Abstract
With the rapid increase in the population of the oldest old (those aged 80 and over), there is some concern how longer life might be associated with a loss of independence in daily living. Addressing the trajectory of loss of independence for the oldest old is challenging, not only because the oldest old are heterogeneous, but also because the health trajectories at the population level may noticeably differ from those at the individual level. We used the 1998, 2000, 2002, 2005 and 2008 waves of the Chinese Longitudinal Healthy Longevity Study to assess the age trajectories of the loss of independence for three cohorts born in 1909–1918, 1899–1908 and 1893–1898, respectively, both at the individual and the population levels. Independence was measured by combining the activities of daily living and the Chinese version of the mini-mental state examination. Controlling for various confounding factors, particularly the selectivity due to death and loss to follow-up, we found that, while more recent cohorts had higher initial levels of independence, this was followed by a faster decline compared to the earlier cohorts. Also, their level of independence fell below that of their earlier born counterparts at the same ages. Decomposition analysis further illustrated that the decline of independence at the population level is more gradual than that at the individual level. This finding can be largely attributed to selective mortality and loss to follow-up. For instance, the population prevalence of independence for the 1893–1898 female cohort declined slightly, from 19.7 to 11.1% in 1998–2008, while the proportion for females who survived from 1998 to 2008 dropped from 66.7 to 11.1%. For the population as a whole, a longer life expectancy does not necessarily result in a rapid decline of independence. For individuals, longer life expectancy accompanies deterioration of independence.
Keywords: Independence in daily living, Chinese oldest old, Age trajectory, Cohort differences, Selection due to attrition, Mortality selection, Decomposition analyses
Introduction
Largely propelled by a remarkable decline in mortality at older ages since the 1950s, and particularly since the 1970s (Vaupel et al. 2006), the oldest old (those aged 80 and over) have become the fastest growing segment of older adults. According to the United Nations (2017), the oldest old currently account for 20% of the total number of older adults (those aged 65 and over). By 2050, this proportion is expected to have risen to 27%.
With the rapid increase in long-lived people, great concern has emerged whether the period of their morbidity would expand (Gruenberg 1977) or compress (Fries 1980) as their lifespan increases. In addition to these two hypotheses, Manton (1982) proposed the model of dynamic equilibrium in which the prevalence of disability may increase, but the severity of disability would decrease. The evidence supporting these three possibilities is mixed, probably due to different research designs, such as longitudinal or cross-sectional data, countries, cohorts, ages under study, social groups and health measurements (Crimmins and Beltrán-Sánchez 2011; Guralnik 2004; Martin and Schoeni 2014; Robine and Michel 2004).
Much is known about the pattern of age-related change in morbidity for people under 80 years old (Crimmins 2004; Freedman et al. 2002, 2013; Manton et al. 2008; Martin et al. 2010; Schaie 2005). The pattern for the oldest old is not sufficiently clear, however. Many studies have proposed that the oldest old are very different from the young old in many ways (Baltes and Smith 2003; Christensen et al. 2009). Typically, the very oldest adults have a lower level of education, a higher level of comorbidity and use more medical and care services. These specific characteristics may play an important role in shaping the health trajectory of long-lived people. Until recently, only a small amount of large-scale and longitudinal data for long-lived people has been available worldwide to reliably estimate their health trajectory. For example, Christensen et al. (2013) compared two Danish cohorts born 10 years apart in 1905 and 1915. They found that in their 90’s, the 1915 cohort had better scores than the 1905 cohort on both the cognitive tests and the activities of daily living scores. These findings suggest more of the oldest old people are living longer and functioning better. Gerstorf et al. (2011) showed that the trend of improvement did not continue during the very last years of life, largely because of the effects of approaching death. Based on another large-scale survey, Zeng et al. (2017) found mixed effects of compression and expansion in trajectories of the physical and cognitive abilities of the Chinese oldest old.
Addressing the health trajectory for the period of very old age is complex. Zeng et al. (2017) showed it is valuable to compare the cohort differences in the health trajectory among the oldest old because more recent cohorts may have had better health benefits from disease prevention and treatment, less disability effects of some major chronic diseases and improved living standards than the earlier born cohorts have had on the one hand, and more recent cohorts include more members who have survived life-threatening conditions but were in relatively poor health than the earlier cohorts do on the other. Smith et al. (2008) suggested that mortality selection influenced the observed health characteristics more among centenarians because of their higher mortality than it did among octogenarians and nonagenarians. Put differently, the oldest old themselves are highly heterogeneous. Their subgroups differ in many aspects such as health benefits, the amount of cumulative age-related changes and mortality (Christensen et al. 2013; Manton 1990; Smith et al. 2008). Therefore, accounting for cohort differences as well as health-related heterogeneity is pivotal in studying the health trajectory among the oldest old.
In addition, in interpreting the health trajectory of old people, it is important to distinguish the differences between the population in general and the individuals themselves (Christensen et al. 2008). It is well recognized that heterogeneity within a group can produce dynamics at the population level that are different from the dynamics within homogenous subgroups or at the individual level. As the frail individuals with a higher death rate tend to die first and leave the robust individuals with a lower death rate in the population, selection occurs and leads to a compositional change in the population. Very often, therefore, conclusions about individual characteristics cannot be drawn directly from observations made at the aggregate population level (Rebke et al. 2010; Vaupel and Yashin 1985). As illustrated by Christensen et al. (2008), the Danish 1905 birth cohort in the aggregate had only a modest decline in the proportion of independence in those aged 92–100 during the period 1998–2005, despite a much faster decline in the proportion of independence at the individual level. This implied that exceptional longevity does not result in exceptional levels of disability for society in general, but long life could bring an increasing risk of loss of independence for an individual. However, the study was based only on a single cohort, leaving it unclear whether or not their findings could be applied to other cohorts of the very old. Even if they could, it still remains unclear whether or not there are cohort differences in age-related changes in independence estimated at the population level and the individual level. Then, it further should be asked whether, and if so how, such cohort differences can be attributed to the mortality selection that plays out differently in various cohorts.
In the current study, we address the age-related loss of independence in daily living among the oldest old both at the individual level and at the population level with an emphasis on cohort differences within the oldest old. To disentangle the effects of cohort from that of aging on the trajectory of loss of independence, we adopted a growth curve model with control for demographic characteristics, health condition and attrition status. To examine the trajectories of independence estimated both at the population level and at the individual level, we employed the method proposed by Christensen et al. (2008). We adopted the decomposition method developed by Rebke et al. (2010) to assess the effect of selection due to attrition on the difference between individuals and the population, in order to examine how the population changes could be separated into individual changes and compositional changes (selection effects).
