Abstract
Malaria and anaemia are key underlying factors for iNTS disease in African children. Knowledge of clinical and epidemiological risk-factors for iNTS disease has not been paralleled by an in-depth knowledge of the immunobiology of the disease. Herein, we review human and animal studies on mechanisms of increased susceptibility to iNTS in children.
Keywords: Salmonella, Malaria, Children, Anaemia, Immunity, Susceptibility
1. Introduction
1.1. Epidemiological association of malaria and iNTS
Both malaria and invasive bacterial infections (IBI) such as Salmonella disease are important causes of death among under-five children in sub Saharan Africa (SSA) [1] with IBI making up about 10% of all infections [1]. Nontyphoidal Salmonella (NTS) serovars S. Typhimurium and S. Enteritidis are among the common causes of IBI in SSA [1], [2]. Invasive NTS (iNTS) is estimated to cause over 2.1 million illnesses and 416,000 deaths per year [2]. The case fatality rate for NTS bacteraemia in children exceeds 20%, even with appropriate antimicrobial treatment [3] while NTS meningitis case fatality reaches 52% in young children and 80% in adults [4]. Key underlying factors of iNTS disease are immature immunity, malaria infection and malnutrition in children and advanced HIV infection in adults [3], [5], [6], [7], [8]. Current or recent Plasmodium falciparum malaria infections have been shown to be strongly associated with iNTS, particularly severe malarial anaemia (SMA) [5], [9]. Case fatality rates are higher in children admitted to hospital with SMA and NTS bacteraemia (24%) compared to malaria alone (10%) [10], [11]. In addition, it is estimated that 6.5% of IBI occurs in malaria-infected children [10], [11]. In view of the low sensitivity of blood cultures, it has been suggested that P. falciparum infection could account for more than 50% of IBI in those children who live in malaria-endemic settings [6]. Co-infection with malaria and iNTS is common in febrile children from high malaria transmission areas compared to those from low malaria transmission areas [12], [9]; conversely typhoid fever is uncommon in febrile children from high malaria transmission areas [12]. These findings indicate differences in childhood susceptibility to S. Typhi and iNTS.
1.2. Challenges in the clinical management of iNTS
The clinical presentation of NTS bacteraemia is poorly defined in young children and exhibits clinical overlap with the presentation of both pneumonia and malaria, posing diagnostic challenges and highlighting the need for improved diagnostic tests. iNTS disease typically presents as a febrile systemic illness similar to enteric fever. Lack of blood culture facilities and delays in determining aetiological agents remain a challenge in resource-limited settings. Poorly defined clinical features and lack of appropriate diagnostic facilities often results in children being wrongly diagnosed and treated for malaria infection while iNTS is unattended, thus leading to poor clinical outcomes. WHO recommends administration of antimalarial and antibiotics drugs in children with severe malaria regardless of proven IBI [11], but antibiotic prescriptions supported by laboratory findings must be encouraged to prevent further development of antibiotic resistance. Gordon et al. described MDR prevalence of NTS in Malawi, defined as resistance to ampicillin, chloramphenicol, and co-trimoxazole [3]. Currently, iNTS is treated using third generation cephalosporins and fluoroquinolones such as ciprofloxacin are too expensive for routine use in endemic areas [13].
2. Immunity to iNTS: lessons from humans and animal models
2.1. Spread of ingested Salmonella to distant tissues via the gastrointestinal tract (GIT)
Salmonella infections start with the ingestion of contaminated food, water and fomites [14] and then the bacteria reach the distal ileum or caecum. A proportion of the infectious load survives the low gastric pH and the competition with normal flora [15]. Salmonella then invade Microfold (M) cells of the Peyer Patches (PP) using the Type III secretion system (T3SS) encoded by the Salmonella pathogenicity island 1 (SPI-1) [16]. The bacteria reach the blood stream from the GIT either extracellularly or transported via CD18+ cells [17]. Salmonella can also penetrate the gut epithelial barrier through dendritic cells (DC) which extend their dendrites between epithelial cells, overlying villi and capture gut luminal Salmonella [18]. Resident macrophages ingest Salmonella in the PP and mesenteric lymph nodes (MLN). However, virulent Salmonella may evade macrophage immunity by inducing macrophage cell death through the SPI-1-encoded Sip B protein which activates caspase 1 [16]. Salmonella can remain restricted to the MLN or disseminate via the thoracic duct to systemic tissues including peripheral blood, spleen, liver and bone marrow [15].
2.2. Phagocytes and host resistance to Salmonella
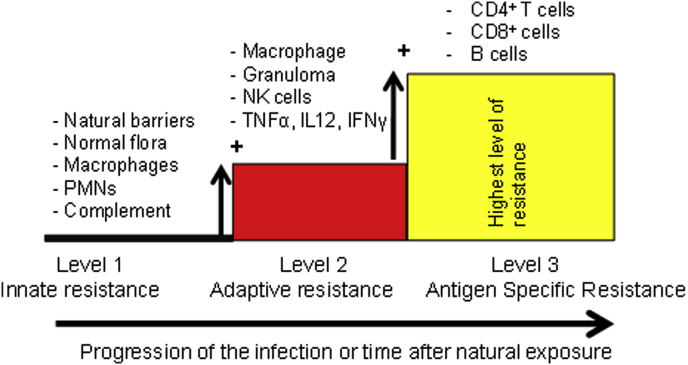
Monocytes and neutrophils play important roles in controlling Salmonella during the early phase of infection (Fig. 1) [19], [20]. Mice rendered neutropenic by administration of granulocyte-depleting monoclonal antibodies have been shown to be more susceptible to Salmonella infection compared to wild type mice [21]. Neutrophils and monocytes efficiently ingest Salmonella opsonised by complement factor C3b, through surface membrane complement receptor 3 (CR3) [22]. However, non-opsonised Salmonella can also be ingested by macrophages and neutrophils through CD14 and LPS interactions [23].
Fig. 1.
Key players of host resistance to Salmonella: Lessons from humans and mice. Immunity to Salmonella builds up gradually during primary infection or subclinical exposure to the pathogens. Each level of resistance adds to the previous one. Therefore, full protection relies on the activity of the immune mechanisms from each one of the levels of resistance. Co-morbidities that undermine any of these levels of resistance would result in impaired overall resistance to iNTS disease.
Killing of engulfed Salmonella is achieved through metabolic reactions within the phagosome membrane and cytosol [15]. Phagocytes killing mechanisms include; acidification (pH ranging 5–4.5), through glucose consumption, generation of phagolysosomes, generation of reactive oxygen intermediates (ROI) and reactive nitrogen intermediates (RNI) [24], [25]. In mice Salmonella growth in the tissues is controlled by macrophage associated Nramp1 (natural resistance-associated macrophage protein one) gene (also known as Slc11a1) during the first few days of infection [26]. Slc11a1 encodes divalent metal (Fe2+, Zn2+ and Mn2+) pump phosphoglycoproteins, which are recruited to the Salmonella-containing phagosome [15], [27]. The importance of ROI in controlling Salmonella infection is shown by the fact that chronic granulomatous disease (CGD) patients, whose phagocytes exhibit defective production of ROI are very susceptible to infections with intracellular pathogens, including Salmonella [28].
