Abstract
AIM
To investigate the specific biomarkers and potential pathogenesis of colorectal cancer-related ischemic stroke (CRCIS).
METHODS
A retrospective study was conducted on CRCIS patients (colorectal cancer patients with ischemic stroke without conventional stroke risk factors) registered at seven centers between January 2007 and December 2017. Clinical data and laboratory and imaging findings were compared with age- and sex- matched patients with colorectal cancer (CRC) without ischemic stroke that were admitted to the same hospital during the same period. Univariate and multivariate analyses were performed to analyze the independent risk factors for CRCIS. A receiver operator characteristic curve was configured to calculate the optimal cut-off value of the products of the independent risk factors for CRCIS.
RESULTS
A total of 114 CRCIS patients and 114 CRC patients were included. Multiple lesions in multiple vascular territories were common in CRCIS patients (71, 62.28%). The levels of plasma D-dimer, carcinoembryonic antigen (CEA), cancer antigen 125, and neutrophil count were significantly higher in CRCIS patients than in CRC patients. Multiple logistic regression analysis revealed that plasma D-dimer levels [odds ratio (OR) = 1.002, 95% confidence interval (CI): 1.001-1.003, P < 0.001], CEA levels (OR = 1.011, 95%CI: 1.006-1.015, P < 0.001), and neutrophil count levels (OR = 1.626, 95%CI: 1.268-2.087, P < 0.001) were independent risk factors for CRCIS. In addition, receiver operator characteristic curve revealed that the area under curve for the products of plasma D-dimer, CEA, and neutrophil count was 0.889 ± 0.022 (95%CI: 0.847-0.932, P < 0.001), and the optimal cut-off value for the product was 252.06, which was called the CRCIS Index, with a sensitivity of 86.0% and specificity of 79.8%.
CONCLUSION
Hypercoagulability induced by elevated CEA and neutrophils may be an important cause of CRCIS. The CRCIS index, which serves as a biomarker of CRCIS, needs further study.
Keywords: Colorectal cancer, Ischemic stroke, Biomarker, Pathogenesis
Core tip: Although cancer-related stroke has long been known, its biomarkers and underling pathogenesis are still unclear. It was hypothesized that a specific cancer type may affect the development of ischemic stroke (IS) according to its primary site, pathological type, and growth stage. It is reported that colorectal cancer can increase the risk of IS. We conducted a retrospective study on colorectal cancer-related IS (CRCIS) patients. We suggest that hypercoagulability induced by elevated carcinoembryonic antigen and increased neutrophil count are the main pathogenic factors in CRCIS, and the CRCIS Index, which serves as a biomarker of CRCIS, needs further study.
INTRODUCTION
Cancer and ischemic stroke (IS) are major causes of morbidity and mortality. In addition, cerebrovascular disease is a common complication in cancer patients, with 15% of patients experiencing thromboembolic events during their clinical course[1]. Moreover, up to 40% of cancer patients with IS lack conventional stroke risk factors, indicating that cancer itself may directly or indirectly lead to the development of IS[2,3]. Previous studies have suggested that cancer-related IS is characterized by elevated plasma D-dimer levels and multiple lesions in multiple arterial territories on diffusion-weighted magnetic resonance imaging[4-6]. Although cancer is associated with IS through tumor compression, invasion of the blood vessels and left atrium, or nonbacterial thrombotic endocarditis[7-9], hypercoagulability is considered to be the most important pathogenic factor of cancer-related IS[10,11]. Furthermore, elevated levels of plasma D-dimer, high-sensitivity C-reactive protein, fibrinogen, and pro-brain natriuretic peptide are thought to be potential biomarkers of cancer-related IS[12,13]. However, despite accumulating knowledge, the specific biomarkers and definitive pathogenesis of cancer-related IS have remained unclear. Most previous studies have been conducted on several types of cancer. The different characteristics of cancer, including primary growth zone, pathological type, and growth stage, may affect IS in different ways[14]. We speculated that studies targeting patients with a specific cancer may be more conducive to illuminate the specific biomarkers and pathogenesis of cancer-related IS.
Colorectal cancer (CRC) is the third most common cancer among men and the second most common among women globally[15]. The incidence of CRC has shown a steady upward trend in China over the past decade, from 12.8 per 100000 in 2003 to 16.8 per 100000 in 2011, and the incidence will reach 20.7 per 100000 in 2020[16]. Notably, previous studies have reported that CRC is associated with IS[17,18]. The risk of IS increased 1.61 times during the first 6 mo after diagnosis of CRC compared to that in the general population[19], indicating that CRC itself could lead to IS, or so-called CRC-related IS (CRCIS). In the present study, we aimed to investigate the specific biomarkers and potential pathogenesis of CRCIS by comparing the clinical data of CRCIS patients with age- and sex-matched CRC patients, and calculating the CRCIS Index from its independent risk factors.
