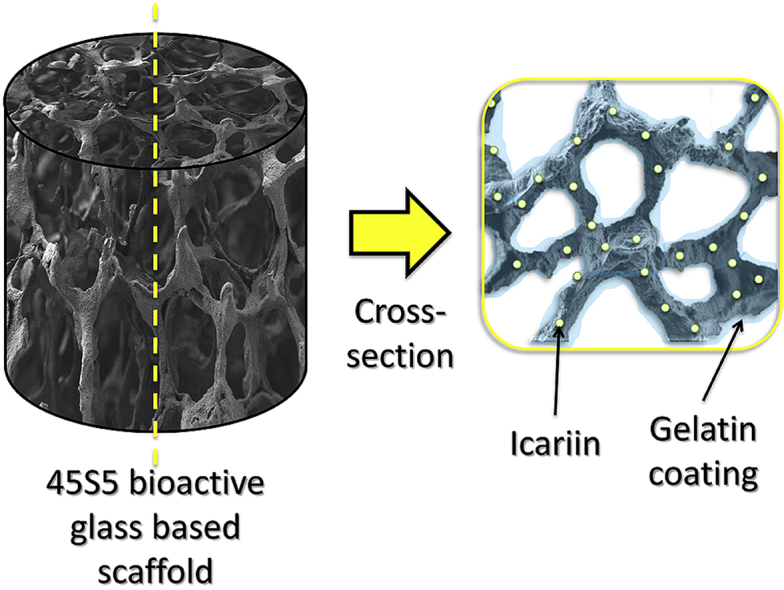
Abstract
Gelatin-coated, 3D sponge-like scaffolds based on 45S5 bioactive glass were produced using the foam replication technique. Compressive strength tests of gelatin-coated samples compared to uncoated scaffolds showed significant strengthening and toughening effects of the gelatin coating with compressive strength values in the range of cortical bone. Additionally, the crosslinked gelatin network (using either caffeic acid or N-(3-Dimethylaminopropyl)-N′-ethylcarbodiimide hydrochloride (EDC)/N-hxdroxysuccinimide (NHS) as crosslinking agent) was shown to be a suitable candidate for the sustained release of the bioactive molecule icariin. Concerning bioactivity of the produced scaffolds, characterization by FTIR and SEM indicated the formation of hydroxyapatite (HA) in all samples after immersion in simulated body fluid (SBF) for 14 days, highlighting the favorable combination of mechanical robustness, bioactivity and drug delivery capability of this new type of scaffolds.
Keywords: Bioactive glass, Icariin, Drug release, Gelatin, Scaffold
Graphical abstract
Highlights
-
•
Foam like bioactive glass scaffolds produced by replication technique.
-
•
Gelatin coatings confer increased compression strength to scaffolds.
-
•
Crosslinked gelatin coating is suitable candidate for the sustained release of icariin.
-
•
Favorable combination of bioactivity, gelatin coating and icariin release demonstrated.
1. Introduction
For applications in tissue engineering bioactive glasses are being widely researched due to their bone bonding ability as well as osteogenic and angiogenic properties [[1], [2], [3]]. By using the foam replica technique, bioactive glass based constructs can be fabricated which exhibit the basic characteristics required for bone tissue engineering scaffolds, e.g. interconnected pores and high porosity (∼90%), osteoconductivity and temporary mechanical stability [4]. However, bioactive glass based scaffolds can be further functionalized to achieve higher mechanical stability, particularly toughness, and to provide drug-delivery capability [[5], [6], [7], [8]]. Synthetic and natural polymer-coatings come into operation to increase mechanical stability and serve as carrier matrix for the local release of growth factors or antibiotics to support bone tissue formation [8]. Usually, bone morphogenetic proteins (BMP), and other growth factors such as TGF-β, IGFs or PDGFs are used for the functionalization of scaffolds [9,10]. However, there are some limitations associated with the use of growth factors due to their rapid degradation during processing and relatively high costs [11]. Alternatives to the use of growth factors are being sought for example focusing on biological active ions [12,13] and natural biological agents (e.g. phytotherapeutics) [14,15]. An inexpensive, highly abundant alternative is the flavonoid compound of Epimedium sagitattum, a traditional Chinese herb, which has been used for the treatment of fractures, bone and joint diseases for hundreds of years, known as icariin [16]. Studies have shown that icariin is capable of accelerating cell cycle and promoting osteogenic differentiation of bone marrow stem cells (BMSCs), furthermore it enhances mineralization and promotes bone defect repair [[17], [18], [19], [20], [21]]. Additionally, icariin has neuroprotective, cardiovascular protective, anti-cancer and anti-inflammation effects [22]. Icariin has been combined with gelatin/hyaluronic acid composite microspheres [21], with porous PHBV scaffolds [18], chitosan/hydroxyapatite scaffolds [19] and, more recently, with bioactive glasses [23]. In this study icariin is considered for the first time in combination with gelatin/bioactive glass (45S5 composition) based scaffolds.
The relative efficiencies of different gelatin crosslinking agents, namely caffeic acid and N-(3-Dimethylaminopropyl)-N′-ethylcarbodiimide hydrochloride (EDC)/N-hxdroxysuccinimide (NHS), are investigated and the mechanical properties, bioactive behavior and drug release capability of the different scaffold types were considered. The main goal of this study was thus to investigate the double function of gelatin coating on BG based scaffolds, namely achieving improved mechanical properties and providing a suitable vehicle for the delivery of icariin.