Up until now, the majority of research on the oldest old has come from developed countries. Since most of the population of the world is aging, more studies are required under different social conditions in other countries, such as China. The population of China is experiencing rapid aging (e.g., Li et al. 2009; Poston and Duan 2000). The oldest old subpopulation is growing much faster in China than in many developed countries. Together with China’s huge population, this fast growth has made China the most populous nation with regard to the oldest old. According to United Nations (2017), about 18% of the world’s oldest old (23.25 million) in 2015 were from China and this number is likely to increase to 26% (110 million) by 2050. China’s oldest old have a higher mortality than their counterparts in most developed countries such as Japan and Sweden (Zeng and Vaupel 2003), yielding greater selection effects that may impact their late life in many ways, including their lifestyle and health outcomes. Fortunately, China has a national survey of the oldest old with a big sample size of more than 8000 respondents, the Chinese Longitudinal Healthy Longevity Survey (CLHLS). This rich data source allowed us to examine cohort differences in the age trajectories of independence among the Chinese oldest old and shed new light on this field of research.
Methods
Sample
The data in this study were collected from the CLHLS. Detailed information about the sample, design and assessment battery is published elsewhere (Zeng et al. 2002). In this study, five waves of data collection are included: a baseline in 1998 and second, third, fourth and fifth waves in 2000, 2002, 2005 and 2008–2009, respectively. The baseline survey was conducted on 631 randomly selected counties and cities from the 22 provinces in China. These provinces covered 85% of the total population in China. All centenarians in these counties and cities were interviewed on a voluntary basis. For each centenarian, one octogenarian (aged 80–89) and one nonagenarian (aged 90–99) who lived nearby, with a pre-designated age and sex, were randomly sampled from the data provided by the local government. “Nearby” is loosely defined. It could mean in the same village or street if such an individual was available or it could be in the same town, the same sampled county or city (Zeng et al. 2002). For example, for a centenarian aged 102, a nearby octogenarian aged 82 and a nearby nonagenarian aged 92 were interviewed. The sex of the octogenarians and nonagenarians to be interviewed was randomly determined with the goal of having approximately equal numbers of males and females at each age from 80 to 99. Absent such individuals, an alternative individual of the same sex and in the same 5-year age group (80–84, 85–89, 90–94, 95–99) could be selected. The total valid sample size of the baseline data is 8805 older persons aged from 80 to 105.
To ensure large enough sample sizes without a loss of meaningful statistical analysis, the respondents were grouped into three cohorts born in 1909–1918, 1899–1908 and 1893–1898, respectively, corresponding to the age groups 80–89, 90–99 and 100–105 at the baseline survey. Because of the over-sampling approach described above, the proportions of the two earlier cohorts represented were higher than what they would have been in the real population.
Like many studies on the oldest old with longitudinal data, the CLHLS encountered problems with response rates and missing data due to attrition from failure to follow-up and missing values in the variables of interest. We did not find the information about the response rates from the data we used, but Gu et al. (2009) reported that the unit non-response rate among the Chinese oldest old was only about 4% in the first three waves. In the present study, the respondents originally recruited into the study who failed to participate in one or more of the subsequent waves were retained until the last wave in which they appeared. The respondents with missing values in the variables of interest were removed from this study. In total, the final sample was thus reduced to 7955 with 3295 men and 4660 women, respectively. The sex and urban–rural compositions of the final sample were somewhat different from that of the non-respondents. For the 1909–1918 birth cohort, non-respondents were more likely to be male and urban residents, while for the 1899–1908 and 1893–1898 birth cohorts, the non-respondents had fewer men and urban residents than the respondents did (see “Appendix 1”).
Measures
Despite plenty of information on health outcomes available in the CLHLS data, we were mainly interested in the physical and cognitive functioning data that had been frequently used to portray the health status of the oldest old in earlier studies. Instead of analyzing physical and cognitive functioning separately, we combined them to obtain a composite measure we called “independence in daily living.” We chose to do so because if one of the oldest old is both physically and cognitively independent, he/she can take care of himself/herself and live alone on a daily basis (Christensen et al. 2008). This information may then become very helpful in the formation of relevant public policy.
Physical functioning
Self-reported limitations in activities of daily living (ADL) were used to measure physical functioning. The respondents were asked whether they received assistance or not in performing any of five ADLs: eating, dressing, bathing, going to bed and using the toilet (continence is not included in the analysis, for the previous study showed that the information on this item is not reliable; Jeune et al. 2006). The total scores ranged 5–10, with higher values indicating higher physical ability. The respondents were also defined as physically independent if they were able to perform all five items without any assistance, and physically dependent otherwise.
Cognitive functioning
The mini-mental state examination (MMSE) was used to assess the cognitive functioning of the Chinese oldest old under study. Translated into the Chinese language, the MMSE is culturally adapted based on established international standards for the MMSE questionnaire, and carefully tested in a pilot survey (Zeng et al. 2002). The MMSE included the following elements: brief measures of orientation, registration, attention, calculation, recall and language, with scores ranging from 0 to 23 with higher scores indicating higher cognitive ability.
The MMSE commonly uses a range of scores from 0 to 30. For the sake of brevity, the CLHLS used 23 items with one point each to measure these five domains of cognitive functioning, resulting in a score ranging from 0 to 23. Incomplete measures mainly occurred in the assessment of orientation, reading and writing abilities. The standard MMSE uses ten items to measure orientation, while the CLHLS uses five items. In addition, the CLHLS does not collect information on reading and writing abilities. The respondents were categorized as cognitively independent if they had a MMSE score of 17 or greater. We chose 17 as a split because the percentage of an MMSE score greater or equal to 17 in the Chinese version was similar to the percentage of an MMSE score greater or equal to 23 in the standard MMSE scores in other biomedical studies of the oldest old such as the Australian Longitudinal Study of Aging (please see “Appendix 2”). It turned out that the cutoff of 17 in the Chinese data also gave a similar distribution of MMSE scores to the cutoff of 23 in the Danish 1905 cohort study.
Independence in daily living
The composite measure of independence was obtained by combining the physical and cognitive functioning introduced above. To estimate the trajectories of independence across cohorts, a total score of independence was calculated by adding the T scores of MMSE and the T scores of ADLs. These scores ranged from 46 to 106, with the higher values indicating a higher level of independence.
To calculate population prevalence, the respondents were categorized as independent if they were both physically and cognitively independent as defined above. This category conforms to the definition of independence in daily living put forward by Christensen et al. (2008).
