Abstract
Background
Cervical foraminal stenosis (CFS) is one of the degenerative changes of the cervical spine; however, correlations between the severity of stenosis and that of symptoms are not consistent in the literature. Studies to date on the prevalence of stenosis are based on images obtained from the departments treating cervical lesions, and thus patient selection bias may have occurred. The purpose of this study was to investigate the prevalence of CFS according to the site, extent, and morphology of stenosis using cervical computed tomography (CT) images obtained from patients who were visiting not because of symptoms related to the cervical spine, cervical pain, or upper limb pain.
Methods
Among patients who underwent CT from January 2016 to March 2016 for reasons other than cervical spine symptoms, a total of 438 subjects were enrolled, and 2,628 cervical disc images (C4–5, C5–6, and C6–7; left and right sides) were examined. Three orthopedic surgeons performed two measurements each at 4-week intervals. Values were used for analysis if matched by more than two surgeons; if no match was found, the median values were used for analysis. The left and right sides on the same axial image were independently classified.
Results
Left C5–6 stenosis was most common (24.66%) among patients. At the left C6–7, there were 20 focal types and 33 diffuse types. At bilateral C4–5 and right C6–7, the focal type was more common, whereas at bilateral C5–6 and left C6–7, the diffuse type was more common. Age and the severity of stenosis showed statistically significant correlation at all cervical levels.
Conclusions
The prevalence of CFS was highest at the C5–6 level (19.06%). Compared to other levels, focal stenosis was more frequent at C4–5 and diffuse stenosis was more common at C5–6. At C6–7, the incidence of focal stenosis was higher on the right side and that of diffuse stenosis was higher on the left side.
Keywords: Cervical vertebrae, Spinal stenosis, Incidence, Community participant
Cervical foraminal stenosis (CFS) is a common diagnosis that causes cervical radiculopathy and is one of the most common diseases requiring surgical treatment in the cervical spine. The presence of CFS and associated upper extremity symptoms are important factors in determining surgical treatment. Postoperative outcomes of CFS are better than those of cervical spine myelopathy caused by spinal cord compression. In spite of being one of the common degenerative changes of the cervical spine,1,2,3,4) however, correlations between the severity of stenosis and severity of symptoms are inconsistent in the literature.5,6)
In the presence of spinal nerve root symptoms and radiographic compression of the same nerve root, surgery for CFS is determined based on the severity of symptoms.7) However, considering that appropriate patient selection is most important for a successful operation, data on the prevalence of CFS without symptoms would be helpful to avoid excessive surgical treatment. There are many studies on symptomatic cervical disc herniation and spinal root compression, but there have been few studies on the diagnosis of CFS in asymptomatic populations.8,9) In addition, studies to date on the incidence of stenosis used images obtained from departments treating cervical lesions (orthopedic surgery, neurological surgery, rehabilitation, pain clinic, and neurology), and thus patient selection bias may have occurred. Therefore, we analyzed the prevalence of CFS using cervical computed tomography (CT) scans taken during a certain period from one university hospital regardless of the department to identify patients without clinical symptoms of CFS. The purpose of this study was to investigate the prevalence of CFS according to the site, extent, and morphology using cervical CT images obtained from patients who had no clinical symptoms of CFS.
METHODS
Patients
We prospectively evaluated all cervical CT scans taken from January 2016 to March 2016 from Daegu Catholic University Hospital regardless of department. Before performing CT, we checked whether the patient had clinical symptoms or a past history of CFS and then collected the data prospectively. We excluded patients with clinical symptoms or a past history of cervical spine problems. All included patients underwent cervical CT for reasons other than cervical lesion examination in medical departments such as otolaryngology, digestive medicine, and general surgery; CT scans from departments where patients are treated for cervical lesions such as orthopedic surgery, neurosurgery, rehabilitation medicine, and pain medicine were not used for the study. A total of 438 subjects were included, and a total of 2,628 cervical disc (C4–5, C5–6, and C6–7; left and right) images were examined. We conducted this study in compliance with the principles of the Declaration of Helsinki. The protocol of this study was reviewed and approved by the Institutional Review Board of Daegu Catholic University (IRB No. CR-16-086). Written informed consents were obtained.
