. J Am Geriatr Soc. 2005;53:1675–1680. doi: 10.1111/j.1532-5415.2005.53501.x. [DOI] [PubMed] [Google Scholar]
- Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- Düzel S, Völkle M, Düzel E, Gerstorf D, Drewelies J, Steinhagen-Thiessen E, Demuth I, Lindenberger U. The subjective health horizon questionnaire (SHH-Q): assessing future time perspectives for facets of an active lifestyle. Gerontology. 2016;62:345–353. doi: 10.1159/000441493. [DOI] [PubMed] [Google Scholar]
- Freter SH, Fruchter N. Relationship between timed, up and go‘and’ gait time in an elderly orthopaedic population. Clin Rehabil. 2000;14:96–101. doi: 10.1191/026921500675545616. [DOI] [PubMed] [Google Scholar]
- Gerstorf D, Hülür G, Drewelies J, Eibich P, Düzel S, Demuth I, Ghisletta P, Steinhagen-Thiessen E, Wagner GG, Lindenberger U. Secular changes in late-life cognition and well-being: towards a long bright future with a short brisk ending? Psychol Aging. 2015;30:301–310. doi: 10.1037/pag0000016. [DOI] [PubMed] [Google Scholar]
- Gerstorf D, Bertram L, Lindenberger U, Pawelec G, Demuth I, Steinhagen-Thiessen E, Wagner GG. The Berlin aging study II–an overview. Gerontology. 2016;62:311–315. doi: 10.1159/000441495. [DOI] [PubMed] [Google Scholar]
- Gouveia ÉR, Gouveia BR, Ihle A, Kliegel M, Maia JA, Badia SB, Freitas DL. Correlates of lifestyle and functional fitness status influencing health-related quality of life in community-dwelling older people. Qual Life Res. 2017;26:1561–1569. doi: 10.1007/s11136-017-1502-z. [DOI] [PubMed] [Google Scholar]
- Ihira H, Furuna T, Mizumoto A, Makino K, Saitoh S, Ohnishi H, Makizako H. Subjective physical and cognitive age among community-dwelling older people aged 75 years and older: differences with chronological age and its associated factors. Aging Ment Health. 2015;19:756–761. doi: 10.1080/13607863.2014.967169. [DOI] [PubMed] [Google Scholar]
- Kotter-Grühn D, Kleinspehn-Ammerlahn A, Gerstorf D, Smith J. Self-perceptions of aging predict mortality and change with approaching death: 16-year longitudinal results from the Berlin Aging Study. Psychol Aging. 2009;24:654. doi: 10.1037/a0016510. [DOI] [PubMed] [Google Scholar]
- Lindenberger U, Von Oertzen T, Ghisletta P, Hertzog C. Cross-sectional age variance extraction: what’s change got to do with it? Psychol Aging. 2011;26:34–47. doi: 10.1037/a0020525. [DOI] [PubMed] [Google Scholar]
- Marshall SJ, Levy SS, Tudor-Locke CE, Kolkhorst FW, Wooten KM, Ji M, Macera CA, Ainswort BE. Translating physical activity recommendations into a pedometer-based step goal: 3000 steps in 30 minutes. Am J Prev Med. 2009;36:410–415. doi: 10.1016/j.amepre.2009.01.021. [DOI] [PubMed] [Google Scholar]
- Meyer A, Salewsky B, Spira D, Steinhagen-Thiessen E, Norman K, Demuth I. Leukocyte telomere length is related to appendicular lean mass: cross-sectional data from the Berlin aging study II (BASE-II) Am J Clin Nutr. 2016;103:178–183. doi: 10.3945/ajcn.115.116806. [DOI] [PubMed] [Google Scholar]
- Middleton A, Fritz SL, Lusardi M. Walking speed: T the functional vital sign. J Aging Phys Act. 2015;23:314–322. doi: 10.1123/japa.2013-0236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motl RW, Weikert M, Suh Y, Sosnoff JJ, Pula J, Soaz C, Daumer M. Accuracy of the actibelt ® accelerometer for measuring walking speed in a controlled environment among persons with multiple sclerosis. Gait Posture. 2012;35:192–196. doi: 10.1016/j.gaitpost.2011.09.005. [DOI] [PubMed] [Google Scholar]
- Mueller S, Wagner J, Drewelies J, Duezel S, Eibich P, Specht J, Demuth I, Steinhagen-Thiessen E, Wagner GG, Gerstorf D. Personality development in old age relates to physical health and cognitive performance: evidence from the Berlin aging study II. J Res Pers. 2016;65:94–108. doi: 10.1016/j.jrp.2016.08.007. [DOI] [Google Scholar]