MATERIALS AND METHODS
Patient selection
This study was approved by the Guangxi Medical University Review Board. Patients with acute IS with the additional diagnosis of active CRC but without conventional stroke risk factors were recruited from seven centers in Guangxi Province between January 2007 and December 2017. Referring to the definition of active cancer in the study of Lee and his colleagues[20], active CRC was defined as a diagnosis of CRC within 6 mo before enrollment, any treatment for CRC within the previous 6 mo, or recurrent or metastatic CRC. The diagnosis of acute IS was based on the American Heart Association diagnostic criteria for stroke[21]. It was difficult to identify CRCIS in clinical practice. Referring to the definition of cancer-related stroke[2,3,5], CRCIS in the present study was defined as patients with acute IS and active CRC without conventional stroke risk factors. Patients with primary or metastatic brain cancer or hematological malignancies were excluded because these patients were considered to represent a subgroup with different underlying stroke mechanisms. Patients with cerebral hemorrhage or other cerebrovascular disease and those with incomplete clinical data were also excluded. The control group was age- and sex- matched patients with CRC but without IS who were admitted to the same hospital during the same period. The exclusion criteria included the presence of conventional stroke risk factors, brain metastasis, and other primary cancers (Figure 1).
Figure 1.
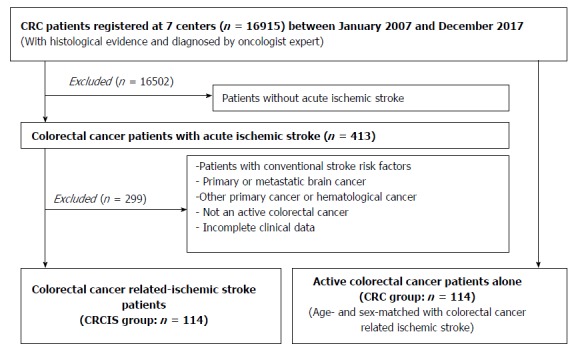
Patient selection. CRC: Colorectal cancer.
Previous studies found that cancer-related IS was associated with elevated plasma D-dimer[10,11]. However, whether it was associated with CRCIS is not clearly defined. Therefore, we hypothesized that D-dimer may also be associated with the occurrence of CRCIS. It was reported that the positive rate of D-dimer in CRC was 15% to 31%. In our 1:1 matched case-control study, for sample size estimation, we assumed the positive rate of D-dimer in CRC patients was 20%, and the risk of CRCIS was 3.5. Results showed that it needed 97 pairs when α = 0.05 and β = 0.2 (two-tailed).
Clinical data collection
Clinical data of all patients, including age, gender, stroke risk factors, pathological type of cancer, metastasis, treatment methods, IS etiology, IS lesion patterns, severity of focal neurological deficits, and time interval from diagnosis of CRC to the occurrence of IS were collected consistently. In addition, laboratory findings including routine blood tests, blood biochemistry, coagulation function, and levels of plasma D-dimer and tumor markers such as carcinoembryonic antigen (CEA), cancer antigen (CA)125, and CA199 were collected. Findings of imaging examinations such as echocardiography, transcranial Doppler ultrasound, cranial computed tomography, computed tomography angiography, magnetic resonance imaging, and magnetic resonance angiography were also collected. The severity of focal neurological deficits were assessed by the National Institutes of Health Stroke Scale (NIHSS).
Statistical analysis
Statistical analysis was performed using SPSS version 20.0 software. An independent sample t test was used to compare continuous variables between groups, while Pearson’s χ2 or Fisher’s exact test was used to compare categorical variables. Multivariate logistic regression analysis was performed to identify the independent risk factors of IS in CRC patients. Variables with P < 0.05 in univariate analyses were considered explanatory variables and were entered to multivariate models. To explore the specific biomarkers of CRCIS, the CRCIS Index was calculated as follows: First, we calculated the products of the independent risk factors for IS in the two groups; second, the cut-off value of the products of independent risk factors for IS that were used to identify IS in CRC patients was calculated by a receiver operator characteristic (ROC) curve; finally, the optimum cut-off value of the products was determined and called the CRCIS Index. All P-values were two-sided, and P < 0.05 was considered statistically significant.
RESULTS
A total of 114 CRCIS patients (78 male, 36 female, mean age = 65.33 ± 12.45 years) were included, accounting for 0.67% of 16915 CRC patients. There were 114 age- and sex-matched CRC patients that served as the control group. The most common pathological type of CRC in both groups was adenocarcinoma. There were no significant differences in cancer systemic metastasis and oncological treatment including surgery and chemoradiotherapy, and no treatment in CRCIS patients compared with CRC patients (Table 1).
Table 1.
