Abstract
Background:
Collagen organization within tissues has a critical role in wound regeneration. Collagen fibril diameter, arrangements and maturity between connective tissue growth factor (CTGF) small interfering RNA (siRNA) and mismatch scrambled siRNA-treated wound were compared to evaluate the efficacy of CTGF siRNA as a future implement for scar preventive medicine.
Methods:
Nanocomplexes of CTGF small interfering RNA (CTGF siRNA) with cell penetrating peptides (KALA and MPG∆NLS) were formulated and their effects on CTGF downregulation, collagen fibril diameter and arrangement were investigated. Various ratios of CTGF siRNA and peptide complexes were prepared and down-regulation were evaluated by immunoblot analysis. Control and CTGF siRNA modified cells-populated collagen lattices were prepared and rates of contraction measured. Collagen organization in rabbit ear 8 mm biopsy punch wound at 1 day to 8 wks post injury time were investigated by transmission electron microscopy and histology was investigated with Olympus System and TS-Auto software.
Conclusion:
CTGF expression was down-regulated to 40% of control by CTGF siRNA/KALA (1:24) complexes (p < 0.01) and collagen lattice contraction was inhibited. However, down-regulated of CTGF by CTGF siRNA/MPG∆NLS complexes was not statistically significant. CTGF KALA-treated wound appeared with well formed-basket weave pattern of collagen fibrils with mean diameter of 128 ± 22 nm (n = 821). Mismatch siRNA/KALA-treated wound showed a high frequency of parallel small diameter fibrils (mean 90 ± 20 nm, n = 563).
Conclusion:
Controlling over-expression of CTGF by peptide-mediated siRNA delivery could improve the collagen orientation and tissue remodeling in full thickness rabbit ear wound.
Keywords: Connective tissue growth factor siRNA, Scar, Collagen, Dermal matrix
Introduction
Serious scars from major burns and surgery cause tremendous burden on individual patients, society and healthcare [1]. Long term physical dysfunction and psychological trauma left the patient in stigma. Current treatment options for scars have significant limitations. In present, mitomycin C and imiquimod have been employed as topical scar medications. In addition, bleomycin, interferon, botulinum toxin A and corticosteroids are prescribed as an intra lesional scar treatment. Triamcinolone acetonide (10–40 mg) injections every 3–4 weeks is one of the most prescribed treatment. However, well controlled double blind study could not prove the favorable scar preventive effect of these medications. In addition, corticosteroid injections cause many adverse effects such as hypo pigmentation and skin atrophy [2, 3].
Scarring involves many aspects that are interrelated with multiple pathways. Tissue repair process is controlled by various peptides including cytokines and growth factors and these peptides are up-regulated after injury [4–6]. Scar tissue is formed by excess collagen synthesized by fibroblasts, in which regulatory cytokines are up-regulated after injury. CTGF functions as a key mediator in hyper-scarring formation after tissue injury [7–10]. Prolonged over expression of CTGF has been associated with tissue fibrosis in human and down regulation CTGF could inhibit tissue fibrosis [11–13]. CTGF, a cystein-rich peptide (MW: 38 kDa) is excreted by fibroblasts, myofibroblasts and endothelial cells. CTGF is also over-expressed in keloid and hypertrophic scars [14, 15].
Recently, gene silencing through siRNA has appeared as a promising approach [16]. siRNA holds promise in the study of gene expression by primary mammalian cells, and provides optimism for the potential future use of dsRNA-mediated silencing in vivo to regulate normal and pathologic processes. However, siRNA cannot penetrate the plasma membrane due to its high molecular size (~ 14,000 daltons) and the strong negative charge of the phosphate [17]. Due to very fast degradation of the 21-nt siRNA on cells in vitro, the application of siRNA in vivo may need chronic expression by an appropriate vector. The desirable siRNA delivery system should possess the capacity to protect siRNA from degradation and target to cell and release siRNA into the cytoplasm [18].
In this study, peptide-based siRNA-mediated modification of CTGF and its implication in wound repair courses focusing on dermal collagen disposition and diameter was investigated. Collagen organization within tissues is a critical parameter that evaluates the efficacy of scar preventive therapies [19, 20]. To analyze the architecture of CTGF siRNA/KALA-treated full thickness skin wound as compared to mismatch siRNA-treated one, transmission electron microscopy (TEM) to measure the fibril diameters in skin at 0–8 wks post-wound. Changes in the homogeneity of fibril diameter were evaluated in relation with scarring. Comparison of fibril diameters, collagen arrangement and maturity between mismatch scrambled CTGF siRNA treatment groups and CTGF siRNA/KALA-treated groups were conducted aiming to evaluate the efficacy of CTGF siRNA as a future implement for scarless medication.
Materials and methods
Materials
All chemicals were purchased from Sigma chemicals (St.Louis, MO, USA). Human dermal fibroblast was provided from Dept. Dermatology, Seoul National University. CTGFsiRNA was purchased from Invitrogen Co., (Carlsbad, CA, USA). KALA and MPG-NLS were synthesized at Peptron Co. (Daejeon, Korea). KALA has an amino acid sequence of WEAKLAKALAKALAKHLAKALAKALKACEA. MPG-NLS has a single mutation on the second lysine residue of the nuclear localizing sequence (NLS) to serine (GALFLGFLGAAGSTMGAWSQPKSKRKV), was reported to abolish the nuclear translocation and facilitate a rapid release of the siRNA in the cytoplasm. DMEM (Dulbecco’s Modified Eagle Medium) culture medium and FBS (fetal bovine serum) were purchased from Cellgro (Washington DC, USA), PS (penicillin streptomycin) was purchased from Gibco (Grand Island, NY, USA) and Cultrex®Rat collagen I was purchased from Trevigen (Geithersburg, MD, USA).