2.3. Role of cytokines and chemokines in immunity to Salmonella
Innate immune cells such as macrophages, neutrophils and natural killer cells (NK) produce cytokines that allow co-ordination of immune responses and subsequent Salmonella killing (Fig. 1). IL-8 and MIP-1 enhance the recruitment of leukocytes to the sites of infection [29]. TNF-α promotes phagocyte recruitment and the generation of multicellular pathological lesions at the foci of infection. The importance of TNF-α has been shown in mice treated with anti-TNF-α antibodies or TNFR 55 knockout; these mice fail to restrict bacterial growth and exhibit poorly organised lesions [30]. IFN-γ is required for the activation and expression of antimicrobial activity against intracellular Salmonella [31]. NK cells and gamma-delta T cells are the main producers of IFN-γ in the early stages of the infection as has been shown in Rag 1 knockout mice (lack mature CD4+ T cells, CD8+ T cells and B cells) which are still capable of producing IFN-γ in response to Salmonella infection [32]. Later in infection IFN-γ is produced by T-cells [33]. Individuals with genetic defects in the IL-12/IFN-γ axis are more susceptible to Salmonella indicating the importance of these cytokines in resistance to Salmonella infection in humans [34].
2.4. Complement-mediated immunity to Salmonella
Complement is important in controlling Salmonella infection (Fig. 1) [35]. C1q deficient mice are more susceptible to infection with S. Typhimurium [35]. Human patients with sickle cell disease are also more susceptible to Salmonella bacteraemia compared to healthy controls [36] with the susceptibility being attributed to reduced serum bactericidal activity as a result of defective function of the alternative complement pathway and low concentration of C3 [37].
2.5. T-helper type 1 CD4+ T cell immunity to Salmonella
Reduced numbers of CD4 T-cells are associated with increased susceptibility to systemic Salmonella disease in animal models [38] or humans (Fig. 1) (e.g. advanced HIV infection) [39]. Acute Salmonella infection is associated with increased CD4+ T helper 1 transcriptional factor Tbet and IL-2, and decreased T helper 2 transcriptional factor Gata 3 and IL-4 indicating the onset of Th1 immunity [40]. In simian immunodeficiency virus (SIV) infected macaques (human HIV infected model), Th17 cells in the ileal mucosa of rhesus macaques are depleted and this is associated with impaired mucosal barrier functions (blunted Th17 responses) resulting in increased systemic dissemination of S. Typhimurium from the gut [41].
2.6. B cell immunity to Salmonella infection
B cells play an important role in protection against Salmonella via antibody production (Fig. 1) [42]. The interaction between CD4+ T cells and B cells is also important for establishment long-term and robust Th1 type immunity to Salmonella infection [33], [43], [44], [45].
Salmonella are facultative intracellular organism and are capable of surviving in both the extracellular and intracellular space. Salmonella grow intracellularly and disperse in the tissues by escaping infected phagocytes and establishing new infection foci at distant sites [46], [47]. This process requires the T3SS encoded by the Salmonella pathogenicity island 2 (SPI-2) [48].
In their extracellular phase Salmonella becomes the target of antibody-mediated immunity [8]. Opsonic IgG or IgM antibodies specific for S. Typhimurium control Salmonella bacteraemia by activating complement cascade through the classical pathway which are ultimately killed through membrane attack complex [8]. Opsonic IgG antibodies also control Salmonella bacteraemia by facilitating neutrophils and monocytes phagocytosis through their surface membrane FcR [49] and increasing the production of ROI [50]. T-cells modulate antibody responses to Salmonella facilitating isotype switching and being necessary for the production of anti-protein antibodies [51], [52].
In Malawian children antibody-mediated serum killing of invasive NTS strain occurs in children older than 16 months and not in younger children [8], indicating a correlation between NTS-specific antibodies (IgG and IgM) and resistance [8]. Interestingly, a similar trend (age related development) of antibody mediated serum immunity to S. Typhi was reported in children from Nepal [53]. These findings support the exploration of antibody-based vaccines for NTS bacteraemia.
3. Immunity to malaria: lesson from humans and animal models
3.1. Burden of malaria
Malaria is an important cause of morbidity and mortality especially in children under the age of 5 years. Nearly 214 million clinical episodes of malaria were reported in 2015 leading to 438,000 deaths, the majority of which were among African children [54]. Although there are four different species of the Plasmodium parasite capable of causing disease in humans, it is P. falciparum which is associated with the highest morbidity and mortality rates especially in children aged twelve and less [55].
3.2. Plasmodium falciparum life cycle
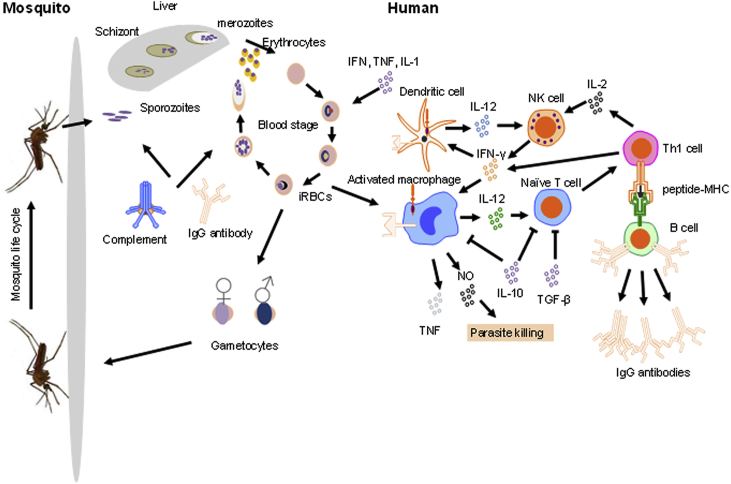
P. falciparum has a complex life cycle with some of the developmental stages occurring in humans and other stages in anopheles mosquitos, the vector for the parasite. The cycle begins when an infected female Anopheline mosquito probes human skin in preparation for a blood meal (Fig. 2). The saliva of infectious mosquitos contains sporozoites, a small number of which, ranging between 10 and 100, are injected into the skin of the patient where they may remain for hours or days [56]. The sporozoites then cross the endothelium of the capillaries in the skin, enter the blood and infect the hepatocytes in the liver [57]. The sporozoites replicate within liver cells, and differentiate into asexual blood-stage parasites called merozoites. This stage of the infection is asymptomatic. Merozoites are then released into the blood stream where they begin 48 h cycles of invasion of red blood cells (RBCs), replication, rupturing of RBCs, release of more merozoites and invasion of new intact RBCs. At this stage parasitemia can increase to over 50,000 infected RBCs (iRBCs) per microliter of blood. Different types of parasite components are released once the iRBCs lyse. The release of these parasite components is usually associated with the onset symptomatic malaria, characterized by headaches, fever and lethargy. The parasite life cycle in humans is completed when the asexual merozoites further differentiate into male and female gametocytes which are then ingested by the mosquito and reach the gut when it feeds on the blood of a human host carrying stage V gametocytes [58]. Male and female gametocytes then fuse in the midgut of the mosquito forming ookinetes which then cross the midgut epithelium before further differentiating to form sporozoites that invade the mosquito's salivary glands ready to be injected into the next human they feed on [58].
Fig. 2.
The Plasmodium falciparum life cycle and human host immunity Macrophages, DCs, T cells, and humoral immunity (antibody and complement) are involved in mounting stage-specific immune responses against the Plasmodium parasite. These are usually divided into either pre-erythrocytic immune responses directed against sporozoites in the skin and liver-stage parasites, or erythrocytic immune responses, directed against merozoites and intra-erythrocytic parasites.
3.3. The immune systems of humans and mosquitos mount responses to P. falciparum infections
In humans the timing, nature and quality of immune responses determines the outcome of the disease, whereas in mosquitoes immunity influences the ability of the vector to transmit the parasite [59]. Human immune resistance to malaria develops with age and with level of exposure, such that individuals living in malaria-endemic countries tend to become resistant to symptomatic malaria, but not to P. falciparum infection [56]. Immunity is both cellular and antibody-mediated (Fig. 2) [57], Innate cells, such as monocytes and natural killer (NK) cells and adaptive cells, such as CD4+ and CD8+ T cells and B cells, are involved in the immune response against the different stages of the parasite cell cycle and against the development of malaria as a disease [60].