2. Materials and methods
2.1. Chemicals and reagents
As scaffold material 45S5 bioactive glass (BG) powder (chemical composition: 45 wt% SiO2, 24.5 wt% Na2O, 24.5 wt% CaO and 6 wt% P2O5) [4] (particle size ∼5 μm) was used. For the foam replication technique, polyurethane (PU) foam, supplied by Eurofoam (Troisdorf, Germany), was used as sacrificial template and icariin was obtained from Changsha Herbway Biotech Co. Ltd (China). Gelatin type A from porcine skin (∼300 bloom), type B from bovine skin (∼225 bloom), 2-(N-morpholino) ethanesulfonic acid hydrate (MES), N-hxdroxysuccinimide (NHS) and caffeic acid (CA) were obtained from Sigma (St. Louis, MO, USA). Phosphate buffered saline (PBS) tablets were purchased from VWR Life Science AMRESCO (Cochran Road, OH, USA) and N-(3-Dimethylaminopropyl)-N′-ethylcarbodiimide hydrochloride (EDC) was purchased from Merck (Billerica MA, USA). All other reagents used were of analytical grade.
2.2. Scaffold fabrication
All scaffolds were fabricated by the foam replication technique as described by Chen et al. [4]. Briefly, a slurry was prepared by dissolving 0.136 mol/l polyvinyl alcohol in DI water at 80 °C. After cooling down, bioactive glass powder was added up to concentration of 50 wt%. The slurry was mixed for one hour and vigorous stirring was carried out by using a magnet stirrer. Cylindrical polyurethane foam samples, which served as sacrificial templates, were immersed in the above-described slurry for one minute, were then retrieved and the extra slurry was squeezed out. The immersed foams were dried at 60 °C for one hour. The procedure was repeated two more times to obtain triple-coated green bodies. Then heat treatment was applied as follows: to burnout the sacrificial templates, samples were hold at 400 °C for 1 h, subsequently, sintering was carried out at 1050 °C for 2 h. The heating rate was 2 °C/min in both cases. After sintering, the furnace was left for natural cool down.
2.3. Coating procedure
Uncoated scaffolds were used as reference. In general, coating of scaffolds with gelatin was executed as described by Metze et al. [24]. Here, the scaffolds were immersed in the respective solution for 30 s, retrieved and then rolled over a tissue paper to remove excess solution. The so-coated scaffolds were left to dry at ambient temperature in air for at least 24 h.
2.3.1. Gelatin solution (G)
Gelatin type A and type B, respectively, were dissolved in DI water at a concentration of 7.5 wt% under vigorous stirring at 40 °C by using a magnetic stirrer. If gelatin type B was used, samples were labelled as GB, whereas gelatin type A samples were labelled as GA.
2.3.2. Crosslinking with caffeic acid (G/CA)
For the crosslinker solution, caffeic acid was dissolved in DI water in a concentration of 20 wt% and the pH was adjusted to 9 by adding 1 M NaOH. This stock solution was stirred for at least 30 min. Separately, a gelatin solution prepared as mentioned above was set to pH 9 prior to adding crosslinking solution to a ratio of 1.5% w/w (related to dry gelatin). The procedure was adapted from studies of Zhang et al. [25] and Kosaraju et al. [26].
2.3.3. Caffeic acid crosslinked gelatin loaded with icariin (GA/CA/I, GB/CA/I)
Icariin was dissolved in DI water to obtain a 2% (w/v to gelatin sol.) solution under vigorous stirring for several minutes. Gelatin solutions with gelatin type A and gelatin type B, respectively, were prepared as mentioned above and the icariin solution was added. After stirring for several minutes, the crosslinking procedure was carried out as described above.
2.3.4. Crosslinking with EDC and NHS (G50, G100)
For the crosslinking with EDC in combination with NHS, MES was dissolved in 40% ethanol to create a 50 mM stock solution (pH 5.5) first. By adding different amounts of EDC and NHS (50 and 100 mM) (EDC/NHS, 1:1) to the MES solution, two different crosslinking solutions were prepared. The mixtures were stirred using a magnetic stirrer until completely solved. To carry out the crosslinking process, the gelatin-coated BG scaffolds were immersed in the prepared solutions and put in the refrigerator at 5 °C for 4 h. By suspending the scaffolds in a 0.1 M Na2HPO4 solution for 2 h, the crosslinking reaction was stopped. The samples were then extensively washed several times with DI water and left to dry for 72 h at ambient temperature [[27], [28], [29]].
2.3.5. EDC crosslinked gelatin loaded with icariin (G + I50, G + I100, G50I, G100I)
To load the bioactive glass scaffolds with icariin, two different procedures were conducted to examine the effects of loading the drug before or after crosslinking. For the first method (before crosslinking, labelled G+), icariin solution was directly added to gelatin solution as described in section 2.3.3. Then the coating and crosslinking procedures were carried out as described above. In the second method (after crosslinking), an icariin solution (2 wt%) was prepared by dissolving the powder in DI water. The coated and crosslinked scaffolds were then immersed in the solution for 1 h, extracted and left to dry at ambient temperature.
Table 1 shows a summary of the scaffolds finally prepared.
Table 1.