Cell lines
Primary human foreskin fibroblasts were kindly provided from dept. dermatology, medical school, Seoul National University. Fibroblasts were cultured in a 6 well plate (5 × 105cells/well) in DMEM medium supplemented with 10% heat inactivated fetal bovine serum and 100 units/mL penicillin/streptomycin. Cells were maintained in a humidified atmosphere with 95% air and 5% CO2 at 37 °C.
Injury of human foreskin fibroblast in vitro
Previously reported in vitro wound model was employed [21]. In brief, cells were PBS washed and scratched with a sterile 100 L plastic pipette. One line was scratched on confluent cultured human primary dermal fibroblast. After washing with PBS, cell media was replaced with complete media.
Preparation of CTGFsiRNA/peptide (KALA and MPGΔNLS) and transfection
For CTGF siRNA delivery, siRNA/KALA or siRNA/MPGΔNLS complexes with different(N/P) ratios (1:1, 1:6, 1:12, and 1:24) were formed in OPTI-MEM reduced serum medium (Invitrogen, Carlsbad, CA) and incubated for 30 min at RT. In brief, CTGF siRNA (5 µL) was placed in vial first and add fusion peptide solution (KALA or MPGΔNLS peptide (36 µg/3 µl) with 1.5 mL of 25 mM Na-acetate buffer into the siRNA. The mixture was then vortexed and incubated for 30 min to form nano complexes. After 30 min incubation, cells grown to 60% confluence were then transfected with each siRNA—KALA or MPGΔNLS (N/P ratio 1, 6, 12 and 24) complexes for 6 h. After 6 h incubation at 37 °C, 2 mL of fresh DMEM supplemented with 10% FBS was added to the cells, without removing the overlay of carrier/siRNA.
CTGF small-interfering RNA (siRNA) and transfection
The siRNA molecules targeting CTGF (5′-UUAGCUCGGUAUGUCUUCAUGCUGG-3′) were complexed with tested peptides. Cells were plated in six-well plates at 1 × 105 cells/well, grown for 24 h then transfected with each 100 pmol siRNA for 6 h using lipofectamine and OPTI-MEMI reduced serum medium (Invitrogen, Carlsbad, CA, USA). Control cell were treated with StealthTM RNAi negative control duplex (Invitrogen, Carlsbad, CA, USA). One day before transfection, cells were plated in six-well plates at 1 × 105 cells/well. Cells should be 60–70% confluent at the time of transfection. For each transfection sample, Stealth™ RNAi-Lipofectamine™ 2000 complexes were prepared. After transfected with CTGF siRNA, cells were changed into the fresh medium with serum and incubated for another 18 h for maximum transfection efficiency. Then cells were PBS-washed and scratched as described previously [21].
Immunoblot analysis
After 6 h (scratched), cells were rinsed with cold PBS, lysed in SDS-lysis buffer. Equal amounts of protein extracts in SDS-lysis buffer were subjected to 12% SDS-PAGE analysis and electrophoretically transferred to nitrocellulose membrane. Anti-CTGF antibody was purchased from Santa Cruz (Santa Cruz, CA, USA). β-actin antibody was from Sigma-Aldrich (St. Louis, MO, USA). Enhanced chemiluminescence (Amersham-Pharmacia, Buckinghamshire, U.K.) system was used for detection. Relative band intensities were determined by quantitation of each band with an Image Analyzer Gel Logic 200 Imaging System (Kodak, Rochester, NY, USA).
Measurement of collagen lattice contraction by fibroblasts
The control and siRNA/peptide modified cells were trypsinized and 0.2 mL of the cell suspension (containing 5 × 105 cells) was added to the dermal collagen solution, gently mixed and poured into a well of the six-well plate. Collagen lattices were allowed to gel for 60 min in a 5% CO2 at 37 °C. After 60 min, the collagen lattices were detached from the surface of the well by rimming the lattice with a sterile spatula. 4 mL of serum–free medium was added to each well and the rate of contraction was determined by measuring lattice diameter.