Clinical characteristics of the patients
| Characteristic | CRCIS (n = 114) | CRC (n = 114) | P value |
| Age | 65.33 ± 12.45 | 63.67 ± 9.18 | 0.748a |
| Gender | |||
| Male, n (%) | 78 (68.42) | 78 (68.42) | 1.000b |
| Female, n (%) | 36 (31.58) | 36 (31.58) | |
| Blood tests | |||
| WBC (109/L) | 7.71 ± 2.27 | 7.18 ± 1.97 | 0.058a |
| HGB (g/L) | 114.96 ± 23.09 | 122.57 ± 85.55 | 0.360a |
| PLT (109/L) | 242.28 ± 116.46 | 235.51 ± 82.35 | 0.613a |
| NC (109/L) | 5.65 ± 2.28 | 4.16 ± 1.49 | < 0.001a |
| PT (s) | 10.94 ± 1.40 | 10.82 ± 0.98 | 0.474a |
| INR | 0.95 ± 0.13 | 0.92 ± 0.08 | 0.137a |
| APTT (s) | 29.52 ± 6.79 | 30.11 ± 6.20 | 0.496a |
| FIB (g/L) | 5.42 ± 4.42 | 5.32 ± 4.74 | 0.862a |
| D-dimer (μg/mL) | 1.40 ± 0.93 | 0.56 ± 0.47 | < 0.001a |
| CRP (mg/L) | 33.54 ± 35.50 | 28.79 ± 34.38 | 0.431a |
| CEA (U/mL) | 222.31 ± 175.85 | 88.48 ± 55.04 | < 0.001a |
| CA125 (U/mL) | 47.13 ± 54.32 | 22.70 ± 34.61 | < 0.001a |
| CA199 (U/mL) | 293.41 ± 1428.18 | 24.83 ± 34.212 | 0.066a |
| Type of CRC | |||
| Adenocarcinoma | 107 | 110 | 0.235b |
| Nonadenocarcinoma | 7 | 4 | |
| Systemic metastasis, n (%) | 0.080b | ||
| Yes | 74 (64.9) | 61 (53.5) | |
| No | 40 (35.1) | 53 (46.5) | |
| Methods of therapy, n (%) | |||
| Surgery | 76 (66.7) | 87 (76.3) | 0.107b |
| Chemotherapy | 46 (40.4) | 33 (28.9) | 0.070b |
| No treatment | 28 (24.6) | 24 (21.2) | 0.528b |
With two independent samples t-test;
With chi-square test. CRCIS: Colorectal cancer related-ischemic stroke; CRC: Colorectal cancer; WBC: White blood cell; HGB: Hemoglobin; PLT: Platelet; NC: Neutrophil count; PT: Prothrombin time; INR: International normalized ratio; APTT: Activated partial thromboplastin time; FIB: Fibrinogen; CRP: C-reactive protein; CEA: Carcinoembryonic antigen; CA: Cancer antigen.
After diagnosis of CRC, 60 (52.63%), 19 (16.67%), and 13 (11.40%) patients experienced acute IS in the first 6 mo, 6 mo to 1 year, and > 1 year, respectively. In addition, 22 (19.30%) patients presented with IS as the initial manifestation of occult CRC, which was confirmed as CRC during anti-stroke therapy. Multiple lesions in multiple arterial territories in the brain on diffusion-weighted magnetic resonance imaging were observed in 71 (62.28%) CRCIS patients. NIHSS score ranged from 0 to 21 on the day of IS onset (Table 2 and Figure 2).
Table 2.
Data of ischemic stroke onset, n (%)
| Characteristic | No. of patients (n =114) |
| IS lesion pattern | |
| Single arterial lesion | 43 (37.72) |
| Multiple arterial lesions | 71 (62.28) |
| NIHSS scores at the day of IS onset | |
| 0-5 | 31 (27.19) |
| 6-15 | 66 (57.90) |
| 16-20 | 13 (11.40) |
| > 20 | 4 (3.51) |
| Time interval between CRC diagnosis and IS onset | |
| IS as the first manifestation of CRC | 22 (19.30) |
| IS onset after CRC diagnosis | |
| 0-6 mo | 60 (52.63) |
| 7-12 mo | 19 (16.67) |
| > 12 mo | 13 (11.40) |
IS: Ischemic stroke; NIHSS: National Institutes of Health Stroke Scale; CRC: Colorectal cancer.
Figure 2.
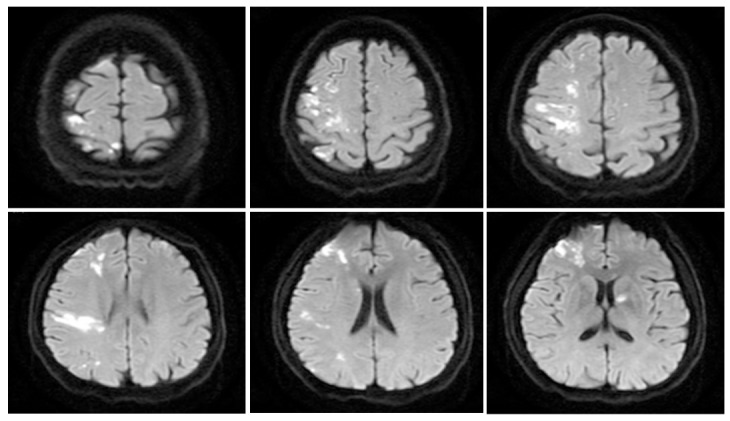
Typical presentation of ischemic stroke in a colorectal cancer patient without conventional stroke mechanism. A 47-year-old male patient experienced acute ischemic stroke in the first 3 mo after diagnosis of colorectal cancer. Diffusion-weighted magnetic resonance imaging showed high signal changes in the middle cerebral and anterior cerebral and anterior choroidal arteries. Echocardiography results were normal.
In terms of laboratory findings, there was no significant difference in most items between the two groups. However, the levels of plasma D-dimer, neutrophil count (NC), CEA, and CA125 were significantly higher in the CRCIS patients compared to the CRC patients (P < 0.05). Multiple logistic regression analysis revealed that the D-dimer levels [odds ratio (OR) = 1.002, 95% confidence interval (CI): 1.001-1.003, P < 0.001], CEA levels (OR = 1.011, 95%CI: 1.006-1.015, P < 0.001) and NC levels (OR = 1.626, 95%CI: 1.268-2.087, P < 0.001) were independent risk factors of CRCIS (Table 3).