Overview of all produced samples. (BGS = bioactive glass scaffold, icariin added b = before and a = after crosslinking).
| Label | Description | Crosslinker | Ratio | Icariin |
|---|---|---|---|---|
| uncoated | Uncoated BGS | – | – | – |
| G | Gelatin coated BGS | – | – | – |
| G50 | Crosslinked gelatin coated BGS | EDC/NHS | 50 mM | – |
| G100 | Crosslinked gelatin coated BGS | EDC/NHS | 100 mM | – |
| G + I50 | Crosslinked gelatin coated BGS, Icariin | EDC/NHS | 50 mM | x (b) |
| G + I100 | Crosslinked gelatin coated BGS, Icariin | EDC/NHS | 100 mM | x (b) |
| G50 I | Crosslinked gelatin coated BGS, Icariin | EDC/NHS | 50 mM | x (a) |
| G100 I | Crosslinked gelatin coated BGS, Icariin | EDC/NHS | 100 mM | x (a) |
| G/CA | Crosslinked gelatin coated BGS | Caffeic acid | 1.5% | – |
| GA/CA/I | Crosslinked gelatin (A) coated BGS, Icariin | Caffeic acid | 1.5% | x (b) |
| GB/CA/I | Crosslinked gelatin (B) coated BGS, Icariin | Caffeic acid | 1.5% | x (b) |
2.4. Characterization
2.4.1. Scanning Electron Microscopy
The morphology of the scaffolds before and after immersion in SBF for 7 and 14 d was determined by Scanning Electron Microscopy (SEM) (Zeiss Auriga 4750). The scaffolds were fixed with conductive silver and sputter coated with gold (Sputter Coater Q150 TS).
2.4.2. Fourier transform infrared spectroscopy
Fourier transform infrared spectra were obtained using FTIR Spectrometer (IRAffinity-1S, SHIMADZU, Germany). The data was recorded for samples immersed in SBF for 0 days, 7 days and 14 days in the range of 1700–400 cm-1 with a resolution of 4 cm-1.
2.4.3. Mechanical properties
The mechanical behavior of the cylindrical scaffolds (diameter = 16 mm, height = 12 mm) was analyzed in compression using a universal testing machine (Zwick Z 050) at a crosshead speed of 5 mm/min with an initial load of 0.1 N. The load cell used was of 50 N for uncoated and 1 kN for coated samples. The test was conducted for five samples of each scaffold type.
2.4.4. Drug release studies
To study the released amount of icariin, 3 scaffolds of each sample type were submerged in 5 mL of PBS and incubated at 37 °C. After 0.5 h, 1 h, 4 h, 6 h, 24 h, 3 d, 7 d and 14 d, and additionally 22 d and 36 d for CA crosslinked ones, the PBS solution was extracted from the sample container and stored at ambient temperature. The containers with the samples were refilled with 5 ml of fresh PBS solution immediately and put back into the shaking incubator after each time point. The icariin concentration released from scaffolds was measured by UV–Vis-spectrometry (Analytic Jena, Specord 40) at λ = 270 nm.
2.4.5. Assessment of bioactivity in vitro
The in vitro bioactivity (or surface bioreactivity) of scaffolds was tested following the standard procedure described by Kokubo et al. [30]. The scaffolds immersed in 50 mL simulated body fluid (SBF) were placed in a shaking incubator at 37 °C for 7 and 14 d. Afterwards they were washed with deionized water and left to dry at room temperature for at least 3 d prior to FTIR analysis and SEM observation.
3. Results and discussion
3.1. Scanning electron microscopy
SEM images confirmed the highly porous structure of the BG scaffolds exhibiting interconnected porosity and pore sizes ranging from about 300 to 500 μm.
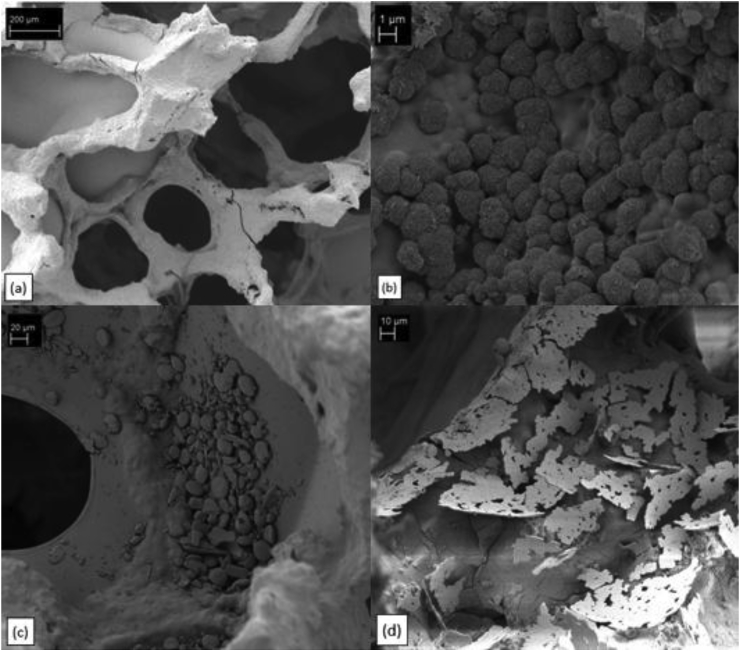
Fig. 1 shows scaffolds coated with EDC/NHS-crosslinked gelatin (type A). It should be noted that all samples, regardless of the used type of gelatin or crosslinking agent, were successfully covered with a well attached and smooth gelatin layer (Fig. 1a). However, it can be seen that some pores are clogged which can probably be attributed to the removal procedure of excess solution by simply rolling the specimen over tissue paper. For all samples immersed in SBF for 7 and 14 d, cauliflower-like shaped structures (Fig. 1b) could be detected, which indicate the formation of hydroxyapatite (HA), as expected.
Fig. 1.
SEM images of gelatin (type A) coated scaffolds crosslinked with EDC/NHS: a) struts of a scaffold coated with gelatin, b) cauliflower shaped structures indicating HA formation after immersion in SBF for 14 d, c) icariin particles situated on top of gelatin coating, d) plate-shaped morphology of HA particles after immersion in SBF for 14 d.