Preparation of whole thickness wound
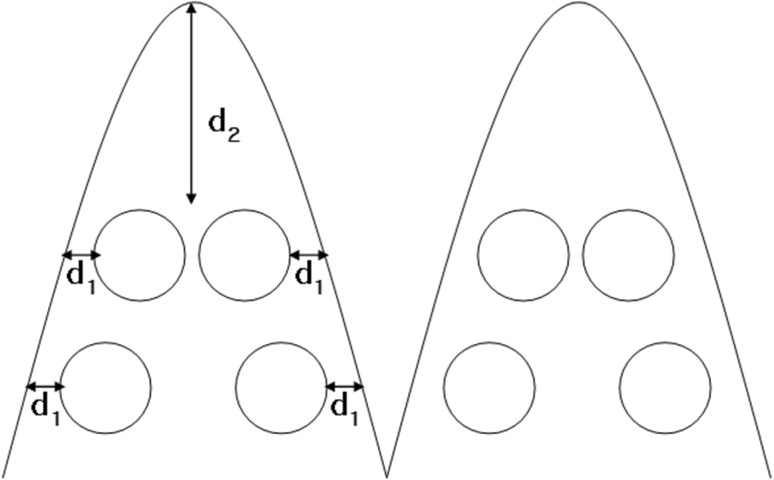
New Zealand White rabbit (7–8 wks old; average body weight, 2.0 ± 0.2 kg) was employed as an animal model in this study [14]. Rabbits were anesthetized by ethyl ether inhalation (4–8 mL) for 30 s and rabbits became sedated. Full thickness biopsy punch wound model was prepared as reported previously [22]. In brief, four sites of full thickness excisional circular wounds (8 mm diameter) were created down to the cartilage on the peritoneal side of each ear employing 8 mm biopsy punch. And the punched area was cooled and disinfected with 5 mL of ice-cold 0.5% acrynol solution for 30 s. The inflicted animals were placed in fixant during the tested periods. Biopsy punch wound induced full thickness wound, in which epidermis, dermis and subcutaneous fat completely removed as shown in Scheme 1. To prevent infection, Cephadex (50 mg/kg) was injected subcutaneously daily. The animal experiments were performed under animal protocol number (2018-003-009) approved by the Duksung Women’s University Institutional Animal Care and Use Committee.
Scheme 1.
Preparation of full thickness biopsy punch wound model. Four circular wounds (diameter: 8 mm) were inflicted on the anterior part of rabbit ear. For a reliable comparative wound healing study, the wound position should be well controlled to have same distance from the top (d2) and the side (d1)
Application of CTGF siRNA/peptide nanocomplexes on wound sites
Each tested solutions was injected dermally into four different wound sites (× 4) with 10 µL injection volume at 1 h pre injury, 24 h, 48 and 96 h post injury time. Duoderm (Convatec Inc., Bridgewater, NJ, USA) dressings were applied at least 2–3 days for controlling exudates and blood.
Histological observation
The skin specimens were excised and processed for histological evaluation at predetermined post injury time. On pre-determined post wounding time, the tested areas were excised and fixed in 10% neutral buffered formaldehyde solution, and paraffin embedded. Skin specimens were sectioned (5 µm) perpendicular to the surface and stained with hematoxylin and eosin. Collagen arrangements of dermal skin of rabbit ear at time was also investigated. Olympus microscope (Model BX 41, Tokyo, Japan) and image recording equipment (Models DP11 and PM 10SP, Tokyo, Japan) were employed.
Transmission electron microscopy (TEM)
For ultrastructural collagen analysis, normal skin and wound tissues were fixed overnight in a mixture of cold 2.5% glutaraldehyde in 0.1 M phosphate buffer (pH 7.2), and 2% paraformaldehyde in 0.1 M phosphate or cacodylate buffer (pH 7.2). These tissues were post-fixed for 1.5 h in 2% osmium tetroxide in 0.1 M phosphate or cacodylate buffer for 1.5 h at room temperature. The samples were then washed briefly with deuterated H2O2, dehydrated throughout a graded 50, 60, 70, 80, 90, 95, and 100% ethanol (X2) series, infiltrated by using propylene oxide and, infiltrated by using propolyne oxide and epoxy embedding medium (Epon_812 substitute) as an embedding resin for electron microscopy. The epoxy resin-mixed samples were loaded into capsules and polymerized at 38 °C for 12 h and 60 °C for 48 h. Sections for light microscopy were cut at 500 nm and stained with 1% toluidine blue for 45 s on a hot plate at 80 °C. Thin sections were made using an ultramicrotome (Model: RMC MT-XL) by Boeckeler Instruments, Inc. (Tuscon, AZ, USA) and collected on copper grid. Appropriate areas for thin sectioning were cut at 65 nm and stained with saturated 6% uranyl acetate and 4% lead citrate before examination with a transmission electron microscope (Model JEM-1400) by JEOL USA, Inc. (Peabody, MA, USA) at 80kV.
Statistical analysis
At least, triplicate experimental values were averaged for the calculation of the experimental results and data are expressed as mean ± standard deviation. Error bars in figures represent the standard deviation. Student’s t test was employed for the calculation of p-values to verify the difference between experimental data. p value < 0.01 was accepted as denoting statistical significance. In this study, statistical probability (p) in figures was expressed as **p < 0.01.