Table 3.
Multivariate logistic regression analysis
| Factors | β | SE | Wals (β) | Df | P value | OR | 95%CI |
| D-dimer | 0.002 | 0.001 | 26.660 | 1 | < 0.001 | 1.002 | 1.001-1.003 |
| NC | 0.486 | 0.127 | 14.617 | 1 | < 0.001 | 1.626 | 1.268-2.087 |
| CEA | 0.011 | 0.002 | 20.104 | 1 | < 0.001 | 1.011 | 1.006-1.015 |
| CA125 | 0.007 | 0.004 | 2.595 | 1 | 0.107 | 1.007 | 0.998-1.016 |
| Constant | -5.843 | 0.866 | 45.484 | 1 | < 0.001 | 0.003 |
NC: Neutrophil count; CEA: Carcinoembryonic antigen; CA125: Cancer antigen 125; SE: Standard error; OR: Odds ratio; CI: Confidence interval.
The ROC curves for identifying CRCIS from the products of D-dimer, CEA, and NC were shown in Figure 1. The mean ± standard error of the area under curve for the product was 0.889 ± 0.022 (95%CI: 0.847-0.932, P < 0.001), indicating good overall accuracy of the test. The optimum diagnostic cut-off value for the products calculated from the ROC was 252.06, which was called the CRCIS Index, with a sensitivity of 84.3% and specificity of 80.9% (Figure 3).
Figure 3.
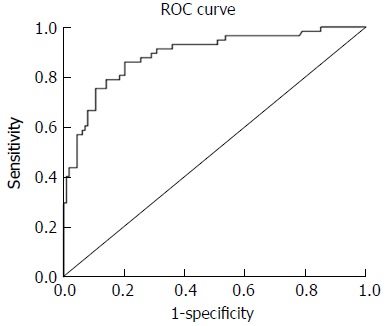
Receiver operator characteristic curve analysis of the product of D-dimer, carcinoembryonic antigen, and neutrophil count. The area under the curve of the products was 0.889 ± 0.022 (95%CI: 0.847-0.932, P < 0.001). The optimum cut-off point was 252.06. At this cut-off value, the sensitivity was 86.0% and the specificity was 79.8%. CI: Confidence interval; ROC: Receiver operator characteristic.
DISCUSSION
IS is a common complication in patients with cancer, and it has received increasing attention[22,23]. Previous studies showed that the incidence of IS was 1.6 times higher in cancer patients than in patients without cancer, especially during the first 6 mo after cancer diagnosis[19]. In the present study, the incidence of IS in hospitalized CRC patients was 0.67%, and most of them (52.63%) developed IS within the first 6 mo after diagnosis of CRC. It is suggested that as soon as CRC diagnosis is established, measures should be taken to prevent IS. In addition, IS as an initial manifestation of occult cancer has been reported in previous studies[17,24]. In the present study, there were 22 (19.3%) patients who were hospitalized for acute IS and then diagnosis of CRC was confirmed, indicating that measures should be taken to screen out occult cancer, including CRC, in patients with unexplained IS.
Previous studies have suggested that certain clinical, laboratory, and radiological features could help to distinguish cancer-related IS from stroke of other etiology[3,25]. Many studies have demonstrated that cancer-related IS is characterized by markedly elevated plasma D-dimer levels and multiple lesions in multiple arterial territories on diffusion-weighted magnetic resonance imaging[4-6]. In the present study, CRCIS patients had similar features to general cancer-related IS such as elevated plasma D-dimer levels and multiple lesions in multiple cerebral arterial territories, as well as distinctive features including elevated plasma CEA and increased NC, which may be useful clues to identify CRCIS.
The unveiling of underlying pathogenesis of cancer-related IS is an important issue because pathogenesis may influence the choice of management, stroke prognosis, and risk of recurrence. In some previous studies, cancer was found to be associated with IS through tumor compression, invasion of the blood vessels and left atrium, and nonbacterial thrombotic endocarditis[7-9]. However, increasing studies have suggested that cancer-related IS were characterized by elevated plasma D-dimer levels[4-6]. Recently, Wang and his colleagues found that the plasma D-dimer value of 2.785 μg/mL was the cutoff in identifying cancer-related IS patients[26]. Moreover, the frequency of microembolic signals in the internal carotid arteries on transcranial Doppler images correlated linearly with D-dimer levels in patients with IS and cancer, which indicated that elevated D-dimer levels are an independent predictor for the detection of embolic signals[11]. Moreover, the D-dimer level is a direct measure of activated coagulation and has been used as a biomarker of hypercoagulability[27]. The elevated D-dimer level suggests that hypercoagulability plays a major role in the pathogenesis of cancer-related IS. In the present study, plasma D-dimer level was significantly higher in CRCIS patients, indicating that hypercoagulability is an important factor in the pathogenesis of CRCIS.
