Abstract
Background
Lung cancer is the leading cause of cancer death worldwide. Routine UK lung cancer screening is not yet available, thus understanding barriers to participation in lung screening could help maximize effectiveness if introduced.
Methods
Population‐based survey of 1007 adults aged 16 and over in Wales using random quota sampling. Computer‐assisted face‐to‐face interviews included demographic variables (age, gender, smoking, social group), four lung cancer belief statements and three lung screening attitudinal items. Determinants of lung screening attitudes were examined using multivariable regression adjusted for age, gender, social group and previous exposure to lung campaign messages.
Results
Avoidance of lung screening due to fear of what might be found was statistically significantly associated with negative lung cancer beliefs including fatalism (aOR = 8.8, 95% CI = 5.6‐13.9, P ≤ 0.001), low perceived value of symptomatic presentation (aOR = 2.4, 95% CI = 1.5‐3.9, P ≤ 0.001) and low treatment efficacy (aOR = 0.3, CI = 0.2‐0.7, P ≤ 0.01).
Low perceived effectiveness of lung screening was significantly associated with fatalism (aOR = 6.4, 95% CI = 3.5‐11.7, P ≤ 0.001), low perceived value of symptom presentation (aOR = 4.9, 95% CI = 2.7‐8.9, P ≤ 0.001) and low treatment efficacy (aOR = 0.1, 95% CI = 0.1‐0.3, P ≤ 0.001). In contrast, respondents who thought lung screening could reduce cancer deaths had positive beliefs about lung cancer (aOR = 0.4, 95% CI = 0.2‐0.7, P ≤ 0.001) and its treatment (aOR = 6.1, 95% CI = 3.0‐12.6, P ≤ 0.001).
Conclusion
People with negative beliefs about lung cancer may be more likely to avoid lung screening. Alongside the introduction of effective early detection strategies, interventions are needed to modify public perceptions of lung cancer, particularly for fatalism.
Keywords: attitudes, beliefs, cancer, lung cancer, quantitative, screening
1. INTRODUCTION
Lung cancer is the leading cause of cancer‐related death worldwide.1 Five‐year cancer survival rapidly decreases the later lung cancer is diagnosed, due to limited treatments options. In the UK, when lung cancer is diagnosed at the earliest stage (Stage I), 56% of patients can expect to survive for one year or more, in comparison with 14% of patients diagnosed at the most advanced stage (Stage IV).2 With 78% of UK non‐small‐cell lung cancer cases diagnosed in the later stages of disease (Stage III or IV),2 there is a need to explore strategies to diagnose lung cancer earlier.
Currently, diagnostic testing for suspected lung cancer in the UK requires symptomatic patients to present to a healthcare professional for referral for further investigation. This approach relies on the patient and healthcare professional accurately appraising symptoms, which may be problematic for early diagnosis of lung cancer.3 Lung cancer symptoms are hard to detect in the early stages, due to misattribution to smoking habit, comorbidities or other benign causes.4, 5
Evidence suggests that low‐dose computed tomography (CT) screening is effective in detecting early‐stage lung cancer.6 The US National Lung Screening Trial reported a 20% reduction in lung cancer mortality6 and is currently the standard of care in the United States.7 Although not routinely available in the UK, trials are ongoing across Europe to assess the effectiveness of CT lung screening among high‐risk groups.8, 9, 10, 11 In the event that lung screening for high‐risk populations is introduced routinely in the UK, it is important to understand the barriers to participation and develop interventions to encourage those who are eligible to engage in lung screening in order to optimize its impact and maintain cost‐effectiveness.
Previous studies of attitudes towards lung cancer screening suggest that smokers from socio‐economically deprived groups place lower value on the benefits of lung cancer screening, hold fatalistic beliefs about lung cancer as an untreatable disease or report stigma as a barrier to screening participation.12, 13 In addition, emotional barriers such as fear of lung cancer14 and the belief that the lungs are an untreatable organ12, 15 were reported to deter participation in lung screening trials. However, these studies have been restricted to samples of people over the age of 40.12, 13, 15, 16, 17, 18 Therefore, little is known about attitudes to lung screening in a population sample including younger age groups who may eventually become eligible for programmatic CT lung screening. Furthermore, there is limited evidence regarding the influences of general beliefs about lung cancer symptomatic presentation, survival and treatment on attitudes towards lung screening.
A population‐based survey was conducted to assess the influence of demographic variables, smoking status and beliefs about lung cancer and early symptomatic detection on lung cancer screening attitudes in a Welsh population sample. It was anticipated that current smokers, respondents from the lowest socio‐economic group and those with negative beliefs about lung cancer would have more negative attitudes towards lung cancer screening.
2. MATERIALS AND METHODS
2.1. Participants
Ethical approval was obtained to undertake a secondary analysis of population‐representative survey data gathered during February and March 2016, prior to the launch of the Welsh lung cancer awareness campaign in July 2016 (http://www.cancerresearchuk.org/health-professional/awareness-and-prevention/be-clear-on-cancer/lung-cancer-awareness-campaign-wales). Cancer Research UK commissioned a survey provider (Beaufort Research) to carry out a nationally representative survey of adults resident in Wales aged 16 years and over, as part of a commercial Omnibus survey to examine the impact of the campaign on lung symptom awareness.
