Abstract
AIM
To uncover the roles of tumor-promoting gene ZEB1 in aerobic glycolysis regulation and shed light on the underlying molecular mechanism.
METHODS
Endogenous zinc finger E-box binding homeobox-1 (ZEB1) was silenced using a lentivirus-mediated method, and the impact of ZEB1 and methyl-CpG binding domain protein 1 (MBD1) on aerobic glycolysis was measured using seahorse cellular flux analyzers, reactive oxygen species quantification, and mitochondrial membrane potential measurement. The interaction between ZEB1 and MBD1 was assessed by co-immunoprecipitation and immunofluorescence assays. The impact of ZEB1 and MBD1 interaction on sirtuin 3 (SIRT3) expression was confirmed by quantitative polymerase chain reaction, western blotting, and dual-luciferase and chromatin-immunoprecipitation assays.
RESULTS
ZEB1 was a positive regulator of aerobic glycolysis in pancreatic cancer. ZEB1 transcriptionally silenced expression of SIRT3, a mitochondrial-localized tumor suppressor, through interaction with MBD1.
CONCLUSION
ZEB1 silenced SIRT3 expression via interaction with MBD1 to promote aerobic glycolysis in pancreatic cancer.
Keywords: Pancreatic cancer, Zinc finger E-box binding homeobox-1, Sirtuin 3, Methyl-CpG binding domain protein 1, Glycolysis
Core tip: Recent studies have demonstrated the impact of aerobic glycolysis on oncogenesis, proliferation, progression, and metastasis of cancer cells. Zinc finger E-box binding homeobox-1 (ZEB1) is an important regulator of metastasis and progression of pancreatic cancer, but its role in aerobic glycolysis has seldom been discussed. Our results demonstrated that ZEB1 is an important regulator of aerobic glycolysis. It has been demonstrated that ZEB1 regulates aerobic glycolysis by suppression of sirtuin 3 via interaction with methyl-CpG binding domain protein 1. Our results shed light on novel aspects and targets for treatment of pancreatic cancer.
INTRODUCTION
Pancreatic cancer is an aggressive and lethal cancer, and its incidence is equal to its mortality rate. Although significant progress has been made in the diagnosis and treatment of pancreatic cancer, the overall 5-year survival rate of the disease remains unchanged at about 6%[1]. One critical reason for the high fatality rate of pancreatic cancer is that pancreatic cancer cells have already metastasized to local and distant organs at the time of diagnosis. Furthermore, resistance to chemotherapy and radiotherapy have made traditional treatment strategies futile[2]. Thus, there is an urgent need for a better understanding of the biological aspects of pancreatic cancer.
Epithelial-mesenchymal transition (EMT) is a morphological cellular program that is defined as the phenotypic transition from an epithelial to mesenchymal state. The epithelial state is considered to be stable and capable of colonization, while the mesenchymal phenotype is regarded as metastable. EMT has long been regarded as an important contributor to cancer metastasis[3]. EMT is regulated by a complex of regulatory networks that involve epigenetic modification and transcription control[4]. Zinc finger E-box-binding homeobox 1 (ZEB1) is an important regulator of EMT and plays vital roles in regulating metastasis to exert a negative impact on malignancy[5]. ZEB1 also participates in the regulation of stroma-related properties of pancreatic cancer[6]. Furthermore, ZEB1 has been reported to regulate genotoxic response and drug resistance in pancreatic cancer and is an important target for improving chemotherapy resistance[7-9]. ZEB1 is an important factor in maintaining the malignant properties of pancreatic cancer cells.
The functions of aberrant cancer cell metabolism in oncogenesis and cancer progression has received increasing attention in recent years[10]. Solid tumor cells reside in a microenvironment that is characterized by dense stroma and a limited vascular system, leading to severe hypoxic conditions. To survive under such a hostile environment, cancer cells must shift their metabolic pattern[11]. By shifting their metabolic program, cancer cells use limited oxygen and nutrient supply to meet the demands for uncontrolled proliferation and metastasis. The best-characterized metabolism reprogramming is for glucose metabolism. Cancer cells utilize glucose by glycolysis instead of mitochondrial oxidative phosphorylation. From the adenosine triphosphate (commonly known as ATP) generation aspect, this seems less efficient, but through glycolysis, cancer cells break down glucose into small molecules for the synthesis of other macromolecules[12]. Furthermore, lactate produced by glycolysis creates an acidic microenvironment, leading to the destruction of extracellular matrix to provide a metastatic advantage[13]. Glycolysis is catalyzed by a series of enzymatic reactions, and some glycolytic enzymes like lactate dehydrogenase and pyruvate kinase play vital roles in metastasis and EMT[14,15]. However, the impact of EMT regulators on glycolysis has seldom been reported.
In the present study, we demonstrate that the EMT regulator ZEB1 plays positive roles in maintaining glycolysis. ZEB1 mediates glycolysis by suppression of sirtuin 3 (SIRT3), a tumor suppressor that localizes to the mitochondria and negatively regulates glycolysis. Our present study sheds light on the novel functions of ZEB1 in regulating glycolysis and metastasis and provides insights into the crosstalk between EMT and glycolysis. Our data further suggest that it may be possible to reverse pancreatic cancer metastasis by cutting its fuel supply.
MATERIALS AND METHODS
Cell culture
The human pancreatic cancer cell lines PANC-1 and MIA PaCa-2 were obtained from the American Type Culture Collection. PANC-1 cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM) containing fetal bovine serum (FBS) at a final concentration of 10%. MIA PaCa-2 cells were cultured in DMEM, with 10% FBS and 2.5% horse serum.
Generation of stable knockdown cell lines
To silence ZEB1 expression in pancreatic cancer cells, the pLKO.1 TRC cloning vector (Addgene plasmid 10878) was used[16]. Targets (21 bp) against ZEB1 were CCTCTCTGAAAGAACACATTA and GCTGTTGTTCTGCCAACAGTT. Lentiviral particles were generated by collectively transfecting pLKO.1-ZEB1 constructs with the lentiviral packaging vectors psPAX2 and pMD2.G in a ratio of 4:3:1. Stable cell lines were generated by infecting target cells with lentivirus followed by puromycin selection. The silencing knockdown efficiency was confirmed by quantitative real-time polymerase chain reaction (PCR) and western blotting.
RNA isolation and quantitative real-time PCR
RNA was extracted using TRIzol reagent (Invitrogen, Carlsbad, CA, United States), and cDNA was obtained by reverse transcription with PrimeScript RT Reagent Kit (TaKaRa, Dalian, China). The expression status of designated genes and the control β-actin gene was determined by quantitative real-time PCR. All reactions were run in triplicate. Primer sequences are listed in Table 1.
Table 1.