3.4. Human immune response against P. falciparum
Innate and acquired immune responses against the Plasmodium parasite are complex and stage-specific. These are usually divided into either pre-erythrocytic responses directed against sporozoites in the skin and liver-stage parasites, and erythrocytic responses, directed against merozoites and intra-erythrocytic parasites (Fig. 2).
3.5. Immune response against the parasite in the human skin and in the liver
The initial introduction of sporozoites into the human skin is merely associated with itching and swelling, but is clinically not linked to any symptomatic disease or any systemic inflammation. In addition there is very little, if any, stimulation of innate immunity in the skin, which in turn, impairs the subsequent activation of adaptive (CD4+- and CD8+-mediated) immunity. This being the case, even after years of repeated exposure to P. falciparum the risk of adults residing in malaria-endemic to becoming infected with P. falciparum parasites is just as high as that of young children [60], although adults tend to become more resistant to symptomatic disease. Some investigators have attributed the poor innate immune response in the skin to the low initial sporozoite inoculum and the relatively high proportion of regulatory T cells [61]. It has been suggested that malaria parasites may use various mechanisms to evade the innate immune system in the skin, such as the delivery of very small numbers of sporozoites during the time the infected mosquito feeds on the human, thus minimizing the activation that the skin-based immune system [62].
3.6. Immune response against blood stage infection
The merozoites that survive during the pre-erythocytic stage are responsible for the modification of iRBCs cells in terms of the array of parasite proteins expressed on the cell surface and triggering of the immune response against the P. falciparum parasite, resulting in the clinical manifestations that characterize malaria [63]. The pathogenic manifestations associated with severe malaria are thought to be due to pro-inflammatory cytokines released by T cells and macrophages in response to malaria parasites and their products, including glycosylphosphatidyl-inositol (GPI) moieties [55], malaria pigment [64] and Plasmodium-derived nitric oxide synthase (NOS)-inducing factor [65].
Antibody-mediated responses against extracellular merozoites and intraerythrocytic parasites have previously been regarded as the most fundamental component of erythrocytic stage immunity, with their beneficial roles first demonstrated by the therapeutic effects of the passive transfer of adult immune IgG to infected children in the sixties [66]. An antibody binding to the surface of the merozoite and to proteins that are externalized from the apical complex of organelles involved in erythrocyte recognition and invasion, has been reported to have an important role in immunity to asexual blood stages. This antibody could neutralize parasites or lead to Fc dependent mechanisms of parasite killing by macrophages [67]. To date little is known about T cell responses against iRBCs, partly because erythrocytes lack MHC class I or class II presentation capacity. Nevertheless, cellular responses against iRBC have been suggested to contribute to protection in humans in the absence of antibodies [68], [69]. In addition, monocyte/macrophage-mediated responses, in particular phagocytosis and antibody-dependent cellular inhibition, also form an important component of blood-stage immunity [70]. IFN-γ is now known to play a crucial role in immunity against blood-stage Plasmodium parasites and requires coordinated and timely innate and adaptive immune responses involving dendritic cells (DC), NK cells, CD4+ T helper cells, and B cells [70]. With the pathogenesis associated with severe malaria thought to be linked to unregulated production of pro-inflammatory cytokines, the balance between pro-inflammatory and anti-inflammatory responses is therefore essential to limit the development of life-threatening immune-mediated pathology such as cerebral malaria (CM) and SMA. A better understanding of regulatory mechanisms required to maintain the balance between beneficial and deleterious responses during blood-stage malaria infection remains elusive but would be extremely useful in the prevention of severe malaria [71].
4. Mechanisms of increased susceptibility to iNTS in children with malaria and anaemia
4.1. Malaria-related impairment of gut barrier defences to NTS
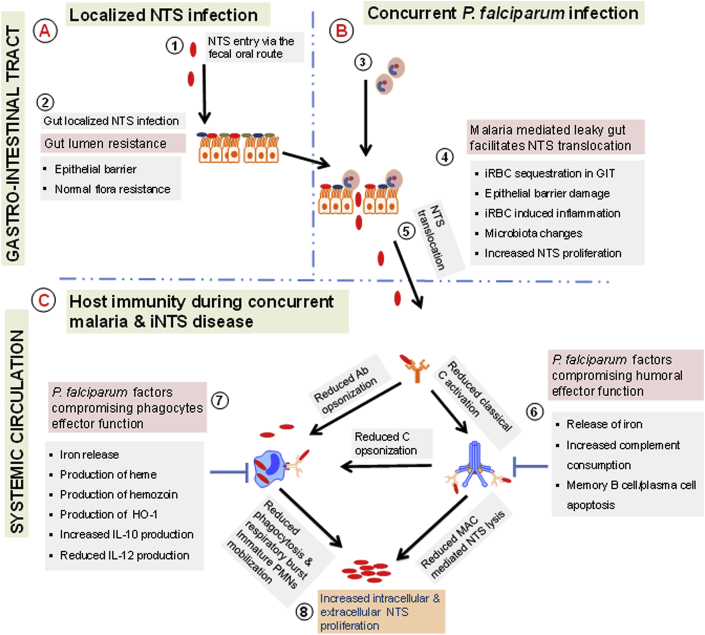
The gut barrier provides the first line of defence to iNTS. Gut mucosal resistance to NTS is complex, potentially involving of both cellular and humoral arms of immunity, the gut epithelial layer and microbiota. From histological studies it has long been known that the sequestration of malaria parasites to the gut is common in humans [72]. Intestinal fatty acid binding protein (IFABP), a biomarker of intestinal barrier ischemia, is elevated in malaria-infected children and this is associated with increased malaria-infected RBC binding to ICAM [73]. In malaria-infected mice, l-arginine deficiency has been reported to mediate the disruption of the intestinal barrier [74]. Alterations in the gut barrier during malaria have been reported to promote microbial translocation from gut to the bloodstream (Fig. 3) [74], [75]. Recently, in malaria-infected mice, it has been shown that inflammation mediates changes in the gut microbiota, leading to increased colonization of the gut by NTS [76]. It is still unclear how different clinical forms of malaria affect the gut barrier to NTS.
Fig. 3.
Malaria and anaemia increase susceptibility to iNTS in children. Healthy children are capable of restricting NTS colonisation and invasion within the gastro-intestinal tract (GIT) through due to their normal flora and efficient epithelial barrier (Fig. 3A). Malaria can impair the gut-epithelial barrier, thus favouring NTS translocation to the blood stream (Fig. 3B). Humoral (antibody and complement) and cellular (monocytes and neutrophils) immunity to NTS can be compromised due to malaria-induced anaemia, products from malaria parasites and also immune responses to malaria parasites which concomitantly favour the proliferation and dissemination of NTS (Fig. 3C).
4.2. Malaria-related compromise of complement mediated immunity to NTS
Complement consumption is known to occur in acute malaria (Fig. 3) [77], [78]. The levels of complement components return to normal during convalescence. Classical pathway components including C1q, C4, C3 are particularly reduced during malaria and this leads to reductions in complement dependent-antibody mediated NTS killing in children [79]. Loss in complement mediated cell-free killing during malaria occurs in children regardless of high antibody titre to LPS [79]. Malaria-induced complement consumption may contribute to reductions in phagocytosis and phagocytes-mediated killing via ROI but this remains to be fully explored in humans. Furthermore, others have shown that complement consumption is higher in severe malaria compared to uncomplicated malaria [77], [78]. However, the relationship between complement consumption in various clinical forms of malaria and susceptibility to iNTS has not been explored.