Pre‐campaign survey data were collected from a total of 1007 adults. The number of people who declined to participate was not recorded, thus the characteristics of survey decliners are unknown.
2.2. Design/procedure
The survey used random quota sampling based on neighbourhoods classified according to census characteristics. The Omnibus sample is designed to be representative of the adult population resident in Wales aged 16 and over, with Lower Layer Super Output Area (LSOA) as the unit of sampling. Sixty‐nine interviewing points throughout Wales were selected with probability proportional to resident population, after stratification by local authority and social group based on occupation. Social group was recorded in four categories using the National Readership Survey grades, based on the occupation of the household's chief income earner: AB (higher and intermediate managerial, administrative and professional), C1 (supervisory, clerical and junior managerial, administrative and professional), C2 (skilled manual workers) and DE (semiskilled and unskilled manual workers, state pensioners, casual and lowest grade workers, and unemployed with state benefits only). Categories were combined to cluster participants by social group: ABC1 participants were considered high socio‐economic status, and the C2DE participants were considered low socio‐economic status.
Within each sampling point, quota sample controls of age and social group within gender were set for the selection of respondents. Quotas were set to reflect the individual demographic profile of each selected point. A fresh sample of interviewing locations and individuals was selected for each survey, and no more than one person per household was interviewed. Respondents completed a computer‐assisted interview in the presence of a trained interviewer. Data were weighted by age group within gender within local authority grouping, so that the sample profiles matched those of people aged 16 years and over in Wales derived from the 2011 Census.
2.3. Measures
Survey measures included demographic characteristics (age, gender, social group), smoking history (smoke up to 20 cigarettes a day, smoke 20 or more cigarettes a day, used to smoke, never smoked), beliefs about lung cancer and attitudes towards lung cancer screening. Prior exposure to lung campaign messages was measured by the following questions: “Have you seen, heard or read any adverts, publicity or other types of information in the last couple of months which focused on the subject of lung cancer?” Response options were “yes,” “no” and “don't know/can't remember.” A brief description of lung screening was given: Now I'm going to read you some statements that are sometimes made about cancer screening (eg, a mammogram for breast cancer screening, a poo testing kit for bowel cancer screening). Thinking about lung screening (ie, a chest scan or X‐ray), can you tell me how much you agree or disagree with each of the following statements? Items relating to lung cancer screening attitudes and beliefs about lung cancer were adapted from the ABC measure.19 Lung cancer beliefs were assessed with four items: “I would not want to know if I had lung cancer” reflecting cancer fatalism; “Going to my GP/doctor early with a symptom of lung cancer makes no difference to my chances of surviving lung cancer” reflecting perceived value of symptom presentation; “If lung cancer is diagnosed early, it is more likely to be treatable” reflecting beliefs about treatment; and “If I had a cough, I would be worried about wasting the GP/doctor's time” reflecting beliefs about symptomatic presentation. Attitudes towards lung cancer screening were assessed using three items: “I would be so worried about what might be found at lung cancer screening that I would prefer not to go”; “I don't think there is any point going for lung cancer screening because it won't affect the outcome”; and “Lung screening could reduce my chances of dying from cancer.” Response options were strongly agree, agree, disagree and strongly disagree. “Don't know” responses were recorded. Responses were recoded for analysis purposes, with strongly agree and agree combined to create “agree,” disagree and strongly disagree combined to create “disagree.” “Don't know” responses were counted as missing.
2.4. Statistical analysis
Descriptive statistics were used to summarize the demographic characteristics of the sample and to assess missing and “don't know” responses. Chi‐square univariable tests were used to examine the influence of smoking history, age, gender, social group and lung cancer beliefs on endorsement of attitudes to individual lung cancer screening items. Multivariable regression modelling was carried out to examine the influence of smoking history and lung cancer beliefs on lung cancer screening attitudes (individual items), adjusting for age, gender, social group combined and prior exposure to lung cancer messages. The significance level was set at P < 0.01 to adjust for multiple testing. To account for nonrepresentativeness, a weight was applied to the data based on age and gender within local authority in Wales.
3. RESULTS
Of a total of 1007 participants, 295 (29%) were aged 16‐34, 328 (33%) aged 33‐54 and 383 (38%) aged over 55 (see Table 1). There were 518 females (51%) and 489 males (49%), with 406 (41%) from the social group ABC1 and 596 (60%) from the social group C2DE. Most of the sample had never smoked (n = 433, 43%), 286 (28%) used to smoke, 259 (26%) currently smoked up to 20 cigarettes a day and 28 (3%) currently smoked more than 20 cigarettes a day (see Table 1). For univariable and multivariable analysis purposes, “smoke up to 20 a day” and “smoke over 20 a day” were combined to create a “currently smoke” category.
Table 1.