CT Scan Parameters
CT was performed using one of three 16-multidetector computed tomography (MDCT) machines or a dual source 64-MDCT system (Lightspeed Ultra; GE Healthcare, Milwaukee, WI, USA). The axial slice thickness varies from 0.75 to 2.5 mm because the images were obtained from patients with different indications and by different protocols.
Radiological Grade Measurement
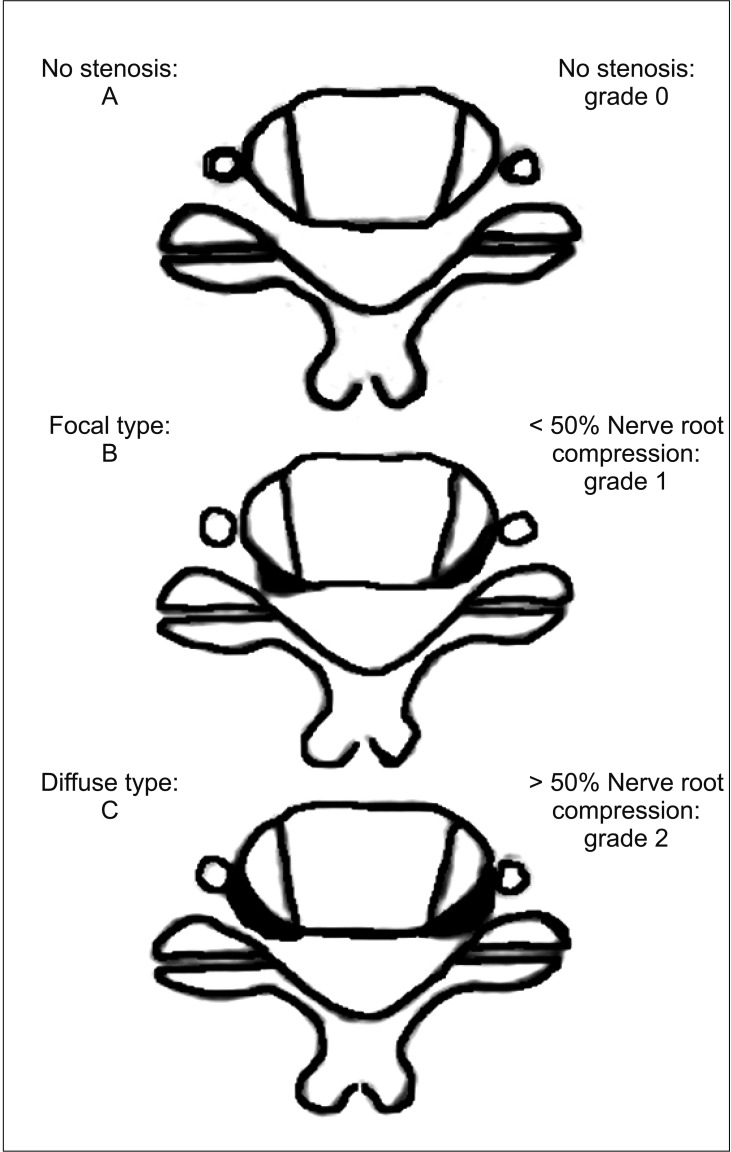
We performed radiographic classification by analyzing axial images at the cervical disc level, and we assessed morphology and extent of compression related to foraminal stenosis at each site (Fig. 1) evaluating the uncovertebral joints and osteophytes of an uncovertebral joint and a facet joint. The shape and extent of stenosis were classified using the Lee et al.'s classification system:10) grade 0, no stenosis; grade 1, less than 50% nerve root compression; and grade 2, more than 50% nerve root compression; focal or diffuse type by shape. Three orthopedic surgeons (JL, a senior resident; SK, a full-time orthopedic spine specialist; and WC, a general orthopedic practitioner) who were blind to the patients' epidemiologic data and who were unaware that the patients were not being treated for spinal problems conducted classification. Each surgeon performed measurement twice with a 4-week interval, and if two measurement values were different, a severe one was selected. If more than two of the three surgeons had the same measurement values, the values were used for analysis. If all measurement values were unmatched, the median values were used for analysis. The left and right sides were independently classified on the same axial image.