Primer sequences used in the text
| ZEB1 forward | 5’-CTGCAGTCCAAGAACCACCCTTG-3’ |
| ZEB1 reverse | 5’-CCACACTCATGAGGTCTTTTACC-3’ |
| SIRT3 forward | 5‘-AGCCCTCTTCATGTTCCGAAGTGT-3’ |
| SIRT3 reverse | 5‘-TCATGTCAACACCTGCAGTCCCTT-3’ |
| SIRT4 forward | 5’-ATGTGGATGCTTTGCACACCAAGG-3’ |
| SIRT4 reverse | 5’-TTCAGGACTTGGAAACGCTCTTGC-3’ |
| SIRT5 forward | 5’-AGAGAGCTCGCCCACTGTGATTTA-3’ |
| SIRT5 reverse | 5’-AGGGTCCCTGGAAATGAAACCTGA-3’ |
| β-actin forward | 5’-CTACGTCGCCCTGGACTTCGAGC-3’ |
| β-actin reverse | 5’-GATGGAGCCGCCGATCCACACGG-3’ |
Protein extraction and western blotting
Pancreatic cancer cells were washed twice with ice-cold phosphate buffer solution (PBS) and lysed in RIPA buffer for 10 min, followed by sonication to ensure complete lysis. Cell debris was removed by centrifugation at 10000 g for 20 min at 4 °C. Whole cell lysates (20 μg) were denatured in sodium dodecyl sulfate (SDS) loading buffer and subjected to denaturing 10% SDS-PAGE. The samples were transferred to a membrane for subsequent blotting with specific antibodies. ZEB1, SIRT3 and β-actin antibodies were purchased from Proteintech (Rosemont, IL, United States).
Extracellular acidification rate and oxygen consumption rate
To assess the impact of ZEB1 on glycolytic capacity and mitochondrial respiration of pancreatic cancer cells, a Seahorse Bioscience XF96 Extracellular Flux Analyzer was used according to the manufacturer’s instructions for the Seahorse XF Glycolysis Stress Test Kit and Cell Mito Stress Test Kit, as described previously[17].
ROS generation analysis
To assess the impact of ZEB1 and SIRT3 on reactive oxygen species (ROS) generation in pancreatic cancer cells, the Beyotime Reactive Oxygen Species Assay Kit (Nantong, China) was used. ROS within a living cell were labeled using the cell-permeable fluorogenic probe 2’,7’-dichlorodihydrofluorescein diacetate (DCF-DA). Once DCF-DA had diffused into cells, it was deacetylated by cellular esterases to a nonfluorescent compound and rapidly oxidized by ROS into DCF. DCF was highly fluorescent and could be detected by cytometry. The fluorescence intensity was proportional to the ROS levels within the cell.
Mitochondrial membrane potential measurement
Cell membrane potential was measured using the Beyotime Mitochondrial Membrane Potential Assay Kit with JC-1 dye. JC-1 showed potential-dependent accumulation in the mitochondria, indicated by a fluorescence emission shift from green (approximately 529 nm) to red (approximately 590 nm). Consequently, mitochondrial depolarization was indicated by a decrease in the red/green fluorescence intensity ratio, which could be measured by cytometry.
Dual-luciferase assay
To assess the impact of ZEB1 on SIRT3 promoter activity, the Promega Dual-Luciferase Assay Kit (Madison, WI, United States) was used. The SIRT3 promoter was amplified from genomic DNA of PANC-1 cells and ligated into the pGL3-Basic vector to generate the pGL3-SIRT3 construct. pGL3-SIRT3 was transfected with a Renilla luciferase vector into pancreatic cancer cells. The impact of ZEB1 on SIRT3 promoter activity was assessed with the Dual-Luciferase Assay Kit.
ChIP assay
To test whether ZEB1 occupied the SIRT3 promoter region, chromatin immunoprecipitation (ChIP) assays were performed. Cells were fixed with formaldehyde and harvested. The ChIP assay was carried out using Millipore’s EZ ChIP kit (Burlington, MA, United States) with a ZEB1 antibody (Cell Signaling Technologies, 3396). The primer sequences to detect ZEB1-bound SIRT3 promoter were forward: 5’-AGTAGCAGGGATTACAGGCATGAG-3’ and reverse: 5’-TGCCTTCCCTGAGATACTCAGCT-3’.
Immunohistochemistry
Expression of ZEB1 and SIRT3 in pancreatic cancer patient samples were assessed by immunohistochemistry (IHC) staining. Paraffin sections were incubated for 1 h at 70 °C, deparaffinized in xylene, and rehydrated in graded ethanol. The slides were neutralized with 3% H2O2 for 30 min. The antigen retrieval was processed with citrate buffer (pH = 6.0) in an incubator at 95 °C. After antigen retrieval, the slides were incubated with primary and secondary antibodies. The ZEB1 antibody (ab181451; Abcam, Cambridge, MA, United States) was used at a dilution of 1:100, and the SIRT3 antibody (ab217319; Abcam) was used at a dilution of 1:50. The sections were stained with 3,3-diaminobenzidine, terminated in PBS, and counterstained with hematoxylin.
The Cancer Genome Atlas dataset analysis
The Cancer Genome Atlas - pancreatic adenocarcinoma (TCGA-PAAD) on RNA expression (Level 3) of pancreatic cancer patients in terms of RNA-seq by Expectation-Maximization was downloaded from the Cancer Genomics Browser of the University of California, Santa Cruz (https://genome-cancer.ucsc.edu/). In total, 160 primary pancreatic cancer samples from patients with detailed expression data were chosen from the updated TCGA database according to the parameters mentioned. Detailed demographics of these patients were characterized by the TCGA consortium.
Statistical analysis
Statistical analysis was performed in SPSS version 17.0 (IBM Corp., Armonk, NY, United States) using an independent Student’s t test (two-tailed) or one-way analysis of variance. Logistic regression was used to determine the correlation between ZEB1 and SIRT3 expression level in the TCGA cohorts. Statistical significance was based on two-sided P values < 0.05.
RESULTS
ZEB1 mediates aerobic glycolysis in pancreatic cancer
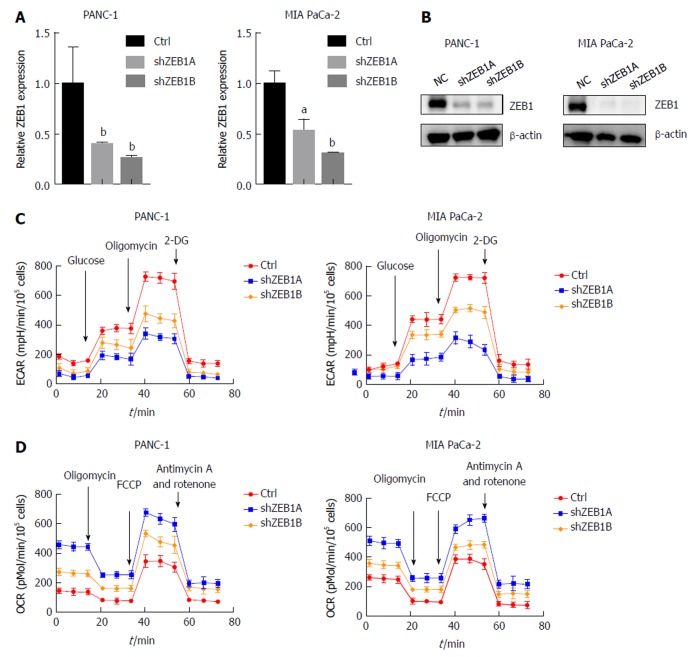
ZEB1 is an important regulator of EMT and stem cell properties, but its roles in aerobic glycolysis and mitochondria-related properties have seldom been discussed in pancreatic cancer. To explore these, we first silenced ZEB1 expression in the PANC-1 and MIA PaCa-2 pancreatic cancer cell lines. The silencing effect was validated by quantitative real-time PCR and western blotting (Figure 1A and B). Aerobic glycolysis was assessed by extracellular acidification rate (ECAR) measurement. In ZEB1-silenced PANC-1 and MIA PaCa-2 cells, we observed a decrease in ECAR rates, suggesting that ZEB1 is a negative regulator of glycolysis (Figure 1C). In the process of metabolism reprogramming from mitochondria to glycolysis, mitochondrial respiration was impaired and could be tested by oxygen consumption rate (OCR) measurement. In ZEB1 knockdown PANC-1 and MIA PaCa-2 cells, the OCR levels increased, which demonstrated that ZEB1 is a negative regulator of mitochondrial respiration (Figure 1D). Collectively, these results suggest that ZEB1 is a positive regulator of aerobic glycolysis in pancreatic cancer.