Participant characteristics
| Variable | Descriptive statistic n (%) | |
|---|---|---|
| Unweighted | Weighted | |
| Age | ||
| 16‐34 | 285 (28%) | 295 (29%) |
| 33‐54 | 282 (28%) | 328 (33%) |
| 55+ | 439 (44%) | 383 (38%) |
| Gender | ||
| Male | 439 (44%) | 489 (49%) |
| Female | 568 (56%) | 518 (51%) |
| Social group | ||
| ABC1 | 412 (41%) | 406 (40%) |
| C2DE | 590 (59%) | 596 (60%) |
| Smoking status | ||
| Never smoked | 445 (44%) | 433 (43%) |
| Used to smoke | 291 (29%) | 286 (28%) |
| Smoke up to 20 a day | 243 (24%) | 259 (26%) |
| Smoke over 20 a day | 27 (3%) | 28 (3%) |
| Exposure to lung messages | ||
| Yes | 511 (51%) | 515 (52%) |
| No | 486 (49%) | 483 (48%) |
| Lung cancer beliefs | ||
| I would not want to know if I had lung cancer | ||
| Agree | 164 (17%) | 168 (17%) |
| Disagree | 802 (83%) | 801 (83%) |
| Going to my GP/doctor early with a symptom of lung cancer makes no difference to my chances of surviving cancer | ||
| Agree | 172 (18%) | 170 (18%) |
| Disagree | 771 (82%) | 777 (82%) |
| If lung cancer is diagnosed early, it is more likely to be treatable | ||
| Agree | 897 (94%) | 905 (94%) |
| Disagree | 60 (6%) | 55 (6%) |
| If I had a cough, I would be worried about wasting the GP/doctor's time | ||
| Agree | 361 (37%) | 358 (37%) |
| Disagree | 609 (63%) | 615 (63%) |
3.1. Univariate analysis
3.1.1. Avoidance of lung screening
Fifteen per cent (n = 144) of the sample endorsed avoidance of lung screening due to fear of what might be found (Figure 1). Avoidance of lung screening was statistically significantly associated with fatalism (P ≤ 0.001), low perceived value of symptom presentation (P ≤ 0.001), having negative views about treatment (P ≤ 0.001) and worry about wasting the doctor's time (P ≤ 0.001) (see Table 2). Associations between lung screening attitudes and age, gender, social group, smoking status and exposure to lung messages were not statistically significant.
Figure 1.
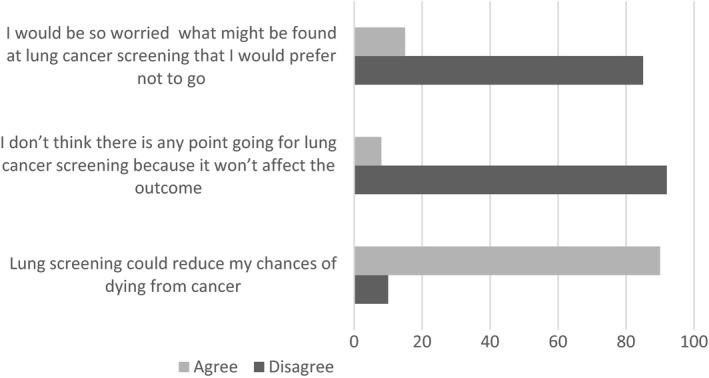
Summary of lung screening attitudes (data presented represents weighted data)
Table 2.
Frequencies and univariate analysis for lung screening attitude “I would be so worried about what might be found at lung cancer screening that I would prefer not to go”
| Unweighted | Weighted | |||||
|---|---|---|---|---|---|---|
| Agree (n = 141) | Disagree (n = 836) | Statistic | Agree (n = 144) | Disagree (n = 832) | Statistic | |
| Age | ||||||
| 16‐34 | 43 (16%) | 233 (84%) | Χ2 (2) = 0.78, P = 0.68 | 43 (15%) | 242 (85%) | Χ2 (2) = 0.89, P = 0.64 |
| 33‐54 | 41 (15%) | 232 (85%) | 51 (16%) | 268 (84%) | ||
| 55+ | 57 (13%) | 370 (87%) | 50 (13%) | 321 (87%) | ||
| Gender | ||||||
| Male | 65 (15%) | 360 (85%) | Χ2 (1) = 0.34, P = 0.56 | 73 (15%) | 400 (85%) | Χ2 (1) = 0.24, P = 0.62 |
| Female | 76 (14%) | 476 (86%) | 71 (14%) | 432 (86%) | ||
| Social group | ||||||
| ABC1 | 51 (12%) | 357 (88%) | Χ2 (1) = 1.98, P = 0.16 | 52 (13%) | 350 (87%) | Χ2 (1) = 1.49, P = 0.22 |
| C2DE | 90 (16%) | 475 (84%) | 91 (16%) | 479 (84%) | ||
| Smoking status | ||||||
| Never smoked | 54 (12%) | 383 (88%) | Χ2 (2) = 3.79, P = 0.15 | 55 (13%) | 370 (87%) | Χ2 (2) = 2.22, P = 0.33 |
| Used to smoke | 40 (15%) | 234 (85%) | 41 (15%) | 227 (85%) | ||
| Currently smoke | 47 (18%) | 219 (82%) | 48 (17%) | 236 (83%) | ||
| Exposure to lung messages | ||||||
| Yes | 73 (15%) | 431 (85%) | Χ2 (1) = 0.00, P = 0.99 | 76 (15%) | 432 (85%) | Χ2 (1) = 0.01, P = 0.94 |
| No | 67 (15%) | 396 (85%) | 67 (15%) | 392 (85%) | ||
| Lung cancer beliefs | ||||||
| I would not want to know if I had lung cancer | ||||||