Fig. 1. Lee's classification system.10).
Statistical Analysis
We determined inter- and intraobserver reliability of the assessment of relationships between the extent of stenosis compression and morphology using Kappa statistics. We evaluated the correlation between age and the severity of CFS using cross tabulation analysis. For statistical analysis, we used IBM SPSS ver. 20.0 (IBM Corp., Armonk, NY, USA) and a p < 0.05 was considered statistically significant.
RESULTS
Epidemiological Results
Of the 438 patients, 195 were female and 243 were male. The mean age of the patients was 54.3 years (range, 20 to 84 years); by sex, the mean age was 55.4 years (range, 21 to 82 years) for males and 53 years (range, 20 to 84 years) for females. Among the total patients, 249 patients were from otorhinolaryngology, 128 patients from general surgery, 31 patients from chest surgery, and 30 patients from digestive medicine. Interobserver reliability was 0.86 and intraobserver reliability was 0.93 for the assessment of the extent of compression; interobserver reliability was 0.84 and intraobserver reliability was 0.91 for the assessment of morphology.
Extent of Compression
Among all 438 patients, left C5–6 stenosis was most common (24.66%), and the most severe stenosis of grade 2 was found in the left C5–6 (2.97%). The prevalence of stenosis at C4–5 was 10.50% on the right side and 13.47% on the left side; at C5–6, it was 13.47% on the right side and 24.66% on the left side; at C6–7, it was 10.96% on the right side and 12.10% on the left side. The prevalence of CFS was high in the following order: left C5–6, left C4–5 and right C5–6, and left C6–7. Overall, the incidence of CFS was greater on the left side than on the right side (Table 1).
Table 1. Foraminal Stenosis According to Degree of Compression.
| Spine level | Side | Grade | No. (%) | Total (%) |
|---|---|---|---|---|
| C4–5 | Right | 0 | 392 (89.50) | |
| 1 | 39 (8.90) | 10.50 | ||
| 2 | 7 (1.60) | |||
| Left | 0 | 379 (86.53) | ||
| 1 | 59 (13.47) | 13.47 | ||
| 2 | 0 | |||
| C5–6 | Right | 0 | 379 (86.53) | |
| 1 | 52 (11.87) | 13.47 | ||
| 2 | 7 (1.60) | |||
| Left | 0 | 330 (75.34) | ||
| 1 | 95 (21.69) | 24.66 | ||
| 2 | 13 (2.97) | |||
| C6–7 | Right | 0 | 390 (89.04) | |
| 1 | 41 (9.36) | 10.96 | ||
| 2 | 7 (1.60) | |||
| Left | 0 | 385 (87.90) | ||
| 1 | 46 (10.50) | 12.10 | ||
| 2 | 7 (1.60) |
On morphological characteristics, the focal type was more common at C4–5, whereas the diffuse type was more common at C5–6. At C6–7, the focal type was more common on the right side, whereas the diffuse type was more common on the left side (Table 2).
Table 2. Foraminal Stenosis According to Morphology.
| Spine level | Side | Type | No. (%) |
|---|---|---|---|
| C4–5 | Right | Focal | 34 (73.91) |
| Diffuse | 12 (26.09) | ||
| Left | Focal | 41 (69.49) | |
| Diffuse | 18 (30.51) | ||
| C5–6 | Right | Focal | 16 (27.12) |
| Diffuse | 43 (72.88) | ||
| Left | Focal | 29 (26.85) | |
| Diffuse | 79 (73.15) | ||
| C6–7 | Right | Focal | 29 (60.42) |
| Diffuse | 19 (39.58) | ||
| Left | Focal | 20 (37.74) | |
| Diffuse | 33 (62.26) |
Correlation between Age and Extent of CFS
The 438 patients were divided by age into two groups (> 55 years and ≤ 55 years). We evaluated the correlation between age and the extent of CFS using cross tabulation analysis. At all cervical levels, age and the extent of CFS showed statistically significant correlation.
