Abstract
Background:
Cartilage tissue engineering (CTE) aims to obtain a structure mimicking native cartilage tissue through the combination of relevant cells, three-dimensional scaffolds, and extraneous signals. Implantation of ‘matured’ constructs is thus expected to provide solution for treating large injury of articular cartilage. Type I collagen is widely used as scaffolds for CTE products undergoing clinical trial, owing to its ubiquitous biocompatibility and vast clinical approval. However, the long-term performance of pure type I collagen scaffolds would suffer from its limited chondrogenic capacity and inferior mechanical properties. This paper aims to provide insights necessary for advancing type I collagen scaffolds in the CTE applications.
Methods:
Initially, the interactions of type I/II collagen with CTE-relevant cells [i.e., articular chondrocytes (ACs) and mesenchymal stem cells (MSCs)] are discussed. Next, the physical features and chemical composition of the scaffolds crucial to support chondrogenic activities of AC and MSC are highlighted. Attempts to optimize the collagen scaffolds by blending with natural/synthetic polymers are described. Hybrid strategy in which collagen and structural polymers are combined in non-blending manner is detailed.
Results:
Type I collagen is sufficient to support cellular activities of ACs and MSCs; however it shows limited chondrogenic performance than type II collagen. Nonetheless, type I collagen is the clinically feasible option since type II collagen shows arthritogenic potency. Physical features of scaffolds such as internal structure, pore size, stiffness, etc. are shown to be crucial in influencing the differentiation fate and secreting extracellular matrixes from ACs and MSCs. Collagen can be blended with native or synthetic polymer to improve the mechanical and bioactivities of final composites. However, the versatility of blending strategy is limited due to denaturation of type I collagen at harsh processing condition. Hybrid strategy is successful in maximizing bioactivity of collagen scaffolds and mechanical robustness of structural polymer.
Conclusion:
Considering the previous improvements of physical and compositional properties of collagen scaffolds and recent manufacturing developments of structural polymer, it is concluded that hybrid strategy is a promising approach to advance further collagen-based scaffolds in CTE.
Keywords: Cartilage tissue engineering, Type I collagen, Articular chondrocytes, Mesenchymal stem cells, Hybrid scaffolds
Introduction to articular cartilage tissue engineering
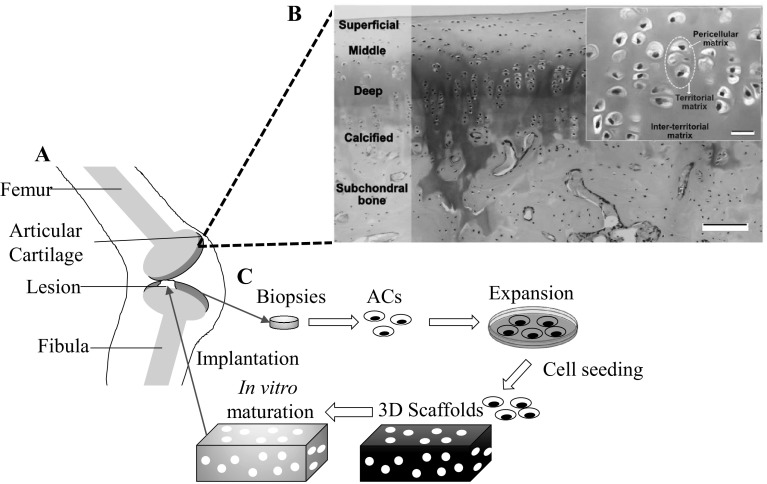
Articular cartilage is an avascular tissue mainly function to allow frictionless movement between articulated bones. Due to the mechanical loads, extracellular matrices (ECMs) of articular cartilage are highly organized and maintained in homeostatic state by the residing articular chondrocytes (ACs) (Fig. 1A, B). However, the avascular nature of articular cartilage leads to the poor self-healing capability [1, 2]. Healing process is also impaired as progenitor cells gain no access to the injury site [3]. Host ACs near lesions are burdened with simultaneous tasks to replace the dead cells and rebuild the secreted matrices at the same time [4]. Spontaneous healing is generally absence for chondral lesions beyond critical size (≥ 3 cm2) and thus surgical interventions are necessary [5].
Fig. 1.
Illustration of articular cartilage tissues and concept of cartilage tissue engineering. A Schematic representation of articular cartilage with a lesion in knee. B Articular cartilage consists of multiple zones and is separated with subchondral bone by calcified zones. Some areas are stained with red Safranin O/Fast staining, indicating presence of proteoglycan. Inset shown AC (dotted circle) embedded in cartilage matrix. Scale bar: 20 μm. C Flow of cartilage tissue engineering started with biopsy of cartilage tissue and ended with implantation of mature scaffolds into lesions.
(Parts of figure B adapted with permission from Armiento et al. [6])
Several medical interventions are available for treating large chondral lesions: microfracture (MF), osteochondral transplant (OCT), and autologous chondrocytes implantation (ACI) [3, 5, 6]. MF relies on the innate healing capability of patients to repair the injury. In this procedure, hole is drilled near lesions area to penetrate subchondral bone and to allow influx of stem cells and/or growth factors to the defect site. However, MF was inconsistent in clinical results and inappropriate for treating elder patients [5]. In contrast, OCT and ACI essentially exploit healthy cartilage tissue or ACs for treating chondral lesions. Despite relatively good initial clinical results, several complications are associated with these treatments, such as donor site morbidity, graft hypertrophy, and inconsistent repair tissue [5, 6].
To overcome these limitations, a concept of cartilage tissue engineering (CTE) is introduced (Fig. 1C) [6]. CTE was defined as the attempt to reconstitute damaged cartilages by combining relevant cells, three-dimensional (3-D) scaffolds and extraneous signals in a harmonious manner [3, 6]. Before implantation, CTE constructs can be matured partially or fully in vitro to achieve similar level of biophysical and biochemical properties with native cartilage tissue. The obtained CTE products are expected to eliminate the necessity of using whole-tissue transplant [3, 7].
There are two relevant cell types in regards to CTE: ACs and mesenchymal stem cells (MSCs), each associated with potencies and challenges. ACs are the preferable option for treating chondral defects because their molecular profile is similar with surrounding host cells [8]. As the adult cells, ACs are also actively secreting cartilage-related ECMs. Prevalent usage of ACs suffers from their scarce availability. The cell number of isolated AC per biopsy of articular cartilage (5 mm × 10 mm of full-thickness area) is around 200,000–300,000 cells [8]. The numbers are relatively small compared with the required cells per cm2 of lesion (0.5–5 million cells/cm2) [6, 9]. Thus, it is common to expand cells for several passages before seeding into 3-D scaffolds. Nonetheless, ACs gradually lose their chondrogenic characteristics with passage numbers (de-differentiation), effectively limiting the cell number of feasible passages [10]. In contrast, MSCs serve as the more abundant alternative of ACs, owing to their numerous extraction sites (e.g., bone marrow [11], adipose tissues [12]).
To be useful in CTE, it is corollary to differentiate MSCs in the chondrogenic lineage through culture in inducing medium. Yet, MSCs apparently possessed variety of chondrogenic capacity dependent on its extraction sources [13]. MSCs might also undergo hypertrophic differentiation that will be deemed detrimental for cartilage regeneration. Important markers of ACs and MSCs corresponding to CTE are listed in Table 1.
Table 1.
Important markers of ACs and MSCs in regards to the chondrogenic performance
| Cells | Markers |
|---|---|
| ACs | Differentiated markers |
| 1. Cellular rounded morphology [14, 15], high proteoglycan synthesize rate [14, 16] | |
| 2. Gene upregulation of Sox9 [17], Col2A1 [18], aggrecan (ACAN) [19], and cartilage oligomeric protein (COMP) [20] | |
| 3. Protein high secretion of type II collagen (Type II/I collagen ratio) [21, 22] and sulfated glycosaminoglycan (sGAG) [14, 23], high expression of integrin α10β1 [24, 25], diffused actin organization [26, 27] | |
| De-differentiated markers | |
| 1. Cellular flattened morphology [14, 15], high proliferation rate [18, 28] | |
| 2. Gene upregulation of Col1A2, Col2A1 [29] | |
| 3. Protein organized actin filament [26, 27] | |
| MSCs | Chondrogenic markers |
| 1. Gene expression of Sox9, Col2A1, ACAN, and COMP [30–32] | |
| 2. Protein secretion of type II collagen (Type II/I collagen ratio) [33, 34] and sGAG [35, 36] | |
| Hypertrophic markers | |
| Transcription and synthesize of type X collagen [37, 38], confirmed with analysis of osteogenic marker (e.g., type I collagen, alkaline phosphatase (ALP), mineral deposition) [34, 39] |
Type I collagen is the crucial material commonly used as scaffolds for CTE products in clinical market [7]. Preference of type I collagen in CTE is largely attributed to its general biocompatibility and safety approvals granted by various agencies (e.g. Food and Drug Agency, Pharmaceuticals and Medical Devices Agency). Short- and mid-term outcomes of the collagen-based CTE generally showed improvement in clinical and histological outcomes compared with the control group (MF) (Table 2). Nonetheless, long-term performance of pure type I collagen may be compromised by significant shrinkage [19], weak mechanical property [40], and limited chondrogenic capacity [23]. Furthermore, recent investigation revealed the continuing de-differentiation of chondrocytes in collagen-based CTE constructs even after implantation [41], suggesting the necessity for future development.
Table 2.
Clinical trials of CTE products employing type I collagen scaffolds
| Product name | Markers | Main results | References |
|---|---|---|---|
| NOVOCART®3D | Type I collagen sponges with bilayer structure | (n = 28) Significant improvement of clinical results after 24 months follow up compared to baseline, based on subjective scoring system: IKDCa (36.0 ± 15.0 ) and Noyes sport ratinga (38.9 ± 31.0) MOCARTb showed low score up to 6 months (60.3 ± 17.4), but it exhibited development of cartilage repair after 24 months (73.2 ± 12.4) | [42] |
| MACI® | Membrane of type I/III collagen‡ | (n = 144) MACI® showed more effective cartilage healing than microfracture (MF) based on KOOSa scoring system of pain (MACI®: 82.5 ± 16.2 vs MF 70.9 ± 24.2) and function (MACI®: 60.9 ± 27.8 vs MF: 48.7 ± 30.3) | [43] |
| CaReS® | Type I collagen gel‡ | (n = 17) All three scoring systems of clinical outcome showed significant improvement of CaReS® treated patient from baseline IKDCa (31.7 ± 12.7 to 61.3 ± 18.2), Lysholma (43.6 ± 11.4 to 64.7 ± 17.1), Cincinnatia (31.5 ± 13.1 to 56.2 ± 13.9)). No significant differences (IKDCa) were observed between CaReS® (61.3 ± 18.2) and MF groups (50.1 ± 24.9) | [44] |
| NeoCart® | Type I collagen gel loaded into sponges of same material‡ | (n = 49) Pain scoring system of KOOSa showed the significant improvement for NeoCart®-treated group compared to baseline at six, twelve, and twenty-four months. The improvement from baseline is greater for NeoCart®-treated group than MF-treated group | [45] |
| Transferred to Japan Tissue Engineering Co., Ltd. with brand name JACC® | Type I atelocollagen gel | (n = 28) Lysholm scoresa showed significant improvement from the baseline over 25 months. Arthroscopic evaluation revealed 26 of 28 knees (93%) were graded as good or excellent. Biomechanical test revealed that transplants had similar stiffness with nearby cartilage | [46] |
‡Controlled with MF
aClinical evaluation: KOOS, Knee injury and Osteoarthritis Outcome Score; IKDC, International Knee Documentation Committee; Lysholm; Cincinnati; Noyes sport rating
bCartilage evaluation: MOCART®, Modified magnetic resonance observation of cartilage repair tissue
Considering the aforementioned works, type I collagen serves as an essential platform for next generation of CTE scaffolds. This paper aims to give insights necessary for advancing further type I collagen-based scaffolds, especially in the context of CTE. Firstly, the interaction of type I/II collagen with ACs and MSCs is discussed. Secondly, physical features of scaffolds necessary to optimize chondrogenic activities of ACs and MSCs are highlighted. Thirdly, studies aspiring to improve collagen matrices through blending with other materials are discussed. Lastly, concept of hybrid scaffolds as the next promising strategy for CTE is detailed.
Interaction of extracellular matrix and cells
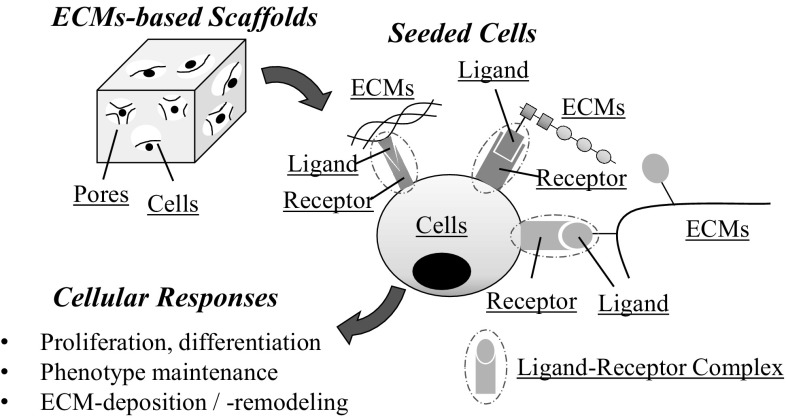
Unlike synthetic materials, ECMs-based scaffolds allow direct attachment of cells, owing to the presence of unique ligand (e.g. amino acid sequences) capable of binding the specific cell receptor (Fig. 2). The activated cell receptor triggers intracellular signaling pathway responsible for a distinct cellular response (e.g., active proliferation, phenotype maintenance) [47–49]. As tissue engineering mainly depends on the particular cell activities (e.g. matrix deposition or remodeling), prudent choice of ECMs substrates is essential to draw out desirable cellular responses. The following sections mainly focus on studies investigating interaction of type I/II collagen with AC/MSC and the relevant cellular responses in regards of cartilage tissue engineering.
Fig. 2.
Schematic illustration of the attachment of cells to ECMs-based scaffolds. Cells attach to the ECMs molecules by formation of ligand-receptor complex. Cells express various receptors capable of recognizing various type of ligand presented by ECMs molecules. Activated cell receptor subsequently modify various responses of cells (proliferation, ECMs deposition)
Interaction of articular chondrocytes (ACs) with type I/II collagen
Type I collagen is a fibril forming protein mainly distributed as the major component in mammalian flesh and connective tissues (e.g., bone, skin, or scar tissue) [45, 50]. The collagen is a heterotrimeric molecule consisting of two α1 chains and one α2 chain [51]. The former chain is encoded by COL1A1 gene, while the latter chain is encoded by COL1A2 gene [51]. Adhesion of type I collagen and ACs is mediated by integrin: strongly by α2β1 integrin and weakly by α10β1 integrin [52, 53]. Type I collagen possess multiple binding sites, such as GFOGER and GROGER (amino acid sequences with single letter amino acid nomenclature), capable to be recognized by α2 I and α10 I domains of integrin [52, 53], respectively.
Type I collagen has benefit of promoting the cell proliferation of ACs without significantly compromising their chondrogenic traits [17, 54]. Kino-Oka et al. [54] investigated ACs on the different substrates [uncoated monolayer polystyrene (PS) and type I collagen-coated PS substrates (CL)]. ACs cultured both on PS and CL groups showed similar level of population doubling for a period of 18 days. Yet, chondrocytes in CL group retained higher fraction of rounded cells compared with majority of flattened chondrocytes in the PS group. To assess the preservation of chondrogenic phenotype, passaged cells were further transferred into collagen gel (3-D environment). ACs of CL group significantly secreted glycosaminoglycans (GAG) and type II collagen in contrast with ACs of PS group, demonstrating the capacity of type I collagen in suppressing de-differentiation of chondrocytes. Nonetheless, type I collagen could not fully stabilize ACs for a long-term culture. The loss of chondrogenic phenotype (altering of COL2A1 and COL1A2 expression) was found after 1000-fold expansion (~ 35 to 50 days) in type I collagen gels [18]. ACs only regained its capability on producing type II collagen after application of re-differentiating factors (insulin and bone morphogenic protein-2) [18].
The unnatural existence of type I collagen in articular cartilage raises concern whether it could destabilize the differentiated phenotype of chondrocytes. It was described that chondrocytes greatly destabilized when it was seeded in type I collagen gels of high concentration (1 g/ml) and slightly destabilized in the gels at lower concentration (10 mg/ml) [55]. The negative impact of type I collagen can be explained by extensive contraction of collagen gel at low concentration, which in turn would encourage the condensation of chondrocytes [56]. It requires experiments capable of decoupling the concentration and shrinkage effects to elucidate whether type I collagen would be detrimental for differentiated phenotype of chondrocytes.
Type II collagen is a major component of articular cartilage. Helical part of the collagen is homotrimer consisting of three α1(II) chains encoded by COL2A1 gene [51]. Due to its native nature, type II collagen has been expected to provide signaling cues necessary for ACs to retain its differentiated morphology and related secretion activities.
ACs possess several receptors capable of recognizing type II collagen: (1) integrin receptor (α10β1 and α2β1) and (2) non-integrin receptor (human discoidin domain receptor-2 (DDR-2) or annexin V) [22, 54, 55]. β1-integrins mainly bind to the D-4 segment of type II collagen [57]. Removal of D-4 section significantly reduced attachment of ACs onto type II collagen and diminished their ability to migrate to the inner part of 3-D scaffolds [57]. Annexin-V and DDR-2 largely adhered to N-propeptide [58] and D-2 segment of type II collagen [59], respectively.