Fig. 1c illustrates a specimen where icariin was loaded after crosslinking with EDC. Apparently, the icariin particles (formed by agglomeration) are not incorporated into the gelatin layer. Instead, they are mostly situated on top of the gelatin surface. Based on this observation it is likely that the formation of the HA-like layer is affected by the presence of icariin in the SBF solution after immersion. The HA particles after 7 or 14 d show plate-shaped morphologies as presented in Fig. 1d, which can be attributed to the icariin content. These results evidence the stimulating effect of icariin on HA formation and are in good agreement with findings from Zhao et al. [16].
3.2. Fourier transform infrared spectra
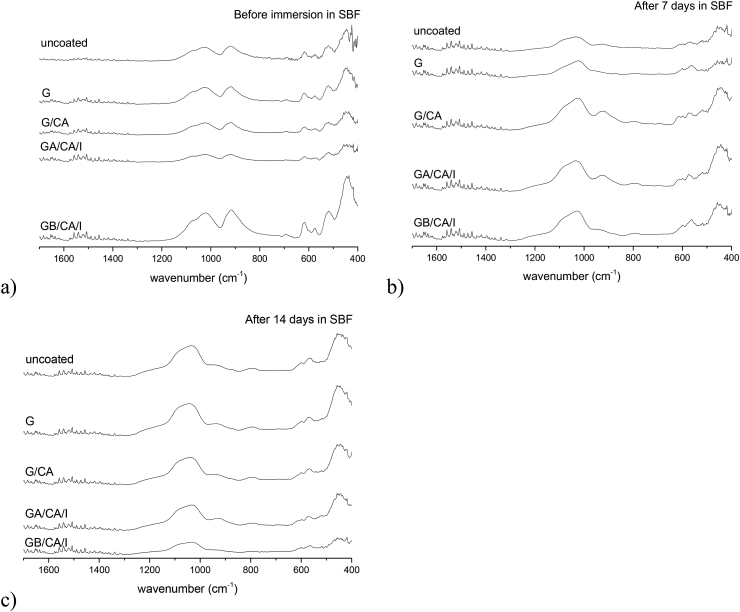
In Fig. 2a, the FTIR spectra of the different uncoated and gelatin coated samples can be seen. Each spectrum exhibits the characteristic absorbance profile of BG by showing the main absorption bands at 1024 cm−1, which is attributed to Si—O—Si stretching mode, at 926 cm−1, which is attributed to Si—O stretching mode and at 480 cm−1, which is attributed to Si—O—Si bending mode [31]. Additionally, the FTIR spectra of gelatin coated and crosslinked gelatin coated samples demonstrate the typical amide bands at ∼1630 (C O stretching), ∼1540 (N—H deformation) and ∼1250 (N—H deformation) cm−1, corresponding to vibrations in amide I, II and III bands. Additionally, the bands at ∼1380 and ∼1440 cm−1 are characteristic of amino acids in the gelatin structure [32], [33]. The presence of caffeic acid and icariin is not evident in these spectra.
Fig. 2.
FTIR spectra of uncoated, gelatine coated, gelatine crosslinked with caffeic acid coated and icariin-gelatine (A or B) crosslinked coated BG scaffolds before (a), after 7 days (b) and 14 days (c) immersion in SBF.
The FTIR spectra of uncoated and coated samples immersed for 7 (Figs. 2b) and 14 (Fig. 2c) days in SBF indicate the formation of HA by a peak appearing at 1036 cm−1 corresponding to P—O stretch of a phosphate enriched layer. Additionally, the vibrational band between 850 and 900 cm−1, corresponding to C—O stretch of CO32-, and bands at ∼600 and 563 cm−1, corresponding to P—O bending of PO43−, can be detected after 7 and 14 days in SBF [24,34].