| Agree | 78 (49%) | 82 (51%) | Χ2 (1) = 188.05, P = 0.000*** | 79 (48%) | 85 (52%) | Χ2 (1) = 180.78, P = 0.000*** |
| Disagree | 56 (7%) | 737 (93%) | 58 (7%) | 732 (93%) | ||
| Going to my GP/doctor early with a symptom of lung cancer makes no difference to my chances of surviving cancer | ||||||
| Agree | 52 (31%) | 115 (69%) | Χ2 (1) = 46.97, P = 0.000*** | 51 (31%) | 114 (69%) | Χ2 (1) = 41.85, P = 0.000*** |
| Disagree | 79 (10%) | 682 (90%) | 84 (11%) | 681 (89%) | ||
| If lung cancer is diagnosed early, it is more likely to be treatable | ||||||
| Agree | 109 (12%) | 772 (88%) | Χ2 (1) = 16.84, P = 0.000*** | 114 (13%) | 774 (87%) | Χ2 (1) = 18.39, P = 0.000*** |
| Disagree | 19 (32%) | 40 (68%) | 19 (35%) | 36 (66%) | ||
| If I had a cough, I would be worried about wasting the GP/doctor's time | ||||||
| Agree | 74 (21%) | 283 (79%) | Χ2 (1) = 18.08, P = 0.000*** | 73 (21%) | 281 (79%) | Χ2 (1) = 14.77, P = 0.000*** |
| Disagree | 63 (11%) | 535 (89%) | 68 (11%) | 535 (89%) | ||
***p≤0.001
3.1.2. Low perceived effectiveness of lung screening
A total of 8% (n = 78) endorsed low perceived effectiveness of lung screening (see Figure 1). Low perceived effectiveness of lung screening was statistically significantly associated with fatalism (P ≤ 0.001), low perceived value of symptom presentation (P ≤ 0.001), having negative views about treatment (P ≤ 0.001) and worry about wasting the doctor's time (P ≤ 0.001) (see Table 3). Effects of age, gender, smoking status, social group and exposure to lung messages were not statistically significant.
Table 3.
Frequencies and univariate analysis for lung screening attitude “I don't think there is any point going for lung cancer screening because it won't affect the outcome”
| Unweighted | Weighted | |||||
|---|---|---|---|---|---|---|
| Agree (n = 83) | Disagree (n = 876) | Statistic | Agree (n = 78) | Disagree (n = 882) | Statistic | |
| Age | ||||||
| 16‐34 | 20 (7%) | 251 (93%) | Χ2 (2) = 0.84, P = 0.66 | 20 (7%) | 262 (93%) | Χ2 (2) = 0.66, P = 0.72 |
| 33‐54 | 24 (9%) | 247 (91%) | 26 (8%) | 290 (92%) | ||
| 55+ | 39 (9%) | 377 (91%) | 32 (9%) | 330 (91%) | ||
| Gender | ||||||
| Male | 38 (9%) | 374 (91%) | Χ2 (1) = 0.18, P = 0.67 | 39 (9%) | 422 (91%) | Χ2 (1) = 0.61, P = 0.81 |
| Female | 45 (8%) | 502 (92%) | 39 (8%) | 460 (92%) | ||
| Social group | ||||||
| ABC1 | 26 (6%) | 374 (94%) | Χ2 (1) = 3.70, P = 0.05 | 25 (6%) | 370 (94%) | Χ2 (1) = 2.61, P = 0.11 |
| C2DE | 57 (10%) | 498 (90%) | 53 (9%) | 508 (91%) | ||
| Smoking status | ||||||
| Never smoked | 28 (7%) | 402 (93%) | Χ2 (2) = 7.94, P = 0.02* | 25 (6%) | 394 (94%) | Χ2 (2) = 6.96, P = 0.03* |
| Used to smoke | 22 (8%) | 247 (92%) | 21 (8%) | 244 (92%) | ||
| Currently smoke | 33 (13%) | 227 (87%) | 32 (12%) | 245 (88%) | ||
| Exposure to lung messages | ||||||
| Yes | 42 (9%) | 454 (91%) | Χ2 (1) = 0.04, P = 0.85 | 39 (8%) | 462 (92%) | Χ2 (1) = 0.13, P = 0.71 |
| No | 41 (9%) | 413 (91%) | 39 (9%) | 412 (91%) | ||
| Lung cancer beliefs | ||||||
| I would not want to know if I had lung cancer | ||||||
| Agree | 47 (30%) | 112 (70%) | Χ2 (1) = 105.84, P = 0.000*** | 45 (27%) | 119 (73%) | Χ2 (1) = 97.64, P = 0.000*** |
| Disagree | 33 (4%) | 749 (96%) | 31 (4%) | 749 (96%) | ||
| Going to my GP/doctor early with a symptom of lung cancer makes no difference to my chances of surviving cancer | ||||||
| Agree | 40 (24%) | 123 (76%) | Χ2 (1) =70.07, P = 0.000*** | 37 (23%) | 124 (77%) | Χ2 (1) = 64.98, P = 0.000*** |
| Disagree | 34 (4%) | 722 (96%) | 32 (4%) | 729 (96%) | ||