ACs may elicit different responses dependent on the type of activated cellular receptors. The α10β1 is the newly discovered integrin indicated to play important roles in proper cartilage development [60]. In the knock-out study, mice lacking α10β1 integrin showed abnormal chondrocytes (increased apoptosis, reduced proliferation, change of shapes) and imperfect cartilage tissue [60]. However, it is currently unknown whether cell–matrix interaction involving α10β1 integrin is beneficial to stabilize phenotype of ACs. Binding of annexin-V and type II collagen was shown to increase uptake of Ca2+ by chondrocyte, suggesting its roles in regulating endochondral ossification [61]. Interaction of DDR-2 and monomeric type II collagen apparently induce ACs to upregulate the expression of cartilage-degrading enzymes (e.g. matrix metallopeptidase (MMP-13, MMP-14) and pro-inflammatory cytokines (e.g. interleukin (IL)-1β, IL-6), suggesting signaling role of type II collagen in physiological cartilage turnover process [62, 63].
Shakibaei et al. [15] showed that ACs quickly adopt fibroblastic morphology over 5 days of monolayer culture on the plastic substrate, whereas it retained round morphology for more than 2 weeks culture on type II collagen coated substrate. Furthermore, type II collagen-based scaffolds apparently induced favorable chondrocyte phenotype in 3-D culture, on the basis of higher deposition of type II collagen, GAG, larger fraction of spherical chondrocytes, and higher expression of GAG/DNA ratio, compared with its type I collagen counterpart [13, 15, 27, 64]. Nevertheless, conflicting results were reported in which chondrocytes were indifferent when cultured on monolayer or 3-D scaffold of type I/II collagen [23, 65, 66]. Such inconsistencies could originate from the experimental variabilities (interspecies difference, culture procedure, etc.) [67] or it might indicate that complete interactions between pure type II collagen and ACs requires additional factors [68]. It is interesting to note that previous studies demonstrating the superior chondrogenic performance of type II collagen were conducted by adding chondroitin sulfate (CS) into the final matrices, resulting in scaffolds with composition approaching native cartilage tissue [13, 22].
Interaction of mesenchymal stem cells (MSCs) with type I/II collagen
Either type I or II collagen can support the attachment, proliferation and chondrogenic differentiation of MSCs [33, 69], whereas head-to-head studies revealed that type II collagen matrices grant more extensive chondrogenic differentiation of MSCs on the basis of higher secretion of type II collagen, proteoglycan and larger production ratio of GAG/DNA compared with its type I counterpart [29, 33, 70]. Nevertheless, the chondrogenic stimulation of type II collagen for MSCs was absence at the gene level, meaning no significant differences of chondrogenic RNA expression (Sox9, COMP, COL1, COL2, COL10) for a period of 21 days in type I and II collagen gels [30]. Other studies confirmed the similar trend in which upregulation of chondrogenic markers were prominent at protein level, yet insignificant at gene level [33, 70]. The discrepancy between gene and protein production is frequently occurred in in vitro [71] or some of the early upregulated genes, such as Sox9 and Runx2, are returned to the basal level at later time, thus giving no difference at late stage analysis [34]. Chondro-inductive properties of type II collagen would originate through β1 integrin-mediated Rho A/Rock signaling, which in turn promote the morphological change of MSCs to round shapes [16, 35, 45]. Type II collagen could promote the chondrogenesis by aggregation of MSCs; however, denaturated type II collagen never showed such aggregation, suggesting the importance of maintaining native helical structure of type II collagen [36].
In the same manner with ACs, type II collagen apparently requires additional factors to unlock the chondrogenic potency. Lu et al. compared MSCs cultured in type I and II collagen gels in the absence and presence of nucleus pulposus cells (NPCs). No difference of chondrogenic markers were observed for MSCs cultured in these gels when NPCs were absence [72]. Strikingly, co-culturing with NPCs caused MSCs grown in type II collagen to significantly upregulate the chondrogenic markers over MSCs of type I collagen group. The selective improvements may originate from soluble factors released by NPCs. These factors were assumed to work specifically with type II collagen to promote chondrogenic differentiation [72].
In summary, type II collagen is a promising CTE scaffold alternative of type I collagen (Table 3). However, type II collagen is a potential arthritogenic agent [60, 61] and has not been widely approved by various health agencies, thus limiting its usage in clinical setting [40].
Table 3.
Advantages and disadvantages of type I and II collagen in CTE
| Type I collagen | Type II collagen | |
|---|---|---|
| Advantages | Promotion of proliferation of chondrocytes [18] | Stabilization of the morphology of AC [23] |
| Wide safety approvals [3, 40] | Support of chondrogenic differentiation of MSCs [72] | |
| Disadvantages | Unable to support the chondrogenic performance of ACs and MSCs [55, 72] | Potentially arthritogenic [62, 63] |
| Limited safety approvals [40] |
Influence of physical features of collagen scaffolds to chondrogenic activities of ACs and MSCs
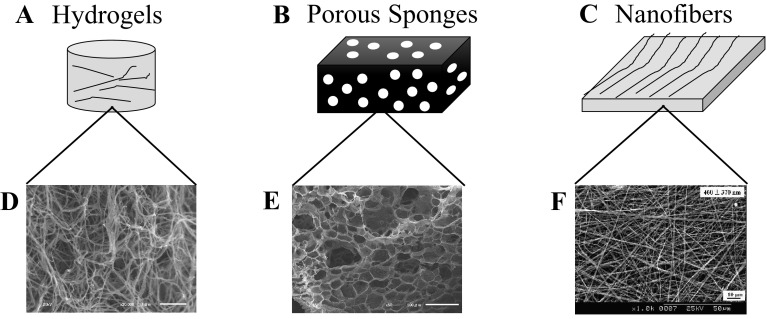
3-D collagen scaffolds are fabricated in three forms, each possessing unique internal structure: (1) hydrogels, (2) porous sponges, and (3) nanofibers (Fig. 3A, C, E) [32, 50, 73–78]. Hydrogels include loose bundle of collagen fibers forming the branched three-dimensional network [50, 74, 75]. Porous sponges consist of membrane-like wall and microscopic pores [32, 73], and nanofibers have the nonwoven network of collagen fibers with tenth to hundredth nanometer in diameter, mimicking structural protein fibers in the human body [76–78].
Fig. 3.
Schematic illustration of macroscopic and internal structures of collagen A, B hydrogels, C, D sponges, and E, F nanofibers.
(F was reprinted with permission from Yeo et al. [123]. Copyright (2008) American Chemical Society)
Hydrogels and porous sponges are fabricated by a common initial route (fibrillogenesis), in which pH of collagen solution is neutralized and followed with incubation below the denaturation temperature [32, 45, 73, 79]. Collagen molecules are self-assembled as nano-sized fibrils in a staggered arrangement [51]. Since collagen possesses abundant water-binding amino acids [50, 80], collagen attracts a lot of water molecules and subsequently formed hydrogels [80, 81]. Porous sponges is obtained by freeze-drying the hydrogels or directly freeze-drying collagen solution [73, 79]. Microstructural observations of both hydrogel and porous sponges generally require freeze-drying as sample preparation step, therefore it is common to dehydrate the hydrogels with ethanol series and t-butanol to preserve the fibrillar structure (Fig. 3B, D). On the other hand, nanofibers of collagen are obtained through the electrospinning process [45, 77, 78]; collagen solution was ejected through a spinning nozzle towards a metal collector by applying high voltage between them and the stable jets of collagen gradually accumulated on the collector to form the nonwoven nanofibers (Fig. 3F) [40].
Several physical features of the scaffolds are controlled by modifying fabrication parameters [32, 77, 82], such as collagen concentration to change diameters of fibers [77], or freezing temperature to change pore sizes in the hydrogels of the porous sponges [32, 82]. These scaffolds might be subjected to various types of crosslinking in order to impart sufficient stiffness and robustness for the future handling [83, 84].
The internal structures of the scaffolds are of great importance in designing ideal scaffolds because they influence behavior of cell by presenting cells with unique microenvironment and distinct bulk properties (e.g., surface area, pore interconnectivity, gas or fluid permeability) [74–76, 85, 86]. ACs, environmentally sensitive cells, would be easily affected by the internal structures because it would lose or regain their differentiated phenotype depends on two dimensional, two-dimensional (2-D), (flattened morphology) or 3-D, (rounded morphology) culturing system [10].
Hydrogels are more superior to porous sponges in supporting differentiated traits of ACs. The wall in porous sponges would be considered as flat area for ACs, while the network structure forced the embedded ACs to assume round shape [74, 87]. In contrast, porous sponges were superior to hydrogels in promoting the viability and chondrogenic differentiation of MSCs [85]. It was argued that hydrogels lacked proper diffusion of nutrient which was vital for sustaining activities of MSCs [85]. On the other hands, fiber diameters in nanofibers dictated whether ACs attached in the flattened or rounded morphologies, which in turn determined the secretion amounts of GAG and cartilage-specific ECMs molecules (i.e., type II and IX collagen, aggrecan, and link protein) [21]. Aligned nanofibers were also shown to be beneficial in promoting the chondrogenic differentiation of MSCs as it caused the alignment of MSC cytoskeleton reminiscent of matured articular ACs [76, 88].
In addition to the internal structures, other physical aspects such as stiffness and pore features (e.g., pore size, porosity, interconnectivity) are of importance for cell fates. Adult stem cells have been found to sense stiffness of their surrounding substrates and to adjust their morphologies and activities accordingly (mechano-transduction) [12, 89]. On the other hands, well-designed pore features are basic requirements of the scaffolds [90]. The optimum state for these two aspects are rewarding in designing scaffolds intended for CTE. However, as each form of scaffolds shows different internal structures, it is not feasible to directly compare one scaffold and the others.
In the next sections, we addressed the effects of stiffness on three forms of the scaffolds to the differentiated phenotype and secretion activity of ACs and MSCs. In the same manner, we also focused on pore size effect of scaffolds and discussed its importance to ACs and MSCs.
Influence of matrices stiffness to differentiated phenotype of ACs
Stiffness—intrinsic resistance of materials to deformation—is an important environmental cue to control cellular activities [89]. It is common practice to express stiffness (elasticity) of elastic or viscoelastic scaffolds with Young’s modulus and shear modulus [91]. Generally, anchorage-dependent cells exert contraction forces onto substrates and respond to the stiffness of the substrates by adjusting its adhesion strength and cytoskeletal formation [89]. Adjustment of binding state subsequently affects the cellular activities, such as proliferation, differentiation, and secretion activities [89].
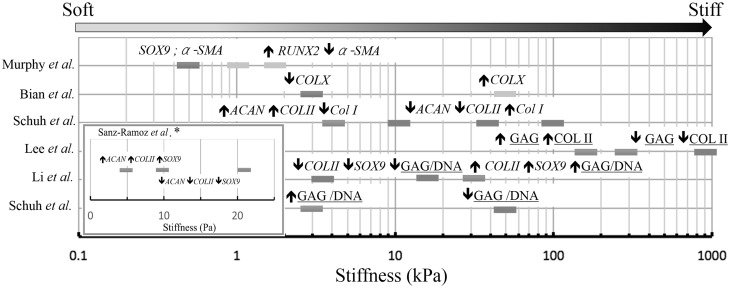
Since the freshly isolated ACs had stiffness of 0.7–4 kPa, scaffolds with the stiffness at these values were expected to provide correct cues for ACs [25, 92, 93]. Schuh et al. [26] cultured monolayer ACs on polyacrylamide with different stiffness (~ 4, ~ 10, ~ 40, ~ 100 kPa) for 7 days; only ACs on the gel at ~ 4 kPa retained the differentiated phenotype as evidenced by the diffused organization of actin, round morphology, and significantly higher expression of type II collagen and aggrecan, and lower expression of type I collagen [26]. ACs seeded on the gel at ~ 4 kPa also showed the lowest cell number, indicating chondrocyte commitment to the secretion activities and not to proliferation [26]. Based on tensegrity hypothesis, it is assumed that ACs adjust stiffness to match the substrates and subsequently alter physiological and differentiated phenotype [26].
Collagen sponges with low initial stiffness would be beneficial in secretion activity of cartilage-related molecules [83, 84]. Lee et al. [83] obtained collagen sponges with different stiffness (145, 346, 369, and 1117 Pa) by crosslinking with dehydrothermal (DHT), ultraviolet (UV), glutaraldehyde (GTA), and carbodiimide (EDC). They described that only soft scaffolds (i.e., DHT and UV crosslinked) exhibited positive staining for type II collagen [83]. Vickers et al. [84] also investigated that collagen sponges crosslinked with DHT exhibited intense staining for proteoglycan and type II collagen compared with sponges heavily crosslinked with EDC. Such favorable results are mainly attributed to the acute contraction of scaffolds with low stiffness (reduced down to ~ 50% of original diameter) after several times of culture [84]. At such situation, cell–cell interactions are largely promoted and in turn have benefit of the phenotype maintenance. Interestingly, stiffness in 3-D environment seems to affect ACs in the indirect manner, in contrast with ACs in 2-D environment.
In the case of hydrogels, effect of stiffness is much subtle, because different studies reported conflicting optimal stiffness values. Sanz-Ramos et al. [94] showed that collagen hydrogels with elastic modulus of ~ 5 Pa were superior in enhancing expression of aggrecan, type II collagen, and Sox9 than stiffer hydrogels (~ 10 and ~ 20 Pa). On the other hand, Li et al. [27] described that stiff polyacrylamide gel (29.9 kPa), not the softer gels (3.8 or 17.1 kPa), promoted the redifferentiation of ACs and subsequent secretion activities. Schuh et al. [95] investigated ACs cultured in two agarose gels with different stiffness ~ 3.7 (soft) and ~ 53 (stiff) kPa) for 2 weeks. They discovered indifferent results for type II and I collagen staining for both of soft and stiff gels, although higher ratio of GAG/DNA and greater cell number were observed for the soft gel [95]. Further analysis revealed that the soft gel significantly absorb bovine serum albumin (BSA), implying the favorable results due to better nutrient permeability [95]. In addition, RGD-binding motif sometimes caused complicated results as it effectively induced dedifferentiation of ACs both in soft and stiff gels [95]. To reduce the perplexity of such issues in hydrogel system, it would be useful to use natural protein-based matrices (collagen) and chemically defined medium.
Skotak et al. [96] investigated ACs cultured on gelatin nanofibers with different stiffness, crosslinked by glutaraldehyde of various concentrations (0–5 wt%). They found that stiffer nanofibers supported higher cell density and greater ratio of type II/type I collagen expression compared to softer substrates [96]. Nonetheless, such improvement could be attributed to the improved structural stability imparted by extensive crosslinking, since easily degraded nanofibrous caused negative effect for ACs [97].
Influence of matrices stiffness to chondrogenic differentiation of MSCs
Stiffness of monolayer substrate affects the differentiation lineage of MSCs at initial stage. Park et al. [98] investigated MSCs on the relatively thick collagen gel (soft substrate) and thinly collagen-coated dish (stiff substrate). MSCs on soft substrate exhibited lower expression of smooth muscle markers (α-actin) and significantly higher type II collagen compared with MSCs seeded on stiff substrate [98]. Subsequent addition of transforming growth factor beta (TGF-β) significantly promoted either α-actin or type II collagen dependent on whether MSCs were seeded on stiff or soft substrates, indicating the role of stiffness in diverging the lineage specification of MSCs [98].
Chondro-inductivity of soft substrate was attributed to the weaker cellular adhesions, which in turn prevent the extensive formation of stress fiber (α-actin) [98]. Diffused organization of α-actin has long been associated with secretion of type II collagen and proteoglycan [99]. Nevertheless, when MSC adhesion was largely inhibited, MSCs failed to differentiate in the chondrogenic pathway despite being cultured in chondro-inductive medium [100]. Such result demonstrates the importance of proper cellular attachment [100].
Benefit of low stiffness in promoting initial stage of chondrogenic differentiation for MSCs is also observed in 3-D environment. Murphy et al. discovered that MSCs cultured in the non-differentiating medium in the soft collagen-based sponge (compressive modulus of 0.5 kPa) promoted upregulation of Sox9 (early chondrogenic marker), while stiff collagen-based sponges (1 and 1.5 kPa) favored upregulation of Runx2 (early osteogenic marker) by MSCs [31]. However, no significant differences were observed for any types of scaffolds for terminal differentiation makers [i.e., Collagen I, Collagen II, and ALP (alkaline phosphatase)], suggesting the necessity of differentiating-medium for lineage commitment [31]. Preference of MSCs for chondrogenic or osteogenic differentiation was associated with expression of α-smooth muscle actin (SMA) which was linearly dependent with compressive modulus [31].
Other studies involving hydrogels and nanofibers also confirmed the advantages of low stiffness in favoring chondrogenic differentiation [101, 102]. During cell culture, soft hydrogels undergo extensive dimensional contraction, which in turn promote cellular condensation and the subsequent chondrogenic differentiation of MSCs [103]. Soft nanofibers may provide ‘dynamic environment’, in which flexible fibers are easily deformed by the cultured cells. Pliable fibers prevent MSCs to develop stress fibers, thus favoring chondrogenic differentiation of MSCs.