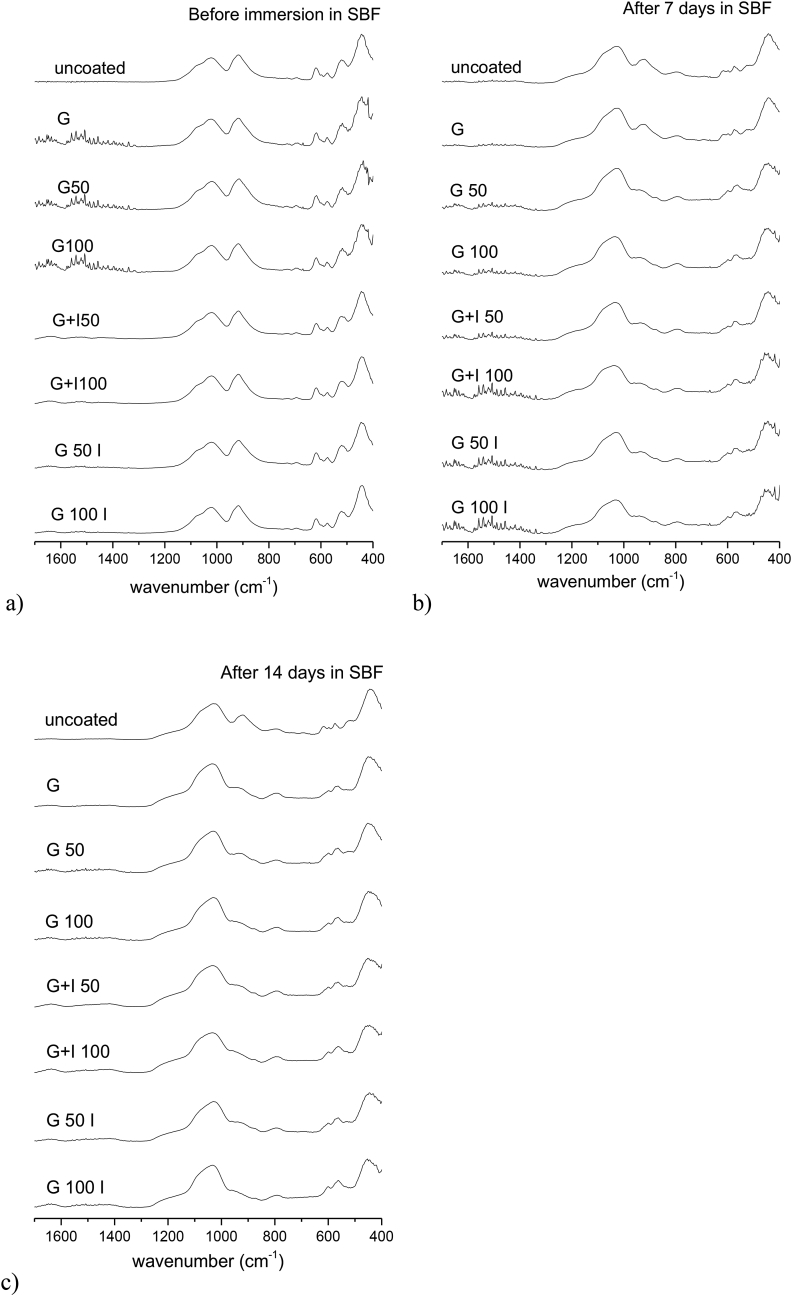
According to Fig. 3, the results obtained for gelatine coated BG scaffolds crosslinked with 50 mM and 100 mM EDC are similar to the ones obtained from samples crosslinked with caffeic acid. The same characteristic peaks for BG can be found in Fig. 3a. Moreover, as already found for caffeic acid-gelatine coated scaffolds with and without icariin, the presence of EDC and icariin cannot be clearly detected. Additionally, according to Fig. 3b and c, the characteristic peaks indicating the formation of HA can be detected after 7 and 14 days in SBF.
Fig. 3.
FTIR spectra of uncoated, gelatine coated, gelatine crosslinked with 50 mM or 100 mM EDC coated and icariin-gelatine (A or B) crosslinked coated BG scaffolds before (a), after 7 days (b) and 14 days (c) immersion in SBF.
3.3. Mechanical properties
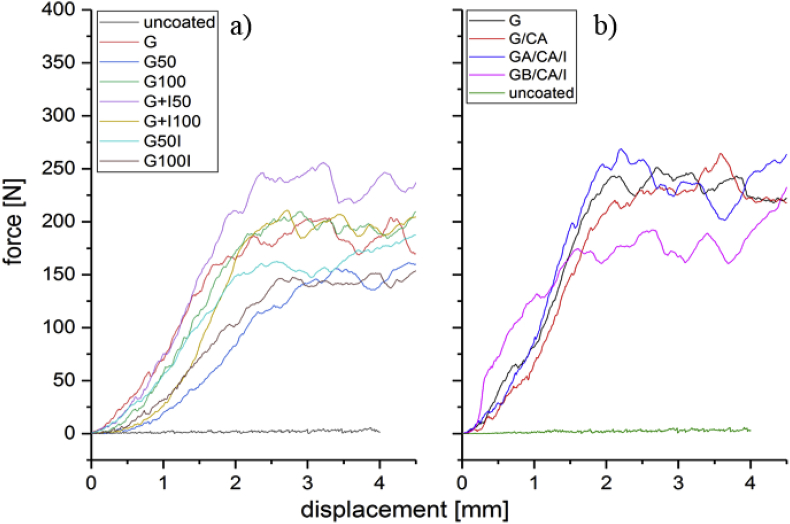
Fig. 4 shows typical force vs. displacement curves in compression of all types of scaffolds. The highly scattered curve at the bottom is characteristic for uncoated BG scaffolds [4]. Due to their brittleness, the samples collapse almost instantly under load and reach maximum compressive strength values of 0.1 MPa. The scattering arises from the continuous fracture of individual struts during the test. The compressive strength as well as the work of fracture (related to the area under the curve) increased drastically when the scaffolds were coated with gelatin. Independently of the used crosslinking agent, the maximum compressive strength reached values in the range 1.8 MPa–2.9 MPa for all samples with crosslinked and uncrosslinked gelatin-coating, indicating a significant improvement of the mechanical property over the uncoated scaffolds. The increase of the mechanical properties of brittle scaffolds with the application of polymer coatings has been previously discussed in detail [7,8]. A crack bridging mechanism is likely active, which leads to an increase of the work of fracture, with scaffolds becoming more resistant to catastrophic fracture under loads.
Fig. 4.
Force vs. displacement curves of coated and uncoated scaffolds crosslinked with a) EDC/NHS and b) caffeic acid.