Results
CTGF siRNA/KALA nanocomplexes inhibited CTGF expression
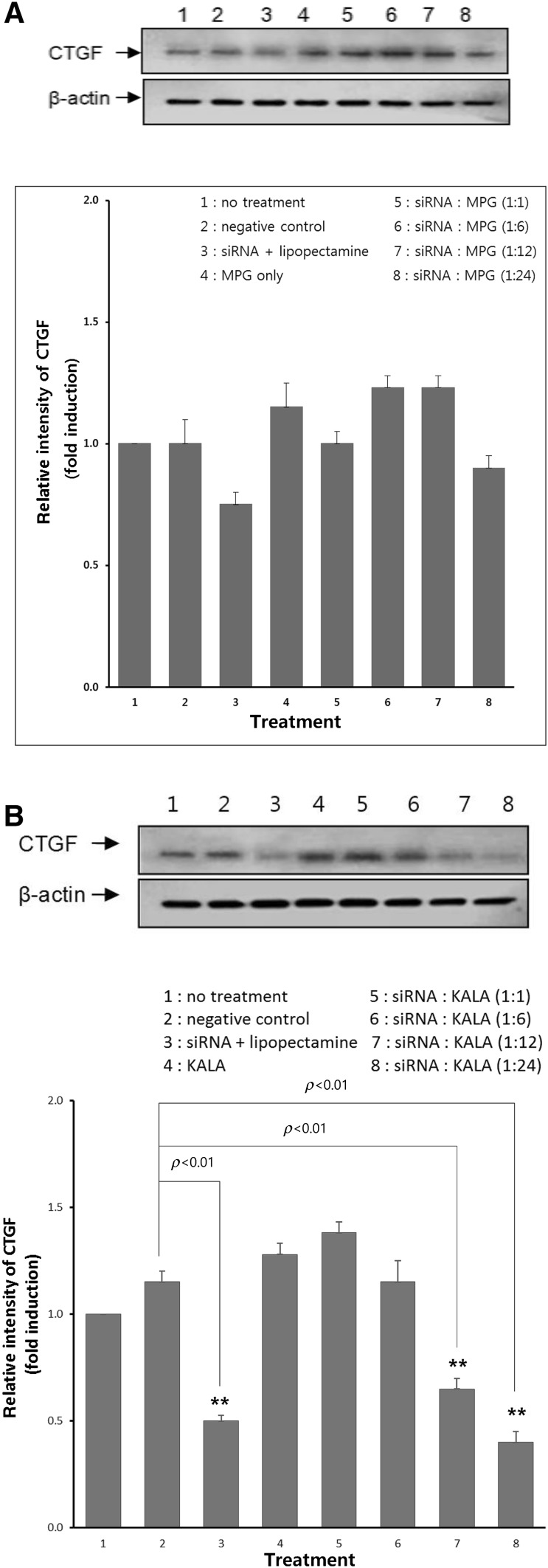
Figure 1A shows Western blot analysis of CTGF expression in fibroblasts: no treatment, negative control, siRNA/Lipofectamine, MPGΔNLS only and siRNA/MPGΔNLS (1:1, 1:6, 1:12 and 1:24) complexes treatment. As a negative and positive control, mismatch siRNA/Lipofectamine and CTGF siRNA/Lipofectamine were employed, respectively. The cell viability was tested by comparing actin levels in tested cells. An insignificant silencing effect was observed for naked siRNA as well as mismatch siRNA/Lipofectamine complexes. CTGF siRNA/MPGΔNLS did not show significant down regulation of CTGF.
Fig. 1.
A Effect of CTGF siRNA/MPGΔNLS ratio (1:1–1:24) on down-regulation of CTGF in fibroblast wound model: mismatch siRNA was served as a negative control and Lipofectamine was served as a positive control. CTGFsiRNA/MPGΔNLS showed no significant silencing effect on CTGF level. B Effect of CTGF siRNA/KALA ratio (1:1–1:24) on down-regulation of CTGF expression in fibroblast wound model. CTGFsiRNA/KALA showed 40–60% down-regulation of CTGF at 1:12 and 1:24 ratios. (*denotes statistically different from control, p < 0.01)
Figure 1B shows Western blot analysis of CTGF expression on fibroblasts: no treatment, negative control, siRNA/lipofectamine, KALA only and siRNA/KALA (1:1, 1:6, 1:12 and 1:24) complexes treatment. As a negative and positive control, mismatch siRNA/Lipofectamine and CTGF siRNA/Lipofectamine were employed, respectively. A negligible silencing effect was shown for naked siRNA as well as mismatch siRNA/Lipofectamine complexes. CTGFsiRNA/KALA showed 40–60% down regulation (p < 0.01) of CTGF at 1:12 and 1:24 ratios; KALA showed slightly higher down regulation of CTGF than Lipofetamine (p < 0.01).
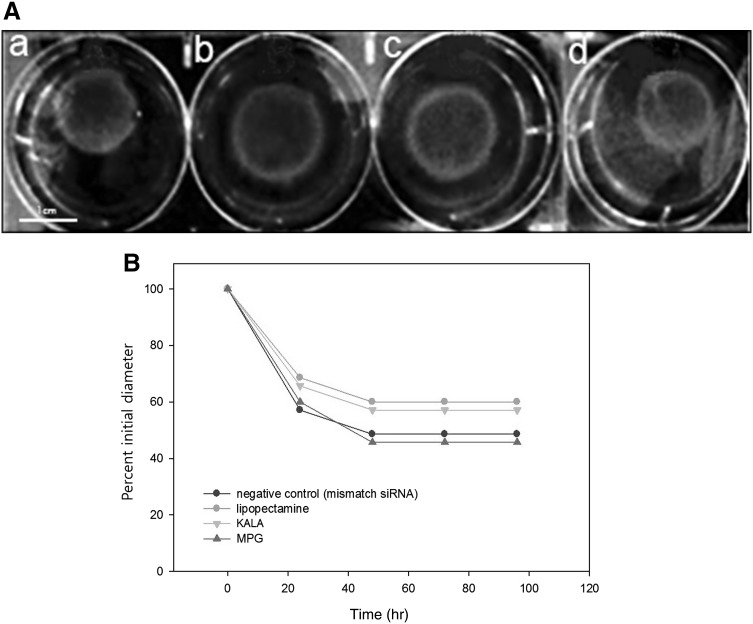
Collagen lattice contraction was inhibited by CTGF siRNA/KALA nanocomplexes
Downregulation of CTGF expression by siRNA transfection on the inhibition of collagen lattice contraction was evaluated by comparing time course of contraction of control and siRNA modified fibroblast populated collagen lattices. Figure 2A shows photographs of the collagen lattices after 48 h contraction by cultured fibroblasts. The lattices were modified by A: mismatch siRNA/lipofectamine, B: CTGF siRNA/lipofectamine, C: CTGF siRNA/KALA, and D: CTGF siRNA/MPGΔNLS. Figure 2B shows time course of contraction of collagen lattices for 96 h: to evaluate the ability of fibroblasts to contract collagen lattices, negative control(mismatch), siRNA/KALA, lipofectamine, MPG-modified cells were cast into floating collagen lattices and monitored contraction over time (Fig. 2A). Collagen lattices were rapidly contracted by transfected cells modified either with mismatch siRNA or CTGF siRNA/MPGΔNLS from a starting diameter of 32mm to less than 18mm (56%) in the first 24 h; whereas lattices with CTGF siRNA/KALA-modified cells contracted from 32mm to 23mm (72%) in the first 24h. The rate of contraction slowed down to 48 h and no further significant contraction observed to 96 h.