Hypertrophic differentiation of MSCs is apparently suppressed in low stiffness matrices [36, 104]. Bian et al. showed that soft hydrogels (~ 3 kPa) exhibited significantly lower expression of type X collagen (hypertrophic marker) than stiffer hydrogels (~ 50 kPa). However, such results were not assigned to mechanotransduction since no blocking of ROCK (Rho-associated protein kinase) and myosin II suppressed matrix calcification at stiffer hydrogels [37]. It was argued that softer hydrogels retain more cartilage-related ECMs (i.e., proteoglycan and type II collagen) and these ECMs may curb the hypertrophic differentiation and matrix calcification [104]. Effect of stiffness on ACs and MSCs are summarized in Fig. 4.
Fig. 4.
Effect of substrate stiffness (colored boxes) on gene transcription (italic name; Sox9, α-SMA, COL II, Col I, Col X, ACAN) and protein synthesize (underlined name; GAG/DNA, COLII) of ACs (blue and red colored) and MSCs (green and yellow colored). Blue and red boxes respectively indicate positive and negative changes of differentiated phenotype of ACs, as reported by Sanz-Ramoz et al. [94], Schuh et al. [26, 95], Li et al. [27] and Lee et al. [83]. In a similar manner, green and yellow boxes are associated with changes of chondrogenic markers of MSCs as reported by Bian et al. [37] and Murphy et al. [31]. (Color figure online)
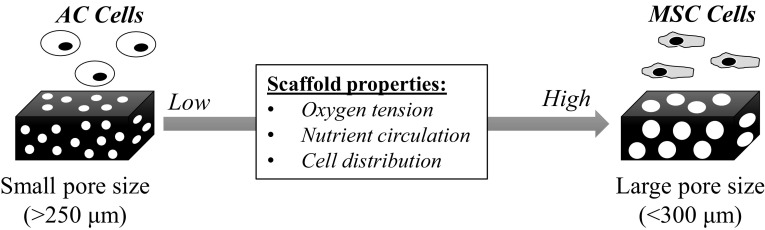
Influence of pore sizes to differentiated phenotype of ACs
Pore sizes smaller than 225 μm in collagen sponges are generally beneficial to support ACs to retain the differentiated phenotype and to promote the biosynthetic activities of cartilage-related ECMs [22, 105]. Nehrer et al. [23] demonstrated that major fraction of ACs retained the rounded morphology in collagen sponge with small pore size at 20 μm compared with large one at 80 μm. Significantly higher ratio of GAG/DNA was also observed for ACs in sponges with smaller pore size at 20 μm than those with 80 μm (GAG/DNA: 11.08–4.03) [23]. Nonetheless, it was argued that scaffolds with pore size in the range of tenth micrometer (13–85 μm) limited cellular migration and medium diffusion, which caused formation of shallow depth of cartilaginous film and might be detrimental for future clinical application [106].
To overcome these issues, Zhang et al. [105] investigated pore sizes of collagen sponges in the larger range (150–250, 250–355, 355–425, and 425–500 μm). Initially, they cultured ACs in the scaffold in vitro for 1 week followed by in vivo implantation for 8 weeks [105]. Cells were homogenously distributed for all scaffolds; however, only ACs seeded in the scaffold with 150–250 μm of pore size showed improvement of differentiated expression of COL2A1 and ACAN, and the higher ratio of sGAG (sulfated GAG)/DNA, compared with ACs in other scaffolds [105]. Other studies employing porous sponges of non-collagenous origin also exhibited the advantages of scaffolds with relatively small pore sizes in supporting the secretion activities of ACs [106, 107]. Small pore size was speculated to be easier to be filled with cells, which would thus promote cell–cell interactions for maintenance of chondrocyte phenotype [22, 105, 107]. Pore size might also indirectly affect ACs by altering stiffness of scaffolds [105]. Other factors such as pore interconnectivity and material composition also significantly affected the chondrocyte behaviors [86]; thus prudence should be necessary in interpreting the pore size effect.
In collagen hydrogels, pores or meshes are defined to be spaces between entangled fibers, and the sizes can be enlarged from 2 to 12 μm by increasing collagen concentration or gelation temperature [82]. Effect of mesh size to chondrogenic phenotype of ACs is mainly investigated in the system of synthetic polymer gels (i.e., poly(ethylene glycol) (PEG) or PEG-based polymer), as their mesh size can be finely tuned by modifying the molecular weight of precursor or crosslinker concentration during polymerization [108, 109]. It was found that the mesh size around 70–100 Å could be optimal to enhance the deposition of type II collagen and aggrecan, and to promote accumulation and distribution of secreted proteoglycan [108, 109].
Mesh sizes rarely influence the distribution of cells in hydrogels because cells are usually encapsulated in the hydrogels by mixing with the initial solution [31, 50, 74]; however, smaller mesh sizes significantly inhibited cell migration and circulation of nutrients [50, 82], which in turn might impede the interaction with host tissue after implantation. Studies involving collagen nanofibers rarely detailed the effect of pore size to activities of ACs, however it was indicated that ACs readily penetrated collagen nanofibers of various pore sizes [77, 78].
Influence of pore sizes to chondrogenic differentiation of MSCs
Pore sizes larger than 300 μm are advantageous to support the chondrogenic differentiation of MSCs and their subsequent production of cartilage-related matrices. Matsiko et al. [33] investigated chondrogenic differentiation of MSCs in collagen-based sponges at different pore sizes (94, 130, and 300 μm) with relatively similar elastic modulus. The scaffold with the largest pore size at 300 μm was superior in supporting the chondrogenic differentiation of MSCs compared with the others on the basis of significantly higher expression of COL2, SOX9 and lower expression of COL1 for 28 days [33]. Moreover, larger amounts of secreted GAG and the gradual increase of Young’s modulus were observed for the scaffolds with the pore size at 300 μm, indicating further improvement in biosynthetic activities of cartilage-related ECMs [33]. Such favorable results of sponges with large pore sizes (> 300 μm) were also confirmed by other studies which employed non-collagenous materials [32, 110–112]. Expression of type X collagen was suppressed with the increasing pore size probably due to the improved chondrogenesis [111]. Chondrogenic differentiation of MSCs was strongly inhibited in hypoxia condition [113]; low oxygen tension in small pore sized scaffolds may thus hamper the chondrogenic differentiation of seeded-MSCs [111]. This is interesting because the trend of optimum size for collagen-based sponges is completely reversed for ACs and MSCs.
Unlike sponges, it is difficult to isolate the pore size effect in hydrogels and nanofibers because it is not possible to alter their pore size without grossly affecting other related properties such as stiffness or fiber size [114–116]. Study involving PEG-based hydrogels indicated that pore size effect would not be as influential as hydrogel compositions in affecting the chondrogenic differentiation [114]. On the other hand, nanofibers mat with larger pore size and fiber diameter apparently supported the change of chondrogenic differentiation of MSCs possibly due to cell penetration and cell–cell interactions [115, 116]. The effect of pore size on ACs and MSCs are summarized in Fig. 5.
Fig. 5.
Schematic illustration of change of scaffold properties with evolution of pore or mesh sizes. Small pore sized scaffolds support the differentiated phenotype of ACs due to non-even cell distribution (induce cell aggregation) and low oxygen tension. On the other hand, large pore sized scaffolds enhance the chondrogenic differentiation of MSCs due to higher oxygen tension and better nutrition circulation
Blending strategy of type I collagen
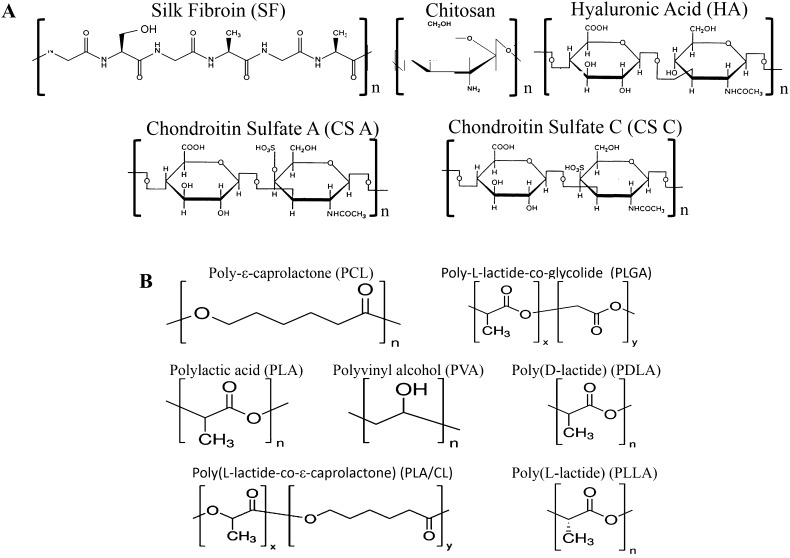
Application of pure type I collagen as a scaffold for CTE has two major issues: (1) weak mechanical robustness which heavily impair scaffold handling before and after implantation [117], (2) limited chondrogenic properties in terms of supporting ACs phenotype and chondrogenic differentiation of MSCs [22, 118]. To overcome the first limitation, collagen crosslinking using carbodiimide or glutaraldehyde has been conducted; however, introduction of a lot of crosslinking points would consume the cell-binding motives (e.g. GFOGER) [119], nullifying the initial purpose of utilizing collagen. Physical mixing (blending) of collagen with other natural polymers, such as silk fibroin, chitosan, hyaluronic acid is useful to obtain more ideal matrices composition without sacrificing desirable features of collagen (e.g., cellular adhesion and biocompatibility) [45, 120]. In section IV, we mainly discussed investigations for applying the blending strategy of collagen with several polymers, as described in Fig. 6, to support phenotypes or activities of ACs and MSCs. Particular instances of materials, such as synthetic polymer or inorganic materials, are only discussed shortly because it is more common to combine them in non-blending strategy, as will be given in Sect. 5.
Fig. 6.
Chemical structures of A natural polymers [silk fibroin (SF), chitosan, hyaluronic acid (HA), chondroitin sulfate-A (CS-A), chondroitin-6 sulfate (CS-C)] commonly blended with collagen matrices (sponges, hydrogel, nanofibers) and B synthetic polymers [poly(lactic acid) (PLA), poly(L-lactide) (PLLA), poly(ε-caprolactone) (PCL), poly(vinyl alcohol) (PVA), poly(D-lactide) (PDLA), poly(L-lactide-co-e-caprolactone) (PLA/CL), poly(L-lactideco-glycolide) (PLGA)] incorporated with collagen matrices in non-blended manner
Blending with silk fibroin
Silk fibroin (SF) extracted from Bombyx mori has long been used as a medical suture due to its excellent toughness and structural stability [121, 122]. In terms of mechanical toughness, SF exhibits larger values (ultimate tensile strength (UTS) at 740 MPa, Young’s modulus at 10 GPa, elongation at 20%), in contrast with crosslinked collagen (UTS at 47–72 MPa, Young’s modulus at 0.4–0.8 GPa, elongation at 12–16%) or with mammal bone (UTS at 160 MPa, Young’s modulus at 20 GPa, elongation at 13%) [122]. SF is environmentally stable fibrous protein owing to its extensive hydrogen bonding, hydrophobic nature, and high degree of protein crystallinity (β-sheet crystals) [121]. Despite the insolubility of SF in mild aqueous solution, various methods have been well established to form SF into sponges, hydrogels, and nanofibers [87, 122–124]. Nonetheless, the high stability of pure SF might impede its application as tissue engineering scaffolds, as it slowly degrades after implantation. SF also lacks cell-binding motives or other mechanism beneficial in supporting cell activities [122]. Thus, it is advantageous to blend SF with collagen [125–129].
Generally, collagen was blended with silk by separately adjusting each solution and subsequently mixing them before next processing (e.g., freeze-drying or electrospinning) (Fig. 7A, B) [125, 128, 129]. Chomcalao et al. [129] synthesized sponges of pure SF and SF-collagen; the addition of collagen improved compressive modulus from 148 to 1532 kPa. The result was less intuitive as why addition of mechanically weak collagen enhanced relatively stronger silk. It turned out that SF suffered from aggregation during freezing, thus yielding mechanically fragile scaffolds [128]. The addition of collagen would prevent the aggregation and produce final scaffolds with undoubtedly better mechanical properties [128].
Fig. 7.
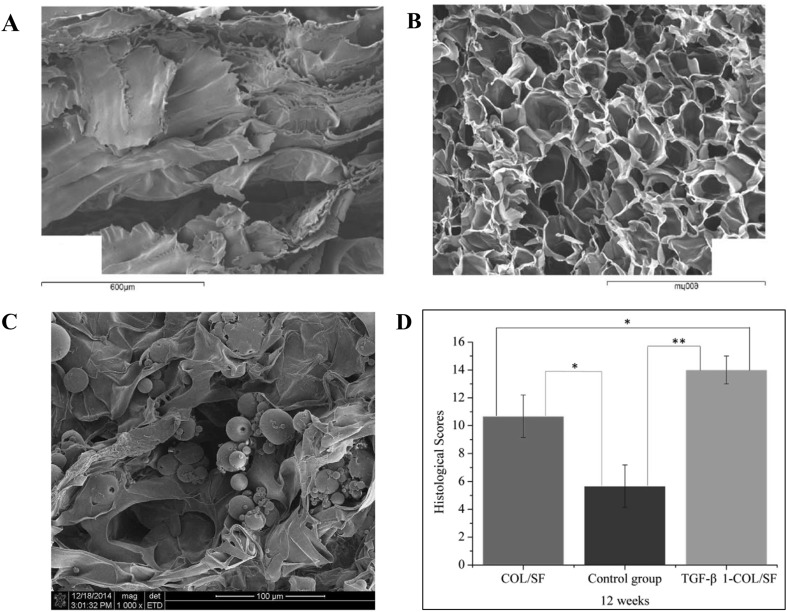
Structure and properties of collagen/silk fibroin (Col/SF) scaffolds. A Microstructure of freeze-dried SF scaffold. B Microstructure of freeze-dried Col/SF scaffolds with 20 wt% Col and 4 wt% SF. C Microstructure of Col/SF scaffolds incorporated with TGF-β1 containing poly(lactic-co-glycolic-acid) microsphere (TGF-β1- COL/SF). D Histological evaluation of Col/SF scaffolds after implantation in vivo for 12 weeks. COL/SF and no scaffold as a control group.
(Parts of figure are adapted with the permission from [128] A, B and [125] C and D)
Blend of SF with collagen is generally superior in supporting cellular viability in contrast with pure collagen and SF scaffolds [125, 128–130]. Wang et al. [125] blended collagen with SF at different ratio in sponge matrices and investigated the subsequent MSC proliferation rate. They found that ratio of 7:3 (collagen:SF) provided the highest proliferation rate of MSCs. Similar finding was also confirmed for other type of cells, such as chondrocytes and HepG2 cells [125, 129]. It was previously described that silk provided stabile porous structures in scaffolds, which would be important to maintain favorable environment during culture [124]. No SF apparently possesses chondro-inductive capabilities, thus disfavoring its application in cartilage tissue engineering. However, some authors proposed the incorporation of encapsulated TGF-β into SF-collagen scaffolds to overcome this limitation (Fig. 7C, D) [125].
Blending with chitosan
Chitosan is a linear polysaccharide of glucosamine and N-acetyl glucosamine mainly derived from deacetylation of chitin which commonly finds in crustacean (crabs, shrimp, and lobster) shells or cell wall of fungi [131]. Long chain of amine endows chitosan with various unique properties, such as antibacterial, cationic nature, and resistance to enzymatic degradation [131, 132]. Chitosan is widely used as biomaterial or drug-delivery agent due to its non-toxicity, low immunogenicity, and antibacterial activities. Several studies have also indicated the capacity of chitosan in supporting synthetic activity of chondrocytes, suggesting its potential in CTE [131–134]. Nevertheless, chitosan resists enzymatic hydrolysis and hardly degrades in vivo [131]. Thus it might be useful to combine chitosan with easily degraded biopolymer to ensure appropriate tissue remodeling.
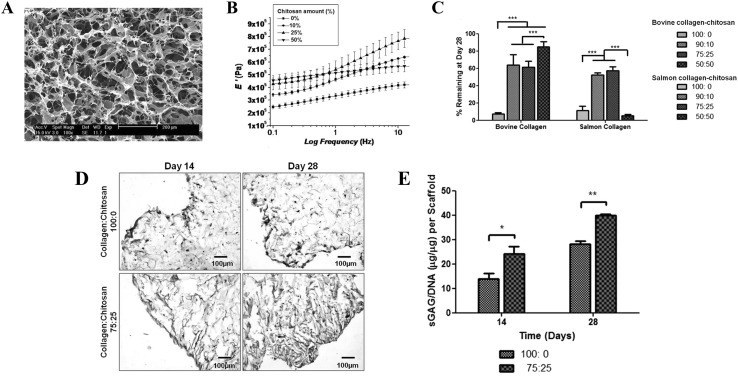
Chitosan can be easily mixed with collagen to obtain the final forms of sponges, hydrogels, and nanofibers [133, 135, 136]. Moderate addition of chitosan is beneficial to enhance the overall mechanical properties and structural stability of scaffolds (Fig. 8A–C) [131, 133–137]. Chen et al. blended chitosan into collagen nanofibers and reported the increase of UTS, strain, and Young’s modulus with contents of chitosan up to 20% (w/v). Further addition of chitosan embrittled the scaffold as UTS and Young’s modulus grossly increased without improvement of tensile strain [136, 138]. Analysis of Fourier transform infrared (FTIR) spectra assigned the increase of mechanical properties to the formation of hydrogen bond of collagen and chitosan, possibly between –C=O and –NH2 groups of collagen with –OH and –NH2 groups of chitosan [136]. At higher contents of chitosan, stronger ionic bond could form and subsequently contribute to the brittleness of scaffold [135, 136]. Excessive amounts of chitosan blend formed clumps, which would block the pores of scaffolds and inhibit penetration of cells into the scaffolds [135]. Chitosan-collagen sponges retained ~ 40% of their original weight after enzyme treatment in contrast with fully degraded pure collagen matrices, indicating improvement of in vitro structural stability [134].