Covariates
The variable sex was coded as 0 for female and 1 for male. The place of residence was coded dichotomously as urban versus rural according to whether the respondents lived in the city or the countryside. Educational attainment was grouped into three levels based on the number of years of schooling: no schooling, 1–6 years and 7 and over years of schooling. These three covariates are time-constant variables. The current marital status was composed of two groups: married and unmarried, where the latter included widowed, separated, divorced and never married people. The number of self-reported diseases was coded as 0 for no disease, 1 for one disease and 2 for two and more diseases. Self-reported diseases included hypertension, diabetes, heart disease, stroke, cerebrovascular diseases, bronchitis, pulmonary emphysema, asthma, pneumonia, cataract, glaucoma, cancer, prostate tumor, gastric or duodenal ulcer, Parkinson’s disease, bedsores and others. Whether or not respondents received adequate medical service when sick included three categories: yes, no and never been sick. The attrition status was defined as alive, deceased and lost to follow-up at the next wave. These four covariates are time-varying variables.
Statistical analysis
The present study employed three steps of analyses to investigate the cohort differences in the trajectories of independence. First, we used growth curve models (or mixed effects models, multilevel models) to reveal cohort differences in the age pattern of loss of independence. This allowed the disentangling of the effects of aging and cohort, controlling for population heterogeneity and selection due to death and loss to follow-up, but it did not allow one to distinguish the differences in trajectories estimated at the population and the individual level. Second, we examined the cohort differences in independence both at the population level and at the individual level. In this step, we employed the same approach as in Christensen et al. (2008), calculating the proportion of independent participants in each wave for the overall population and for the individuals who completed at least 2, 3, 4 and 5 waves, respectively. A comparison was made between the independence trajectory estimated at the population level and that at the individual level. In a third step, we decomposed the differences in the trajectories of independence estimated at the individual level and at the population level with the equation developed by Rebke et al. (2010). Such decomposition could reveal how the different patterns of change in independence at the population level could be attributed to individual changes and compositional changes.
The growth curve models
In order to address cohort differences in the age pattern of loss of independence, we specified the growth curve model in a multilevel framework as follows:
Level-2 model:
For the intercept,
For the linear slope,
For the quadratic growth rate,
where Yti is the score of independence for the respondent i at time t, for i = 1, …, n and t = 1, …, Ti; Ti is the measurement time and ranges from 1 to 5; Ageti is the age of the respondent i at time t. Previous studies suggested that the trajectory of health status is a quadratic function of age (e.g., Yang and Land 2013; Marshall et al. 2015). In this model, we tested both linear and nonlinear specifications of age effects with five waves of longitudinal data. The current parameterization was chosen based on significant tests of coefficients and a comparative model fit. We centered the age variable on the mean age of the sample (i.e., 92.5 years), for which the intercept β0i is the expected independence score of person i at the mean age. β1i and β2i are the expected linear and quadratic rates of increase per year of age for person i, respectively. are time-varying variables, including current marital status, the number of diseases, adequate provision of medical services and attrition status at the next wave. Attrition status at the next wave was included to control for potential effects of the selection due to death and loss to follow-up. The random within-person error for person i at t, eti, is assumed normally distributed.
The level-2 model specifies a distinct trajectory for cohorts and other time-constant covariates associated with each individual, i.e., sex, educational attainment and place of residence. In this model, we included both the linear and the squared cohort terms. The results showed that they were significant for the intercept and the linear rates rather than for the quadratic rates (see below). Operationalization of cohorts using dummy variables shows similar significant tests and models. We experimented with including the interaction terms of cohort by sex, educational attainment and place of residence, and we found that only the interaction between cohort and sex was significant. ΣZji are the time-constant variables educational attainment and place of residence. As the residual random effects, μ0i and μ1i are assumed to have a multivariate normal distribution.
Decomposition analysis
The proportion of independent individuals in a cohort is a measure at the aggregate level. Its change may be due not only to the true change in individuals’ independence, but also due to compositional change. Rebke et al. (2010) show that population change P can be exactly decomposed into the average change for the surviving individual s, which is called the average individual change, plus compositional change due to selective disappearance d:
| 1 |
where is the difference between the averages of a characteristic of all individuals at one time point of an explanatory variable ( at age x) and the next instance Vx+k with k being the time interval under study, gives the respective difference for the survivors at one instance (vx) and the next instance (vx+k) and d = vx − Vx refers to the difference between the two means at the first time point. The change due to selective disappearance is zero if the population is homogenous or if there is no individual who drops out. This approach works for means in general. In the special case of a binary variable (e.g., independence), the mean number of successes, or in other words, the proportion of successes can be used for Vx, Vx+k, vx and vx+k.
Results
Table 1 shows the sample composition at the baseline survey and its subsequent attrition over time. The number of respondents for each cohort declined with age mainly due to high mortality, loss to follow-up and other missing data among the oldest old. For example, only 38 respondents in the 1899–1908 cohort, both sexes combined, were still observed at the fifth wave and none of the male respondents in the 1893–1898 cohort survived up to that wave. As time went by, the oldest old became older with a decrease in the independence (as shown in the percentage and scores below the number of respondents). For all three cohorts, the males were more likely to be independent than were their female counterparts at all waves. It is noteworthy that, as frail people were selected out, the sample size shrank quickly.
Table 1.