Fig. 2.
A Photographs of the fibroblast populated collagen lattices after 48 h contraction. The lattices were modified by (a) mismatch siRNA/lipofectamine, (b) CTGF siRNA/Lipofectamine, (c) CTGF siRNA/KALA, and (d) CTGF siRNA/MPGΔNLS. B Time course of contraction of fibroblast-populated collagen lattices: control cells and siRNA-modified cells were cast in floating collagen gels and the rate of contraction determined by measuring lattice diameter at predetermined time
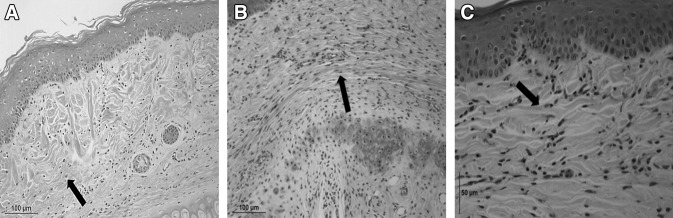
Weave-like collagen arrangement observed in CTGF siRNA/KALA nanocomplex-treated wound
Figure 3A shows histology of normal skin which shows well-developed epidermis and dermal collagen matrix (arrow) and basement membrane (arrow) between dermal-epidermal junctions. A typical basket weave-like pattern of collagen arrangements were observed. Figure 3B shows histologic cross-section of mismatch siRNA/KALA-treated 8 mm full thickness surgical wound at 61 day post wounding time: dense and parallel abnormal arrangements of collagen fibrils observed (indicated as an arrow); damaged cartilage (arrow) has not been repaired yet (hematoxylin & eosin stain, x100 and x200). Figure 3C shows the histology of 61 day post injury after treatment of siRNA/KALA. Collagen fibril bundles arranged in a basket weave pattern similar to normal dermis were observed.
Fig. 3.
Histological cross section of H-E stained 8 mm biopsy punch wound in rabbit ear with A Normal skin. B H&E stained sections of mismatch siRNA/KALA-treated 8 mm biopsy punch wound after 56 post injury. As compared with weave-like collagen structure, parallel and less developed collagen bundles observed (depicted as an arrow). C CTGF siRNA/KALA-treated wound showed well stained epidermis layer as denoted an arrow and high density of fibroblasts and collagen bundles as weave-like in dermis layer (Original magnification × 400)
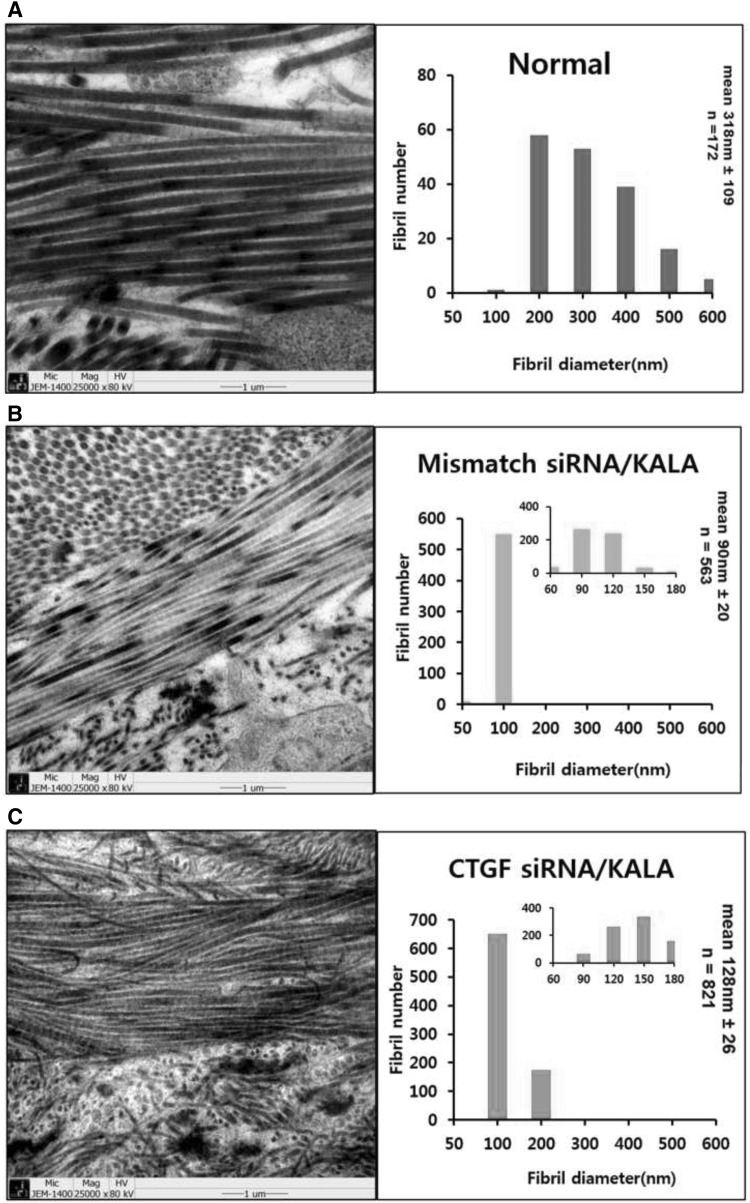
Collagen fibril diameter and organization were analyzed by TEM
Ultra-structural collagen organization was examined as an indication of the quality of the scar tissue. The relative frequences within a specific range of diameters were evaluated and a histogram to demonstrate the differences in the disposition of collagen fibril diameters was prepared. Figure 4A shows strong well developed collagen fibrils in dermal matrix of rabbit ear skin. The interwoven organization is the key to a functional connective tissue matrix. In normal skin, the extracellular matrix was made of collagen fibril bundles organized in a basket weave pattern. Normal skin contained relatively large collagen with average diameter 318 ± 109 nm (n = 172). Scar is composed of densely packed, disorganized collagen fiber bundles. An impaired collagen fibril organization with fibril bundles oriented parallel to each other in contrast with a basket weave pattern arrangement in normal skin are typical characterization of scar tissue [19, 20]. Figure 4B shows a parallel arrangement of collagen bundles in 61 day post 8 mm full thickness wounding after mismatch siRNA/KALA-treated wound. Parallel collagen fibril arrangement is a characteristic of scar tissue and causes the matrix to retract and harden. Scar tissue from mismatch siRNA/KALA-treated wound contained a high frequency of very small diameter fibrils. This fibril diameter is significantly lower than that of normal skin; with average diameter 90 ± 20 nm (n = 563). Figure 4C shows TEM of CTGF siRNA/KALA-treated wound at 61 day post wounding. It shows extracellular matrix was made of collagen fibril bundles organized in a basket weave pattern. CTGF siRNA/KALA-treated wound showed well developed collagen arrangements with the mean diameter of 128 ± 22 nm (n = 821).