Figure 1.
Zinc finger E-box binding homeobox-1 mediates aerobic glycolysis in pancreatic cancer. A: Zinc finger E-box binding homeobox-1 (ZEB1) knockdown efficiency was assessed by quantitative real-time polymerase chain reaction in PANC-1 and MIA PaCa-2 cells; B: Immunoblot with ZEB1 antibody confirmed that ZEB1 was effectively downregulated in PANC-1 and MIA PaCa-2 cells; C: ZEB1 knockdown decreased glycolysis, as reflected by extracellular acidification rate measurements; D: ZEB1 knockdown increased mitochondrial respiration, measured by oxygen consumption rate; E and F: Decreased ZEB1 expression in PANC-1 and MIA PaCa-2 cells decreased reactive oxygen species generation; G and H: Downregulation of ZEB1 increased mitochondrial membrane potential in PANC-1 and MIA PaCa-2 cells. aP < 0.05, and bP < 0.01 vs NC or mock group. Error bars indicate mean ± SD. ZEB1: Zinc finger E-box binding homeobox-1; ECAR: Extracellular acidification rate; OCR: Oxygen consumption rate.
ZEB1 maintains ROS generation and mitochondrial membrane potentials
ROS production is a net result of glycolysis, which in turn exerts a positive impact on aerobic glycolysis. When ZEB1 expression was silenced, ROS production decreased, suggesting that ZEB1 functions as a positive regulator of ROS production in pancreatic cancer cells (Figure 2A and B). Cancer cells utilize glucose through aerobic glycolysis, and in this process, mitochondrial membrane potentials decrease. Upon ZEB1 silencing, the mitochondrial membrane potential increased (Figure 2C and D). Thus, ZEB1 can maintain ROS generation and regulate mitochondrial membrane potentials in pancreatic cancer cells.
Figure 2.
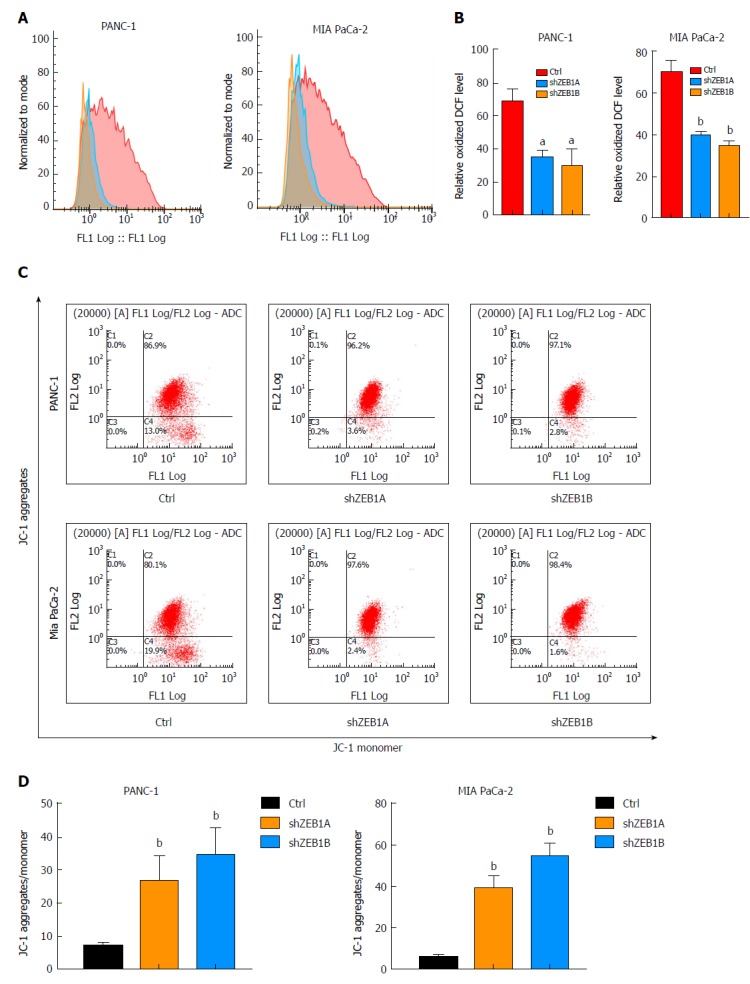
Zinc finger E-box binding homeobox-1 regulates reactive oxygen species generation and mitochondrial membrane potentials. A and B: Decreased zinc finger E-box binding homeobox-1 (ZEB1) expression in PANC-1 and MIA PaCa-2 cells decreased reactive oxygen species generation, as demonstrated by using ROS assay kit analysis; C and D: Downregulation of ZEB1 increased mitochondrial membrane potential in PANC-1 and MIA PaCa-2 cells, which were confirmed by membrane potential probe JC-1 measurements. aP < 0.05, and bP < 0.01 vs NC or mock group. Error bars indicate mean ± SD. ROS: Reactive oxygen species; ZEB1: Zinc finger E-box binding homeobox-1.
ZEB1 negatively correlates with SIRT3 expression in pancreatic cancer
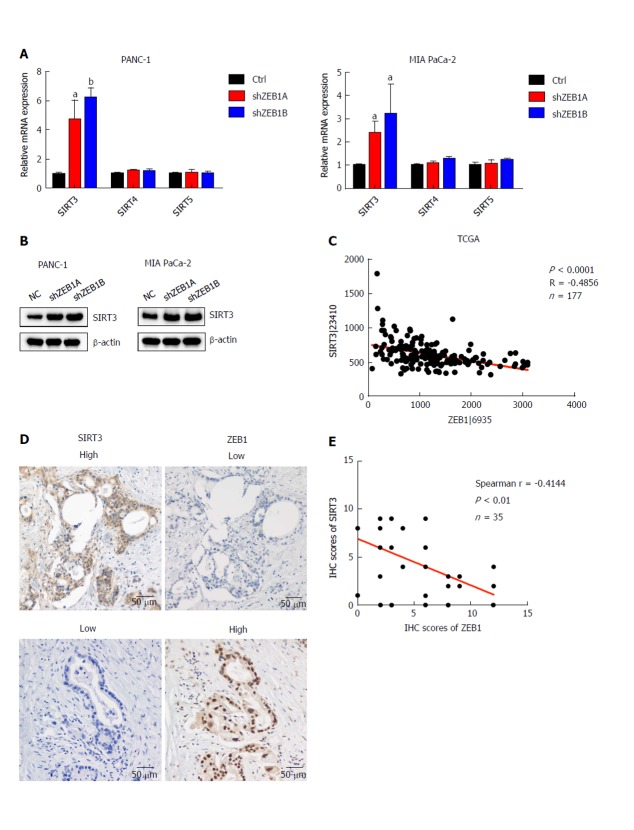
Sirtuin family members, including SIRT1-7, are deacetylases and regulate metabolism, aging, energy and redox homeostasis in cells. Among them, SIRT3, SIRT4 and SIRT5 localize to mitochondria and negatively regulate glycolysis. Thus, we examined the impact of ZEB1 on SIRT3, SIRT4 and SIRT5 expression in PANC-1 and MIA PaCa-2 cells. SIRT3 mRNA and protein levels significantly increased in ZEB1-silenced PANC-1 and MIA PaCa-2 cells (Figure 3A and B). To further explore the correlation between ZEB1 and SIRT3, we analyzed ZEB1 and SIRT3 expression in TCGA-included pancreatic cancer patients. ZEB1 negatively and significantly correlated with SIRT3 expression in pancreatic cancer patients (Figure 3C). We performed IHC staining to examine the expression correlation between ZEB1 and SIRT3. Pancreatic cancer patients that displayed higher ZEB1 expression exhibited lower SIRT3 expression (Figure 3D). Furthermore, the negative correlation was statistically significant (Figure 3E).