4.3. Malaria-related impairment of phagocytes effector functions to NTS
Malaria-derived products have been shown to compromise the effector functions of neutrophils, monocytes and macrophage in humans and animals [80], [81], [82]. Uptake of malaria-infected RBC and hemozoin renders monocytes unable to degrade the internalised products and reduces their phagocytic functions (Fig. 3) [81]. Robust production of ROI does not occur in phagocytes following the ingestion of malaria-derived products (Fig. 3) [81]. In line with this, hemozoin has been implicated in the suppression of the function of NADPH oxidase in human monocyte-derived macrophage via inhibition of PKC [82]. Malaria-mediated hemolysis releases hemoglobin and increases the levels of hemoglobin-derived heme. The latter induces heme oxygenase-1 (HO-1) in immature myeloid cells; heme is further degraded to biliverdin, carbon monoxide and iron. Heme degradation products in chronic hemolysis drive the release of functionally immature granulocytes from the bone marrow into the blood and these immature phagocytes have reduced ability to produce ROI and are thus less efficient at controlling bacterial infections including NTS [80], [83].
4.4. Malaria-related cytokine changes favouring the proliferation of NTS
Malaria induces the elevated levels of anti-inflammatory IL-10, which plays a role in avoiding malaria-related tissue damage by prolonged pro-inflammatory response. However IL-10 has been shown in animal studies to exacerbate bacterial infections including salmonellosis [84], [85]. In severe malaria anaemia increased production of IL-10 has been implicated in down-regulating the production of IL-12. Lower levels of IL-12 have been shown to favour the growth of microbes such as NTS (Fig. 3) [86], [87]. This anti-inflammatory environment favourable for the growth of NTS occurs within the gastro-intestinal tract and systemic circulation [84], [85]. The impact of malaria related anti-inflammatory responses on susceptibility to iNTS has not been investigated in endemic settings.
4.5. Malaria and non-malaria related anaemia favours the proliferation of NTS
Epidemiological studies have shown that malaria, particularly severe malaria anaemia, is a key risk factor for iNTS [36], [11]. Malaria-induced destruction of iRBC allows the release of iron and heme, which promote intracellular bacterial growth and inhibit the effector functions of phagocytes respectively (Fig. 3) [88], [80], [89]. Individuals with sickle cell anaemia are more susceptible to Salmonella bacteraemia and this susceptibility appears to be mediated by defective complement immunity particularly the alternative pathway [37], [90].
4.6. Age-related susceptibility to iNTS
Young children below the age of 2 are more susceptible to iNTS disease in endemic settings [7] with peak susceptibility around 13 months of age. The peak incidence of iNTS disease coincides low titres of antibodies, suggesting that antibodies play a crucial role in controlling NTS infection [7], [8].
4.7. Natural and vaccine-acquired memory B cell and antibody responses to Salmonella during malaria
In malaria-endemic regions it has long been suspected that P. falciparum infection reduces vaccine efficacy in children [91]. Most licensed vaccines confer protection through antibodies [92]. Long-lived antibody responses depend on memory B cells (MBCs) and long-lived plasma cells (LLPCs) [93]. In mice, malaria-mediated apoptosis has been implicated to loss of memory B and plasma cells [94]. Whether this contributes to the suppression of pre-existing antibody responses remains to be explored.
It has been shown in children that malaria suppresses antibody responses to polysaccharide vaccine antigens while evidence on suppression of antibody responses to vaccine protein antigens is weak [95]. We found in Malawian children that levels of pre-existing IgG antibodies targeting NTS-LPS were similar during acute malaria, at days 14 and 30 malaria convalescence compared to age match healthy controls [79]. In line with this, mice immunized with live attenuated Salmonella vaccine had similar levels of antibody titres pre and post malaria infection [96]. GMMA and new generations of glycoproteins-based NTS vaccines are currently in the pre-clinical phase of evaluation [97], [98]. Field studies are required to gain better understanding of the effect of malaria on NTS-specific vaccine responses currently in pre-clinical phase; this is a crucial step in the development of these new vaccines aimed to be implemented in settings where malaria is endemic.
4.8. Vaccine-acquired memory CD4+ T cell and effector function to Salmonella during malaria
Strong evidence on how malaria suppresses vaccine induced memory CD4+ T cell immune responses to NTS comes from mice model of malaria and NTS co-infection. Protective immune responses to NTS conferred by live attenuated Salmonella vaccine is lost following challenge with non-lethal Plasmodium yoelii 17XNL coinfection [96]. This loss in protection to NTS is associated with increased levels of IL-10 and Salmonella specific effector CD4 T cell responses are reduced [96]. Blocking IL-10 with antibodies partially restored protection to NTS. Other key contributors to this malaria-related susceptibility to NTS include increased expression of CTLA-4, LAG3 and PD1 on CD4+ T cells [96].
5. Strategies to overcome malaria and iNTS disease in children
5.1. Prevention of both malaria and iNTS
In SSA, where both malaria and iNTS are endemic, prevention of these fatal diseases through vaccination has not been possible due to the delay in vaccine development. The RSTS,S malaria vaccine has shown promising results in clinical studies. It is anticipated that when this vaccine is implemented, it will supplement existing malaria reduction strategies including early treatment of malaria cases with artemisinin-based combination therapies (ACTs), indoor residual spraying (IRS), long-lasting insecticide treated bed nets (LLINS) and intermittent treatment therapy for pregnant women (IPTp). As observed in some countries that have experienced epidemiological changes in the incidence of malaria and iNTS disease, we anticipate that following the rollout of malaria vaccines, cases of iNTS will be reduced in endemic settings. It will take several years before the implementation of a vaccine against iNTS disease as most candidates are still at the pre-clinical phase [98].
5.2. Population tailored interventions
WHO guidelines recommend the administration of both anti-malaria drug and antibiotic in case of severe malaria in children without evidence of concurrent invasive microbial infection [11]. This recommendation is widely accepted by researchers on malaria and bacterial co-infections. However, improvements on this recommendation are necessary as field studies are showing that even uncomplicated malaria renders children susceptible to invasive bacterial infections [79]. Revised guidelines that will also cover uncomplicated malaria and bacterial infection are warranted. Field epidemiological studies have also suggested that malaria plays a role in the poor vaccine efficacy observed in malaria endemic areas. Recommendations by other investigators to avoid childhood vaccination during malaria episodes and only administer vaccines when malaria has been cleared have not materialised probably due to limited scientific evidence supporting these recommendations. In regions where NTS colonization is endemic, iron supplementation to address malaria or non-malaria induced anaemia should be administered with caution to avoid creating favourable niche for the growth of microbes such as NTS. The use of adjunct therapy in order to correct factors that promote NTS growth such as HO-1 needs to be explored further in field settings.
5.3. Field studies
Recent evidence from field studies that malaria compromises both the humoral and cellular immunity is interesting [79]. While several studies have focussed on the malaria-induced compromise of the cell mediated host immunity, studies on malaria-related impairment of humoral immunity are limited. We particularly recommend field studies to establish reference levels of complement in various forms of malaria and also areas of malaria endemicity. Such studies will help to inform immunological interventions to correct malaria induced hypocomplentaemia.