Fig. 4.
Representative TEM images of collagen fibrils and histogram. Average collagen fibril diameter in CTGF siRNA/KALA and mismatch siRNA/KALA-treated wounds as compared with those of normal (unwounded) skin. Graph inserts indicate treatment type, average fibril diameter (nm), and the total range of fibril diameters (nm). A TEM of normal (unwounded) skin. It shows well matured collagen fibrils are formed as a weave-like pattern. The mean collagen fibril diameter in normal skin is 318 ± 109 nm (n = 172). B TEM of mismatch siRNA/KALA-treated wound at 61 day post injury. It shows random appearance of collagen fibers and disorganized arrangement. Collagen fibers formed in scar tissue are much smaller. This defected collagen arrangement contributes that scar tissue is always weaker and will break apart before the surrounding normal tissue. Disruption of collagen fibril arrangements observed. The mean collagen fibril diameter is 90 ± 20 nm (n = 563). C TEM of CTGF siRNA/KALA-treated wound at 61 day post injury. It shows more organized collagen fibers and larger collagen diameter. CTGF siRNA/KALA-treated tissues show more closely resembled normal skin with a narrower distribution of collagen fibril diameters. The mean collagen fibril diameter is 128 ± 22 nm (n = 182)
Discussion
Collagen remodeling occurs during wound healing. Regulation of collagen synthesis and disposition is an efficient approach to control scar tissue formation [23]. Cell penetrating peptides (CPPs) such as KALA and MPGΔNLS with siRNA complexes were prepared and the down-regulation of CTGF and its effect on the collagen lattice contraction rate were investigated. In this investigation, as a vector to deliver siRNA into the cytosol, fusion peptides KALA and MPG∆NLS were investigated. In 1997, Szoka group at UCSF proposed a low-molecular weight cationic amphipathic peptide KALA that binds to DNA, destabilizes membranes, and mediates DNA transfection [24]. KALA has been recognized for its membrane disrupting property: KALA enters the endosomal pathway and then disrupt the acidic compartment and introduce siRNA into the cytoplasm prior to fusion of the endosome with the lysosome [25]. MPG peptide derived from the fusion peptide of HIV-1 gp41 protein is a 27-residue-long primary amphipathic peptide contains the fusion sequence, nuclear localizing sequence and linker domain. MPG carries a cysteamide group at its C-terminus, which is essential for both cell entry and stabilization of the complexes with siRNA. A single mutation on the second lysine residue of the NLS to serine (MPGΔNLS) could abolish the nuclear translocation and facilitate a rapid release of the siRNA in the cytoplasm [17]. MPG∆NLS lysine at 18th amino acid replaced with serine residue for an enhanced cytosolic delivery was investigated for its CTGF down-regulation efficacy.