Fig. 8.
Structure and properties of collagen/chitosan scaffolds. A Microstructure of freeze-dried collagen/chitosan scaffold. B Change of mechanical properties of collagen/chitosan scaffold caused by the variation of chitosan concentration (0–50 wt%). C Remaining of collagen/chitosan scaffold after incubation for 28 days in serum containing media. Data (n = 3) is plotted in mean ± standard deviation. Significance is indicated by *p < 0.05; **p < 0.01; ***p < 0.001. D, E Determination of sGAG deposition of collagen (collagen:chitosan at 100:0) versus collagen/chitosan (collagen:chitosan at 75:25) scaffolds, by Safranin O staining (D) and sGAG quantification (E). Data (n = 3) is plotted in mean ± standard deviation. Significance is indicated by *p < 0.05; **p < 0.01; ***p < 0.001.
(Parts of figure are adapted, with permission, from [137] A, B and [139] C, D, E)
Biosynthetically active ACs mainly produced anionic GAG (e.g., chondroitin sulfate, heparan sulfate) during cell culture, and the presence of chitosan could be useful to retain such GAG secretion, since the cationic nature of chitosan allows interaction with negatively charged GAG or other anionic proteoglycan [131]. Yan et al. [134] cultured AC in a scaffold of chitosan-blended collagen and described higher amounts of GAG content compared with pure collagen scaffolds. The bioactive signals of chitosan can be attributed to its structural similarity with GAG molecules [131, 133]. Build-up of GAG in scaffolds is highly desirable as it may contribute further to the chondro-inductive environment and gradual improvements of mechanical properties of final structures (Fig. 8D, E) [36, 139, 140].
Blending with hyaluronic acid and chondroitin sulfate
Hyaluronic acid (HA) and chondroitin sulfate (CS) are members of long unbranched polysaccharides (GAG) commonly found in native articular cartilage tissues. A lot of CS and other sulfated GAG are attached with the core protein, which forms gigantic and bottlebrush-like molecules called aggrecan [141]. Due to the anionic nature of CS, aggrecan attracts huge amounts of water and provide osmotic resistance of articular cartilage during compression [141]. Aggrecan associates with a link protein and binds to a single molecule HA to form larger proteoglycan complexes, which together with type II collagen provide tensile and compressive resilience of articular cartilage [6].
Roles of blending HA with collagen in modulating phenotype and bioactivities of ACs and MSCs have been investigated [109, 142–147]. HA-collagen composites supported higher fractions of ACs to retain rounded shapes and to promote the secretion of cartilage-related ECMs, much better than pure collagen counterpart (Fig. 9A, B) [35]. In the case of MSCs, addition of HA into collagen sponges apparently promoted the cellular infiltration and favored expression of chondrogenic markers (i.e. Sox9 and COLII) in the short term culture period (≤ 14 days) [30, 35, 148]. CD44 is a main receptor for HA and their corresponding bindings have been shown to be crucial to maintain chondrogenic states and cellular motility [142, 143, 148].
Fig. 9.
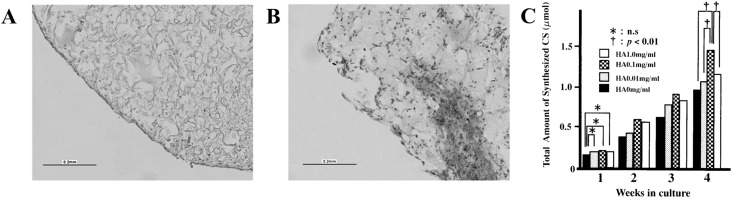
A, B Histological sections of scaffolds of collagen (A) and collagen/HA (B). Red color indicates sGAG staining by Safranin-O. C Effect of HA concentration of the collagen/HA scaffolds on the total amount of deposited chondroitin sulfate. Data (n = 6) is plotted in mean ± standard deviation. *indicates insignificant differences. † indicates p < 0.01.
(Parts of figure are adapted from [35] A, B and [144] C). (Color figure online)
HA seems to require optimum concentration in order for its addition to elicit the expected benefits. Kawasaki et al. [144] incorporated HA at different concentrations (0, 0.01, 0.1, 1.0 mg/ml) in type I collagen gel, and they found that HA at 0.1 mg/ml was optimal for enhancing chondrocyte proliferation and CS secretion (Fig. 9C). In contrast, HA caused no change at higher or lower concentration (0.01 or 1.0 mg/ml). Further analysis also revealed that only 0.1 mg/ml HA-collagen hydrogel altered the ratio of secreted CS in favor of higher production of cartilage-related chondroitin-6 sulfate (CS-C), instead of ligament-related chondroitin-4 sulfate (CS-A). Other study reported similar findings, in which excessive addition of HA (> 2% w/w) suppressed the secreted amounts of cartilage-related by ACs [149]. In the aqueous solution, HA is known to form polyion complexes with collagen molecules, thus preventing the formation of homogeneous scaffolds [150]. Furthermore, HA in high concentration may entangle and form hydrophobic patches which are detrimental for secretion of cartilage-related ECMs [151].
Collagen-CS scaffolds are frequently employed for culturing chondrocyte aiming to maintain the round morphology and its biosynthetic activities [13, 22, 38, 83, 84]. Surprisingly, studies aimed to scrutinize chondrogenic benefits of CS addition showed mixed results [18, 146]. Van Susante et al. [146] crosslinked CS into type I collagen sponges and observed no difference in chondrogenic expression (i.e., aggrecan, biglycan, decorin), despite the improved amount of secreted GAG. In similar study, Pieper et al. [19] described that no major difference was caused by the absence or presence of CS in type I collagen scaffold. These contradictory results would be explained by difference of CS types used. Nishimoto et al. [152] investigated that CS-C, and not CS-A, upregulated chondrogenic expression (i.e., type II collagen and aggrecan) in 3-D collagen culture, possibly due to the binding with specific cell receptor. Other reasons would be the gradual release of added-CS into the medium throughout the period of cell cultures [34].
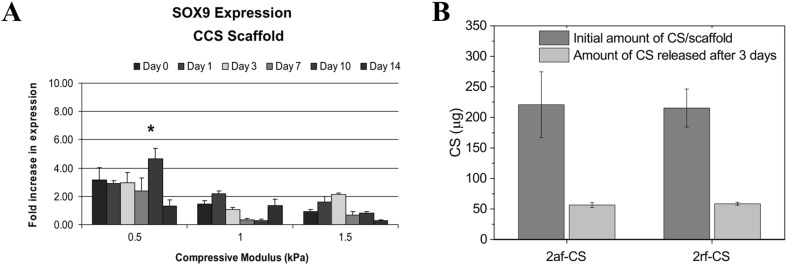
Incorporation of CS apparently benefit the chondrogenic differentiation of MSCs in the low stiffness environment (Fig. 10A) [30, 38]. In the absence of differentiating medium, blending of CS into collagen sponges with a low stiffness (~ 0.5 kPa) promoted early upregulation of Sox9. Meanwhile, CS caused a favorable expression of osteogenic marker (Runx2) in the high stiffness sponges (~ 1 and 1.5 kPa) [31]. Furthermore, CS addition seems to suppress hypertrophic differentiation of MSCs, as evidenced by the downregulation of type X collagen and Alizarin staining [33, 37, 38].
Fig. 10.
A Sox9 expression of MSCs seeded in scaffold of collagen/chondroitin sulfate (CCS) with different compressive modulus (stiffness). B Amount of CS (chondroitin sulfate) in two different type of collagen scaffolds at initial time and after 3 days of incubation in cell culture media. 2af-CS and 2rf CS indicate afibrillar type II collagen-CS and fibrillar type II collagen-CS scaffolds, respectively.
(Parts of figure are adapted from [31] A with permission and [34] B under a Creative Commons Attribution License)
Cautions are necessary to interpret synergistic effects of HA and CS in a single collagen scaffold. Final compositions of trimeric scaffold (HA/CS/collagen) are easily altered during preparation of scaffolds or cell-culture stages [153, 154]. HA strongly interacts with CS when mixed in aqueous solution, preventing considerable amounts of CS to be incorporated in collagen hydrogel [153]. CS are also easily washed away during cell culture due to their high water-solubility (Fig. 10B) [34, 154].
Addition of HA or CS would hardly cause any change to mechanical properties of collagen matrices [35], thus several authors have proposed additional blending with more robust polymer (e.g. chitosan, methacrylic anhydride) [35, 134, 155].
Blending with synthetic polymer and inorganic materials
Organic solvents are generally required to process biocompatible synthetic polymers, such as poly-ε-caprolactone (PCL), poly-L-lactide-co-glycolide (PLGA), and polylactic acid (PLA), and thus blending collagen in such solvents is not feasible due to denaturation of collagen. Lack of common solvent limits the feasible option to hydrophilic synthetic polymers [156].
Poly(vinyl alcohol) (PVA) is one of the synthetic and biocompatible polymers feasible to be blended with collagen [157, 158]. Mechanical properties of PVA can be altered by several processing methods (e.g., freeze thawing, or crosslinking) [159, 160]. Since PVA incapable of supporting cell adhesion, it gains benefit by mixing with collagen [157]. Moreover, PVA also provides cheaper alternative to fill the bulk volume of collagen matrices.
Mehrasa et al. [157] electrospun pure PVA and collagen-mixed PVA, and discovered that the addition of collagen increased the tensile strength, Young’s modulus, and elasticity of the final scaffolds. PVA and collagen composites form strong hydrogen bonding in the composites, which improves thermal stability and mechanical toughness. Nonetheless, PVA-collagen composites apparently showed no improvement of bioactivities for chondrocytes [157].
Inorganic materials are rarely used for the application of articular cartilage regeneration, because its excessive stiffness might not be suitable for chondrocytes and most of composites involving collagen and inorganic materials are commonly intended for osteochondral applications [161]. Nevertheless, Ohyabu et al. [162] demonstrated that hydroxyapatite (HAp) nanoparticles of moderate amounts were beneficial to sensibly improve mechanical properties of collagen sponges. HAp nanoparticles also increased significantly surface area of the collagen sponges (6.52–34.5 m2/g), thus enhancing cells (MSCs) distribution and adhesion [163].
Aforementioned effects of collagen blending to final mechanical properties were summarized in Table 4.
Table 4.
The effect of blending collagen on mechanical properties of final matrices
| Matrices composition [form] | Initial strength | Initial elasticity | References |
|---|---|---|---|
| SF (6 wt%); SF/collagen (25:75) [Sponges] | Not mentioned | 148 ± 12 kPa; 1532 ± 697 kPac | [129] |
| SF (4 wt%); SF/collagen (10, 20 wt%) [Sponges] | 20 ± 1 kPa; 310 ± 10, 354 ± 25 kPab | 0.43 ± 0.055 MPa; 10 ± 0.1, 30 ± 0.1 MPac | [128] |
| Collagen (0.6%w/v); collagen/chitosan (1%w/v with 80:20 w/w) [Sponges] | 0.37 ± 0.05 MPa; 0.51 ± 0.08 MPaa | Not mentioned | [134] |
| Collagen (2.10 mg/ml); collagen/chitosan (2:1, 1:1 w/w) [Hydrogels] | Not mentioned | ~ 10 kPa; ~ 15, 20 kPac | [133] |
| Collagen/chitosan (100/0; 80/20; 60/40; 50/50; 40/60; 20/80% content in complex) [Nanofibers] | 23.7 ± 5.8; 21.7 ± 19.3; 62.8 ± 14.1; 61.8 ± 26.0, 47.0 ± 19.1; 10.5 ± 8.0 MPaa | 1371 ± 225; 1611 ± 793; 5966 ± 2137; 6801 ± 3256; 4159 ± 1195; 3601 ± 485 MPac | [136] |
| Collagen (2 wt%); collagen/HA (9:1 v/v) [Sponges] | Not mentioned | ~ 15 kPa; ~ 15 kPac | [164] |
| Collagen; collagen/HA (0.5, 1.0 mg/ml) [Hydrogel] | Not mentioned |
24.3 ± 5.2 Pa; 19.8 ± 3.1, 15.8 ± 3.4 Pad 4.8 ± 2.0 kPa; 11.6 ± 8.3, 41.6 ± 20.9 kPac |
[165] |
| Collagen (0.5%w/v); collagen/CS (0.05%w/v) [Sponges] | Not mentioned | ~ 0.2 kPa; 0.2 kPa | [35] |
| PVA; collagen/PVA (60:40 v/v) | 0.98 ± 0.75 MPa; 1.32 ± 0.33 MPaa | 3.12 ± 0.78 MPa; 5.40 ± 1.32 MPac | [157] |
| Collagen; collagen/hydroxyapatite nanoparticles | ~ 0.6 kPa; ~ 2.8 kPab | ~ 1 kPa; ~ 4.9 kPac | [166] |
aTensile strength
bCompression strength
cElastic modulus
dStorage modulus
Collagen denaturation in blending strategy
Collagen is denatured by physical stress [167], temperature increase [168], or exposure to strong organic solvent [169]. Denatured collagen losses the native helical structure (triple helix) and subsequently forms a hydrolyzed product (gelatin) [169]. Gelatin still supports the cellular activities (cell attachment and cell proliferation) [96]; however physical properties of gelatin are different from collagen, in which it shows rapid degradation and inferior mechanical properties [170]. Loss of native structure of collagen scaffolds is associated with the altered attachment behavior of ACs [171] and the diminished capacity of type II collagen to stimulate chondrogenic differentiation of MSCs [118]. In other words, denaturation of collagen may diminish the mechanical and biological performance of scaffolds in CTE.
Denaturation limits the options of materials able to be processed by blending strategy. Attempts to achieve the homogeneous blending of collagens requires exposure of collagen to high temperature or strong inorganic solvent, inadvertently lead to the collagen denaturation [170, 172–175]. Silk fibroin is a hydrophobic polymer [122], thus silk shows limited miscibility with collagen. Several investigators attempted to blend silk and collagen solution at high temperature (60 °C) [128, 172], inadvertently damaged the native structure of collagen in the process. Other investigators used fluoroalcohol-based solvent [(1,1,1,3,3,3-hexafluoro-2-propanol (HFP)) to electro-spin the blend of collagen/synthetic polymers PCL [175] or poly(L-lactic acid) (PLLA)] [176]. HFP was previously showed to be detrimental for helical conformation of collagen, effectively transforming the collagen in final scaffolds into gelatin [169]. The final polymer/collagen scaffolds exhibit no negative effects on the biological activities of cells [175, 176]; however, collagen scaffolds without native structures might not be suitable for CTE applications.
Non-blending strategy (hybrid scaffolds) of type I collagen
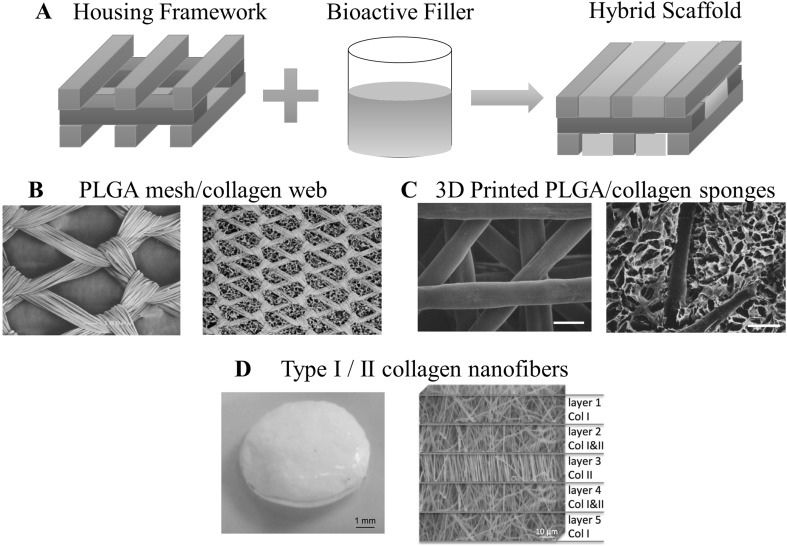
Non-blending strategy involves combination of different forms of matrices (i.e., sponges, hydrogels, and nanofibers) into a single scaffold without altering the chemical composition of matrices (Fig. 11A). Components of hybrid scaffolds were classified into two parts: (1) external housing framework and (2) internal bioactive filler. Housing framework, as the name suggested, mainly function to shelter the bioactive filler and to provide necessary mechanical integrity. Meanwhile, the bioactive filler is composed of collagen or blended-collagen matrices including ACs or MSCs. In this way, conventional collagen matrices may receive benefits from recent breakthroughs in synthetic polymer forming process (e.g., 3-D printing, etc.). Moreover, collagen denaturation can be minimized as collagen is handled under appropriate conditions, such as processing by water-based solvent at pH ~ 3 and below room temperature.
Fig. 11.
A Conceptual illustration of hybrid scaffolds in which it is composed of two different components: housing framework and bioactive filler. B–D are the examples of collagen-based hybrid scaffolds. B PLGA mesh (left figure) was furnished with collagen web to form hybrid scaffold (right figure). C 3D printed PLGA (left figure) was used as housing framework for collagen sponges to obtain hybrid scaffold (right figure). D Appearance of hybrid scaffold of nanofibers type I/II collagen (left figure) and the corresponding internal structure (right figure).