Although hypercoagulability may be associated with cancer-related IS, its underlying mechanism remains unclear. Interestingly, recent studies have suggested that mucins generated from mucinous cancer are associated with hypercoagulability and increase the risk of IS[28-30]. A study by Jovin et al[28] reported four patients with metastatic cancer, brain infarcts, and markedly elevated mucinous serum marker CA125 levels, and suggested a possible association between this protein and stroke. Moreover, the relationship between mucins and hypercoagulability were further confirmed by necropsy evidences from mucinous cancer patients with widespread intracranial arteriovenous thrombosis and multiple cerebral infarctions, in which the mucin within vessels and in microthrombus in the regions of infarction were found by microscopic examination[29]. Furthermore, animal experiments demonstrated that mucins secreted by cancer cells could trigger the reciprocal activation of platelets and neutrophils and led to the formation of thrombus in the blood[30]. These findings indicated that mucins associated hypercoagulability played an important role in cancer-related IS. In the present study, CRCIS patients had higher CEA and CA125, and elevated CEA was an independent risk factor for IS. CEA, a general oncofetal antigen, is also a mucinous marker similar to CA125[31]. Therefore, we speculated that CEA, a mucinous substance generated from CRC cells may also lead to hypercoagulability by triggering the reciprocal activation of platelets and neutrophils.
Additionally, neutrophil extracellular traps (NETs) generated from neutrophils were also found to be associated with hypercoagulability and thrombosis diseases (including IS) in patients with cancer[32,33]. In solid tumor models, it was found that cancer can induce an increase of peripheral blood neutrophils. Increased neutrophils may release NETs in the vascular, which may promote hypercoagulability by stimulating platelet activation[34]. Therefore, NETs generated from neutrophils were considered to be a novel coagulation-promoting mechanism in cancer patients[35,36]. In the present study, NC was significantly increased in CRCIS patients, and multivariate logistic regression analysis revealed that the increased NC may independently increase the risk of IS. Therefore, the increase in neutrophils may also lead to hypercoagulability by releasing NETs to stimulate the activation of platelets and thus increase the risk of IS in CRC patients.
In the present study, multivariate logistic regression analysis revealed that elevated plasma D-dimer and CEA and increased NC were independent risk factors for CRCIS, indicating that these parameters could be used as potential biomarkers for CRCIS. However, as a common coagulation marker, plasma D-dimer lack specificity and sensitivity. Elevated levels of CEA are also found in other cancers and increased NC is common in systemic inflammation. Therefore, elevated plasma D-dimer and CEA and increased NC were not specific biomarkers for CRCIS. Considering that development of CRCIS may be caused by the combined effects of elevated plasma D-dimer and CEA levels and increased NC, we calculated the CRCIS Index. As the area under the ROC curve of the CRCIS Index was highly accurate, and the sensitivity and specificity of the CRCIS Index were high, we suggest that the CRCIS Index could serve as a potential biomarker. However, due to the retrospective design of the present study, the other biomarkers mentioned in previous studies that may be related to cancer-related IS were not studied[12,13]. The role of the CRCIS Index in CRCIS patients will need to be confirmed in future studies with larger sample sizes and more biomarkers. Nevertheless, our study provided a meaningful method to further the investigation about the index of CRCIS in future studies.
The strengths of our study were multicenter enrollment and comparison with CRC patients without IS. The main limitations of this study included the relatively small sample size and some uncontrollable settings. Still, our results could promote additional larger prospective population studies, which would better illuminate the biomarkers and pathogenesis of IS in CRC patients.
In summary, our findings suggest that hypercoagulability induced by elevated CEA and neutrophils may be an important cause of CRCIS. The CRCIS index, which serves as a novel biomarker of CRCIS, needs to be confirmed in future studies.
ARTICLE HIGHLIGHTS
Research background
Cancer is associated with an increased risk of ischemic stroke (IS), and IS can be the first manifestation of an occult cancer. Several biomarkers and mechanisms in cancer related-IS have been reported. However, most previous studies had been conducted on several types of cancer with few studies focusing on one cancer. The specific biomarkers and mechanisms of colorectal cancer related-IS (CRCIS) have not been fully investigated yet. Therefore, the aim of the study was to investigate the specific biomarkers and potential pathogenesis of CRCIS, which may contribute to the establishment of CRCIS therapeutic strategy.
Research motivation
Cancer related-IS has received widespread attention. However, biomarkers and the pathogenesis of CRCIS remain unclear. The key problems to be solved are how to identify CRCIS patients in clinical practice and whether hypercoagulability is the major cause and mechanism of IS. Identifying the biomarkers of CRCIS patients, which combined with clinical manifestation may be helpful to distinguish patients with other stroke etiology and other types of cancer-related stroke. Understanding the specific mechanisms in stroke of CRC patients is crucial to its therapeutic strategy.
Research objectives
The main objective of the retrospective study was to investigate the specific biomarkers and potential pathogenesis of CRCIS.
Research methods
The clinical data of 114 CRC patients with IS but without conventional stroke risk factors were retrospectively analyzed and compared with those of CRC patients without IS. Univariate and multivariate analyses were used to identify independent risk factors for CRCIS. The products of the independent risk factors in the two groups were calculated. The receiver operator characteristic curve was used to determine the area under the curve and the optimal value of the products.