Figure 3.
Zinc finger E-box binding homeobox-1 expression is negatively correlated with sirtuin 3 expression in pancreatic cancer. A: Zinc finger E-box binding homeobox-1 (ZEB1) knockdown increased sirtuin 3 (SIRT3) mRNA levels in PANC-1 and MIA PaCa-2 cells, but had only a small impact on SIRT4 and SIRT5 expression; B: SIRT3 protein levels increased in ZEB1-silenced PANC-1 and MIA PaCa-2 cells; C: SIRT3 expression negatively and significantly correlated with ZEB1 expression in The Cancer Genome Atlas cohort of pancreatic cancer patients (P < 0.0001, R = -0.4856); D: SIRT3 expression was lower in patients with higher ZEB1 expression, as suggested by immunohistochemistry (IHC) staining; E: SIRT3 expression was negatively and significantly correlated with ZEB1 expression, as demonstrated by IHC staining in pancreatic cancer patients (P = 0.0078, r = -0.4144). aP < 0.05, and bP < 0.01 vs NC or mock group. Error bars indicate mean ± SD. ZEB1: Zinc finger E-box binding homeobox-1; SIRT3: Sirtuin 3; IHC: Immunohistochemistry.
SIRT3 is a transcriptional target of ZEB1
To confirm the negative correlation between ZEB1 and SIRT3, we asked whether SIRT3 was a downstream target of ZEB1 in pancreatic cancer cells. ZEB1 specifically binds to E-box (CANNTG) or Z-box (CAGGTA) sequences in the promoter region of its downstream transcriptional targets. First, we analyzed the promoter region of SIRT3 that covered from -2500 to +200 and identified a potential Z box in the promoter region (Figure 4A). We performed a dual-luciferase assay to examine the impact of ZEB1 on SIRT3 promoter luciferase activity. ZEB1 inhibited SIRT3 promoter activity in a dose-dependent manner (Figure 4B). To confirm the impact of ZEB1 on SIRT3 promoter activity, we mutated the Z-box in the SIRT3 promoter region from CAGGTA to GTGGTA (pGL3-SIRT3Mut) (Figure 4C). Subsequent promoter activity indicated that ZEB1 had an impact on pGL3-SIRTMut activity. Collectively, these results suggested that SIRT3 is a downstream transcription target of ZEB1.
Figure 4.
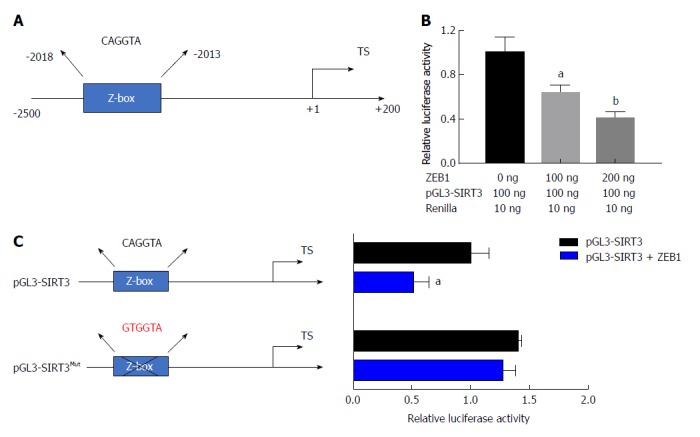
Sirtuin 3 is a transcriptional target of zinc finger E-box binding homeobox-1. A: There is a specific zinc finger E-box binding homeobox-1 (ZEB1) binding element (Z-box: CAGGTA) in the promoter region of sirtuin 3 (SIRT3); B: ZEB1 suppressed SIRT3 promoter luciferase activity in a dose-dependent manner; C: The Z-box in the SIRT3 promoter was mutated from CAGGTA to GTGGTA to confirm that ZEB1 specifically regulated SIRT3 promoter activity; D: ZEB1 had a slight impact on mutated SIRT3 promoter luciferase activity, suggesting that ZEB1 regulated SIRT3 expression by Z-box specific binding. aP < 0.05, and bP < 0.01 vs NC or mock group. ZEB1: Zinc finger E-box binding homeobox-1; SIRT3: Sirtuin 3.
Epigenetic factor MBD1 can interact with ZEB1 in pancreatic cancer cells
Our previous studies demonstrated that epigenetic factor methyl-CpG binding domain protein 1 (MBD1) interacts with TWIST in pancreatic cancer cells to induce EMT. ZEB1 and TWIST share common downstream targets in promoting metastasis. Thus, we asked whether ZEB1 could interact with MBD1 in pancreatic cancer patients. First, we performed a co-immunoprecipitation (Co-IP) assay to check whether these two nuclear proteins interacted with each other. As demonstrated by Co-IP, we found that ZEB1 interacted with MBD1 (Figure 5A and B). We then performed an immunofluorescence assay using ZEB1 and MBD1 antibodies to examine their localization. ZEB1 colocalized with MBD1 in the nucleus in PANC-1 and MIA PaCa-2 cells (Figure 5C).
Figure 5.