(Parts of figure are adapted, with permission, from [177] B, [178] C and [179] D)
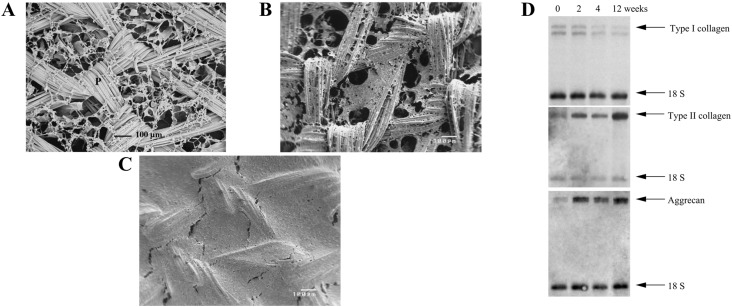
Chen et al. [177] pioneered the first generation of hybrid scaffolds, in which they formed type I collagen webs at the interstices of highly porous woven PLGA (Fig. 11B). By this configuration, PLGA played a role of housing framework, while collagen provided 3-D environment for PLGA-collagen structure was used to produce tissue engineered cartilage by initially culturing chondrocytes in the scaffolds in vitro for 1 week, then subcutaneously implanted the scaffolds in the dorsum of athymic nude mice for 12 weeks [177]. During the in vitro culture, chondrocytes were mainly suspended on the collagen web and gradually occupied the openings of scaffolds (Fig. 12A–C) [180]. Stabilization of chondrocytes phenotype was indicated by northern blot analysis in which type II collagen and aggrecan were upregulated while type I collagen was diminished over the course of 12 weeks (Fig. 12D) [180]. Such favorable result originated from accumulation of ACs on the web like-collagen, which in turn helps to maintain its differentiated phenotype [180]. After implantation in vivo, glistening white appearance of scaffolds was observed, suggesting large secretion of proteoglycan and GAG. These findings were further supported by staining with Safranin-O and toluidine blue [177]. Secretion of type II collagen was also positive for the implanted scaffolds, implying the formation of hyaline-like tissues [177].
Fig. 12.
Hybrid scaffold of collagen PLGA mesh/collagen web. A Initial microstructure of PLGA mesh/collagen web, B, C microstructure of PLGA mesh/collagen web after 1 and 4 weeks of chondrocyte incorporation. Chondrocyte was attached and suspended on the collagen web. P and C indicate PLGA mesh and collagen web, respectively. D Northern blot analyses of chondrocytes seeded in PLGA mesh/collagen web scaffolds for the gene encoding ColI, COLII, and ACAN for the period of 0, 2, 4, 12 weeks.
(Parts of figure are adapted from [180] A, B, C, and D with permission)
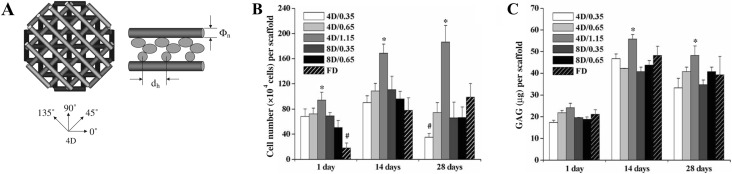
Usage of knitted meshes may be limited by pre-fixed microstructures and macroscopic shapes, thus fabrication of housing framework by 3D printing may provide better alternatives. Chen et al. employed selective laser deposition method to fabricate PCL with unique microstructures. They vertically stacked layers of PCL struts at angles of 0°/90°/0°/90° between each layer [181]. The structures obtained were then immersed in type I collagen solution containing AC suspension, then subsequently gelled to form the hybrid scaffolds [181]. Compared with single PCL matrices, hybrid scaffolds of PCL/collagen exhibited significantly higher cell proliferation and secretion of GAG and type II collagen for a period of 28 days [181]. Yen et al. constructed hybrid scaffolds of PLGA/type II collagen and easily controlled the physical features of hybrid scaffolds (i.e., compressive modulus and porosity) by modifying the spacing of PLGA fibers or stacking angles (Figs. 11C, 13A). These parameters can be further adjusted to optimize the proliferation and bioactivities of chondrocytes (Fig. 13B, C) [178].
Fig. 13.
Hybrid scaffolds of type II collagen/PLGA obtained by fused deposition manufacturing (FDM). A Illustration of PLGA scaffolds obtained by stacking four layers of extruded polymer fiber in different angle (4D:0°, 45°, 90°, 135°). dh and Φn indicate fiber distance and nozzle aperture, respectively. B Cell number of chondrocytes and C deposited amount of GAG by chondrocytes seeded on hybrid scaffold of different parameters. Sample naming is 4D/dh and 8D/dh. FD is freeze-dried type II collagen. n = 3, p < 0.05, * and # are significantly higher and lower from other scaffolds.
(Parts of figure are adapted from [178] A, B, C with permission)
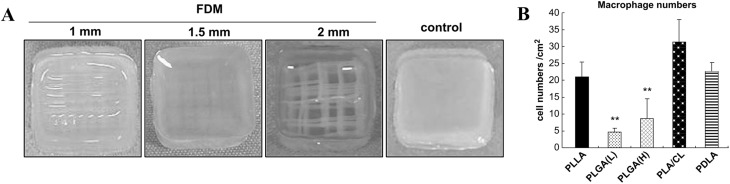
Housing frameworks determined biocompatibility of the hybrid scaffolds (Fig. 14A). Tanaka et al. investigated the immunogenicity of AC-laden hybrid scaffolds with housing framework made by five different synthetic polymers: PLGA with low molecular weight (PLGA-L), PLGA with high molecular weight (PLGA-H), poly(D-lactide) (PDLA), poly(L-lactide-co-ε-caprolactone) (PLA/CL), and poly(L-lactide) (PLLA) [182]. Two months post-implantation in the back of nude mice showed PLGA-L and PLGA-H were deemed to be biocompatible compared with other polymers, since these two matrices largely degraded without attracting large number of macrophages (Fig. 14B) [182]. Macrophages secrete several cytokines [e.g., TNF-α (tumor necrosis factor-α), IL-1β (interleukin-1)] capable of inducing degradation of healthy cartilage matrices, thus correct selection of housing framework material is crucial to avoid unnecessary complication.
Fig. 14.
A Hybrid scaffolds of PLLA obtained by fused-deposition method (FDM) with different aperture size (1, 1.5, 2 mm) and collagen. Control is a collagen gel. B Number of macrophages accumulated in the hybrid scaffolds of collagen and various synthetic polymer (PLLA, PLGA(L), PLGA(H), PLA/CL, and PDLA after 2 months of subcutaneous implantations in mice.
(Parts of figure are adapted from [182] with permission)
Internal bioactive filler may reap benefits from previously optimized collagen-blended composition. Liao et al. [151] mixed HA with type I collagen solution, then filled the 3D printed poly(propylene fumarate). ACs cultured on HA-collagen hybrid scaffolds exhibited strong staining of GAG, type II collagen and weak staining of type I collagen, compared with pure collagen hybrid scaffolds [151]. Fibrin gels can be formed by adding thrombin into fibrinogen solution and gelation can be conducted quickly without harming cells; however, similar with other hydrogels, mechanical strength of fibrin gels is weak. To overcome this problem, Deponti et al. [183] proposed a hybrid scaffold of fibrin and collagen. Initially, they suspended ACs into fibrinogen solution, dropped the mixture into collagen sponges, and subsequently added thrombin to harden fibrinogen. After 3 weeks of culture, ACs in the hybrid scaffolds showed homogeneous cell distribution and higher production of GAG and type II collagen compared with ACs cultured in sponges without fibrin glue. Interestingly, the hybrid scaffolds seem to rescue chondrocyte phenotype during 3 weeks culture, as evidenced in the gradual increasing of GAG/DNA ratio and gene upregulation of COLII, ACAN, and Sox9 [183].
Xu et al. [184] developed nanofibers hybrid scaffolds by employing PCL nanofiber as a housing framework and chondrocyte/fibrinogen/collagen gels as a bioactive layer. Electrospinning was used to deposit the fibrous PCL on the 1st, 3rd, and 5th layers, while inkjet printing was used to dispense the solution of Col-layer at 2nd and 4th layers, together with thrombin to allow the gelation of fibrinogen [184]. The multilayered constructs showed higher ultimate tensile strength and Young’s modulus (1.1 and 1.8 MPa) compared with isolated component; PCL (0.9 and 0.8 MPa) or collagen/fibrinogen matrix (incapable for testing) [184]. It indicated that the hybrid scaffolds did not affect viability and bioactivity of ACs, as evidenced by the live/dead assay (82%) and positive staining for type II collagen and GAG after in vitro and in vivo experiments. On the other hand, Reboredo et al. [179] used electrospinning to obtain hybrid scaffolds with the structure mimicking native cartilage tissues; the scaffolds had five layers, 1st and 5th layers included type I collagen nanofibers aligned randomly, 2nd and 4th layers consisted of mixture of type I and II collagen nanofibers, and 3rd layer of aligned type II collagen nanofibers (Fig. 10D). The hybrid scaffolds showed average elastic modulus and tensile strength of ~ 1046 and ~ 32 MPa, respectively. MSCs seeded on the hybrid scaffolds synthesized proteoglycan and type II collagen in contrast with human cell pellets culture, indicating the layer-by-layer scaffolds would induce chondrogenic differentiation of MSCs [179].
Conclusion
Type I collagen is a compelling platform for next development of CTE scaffolds, considering its proven short- and mid-term clinical efficacy and the potentially smooth access to enter the market of health products. Nonetheless, long-term clinical performance of type I collagen scaffolds may be hampered by its limited chondrogenic capacity, weak mechanical strength, and significant shrinkage.
If one considers chondrogenic performance, type II collagen-based scaffolds would be a promising alternative of type I collagen; however additional studies are necessary to confirm safety issues of type II collagen. Therefore, in the foreseeable future, type I collagen is the more feasible option of CTE scaffold in clinical setting.
In designing scaffold of CTE, physical (i.e., internal structure, pore size, stiffness) and chemical features of scaffold (i.e., blend composition) are crucial in influencing the differentiation fate and synthetic activities of ACs and MSCs.
Different forms of 3-D collagen scaffolds (i.e., sponges, hydrogel, nanofibers) possess unique internal structure, which may affect chondrogenic activities of ACs and MSCs in the particular ways. For examples, ACs are suspended on the fiber network of hydrogel, while ACs assume flat appearance on cell wall of sponges. Physical features of scaffolds also influence phenotype and activities of ACs/MSCs. Scaffolds with low stiffness favor chondrogenic phenotype of ACs and chondrogenic differentiation of MSCs by discouraging the formation of organized stress fibers or promoting scaffold shrinkage. Strikingly, positive influence of pore size apparently depends on cell types. Small pore size (< 225 μm) is desirable for ACs, whereas large pore size (> 300 μm) is suitable for MSCs. Therefore, the matrices in CTE should be optimized by considering type of scaffold and cells used.
Composition of collagen scaffold can be altered by blending with other molecules (e.g., chitosan, HA, CS) to improve mechanical properties and biological activities of final scaffold. Despite the versatility of collagen bending, options of feasible additive materials are limited, as type I collagen easily denature at harsh processing condition.
Synthetic polymer is a structurally robust and easy-to-be fabricated material. However, physical and chemical nature of synthetic polymer do not allow direct blending with type I collagen. To overcome these problems, the concept of hybrid scaffold was introduced. Hybrid scaffolds consist of two parts: (1) housing framework and (2) bioactive filler. Mechanically robust and easy-to-be processed polymer may constitute the housing framework. Additionally, physically and compositionally optimized type I collagen may serve as the bioactive filler. Configuration of hybrid scaffolds provides a promising solution to maximize requirements of mechanical and structural integrity of type I collagen without compromising its bioactivity.
Compliance with ethical standards
Conflict of interest
The authors have no financial conflict of interest.
Ethical statement
There are no human experiments conducted for this article and approval of institutional review board (IRB) is not necessary for this article. There are no animal experiments carried out for this article.
References
- 1.Mankin HJ. The response of articular cartilage to mechanical injury. J Bone Joint Surg Am. 1982;64:460–466. [PubMed] [Google Scholar]
- 2.Pearle AD, Warren RF, Rodeo SA. Basic science of articular cartilage and osteoarthritis. Clin Sports Med. 2005;24:1–12. doi: 10.1016/j.csm.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 3.Hunziker EB. Articular cartilage repair: basic science and clinical progress. A review of the current status and prospects. Osteoarthritis Cartilage. 2002;10:432–463. doi: 10.1053/joca.2002.0801. [DOI] [PubMed] [Google Scholar]
- 4.O’Driscoll SW. The healing and regeneration of articular cartilage. J Bone Joint Surg Am. 1998;80:1795–1812. [PubMed] [Google Scholar]
- 5.Camp CL, Stuart MJ, Krych AJ. Current concepts of articular cartilage restoration techniques in the knee. Sports Health. 2014;6:265–273. doi: 10.1177/1941738113508917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Armiento AR, Stoddart MJ, Alini M, Eglin D. Biomaterials for articular cartilage tissue engineering: learning from biology. Acta Biomater. 2018;65:1–20. doi: 10.1016/j.actbio.2017.11.021. [DOI] [PubMed] [Google Scholar]
- 7.Huang BJ, Hu JC, Athanasiou KA. Cell-based tissue engineering strategies used in the clinical repair of articular cartilage. Biomaterials. 2016;98:1–22. doi: 10.1016/j.biomaterials.2016.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cole BJ, D’Amato M. Autologous chondrocyte implantation. Oper Tech Orthop. 2001;11:115–131. [Google Scholar]
- 9.Brittberg M. Autologous chondrocyte implantation—technique and long-term follow-up. Injury. 2008;39(Suppl 1):S40–S49. doi: 10.1016/j.injury.2008.01.040. [DOI] [PubMed] [Google Scholar]
- 10.Benya PD, Shaffer JD. Dedifferentiated chondrocytes reexpress the differentiated collagen phenotype when cultured in agarose gels. Cell. 1982;30:215–224. doi: 10.1016/0092-8674(82)90027-7. [DOI] [PubMed] [Google Scholar]
- 11.Higuchi A, Kumar SS, Benelli G, Alarfaj AA, Munusamy MA, Umezawa A, et al. Stem cell therapies for reversing vision loss. Trends Biotechnol. 2017;35:1102–1117. doi: 10.1016/j.tibtech.2017.06.016. [DOI] [PubMed] [Google Scholar]
- 12.Higuchi A, Ling QD, Ko YA, Chang Y, Umezawa A. Biomaterials for the feeder-free culture of human embryonic stem cells and induced pluripotent stem cells. Chem Rev. 2011;111:3021–3035. doi: 10.1021/cr1003612. [DOI] [PubMed] [Google Scholar]
- 13.Somoza RA, Welter JF, Correa D, Caplan AI. Chondrogenic differentiation of mesenchymal stem cells: challenges and unfulfilled expectations. Tissue Eng Part B Rev. 2014;20:596–608. doi: 10.1089/ten.teb.2013.0771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nehrer S, Breinan HA, Ramappa A, Shortkroff S, Young G, Minas T, et al. Canine chondrocytes seeded in type I and type II collagen implants investigated in vitro. J Biomed Mater Res. 1997;38:95–104. doi: 10.1002/(sici)1097-4636(199722)38:2<95::aid-jbm3>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 15.Shakibaei M, De Souza P, Merker HJ. Integrin expression and collagen type II implicated in maintenance of chondrocyte shape in monolayer culture: an immunomorphological study. Cell Biol Int. 1997;21:115–125. doi: 10.1006/cbir.1996.0118. [DOI] [PubMed] [Google Scholar]
- 16.van Susante JL, Buma P, van Osch GJ, Versleyen D, van der Kraan PM, van der Berg WB, et al. Culture of chondrocytes in alginate and collagen carrier gels. Acta Orthop Scand. 1995;66:549–556. doi: 10.3109/17453679509002314. [DOI] [PubMed] [Google Scholar]
- 17.Lu Z, Doulabi BZ, Huang C, Bank RA, Helder MN. Collagen type II enhances chondrogenesis in adipose tissue-derived stem cells by affecting cell shape. Tissue Eng Part A. 2010;16:81–90. doi: 10.1089/ten.TEA.2009.0222. [DOI] [PubMed] [Google Scholar]
- 18.Takahashi T, Ogasawara T, Asawa Y, Mori Y, Uchinuma E, Takato T, et al. Three-dimensional microenvironments retain chondrocyte phenotypes during proliferation culture. Tissue Eng. 2007;13:1583–1592. doi: 10.1089/ten.2006.0322. [DOI] [PubMed] [Google Scholar]
- 19.Pieper JS, van der Kraan PM, Hafmans T, Kamp J, Buma P, van Susante JL, et al. Crosslinked type II collagen matrices: preparation, characterization, and potential for cartilage engineering. Biomaterials. 2002;23:3183–3192. doi: 10.1016/s0142-9612(02)00067-4. [DOI] [PubMed] [Google Scholar]
- 20.Galois L, Hutasse S, Cortial D, Rousseau CF, Grossin L, Ronziere MC, et al. Bovine chondrocyte behaviour in three-dimensional type I collagen gel in terms of gel contraction, proliferation and gene expression. Biomaterials. 2006;27:79–90. doi: 10.1016/j.biomaterials.2005.05.098. [DOI] [PubMed] [Google Scholar]