Research results
Our study found that multiple lesions in multiple vascular territories were common in CRCIS patients (71, 62.28%). In addition, the level of plasma D-dimer, neutrophil count, carcinoembryonic antigen (CEA), and cancer antigen 125 were significantly higher in CRCIS patients compared to CRC patients. D-dimer, neutrophil count, and CEA were found to independently associated with CRCIS. Considering that the combined effects of D-dimer, neutrophil count, and CEA may be the major cause of hypercoagulability, the products of each were calculated. The area under the curve of the product was 0.889 ± 0.022, optimal cut-off value of the product was 252.06, which was called the CRCIS Index in the study, with a sensitivity of 86.0% and specificity of 79.8%.
Research conclusions
Neutrophil extracellular traps generated from active neutrophils and CEA secreted by CRC cells that resulted in hypercoagulability may be the major cause of CRCIS. The CRCIS index, which serves as a biomarker for CRCIS, may need to be confirmed by future studies.
Research perspectives
In the future, the detailed mechanism of hypercoagulability in CRCIS patients may need to further illuminated. The CRCIS index that serves as a useful biomarker for the identification of CRCIS should be confirmed in larger prospective population studies.
Footnotes
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
Institutional review board statement: This study was reviewed and approved by the First Affiliated Hospital of Guangxi Medical University Institutional Review Board.
Informed consent statement: Patients were not required to give informed consent to the study because the analysis used anonymous clinical data that were obtained after each patient agreed to treatment by written consent. For full disclosure, the details of the study are published on the home page of the First Affiliated Hospital of Guangxi Medical University.
Conflict-of-interest statement: Authors declare no conflict of interest for this article.
Data sharing statement: No additional data are available.
Peer-review started: September 4, 2018
First decision: October 24, 2018
Article in press: November 8, 2018
P- Reviewer: Abd-Elsalam S, Azer AS S- Editor: Ma RY L- Editor: Filipodia E- Editor: Bian YN
Contributor Information
Qi-Xiong Qin, Department of Neurology, The First Affiliated Hospital of Guangxi Medical University, Nanning 530021, Guangxi Province, China.
Xue-Min Cheng, Department of Neurology, The First Affiliated Hospital of Guangxi Medical University, Nanning 530021, Guangxi Province, China.
Li-Zhi Lu, Department of Neurology, The First Affiliated Hospital of Guangxi Medical University, Nanning 530021, Guangxi Province, China.
Yun-Fei Wei, Department of Neurology, The Second Affiliated Hospital of Guangxi Medical University, Nanning 530007, Guangxi Province, China.
Da-Cheng Wang, Department of Neurology, The Ninth Affiliated Hospital of Guangxi Medical University, Beihai 536000, Guangxi Province, China.
Hai-Hua Li, Department of Neurology, Fusui County People’s Hospital, Chongzuo 532100, Guangxi Province, China.
Guo-Hui Li, Department of Neurology, Wuzhou Red Cross Hospital, Wuzhou 543002, Guangxi Province, China.
Hong-Bin Liang, Department of Neurology, Cenxi People’s Hospital, Cenxi 543200, Guangxi Province, China.
Sheng-Yu Li, Department of Neurology, Wuming County People’s Hospital, Nanning 530100, Guangxi Province, China.
Li Chen, Department of Neurology, The First Affiliated Hospital of Guangxi Medical University, Nanning 530021, Guangxi Province, China.
Zhi-Jian Liang, Department of Neurology, The First Affiliated Hospital of Guangxi Medical University, Nanning 530021, Guangxi Province, China. lzj200415@126.com.
References
- 1.Graus F, Rogers LR, Posner JB. Cerebrovascular complications in patients with cancer. Medicine (Baltimore) 1985;64:16–35. doi: 10.1097/00005792-198501000-00002. [DOI] [PubMed] [Google Scholar]
- 2.Kim SG, Hong JM, Kim HY, Lee J, Chung PW, Park KY, Kim GM, Lee KH, Chung CS, Bang OY. Ischemic stroke in cancer patients with and without conventional mechanisms: a multicenter study in Korea. Stroke. 2010;41:798–801. doi: 10.1161/STROKEAHA.109.571356. [DOI] [PubMed] [Google Scholar]