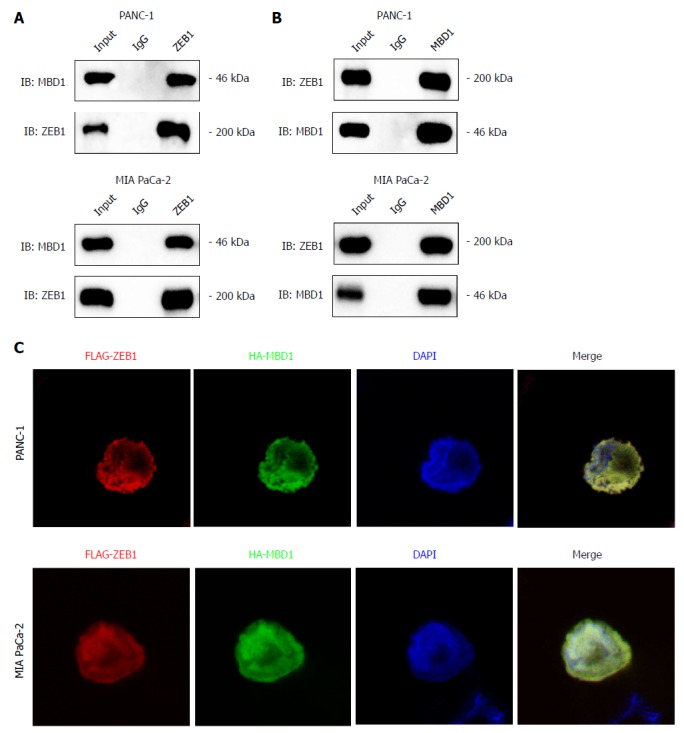
Epigenetic factor methyl-CpG binding domain protein 1 interacts with zinc finger E-box binding homeobox-1 in pancreatic cancer cells. A and B: Co-immunoprecipitation assay with methyl-CpG binding domain protein 1 (MBD1) or zinc finger E-box binding homeobox-1 (ZEB1) antibody demonstrated that MBD1 interacted with ZEB1 in PANC-1 and MIA PaCa-2 cells; C: Immunofluoresence assay indicated that MBD1 colocalized with ZEB1 in the nucleus in PANC-1 and MIA PaCa-2 cells. MBD1: Methyl-CpG binding domain protein 1; ZEB1: Zinc finger E-box binding homeobox-1.
MBD1 positively regulates aerobic glycolysis and ROS production in pancreatic cancer cells
We then asked whether MBD1 could regulate aerobic glycolysis like its interaction partner ZEB1. First, we generated stable MBD1-silenced PANC-1 and MIA PaCa-2 cell lines. Knockdown efficiency was validated by quantitative PCR and western blotting (Figure 6A and B). Next, using a Seahorse energy flux analyzer, we tested the impact of MBD1 on glycolysis. MBD1 knockdown inhibited ECAR values in PANC-1 and MIA PaCa-2 cells, indicating that MBD1 functioned as a positive regulator of glycolysis (Figure 6C). Next, we observed an increase in OCR values in MBD1 knockdown cell lines, suggesting that MBD1 acts as a negative mitochondrial respiration regulator (Figure 6D). In the end, we examined the influence of MBD1 on ROS generation in PANC-1 and MIA PaCa-2 cells. MBD1 knockdown decreased ROS production in pancreatic cancer cells (Figure 6E). These results confirm that MBD1 positively regulate aerobic glycolysis and ROS generation in pancreatic cancer cells.
Figure 6.
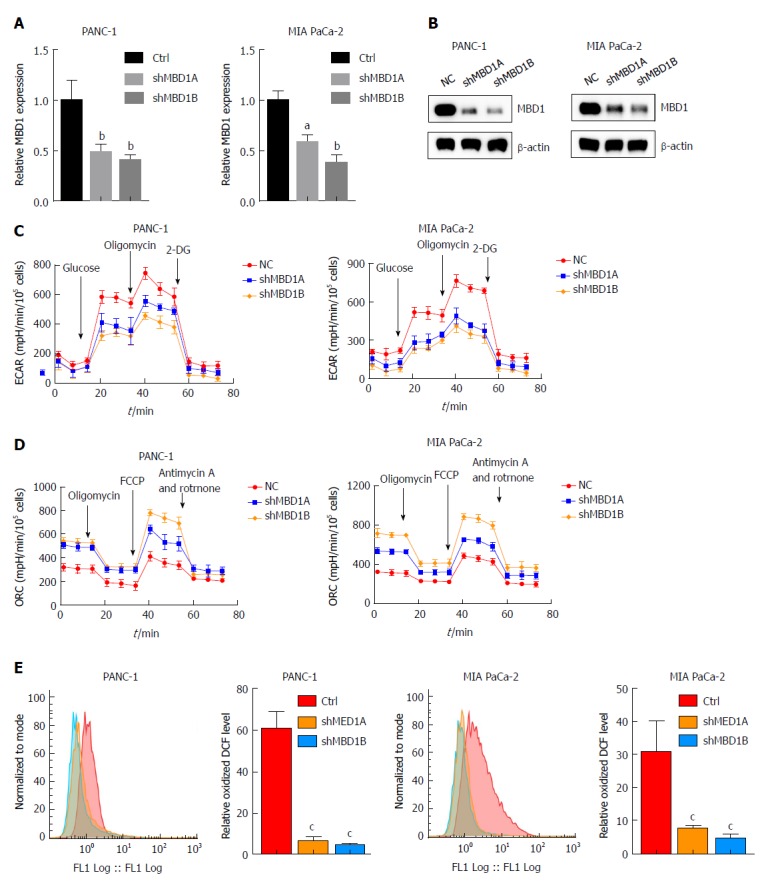
Methyl-CpG binding domain protein 1 positively regulates aerobic glycolysis and reactive oxygen species production in pancreatic cancer cells. A and B: Real-time polymerase chain reaction and western blotting demonstrated that methyl-CpG binding domain protein 1 (MBD1) was silenced in PANC-1 and MIA PaCa-2 cells; C: Knockdown of MBD1 decreased extracellular acidification rate values, indicating that MBD1 was a positive regulator of glycolysis; D: Silencing MBD1 in PANC-1 and MIA PaCa-2 cells increased oxygen consumption rate values, suggesting that MBD1 negatively regulated mitochondrial respiration; E: Downregulation of MBD1 decreased reactive oxygen species generation in PANC-1 and MIA PaCa-2 cells. aP < 0.05, bP < 0.01, and cP < 0.001 vs NC or mock group. MBD1: Methyl-CpG binding domain protein 1; ECAR: Extracellular acidification rate; OCR: Oxygen consumption rate.
ZEB1 interacts with MBD1 to suppress SIRT3 expression in pancreatic cancer cells
As demonstrated above, ZEB1 negatively regulated SIRT3 expression in pancreatic cancer cells. Thus, we asked whether MBD1 functioned as a cofactor in ZEB1-mediated SIRT3 repression. First, we performed a dual luciferase assay, which showed that MBD1 repressed SIRT3 promoter activity, and co-transfection of ZEB1 with MBD1 more dramatically repressed SIRT3 promoter activity (Figure 7A). Then, we performed a ChIP assay to validate that MBD1 and ZEB1 occupied the Z-box region in SIRT3 promoter. ZEB1 and MBD1 were enriched in the SIRT3 promoter region (Figure 7B and C). Subsequently, we performed a reChIP assay to examine whether ZEB1 and MBD1 jointly occupied the Z-box in SIRT3 promoter. As shown by ChIP and reChIP assay, ZEB1 and MBD1 simultaneously occupied the same region in the SIRT3 promoter (Figure 7D). In conclusion, the present study demonstrates that ZEB1 and MBD1 interact to suppress SIRT3 expression to induce aerobic glycolysis in pancreatic cancer (Figure 7E).
Figure 7.