- 21.Li WJ, Jiang YJ, Tuan RS. Chondrocyte phenotype in engineered fibrous matrix is regulated by fiber size. Tissue Eng. 2006;12:1775–1785. doi: 10.1089/ten.2006.12.1775. [DOI] [PubMed] [Google Scholar]
- 22.Noriega SE, Hasanova GI, Schneider MJ, Larsen GF, Subramanian A. Effect of fiber diameter on the spreading, proliferation and differentiation of chondrocytes on electrospun chitosan matrices. Cells Tissues Organs. 2012;195:207–221. doi: 10.1159/000325144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nehrer S, Breinan HA, Ramappa A, Young G, Shortkroff S, Louie LK, et al. Matrix collagen type and pore size influence behaviour of seeded canine chondrocytes. Biomaterials. 1997;18:769–776. doi: 10.1016/s0142-9612(97)00001-x. [DOI] [PubMed] [Google Scholar]
- 24.Freyria AM, Ronzière MC, Cortial D, Galois L, Hartmann D, Herbage D, et al. Comparative phenotypic analysis of articular chondrocytes cultured within type I or type II collagen scaffolds. Tissue Eng Part A. 2009;15:1233–1245. doi: 10.1089/ten.tea.2008.0114. [DOI] [PubMed] [Google Scholar]
- 25.Camper L, Hellman U, Lundgren-Akerlund E. Isolation, cloning, and sequence analysis of the integrin subunit alpha10, a beta1-associated collagen binding integrin expressed on chondrocytes. J Biol Chem. 1998;273:20383–20389. doi: 10.1074/jbc.273.32.20383. [DOI] [PubMed] [Google Scholar]
- 26.Schuh E, Kramer J, Rohwedel J, Notbohm H, Müller R, Gutsmann T, et al. Effect of matrix elasticity on the maintenance of the chondrogenic phenotype. Tissue Eng Part A. 2010;16:1281–1290. doi: 10.1089/ten.TEA.2009.0614. [DOI] [PubMed] [Google Scholar]
- 27.Li X, Chen S, Li J, Wang X, Zhang J, Kawazoe N, et al. 3D culture of chondrocytes in gelatin hydrogels with different stiffness. Polymers (Basel) 2016;8:269. doi: 10.3390/polym8080269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bates GP, Schor SL, Grant ME. A comparison of the effects of different substrata on chondrocyte morphology and the synthesis of collagen types IX and X. In Vitro Cell Dev Biol. 1987;23:374–380. doi: 10.1007/BF02620995. [DOI] [PubMed] [Google Scholar]
- 29.Duval E, Leclercq S, Elissalde JM, Demoor M, Galéra P, Boumédiene K. Hypoxia-inducible factor 1alpha inhibits the fibroblast-like markers type I and type III collagen during hypoxia-induced chondrocyte redifferentiation: hypoxia not only induces type II collagen and aggrecan, but it also inhibits type I and type III collagen in the hypoxia-inducible factor 1alpha-dependent redifferentiation of chondrocytes. Arthritis Rheum. 2009;60:3038–3048. doi: 10.1002/art.24851. [DOI] [PubMed] [Google Scholar]
- 30.Bosnakovski D, Mizuno M, Kim G, Takagi S, Okumura M, Fujinaga T. Chondrogenic differentiation of bovine bone marrow mesenchymal stem cells (MSCs) in different hydrogels: influence of collagen type II extracellular matrix on MSC chondrogenesis. Biotechnol Bioeng. 2006;93:1152–1163. doi: 10.1002/bit.20828. [DOI] [PubMed] [Google Scholar]
- 31.Murphy CM, Matsiko A, Haugh MG, Gleeson JP, O’Brien FJ. Mesenchymal stem cell fate is regulated by the composition and mechanical properties of collagen-glycosaminoglycan scaffolds. J Mech Behav Biomed Mater. 2012;11:53–62. doi: 10.1016/j.jmbbm.2011.11.009. [DOI] [PubMed] [Google Scholar]
- 32.Higuchi A, Ling QD, Kumar SS, Chang Y, Alarfaj AA, Munusamy MA, et al. Physical cues of cell culture materials lead the direction of differentiation lineages of pluripotent stem cells. J Mater Chem B. 2015;3:8032–8058. doi: 10.1039/c5tb01276g. [DOI] [PubMed] [Google Scholar]
- 33.Matsiko A, Gleeson JP, O’Brien FJ. Scaffold mean pore size influences mesenchymal stem cell chondrogenic differentiation and matrix deposition. Tissue Eng Part A. 2015;21:486–497. doi: 10.1089/ten.TEA.2013.0545. [DOI] [PubMed] [Google Scholar]
- 34.Tamaddon M, Burrows M, Ferreira SA, Dazzi F, Apperley JF, Bradshaw A, et al. Monomeric, porous type II collagen scaffolds promote chondrogenic differentiation of human bone marrow mesenchymal stem cells in vitro. Sci Rep. 2017;7:43519. doi: 10.1038/srep43519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Matsiko A, Levingstone TJ, O’Brien FJ, Gleeson JP. Addition of hyaluronic acid improves cellular infiltration and promotes early-stage chondrogenesis in a collagen-based scaffold for cartilage tissue engineering. J Mech Behav Biomed Mater. 2012;11:41–52. doi: 10.1016/j.jmbbm.2011.11.012. [DOI] [PubMed] [Google Scholar]
- 36.Choi B, Kim S, Lin B, Wu BM, Lee M. Cartilaginous extracellular matrix-modified chitosan hydrogels for cartilage tissue engineering. ACS Appl Mater Interfaces. 2014;6:20110–20121. doi: 10.1021/am505723k. [DOI] [PubMed] [Google Scholar]
- 37.Bian L, Hou C, Tous E, Rai R, Mauck RL, Burdick JA. The influence of hyaluronic acid hydrogel crosslinking density and macromolecular diffusivity on human MSC chondrogenesis and hypertrophy. Biomaterials. 2013;34:413–421. doi: 10.1016/j.biomaterials.2012.09.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Varghese S, Hwang NS, Canver AC, Theprungsirikul P, Lin DW, Elisseeff J. Chondroitin sulfate based niches for chondrogenic differentiation of mesenchymal stem cells. Matrix Biol. 2008;27:12–21. doi: 10.1016/j.matbio.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 39.Chen WC, Yao CL, Chu IM, Wei YH. Compare the effects of chondrogenesis by culture of human mesenchymal stem cells with various type of the chondroitin sulfate C. J Biosci Bioeng. 2011;111:226–231. doi: 10.1016/j.jbiosc.2010.10.002. [DOI] [PubMed] [Google Scholar]
- 40.Higuchi A, Ling QD, Hsu ST, Umezawa A. Biomimetic cell culture proteins as extracellular matrices for stem cell differentiation. Chem Rev. 2012;112:4507–4540. doi: 10.1021/cr3000169. [DOI] [PubMed] [Google Scholar]
- 41.Albrecht C, Tichy B, Nürnberger S, Hosiner S, Zak L, Aldrian S, et al. Gene expression and cell differentiation in matrix-associated chondrocyte transplantation grafts: a comparative study. Osteoarthritis Cartilage. 2011;19:1219–1227. doi: 10.1016/j.joca.2011.07.004. [DOI] [PubMed] [Google Scholar]
- 42.Zak L, Albrecht C, Wondrasch B, Widhalm H, Vekszler G, Trattnig S, et al. Results 2 years after matrix-associated autologous chondrocyte transplantation using the Novocart 3D scaffold. Am J Sports Med. 2014;42:1618–1627. doi: 10.1177/0363546514532337. [DOI] [PubMed] [Google Scholar]
- 43.Saris D, Price A, Widuchowski W, Bertrand-Marchand M, Caron J, Drogset JO, et al. Matrix-applied characterized autologous cultured chondrocytes versus microfracture two-year follow-up of a prospective randomized trial. Am J Sports Med. 2014;42:1384–1394. doi: 10.1177/0363546514528093. [DOI] [PubMed] [Google Scholar]
- 44.Petri M, Broese M, Simon A, Liodakis E, Ettinger M, Guenther D, et al. CaReS®(MACT) versus microfracture in treating symptomatic patellofemoral cartilage defects: a retrospective matched-pair analysis. J Orthop Sci. 2013;18:38–44. doi: 10.1007/s00776-012-0305-x. [DOI] [PubMed] [Google Scholar]
- 45.Crawford DC, DeBerardino TM, Williams RJ., 3rd NeoCart, an autologous cartilage tissue implant, compared with microfracture for treatment of distal femoral cartilage lesions: an FDA phase-II prospective, randomized clinical trial after two years. J Bone Joint Surg Am. 2012;94:979–989. doi: 10.2106/JBJS.K.00533. [DOI] [PubMed] [Google Scholar]
- 46.Ochi M, Uchio Y, Kawasaki K, Wakitani S, Iwasa J. Transplantation of cartilage-like tissue made by tissue engineering in the treatment of cartilage defects of the knee. J Bone Joint Surg Am. 2002;84:571–578. doi: 10.1302/0301-620x.84b4.11947. [DOI] [PubMed] [Google Scholar]
- 47.Keselowsky BG, Collard DM, García AJ. Surface chemistry modulates focal adhesion composition and signaling through changes in integrin binding. Biomaterials. 2004;25:5947–5954. doi: 10.1016/j.biomaterials.2004.01.062. [DOI] [PubMed] [Google Scholar]
- 48.Salasznyk RM, Klees RF, Hughlock MK, Plopper GE. ERK signaling pathways regulate the osteogenic differentiation of human mesenchymal stem cells on collagen I and vitronectin. Cell Commun Adhes. 2004;11:137–153. doi: 10.1080/15419060500242836. [DOI] [PubMed] [Google Scholar]
- 49.Franceschi RT. The developmental control of osteoblast-specific gene expression: role of specific transcription factors and the extracellular matrix environment. Crit Rev Oral Biol Med. 1999;10:40–57. doi: 10.1177/10454411990100010201. [DOI] [PubMed] [Google Scholar]
- 50.Wallace DG, Rosenblatt J. Collagen gel systems for sustained delivery and tissue engineering. Adv Drug Deliv Rev. 2003;55:1631–1649. doi: 10.1016/j.addr.2003.08.004. [DOI] [PubMed] [Google Scholar]
- 51.Gelse K, Pöschl E, Aigner T. Collagens—structure, function, and biosynthesis. Adv Drug Deliv Rev. 2003;55:1531–1546. doi: 10.1016/j.addr.2003.08.002. [DOI] [PubMed] [Google Scholar]
- 52.Tulla M, Pentikäinen OT, Viitasalo T, Käpylä J, Impola U, Nykvist P, et al. Selective binding of collagen subtypes by integrin α1I, α2I, and α10I domains. J Biol Chem. 2001;276:48206–48212. doi: 10.1074/jbc.M104058200. [DOI] [PubMed] [Google Scholar]
- 53.Hamaia SW, Luff D, Hunter EJ, Malcor JD, Bihan D, Gullberg D, et al. Unique charge-dependent constraint on collagen recognition by integrin α10β1. Matrix Biol. 2017;59:80–94. doi: 10.1016/j.matbio.2016.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kino-Oka M, Yashiki S, Ota Y, Mushiaki Y, Sugawara K, Yamamoto T, et al. Subculture of chondrocytes on a collagen type I-coated substrate with suppressed cellular dedifferentiation. Tissue Eng. 2005;11:597–608. doi: 10.1089/ten.2005.11.597. [DOI] [PubMed] [Google Scholar]
- 55.Farjanel J, Schürmann G, Bruckner P. Contacts with fibrils containing collagen I, but not collagens II, IX, and XI, can destabilize the cartilage phenotype of chondrocytes. Osteoarthritis Cartilage. 2001;9:S55–S63. doi: 10.1053/joca.2001.0445. [DOI] [PubMed] [Google Scholar]
- 56.Jin GZ, Kim HW. Effects of type I collagen concentration in hydrogel on the growth and phenotypic expression of rat chondrocytes. Tissue Eng Regen Med. 2017;14:383–391. doi: 10.1007/s13770-017-0060-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fertala A, Han WB, Ko FK. Mapping critical sites in collagen II for rational design of gene-engineered proteins for cell-supporting materials. J Biomed Mater Res. 2001;57:48–58. doi: 10.1002/1097-4636(200110)57:1<48::aid-jbm1140>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 58.Lucic D, Mollenhauer J, Kilpatrick KE, Cole AA. N-telopeptide of type II collagen interacts with annexin V on human chondrocytes. Connect Tissue Res. 2003;44:225–239. [PubMed] [Google Scholar]
- 59.Leitinger B, Steplewski A, Fertala A. The D2 period of collagen II contains a specific binding site for the human discoidin domain receptor, DDR2. J Mol Biol. 2004;344:993–1003. doi: 10.1016/j.jmb.2004.09.089. [DOI] [PubMed] [Google Scholar]
- 60.Bengtsson T, Aszodi A, Nicolae C, Hunziker EB, Lundgren-Akerlund E, Fässler R. Loss of alpha10beta1 integrin expression leads to moderate dysfunction of growth plate chondrocytes. J Cell Sci. 2005;118:929–936. doi: 10.1242/jcs.01678. [DOI] [PubMed] [Google Scholar]
- 61.von der Mark K, Mollenhauer J. Annexin V interactions with collagen. Cell Mol Life Sci. 1997;53:539–545. doi: 10.1007/s000180050069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Klatt AR, Paul-Klausch B, Klinger C, Kühn C, Renno JH, Banerjee M, et al. A critical role for collagen II in cartilage matrix degradation: collagen II induces pro-inflammatory cytokines and MMPs in primary human chondrocytes. J Orthop Res. 2009;27:65–70. doi: 10.1002/jor.20716. [DOI] [PubMed] [Google Scholar]
- 63.Goldring MB. Chondrogenesis, chondrocyte differentiation, and articular cartilage metabolism in health and osteoarthritis. Ther Adv Musculoskelet Dis. 2012;4:269–285. doi: 10.1177/1759720X12448454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gigante A, Bevilacqua C, Cappella M, Manzotti S, Greco F. Engineered articular cartilage: influence of the scaffold on cell phenotype and proliferation. J Mater Sci Mater Med. 2003;14:713–716. doi: 10.1023/a:1024915817061. [DOI] [PubMed] [Google Scholar]
- 65.Brodkin KR, García AJ, Levenston ME. Chondrocyte phenotypes on different extracellular matrix monolayers. Biomaterials. 2004;25:5929–5938. doi: 10.1016/j.biomaterials.2004.01.044. [DOI] [PubMed] [Google Scholar]
- 66.Ohno T, Tanisaka K, Hiraoka Y, Ushida T, Tamaki T, Tateishi T. Effect of type I and type II collagen sponges as 3D scaffolds for hyaline cartilage-like tissue regeneration on phenotypic control of seeded chondrocytes in vitro. Mater Sci Eng C Mater Biol Appl. 2004;24:407–411. [Google Scholar]
- 67.Hubka KM, Dahlin RL, Meretoja VV, Kasper FK, Mikos AG. Enhancing chondrogenic phenotype for cartilage tissue engineering: monoculture and coculture of articular chondrocytes and mesenchymal stem cells. Tissue Eng Part B Rev. 2014;20:641–654. doi: 10.1089/ten.teb.2014.0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Woltersdorf C, Bonk M, Leitinger B, Huhtala M, Käpylä J, Heino J, et al. The binding capacity of α1β1-, α2β1- and α10β1-integrins depends on non-collagenous surface macromolecules rather than the collagens in cartilage fibrils. Matrix Biol. 2017;63:91–105. doi: 10.1016/j.matbio.2017.02.001. [DOI] [PubMed] [Google Scholar]
- 69.Nöth U, Rackwitz L, Heymer A, Weber M, Baumann B, Steinert A, et al. Chondrogenic differentiation of human mesenchymal stem cells in collagen type I hydrogels. J Biomed Mater Res A. 2007;83:626–635. doi: 10.1002/jbm.a.31254. [DOI] [PubMed] [Google Scholar]
- 70.Rutgers M, Saris DB, Vonk LA, van Rijen MH, Akrum V, Langeveld D, et al. Effect of collagen type I or type II on chondrogenesis by cultured human articular chondrocytes. Tissue Eng Part A. 2013;19:59–65. doi: 10.1089/ten.TEA.2011.0416. [DOI] [PubMed] [Google Scholar]
- 71.Schedel J, Distler O, Woenckhaus M, Gay RE, Simmen B, Michel BA, et al. Discrepancy between mRNA and protein expression of tumour suppressor maspin in synovial tissue may contribute to synovial hyperplasia in rheumatoid arthritis. Ann Rheum Dis. 2004;63:1205–1211. doi: 10.1136/ard.2003.006312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lu ZF, Doulabi BZ, Wuisman PI, Bank RA, Helder MN. Influence of collagen type II and nucleus pulposus cells on aggregation and differentiation of adipose tissue-derived stem cells. J Cell Mol Med. 2008;12:2812–2822. doi: 10.1111/j.1582-4934.2008.00278.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Vincent I, Tomomei S, Toshiyuki I. Magnetite/hydroxyapatite composite particles-assisted pore alignment of tilapia fish scale collagen-based scaffold. Key Eng Mater. 2016;696:121–128. [Google Scholar]
- 74.Zhang J, Yang Z, Li C, Dou Y, Li Y, Thote T, et al. Cells behave distinctly within sponges and hydrogels due to differences of internal structure. Tissue Eng Part A. 2013;19:2166–2175. doi: 10.1089/ten.tea.2012.0393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Nuernberger S, Cyran N, Albrecht C, Redl H, Vécsei V, Marlovits S. The influence of scaffold architecture on chondrocyte distribution and behavior in matrix-associated chondrocyte transplantation grafts. Biomaterials. 2011;32:1032–1040. doi: 10.1016/j.biomaterials.2010.08.100. [DOI] [PubMed] [Google Scholar]
- 76.Wise JK, Yarin AL, Megaridis CM, Cho M. Chondrogenic differentiation of human mesenchymal stem cells on oriented nanofibrous scaffolds: engineering the superficial zone of articular cartilage. Tissue Eng Part A. 2009;15:913–921. doi: 10.1089/ten.tea.2008.0109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Matthews JA, Wnek GE, Simpson DG, Bowlin GL. Electrospinning of collagen nanofibers. Biomacromolecules. 2002;3:232–238. doi: 10.1021/bm015533u. [DOI] [PubMed] [Google Scholar]