Number of participants and percentage of independent individuals for three cohorts at five assessments during 1998–2008
| Cohorts | Survey years | ||||
|---|---|---|---|---|---|
| 1998 | 2000 | 2002 | 2005 | 2008 | |
| 1909–1918 birth cohort (aged 80–89 at the first wave in 1998) | |||||
| Male (n) | 1667 | 1121 | 687 | 293 | 100 |
| % Independent | 80.32 | 72.08 | 64.77 | 58.70 | 55.00 |
| Independence scores: mean (SD) | 109.94 (9.04) | 107.49 (11.49) | 105.56 (12.85) | 104.69 (13.45) | 101.10 (17.11) |
| Female (n) | 1685 | 1163 | 736 | 369 | 132 |
| % Independent | 66.47 | 61.05 | 46.47 | 29.58 | 37.88 |
| Independence scores: mean (SD) | 106.75 (11.02) | 104.19 (12.73) | 100.62 (14.76) | 98.83 (15.40) | 97.05 (17.21) |
| 1899–1908 birth cohort (aged 90–99 at the first wave in 1998) | |||||
| Male (n) | 1234 | 570 | 268 | 71 | 17 |
| % Independent | 55.75 | 50.88 | 39.55 | 26.40 | 29.41 |
| Independence scores: mean (SD) | 103.21 (14.57) | 101.29 (15.98) | 97.59 (15.85) | 96.10 (19.03) | 93.72 (19.17) |
| Female (n) | 1554 | 730 | 335 | 95 | 21 |
| % Independent | 34.11 | 31.64 | 23.28 | 23.16 | 28.57 |
| Independence scores mean (SD) | 96.60 (15.83) | 95.07 (17.21) | 92.09 (17.26) | 93.35 (16.59) | 97.01 (13.79) |
| 1893–1898 birth cohort (aged 100–105 at the first wave in 1998) | |||||
| Male (n) | 394 | 115 | 33 | 3 | 0 |
| % Independent | 35.79 | 33.04 | 39.39 | 0.00 | – |
| Independence scores: mean (SD) | 96.34 (16.88) | 93.17 (19.00) | 97.66 (15.84) | 89.67 (21.21) | |
| Female (n) | 1421 | 493 | 201 | 32 | 10 |
| % Independent | 19.70 | 22.52 | 16.92 | 18.75 | 10.00 |
| Independence scores: mean (SD) | 87.81 (18.61) | 89.33 (18.48) | 87.83 (16.49) | 86.13 (19.09) | 87.11 (20.06) |
Table 2 presents a description of the sample based on the covariates for the three cohorts at the baseline survey. The majority of the Chinese oldest old had low levels of educational attainment, and the older, the lower: 56% of the 1909–1918 cohort had no schooling at all, compared to 84% of the 1893–1898 cohort who had none. A large proportion of the oldest old was unmarried (mostly widowed). Two thirds lived in a rural area. No obvious differences were seen in the number of diseases across the three cohorts, probably because the oldest old could not tell if they had certain diseases with the poor medical services available to them for diagnosis. For a similar reason, the different cohorts did not show much difference in their access to adequate medical services when they were sick. Corresponding to increasing mortality with age, more of the earlier cohorts died than the more recent ones did during the study period.
Table 2.
Descriptive statistics for the covariates at baseline survey across cohorts (%)
| Cohorts | ||||
|---|---|---|---|---|
| 1909–1918 birth cohort | 1899–1908 birth cohort | 1893–1898 birth cohort | Total | |
| Educational attainment | ||||
| No schooling | 55.85 | 67.22 | 83.75 | 66.15 |
| 1–6 years of schooling | 32.04 | 24.69 | 13.28 | 25.21 |
| 7+ years of schooling | 12.11 | 8.10 | 2.98 | 8.63 |
| Current marital status | ||||
| Married | 28.80 | 11.29 | 3.03 | 16.83 |
| Unmarried | 71.20 | 88.71 | 96.97 | 83.17 |
| Place of residence | ||||
| Urban | 43.95 | 36.80 | 27.05 | 37.61 |
| Rural | 56.05 | 63.20 | 72.95 | 62.39 |
| Number of diseases | ||||
| No disease | 46.52 | 48.69 | 47.55 | 47.51 |
| 1 disease | 34.44 | 31.46 | 33.22 | 33.12 |
| 2+ diseases | 19.05 | 19.85 | 19.23 | 19.37 |
| Whether or not get adequate medical services when sick | ||||
| Yes | 81.72 | 80.47 | 78.62 | 80.58 |
| No | 3.60 | 3.05 | 1.98 | 3.04 |
| Never been sick | 14.68 | 16.48 | 19.39 | 16.38 |
| Attrition status at the next wave | ||||
| Alive | 69.73 | 50.99 | 39.61 | 56.34 |
| Lost to follow-up | 10.90 | 8.17 | 5.56 | 8.73 |
| Deceased | 19.37 | 40.85 | 54.82 | 34.92 |
Cohort differences in the trajectories of independence over age
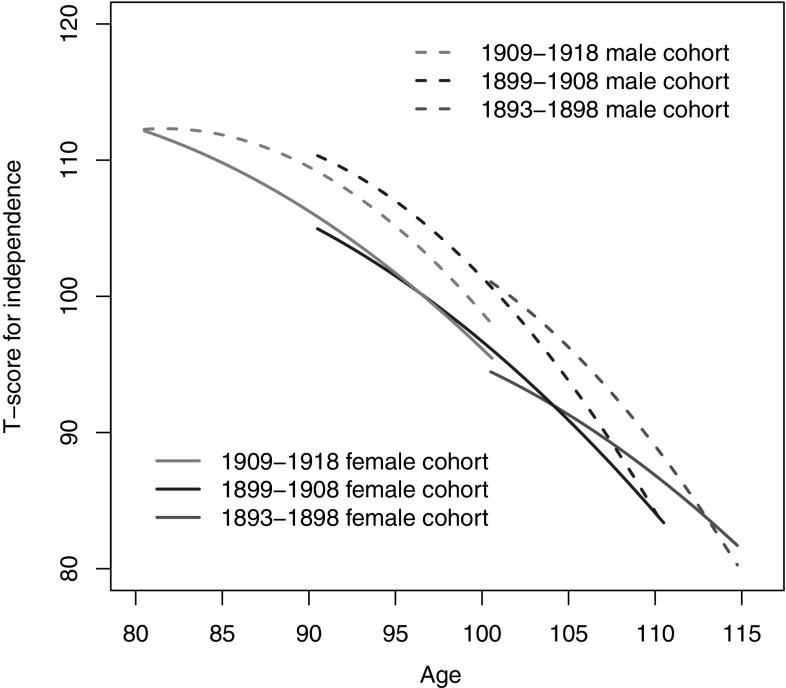
As the first step of analysis, a growth curve model was used to check whether there were cohort differences in the age trajectories of independence controlled for the related components of heterogeneity including sex, educational attainment, current marital status, place of residence, diseases, medical service and attrition status. Table 3 presents the estimates of the model from which we calculated the predicted levels of independence by cohorts for both men and women (see Fig. 1).
Table 3.