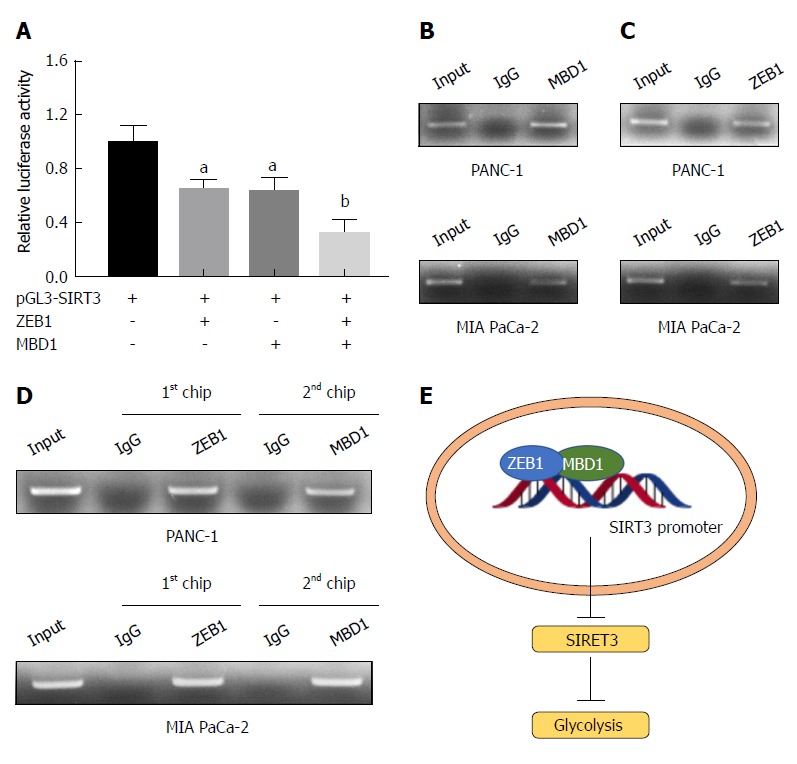
Zinc finger E-box binding homeobox-1 interacts with methyl-CpG binding domain protein 1 to suppress sirtuin 3 expression in pancreatic cancer cells. A: Methyl-CpG binding domain protein 1 (MBD1) and zinc finger E-box binding homeobox-1 (ZEB1) repressed sirtuin 3 (SIRT3) promoter luciferase activity, and when cotransfected into HEK-293T cells, SIRT3 promoter activity more dramatically decreased than transfecting MBD1 or ZEB1 alone; B and C: Chromatin immunoprecipitation (ChIP) assay demonstrated that MBD1 and ZEB1 bound the Z-box region of the SIRT3 promoter; D: ChIP and re-ChIP assays demonstrated that MBD1 and ZEB1 jointly occupied the same Z-box region in the SIRT3 promoter; E: Schematic representation of the working model. aP < 0.05, and bP < 0.01 vs NC or mock group. Error bars indicate mean ± SD. ChIP: Chromatin immunoprecipitation; MBD1: Methyl-CpG binding domain protein 1; SIRT3: Sirtuin 3; ZEB1: Zinc finger E-box binding homeobox-1.
DISCUSSION
In the present study, we demonstrated that the EMT regulator ZEB1 could positively regulate aerobic glycolysis in pancreatic cancer. Mechanistic studies demonstrated that ZEB1 interacted with the epigenetic factor MBD1 to suppress SIRT3 expression. The present study sheds light on a novel link between EMT and glycolysis in cancer.
Accumulating evidence suggests that EMT plays important roles in tumor progression[18]. In pancreatic cancer, EMT also plays pivotal roles in tumorigenesis[19]. ZEB1 plays an important role in inducing and maintaining EMT and is regarded as a gatekeeper of the EMT process and maintenance of cancer malignancy[20]. ZEB1 expression is increased in poorly differentiated pancreatic cancer samples and in invasive cells derived from differentiated pancreatic cancer tumors and can be used as a prognostic marker for overall survival[21]. ZEB1 is also reported to regulate drug resistance, and pancreatic cancer cells that develop resistance to gemcitabine have increased ZEB1 expression. However, the impact of ZEB1 on pancreatic cancer cell metabolism has seldom been reported. Recent years have witnessed the impact of cancer cell metabolism on maintaining the malignant properties of cancer cells, and this is regarded as one of the hallmarks of cancer. However, the impact of ZEB1 on cancer cell metabolism has seldom been discussed. In breast cancer, the EMT regulator SNAIL has been reported to play a positive role in inducing aerobic glycolysis by suppressing fructose-bisphosphatase 1 (FBP1). FBP1 is a negative regulator of glycolysis and catalyzes gluconeogenesis. In breast cancer, clear renal cell carcinoma and gastric cancer, FBP1 acts a tumor suppressor. Thus, SNAIL-mediated repression of FBP1 renders a metabolic advantage to invasive cancer cells[22]. In line with breast cancer studies, our present study suggests a novel function of ZEB1 in regulating glycolysis. Mechanistic studies have demonstrated that ZEB1 represses SIRT3 expression. SIRT3 is a mitochondrial tumor suppressor gene, which negatively regulates glycolysis[23]. For example, SIRT3 opposes metabolic reprogramming by destabilizing hypoxia-inducible factor 1α (HIF1α)[24]. Beyond its role in regulating glucose metabolism, SIRT3 has also been reported to regulate EMT and metastasis in cancer cells. For example, as a deacetylase, SIRT3 can deacetylate S-phase kinase-associated protein 2 (SKP2). Deacetylated SKP2 exhibits decreased ligase activity and stabilizes its substrate E-cadherin, thus reversing EMT[25]. Thus, SIRT3 functions as a negative regulator of EMT. Based on these observations, we put forward the hypothesis that ZEB1-mediated repression of SIRT3 renders a metabolic advantage to highly metastatic pancreatic cancer cells.
SIRT3 is a tumor suppressor and negatively regulates glycolysis and EMT. However, the regulatory mechanism for SIRT3 dysregulation in cancer has seldom been discussed. Peroxisome proliferator-activated receptor γ co-activator-1α, a transcription factor that regulates mitochondrial biogenesis, also regulates SIRT3 expression via estrogen-related receptor-α (ERR-α), and there exists a putative ERR-α biding element in the promoter region of SIRT3[26]. Another observation is that SIRT3 is specifically upregulated in response to increased ROS production[27]. This upregulation could be controlled by an oxygen-sensing mechanism involving transcription factors like HIF1α or antioxidant response factor nuclear factor E2-related factor 2 (Nrf2)[28,29]. In the process, variations in ROS levels are also observed, thus, it is natural to conceive that the EMT regulator could exert some effect on SIRT3 expression. Consistent with this hypothesis, we validated that the EMT regulator ZEB1 is a transcription factor for SIRT3 in pancreatic cancer, and there is a specific ZEB1-binding element CAGGTA in the SIRT3 promoter region. In Alzheimer’s disease, DNA methylation in the SIRT3 promoter region has been observed, suggesting that SIRT3 expression is under epigenetic control[30]. MBD1, an epigenetic factor that can specifically bind to methylated CpG islands, has been reported to be upregulated in pancreatic cancer and to regulate EMT and chemotherapy and radiotherapy resistance[31,32]. In the present study, we demonstrated that ZEB1 interacts with MBD1, and these two proteins jointly occupy the SIRT3 promoter region, indicating that the ZEB1/MBD1 complex regulates SIRT3 expression. However, further investigation is required. For example, it is unclear whether SIRT3 expression changes during the process of EMT or whether interrupting SIRT3 expression or SIRT3 activity could improve glycolysis during EMT. Another indication that needs to be confirmed is whether DNA methylation regulates SIRT3 expression in pancreatic cancer and whether treating pancreatic cancer cells with DNA methyltransferase inhibitors reverses SIRT3 expression.