- 78.Matthews JA, Boland ED, Wnek GE, Simpson DG, Bowlin GL. Electrospinning of collagen type II: a feasibility study. J Bioact Compat Polym. 2003;18:125–134. [Google Scholar]
- 79.Yunoki S, Ikoma T, Monkawa A, Ohta K, Kikuchi M, Sotome S, et al. Control of pore structure and mechanical property in hydroxyapatite/collagen composite using unidirectional ice growth. Mater Lett. 2006;60:999–1002. [Google Scholar]
- 80.Ikoma T, Kobayashi H, Tanaka J, Walsh D, Mann S. Physical properties of type I collagen extracted from fish scales of Pagrus major and Oreochromis niloticas. Int J Biol Macromol. 2003;32:199–204. doi: 10.1016/s0141-8130(03)00054-0. [DOI] [PubMed] [Google Scholar]
- 81.Kawamata H, Kuwaki S, Mishina T, Ikoma T, Tanaka J, Nozaki R. Hierarchical viscosity of aqueous solution of tilapia scale collagen investigated via dielectric spectroscopy between 500 MHz and 2.5 THz. Sci Rep. 2017;7:45398. doi: 10.1038/srep45398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yang YL, Motte S, Kaufman LJ. Pore size variable type I collagen gels and their interaction with glioma cells. Biomaterials. 2010;31:5678–5688. doi: 10.1016/j.biomaterials.2010.03.039. [DOI] [PubMed] [Google Scholar]
- 83.Lee CR, Grodzinsky AJ, Spector M. The effects of cross-linking of collagen-glycosaminoglycan scaffolds on compressive stiffness, chondrocyte-mediated contraction, proliferation and biosynthesis. Biomaterials. 2001;22:3145–3154. doi: 10.1016/s0142-9612(01)00067-9. [DOI] [PubMed] [Google Scholar]
- 84.Vickers SM, Squitieri LS, Spector M. Effects of cross-linking type II collagen-GAG scaffolds on chondrogenesis in vitro: dynamic pore reduction promotes cartilage formation. Tissue Eng. 2006;12:1345–1355. doi: 10.1089/ten.2006.12.1345. [DOI] [PubMed] [Google Scholar]
- 85.Zhang J, Wu Y, Thote T, Lee EH, Ge Z, Yang Z. The influence of scaffold microstructure on chondrogenic differentiation of mesenchymal stem cells. Biomed Mater. 2014;9:035011. doi: 10.1088/1748-6041/9/3/035011. [DOI] [PubMed] [Google Scholar]
- 86.Miot S, Woodfield T, Daniels AU, Suetterlin R, Peterschmitt I, Heberer M, et al. Effects of scaffold composition and architecture on human nasal chondrocyte redifferentiation and cartilaginous matrix deposition. Biomaterials. 2005;26:2479–2489. doi: 10.1016/j.biomaterials.2004.06.048. [DOI] [PubMed] [Google Scholar]
- 87.Chao PG, Yodmuang S, Wang X, Sun L, Kaplan DL, Vunjak-Novakovic G. Silk hydrogel for cartilage tissue engineering. J Biomed Mater Res B Appl Biomater. 2010;95:84–90. doi: 10.1002/jbm.b.31686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lee P, Tran K, Chang W, Shelke NB, Kumbar SG, Yu X. Influence of chondroitin sulfate and hyaluronic acid presence in nanofibers and its alignment on the bone marrow stromal cells: cartilage regeneration. J Biomed Nanotechnol. 2014;10:1469–1479. doi: 10.1166/jbn.2014.1831. [DOI] [PubMed] [Google Scholar]
- 89.Discher DE, Janmey P, Wang YL. Tissue cells feel and respond to the stiffness of their substrate. Science. 2005;310:1139–1143. doi: 10.1126/science.1116995. [DOI] [PubMed] [Google Scholar]
- 90.Hutmacher DW. Scaffolds in tissue engineering bone and cartilage. Biomaterials. 2000;21:2529–2543. doi: 10.1016/s0142-9612(00)00121-6. [DOI] [PubMed] [Google Scholar]
- 91.Lv H, Wang H, Zhang Z, Yang W, Liu W, Li Y, et al. Biomaterial stiffness determines stem cell fate. Life Sci. 2017;178:42–48. doi: 10.1016/j.lfs.2017.04.014. [DOI] [PubMed] [Google Scholar]
- 92.Koay EJ, Shieh AC, Athanasiou KA. Creep indentation of single cells. J Biomech Eng. 2003;125:334–341. doi: 10.1115/1.1572517. [DOI] [PubMed] [Google Scholar]
- 93.Freeman PM, Natarajan RN, Kimura JH, Andriacchi TP. Chondrocyte cells respond mechanically to compressive loads. J Orthop Res. 1994;12:311–320. doi: 10.1002/jor.1100120303. [DOI] [PubMed] [Google Scholar]
- 94.Sanz-Ramos P, Mora G, Vicente-Pascual M, Ochoa I, Alcaine C, Moreno R, et al. Response of sheep chondrocytes to changes in substrate stiffness from 2 to 20 Pa: effect of cell passaging. Connect Tissue Res. 2013;54:159–166. doi: 10.3109/03008207.2012.762360. [DOI] [PubMed] [Google Scholar]
- 95.Schuh E, Hofmann S, Stok K, Notbohm H, Müller R, Rotter N. Chondrocyte redifferentiation in 3D: the effect of adhesion site density and substrate elasticity. J Biomed Mater Res A. 2012;100:38–47. doi: 10.1002/jbm.a.33226. [DOI] [PubMed] [Google Scholar]
- 96.Skotak M, Noriega S, Larsen G, Subramanian A. Electrospun cross-linked gelatin fibers with controlled diameter: the effect of matrix stiffness on proliferative and biosynthetic activity of chondrocytes cultured in vitro. J Biomed Mater Res A. 2010;95:828–836. doi: 10.1002/jbm.a.32850. [DOI] [PubMed] [Google Scholar]
- 97.Li WJ, Cooper JA, Mauck RL, Tuan RS. Fabrication and characterization of six electrospun poly(α-hydroxy ester)-based fibrous scaffolds for tissue engineering applications. Acta Biomater. 2006;2:377–385. doi: 10.1016/j.actbio.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 98.Park JS, Chu JS, Tsou AD, Diop R, Tang Z, Wang A, et al. The effect of matrix stiffness on the differentiation of mesenchymal stem cells in response to TGF-β. Biomaterials. 2011;32:3921–3930. doi: 10.1016/j.biomaterials.2011.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Woods A, Wang G, Beier F. RhoA/ROCK signaling regulates Sox9 expression and actin organization during chondrogenesis. J Biol Chem. 2005;280:11626–11634. doi: 10.1074/jbc.M409158200. [DOI] [PubMed] [Google Scholar]
- 100.Li YY, Choy TH, Ho FC, Chan PB. Scaffold composition affects cytoskeleton organization, cell-matrix interaction and the cellular fate of human mesenchymal stem cells upon chondrogenic differentiation. Biomaterials. 2015;52:208–220. doi: 10.1016/j.biomaterials.2015.02.037. [DOI] [PubMed] [Google Scholar]
- 101.Liu SQ, Tian Q, Hedrick JL, Po Hui JH, Ee PL, Yang YY. Biomimetic hydrogels for chondrogenic differentiation of human mesenchymal stem cells to neocartilage. Biomaterials. 2010;31:7298–7307. doi: 10.1016/j.biomaterials.2010.06.001. [DOI] [PubMed] [Google Scholar]
- 102.Nam J, Johnson J, Lannutti JJ, Agarwal S. Modulation of embryonic mesenchymal progenitor cell differentiation via control over pure mechanical modulus in electrospun nanofibers. Acta Biomater. 2011;7:1516–1524. doi: 10.1016/j.actbio.2010.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Toh WS, Lim TC, Kurisawa M, Spector M. Modulation of mesenchymal stem cell chondrogenesis in a tunable hyaluronic acid hydrogel microenvironment. Biomaterials. 2012;33:3835–3845. doi: 10.1016/j.biomaterials.2012.01.065. [DOI] [PubMed] [Google Scholar]
- 104.Lv H, Li L, Sun M, Zhang Y, Chen L, Rong Y, et al. Mechanism of regulation of stem cell differentiation by matrix stiffness. Stem Cell Res Ther. 2015;6:103. doi: 10.1186/s13287-015-0083-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Zhang Q, Lu H, Kawazoe N, Chen G. Pore size effect of collagen scaffolds on cartilage regeneration. Acta Biomater. 2014;10:2005–2013. doi: 10.1016/j.actbio.2013.12.042. [DOI] [PubMed] [Google Scholar]
- 106.Griffon DJ, Sedighi MR, Schaeffer DV, Eurell JA, Johnson AL. Chitosan scaffolds: interconnective pore size and cartilage engineering. Acta Biomater. 2006;2:313–320. doi: 10.1016/j.actbio.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 107.Nava MM, Draghi L, Giordano C, Pietrabissa R. The effect of scaffold pore size in cartilage tissue engineering. J Appl Biomater Funct Mater. 2016;14:e223–e229. doi: 10.5301/jabfm.5000302. [DOI] [PubMed] [Google Scholar]
- 108.Lin S, Sangaj N, Razafiarison T, Zhang C, Varghese S. Influence of physical properties of biomaterials on cellular behavior. Pharm Res. 2011;28:1422–1430. doi: 10.1007/s11095-011-0378-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Bryant SJ, Anseth KS. Hydrogel properties influence ECM production by chondrocytes photoencapsulated in poly(ethylene glycol) hydrogels. J Biomed Mater Res. 2002;59:63–72. doi: 10.1002/jbm.1217. [DOI] [PubMed] [Google Scholar]
- 110.Zhao Y, Tan K, Zhou Y, Ye Z, Tan WS. A combinatorial variation in surface chemistry and pore size of three-dimensional porous poly(ε-caprolactone) scaffolds modulates the behaviors of mesenchymal stem cells. Mater Sci Eng C Mater Biol Appl. 2016;59:193–202. doi: 10.1016/j.msec.2015.10.017. [DOI] [PubMed] [Google Scholar]
- 111.Oh SH, Kim TH, Im GI, Lee JH. Investigation of pore size effect on chondrogenic differentiation of adipose stem cells using a pore size gradient scaffold. Biomacromolecules. 2010;11:1948–1955. doi: 10.1021/bm100199m. [DOI] [PubMed] [Google Scholar]
- 112.Im GI, Ko JY, Lee JH. Chondrogenesis of adipose stem cells in a porous polymer scaffold: influence of the pore size. Cell Transplant. 2012;21:2397–2405. doi: 10.3727/096368912X638865. [DOI] [PubMed] [Google Scholar]
- 113.Malladi P, Xu Y, Chiou M, Giaccia AJ, Longaker MT. Effect of reduced oxygen tension on chondrogenesis and osteogenesis in adipose-derived mesenchymal cells. Am J Physiol Cell Physiol. 2006;290:C1139–C1146. doi: 10.1152/ajpcell.00415.2005. [DOI] [PubMed] [Google Scholar]
- 114.Buxton AN, Zhu J, Marchant R, West JL, Yoo JU, Johnstone B. Design and characterization of poly(ethylene glycol) photopolymerizable semi-interpenetrating networks for chondrogenesis of human mesenchymal stem cells. Tissue Eng. 2007;13:2549–2560. doi: 10.1089/ten.2007.0075. [DOI] [PubMed] [Google Scholar]
- 115.Shanmugasundaram S, Chaudhry H, Arinzeh TL. Microscale versus nanoscale scaffold architecture for mesenchymal stem cell chondrogenesis. Tissue Eng Part A. 2011;17:831–840. doi: 10.1089/ten.TEA.2010.0409. [DOI] [PubMed] [Google Scholar]
- 116.Bean AC, Tuan RS. Fiber diameter and seeding density influence chondrogenic differentiation of mesenchymal stem cells seeded on electrospun poly(ε-caprolactone) scaffolds. Biomed Mater. 2015;10:015018. doi: 10.1088/1748-6041/10/1/015018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Chen G, Ushida T, Tateishi T. Scaffold design for tissue engineering. Macromol Biosci. 2002;2:67–77. [Google Scholar]
- 118.Chen CW, Tsai YH, Deng WP, Shih SN, Fang CL, Burch JG, et al. Type I and II collagen regulation of chondrogenic differentiation by mesenchymal progenitor cells. J Orthop Res. 2005;23:446–453. doi: 10.1016/j.orthres.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 119.Bax DV, Davidenko N, Gullberg D, Hamaia SW, Farndale RW, Best SM, et al. Fundamental insight into the effect of carbodiimide crosslinking on cellular recognition of collagen-based scaffolds. Acta Biomater. 2017;49:218–234. doi: 10.1016/j.actbio.2016.11.059. [DOI] [PubMed] [Google Scholar]
- 120.Raghunath J, Rollo J, Sales KM, Butler PE, Seifalian AM. Biomaterials and scaffold design: key to tissue-engineering cartilage. Biotechnol Appl Biochem. 2007;46:73–84. doi: 10.1042/BA20060134. [DOI] [PubMed] [Google Scholar]
- 121.Hardy JG, Scheibel TR. Composite materials based on silk proteins. Prog Polym Sci. 2010;35:1093–1115. [Google Scholar]
- 122.Altman GH, Diaz F, Jakuba C, Calabro T, Horan RL, Chen J, et al. Silk-based biomaterials. Biomaterials. 2003;24:401–416. doi: 10.1016/s0142-9612(02)00353-8. [DOI] [PubMed] [Google Scholar]
- 123.Yeo IS, Oh JE, Jeong L, Lee TS, Lee SJ, Park WH, et al. Collagen-based biomimetic nanofibrous scaffolds: preparation and characterization of collagen/silk fibroin bicomponent nanofibrous structures. Biomacromolecules. 2008;9:1106–1116. doi: 10.1021/bm700875a. [DOI] [PubMed] [Google Scholar]
- 124.Meinel L, Hofmann S, Karageorgiou V, Zichner L, Langer R, Kaplan D, et al. Engineering cartilage-like tissue using human mesenchymal stem cells and silk protein scaffolds. Biotechnol Bioeng. 2004;88:379–391. doi: 10.1002/bit.20252. [DOI] [PubMed] [Google Scholar]
- 125.Wang J, Yang Q, Cheng N, Tao X, Zhang Z, Sun X, et al. Collagen/silk fibroin composite scaffold incorporated with PLGA microsphere for cartilage repair. Mater Sci Eng C Mater Biol Appl. 2016;61:705–711. doi: 10.1016/j.msec.2015.12.097. [DOI] [PubMed] [Google Scholar]
- 126.Wang J, Zhou W, Hu W, Zhou L, Wang S, Zhang S. Collagen/silk fibroin bi-template induced biomimetic bone-like substitutes. J Biomed Mater Res A. 2011;99:327–334. doi: 10.1002/jbm.a.32602. [DOI] [PubMed] [Google Scholar]
- 127.Chen L, Hu J, Ran J, Shen X, Tong H. Preparation and evaluation of collagen-silk fibroin/hydroxyapatite nanocomposites for bone tissue engineering. Int J Biol Macromol. 2014;65:1–7. doi: 10.1016/j.ijbiomac.2014.01.003. [DOI] [PubMed] [Google Scholar]
- 128.Lv Q, Feng Q, Hu K, Cui F. Three-dimensional fibroin/collagen scaffolds derived from aqueous solution and the use for HepG2 culture. Polymer (Guildf) 2005;46:12662–12669. [Google Scholar]
- 129.Chomchalao P, Pongcharoen S, Sutheerawattananonda M, Tiyaboonchai W. Fibroin and fibroin blended three-dimensional scaffolds for rat chondrocyte culture. Biomed Eng Online. 2013;12:28. doi: 10.1186/1475-925X-12-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Sun K, Li H, Li R, Nian Z, Li D, Xu C. Silk fibroin/collagen and silk fibroin/chitosan blended three-dimensional scaffolds for tissue engineering. Eur J Orthop Surg Traumatol. 2015;25:243–249. doi: 10.1007/s00590-014-1515-z. [DOI] [PubMed] [Google Scholar]
- 131.Di Martino A, Sittinger M, Risbud MV. Chitosan: a versatile biopolymer for orthopaedic tissue-engineering. Biomaterials. 2005;26:5983–5990. doi: 10.1016/j.biomaterials.2005.03.016. [DOI] [PubMed] [Google Scholar]
- 132.Suh JK, Matthew HW. Application of chitosan-based polysaccharide biomaterials in cartilage tissue engineering: a review. Biomaterials. 2000;21:2589–2598. doi: 10.1016/s0142-9612(00)00126-5. [DOI] [PubMed] [Google Scholar]
- 133.Chicatun F, Pedraza CE, Muja N, Ghezzi CE, McKee MD, Nazhat SN. Effect of chitosan incorporation and scaffold geometry on chondrocyte function in dense collagen type I hydrogels. Tissue Eng Part A. 2013;19:2553–2564. doi: 10.1089/ten.tea.2013.0114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Yan J, Li X, Liu L, Wang F, Zhu TW, Zhang Q. Potential use of collagen–chitosan–hyaluronan tri-copolymer scaffold for cartilage tissue engineering. Artif Cells Blood Substit Immobil Biotechnol. 2006;34:27–39. doi: 10.1080/10731190500430024. [DOI] [PubMed] [Google Scholar]
- 135.Tan W, Krishnaraj R, Desai TA. Evaluation of nanostructured composite collagen–chitosan matrices for tissue engineering. Tissue Eng. 2001;7:203–210. doi: 10.1089/107632701300062831. [DOI] [PubMed] [Google Scholar]
- 136.Chen Z, Mo X, He C, Wang H. Intermolecular interactions in electrospun collagen–chitosan complex nanofibers. Carbohydr Polym. 2008;72:410–418. [Google Scholar]
- 137.Yan LP, Wang YJ, Ren L, Wu G, Caridade SG, Fan JB, et al. Genipin-cross-linked collagen/chitosan biomimetic scaffolds for articular cartilage tissue engineering applications. J Biomed Mater Res A. 2010;95:465–475. doi: 10.1002/jbm.a.32869. [DOI] [PubMed] [Google Scholar]
- 138.Haaparanta AM, Järvinen E, Cengiz IF, Ellä V, Kokkonen HT, Kiviranta I, et al. Preparation and characterization of collagen/PLA, chitosan/PLA, and collagen/chitosan/PLA hybrid scaffolds for cartilage tissue engineering. J Mater Sci Mater Med. 2014;25:1129–1136. doi: 10.1007/s10856-013-5129-5. [DOI] [PubMed] [Google Scholar]
- 139.Raftery RM, Woods B, Marques ALP, Moreira-Silva J, Silva TH, Cryan SA, et al. Multifunctional biomaterials from the sea: assessing the effects of chitosan incorporation into collagen scaffolds on mechanical and biological functionality. Acta Biomater. 2016;43:160–169. doi: 10.1016/j.actbio.2016.07.009. [DOI] [PubMed] [Google Scholar]
- 140.Dai W, Kawazoe N, Lin X, Dong J, Chen G. The influence of structural design of PLGA/collagen hybrid scaffolds in cartilage tissue engineering. Biomaterials. 2010;31:2141–2152. doi: 10.1016/j.biomaterials.2009.11.070. [DOI] [PubMed] [Google Scholar]
- 141.Aspberg A. Cartilage proteoglycans. In: Grässel S, Aszódi A, editors. Cartilage. Springer; 2016. p. 1–22.