3.4. Drug release
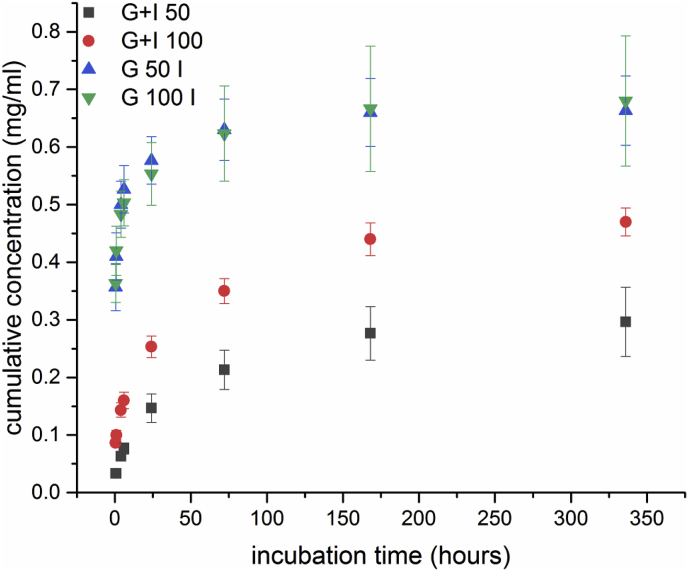
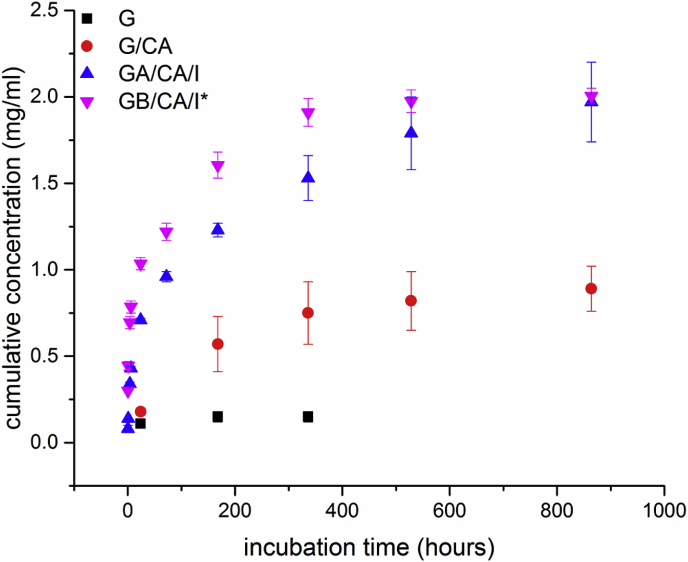
Fig. 5, Fig. 6 show the release profiles of icariin from different scaffold types. An initial burst release after the first 6 h of incubation can be seen for all systems and this result can be attributed to the release of superficially entrapped icariin as swelling of the gelatin coatings occurred, which has been reported in literature [19]. For G50I/G100I scaffolds (Fig. 5) this release mechanism is preferential as icariin is only attached to the surface due to the fact that it was loaded after crosslinking and hence a great amount (0.35 mg/ml) is released after only 0.5 h in a bust release manner. In comparison, scaffolds loaded with icariin before crosslinking show a more sustained profile as released cumulative concentrations are significantly higher after the initial stage. The overall significant lower values can be explained by the unintentional release of icariin during the crosslinking process. In contrast, Fig. 6 shows release profiles and maximum released concentrations for CA crosslinked scaffolds much higher than for EDC/NHS crosslinked ones, namely ∼2.00 mg/ml versus ∼0.45–0.7 mg/ml. Furthermore, CA leads to an interference with drug absorbance. The CA “release” reaches a maximum at ∼0.8 mg/ml after 14 d, from which no significant cumulative amount of CA is further released. However, release profiles for the icariin loaded scaffolds (GA/CA/I, GB/CA/I*) still show an increasing trend. Hence, the individual values of released concentration cannot be assessed (due to interference with CA). Nevertheless profile trends can be interpreted qualitatively. After the initial stage, both profiles for GA and GB increase steadily to a much higher maximum concentration than the concentration released in the initial stage, whereas GB reaches a maximum after 14 d and GA not until 36 d. This result may be attributed to different electrostatically interactions between gelatin type A and type B, respectively, with icariin, referred to as polyion complex in literature [35]. The slower release rates of all samples after the initial stage can be associated to slow diffusion processes and/or gelatin degradation after the swelling equilibrium is reached [19,36]. The obtained results for the release behavior of G+ scaffolds and CA crosslinked ones show a sustained release of icariin. After an initial burst release, release continues with decreasing release rate, which is likely due to a change of the release mechanisms. More work is required to investigate the release behavior of the different scaffold types, especially considering also the possible effect of HA formation on the sample struts on the long-term icariin release kinetics. Indeed the long-term icariin release behavior will have a complex dependence with the characteristics and degradation kinetics of the gelatin coating (linked to the crosslinking method used) and with the kinetics of formation of HA, a quantitative analysis of these factors to control icariin release remains a subject of future research.
Fig. 5.
Cumulative release concentration of icariin from EDC/NHS crosslinked scaffolds.
Fig. 6.
Cumulative release concentrations of icariin, G and G/CA from CA crosslinked scaffolds.
4. Conclusion
Bioactive glass based scaffolds were significantly reinforced by gelatin-coatings, considering both gelatin types (A, B). Furthermore, loading with icariin was shown to enhance the mineralization (formation of HA), as revealed by SEM observations and FTIR measurements. Additionally, different crosslinking methods resulted in different release profiles of icariin. Sustained release profiles for G+50I, G+100I, GA/CA/I and GB/CA/I scaffolds were observed, which confirm the dual effect of the gelatin coating imparting mechanical property improvement and drug delivery function, similarly to recent results in literature on composite scaffolds [37]. However, further investigations should be carried out to quantify the long-term release of the drug as function of crosslinking method.
Acknowledgements
Authors would like to thank Alina Grünewald for experimental support. We thank Prof. Dirk Schubert for granting access to experimental facilities at the Institute of Polymer Materials (FAU).