Coefficient estimates of the growth curve model of independence
| Model 1 | Model 2 | |||
|---|---|---|---|---|
| Fixed effects | ||||
| Intercept | 100.38*** | 97.91*** | ||
| Cohort | 2.16 | 7.59** | ||
| Cohort2 | − 0.83 | − 2.36* | ||
| Sex (male = 1) | 7.27*** | 7.59*** | ||
| Cohort*Sex | − 2.21** | − 2.05** | ||
| For linear growth rate | ||||
| Intercept | − 0.79** | − 0.31 | ||
| Cohort | − 0.26 | − 0.63* | ||
| Cohort2 | 0.03 | 0.16 | ||
| Sex (male = 1) | 0.00 | 0.02 | ||
| For quadratic growth curve rate | ||||
| Intercept | − 0.03*** | − 0.02* | ||
| Sex (male = 1) | − 0.02** | − 0.02** | ||
| Control variables | ||||
| 1–6 years of schooling (no school) | 2.36*** | 2.27*** | ||
| 7+ years of schooling (no school) | 3.32*** | 3.03*** | ||
| Unmarried (married) | − 1.28*** | − 0.82** | ||
| Rural (urban) | − 0.61* | − 0.56* | ||
| 1 disease (no disease) | − 2.69*** | − 2.50*** | ||
| 2+ diseases (no disease) | − 4.84*** | − 4.48*** | ||
| Not get adequate medical service when sick (yes) | − 4.28*** | − 3.84*** | ||
| Never been sick | 1.98*** | 1.68*** | ||
| Lost to follow-up (alive) | − 2.45*** | |||
| Died (alive) | − 5.60*** | |||
| Variances | SE | Variances | SE | |
|---|---|---|---|---|
| Random effects–variance components | ||||
| Level-1: Within-person | 123.28 | 1.96 | 119.67 | 1.91 |
| Level-2: In intercept | 67.34 | 2.71 | 63.50 | 2.59 |
| In growth rate | 0.39 | 0.03 | 0.36 | 0.03 |
| Covariance of intercept and slope | 5.11 | 0.22 | 4.79 | 0.21 |
| Goodness of fit | ||||
| BIC | 125,002 | 124,449 | ||
Reference group is in parentheses
BIC Bayesian information criterion
*p < .05; **p < .01; ***p < .001 (two-tailed test)
Fig. 1.
Predicted independence trajectories over age by cohort and sex
Attrition of respondents due to death and loss to follow-up could distort the estimation of the age trajectories of independence across cohorts because the oldest old who dropped out of observation could have been those who were more likely to have been in worse health. Hence, death and loss to follow-up were treated as control variables to account for such selections. Model 1 includes all covariates except the indicator for attrition status, while Model 2 includes them all. The column of Model 2 in Table 3 shows that the deceased and lost to follow-up at the next wave had significantly lower independence scores than those who had completed the next wave. Controlling for attrition status, the cohort effect on the intercept and its rate of change became significant in Model 2 while it did not in Model 1. The lower BIC of Model 2 compared to Model 1 also confirmed this.
It follows from Model 2 that the age trajectory of independence among the Chinese oldest old can be characterized by a linear decline with some deceleration. To be exact, the mean level of independence of a later born cohort is 7.59 units higher than the consecutive earlier born cohort. As for the linear growth rate, at the mean age, the linear decline for independence is 0.31 units per year of age. The negative coefficients of cohorts suggest that the later born cohorts experienced a faster decline in their level of independence, although they began with higher initial values. Quadratic growth rate is significant, implying that loss in independence decelerated with age at the rate of 0.02 in a quadratic pattern. In short, Model 2 shows pronounced cohort differences in both the mean level of independence and its decline with age, suggesting that the individuals from different cohorts had significantly different age trajectories of independence.
Other covariates are also related to independence in daily living. The estimates show that the oldest old in marriage with a higher level of education and living in an urban area tended to have a higher level of independence. The oldest old who had no diseases and had not been sick during the last year performed better.
Figure 1 depicts the trajectories of predicted independence by sex and age are based on the results of Model 2 with the values of all covariates being set to zero (i.e., the reference categories). The trends of loss of independence for the different cohorts reaffirm the cohort effects as discussed above. A more recent cohort has higher initial values but bends downward much more than an earlier cohort. This is also confirmed by the significant positive estimate of the covariance between the intercept and the linear slope of the random effects in Model 2, indicating that the higher the intercept, the faster the decline. With such a decline, the successive cohorts would have a lower level of independence than their earlier counterparts would at the same ages. The fact is more evident for males.
As shown in Fig. 1, there are remarkable sex differences in both the mean levels and the degree of change in independence. Males generally have a higher independence in daily living than females do, but lose their independence more quickly.1 Such a pattern leads to a convergence with age between the two sexes, which is more obvious for the two earlier cohorts. The 1893–1898 male cohort shows an odd pattern in convergence: It crosses over the curve of their female counterparts, probably since relatively few male participants lived to age 110 and over. Interestingly, due to their faster decline in independence, the age point where the more recent male cohorts drop below the independence score of their earlier male cohorts happens sooner than do their female counterparts.
Cohort differences in the trajectories of independence at both the individual level and the population level
Figure 2 provides us with a very intuitive impression of the pattern of loss of independence with age at the individual level (indicated by dashed lines) and also by cohort. The percentage of independence fell quickly with age, even for the earliest cohort. For example, the proportion of independence dropped from 66.7 to 11.1% for the 1893–1898 female cohort who survived from age 100+ to age 110+. The initial percentage of independence was higher for the more recent cohorts, but was followed by a faster decline, compared to the earlier cohorts. As a result, the more recent cohorts would be expected to have a lower level of independence by the time they reached the same ages as their earlier cohorts. This conclusion is apparent for men, but not for women. It should be noted that the pattern of trajectory for the 1893–1898 birth cohort and the 1899–1908 female cohort appears not very regular mainly because the sample sizes shrank over time.
Fig. 2.

The trajectories of independence by examining the change in the percentage of independent respondents over age. It shows these changes at both the individual level and at the population level. The dashed lines in blue, brown, green and red track the individuals who completed at least 2, 3, 4 and 5 waves, respectively, while the black solid line shows the change in the percentage of independent individuals in each wave. The rate of decline of independence at the individual level was much sharper than it was at the population level. (Color figure online)
At the population level (indicated by black solid lines), the decline in independence took place more slowly than it did for all three cohorts at the individual levels. The population trajectories of independence for the successive cohorts were roughly concatenated among each other, thereby forming a “synthetic” age trajectory of loss of independence. The shape of the independence trajectory shows a decelerating pattern of loss of independence with age. Particularly at the ages above 95, for both males and females, the decline tended to level off, although the data analysis was a little bumpy largely due to the small number of survivors at those ages. For example, for the 1909–1918 birth cohort, the proportion of independence declined rapidly from 80.3 to 56.8% from age 80+ to 90+ for males, and from 66.5 to 38.9% for females. And the proportion of those who were independent changed from 35.8 to 36.7% between the ages of 100+ to 104+ for the 1893–1898 male birth cohort2 and from 19.7 to 11.1% between the ages of 100+ to 110+ for the 1893–1898 female birth cohort.
Comparing the left panel with the right in Fig. 2, we can see an obvious sex difference in the change of the percentage of those who are independent. Initially a higher percentage of males were independent but that percentage dropped more sharply than that of the females. For the two earlier cohorts, the percentage of independent people dropped more sharply for males at the individual level than for females. In addition, the noticeable distances between the solid and dashed lines suggest that the discrepancy between the trajectories of independence for individuals versus that for the population is more pronounced among males than among females.