In this study, fusion peptide KALA could form CTGF siRNA complexes and down-regulate CTGF level to 40–60% as compared with control values (mismatch siRNA/Lipofectamine). However, CTGFsiRNA/MPGΔNLS showed no statistically significant down-regulation of CTGF than those of Lipofectamine at either 1:12 or 1:24 ratio complexes. Inefficiency to down-regulate CTGF expression by CTGFsiRNA/MPGΔNLS could be due to the difficulty of siRNA escaping from endosome or release siRNA from the siRNA-MPG△NLS complexes [17]. Further study to verify this hypothesis should be performed. To analyze the effect of peptide-mediated down regulation of CTGF on full thickness wound healing process (0–61 day post injury time) focusing on collagen arrangement and fibril formation, we employed the rabbit ear model which permits a precise, reproducible, and more comprehensive analysis [14]. Histology and TEM analysis enabled us to understand the changes in the homogeneity of collagen fibril diameter in relation with scar prevention efficacy of CTGFsiRNA/KALA as compared with scrambled mismatch siRNA. Comparison of collage organization and fibril diameter in the tested tissues could be a critical parameter that evaluates the efficacy of scar preventive therapies.
In conclusion, CTGF siRNA/cell penetration peptide nanocomplexes were prepared. Peptide-mediated downregulation of CTGF by siRNA/KALA nanocomplexes modulated collagen fibril organization and diameter in full-thickness wound and showed comparable weave-like collagen arrangements like normal skin. This result suggests the potential application of CTGF siRNA/KALA nanocomplexes for anti-scar therapy. Further physico-chemical characterization and formulation development including long-term stability in different storage condition is required for developing promising siRNA delivery medications [26, 27].
Acknowledgements
This work was supported by the research grant from 2017 Duksung Women’s University. We thank to Ms. Youli Lee for her excellent technical assistance in quantitative analysis of collagen morphology. We thank to Prof. KC Park at Dermatology Department, School of Medicine, Seoul National University for his valuable discussion and donation of primary human fibroblasts.
Compliance with ethical standards
Conflicts of interest
The authors have no financial conflicts of interest.
Ethical statement
The animal study was performed after receiving approval of the Institutional Animal Care and Use Committee in Duksung Women’s University (IACUC no. 2018-003-009).
References
- 1.Block L, Gosain A, King TW. Emerging therapies for scar prevention. Adv Wound Care. 2015;4:607–614. doi: 10.1089/wound.2015.0646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brown BC, Mckenna SP, Siddhi K, McGrouthe DA, Bayat A. The hidden cost of skin scars: quality of life after skin scarring. J Plast Reconstr Aesthet Surg. 2008;61:1049–1058. doi: 10.1016/j.bjps.2008.03.020. [DOI] [PubMed] [Google Scholar]
- 3.Young VL, Hutchison J. Insights into patient and clinician concerns about scar appearance: semi quantitative structured surveys. Plast Reconstr Surg. 2009;124:256–265. doi: 10.1097/PRS.0b013e3181a80747. [DOI] [PubMed] [Google Scholar]
- 4.Cross KJ, Mustoe TA. Growth factors in wound healing. Surg Clin North Am. 2003;83:531–545. doi: 10.1016/S0039-6109(02)00202-5. [DOI] [PubMed] [Google Scholar]
- 5.Reish RG, Eriksson E. Scars: a review of emerging and currently available therapies. Plast Reconstr Surg. 2008;122:1068–1078. doi: 10.1097/PRS.0b013e318185d38f. [DOI] [PubMed] [Google Scholar]
- 6.Campaner AB, Ferreira LM, Gragnani A, Bruder JM, Cusick JL, Morgan JR. Upregulation of TGF-beta1 expression may be necessary but is not sufficient for excessive scarring. J Invest Dermatol. 2006;126:1168–1176. doi: 10.1038/sj.jid.5700200. [DOI] [PubMed] [Google Scholar]
- 7.Blom IE, Goldschmeding R, Leask A. Gene regulation of connective tissue growth factor: new targets for antifibrotic therapy. Matrix Biol. 2002;21:473–482. doi: 10.1016/S0945-053X(02)00055-0. [DOI] [PubMed] [Google Scholar]
- 8.Chujo S, Shirasaki F, Kawara S, Inagaki Y, Kinbara T, Inaoki M, et al. Connective tissue growth factor causes persistent proalpha2(I) collagen gene expression induced by transforming growth factor-beta in a mouse fibrosis model. J Cell Physiol. 2005;203:447–456. doi: 10.1002/jcp.20251. [DOI] [PubMed] [Google Scholar]
- 9.Frazier K, Williams S, Kothapalli D, Klapper H, Grotendors GR. Stimulation of fibroblast cell growth, matrix production and granulation tissue formation by CTGF. J Invest Dermatol. 1996;107:404–4011. doi: 10.1111/1523-1747.ep12363389. [DOI] [PubMed] [Google Scholar]