Footnotes
Peer review under responsibility of KeAi Communications Co., Ltd.
References
- 1.Hench L.L., Polak J.M. Third-generation biomedical materials. Science. 2002;295(5557):1014–1017. doi: 10.1126/science.1067404. [DOI] [PubMed] [Google Scholar]
- 2.Gorustovich A.A., Roether J.A., Boccaccini A.R. Effect of bioactive glasses on angiogenesis: a review of in vitro and in vivo evidences. Tissue Eng. B Rev. 2009;16(2):199–207. doi: 10.1089/ten.TEB.2009.0416. [DOI] [PubMed] [Google Scholar]
- 3.El-Rashidy A.A., Roether J.A., Harhaus L., Kneser U., Boccaccini A.R. Regenerating bone with bioactive glass scaffolds: a review of in vivo studies in bone defect models. Acta Biomater. 2017;62:1–28. doi: 10.1016/j.actbio.2017.08.030. [DOI] [PubMed] [Google Scholar]
- 4.Chen Q.Z., Thompson I.D., Boccaccini A.R. 45S5 Bioglass®-derived glass-ceramic scaffolds for bone tissue engineering. Biomaterials. 2006;27:2414–2425. doi: 10.1016/j.biomaterials.2005.11.025. [DOI] [PubMed] [Google Scholar]
- 5.Fu Q., Saiz E., Tomsia A.P. Direct ink writing of highly porous and strong glass scaffolds for load-bearing bone defects repair and regeneration. Acta Biomater. 2011;7:3547–3554. doi: 10.1016/j.actbio.2011.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li W., Nooeaid P., Roether J.A., Schubert D.W., Boccaccini A.R. Preparation and characterization of vancomycin releasing PHBV coated 45S5 Bioglass®-based glass–ceramic scaffolds for bone tissue engineering. J. Eur. Ceram. Soc. 2014;34:505–514. [Google Scholar]
- 7.Yunos D.M., Bretcanu O., Boccaccini A.R. Polymer-bioceramic composites for tissue engineering scaffolds. J. Mater. Sci. 2008;43(13):4433. [Google Scholar]
- 8.Philippart A., Boccaccini A.R., Fleck C., Schubert D.W., Roether J.A. Toughening and functionalization of bioactive ceramic and glass bone scaffolds by biopolymer coatings and infiltration - a review of the last 5 years. Expet Rev. Med. Dev. 2015;12:93–111. doi: 10.1586/17434440.2015.958075. [DOI] [PubMed] [Google Scholar]
- 9.Liu W.-C., Robu I.S., Patel R., Leu M.C., Velez M., Chu T.-M.G. The effects of 3D bioactive glass scaffolds and BMP-2 on bone formation in rat femoral critical size defects and adjacent bones. Biomed. Mater. 2014;9:45013. doi: 10.1088/1748-6041/9/4/045013. [DOI] [PubMed] [Google Scholar]
- 10.Liu X., Rahaman M.N., Liu Y., Bal B.S., Bonewald L.F. Enhanced bone regeneration in rat calvarial defects implanted with surface-modified and BMP-loaded bioactive glass (13–93) scaffolds. Acta Biomater. 2013;9:7506–7517. doi: 10.1016/j.actbio.2013.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee K., Silva E.A., Mooney D.J. Growth factor delivery-based tissue engineering: general approaches and a review of recent developments. J. R. Soc. Interface. 2011;8:153–170. doi: 10.1098/rsif.2010.0223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hoppe A., Güldal N.S., Boccaccini A.R. A review of the biological response to ionic dissolution products from bioactive glasses and glass-ceramics. Biomaterials. 2011;32:2757–2774. doi: 10.1016/j.biomaterials.2011.01.004. [DOI] [PubMed] [Google Scholar]
- 13.Wu C., Zhou Y., Fan W., Han P., Chang J., Yuen J., Zhang M., Xiao Y. Hypoxia-mimicking mesoporous bioactive glass scaffolds with controllable cobalt ion release for bone tissue engineering. Biomaterials. 2012;33:2076–2085. doi: 10.1016/j.biomaterials.2011.11.042. [DOI] [PubMed] [Google Scholar]
- 14.Cazzola M., Corazzari I., Prenesti E., Bertone E., Vernè E., Ferraris S. Bioactive glass coupling with natural polyphenols: surface modification, bioactivity and anti-oxidant ability. Appl. Surf. Sci. 2016;367:237–248. [Google Scholar]
- 15.Galarraga-Vinueza M.E., Mesquita-Guimarães J., Magini R.S., Souza J.C., Fredel M.C., Boccaccini A.R. Mesoporous bioactive glass embedding propolis and cranberry antibiofilm compounds. J. Biomed. Mater. Res. Part A. 2018;106:1614–1625. doi: 10.1002/jbm.a.36352. [DOI] [PubMed] [Google Scholar]
- 16.Zhao J., Ohba S., Komiyama Y., Shinkai M., Chung U., Nagamune T. Icariin: a potential osteoinductive compound for bone tissue engineering. Tissue Eng. 2010;16:233–243. doi: 10.1089/ten.TEA.2009.0165. [DOI] [PubMed] [Google Scholar]
- 17.Fan J.-J., et al. The dose-effect of icariin on the proliferation and osteogenic differentiation of human bone mesenchymal stem cells. Molecules. 2011;16:10123–10133. doi: 10.3390/molecules161210123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xia L., Li Y., Zhou Z., Dai Y., Liu H., Liu H. Icariin delivery porous PHBV scaffolds for promoting osteoblast expansion in vitro. Mater. Sci. Eng. C. 2013;33:3545–3552. doi: 10.1016/j.msec.2013.04.050. [DOI] [PubMed] [Google Scholar]