6. Conclusion
Malaria has consistently been shown to be a major risk factor for iNTS disease in African children. Knowledge of clinical and epidemiological risk-factors for iNTS disease has not been paralleled by an in depth knowledge of the immunobiology of the disease. Our understanding of resistance/susceptibility to iNTS in Africa is further complicated by overlapping risk factors/comorbidities such as young age, malaria and anaemia that often coexist in the same individual or in the same endemic area, and create complex clinical scenarios for the understanding of immunity and the evaluation of protection.
Although modification of susceptibility factors is important, vaccines against iNTS for African children are a high priority in settings with very limited health systems, but their development is challenging. Both the quality of response, and antigen targets need to be optimised to confer a sufficient level of protection, even in children whose innate immunity may be impaired by widespread comorbidities such as malaria and anaemia.
Therefore better knowledge of how host effector immunological mechanisms interact to control the growth and kill iNTS in humans in the setting of co-morbidities such as malaria is absolutely essential for vaccine development against iNTS in Africa.
Conflict of interest
All authors declared no conflict of interest.
Acknowledgments
TS Nyirenda is supported by a Post-Doctoral Training Fellowship from Wellcome Trust Southern Africa Consortium for Research Excellence (SACORE), WT087537MA and Post-Doctoral Training Fellowship from Consortium for Advance Research Training in Africa (CARTA). CARTA is jointly led by African Population and Health Research Centre and the University of the Witwatersrand and funded by Wellcome Trust (UK) (Grant No: 087547/Z/08/Z), the Carnegie Corporation of New York (Grant No: -B 8696.R02), Sida (Grant No: 54100029).
References
- 1.Reddy E.A., Shaw A.V., Crump J.A. Community-acquired bloodstream infections in Africa: a systematic review and meta-analysis. Lancet Infect Dis. 2010;10:417–432. doi: 10.1016/S1473-3099(10)70072-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ao T.T., Feasey N.A., Gordon M.A., Keddy K.H., Angulo F.J., Crump J.A. Global burden of invasive nontyphoidal Salmonella disease, 2010. Emerg Infect Dis. 2015;21(1) doi: 10.3201/eid2106.140999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gordon M.A., Graham S.M., Walsh A.L., Wilson L., Phiri A., Molyneux E. Epidemics of invasive Salmonella enterica serovar enteritidis and Salmonella enterica serovar typhimurium infection associated with multidrug resistance among adults and children in Malawi. Clin Infect Dis. 2008;46:963–969. doi: 10.1086/529146. [DOI] [PubMed] [Google Scholar]
- 4.Molyneux E.M., Mankhambo L.A., Phiri A., Graham S.M., Forsyth H., Phiri A. The outcome of non-typhoidal Salmonella meningitis in Malawian children, 1997–2006. Ann Trop Paediatr. 2009;29:13–22. doi: 10.1179/146532809X401980. [DOI] [PubMed] [Google Scholar]
- 5.Bronzan R.N., Taylor T.E., Mwenechanya J., Tembo M., Kayira K., Bwanaisa L. Bacteremia in Malawian children with severe malaria: prevalence, etiology, HIV coinfection, and outcome. J Infect Dis. 2007;195:895–904. doi: 10.1086/511437. [DOI] [PubMed] [Google Scholar]
- 6.Scott J.A., Berkley J.A., Mwangi I., Ochola L., Uyoga S., Macharia A. Relation between falciparum malaria and bacteraemia in Kenyan children: a population-based, case-control study and a longitudinal study. Lancet. 2011;378:1316–1323. doi: 10.1016/S0140-6736(11)60888-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nyirenda T.S., Gilchrist J.J., Feasey N.A., Glennie S.J., Bar-Zeev N., Gordon M.A. Sequential acquisition of T cells and antibodies to nontyphoidal Salmonella in Malawian children. J Infect Dis. 2014;210:56–64. doi: 10.1093/infdis/jiu045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.MacLennan C.A., Gondwe E.N., Msefula C.L., Kingsley R.A., Thomson N.R., White S.A. The neglected role of antibody in protection against bacteremia caused by nontyphoidal strains of Salmonella in African children. J Clin Invest. 2008;118:1553–1562. doi: 10.1172/JCI33998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Park S.E., Pak G.D., Aaby P., Adu-Sarkodie Y., Ali M., Aseffa A. The relationship between invasive nontyphoidal Salmonella disease, other bacterial bloodstream infections, and Malaria in sub-Saharan Africa. Clin Infect Dis. 2016;62(Suppl. 1):S23–S31. doi: 10.1093/cid/civ893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bassat Q., Guinovart C., Sigauque B., Mandomando I., Aide P., Sacarlal J. Severe malaria and concomitant bacteraemia in children admitted to a rural Mozambican hospital. Trop Med Int Health. 2009;14:1011–1019. doi: 10.1111/j.1365-3156.2009.02326.x. [DOI] [PubMed] [Google Scholar]
- 11.Church J., Maitland K. Invasive bacterial co-infection in African children with Plasmodium falciparum malaria: a systematic review. BMC Med. 2014;12:31. doi: 10.1186/1741-7015-12-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Biggs H.M., Lester R., Nadjm B., Mtove G., Todd J.E., Kinabo G.D. Invasive Salmonella infections in areas of high and low malaria transmission intensity in Tanzania. Clin Infect Dis. 2014;58:638–647. doi: 10.1093/cid/cit798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Feasey N.A., Dougan G., Kingsley R.A., Heyderman R.S., Gordon M.A. Invasive non-typhoidal Salmonella disease: an emerging and neglected tropical disease in Africa. Lancet. 2012;379:2489–2499. doi: 10.1016/S0140-6736(11)61752-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kariuki S., Revathi G., Kariuki N., Kiiru J., Mwituria J., Muyodi J. Invasive multidrug-resistant non-typhoidal Salmonella infections in Africa: zoonotic or anthroponotic transmission? J Med Microbiol. 2006;55:585–591. doi: 10.1099/jmm.0.46375-0. [DOI] [PubMed] [Google Scholar]
- 15.Mastroeni P., Ugrinovic S., Chandra A., MacLennan C., Doffinger R., Kumararatne D. Resistance and Susceptibility to Salmonella infections: lessons from mice and patients with immunodeficiencies. Rev Med Micro. 2003;14:1–10. [Google Scholar]
- 16.Monack D.M., Mueller A., Falkow S. Persistent bacterial infections: the interface of the pathogen and the host immune system. Nat Rev Microbiol. 2004;2:747–765. doi: 10.1038/nrmicro955. [DOI] [PubMed] [Google Scholar]
- 17.Vazquez-Torres A., Jones-Carson J., Baumler A.J., Falkow S., Valdivia R., Brown W. Extraintestinal dissemination of Salmonella by CD18-expressing phagocytes. Nature. 1999;401:804–808. doi: 10.1038/44593. [DOI] [PubMed] [Google Scholar]
- 18.Rescigno M., Urbano M., Valzasina B., Francolini M., Rotta G., Bonasio R. Dendritic cells express tight junction proteins and penetrate gut epithelial monolayers to sample bacteria. Nat Immunol. 2001;2:361–367. doi: 10.1038/86373. [DOI] [PubMed] [Google Scholar]
- 19.Fierer J. Polymorphonuclear leukocytes and innate immunity to Salmonella infections in mice. Microb Infect. 2001;3:1233–1237. doi: 10.1016/s1286-4579(01)01483-6. [DOI] [PubMed] [Google Scholar]