DISCUSSION
The most important finding of this study is that left C5–6 stenosis was most common (24.66%) in the cervical spine on the CT scan, but the more severe grade of stenosis was found in the left C5–6 level (2.97%). The incidences were evaluated from a selected community-based population that had no clinical symptoms of CFS.
Cervical neuromuscular disease is manifested by symptoms in specific neuromuscular regions of the upper limbs, and radiating pain is mostly caused by cervical nerve root compression due to stenosis of the cervical vertebrae.11,12,13) In the presence of progressive neurological deterioration, intractable pain, signs of myelopathy, fracture, instability, or ligamentous injury, and bone anomalies or destruction are associated with surgical indications. However, CFS can be asymptomatic.14) To determine the affected level that requires cervical spine surgery, the patients undergo neurological and physical examinations and then both CT and magnetic resonance imaging (MRI); if the results do not match, additional neurophysiological testing is required to determine the affected level. However, if neurological examinations are performed after radiological examinations, it is difficult to rule out the possibility of bias; radiological findings may influence surgeon's decision making.
CT and MRI sometimes do not show stenosis of the cervical vertebra in spite of clinical and neurological symptoms.15) CFS is sometimes referred to as silent cervical spinal canal stenosis, and this compression of the cervical nerve roots is mainly caused by degenerative changes in the spine or secondary intervertebral stenosis caused by disc herniation.11,12) Silent cervical spinal canal stenosis has been reported by several authors for the risk of neurological damage at the time of injury and prophylactic surgery,16) but there are very few studies on stenosis of the cervical intervertebral disc.1,17,18,19,20) According to Kuijper et al.,14) false positive nerve root compression is also seen in 45% of cases, mostly in the upper and lower parts of at least one of the clinical abnormalities and even on the opposite side; this asymptomatic nerve root compression is often accompanied by nerve root compression with clinical symptoms, and based on compression findings observed on MRI, patients may be treated unnecessarily. To avoid this, clinicians should take detailed histories of the patient and conduct neurological examinations and interpret MRI results based on these findings. In the case of CFS, it may be more accurate to use CT spine images rather than MRI,21,22,23,24) and we had difficulty gathering and analyzing MRI scans of the general population.
Teresi et al.25) analyzed the MRIs of 100 patients with laryngeal disease and reported symptomatic cervical disc herniation in 57% of patients older than 64 years and in 20% of patients between 45 and 54 years of age. In addition, Boden et al.1) reported major cervical spine abnormalities such as disc herniation, intervertebral stenosis, and stenosis of the cervical intervertebral disc in 28% of patients over age 40 years without symptoms.
In this study, C5–6 was the most common level for CFS, including severe stenosis. Degenerative changes in the cervical spine are known to occur frequently at C5–6,26) and in previous studies, the incidence of CFS was found to be higher at C5–6 than at other sites.2,27) The causes of CFS in this specific area have not yet been elucidated. However, based on the results of cervical motion studies, it may be attributable to the range of flexion and axial rotation in the C5–6 segment that is broader than that in other regions.28) Further investigation is needed to determine whether these biomechanical properties are related to inducing intervertebral disc stenosis. In addition, C5–6 stenosis was more frequent in the left intervertebral foramina than on the right side, and the stenosis also differed from that in other segments in terms of the higher incidence of diffuse type.
The limitations of this study are as follows. First, this study did not evaluate the correlation between the extent of stenosis and clinical symptoms. However, the aim of this study was to evaluate incidence of CFS in CT among a selected community-based population without clinical symptoms of cervical spine. Second, since the study period was not the entire year but only 3 months of 2016, this study may not be free from selection bias. Third, we could not explain why diffuse type was more common on the left side; further investigation is needed to establish its relationship with the dominant side of the body. Last, the neck CT scan, which was performed for other purposes in various departments, does not accurately show the appearance of the foramen of cervical spine. However, to the best of our knowledge, this is the first study on the extent and shape of the intervertebral foramina at each level of the cervical vertebrae, and it is also important to note that this is the first study that addresses the differences between the left and right sides.