- 3.Bang OY, Seok JM, Kim SG, Hong JM, Kim HY, Lee J, Chung PW, Park KY, Kim GM, Chung CS, et al. Ischemic stroke and cancer: stroke severely impacts cancer patients, while cancer increases the number of strokes. J Clin Neurol. 2011;7:53–59. doi: 10.3988/jcn.2011.7.2.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schwarzbach CJ, Fatar M, Eisele P, Ebert AD, Hennerici MG, Szabo K. DWI Lesion Patterns in Cancer-Related Stroke--Specifying the Phenotype. Cerebrovasc Dis Extra. 2015;5:139–145. doi: 10.1159/000439549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kono T, Ohtsuki T, Hosomi N, Takeda I, Aoki S, Sueda Y, Ishihara K, Nakamura T, Yamawaki T, Matsumoto M. Cancer-associated ischemic stroke is associated with elevated D-dimer and fibrin degradation product levels in acute ischemic stroke with advanced cancer. Geriatr Gerontol Int. 2012;12:468–474. doi: 10.1111/j.1447-0594.2011.00796.x. [DOI] [PubMed] [Google Scholar]
- 6.Sorgun MH, Kuzu M, Ozer IS, Yilmaz V, Ulukan C, Cotur Levent H, Tezcan S, Rzayev S, Rawandi A, Bakırarar B, et al. Risk Factors, Biomarkers, Etiology, Outcome and Prognosis of Ischemic Stroke in Cancer Patients. Asian Pac J Cancer Prev. 2018;19:649–653. doi: 10.22034/APJCP.2018.19.3.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gundersen H, Moynihan B. An Uncommon Cause of Stroke: Non-bacterial Thrombotic Endocarditis. J Stroke Cerebrovasc Dis. 2016;25:e163–e164. doi: 10.1016/j.jstrokecerebrovasdis.2015.11.009. [DOI] [PubMed] [Google Scholar]
- 8.Dimitrović A, Breitenfeld T, Supanc V, Roje-Bedeković M, Butković Soldo S, Vargek-Solter V. Stroke Caused by Lung Cancer Invading the Left Atrium. J Stroke Cerebrovasc Dis. 2016;25:e66–e68. doi: 10.1016/j.jstrokecerebrovasdis.2015.12.043. [DOI] [PubMed] [Google Scholar]
- 9.Vlachostergios PJ, Daliani DD, Dimopoulos V, Patrikidou A, Voutsadakis IA, Papandreou CN. Nonbacterial thrombotic (marantic) endocarditis in a patient with colorectal cancer. Onkologie. 2010;33:456–459. doi: 10.1159/000317342. [DOI] [PubMed] [Google Scholar]
- 10.Schwarzbach CJ, Schaefer A, Ebert A, Held V, Bolognese M, Kablau M, Hennerici MG, Fatar M. Stroke and cancer: the importance of cancer-associated hypercoagulation as a possible stroke etiology. Stroke. 2012;43:3029–3034. doi: 10.1161/STROKEAHA.112.658625. [DOI] [PubMed] [Google Scholar]
- 11.Seok JM, Kim SG, Kim JW, Chung CS, Kim GM, Lee KH, Bang OY. Coagulopathy and embolic signal in cancer patients with ischemic stroke. Ann Neurol. 2010;68:213–219. doi: 10.1002/ana.22050. [DOI] [PubMed] [Google Scholar]
- 12.Kim K, Lee JH. Risk factors and biomarkers of ischemic stroke in cancer patients. J Stroke. 2014;16:91–96. doi: 10.5853/jos.2014.16.2.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee EJ, Nah HW, Kwon JY, Kang DW, Kwon SU, Kim JS. Ischemic stroke in patients with cancer: is it different from usual strokes? Int J Stroke. 2014;9:406–412. doi: 10.1111/ijs.12124. [DOI] [PubMed] [Google Scholar]
- 14.Navi BB, Reiner AS, Kamel H, Iadecola C, Okin PM, Elkind MSV, Panageas KS, DeAngelis LM. Risk of Arterial Thromboembolism in Patients With Cancer. J Am Coll Cardiol. 2017;70:926–938. doi: 10.1016/j.jacc.2017.06.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–E386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 16.Zhu J, Tan Z, Hollis-Hansen K, Zhang Y, Yu C, Li Y. Epidemiological Trends in Colorectal Cancer in China: An Ecological Study. Dig Dis Sci. 2017;62:235–243. doi: 10.1007/s10620-016-4362-4. [DOI] [PubMed] [Google Scholar]
- 17.Tsai CC, Wu MN. Frequent Ischemic Stroke as First Manifestation of Occult Colon Cancer: A Rare Case. Am J Case Rep. 2015;16:723–727. doi: 10.12659/AJCR.895130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lin RJ, Amoruso DR. A mysterious stroke in a colon cancer patient. QJM. 2014;107:919–922. doi: 10.1093/qjmed/hcs034. [DOI] [PubMed] [Google Scholar]
- 19.Zöller B, Ji J, Sundquist J, Sundquist K. Risk of haemorrhagic and ischaemic stroke in patients with cancer: a nationwide follow-up study from Sweden. Eur J Cancer. 2012;48:1875–1883. doi: 10.1016/j.ejca.2012.01.005. [DOI] [PubMed] [Google Scholar]
- 20.Lee AY, Levine MN, Baker RI, Bowden C, Kakkar AK, Prins M, Rickles FR, Julian JA, Haley S, Kovacs MJ, Gent M; Randomized Comparison of Low-Molecular-Weight Heparin versus Oral Anticoagulant Therapy for the Prevention of Recurrent Venous Thromboembolism in Patients with Cancer (CLOT) Investigators. Low-molecular-weight heparin versus a coumarin for the prevention of recurrent venous thromboembolism in patients with cancer. N Engl J Med. 2003;349:146–153. doi: 10.1056/NEJMoa025313. [DOI] [PubMed] [Google Scholar]