- 20.Cheminay C., Chakravortty D., Hensel M. Role of neutrophils in murine salmonellosis. Infect Immun. 2004;72:468–477. doi: 10.1128/IAI.72.1.468-477.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Conlan J.W. Critical roles of neutrophils in host defense against experimental systemic infections of mice by Listeria monocytogenes, Salmonella typhimurium, and Yersinia enterocolitica. Infect Immun. 1997;65:630–635. doi: 10.1128/iai.65.2.630-635.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van Bruggen R., Zweers D., van Diepen A., van Dissel J.T., Roos D., Verhoeven A.J. Complement receptor 3 and Toll-like receptor 4 act sequentially in uptake and intracellular killing of unopsonized Salmonella enterica serovar typhimurium by human neutrophils. Infect Immun. 2007;75:2655–2660. doi: 10.1128/IAI.01111-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Heale J.P., Pollard A.J., Stokes R.W., Simpson D., Tsang A., Massing B. Two distinct receptors mediate nonopsonic phagocytosis of different strains of Pseudomonas aeruginosa. J Infect Dis. 2001;183:1214–1220. doi: 10.1086/319685. [DOI] [PubMed] [Google Scholar]
- 24.Mastroeni P., Vazquez-Torres A., Fang F.C., Xu Y., Khan S., Hormaeche C.E. Antimicrobial actions of the NADPH phagocyte oxidase and inducible nitric oxide synthase in experimental salmonellosis. II. Effects on microbial proliferation and host survival in vivo. J Exp Med. 2000;192:237–248. doi: 10.1084/jem.192.2.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vazquez-Torres A., Jones-Carson J., Mastroeni P., Ischiropoulos H., Fang F.C. Antimicrobial actions of the NADPH phagocyte oxidase and inducible nitric oxide synthase in experimental salmonellosis. I. Effects on microbial killing by activated peritoneal macrophages in vitro. J Exp Med. 2000;192:227–236. doi: 10.1084/jem.192.2.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hormaeche C.E. Dead salmonellae or their endotoxin accelerate the early course of a Salmonella infection in mice. Microb Pathog. 1990;9:213–218. doi: 10.1016/0882-4010(90)90023-j. [DOI] [PubMed] [Google Scholar]
- 27.Vidal S.M., Malo D., Vogan K., Skamene E., Gros P. Natural resistance to infection with intracellular parasites: isolation of a candidate for Bcg. Cell. 1993;73:469–485. doi: 10.1016/0092-8674(93)90135-d. [DOI] [PubMed] [Google Scholar]
- 28.Mouy R., Fischer A., Vilmer E., Seger R., Griscelli C. Incidence, severity, and prevention of infections in chronic granulomatous disease. J Pediatr. 1989;114:555–560. doi: 10.1016/s0022-3476(89)80693-6. [DOI] [PubMed] [Google Scholar]
- 29.Tam M.A., Rydstrom A., Sundquist M., Wick M.J. Early cellular responses to Salmonella infection: dendritic cells, monocytes, and more. Immunol Rev. 2008;225:140–162. doi: 10.1111/j.1600-065X.2008.00679.x. [DOI] [PubMed] [Google Scholar]
- 30.Everest P., Roberts M., Dougan G. Susceptibility to Salmonella typhimurium infection and effectiveness of vaccination in mice deficient in the tumor necrosis factor alpha p55 receptor. Infect Immun. 1998;66:3355–3364. doi: 10.1128/iai.66.7.3355-3364.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Muotiala A., Makela P.H. The role of IFN-gamma in murine Salmonella typhimurium infection. Microb Pathog. 1990;8:135–141. doi: 10.1016/0882-4010(90)90077-4. [DOI] [PubMed] [Google Scholar]
- 32.Ramarathinam L., Niesel D.W., Klimpel G.R. Salmonella typhimurium induces IFN-gamma production in murine splenocytes. Role of natural killer cells and macrophages. J Immunol. 1993;150:3973–3981. [PubMed] [Google Scholar]
- 33.Barr T.A., Brown S., Mastroeni P., Gray D. B cell intrinsic MyD88 signals drive IFN-gamma production from T cells and control switching to IgG2c. J Immunol. 2009;183:1005–1012. doi: 10.4049/jimmunol.0803706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sharifi Mood B., Mohraz M., Mansouri S.D., Alavi Naini R., Kouhpayeh H.R., Naderi M. Recurrent non-typhoidal Salmonella bacteremia in a patient with interleukin -12p40 deficiency. Iran J Allergy Asthma Immunol. 2004;3:197–200. [PubMed] [Google Scholar]
- 35.Warren J., Mastroeni P., Dougan G., Noursadeghi M., Cohen J., Walport M.J. Increased susceptibility of C1q-deficient mice to Salmonella enterica serovar typhimurium infection. Infect Immun. 2002;70:551–557. doi: 10.1128/iai.70.2.551-557.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Calis J.C., Phiri K.S., Faragher E.B., Brabin B.J., Bates I., Cuevas L.E. Severe anemia in Malawian children. N Engl J Med. 2008;358:888–899. doi: 10.1056/NEJMoa072727. [DOI] [PubMed] [Google Scholar]
- 37.Hand W.L., King N.L. Deficiency of serum bactericidal activity against Salmonella typhimurium in sickle cell anaemia. Clin Exp Immunol. 1977;30:262–270. [PMC free article] [PubMed] [Google Scholar]
- 38.Hess J., Ladel C., Miko D., Kaufmann S.H. Salmonella typhimurium aroA- infection in gene-targeted immunodeficient mice: major role of CD4+ TCR-alpha beta cells and IFN-gamma in bacterial clearance independent of intracellular location. J Immunol. 1996;156:3321–3326. [PubMed] [Google Scholar]
- 39.Kankwatira A.M., Mwafulirwa G.A., Gordon M.A. Non-typhoidal Salmonella bacteraemia–an under-recognized feature of AIDS in African adults. Trop Doct. 2004;34:198–200. doi: 10.1177/004947550403400404. [DOI] [PubMed] [Google Scholar]
- 40.Lin A.Y., Lin C.Y., Chen C.T., Chen W.L. Host defense against Salmonella and rotaviral gastroenteritis: a serial study of transcriptional factors and cytokines. J Microbiol Immunol Infect. 2008;41:265–271. [PubMed] [Google Scholar]
- 41.Raffatellu M., Santos R.L., Verhoeven D.E., George M.D., Wilson R.P., Winter S.E. Simian immunodeficiency virus-induced mucosal interleukin-17 deficiency promotes Salmonella dissemination from the gut. Nat Med. 2008;14:421–428. doi: 10.1038/nm1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mastroeni P., Villarreal-Ramos B., Hormaeche C.E. Adoptive transfer of immunity to oral challenge with virulent Salmonella in innately susceptible BALB/c mice requires both immune serum and T cells. Infect Immun. 1993;61:3981–3984. doi: 10.1128/iai.61.9.3981-3984.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Barr T.A., Brown S., Mastroeni P., Gray D. TLR and B cell receptor signals to B cells differentially program primary and memory Th1 responses to Salmonella enterica. J Immunol. 2010;185:2783–2789. doi: 10.4049/jimmunol.1001431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mastroeni P., Simmons C., Fowler R., Hormaeche C.E., Dougan G. Igh-6(-/-) (B-cell-deficient) mice fail to mount solid acquired resistance to oral challenge with virulent Salmonella enterica serovar typhimurium and show impaired Th1 T-cell responses to Salmonella antigens. Infect Immun. 2000;68:46–53. doi: 10.1128/iai.68.1.46-53.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ugrinovic S., Menager N., Goh N., Mastroeni P. Characterization and development of T-cell immune responses in B-cell-deficient (Igh-6(-/-)) mice with Salmonella enterica serovar typhimurium infection. Infect Immun. 2003;71:6808–6819. doi: 10.1128/IAI.71.12.6808-6819.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mastroeni P., Grant A., Restif O., Maskell D. A dynamic view of the spread and intracellular distribution of Salmonella enterica. Nat Rev Microbiol. 2009;7:73–80. doi: 10.1038/nrmicro2034. [DOI] [PubMed] [Google Scholar]