Decomposition of the population changes into individual changes and compositional changes
Figure 3 illustrates the results of the decomposition analysis. The dark blue bars indicate the change at the aggregate population level. The light blue bars and the orange bars refer to the changes in independence among the survivors (individuals) and the compositional change, respectively. The results confirm that for all three cohorts and for both sexes, the independent respondents experienced a stronger decrease in independence at the individual level than they did at the aggregate level over age, as indicated by the greater length of the light blue bars than the dark blue bars.
Fig. 3.

Change in the proportion of independent individuals for different cohorts at the population level (dark blue bars), the contributions to this change due to individual change (light blue bars) and compositional change (orange bars) in the period 1998–2008. (Color figure online)
As shown by the light blue bars in Fig. 3, all individuals, no matter what cohort or sex, experienced a loss of independence with age. For the males in the left column, the proportion of the 1909–1918 birth cohort who remained independent declined at a nearly constant rate in between five successive waves during the period 1998–2008. The 1899–1908 male birth cohort had an accelerated drop in the proportion of those who remained independent over the first four waves in 1998–2005, followed by a relatively small decline in the last wave in 2008. The proportion of the 1893–1898 male birth cohort who were independent fluctuated a bit in the first three waves, but between 2002 and 2005 there was a big drop because only a few of the males survived into the higher age range. Females had a pattern similar to the males with respect to the decline in the proportion of them who remained independent. One exception was for the females in the 1899–1908 birth cohort where the individual changes even turned positive in the latest wave due to the small number of survivors left in the cohort.
At the population level, the proportion of independent respondents decreased with age for the 1909–1918 and 1899–1908 birth cohorts, while that of the 1893–1898 cohort fluctuated to some extent. What is remarkable is the discrepancy between the change at the population level and at the individual level. Moreover, these selection effects show different temporal patterns among the different sex and birth cohorts. For both males and females in the 1909–1918 birth cohort, the selection effects became increasingly significant as time went by. However, for females in the 1899–1908 birth cohort, these effects dwindled. There were some fluctuations in the selection effects for the 1893–1898 birth cohort, however.
Because of the selection effects, the change in the proportion of the population that remained independent simply cannot be interpreted as it seems to appear. For instance, for the 1909–1918 birth cohort of both sexes, the dark blue bars become shorter and shorter from 1998 to 2008, meaning that the loss of independence slowed down with age. But in fact, the individuals experienced a loss of their independence at a nearly constant rate, as illustrated by the light blue bars that show no big changes. The compositional changes resulting from the selective dropout caused the discrepancies between the individual and population changes. While we welcome the fact that, in the population taken as a whole, the decline in the proportion of those who are independent appears to level off with age, we are concerned that looked at individually, people still experience a noticeable deterioration of their independence in daily living. The decomposition analysis reaffirms what we found in Fig. 2, but with more details about the mechanism underlying the dynamics of such changes.
Discussion
Our analyses demonstrated that there was a pronounced cohort difference in the loss of independence with age by comparing the three birth cohorts, i.e., the 1909–1918 cohort, the 1899–1908 cohort and the 1893–1898 cohort, during the period of 1998–2008. We found that while the more recent cohorts had a higher level of independence initially, they had a greater decline in their independence when compared with the earlier born cohorts. The more recent cohorts experienced more disability than the earlier cohorts when compared at the same ages. Similar findings were reported in the other studies on the trajectories of various health outcomes in China (Chen et al. 2010; Li and Zhang 2014; Marshall et al. 2015), frailty in the UK (Marshall et al. 2015) and self-perceived general health in the USA (Yang and Land 2013). More importantly, it was found that the decline of independence was much slower at the level of the population than that at the individual level for all three cohorts under study. This finding is consistent with the findings reported by Christensen et al. (2008). With examination of successive birth cohorts, it was further found that the shape of the decline at the population level significantly decelerated with age and tended to level off after the age of 95. Selective mortality and dropout accounted for the discrepancy between the estimates based on the individual trajectories and the population prevalence.
The results based on the growth curve models as well as the individual trajectories of independence across cohorts support the hypothesis of the expansion of morbidity. The possible explanation for this is that the more recent cohorts might have benefitted more from medical advances than the earlier cohorts did, so more of the frailer members were able to live longer although they still had a higher level of disability (Marshall et al. 2015; Zeng et al. 2017). On the other hand, the earlier born cohorts had a higher mortality at the same level of disability and thus more of them were selected out of the population earlier (Manton et al. 1997). Thus, the more recent cohorts would show a higher occurrence of disability when compared with their earlier counterparts at the same ages. This effect is also known as the “costs of success” or the “failure of success” (Christensen et al. 2013; Zeng et al. 2017). However, the population trajectory of independence shows that the survivors at extreme ages, i.e., nonagenarians and centenarians, would not necessarily have a sharp decline in their independence. Although those long-lived persons have a low level of independence, the prevalence of this independence declined slowly. For example, prevalence of independence in the population for the 1893–1898 female cohort declined modestly, from 19.7 to 11.1% in 1998–2008, while the percentage for females who survived from 1998 to 2008 declined from 66.7 to 11.1%. This implies individuals may have to face a higher cost of health care than society as a whole does.
Our analyses show how important the effects of selection due to death and loss to follow-up are for shaping independence and its trajectory over age for the oldest old. Selectivity underestimates the actual disability associated with the age-related change of a given cohort in very old age because the disabled individuals in this cohort are more likely to drop out. This is more evident for the earlier born cohorts because they had a higher mortality and dropout than the later born cohorts. Therefore, the significant differences in independence and its trajectory between the earlier cohorts and the later cohorts can be observed only when controlled for selectivity. Attrition outweighs the other components of heterogeneity such as education, marital status, diseases and medical services in the Chinese context. In fact, selection effects played an important role in shaping the mortality pattern of the Chinese oldest old. Zeng and Vaupel (2003) found that the mortality of the Chinese oldest old was obviously higher before the age of 96 than those of their Swedish and Japanese counterparts, but converged with them after that age. This convergence may be mainly due to the higher death among the Chinese oldest old.
It turns out that there were sex differences in the age trajectory of disability both at the population level and at the individual level. Although men had the initial advantage, they also experienced a sharper decline in independence than women did, particularly among the two earlier born cohorts. This finding is in line with previous research (Christensen et al. 2008; Oksuzyan et al. 2010; Zeng et al. 2017). Oksuzyan et al. (2010) also reported that men in their 90’s had a higher level of physical function accompanied by a greater decline compared to women at the same age. Zeng et al. (2017) reported that women’s functional capacities in the activities of daily living, cognition and physical performance were worse than that of their male counterparts among the oldest old.