- 142.Knudson W, Loeser RF. CD44 and integrin matrix receptors participate in cartilage homeostasis. Cell Mol Life Sci. 2002;59:36–44. doi: 10.1007/s00018-002-8403-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Mahmood TA, Miot S, Frank O, Martin I, Riesle J, Langer R, et al. Modulation of chondrocyte phenotype for tissue engineering by designing the biologic-polymer carrier interface. Biomacromolecules. 2006;7:3012–3018. doi: 10.1021/bm060489+. [DOI] [PubMed] [Google Scholar]
- 144.Kawasaki K, Ochi M, Uchio Y, Adachi N, Matsusaki M. Hyaluronic acid enhances proliferation and chondroitin sulfate synthesis in cultured chondrocytes embedded in collagen gels. J Cell Physiol. 1999;179:142–148. doi: 10.1002/(SICI)1097-4652(199905)179:2<142::AID-JCP4>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 145.Zhang L, Li K, Xiao W, Zheng L, Xiao Y, Fan H, et al. Preparation of collagen–chondroitin sulfate-hyaluronic acid hybrid hydrogel scaffolds and cell compatibility in vitro. Carbohydr Polym. 2011;84:118–125. [Google Scholar]
- 146.van Susante JLC, Pieper J, Buma P, van Kuppevelt TH, van Beuningen H, van Der Kraan PM, et al. Linkage of chondroitin-sulfate to type I collagen scaffolds stimulates the bioactivity of seeded chondrocytes in vitro. Biomaterials. 2001;22:2359–2369. doi: 10.1016/s0142-9612(00)00423-3. [DOI] [PubMed] [Google Scholar]
- 147.Ko CS, Huang JP, Huang CW, Chu IM. Type II collagen–chondroitin sulfate-hyaluronan scaffold cross-linked by genipin for cartilage tissue engineering. J Biosci Bioeng. 2009;107:177–182. doi: 10.1016/j.jbiosc.2008.09.020. [DOI] [PubMed] [Google Scholar]
- 148.Zhu H, Mitsuhashi N, Klein A, Barsky LW, Weinberg K, Barr ML, et al. The role of the hyaluronan receptor CD44 in mesenchymal stem cell migration in the extracellular matrix. Stem Cells. 2006;24:928–935. doi: 10.1634/stemcells.2005-0186. [DOI] [PubMed] [Google Scholar]
- 149.Allemann F, Mizuno S, Eid K, Yates KE, Zaleske D, Glowacki J. Effects of hyaluronan on engineered articular cartilage extracellular matrix gene expression in 3-dimensional collagen scaffolds. J Biomed Mater Res. 2001;55:13–19. doi: 10.1002/1097-4636(200104)55:1<13::aid-jbm20>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 150.Taguchi T, Ikoma T, Tanaka J. An improved method to prepare hyaluronic acid and type II collagen composite matrices. J Biomed Mater Res. 2002;61:330–336. doi: 10.1002/jbm.10147. [DOI] [PubMed] [Google Scholar]
- 151.Liao E, Yaszemski M, Krebsbach P, Hollister S. Tissue-engineered cartilage constructs using composite hyaluronic acid/collagen I hydrogels and designed poly(propylene fumarate) scaffolds. Tissue Eng. 2007;13:537–550. doi: 10.1089/ten.2006.0117. [DOI] [PubMed] [Google Scholar]
- 152.Nishimoto S, Takagi M, Wakitani S, Nihira T, Yoshida T. Effect of chondroitin sulfate and hyaluronic acid on gene expression in a three-dimensional culture of chondrocytes. J Biosci Bioeng. 2005;100:123–126. doi: 10.1263/jbb.100.123. [DOI] [PubMed] [Google Scholar]
- 153.Vázquez-Portalatín N, Kilmer CE, Panitch A, Liu JC. Characterization of collagen type I and II blended hydrogels for articular cartilage tissue engineering. Biomacromolecules. 2016;17:3145–3152. doi: 10.1021/acs.biomac.6b00684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Guo Y, Yuan T, Xiao Z, Tang P, Xiao Y, Fan Y, et al. Hydrogels of collagen/chondroitin sulfate/hyaluronan interpenetrating polymer network for cartilage tissue engineering. J Mater Sci Mater Med. 2012;23:2267–2279. doi: 10.1007/s10856-012-4684-5. [DOI] [PubMed] [Google Scholar]
- 155.Li X, Teng Y, Liu J, Lin H, Fan Y, Zhang X. Chondrogenic differentiation of BMSCs encapsulated in chondroinductive polysaccharide/collagen hybrid hydrogels. J Mater Chem B. 2017;5:5109–5119. doi: 10.1039/c7tb01020f. [DOI] [PubMed] [Google Scholar]
- 156.Sionkowska A. Current research on the blends of natural and synthetic polymers as new biomaterials: review. Prog Polym Sci. 2011;36:1254–1276. [Google Scholar]
- 157.Mehrasa M, Anarkoli AO, Rafienia M, Ghasemi N, Davary N, Bonakdar S, et al. Incorporation of zeolite and silica nanoparticles into electrospun PVA/collagen nanofibrous scaffolds: the influence on the physical, chemical properties and cell behavior. Int J Polym Mater. 2016;65:457–465. [Google Scholar]
- 158.Lin HY, Tsai WC, Chang SH. Collagen-PVA aligned nanofiber on collagen sponge as bi-layered scaffold for surface cartilage repair. J Biomater Sci Polym Ed. 2017;28:664–678. doi: 10.1080/09205063.2017.1295507. [DOI] [PubMed] [Google Scholar]
- 159.Mansur HS, Sadahira CM, Souza AN, Mansur AAP. FTIR spectroscopy characterization of poly (vinyl alcohol) hydrogel with different hydrolysis degree and chemically crosslinked with glutaraldehyde. Mater Sci Eng C Mater Biol Appl. 2008;28:539–548. [Google Scholar]
- 160.Wan WK, Campbell G, Zhang ZF, Hui AJ, Boughner DR. Optimizing the tensile properties of polyvinyl alcohol hydrogel for the construction of a bioprosthetic heart valve stent. J Biomed Mater Res. 2002;63:854–861. doi: 10.1002/jbm.10333. [DOI] [PubMed] [Google Scholar]
- 161.Yousefi AM, Hoque ME, Prasad RGSV, Uth N. Current strategies in multiphasic scaffold design for osteochondral tissue engineering: a review. J Biomed Mater Res A. 2015;103:2460–2481. doi: 10.1002/jbm.a.35356. [DOI] [PubMed] [Google Scholar]
- 162.Ohyabu Y, Adegawa T, Yoshioka T, Ikoma T, Shinozaki K, Uemura T, et al. A collagen sponge incorporating a hydroxyapatite/chondroitinsulfate composite as a scaffold for cartilage tissue engineering. J Biomater Sci Polym Ed. 2009;20:1861–1874. doi: 10.1163/156856208X386462. [DOI] [PubMed] [Google Scholar]
- 163.Ohyabu Y, Adegawa T, Yoshioka T, Ikoma T, Uemura T, Tanaka J. Cartilage regeneration using a porous scaffold, a collagen sponge incorporating a hydroxyapatite/chondroitinsulfate composite. Mater Sci Eng B Solid State Mater Adv Technol. 2010;173:204–207. [Google Scholar]
- 164.Chen S, Zhang Q, Kawazoe N, Chen G. Effect of high molecular weight hyaluronic acid on chondrocytes cultured in collagen/hyaluronic acid porous scaffolds. RSC Adv. 2015;5:94405–94410. [Google Scholar]
- 165.Kreger ST, Voytik-Harbin SL. Hyaluronan concentration within a 3D collagen matrix modulates matrix viscoelasticity, but not fibroblast response. Matrix Biol. 2009;28:336–346. doi: 10.1016/j.matbio.2009.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Jia L, Duan Z, Fan D, Mi Y, Hui J, Chang L. Human-like collagen/nano-hydroxyapatite scaffolds for the culture of chondrocytes. Mater Sci Eng C Mater Biol Appl. 2013;33:727–734. doi: 10.1016/j.msec.2012.10.025. [DOI] [PubMed] [Google Scholar]
- 167.Szczesny SE, Aeppli C, David A, Mauck RL. Fatigue loading of tendon results in collagen kinking and denaturation but does not change local tissue mechanics. J Biomech. 2018;71:251–256. doi: 10.1016/j.jbiomech.2018.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Nakada A, Shigeno K, Sato T, Hatayama T, Wakatsuki M, Nakamura T. Optimal dehydrothermal processing conditions to improve biocompatibility and durability of a weakly denatured collagen scaffold. J Biomed Mater Res B Appl Biomater. 2017;105:2301–2307. doi: 10.1002/jbm.b.33766. [DOI] [PubMed] [Google Scholar]
- 169.Zeugolis DI, Khew ST, Yew ES, Ekaputra AK, Tong YW, Yung LY, et al. Electro-spinning of pure collagen nano-fibres—just an expensive way to make gelatin? Biomaterials. 2008;29:2293–2305. doi: 10.1016/j.biomaterials.2008.02.009. [DOI] [PubMed] [Google Scholar]
- 170.Grover CN, Cameron RE, Best SM. Investigating the morphological, mechanical and degradation properties of scaffolds comprising collagen, gelatin and elastin for use in soft tissue engineering. J Mech Behav Biomed Mater. 2012;10:62–74. doi: 10.1016/j.jmbbm.2012.02.028. [DOI] [PubMed] [Google Scholar]
- 171.Tuckwell DS, Ayad S, Grant ME, Takigawa M, Humphries MJ. Conformation dependence of integrin-type II collagen binding. Inability of collagen peptides to support alpha 2 beta 1 binding, and mediation of adhesion to denatured collagen by a novel alpha 5 beta 1-fibronectin bridge. J Cell Sci. 1994;107:993–1005. doi: 10.1242/jcs.107.4.993. [DOI] [PubMed] [Google Scholar]
- 172.Lv Q, Hu K, Feng Q, Cui F. Fibroin/collagen hybrid hydrogels with crosslinking method: preparation, properties, and cytocompatibility. J Biomed Mater Res A. 2008;84:198–207. doi: 10.1002/jbm.a.31366. [DOI] [PubMed] [Google Scholar]
- 173.Sensini A, Gualandi C, Cristofolini L, Tozzi G, Dicarlo M, Teti G, et al. Biofabrication of bundles of poly (lactic acid)-collagen blends mimicking the fascicles of the human Achille tendon. Biofabrication. 2017;9:015025. doi: 10.1088/1758-5090/aa6204. [DOI] [PubMed] [Google Scholar]
- 174.Maisani M, Ziane S, Ehret C, Levesque L, Siadous R, Le Meins JF, et al. A new composite hydrogel combining the biological properties of collagen with the mechanical properties of a supramolecular scaffold for bone tissue engineering. J Tissue Eng Regen Med. 2018;12:e1489–e1500. doi: 10.1002/term.2569. [DOI] [PubMed] [Google Scholar]
- 175.Klumpp D, Rudisile M, Kühnle RI, Hess A, Bitto FF, Arkudas A, et al. Three-dimensional vascularization of electrospun PCL/collagen-blend nanofibrous scaffolds in vivo. J Biomed Mater Res A. 2012;100:2302–2311. doi: 10.1002/jbm.a.34172. [DOI] [PubMed] [Google Scholar]
- 176.Prabhakaran MP, Venugopal J, Ramakrishna S. Electrospun nanostructured scaffolds for bone tissue engineering. Acta Biomater. 2009;5:2884–2893. doi: 10.1016/j.actbio.2009.05.007. [DOI] [PubMed] [Google Scholar]
- 177.Chen G, Sato T, Ushida T, Hirochika R, Shirasaki Y, Ochiai N, et al. The use of a novel PLGA fiber/collagen composite web as a scaffold for engineering of articular cartilage tissue with adjustable thickness. J Biomed Mater Res A. 2003;67:1170–1180. doi: 10.1002/jbm.a.10164. [DOI] [PubMed] [Google Scholar]
- 178.Yen HJ, Tseng CS, Hsu SH, Tsai CL. Evaluation of chondrocyte growth in the highly porous scaffolds made by fused deposition manufacturing (FDM) filled with type II collagen. Biomed Microdevices. 2009;11:615–624. doi: 10.1007/s10544-008-9271-7. [DOI] [PubMed] [Google Scholar]
- 179.Reboredo JW, Weigel T, Steinert A, Rackwitz L, Rudert M, Walles H. Investigation of migration and differentiation of human mesenchymal stem cells on five-layered collagenous electrospun scaffold mimicking native cartilage structure. Adv Healthc Mater. 2016;5:2191–2198. doi: 10.1002/adhm.201600134. [DOI] [PubMed] [Google Scholar]
- 180.Chen G, Sato T, Ushida T, Hirochika R, Tateishi T. Redifferentiation of dedifferentiated bovine chondrocytes when cultured in vitro in a PLGA-collagen hybrid mesh. FEBS Lett. 2003;542:95–99. doi: 10.1016/s0014-5793(03)00354-5. [DOI] [PubMed] [Google Scholar]
- 181.Chen CH, Shyu VB, Chen JP, Lee MY. Selective laser sintered poly-ε-caprolactone scaffold hybridized with collagen hydrogel for cartilage tissue engineering. Biofabrication. 2014;6:015004. doi: 10.1088/1758-5082/6/1/015004. [DOI] [PubMed] [Google Scholar]
- 182.Tanaka Y, Yamaoka H, Nishizawa S, Nagata S, Ogasawara T, Asawa Y, et al. The optimization of porous polymeric scaffolds for chondrocyte/atelocollagen based tissue-engineered cartilage. Biomaterials. 2010;31:4506–4516. doi: 10.1016/j.biomaterials.2010.02.028. [DOI] [PubMed] [Google Scholar]
- 183.Deponti D, Di Giancamillo A, Gervaso F, Domenicucci M, Domeneghini C, Sannino A, et al. Collagen scaffold for cartilage tissue engineering: the benefit of fibrin glue and the proper culture time in an infant cartilage model. Tissue Eng Part A. 2014;20:1113–1126. doi: 10.1089/ten.TEA.2013.0171. [DOI] [PubMed] [Google Scholar]
- 184.Xu T, Binder KW, Albanna MZ, Dice D, Zhao W, Yoo JJ, et al. Hybrid printing of mechanically and biologically improved constructs for cartilage tissue engineering applications. Biofabrication. 2013;5:015001. doi: 10.1088/1758-5082/5/1/015001. [DOI] [PubMed] [Google Scholar]