- 47.Mastroeni P., Rossi O. Immunology, epidemiology and mathematical modelling towards a better understanding of invasive non-typhoidal Salmonella disease and rational vaccination approaches. Expert Rev Vaccines. 2016;15:1545–1555. doi: 10.1080/14760584.2016.1189330. [DOI] [PubMed] [Google Scholar]
- 48.Grant A.J., Morgan F.J., McKinley T.J., Foster G.L., Maskell D.J., Mastroeni P. Attenuated Salmonella typhimurium lacking the pathogenicity island-2 type 3 secretion system grow to high bacterial numbers inside phagocytes in mice. PLoS Pathog. 2012;8 doi: 10.1371/journal.ppat.1003070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Uppington H., Menager N., Boross P., Wood J., Sheppard M., Verbeek S. Effect of immune serum and role of individual Fcgamma receptors on the intracellular distribution and survival of Salmonella enterica serovar typhimurium in murine macrophages. Immunology. 2006;119:147–158. doi: 10.1111/j.1365-2567.2006.02416.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gondwe E.N., Molyneux M.E., Goodall M., Graham S.M., Mastroeni P., Drayson M.T. Importance of antibody and complement for oxidative burst and killing of invasive nontyphoidal Salmonella by blood cells in Africans. Proc Natl Acad Sci U S A. 2010;107:3070–3075. doi: 10.1073/pnas.0910497107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Harrison J.A., Villarreal-Ramos B., Mastroeni P., Demarco de Hormaeche R., Hormaeche C.E. Correlates of protection induced by live Aro- Salmonella typhimurium vaccines in the murine typhoid model. Immunology. 1997;90:618–625. doi: 10.1046/j.1365-2567.1997.00158.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sinha K., Mastroeni P., Harrison J., de Hormaeche R.D., Hormaeche C.E. Salmonella typhimurium aroA, htrA, and aroD htrA mutants cause progressive infections in athymic (nu/nu) BALB/c mice. Infect Immun. 1997;65:1566–1569. doi: 10.1128/iai.65.4.1566-1569.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pulickal A.S., Gautam S., Clutterbuck E.A., Thorson S., Basynat B., Adhikari N. Kinetics of the natural, humoral immune response to Salmonella enterica serovar Typhi in Kathmandu, Nepal. Clin Vaccine Immunol. 2009;16:1413–1419. doi: 10.1128/CVI.00245-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.WHO . 2016. World Malaria Report.http://www.who.int/malaria/publications/world-malaria-report-2016/report/en/ [Google Scholar]
- 55.Schofield L., Mueller I. Clinical immunity to malaria. Curr Mol Med. 2006;6:205–221. doi: 10.2174/156652406776055221. [DOI] [PubMed] [Google Scholar]
- 56.Gueirard P., Tavares J., Thiberge S., Bernex F., Ishino T., Milon G. Development of the malaria parasite in the skin of the mammalian host. Proc Natl Acad Sci U S A. 2010;107:18640–18645. doi: 10.1073/pnas.1009346107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mota M.M., Pradel G., Vanderberg J.P., Hafalla J.C., Frevert U., Nussenzweig R.S. Migration of Plasmodium sporozoites through cells before infection. Science. 2001;291:141–144. doi: 10.1126/science.291.5501.141. [DOI] [PubMed] [Google Scholar]
- 58.Meibalan E., Marti M. Biology of malaria transmission. Cold Spring Harb Perspect Med. 2017;7:a025452. doi: 10.1101/cshperspect.a025452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rosenberg R., Wirtz R.A., Schneider I., Burge R. An estimation of the number of malaria sporozoites ejected by a feeding mosquito. Trans Roy Soc Trop Med Hyg. 1990;84:209–212. doi: 10.1016/0035-9203(90)90258-g. [DOI] [PubMed] [Google Scholar]
- 60.Tran T.M., Li S., Doumbo S., Doumtabe D., Huang C.Y., Dia S. An intensive longitudinal cohort study of Malian children and adults reveals no evidence of acquired immunity to Plasmodium falciparum infection. Clin Infect Dis. 2013;57:40–47. doi: 10.1093/cid/cit174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Honda T., Miyachi Y., Kabashima K. Regulatory T cells in cutaneous immune responses. J Dermatol Sci. 2011;63:75–82. doi: 10.1016/j.jdermsci.2011.06.004. [DOI] [PubMed] [Google Scholar]
- 62.Usynin I., Klotz C., Frevert U. Malaria circumsporozoite protein inhibits the respiratory burst in Kupffer cells. Cell Microbiol. 2007;9:2610–2628. doi: 10.1111/j.1462-5822.2007.00982.x. [DOI] [PubMed] [Google Scholar]
- 63.Miller L.H., Good M.F., Kaslow D.C. Vaccines against the blood stages of falciparum malaria. Adv Exp Med Biol. 1998;452:193–205. doi: 10.1007/978-1-4615-5355-7_22. [DOI] [PubMed] [Google Scholar]
- 64.Pichyangkul S., Saengkrai P., Webster H.K. Plasmodium falciparum pigment induces monocytes to release high levels of tumor necrosis factor-alpha and interleukin-1 beta. Am J Trop Med Hyg. 1994;51:430–435. [PubMed] [Google Scholar]
- 65.Ghigo D., Todde R., Ginsburg H., Costamagna C., Gautret P., Bussolino F. Erythrocyte stages of Plasmodium falciparum exhibit a high nitric oxide synthase (NOS) activity and release an NOS-inducing soluble factor. J Exp Med. 1995;182:677–688. doi: 10.1084/jem.182.3.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cohen S., Mc G.I., Carrington S. Gamma-globulin and acquired immunity to human malaria. Nature. 1961;192:733–737. doi: 10.1038/192733a0. [DOI] [PubMed] [Google Scholar]
- 67.Saul A. The role of variant surface antigens on malaria-infected red blood cells. Parasitol Today. 1999;15:455–457. doi: 10.1016/s0169-4758(99)01534-3. [DOI] [PubMed] [Google Scholar]
- 68.Pombo D.J., Lawrence G., Hirunpetcharat C., Rzepczyk C., Bryden M., Cloonan N. Immunity to malaria after administration of ultra-low doses of red cells infected with Plasmodium falciparum. Lancet. 2002;360:610–617. doi: 10.1016/S0140-6736(02)09784-2. [DOI] [PubMed] [Google Scholar]
- 69.Roestenberg M., McCall M., Hopman J., Wiersma J., Luty A.J., van Gemert G.J. Protection against a malaria challenge by sporozoite inoculation. N Engl J Med. 2009;361:468–477. doi: 10.1056/NEJMoa0805832. [DOI] [PubMed] [Google Scholar]
- 70.McCall M.B., Sauerwein R.W. Interferon-gamma–central mediator of protective immune responses against the pre-erythrocytic and blood stage of malaria. J Leukoc Biol. 2010;88:1131–1143. doi: 10.1189/jlb.0310137. [DOI] [PubMed] [Google Scholar]