- 21.Powers WJ, Derdeyn CP, Biller J, Coffey CS, Hoh BL, Jauch EC, Johnston KC, Johnston SC, Khalessi AA, Kidwell CS, et al. 2015 American Heart Association/American Stroke Association Focused Update of the 2013 Guidelines for the Early Management of Patients With Acute Ischemic Stroke Regarding Endovascular Treatment: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke. 2015;46:3020–3035. doi: 10.1161/STR.0000000000000074. [DOI] [PubMed] [Google Scholar]
- 22.Sanossian N, Djabiras C, Mack WJ, Ovbiagele B. Trends in cancer diagnoses among inpatients hospitalized with stroke. J Stroke Cerebrovasc Dis. 2013;22:1146–1150. doi: 10.1016/j.jstrokecerebrovasdis.2012.11.016. [DOI] [PubMed] [Google Scholar]
- 23.Selvik HA, Thomassen L, Bjerkreim AT, Næss H. Cancer-Associated Stroke: The Bergen NORSTROKE Study. Cerebrovasc Dis Extra. 2015;5:107–113. doi: 10.1159/000440730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wada Y, Takahashi R, Yanagihara C, Nishimura Y. Paradoxical cerebral embolism as the initial symptom in a patient with ovarian cancer. J Stroke Cerebrovasc Dis. 2007;16:88–90. doi: 10.1016/j.jstrokecerebrovasdis.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 25.Grazioli S, Paciaroni M, Agnelli G, Acciarresi M, Alberti A, D'Amore C, Caso V, Venti M, Guasti L, Ageno W, et al. Cancer-associated ischemic stroke: A retrospective multicentre cohort study. Thromb Res. 2018;165:33–37. doi: 10.1016/j.thromres.2018.03.011. [DOI] [PubMed] [Google Scholar]
- 26.Wang JY, Zhang GJ, Zhuo SX, Wang K, Hu XP, Zhang H, Qu LD. D-dimer >2.785 μg/ml and multiple infarcts ≥3 vascular territories are two characteristics of identifying cancer-associated ischemic stroke patients. Neurol Res. 2018;40:948–954. doi: 10.1080/01616412.2018.1504179. [DOI] [PubMed] [Google Scholar]
- 27.Gon Y, Sakaguchi M, Takasugi J, Kawano T, Kanki H, Watanabe A, Oyama N, Terasaki Y, Sasaki T, Mochizuki H. Plasma D-dimer levels and ischaemic lesions in multiple vascular regions can predict occult cancer in patients with cryptogenic stroke. Eur J Neurol. 2017;24:503–508. doi: 10.1111/ene.13234. [DOI] [PubMed] [Google Scholar]
- 28.Jovin TG, Boosupalli V, Zivkovic SA, Wechsler LR, Gebel JM. High titers of CA-125 may be associated with recurrent ischemic strokes in patients with cancer. Neurology. 2005;64:1944–1945. doi: 10.1212/01.WNL.0000163850.07976.63. [DOI] [PubMed] [Google Scholar]
- 29.Shao B, Wahrenbrock MG, Yao L, David T, Coughlin SR, Xia L, Varki A, McEver RP. Carcinoma mucins trigger reciprocal activation of platelets and neutrophils in a murine model of Trousseau syndrome. Blood. 2011;118:4015–4023. doi: 10.1182/blood-2011-07-368514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Amico L, Caplan LR, Thomas C. Cerebrovascular complications of mucinous cancers. Neurology. 1989;39:522–526. doi: 10.1212/wnl.39.4.522. [DOI] [PubMed] [Google Scholar]
- 31.Thomsen M, Skovlund E, Sorbye H, Bolstad N, Nustad KJ, Glimelius B, Pfeiffer P, Kure EH, Johansen JS, Tveit KM, et al. Prognostic role of carcinoembryonic antigen and carbohydrate antigen 19-9 in metastatic colorectal cancer: a BRAF-mutant subset with high CA 19-9 level and poor outcome. Br J Cancer. 2018;118:1609–1616. doi: 10.1038/s41416-018-0115-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thålin C, Demers M, Blomgren B, Wong SL, von Arbin M, von Heijne A, Laska AC, Wallén H, Wagner DD, Aspberg S. NETosis promotes cancer-associated arterial microthrombosis presenting as ischemic stroke with troponin elevation. Thromb Res. 2016;139:56–64. doi: 10.1016/j.thromres.2016.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Demers M, Wagner DD. NETosis: a new factor in tumor progression and cancer-associated thrombosis. Semin Thromb Hemost. 2014;40:277–283. doi: 10.1055/s-0034-1370765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Demers M, Krause DS, Schatzberg D, Martinod K, Voorhees JR, Fuchs TA, Scadden DT, Wagner DD. Cancers predispose neutrophils to release extracellular DNA traps that contribute to cancer-associated thrombosis. Proc Natl Acad Sci USA. 2012;109:13076–13081. doi: 10.1073/pnas.1200419109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hisada Y, Geddings JE, Ay C, Mackman N. Venous thrombosis and cancer: from mouse models to clinical trials. J Thromb Haemost. 2015;13:1372–1382. doi: 10.1111/jth.13009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Darbousset R, Thomas GM, Mezouar S, Frère C, Bonier R, Mackman N, Renné T, Dignat-George F, Dubois C, Panicot-Dubois L. Tissue factor-positive neutrophils bind to injured endothelial wall and initiate thrombus formation. Blood. 2012;120:2133–2143. doi: 10.1182/blood-2012-06-437772. [DOI] [PubMed] [Google Scholar]