| If lung cancer is diagnosed early, it is more likely to be treatable | ||||||
| Agree | 56 (6%) | 817 (94%) | Χ2 (1) = 47.46, P = 0.000*** | 55 (6%) | 826 (94%) | Χ2 (1) = 42.12, P = 0.000*** |
| Disagree | 19 (33%) | 39 (67%) | 17 (32%) | 37 (68%) | ||
| If I had a cough, I would be worried about wasting the GP/doctor's time | ||||||
| Agree | 45 (13%) | 306 (87%) | Χ2 (1) = 12.67, P = 0.000*** | 43 (12%) | 305 (88%) | Χ2 (1) = 13.85, P = 0.000*** |
| Disagree | 35 (6%) | 557 (94%) | 32 (5%) | 566 (95%) | ||
*p≤0.05 , ***p≤0.001
3.1.3. Lung screening to reduce mortality
Ninety per cent (n = 859) of the sample agreed that lung screening could reduce chances of lung cancer death (Figure 1). Agreeing that lung screening could reduce chances of dying from cancer was associated with positive lung cancer beliefs reflecting lack of fatalism (P ≤ 0.001) and having positive views about treatment (P ≤ 0.001) (see Table 4). There were no statistically significant effects of any demographic variables.
Table 4.
Frequencies and univariate analysis for lung screening attitude “Lung screening could reduce my chances of dying from cancer”
| Unweighted | Weighted | |||||
|---|---|---|---|---|---|---|
| Agree (n = 857) | Disagree (n = 91) | Statistic | Agree (n = 859) | Disagree (n = 92) | Statistic | |
| Age | ||||||
| 16‐34 | 247 (92%) | 22 (8%) | Χ2 (2) = 3.39, P = 0.18 | 258 (92%) | 22 (8%) | Χ2 (2) = 3.60, P = 0.17 |
| 33‐54 | 233 (88%) | 33 (12%) | 273 (88%) | 38 (12%) | ||
| 55+ | 376 (91%) | 36 (9%) | 327 (91%) | 32 (9%) | ||
| Gender | ||||||
| Male | 381 (92%) | 32 (8%) | Χ2 (1) = 2.52, P = 0.11 | 424 (92%) | 38 (8%) | Χ2 (1) = 1.85, P = 0.17 |
| Female | 476 (89%) | 59 (11%) | 435 (89%) | 54 (11%) | ||
| Social group | ||||||
| ABC1 | 366 (92%) | 32 (8%) | Χ2 (1) = 1.49, P = 0.22 | 359 (92%) | 33 (8%) | Χ2 (1) = 0.89, P = 0.35 |
| C2DE | 488 (89%) | 58 (11%) | 496 (89%) | 58 (11%) | ||
| Smoking status | ||||||
| Never smoked | 386 (91%) | 39 (9%) | Χ2 (2) = 4.29, P = 0.12 | 374 (90%) | 40 (10%) | Χ2 (2) = 3.32, P = 0.19 |
| Used to smoke | 250 (93%) | 20 (7%) | 246 (93%) | 20 (7%) | ||
| Currently smoke | 221 (87%) | 32 (13%) | 238 (88%) | 33 (12%) | ||
| Exposure to lung messages | ||||||
| Yes | 438 (90%) | 51 (10%) | Χ2 (1) = 0.47, P = 0.49 | 445 (90%) | 50 (10%) | Χ2 (1) = 0.07, P = 0.79 |
| No | 410 (91%) | 40 (9%) | 406 (91%) | 42 (9%) | ||
| Lung cancer beliefs | ||||||
| I would not want to know if I had lung cancer | ||||||
| Agree | 125 (81%) | 29 (19%) | Χ2 (1) = 15.80, P = 0.000*** | 128 (81%) | 30 (19%) | Χ2 (1) = 16.60, P = 0.000*** |
| Disagree | 712 (92%) | 62 (8%) | 712 (92%) | 62 (8%) | ||
| Going to my GP/doctor early with a symptom of lung cancer makes no difference to my chances of surviving cancer | ||||||
| Agree | 135 (87%) | 21 (13%) | Χ2 (1) = 2.92, P = 0.09 | 133 (86%) | 22 (14%) | Χ2 (1) = 3.80, P = 0.051 |
| Disagree | 685 (91%) | 65 (9%) | 690 (91%) | 66 (9%) | ||
| If lung cancer is diagnosed early, it is more likely to be treatable | ||||||
| Agree | 804 (92%) | 67 (8%) | Χ2 (1) = 42.37, P = 0.000*** | 809 (92%) | 70 (8%) | Χ2 (1) = 34.78 P = 0.000*** |
| Disagree | 35 (65%) | 19 (35%) | 33 (66%) | 17 (34%) | ||
| If I had a cough, I would be worried about wasting the GP/doctor's time | ||||||
| Agree | 306 (89%) | 38 (11%) | Χ2 (1) = 0.92, P = 0.34 | 304 (89%) | 39 (11%) | Χ2 (1) = 1.17, P = 0.28 |
| Disagree | 533 (91%) | 52 (9%) | 539 (91%) | 53 (9%) | ||
***p≤0.001
3.1.4. Logistic regression
Regression analysis was completed using the weighted data, adjusting for age, gender, social group and previous exposure to lung messages.