- 19.Wu T., et al. A new bone repair scaffold combined with chitosan/hydroxyapatite and sustained releasing icariin. Chin. Sci. Bull. 2009;54:2953–2961. [Google Scholar]
- 20.Lai Y., Cao H., Wang X., Chen S., Zhang M., et al. Porous composite scaffold incorporating osteogenic phytomolecule icariin for promoting skeletal regeneration in challenging osteonecrotic bone in rabbits. Biomaterials. 2018;153:1–13. doi: 10.1016/j.biomaterials.2017.10.025. [DOI] [PubMed] [Google Scholar]
- 21.Yan H., Zhou Z., Huang T. Controlled release in vitro of icariin from gelatin/hyaluronic acid composite microspheres. Polym. Bull. 2016;73:1055–1066. [Google Scholar]
- 22.Li C., et al. Pharmacological effects and pharmacokinetic properties of icariin, the major bioactive component in Herba Epimedii. Life Sci. 2015;126:57–68. doi: 10.1016/j.lfs.2015.01.006. [DOI] [PubMed] [Google Scholar]
- 23.Jing X., et al. Icariin doped bioactive glasses seeded with rat adipose-derived stem cells to promote bone repair via enhanced osteogenic and angiogenic activities. Life Sci. 2018;202:52–60. doi: 10.1016/j.lfs.2018.02.026. [DOI] [PubMed] [Google Scholar]
- 24.Metze A.L., et al. Gelatin coated 45S5 bioglass®-derived scaffolds for bone tissue engineering. Key Eng. Mater. 2013;541:31–39. [Google Scholar]
- 25.Zhang X., et al. Chemical cross-linking gelatin with natural phenolic compounds as studied by high-resolution NMR spectroscopy. Biomacromolecules. 2010;11:1125–1132. doi: 10.1021/bm1001284. [DOI] [PubMed] [Google Scholar]
- 26.Kosaraju S.L., Puvanenthiran A., Lillford P. Naturally crosslinked gelatin gels with modified material properties. Food Res. Int. 2010;43:2385–2389. [Google Scholar]
- 27.Adhirajan N., Shanmugasundaram N., Babu M. Gelatin microspheres cross-linked with EDC as a drug delivery system for doxycyline: development and characterization. J. Microencapsul. 2008;24:659–671. doi: 10.1080/02652040701500210. [DOI] [PubMed] [Google Scholar]
- 28.Powell H.M., Boyce S.T. EDC cross-linking improves skin substitute strength and stability. Biomaterials. 2006;27:5821–5827. doi: 10.1016/j.biomaterials.2006.07.030. [DOI] [PubMed] [Google Scholar]
- 29.Olde Damink L., Dijkstra P.J., van Luyn M., van Wachem P.B., Nieuwenhuis P., Feijen J. Cross-linking of dermal sheep collagen using a water-soluble carbodiimide. Biomaterials. 1996;17:765–773. doi: 10.1016/0142-9612(96)81413-x. [DOI] [PubMed] [Google Scholar]
- 30.Kokubo T., Takadama H. How useful is SBF in predicting in vivo bone bioactivity? Biomaterials. 2006;27:2907–2915. doi: 10.1016/j.biomaterials.2006.01.017. [DOI] [PubMed] [Google Scholar]
- 31.Boccaccini A.R., Chen Q., Lefebvre L. Sintering, crystallisation and biodegradation behaviour of bioglass -derived glass–ceramics. Faraday Discus. 2007;136:27–44. doi: 10.1039/b616539g. [DOI] [PubMed] [Google Scholar]
- 32.Kim H., Knowles J.C., Kim H. Porous scaffolds of gelatin – hydroxyapatite nanocomposites obtained by biomimetic approach : Characterization and antibiotic drug release. J. Biomed. Mater. Res. Part B. 2005;74:686–698. doi: 10.1002/jbm.b.30236. [DOI] [PubMed] [Google Scholar]
- 33.Staroszczyk H., Sztuka K., Wolska J., Wojtasz-paja A. Interactions of fish gelatin and chitosan in uncrosslinked and crosslinked with EDC Films : FT-IR study. Spect. Chim. Acta Part A. 2014;117:707–712. doi: 10.1016/j.saa.2013.09.044. [DOI] [PubMed] [Google Scholar]
- 34.Bellucci D., Sola A., Gentile P., Ciardelli G., Cannillo V. Biomimetic coating on bioactive glass-derived scaffolds mimicking bone tissue. J. Biomed. Mater. Res. Part A. 2012;100:3259–3266. doi: 10.1002/jbm.a.34271. [DOI] [PubMed] [Google Scholar]
- 35.Tabata Y., Ikada Y. Protein release from gelatin matrices. Adv. Drug Deliv. Rev. 1998;31:287–301. doi: 10.1016/s0169-409x(97)00125-7. [DOI] [PubMed] [Google Scholar]
- 36.Li Y., Liu T., Zheng J., Xu X. Glutaraldehyde-crosslinked chitosan/hydroxyapatite bone repair scaffold and its application as drug Carrier for icariin. J. Appl. Polym. Sci. 2013;130:1539–1547. [Google Scholar]
- 37.Shuai C., Guo W., Wu P., Yang W., Hu S., Xi Y., Feng P. A graphene oxide-Ag co-dispersing nanosystem: dual synergistic effects on antibacterial activities and mechanical properties of polymer scaffolds. Chem. Eng. J. 2018;347:322–333. [Google Scholar]