In conclusion, we demonstrated that ZEB1 interacts with MBD1 to suppress SIRT3 expression in pancreatic cancer, which might provide metabolic advantages to highly metastatic pancreatic cancer cells. We shed light on the possibility of inhibiting pancreatic cancer metastasis by cutting the fuel supply and inhibiting glycolysis. Collectively, the present study uncovered a novel link between EMT and glycolysis and provided potential therapeutic targets for pancreatic cancer.
ARTICLE HIGHLIGHTS
Research background
Pancreatic cancer is a highly lethal disease that is characterized by metastasis. Uncovering novel functions of metastasis regulators might provide novel predictive and treatment targets.
Research motivation
Aerobic glycolysis has been implicated in multiple processes of cancer progression. However, the impact of the cancer metastasis gene zinc finger E-box-binding homeobox 1 (ZEB1) on aerobic glycolysis has seldom been discussed, and the molecular link is lacking.
Research objectives
To uncover the roles of ZEB1 in aerobic glycolysis regulation and establish the molecular mechanism.
Research methods
ZEB1 was silenced in pancreatic cancer cells to examine its impact on aerobic glycolysis. The impact of ZEB1 on mitochondrion-localized tumor suppressors sirtuin 3 (SIRT3), 4 and 5 was assessed by real-time polymerase chain reaction and western blotting. The transcriptional regulation of ZEB1 on SIRT3 expression was assayed by dual-luciferase and chromatin immunoprecipitation assays. The underlying molecular mechanism that governs the effect of ZEB1 on SIRT3 expression was confirmed by protein interaction, promoter luciferase and chromatin immunoprecipitation assays.
Research results
ZEB1 positively regulated aerobic glycolysis. Mechanistically, ZEB1 regulated aerobic glycolysis by suppression of SIRT3 via interaction with methyl-CpG binding domain protein 1.
Research conclusions
The metastasis gene ZEB1 is an important regulator of aerobic glycolysis.
Research perspectives
The impact of ZEB1 and SIRT3 on the prediction of prognosis and the prospect for targeting metastasis by regulation of glycolysis should be investigated in the future.
Footnotes
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
Conflict-of-interest statement: The authors have declared no conflicts of interest.
Peer-review started: July 9, 2018
First decision: August 31, 2018
Article in press: October 15, 2018
P- Reviewer: Chowdhury P S- Editor: Ma RY L- Editor: Filipodia E- Editor: Bian YN
Contributor Information
Wen-Yan Xu, Department of Pancreatic Surgery, Fudan University Shanghai Cancer Center, Shanghai 200032, China.
Qiang-Sheng Hu, Department of Pancreatic Surgery, Fudan University Shanghai Cancer Center, Shanghai 200032, China.
Yi Qin, Department of Oncology, Shanghai Medical College, Fudan University, Shanghai 200032, China; Pancreatic Cancer Institute, Fudan University, Shanghai Pancreatic Cancer Institute, Shanghai 200032, China.
Bo Zhang, Department of Pancreatic Surgery, Fudan University Shanghai Cancer Center, Shanghai 200032, China; Pancreatic Cancer Institute, Fudan University, Shanghai Pancreatic Cancer Institute, Shanghai 200032, China.
Wen-Sheng Liu, Department of Pancreatic Surgery, Fudan University Shanghai Cancer Center, Shanghai 200032, China.
Quan-Xing Ni, Department of Pancreatic Surgery, Fudan University Shanghai Cancer Center, Shanghai 200032, China.
Jin Xu, Department of Oncology, Shanghai Medical College, Fudan University, Shanghai 200032, China; Pancreatic Cancer Institute, Fudan University, Shanghai Pancreatic Cancer Institute, Shanghai 200032, China.
Xian-Jun Yu, Department of Pancreatic Surgery, Fudan University Shanghai Cancer Center, Shanghai 200032, China. yuxianjun@fudanpci.org.
References
- 1.Muñoz AR, Chakravarthy D, Gong J, Halff GA, Ghosh R, Kumar AP. Pancreatic cancer: Current status and Challenges. Curr Pharmacol Rep. 2017;3:396–408. doi: 10.1007/s40495-017-0112-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Narayanan V, Weekes CD. Molecular therapeutics in pancreas cancer. World J Gastrointest Oncol. 2016;8:366–379. doi: 10.4251/wjgo.v8.i4.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mittal V. Epithelial Mesenchymal Transition in Tumor Metastasis. Annu Rev Pathol. 2018;13:395–412. doi: 10.1146/annurev-pathol-020117-043854. [DOI] [PubMed] [Google Scholar]
- 4.Jiang JH, Liu C, Cheng H, Lu Y, Qin Y, Xu YF, Xu J, Long J, Liu L, Ni QX, et al. Epithelial-mesenchymal transition in pancreatic cancer: Is it a clinically significant factor? Biochim Biophys Acta. 2015;1855:43–49. doi: 10.1016/j.bbcan.2014.11.004. [DOI] [PubMed] [Google Scholar]
- 5.Krebs AM, Mitschke J, Lasierra Losada M, Schmalhofer O, Boerries M, Busch H, Boettcher M, Mougiakakos D, Reichardt W, Bronsert P, et al. The EMT-activator Zeb1 is a key factor for cell plasticity and promotes metastasis in pancreatic cancer. Nat Cell Biol. 2017;19:518–529. doi: 10.1038/ncb3513. [DOI] [PubMed] [Google Scholar]
- 6.Sangrador I, Molero X, Campbell F, Franch-Expósito S, Rovira-Rigau M, Samper E, Domínguez-Fraile M, Fillat C, Castells A, Vaquero EC. Zeb1 in Stromal Myofibroblasts Promotes Kras-Driven Development of Pancreatic Cancer. Cancer Res. 2018;78:2624–2637. doi: 10.1158/0008-5472.CAN-17-1882. [DOI] [PubMed] [Google Scholar]
- 7.Passacantilli I, Panzeri V, Bielli P, Farini D, Pilozzi E, Fave GD, Capurso G, Sette C. Alternative polyadenylation of ZEB1 promotes its translation during genotoxic stress in pancreatic cancer cells. Cell Death Dis. 2017;8:e3168. doi: 10.1038/cddis.2017.562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zheng X, Carstens JL, Kim J, Scheible M, Kaye J, Sugimoto H, Wu CC, LeBleu VS, Kalluri R. Epithelial-to-mesenchymal transition is dispensable for metastasis but induces chemoresistance in pancreatic cancer. Nature. 2015;527:525–530. doi: 10.1038/nature16064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Meidhof S, Brabletz S, Lehmann W, Preca BT, Mock K, Ruh M, Schüler J, Berthold M, Weber A, Burk U, et al. ZEB1-associated drug resistance in cancer cells is reversed by the class I HDAC inhibitor mocetinostat. EMBO Mol Med. 2015;7:831–847. doi: 10.15252/emmm.201404396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Koppenol WH, Bounds PL, Dang CV. Otto Warburg’s contributions to current concepts of cancer metabolism. Nat Rev Cancer. 2011;11:325–337. doi: 10.1038/nrc3038. [DOI] [PubMed] [Google Scholar]