3.1.5. Avoidance of lung screening
Negative lung cancer beliefs including fatalism (aOR = 8.8 CI = 5.6‐13.9, P ≤ 0.001), low perceived value of symptom presentation (aOR = 2.4, CI = 1.5‐3.9, P ≤ 0.001) and having negative views about treatment (aOR = 0.3, CI = 0.2‐0.7, P ≤ 0.01) showed a statistically significant association with not wanting to have lung screening due to being worried about what might be found (see Table 5). Smoking status and being worried about wasting the doctor's time were not statistically significantly associated with lung cancer screening avoidance due to worry about the outcome.
Table 5.
| Q1. I would be so worried about what might be found at lung screening that I would prefer not to go | Q2. I don't think there is any point going for lung cancer screening because it won't affect the outcome | Q3. Lung screening could reduce my chances of dying from cancer | |||||||
|---|---|---|---|---|---|---|---|---|---|
| B (SE) | OR (95% CI) | P | B (SE) | OR (95% CI) | P | B (SE) | OR (95% CI) | P | |
| Smoking status | 0.17 (0.30) | 1.19 (0.66‐2.13) | 0.56 | −0.18 (0.39) | 0.84 (0.39‐1.79) | 0.64 | 0.47 (0.35) | 1.61 (0.81‐3.17) | 0.17 |
| I would not want to know if I had cancer | 2.18 (0.23) | 8.80 (5.58‐13.87) | 0.000*** | 1.85 (0.31) | 6.38 (3.49‐11.66) | 0.000*** | −0.91 (0.28) | 0.40 (0.23‐0.70) | 0.001*** |
| Going to my GP/doctor early with a symptom of lung cancer makes no difference to my chances of surviving cancer | 0.86 (0.25) | 2.37 (1.45‐3.86) | 0.001*** | 1.58 (0.31) | 4.85 (2.65‐8.88) | 0.000*** | −0.17 (0.30) | 0.84 (0.47‐1.53) | 0.58 |
| If lung cancer is diagnosed early, it is more likely to be treatable | −1.08 (0.40) | 0.34 (0.16‐0.74) | 0.007** | −2.01 (0.43) | 0.13 (0.06‐0.31) | 0.000*** | 1.81 (0.37) | 6.12 (2.98‐12.56) | 0.000*** |
| If I had a cough, I would be worried about wasting the GP/doctor's time | 0.47 (0.22) | 1.60 (1.03‐2.49) | 0.04* | 0.71 (0.31) | 2.04 (1.12‐3.72) | 0.02* | 0.14 (0.26) | 1.15 (0.69‐1.90) | 0.60 |
OR, odds ratio; CI, confidence interval
* P ≤ 0.05
** P ≤ 0.01
*** P ≤ 0.001
aWeighting for nonrepresentativeness in age and gender within local authority in Wales
bAdjusting for age, gender, social group and previous exposure to lung messages
3.1.6. Low perceived effectiveness of lung screening
Negative lung cancer beliefs including fatalism (aOR = 6.4 CI = 3.5‐11.7, P ≤ 0.001), low perceived value of symptom presentation (aOR = 4.9, CI = 2.7‐8.9, P ≤ 0.001) and having negative views about treatment (aOR = 0.1, CI = 0.1‐0.3, P ≤ 0.001) showed a statistically significant association with low perceived effectiveness of lung screening (see Table 5). Smoking status and being worried about wasting the doctor's time were not statistically significantly associated with attitudes towards the efficacy of screening.
3.1.7. Lung screening to reduce mortality
Positive lung cancer beliefs reflecting lack of fatalism (aOR = 0.4 CI = 0.2‐0.7, P ≤ 0.001) and positive views about treatment (aOR = 6.1, 95% CI = 3.0‐12.6, P ≤ 0.001) showed a statistically significant association with agreeing that lung screening could reduce chances of dying from cancer (see Table 5). Smoking status, beliefs about early presentation and being worried about wasting the doctor's time were not significantly associated with perceptions that lung screening could reduce lung cancer mortality.