The prevalence of CFS was highest at the C5–6 level: 19.06% for CFS, and 2.29% for severe stenosis (more than 50% nerve root compression). CFS occurred more often on the left side than on the right side (24.66% vs. 13.47%). Focal stenosis was more frequent at C4–5 and diffuse stenosis was more common at C5–6 than at other sites. At C6–7, focal stenosis was more common on the right side and so was diffuse stenosis on the left side. If CT shows normal changes in spite of clinical symptoms of cervical vertebrae stenosis, neurological and neurophysiologic examinations may be important in determining the extent of the lesion.
ACKNOWLEDGEMENTS
This work was supported by a grant from the Research Institute of Medical Science, Catholic University of Daegu (2017).
Footnotes
CONFLICT OF INTEREST: No potential conflict of interest relevant to this article was reported.
References
- 1.Boden SD, Davis DO, Dina TS, Patronas NJ, Wiesel SW. Abnormal magnetic-resonance scans of the lumbar spine in asymptomatic subjects: a prospective investigation. J Bone Joint Surg Am. 1990;72(3):403–408. [PubMed] [Google Scholar]
- 2.Matsumoto M, Fujimura Y, Suzuki N, et al. MRI of cervical intervertebral discs in asymptomatic subjects. J Bone Joint Surg Br. 1998;80(1):19–24. doi: 10.1302/0301-620x.80b1.7929. [DOI] [PubMed] [Google Scholar]
- 3.Wilson JR, Barry S, Fischer DJ, et al. Frequency, timing, and predictors of neurological dysfunction in the nonmyelopathic patient with cervical spinal cord compression, canal stenosis, and/or ossification of the posterior longitudinal ligament. Spine (Phila Pa 1976) 2013;38(22 Suppl 1):S37–S54. doi: 10.1097/BRS.0b013e3182a7f2e7. [DOI] [PubMed] [Google Scholar]
- 4.Gore DR, Sepic SB, Gardner GM. Roentgenographic findings of the cervical spine in asymptomatic people. Spine (Phila Pa 1976) 1986;11(6):521–524. doi: 10.1097/00007632-198607000-00003. [DOI] [PubMed] [Google Scholar]
- 5.Kang Y, Lee JW, Koh YH, et al. New MRI grading system for the cervical canal stenosis. AJR Am J Roentgenol. 2011;197(1):W134–W140. doi: 10.2214/AJR.10.5560. [DOI] [PubMed] [Google Scholar]
- 6.Bednarik J, Kadanka Z, Dusek L, et al. Presymptomatic spondylotic cervical myelopathy: an updated predictive model. Eur Spine J. 2008;17(3):421–431. doi: 10.1007/s00586-008-0585-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Oliveira Vilaca C, Orsini M, Leite MA, et al. Cervical spondylotic myelopathy: what the neurologist should know. Neurol Int. 2016;8(4):6330. doi: 10.4081/ni.2016.6330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sheikh Taha AM, Shue J, Lebl D, Girardi F. Considerations for prophylactic surgery in asymptomatic severe cervical stenosis: review article. HSS J. 2015;11(1):31–35. doi: 10.1007/s11420-014-9426-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fassett DR, Jeyamohan S, Harrop J. Asymptomatic cervical stenosis: to operate or not? Semin Spine Surg. 2007;19(1):47–50. [Google Scholar]
- 10.Lee SH, Park SY, Wang JC, Kang KC, Hwang SP, Jang S. A comprehensive MRI classification system for cervical foraminal stenosis. Spine J. 2015;15(10):S203. [Google Scholar]
- 11.Tanaka N, Fujimoto Y, An HS, Ikuta Y, Yasuda M. The anatomic relation among the nerve roots, intervertebral foramina, and intervertebral discs of the cervical spine. Spine (Phila Pa 1976) 2000;25(3):286–291. doi: 10.1097/00007632-200002010-00005. [DOI] [PubMed] [Google Scholar]