- 11.Tennant DA, Durán RV, Gottlieb E. Targeting metabolic transformation for cancer therapy. Nat Rev Cancer. 2010;10:267–277. doi: 10.1038/nrc2817. [DOI] [PubMed] [Google Scholar]
- 12.Hamanaka RB, Chandel NS. Targeting glucose metabolism for cancer therapy. J Exp Med. 2012;209:211–215. doi: 10.1084/jem.20120162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sun S, Li H, Chen J, Qian Q. Lactic Acid: No Longer an Inert and End-Product of Glycolysis. Physiology (Bethesda) 2017;32:453–463. doi: 10.1152/physiol.00016.2017. [DOI] [PubMed] [Google Scholar]
- 14.Zhao J, Huang X, Xu Z, Dai J, He H, Zhu Y, Wang H. LDHA promotes tumor metastasis by facilitating epithelialmesenchymal transition in renal cell carcinoma. Mol Med Rep. 2017;16:8335–8344. doi: 10.3892/mmr.2017.7637. [DOI] [PubMed] [Google Scholar]
- 15.Fan FT, Shen CS, Tao L, Tian C, Liu ZG, Zhu ZJ, Liu YP, Pei CS, Wu HY, Zhang L, et al. PKM2 regulates hepatocellular carcinoma cell epithelial-mesenchymal transition and migration upon EGFR activation. Asian Pac J Cancer Prev. 2014;15:1961–1970. doi: 10.7314/apjcp.2014.15.5.1961. [DOI] [PubMed] [Google Scholar]
- 16.Moffat J, Grueneberg DA, Yang X, Kim SY, Kloepfer AM, Hinkle G, Piqani B, Eisenhaure TM, Luo B, Grenier JK, et al. A lentiviral RNAi library for human and mouse genes applied to an arrayed viral high-content screen. Cell. 2006;124:1283–1298. doi: 10.1016/j.cell.2006.01.040. [DOI] [PubMed] [Google Scholar]
- 17.Ji S, Qin Y, Liang C, Huang R, Shi S, Liu J, Jin K, Liang D, Xu W, Zhang B, et al. FBW7 (F-box and WD Repeat Domain-Containing 7) Negatively Regulates Glucose Metabolism by Targeting the c-Myc/TXNIP (Thioredoxin-Binding Protein) Axis in Pancreatic Cancer. Clin Cancer Res. 2016;22:3950–3960. doi: 10.1158/1078-0432.CCR-15-2380. [DOI] [PubMed] [Google Scholar]
- 18.Brabletz T, Kalluri R, Nieto MA, Weinberg RA. EMT in cancer. Nat Rev Cancer. 2018;18:128–134. doi: 10.1038/nrc.2017.118. [DOI] [PubMed] [Google Scholar]
- 19.Beuran M, Negoi I, Paun S, Ion AD, Bleotu C, Negoi RI, Hostiuc S. The epithelial to mesenchymal transition in pancreatic cancer: A systematic review. Pancreatology. 2015;15:217–225. doi: 10.1016/j.pan.2015.02.011. [DOI] [PubMed] [Google Scholar]
- 20.Caramel J, Ligier M, Puisieux A. Pleiotropic Roles for ZEB1 in Cancer. Cancer Res. 2018;78:30–35. doi: 10.1158/0008-5472.CAN-17-2476. [DOI] [PubMed] [Google Scholar]
- 21.Bronsert P, Kohler I, Timme S, Kiefer S, Werner M, Schilling O, Vashist Y, Makowiec F, Brabletz T, Hopt UT, et al. Prognostic significance of Zinc finger E-box binding homeobox 1 (ZEB1) expression in cancer cells and cancer-associated fibroblasts in pancreatic head cancer. Surgery. 2014;156:97–108. doi: 10.1016/j.surg.2014.02.018. [DOI] [PubMed] [Google Scholar]
- 22.Dong C, Yuan T, Wu Y, Wang Y, Fan TW, Miriyala S, Lin Y, Yao J, Shi J, Kang T, et al. Loss of FBP1 by Snail-mediated repression provides metabolic advantages in basal-like breast cancer. Cancer Cell. 2013;23:316–331. doi: 10.1016/j.ccr.2013.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Haigis MC, Deng CX, Finley LW, Kim HS, Gius D. SIRT3 is a mitochondrial tumor suppressor: a scientific tale that connects aberrant cellular ROS, the Warburg effect, and carcinogenesis. Cancer Res. 2012;72:2468–2472. doi: 10.1158/0008-5472.CAN-11-3633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Finley LW, Carracedo A, Lee J, Souza A, Egia A, Zhang J, Teruya-Feldstein J, Moreira PI, Cardoso SM, Clish CB, et al. SIRT3 opposes reprogramming of cancer cell metabolism through HIF1α destabilization. Cancer Cell. 2011;19:416–428. doi: 10.1016/j.ccr.2011.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Inuzuka H, Gao D, Finley LW, Yang W, Wan L, Fukushima H, Chin YR, Zhai B, Shaik S, Lau AW, et al. Acetylation-dependent regulation of Skp2 function. Cell. 2012;150:179–193. doi: 10.1016/j.cell.2012.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Giralt A, Hondares E, Villena JA, Ribas F, Díaz-Delfín J, Giralt M, Iglesias R, Villarroya F. Peroxisome proliferator-activated receptor-gamma coactivator-1alpha controls transcription of the Sirt3 gene, an essential component of the thermogenic brown adipocyte phenotype. J Biol Chem. 2011;286:16958–16966. doi: 10.1074/jbc.M110.202390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kong X, Wang R, Xue Y, Liu X, Zhang H, Chen Y, Fang F, Chang Y. Sirtuin 3, a new target of PGC-1alpha, plays an important role in the suppression of ROS and mitochondrial biogenesis. PLoS One. 2010;5:e11707. doi: 10.1371/journal.pone.0011707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hervouet E, Cízková A, Demont J, Vojtísková A, Pecina P, Franssen-van Hal NL, Keijer J, Simonnet H, Ivánek R, Kmoch S, et al. HIF and reactive oxygen species regulate oxidative phosphorylation in cancer. Carcinogenesis. 2008;29:1528–1537. doi: 10.1093/carcin/bgn125. [DOI] [PubMed] [Google Scholar]
- 29.Sporn MB, Liby KT. NRF2 and cancer: the good, the bad and the importance of context. Nat Rev Cancer. 2012;12:564–571. doi: 10.1038/nrc3278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Silva PN, Gigek CO, Leal MF, Bertolucci PH, de Labio RW, Payão SL, Smith Mde A. Promoter methylation analysis of SIRT3, SMARCA5, HTERT and CDH1 genes in aging and Alzheimer’s disease. J Alzheimers Dis. 2008;13:173–176. doi: 10.3233/jad-2008-13207. [DOI] [PubMed] [Google Scholar]
- 31.Xu J, Zhu W, Xu W, Yao W, Zhang B, Xu Y, Ji S, Liu C, Long J, Ni Q, et al. Up-regulation of MBD1 promotes pancreatic cancer cell epithelial-mesenchymal transition and invasion by epigenetic down-regulation of E-cadherin. Curr Mol Med. 2013;13:387–400. [PubMed] [Google Scholar]
- 32.Xu J, Zhu W, Xu W, Cui X, Chen L, Ji S, Qin Y, Yao W, Liu L, Liu C, et al. Silencing of MBD1 reverses pancreatic cancer therapy resistance through inhibition of DNA damage repair. Int J Oncol. 2013;42:2046–2052. doi: 10.3892/ijo.2013.1901. [DOI] [PubMed] [Google Scholar]