4. DISCUSSION
To our knowledge, the present study was the first to test associations with lung cancer screening attitudes using quantitative survey methods in a population sample of adults over the age of 16. Attitudes towards lung cancer screening were generally positive, with over 90% of survey respondents believing that there was benefit to lung cancer screening in terms of lung cancer outcomes and survival, and may encourage participation in lung cancer screening. However, those who endorse negative beliefs about lung cancer may be more likely to avoid lung screening. Respondents who endorsed negative beliefs about lung cancer—reflecting fatalism, low perceived effectiveness of symptom presentation and negative views about treatment—were more likely to hold negative attitudes towards lung cancer screening. Smoking status was not significantly associated with attitudes towards lung cancer screening in the current study.
Our findings mirror those of previous studies that have examined participation in a colorectal cancer screening context, where over 90% of respondents in an Australian population‐based study held positive beliefs about colorectal cancer screening.20 In addition, positive beliefs about the benefits of colorectal cancer screening have been associated with increased anticipated uptake of screening21 and participation in screening.20, 22
The current study suggests that negative beliefs about lung cancer were associated with lung screening avoidance, particularly fatalism, suggesting that those who decline screening would prefer not to know if they have lung cancer, potentially due to fear of treatment and lung cancer death. Our findings are in line with previous research highlighting fear of lung cancer12, 13, 14 and fatalism, including beliefs about the treatment for lung cancer12, 15 as barriers to participation in lung cancer screening. It is likely that avoidance of lung screening and negative beliefs may reflect lung cancer stigma,23 possibly due to the relationship between lung cancer and smoking, and poor lung cancer outcomes.
The absence of an observed association between smoking status and lung cancer screening attitudes in our study contradicts the findings of previous studies, which have highlighted more negative screening attitudes among current smokers.12, 24 In addition, former smokers have been shown to be over‐represented in lung cancer screening trials.25, 26 Our contradictory findings are likely to reflect the limited representation of heavy smokers in our sample and consequent low statistical power. Poor representation from current smokers should be noted as a limitation of this study. Future work focusing on attitudes in heavy, moderate and light smokers would help to further understanding of the influence of nicotine dependence on screening attitudes. It should also be noted that the associations between lung cancer beliefs and lung screening attitudes may partly reflect shared method variance, where associations between variables can be inflated when measures are taken at the same point in time. Prospective longitudinal research should therefore be undertaken to examine the predictors of lung screening uptake and outcomes. Finally, we used an adapted version of the ABC measure in the absence of a validated measure of lung screening at the time of survey development. Future studies could consider using a recently developed and psychometrically validated measure of lung screening health beliefs.27
Our findings suggest that negative beliefs about lung cancer may deter participation in lung cancer screening. Therefore, addressing population beliefs about lung cancer is an important step before the implementation of a lung screening programme. Public awareness campaigns should focus on the benefits of lung cancer screening, where early detection increases survival through access to more effective treatments, to modify fatalistic beliefs about lung cancer survival and treatment.
CONFLICT OF INTEREST
No conflict of interests to declare
ACKNOWLEDGEMENTS
We would like to thank Cancer Research UK, who commissioned the survey from which the relevant data were taken. We would particularly like to thank Jodie Moffat from Cancer Research UK. We would also like to thank Beaufort Research, who collected the data. Thanks also go to Kate Lifford, for providing support and advice for statistical queries.
Smits SE, McCutchan GM, Hanson JA, Brain KE. Attitudes towards lung cancer screening in a population sample. Health Expect. 2018;21:1150–1158. 10.1111/hex.12819
Funding information
This work was undertaken as a secondary analysis of an existing data set that was funded by Cancer Research UK. Dr Stephanie Smits is jointly funded by PRIME Centre Wales and Wales Cancer Research Centre, which are funded by Welsh Government through Health and Care Research Wales. Dr Grace McCutchan is funded by Cancer Research UK on the LUSH study (ref C16377/A22034).