Two limitations in our study should be noted. First, in spite of an initially large sample, the small sample size in the more recent assessments introduced some bumpiness into our estimation, particularly for the 1893–1898 birth cohort. This is because the selective mortality and loss to follow-up might have had a relatively greater impact on the estimates of coefficients for the groups with fewer survivors. Thus, we need to be cautious in interpreting the results in those cases. Second, although the unit non-response rate was very low, about 4% among the Chinese oldest old, the item non-response rate was higher, so we deleted around 9% of the participants due to missing values on the variables explored in the current study. We noticed that the excluded non-respondents had a somewhat different sex and urban–rural composition than the final sample. For instance, two of the later born cohorts consisted of more urban residents than the earliest cohort in the final sample, while the urban–rural composition was just the opposite for the non-respondents. Excluding the non-respondents could affect the estimates of the relevant parameters by raising the share of urban participants in the sample. Moreover, for those who were lost to follow-up, we kept them in the data and treated them as a category (e.g., Hayward and Gorman 2004; Yang and Land 2013). These might be crude treatment techniques, but we made the choice not to impute the missing data because we were unable to assure the accuracy of the imputation.
Despite these limitations, the current study contributes to the ongoing discussion of the cohort differences in the health trajectory as well as in distinguishing the population trajectory from the individual trajectory. The trajectory of independence among the oldest old is found somewhat unfavorable to the more recent cohorts who showed a lower proportion of independence compared to the earlier cohorts at the same ages. The population trajectory shows a less severe loss of independence than the individual trajectory of independence. From a policy perspective, it is important that particular attention must be paid to the differences in the health trajectories of the oldest old across and within cohorts and between individuals and the population as a whole.
Acknowledgements
We thank the editor, Prof. Frans Willekens and two anonymous reviewers for their helpful suggestions and comments. This study was funded by the National Natural Science Foundation of China (71503082, 71473044 and 71490735) and by Shanghai Planning Office of Philosophy and Social Science (2013BSH003).
Appendices
Appendix 1
See Table 4.
Table 4.
Distribution of the subjects by sex and place of residence for the final sample (N = 7955) and non-respondents (N = 850) across three cohorts. Total sample N = 8805
| 1909–1918 birth cohort | 1899–1908 birth cohort | 1893–1898 birth cohort | ||||
|---|---|---|---|---|---|---|
| Non-respondents | Respondents | Non-respondents | Respondents | Non-respondents | Respondents | |
| N | 176 | 3352 | 225 | 2788 | 449 | 1815 |
| % Men | 68.18 | 49.73 | 28.89 | 44.26 | 14.25 | 21.71 |
| % Women | 31.82 | 50.27 | 71.11 | 55.74 | 85.75 | 78.29 |
| % Urban | 72.16 | 56.44 | 37.78 | 63.24 | 31.63 | 72.95 |
| % Rural | 27.84 | 43.56 | 62.22 | 36.76 | 68.37 | 27.05 |
Appendix 2
We used data from the sixth wave of the Australian Longitudinal Study of Aging (ALSA) conducted in 1990–2000 to look for the cutoff point for non-cognitive impairment in the MMSE scores. The study sample of the ALSA was persons 70 years old and over and the total sample size was 791. After we excluded the subsample of those younger than 80 and those cases with missing values on the MMSE, the final sample size was 456. The cutoff of 17 for the Chinese version of MMSE may produce the similar distribution of cognition to what the cutoff of 23 for standard version of MMSE does (please see the following table).
Distribution of cognition by different cutoff for standard and Chinese version of MMSE, using the data from the sixth wave of the Australian Longitudinal Study of Aging.
| Freq. | % | |
|---|---|---|
| Standard MMSE | ||
| MMSE < 23 | 67 | 14.69 |
| MMSE ≥ 23 | 389 | 85.31 |
| Total | 456 | 100.00 |
| Chinese version of MMSE | ||
| MMSE < 17 | 73 | 16.01 |
| MMSE ≥ 17 | 383 | 83.99 |
| Total | 456 | 100.00 |
| Chinese version of MMSE | ||
| MMSE < 16 | 58 | 12.72 |
| MMSE ≥ 16 | 398 | 87.28 |
| Total | 456 | 100.00 |
Footnotes
Males and females began with almost the same levels of independence at the exact age of 80, probably because the study recruited relatively highly functional males and females at that age.
Due to the small sample size after age 104 for the 1893–1898 male birth cohort, we plotted only the trajectory of independence until age 104 for this cohort.