- 10.Sonnylal S, Shi-Wen X, Leoni P, Naff K, Van Pelt CS, Nakamura H, et al. Selective expression of CTGF in fibroblasts in vivo promotes systemic tissue fibrosis. Arthritis Rheum 2010;62:1523–32. [DOI] [PMC free article] [PubMed]
- 11.Leask A, Holmes A, Abraham DJ. Connective tissue growth factor: a new and important player in the pathogenesis of fibrosis. Curr Rheumatol Rep. 2002;4:136–142. doi: 10.1007/s11926-002-0009-x. [DOI] [PubMed] [Google Scholar]
- 12.Li G, Xie Q, Shi Y, Li D, Zhang M, Jiang S, et al. Inhibition of CTGF by siRNA prevents liver fibrosis in rats. J Gene Med. 2006;2006:889–900. doi: 10.1002/jgm.894. [DOI] [PubMed] [Google Scholar]
- 13.Nishio N, Ito S, Suzuki H, Isobe K. Antibodies to wounded tissue enhance cutaneous wound healing. Immunology. 2009;128(3):369–380. doi: 10.1111/j.1365-2567.2009.03119.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sisco M, Kryzer ZB, O’Shaughnessy KD, Kim PS, Schultz GS, Ding XZ, et al. Antisense inhibition of connective tissue growth factor (CTGF/CCN2) mRNA limits hypertrophic scarring without affecting wound healing in vivo. Wound Rep Reg. 2008;16:661–673. doi: 10.1111/j.1524-475X.2008.00416.x. [DOI] [PubMed] [Google Scholar]
- 15.Daniels JT, Schultz GS, Blalock TD, Garrett Q, Grotendorst GR, Dean NM, et al. Mediation of transforming growth factor-beta(1)-stimulated matrix contraction by fibroblasts: a role for connective tissue growth factor in contractile scarring. Am J Pathol. 2003;163:2043–2052. doi: 10.1016/S0002-9440(10)63562-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Whitehead KA, Langer R, Anderson DG. Knocking down barriers: advances in siRNA delivery. Nat Rev Drug Discov. 2009;8:129–138. doi: 10.1038/nrd2742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eguchi A, Dowdy SF. siRNA delivery using peptide transduction domains. Trends Pharmacol Sci. 2009;30:341–345. doi: 10.1016/j.tips.2009.04.009. [DOI] [PubMed] [Google Scholar]
- 18.Wang JF, Olson ME, Ma L, Brigstock DR, Hart DA. Connective tissue growth factor siRNA modulates mRNA levels for a subset of molecules in normal and TGF-beta 1-stimulated porcine skin fibroblasts. Wound Repair Regen. 2004;12:205–216. doi: 10.1111/j.1067-1927.2004.012113.x. [DOI] [PubMed] [Google Scholar]
- 19.Berthod F, Germain L, Li H, Xu W, Damour O, Auger FA. Collagen fibril network and elastic system remodeling in a reconstructed skin transplanted on nude mice. Matrix Biol. 2001;20:463–473. doi: 10.1016/S0945-053X(01)00162-7. [DOI] [PubMed] [Google Scholar]
- 20.White JF, Werkmeister JA, Darby JA, Bisucci T, Birk DE, Ramshaw JA. Collagen fibril formation in a wound healing model. J Struct Biol. 2002;137:23–30. doi: 10.1006/jsbi.2002.4460. [DOI] [PubMed] [Google Scholar]
- 21.Moon H, Yong H, Lee AR. Optimum scratch assay condition to evaluate connective tissue growth factor for anti-scar therapy. Arch Pharm Res. 2012;35:383–388. doi: 10.1007/s12272-012-0220-x. [DOI] [PubMed] [Google Scholar]
- 22.Kim SC, Cho Lee A. Preparation of reproducible and responsive scar model and histology analysis. J Pharm Investig. 2010;40:45–49. doi: 10.4333/KPS.2010.40.1.045. [DOI] [Google Scholar]
- 23.Reid R, Mogford JE, Butt R, deGiorgio-Miller A, Mustoe T. Inhibition of procollagen C-proteinase reduces scar hyperthrophy in a rabbit model of cutaneous scarring. Wound Repair Regen. 2006;14:138–141. doi: 10.1111/j.1743-6109.2006.00103.x. [DOI] [PubMed] [Google Scholar]
- 24.Wyman TB, Nicol F, Zelphati O, Scaria PV, Plank C, Szoka FC., Jr Design, synthesis and characterization of a cationic peptide that binds to nucleic acids and permeabilizes bilayers. Biochemistry. 1997;36:3008–3017. doi: 10.1021/bi9618474. [DOI] [PubMed] [Google Scholar]
- 25.Crombez L, Aldrian-Herrada G, Konate K, Nguyen QN, McMaster GK, Brasseur R, Heitz F, et al. A new potent secondary amphipathic cell-penetrating peptide for siRNA delivery into mammalian cells. Mol Ther. 2009;17:95–103. doi: 10.1038/mt.2008.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mok H, Park TG. Self-crosslinked and reducible fusogenic peptides for intracellular delivery of siRNA. Biopolymers. 2007;89:881–888. doi: 10.1002/bip.21032. [DOI] [PubMed] [Google Scholar]
- 27.Lee SH, Kim SH, Park TG. Intracellular siRNA delivery system using polyelectrolyte complex micelles prepared from VEGF siRNA-PEG conjugate and cationic fusogenic peptide. Biochem Biophys Res Commun. 2007;357:511–516. doi: 10.1016/j.bbrc.2007.03.185. [DOI] [PubMed] [Google Scholar]