REFERENCES
- 1. Cancer Research UK . Lung cancer statistics. 2016. [online] Available at: http://www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/lung-cancer [Accessed 18.07.2017]
- 2. Walters S, Maringe C, Coleman MP, et al. Lung cancer survival and stage at diagnosis in Australia, Canada, Denmark, Norway, Sweden and the UK: a population‐based study, 2004–2007. Thorax. 2013;68:551‐564. [DOI] [PubMed] [Google Scholar]
- 3. Neal R, Robbe IJ, Lewis M, Williamson I, Hanson J. The complexity and difficulty of diagnosing lung cancer: findings from a national primary‐care study in Wales. Prim Health Care Res Dev. 2015;16(5):436‐49. [DOI] [PubMed] [Google Scholar]
- 4. Birt L, Hall N, Emery J, et al. Responding to symptoms suggestive of lung cancer: a qualitative interview study. BMJ Open Resp Res. 2014;1:e000067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Corner J, Hopkinson J, Fitzsimmons D, et al. Is late diagnosis of lung cancer inevitable? Interview study of patients’ recollections of symptoms before diagnosis. Thorax. 2005;60:314‐319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Aberle DR, Adams AM, Berg CD, et al. Reduced lung‐cancer mortality with low‐dose computed tomographic screening. N Engl J Med. 2011;365(5):395‐409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Moyer VA. U.S. preventive services task force. Screening for lung cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2014;160(5):330‐8. [DOI] [PubMed] [Google Scholar]
- 8. Aggestrup LM, Hestbech MS, Siersma V, et al. Psychosocial consequences of allocation to lung cancer screening: a randomised controlled trial. BMJ Open. 2012;2:e000663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rasmussen JF, Siersma V, Pedersen JH, Brodersen J. Psychosocial consequences in the Danish randomised controlled lung cancer screening trial (DLCST). Lung Cancer. 2015;87(1):65‐72. [DOI] [PubMed] [Google Scholar]
- 10. van den Bergh KAM, Essink‐Bot ML, Borsboom GJJM, et al. Long‐term effects of lung cancer computed tomography screening on health‐related quality of life: the NELSON trial. Er Resp J. 2011;38:154‐161. [DOI] [PubMed] [Google Scholar]
- 11. Field JK, Duffy SW, Baldwin DR, Whynes DK, Devaraj A, Brain KE. United Kingdom Lung Cancer RCT Pilot Screening Trial (UKLS): Baseline findings from the screening arm provide evidence for a cost effective exemplar for implementation of lung cancer screening. Thorax. 2015;71(2):161‐70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Quaife S, Marlow LAV, McEwen A, Janes S, Wardle J. Attitudes towards lung cancer screening in socioeconomically deprived and heavy smoking communities: informing screening communication. Health Expect. 2016;. 10.1111/hex.12481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Patel D, Akporobaro A, Chinyanganya N, Hackshaw A, Seale C, Spiro SG. Attitudes to participation in a lung cancer screening trial: a qualitative study. Thorax. 2012;67:418‐25. [DOI] [PubMed] [Google Scholar]
- 14. Ali N, Lifford K, Carter B, et al. Barriers to uptake among high‐risk individuals declining participation in lung cancer screening: a mixed methods analysis of the UK Lung Cancer Screening (UKLS) trial. BMJ Open. 2015;5(7):e008254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Silvestri GA, Nietert PJ, Zoller J, Carter C, Bradford D. Attitudes towards screening for lung cancer among smokers and their non‐smoking counterparts. Thorax. 2007;62:126‐130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jonnalagadda S, Bergamo C, Lin JJ, Wisnivesky JP. Beliefs and attitudes about lung cancer screening among smokers. Lung Cancer. 2012;77(33):526‐531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Carter‐Harris L, Ceppa DP, Hanna N, Rawl SM. Lung cancer screening: what do long‐term smokers know and believe? Health Expect. 2015;20(1):59‐68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Flynn AE, Peters MJ, Morgan LC. Attitudes towards Lung Cancer Screening in an Australian High‐Risk Population. Lung Cancer Int. 2013;2013:789057 10.1155/2013/789059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Simon AE, Forbes LJL, Boniface D, et al. An international measure of awareness and beliefs about cancer: development and testing of the ABC. BMJ Open. 2012;2:e001758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Varlow M, Ingrid A, Stacey A, et al. Self‐reported participation and beliefs about bowel cancer screening in New South Wales. Aus Health Prom J Aus. 2014;25:97‐103. [DOI] [PubMed] [Google Scholar]
- 21. de Wijkerslooth TR, de Hann TR, Stoop MC, et al. Reasons for participation and nonparticipation in colorectal cancer screening: a randomized trial of colonoscopy and CT colonography. Am J Gastroent. 2012;107(12):1777‐1783. [DOI] [PubMed] [Google Scholar]
- 22. Wardle J, Sutton S, Williamson S, et al. Psychosocial influences on older adults’ interest in participating in bowel cancer screening. Prev Med. 2000;31(4):323‐334. [DOI] [PubMed] [Google Scholar]
- 23. Marlow LA, Waller J, Wardle J. Does lung cancer attract greater stigma than other cancer types? Lung Cancer. 2015;88(1):104‐7. [DOI] [PubMed] [Google Scholar]
- 24. Quaife SL, Vrinten C, Ruparel M, et al. Smokers’ interest in a lung cancer screening programme: a national survey in England. BMC Cancer. 2018;18:497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. McRonald FE, Yadegarfar G, Baldwin DR, et al. The UK Lung Screen (UKLS): demographic profile of first 88,897 approaches provides recommendations for population screening. Cancer Prev Res. 2014;7(3):362‐71. [DOI] [PubMed] [Google Scholar]
- 26. Hestbech MS, Siersma V, Dirksen A, Pedersen JH, Brodersen J. Participation bias in a randomised trial of screening for lung cancer. Lung Cancer. 2011;73(3):325‐31. [DOI] [PubMed] [Google Scholar]
- 27. Carter‐Harris L, Slaven JE 2nd, Monohan P, Rawl SM. Development and Psychometric Evaluation of the Lung Cancer Screening Health Belief Scales. Cancer Nurs. 2017;40(30):237‐244. [DOI] [PMC free article] [PubMed] [Google Scholar]