References
- Baltes PB, Smith J. New frontiers in the future of aging: from successful aging of the young old to the dilemmas of the fourth age. Gerontology. 2003;49:123–135. doi: 10.1159/000067946. [DOI] [PubMed] [Google Scholar]
- Chen F, Yang Y, Liu G. Social change and socioeconomic disparities in health over the life course in China: a cohort analysis. Am Sociol Rev. 2010;75:126–150. doi: 10.1177/0003122409359165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen K, McGue M, Petersen I, Jeune B, Vaupel JW. Exceptional longevity does not result in excessive levels of disability. Proc Natl Acad Sci. 2008;105:13274–13279. doi: 10.1073/pnas.0804931105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen K, Doblhammer G, Rau R, Vaupel JW. Ageing populations: the challenges ahead. The Lancet. 2009;374:1196–1208. doi: 10.1016/S0140-6736(09)61460-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen K, et al. Physical and cognitive functioning of people older than 90 years: a comparison of two Danish cohorts born 10 years apart. The Lancet. 2013;382:1507–1513. doi: 10.1016/S0140-6736(13)60777-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crimmins EM. Trends in the health of the elderly Annu Rev Publ. Health. 2004;25:79–98. doi: 10.1146/annurev.publhealth.25.102802.124401. [DOI] [PubMed] [Google Scholar]
- Crimmins EM, Beltrán-Sánchez H. Mortality and morbidity trends: is there compression of morbidity? J Gerontol Ser B. 2011;66B:75–86. doi: 10.1093/geronb/gbq088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman VA, Martin LG, Schoeni RF. Recent trends in disability and functioning among older adults in the united states: a systematic review. JAMA. 2002;288:3137–3146. doi: 10.1001/jama.288.24.3137. [DOI] [PubMed] [Google Scholar]
- Freedman VA, et al. Trends in late-life activity limitations in the united states: an update from five national surveys. Demography. 2013;50:661–671. doi: 10.1007/s13524-012-0167-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fries JF. Aging, natural death, and the compression of morbidity. N Engl J Med. 1980;303:130–135. doi: 10.1056/NEJM198007173030304. [DOI] [PubMed] [Google Scholar]
- Gerstorf D, Ram N, Hoppmann C, Willis SL, Schaie KW. Cohort differences in cognitive aging and terminal decline in the Seattle Longitudinal Study. Dev Psychol. 2011;47:1026–1041. doi: 10.1037/a0023426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruenberg EM. The failures of success. Milbank Meml Fund Q Health Soc. 1977;55:3–24. doi: 10.2307/3349592. [DOI] [PubMed] [Google Scholar]
- Gu D, Dupre ME, Sautter J, Zhu H, Liu Y, Zeng Y. Frailty and mortality among Chinese at advanced ages. J Gerontol Ser B. 2009;64B:279–289. doi: 10.1093/geronb/gbn009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guralnik JM. Population aging across time and cultures: can we move from theory to evidence? J Gerontol Ser A. 2004;59:M606–M608. doi: 10.1093/gerona/59.6.M606. [DOI] [PubMed] [Google Scholar]
- Hayward MD, Gorman BK. The long arm of childhood: the influence of early-life social conditions on men’s mortality. Demography. 2004;41:87–107. doi: 10.1353/dem.2004.0005. [DOI] [PubMed] [Google Scholar]
- Jeune B, et al. Handgrip strength among nonagenarians and centenarians in three European regions. J Gerontol Ser A. 2006;61:707–712. doi: 10.1093/gerona/61.7.707. [DOI] [PubMed] [Google Scholar]
- Li T, Zhang Y. Growth curve trajectories of elderly people’s health indicators in china: cohort variations and rural-urban disparities. Popul Res (Chin) 2014;38:18–35. [Google Scholar]
- Li Q, Reuser M, Kraus C, Alho J. Ageing of a giant: a stochastic population forecast for China, 2006–2060. J Popul Res. 2009;26:21. doi: 10.1007/s12546-008-9004-z. [DOI] [Google Scholar]
- Manton KG. Changing concepts of morbidity and mortality in the elderly population. Milbank Meml Fund Q Health Soc. 1982;60:183–244. doi: 10.2307/3349767. [DOI] [PubMed] [Google Scholar]
- Manton KG. Mortality and morbidity. In: Binstock RH, George LK, editors. Handbook of aging and the social sciences. 3. San Diego, CA: Academic Press; 1990. [Google Scholar]
- Manton KG, Stallard E, Corder L. Changes in the age dependence of mortality and disability: cohort and other determinants. Demography. 1997;34:135–157. doi: 10.2307/2061664. [DOI] [PubMed] [Google Scholar]
- Manton KG, Gu X, Lowrimore GR. Cohort changes in active life expectancy in the U.S. elderly population: experience from the 1982–2004 National Long-Term Care Survey. J Gerontol Ser B. 2008;63:S269–S281. doi: 10.1093/geronb/63.5.S269. [DOI] [PubMed] [Google Scholar]
- Marshall A, Nazroo J, Tampubolon G, Vanhoutte B. Cohort differences in the levels and trajectories of frailty among older people in England. J Epidemiol Commun Health. 2015;69:316. doi: 10.1136/jech-2014-204655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin LG, Schoeni RF. Trends in disability and related chronic conditions among the forty-and-over population: 1997–2010. Disabil Health J. 2014;7:S4–S14. doi: 10.1016/j.dhjo.2013.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin LG, Schoeni RF, Andreski PM. Trends in health of older adults in the United States: past, present, future. Demography. 2010;47:S17–S40. doi: 10.1353/dem.2010.0003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oksuzyan A, Maier H, McGue M, Vaupel JW, Christensen K. Sex differences in the level and rate of change of physical function and grip strength in the Danish 1905-Cohort Study. J Aging Health. 2010;22:589–610. doi: 10.1177/0898264310366752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poston DL, Duan CC. The current and projected distribution of the elderly and eldercare in the People’s Republic of China. J Fam Issues. 2000;21:714–732. doi: 10.1177/019251300021006003. [DOI] [Google Scholar]
- Rebke M, Coulson T, Becker PH, Vaupel JW. Reproductive improvement and senescence in a long-lived bird. Proc Natl Acad Sci. 2010;107:7841–7846. doi: 10.1073/pnas.1002645107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robine J-M, Michel J-P. Looking forward to a general theory on population aging. J Gerontol Ser A. 2004;59:M590–M597. doi: 10.1093/gerona/59.6.M590. [DOI] [PubMed] [Google Scholar]
- Schaie KW. Developmental influences on adult intelligence: the Seattle longitudinal study. Oxford: Oxford University Press; 2005. [Google Scholar]
- Smith J, Gerstorf D, Li Q. Psychological resources for well-being among octogenarians, nonagenarians, and centenarians: differential effects of age and selective mortality. In: Zeng Y, Poston DL Jr, Vlosky DA, Gu D, editors. Healthy longevity in China. Dordrecht: Springer; 2008. pp. 329–346. [Google Scholar]
- Vaupel JW, Yashin AI. Heterogeneity’s ruses: some surprising effects of selection on population dynamics. Am Stat. 1985;39:176–185. [PubMed] [Google Scholar]
- Vaupel JW, Rau R, Jasilionis D. The remarkable, accelerating decline in mortality at older ages and the prospects for further improvement in life expectancy. Biochim Clin. 2006;30:S4–S5. [Google Scholar]
- United Nations (2017) World population prospects, the 2017 revision. https://esa.un.org/unpd/wpp/Publications/Files/WPP2017_KeyFindings.pdf
- Yang Y, Land KC. Age-period-cohort analysis: new models, methods, and empirical applications. Boca Raton: CRC Press; 2013. [Google Scholar]
- Zeng Y, Vaupel JW. Oldest old mortality in China. Demogr Res. 2003;8:215–244. doi: 10.4054/DemRes.2003.8.7. [DOI] [Google Scholar]
- Zeng Y, Vaupel JW, Xiao Z, Zhang C, Liu Y. Sociodemographic and health profiles of the oldest old in China. Popul Dev Rev. 2002;28:251–273. doi: 10.1111/j.1728-4457.2002.00251.x. [DOI] [Google Scholar]
- Zeng Y, Feng Q, Hesketh T, Christensen K, Vaupel JW. Survival, disabilities in activities of daily living, and physical and cognitive functioning among the oldest-old in China: a cohort study. The Lancet. 2017;389:1619–1629. doi: 10.1016/S0140-6736(17)30548-2. [DOI] [PMC free article] [PubMed] [Google Scholar]