- Nelson EA, Dannefer D. Aged heterogeneity: Fact or fiction? the fate of diversity in gerontological research. Gerontologist. 1992;32:17–23. doi: 10.1093/geront/32.1.17. [DOI] [PubMed] [Google Scholar]
- Pasma JH, Stijntjes M, Ou SS, Blauw GJ, Meskers CG, Maier AB. Walking speed in elderly outpatients depends on the assessment method. Age. 2014;36:1–8. doi: 10.1007/s11357-014-9736-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podsiadlo D, Richardson S. The Timed “Up & Go”: A test of basic functional mobility for frail elderly persons. J Am Geriatr Soc. 1991;39:142–148. doi: 10.1111/j.1532-5415.1991.tb01616.x. [DOI] [PubMed] [Google Scholar]
- Radloff LW. The CES-D scale: a self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1:385–401. doi: 10.1177/014662167700100306. [DOI] [Google Scholar]
- Rubin DC, Berntsen D. People over forty feel 20% younger than their age: subjective age across the lifespan. Psychon Bull Rev. 2006;13:776–780. doi: 10.3758/BF03193996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schimpl M, Lederer C, Daumer M. Development and validation of a new method to measure walking speed in free-living environments using the Actibelt® Platform. PLoS ONE. 2011;6:e23080. doi: 10.1371/journal.pone.0023080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonsick EM, Montgomery PS, Newman AB, Bauer DC, Harris T. Measuring fitness in healthy older adults: the health ABC long distance corridor walk. J Am Geriatr Soc. 2001;49:1544–1548. doi: 10.1046/j.1532-5415.2001.4911247.x. [DOI] [PubMed] [Google Scholar]
- Spuling SM, Miche M, Wurm S, Wahl H-W. Exploring the causal interplay of subjective age and health dimensions in the second half of life A cross-lagged panel analysis. Z Gesundheitspsychol. 2013;21:5–15. doi: 10.1026/0943-8149/a000084. [DOI] [Google Scholar]
- Stephan Y, Sutin AR, Terracciano A. “Feeling younger, walking faster”: subjective age and walking speed in older adults. Age. 2015;37:1–12. doi: 10.1007/s11357-015-9830-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephan Y, Sutin AR, Terraciano A. Feeling older and risk of hospitalization: evidence from three longitudinal cohorts. Health Psychol. 2016;35:634–637. doi: 10.1037/hea0000335. [DOI] [PubMed] [Google Scholar]
- Studenski S, Perera S, Patel K, Rosano C, Faulkner K, Inzitari M, Nevitt M. Gait speed and survival in older adults. JAMA. 2011;305:50–58. doi: 10.1001/jama.2010.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swift HJ, Lamont RA, Abrams D. Are they half as strong as they used to be? An experiment testing whether age-related social comparisons impair older people’s hand grip strength and persistence. BMJ Open. 2012;2:e001064. doi: 10.1136/bmjopen-2012-001064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sylvia LG, Bernstein EE, Hubbard JL, Keating L, Anderson EJ. A practical guide to measuring physical activity. J Acad Nutr Diet. 2014;114:199–208. doi: 10.1016/j.jand.2013.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uotinen V, Rantanen T, Suutama T. Perceived age as a predictor of old age mortality: a 13-year prospective study. Age Ageing. 2005;34:368–372. doi: 10.1093/ageing/afi091. [DOI] [PubMed] [Google Scholar]
- Verbrugge LM, Jette AM. The disablement process. Soc Sci Med. 1994;38:1–14. doi: 10.1016/0277-9536(94)90294-1. [DOI] [PubMed] [Google Scholar]
- Viccaro LJ, Perera S, Studenski SA. Is timed up and go better than gait speed in predicting health, function, and falls in older adults? J Am Geriatr Soc. 2011;59:887–892. doi: 10.1111/j.1532-5415.2011.03336.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss D, Lang FR. “They” are old but “I” feel younger: age-group dissociation as a self-protective strategy in old age. Psychol Aging. 2012;27:153–163. doi: 10.1037/a0024887. [DOI] [PubMed] [Google Scholar]