- 71.Stevenson M.M., Ing R., Berretta F., Miu J. Regulating the adaptive immune response to blood-stage malaria: role of dendritic cells and CD4(+)Foxp3(+) regulatory T cells. Int J Biol Sci. 2011;7:1311–1322. doi: 10.7150/ijbs.7.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Seydel K.B., Milner D.A., Kamiza S.B., Molyneux M.E., Taylor T.E. The distribution and intensity of parasite sequestration in comatose Malawian children. J Infect Dis. 2006;194:208–215. doi: 10.1086/505078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Church J.A., Nyamako L., Olupot-Olupot P., Maitland K., Urban B.C. Increased adhesion of Plasmodium falciparum infected erythrocytes to ICAM-1 in children with acute intestinal injury. Malar J. 2016;15:54. doi: 10.1186/s12936-016-1110-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chau J.Y., Tiffany C.M., Nimishakavi S., Lawrence J.A., Pakpour N., Mooney J.P. Malaria-associated L-arginine deficiency induces mast cell-associated disruption to intestinal barrier defenses against nontyphoidal Salmonella bacteremia. Infect Immun. 2013;81:3515–3526. doi: 10.1128/IAI.00380-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wilairatana P., Meddings J.B., Ho M., Vannaphan S., Looareesuwan S. Increased gastrointestinal permeability in patients with Plasmodium falciparum malaria. Clin Infect Dis. 1997;24:430–435. doi: 10.1093/clinids/24.3.430. [DOI] [PubMed] [Google Scholar]
- 76.Mooney J.P., Lokken K.L., Byndloss M.X., George M.D., Velazquez E.M., Faber F. Inflammation-associated alterations to the intestinal microbiota reduce colonization resistance against non-typhoidal Salmonella during concurrent malaria parasite infection. Sci Rep. 2015;5 doi: 10.1038/srep14603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Greenwood B.M., Brueton M.J. Complement activation in children with acute malaria. Clin Exp Immunol. 1974;18:267–272. [PMC free article] [PubMed] [Google Scholar]
- 78.Nyakoe N.K., Taylor R.P., Makumi J.N., Waitumbi J.N. Complement consumption in children with Plasmodium falciparum malaria. Malar J. 2009;8:7. doi: 10.1186/1475-2875-8-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Nyirenda T.S., Nyirenda J.T., Tembo D.L., Storm J., Dube Q., Msefula C.L. Loss of humoral and cellular immunity to invasive nontyphoidal Salmonella during current or convalescent Plasmodium falciparum infection in Malawian children. Clin Vaccine Immunol. 2017;24 doi: 10.1128/CVI.00057-17. e00057–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Cunnington A.J., de Souza J.B., Walther M., Riley E.M. Malaria impairs resistance to Salmonella through heme- and heme oxygenase-dependent dysfunctional granulocyte mobilization. Nat Med. 2012;18:120–127. doi: 10.1038/nm.2601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Schwarzer E., Turrini F., Ulliers D., Giribaldi G., Ginsburg H., Arese P. Impairment of macrophage functions after ingestion of Plasmodium falciparum-infected erythrocytes or isolated malarial pigment. J Exp Med. 1992;176:1033–1041. doi: 10.1084/jem.176.4.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Schwarzer E., Arese P. Phagocytosis of malarial pigment hemozoin inhibits NADPH-oxidase activity in human monocyte-derived macrophages. Biochim Biophys Acta. 1996;1316:169–175. doi: 10.1016/0925-4439(96)00021-x. [DOI] [PubMed] [Google Scholar]
- 83.Cunnington A.J., Njie M., Correa S., Takem E.N., Riley E.M., Walther M. Prolonged neutrophil dysfunction after Plasmodium falciparum malaria is related to hemolysis and heme oxygenase-1 induction. J Immunol. 2012;189:5336–5346. doi: 10.4049/jimmunol.1201028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lokken K.L., Mooney J.P., Butler B.P., Xavier M.N., Chau J.Y., Schaltenberg N. Malaria parasite infection compromises control of concurrent systemic non-typhoidal Salmonella infection via IL-10-mediated alteration of myeloid cell function. PLoS Pathog. 2014;10 doi: 10.1371/journal.ppat.1004049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Mooney J.P., Butler B.P., Lokken K.L., Xavier M.N., Chau J.Y., Schaltenberg N. The mucosal inflammatory response to non-typhoidal Salmonella in the intestine is blunted by IL-10 during concurrent malaria parasite infection. Mucosal Immunol. 2014;7:1302–1311. doi: 10.1038/mi.2014.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mastroeni P., Harrison J.A., Chabalgoity J.A., Hormaeche C.E. Effect of interleukin 12 neutralization on host resistance and gamma interferon production in mouse typhoid. Infect Immun. 1996;64:189–196. doi: 10.1128/iai.64.1.189-196.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Mastroeni P., Harrison J.A., Robinson J.H., Clare S., Khan S., Maskell D.J. Interleukin-12 is required for control of the growth of attenuated aromatic-compound-dependent salmonellae in BALB/c mice: role of gamma interferon and macrophage activation. Infect Immun. 1998;66:4767–4776. doi: 10.1128/iai.66.10.4767-4776.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kaye D., Hook E.W. The influence of hemolysis or blood loss on susceptibility to infection. J Immunol. 1963;91:65–75. [PubMed] [Google Scholar]
- 89.Roux C.M., Butler B.P., Chau J.Y., Paixao T.A., Cheung K.W., Santos R.L. Both hemolytic anemia and malaria parasite-specific factors increase susceptibility to nontyphoidal Salmonella enterica serovar typhimurium infection in mice. Infect Immun. 2010;78:1520–1527. doi: 10.1128/IAI.00887-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wilson W.A., Thomas E.J., Sissons J.G. Complement activation in asymptomatic patients with sickle cell anaemia. Clin Exp Immunol. 1979;36:130–139. [PMC free article] [PubMed] [Google Scholar]
- 91.Greenwood B.M., Bradley-Moore A.M., Bryceson A.D., Palit A. Immunosuppression in children with malaria. Lancet. 1972;1:169–172. doi: 10.1016/s0140-6736(72)90569-7. [DOI] [PubMed] [Google Scholar]
- 92.Plotkin S.A. Correlates of protection induced by vaccination. Clin Vaccine Immunol. 2010;17:1055–1065. doi: 10.1128/CVI.00131-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Tarlinton D., Good-Jacobson K. Diversity among memory B cells: origin, consequences, and utility. Science. 2013;341:1205–1211. doi: 10.1126/science.1241146. [DOI] [PubMed] [Google Scholar]
- 94.Banga S., Coursen J.D., Portugal S., Tran T.M., Hancox L., Ongoiba A. Impact of acute malaria on pre-existing antibodies to viral and vaccine antigens in mice and humans. PLoS One. 2015;10 doi: 10.1371/journal.pone.0125090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Cunnington A.J., Riley E.M. Suppression of vaccine responses by malaria: insignificant or overlooked? Expert Rev Vaccines. 2010;9:409–429. doi: 10.1586/erv.10.16. [DOI] [PubMed] [Google Scholar]
- 96.Mooney J.P., Lee S.J., Lokken K.L., Nanton M.R., Nuccio S.P., McSorley S.J. Transient loss of protection afforded by a live attenuated non-typhoidal Salmonella vaccine in mice co-infected with malaria. PLoS Negl Trop Dis. 2015;9 doi: 10.1371/journal.pntd.0004027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.MacLennan C.A., Levine M.M. Invasive nontyphoidal Salmonella disease in Africa: current status. Expert Rev Anti Infect Ther. 2013;11:443–446. doi: 10.1586/eri.13.27. [DOI] [PubMed] [Google Scholar]
- 98.MacLennan C.A., Martin L.B., Micoli F. Vaccines against invasive Salmonella disease: current status and future directions. Hum Vaccin Immunother. 2014;10:1478–1493. doi: 10.4161/hv.29054. [DOI] [PMC free article] [PubMed] [Google Scholar]