- 12.Radhakrishnan K, Litchy WJ, O'Fallon WM, Kurland LT. Epidemiology of cervical radiculopathy: a population-based study from Rochester, Minnesota, 1976 through 1990. Brain. 1994;117(Pt 2):325–335. doi: 10.1093/brain/117.2.325. [DOI] [PubMed] [Google Scholar]
- 13.Kuijper B, Tans JT, Schimsheimer RJ, et al. Degenerative cervical radiculopathy: diagnosis and conservative treatment. A review. Eur J Neurol. 2009;16(1):15–20. doi: 10.1111/j.1468-1331.2008.02365.x. [DOI] [PubMed] [Google Scholar]
- 14.Kuijper B, Tans JT, van der, Nollet F, Lycklama A, de Visser M. Root compression on MRI compared with clinical findings in patients with recent onset cervical radiculopathy. J Neurol Neurosurg Psychiatry. 2011;82(5):561–563. doi: 10.1136/jnnp.2010.217182. [DOI] [PubMed] [Google Scholar]
- 15.Nakashima H, Yukawa Y, Suda K, Yamagata M, Ueta T, Kato F. Abnormal findings on magnetic resonance images of the cervical spines in 1211 asymptomatic subjects. Spine (Phila Pa 1976) 2015;40(6):392–398. doi: 10.1097/BRS.0000000000000775. [DOI] [PubMed] [Google Scholar]
- 16.Shigematsu H, Cheung JP, Mak KC, Bruzzone M, Luk KD. Cervical spinal canal stenosis first presenting after spinal cord injury due to minor trauma: an insight into the value of preventive decompression. J Orthop Sci. 2017;22(1):22–26. doi: 10.1016/j.jos.2016.09.008. [DOI] [PubMed] [Google Scholar]
- 17.Jensen MV, Tuchsen F, Orhede E. Prolapsed cervical intervertebral disc in male professional drivers in Denmark, 1981-1990: a longitudinal study of hospitalizations. Spine (Phila Pa 1976) 1996;21(20):2352–2355. doi: 10.1097/00007632-199610150-00009. [DOI] [PubMed] [Google Scholar]
- 18.van Rijn JC, Klemetso N, Reitsma JB, et al. Observer variation in the evaluation of lumbar herniated discs and root compression: spiral CT compared with MRI. Br J Radiol. 2006;79(941):372–377. doi: 10.1259/bjr/26216335. [DOI] [PubMed] [Google Scholar]
- 19.van Rijn JC, Klemetso N, Reitsma JB, et al. Observer variation in MRI evaluation of patients suspected of lumbar disk herniation. AJR Am J Roentgenol. 2005;184(1):299–303. doi: 10.2214/ajr.184.1.01840299. [DOI] [PubMed] [Google Scholar]
- 20.Boden SD, McCowin PR, Davis DO, Dina TS, Mark AS, Wiesel S. Abnormal magnetic-resonance scans of the cervical spine in asymptomatic subjects: a prospective investigation. J Bone Joint Surg Am. 1990;72(8):1178–1184. [PubMed] [Google Scholar]
- 21.Fortin J, Riethmiller DW, Vilensky JA. No clear winner in differing imaging modalities for cervical radiculopathy. Pain Physician. 2002;5(3):285–287. [PubMed] [Google Scholar]
- 22.Bartlett RJ, Hill CR, Gardiner E. A comparison of T2 and gadolinium enhanced MRI with CT myelography in cervical radiculopathy. Br J Radiol. 1998;71(841):11–19. doi: 10.1259/bjr.71.841.9534693. [DOI] [PubMed] [Google Scholar]
- 23.Birchall D, Connelly D, Walker L, Hall K. Evaluation of magnetic resonance myelography in the investigation of cervical spondylotic radiculopathy. Br J Radiol. 2003;76(908):525–531. doi: 10.1259/bjr/99259611. [DOI] [PubMed] [Google Scholar]
- 24.Kaiser JA, Holland BA. Imaging of the cervical spine. Spine (Phila Pa 1976) 1998;23(24):2701–2712. doi: 10.1097/00007632-199812150-00009. [DOI] [PubMed] [Google Scholar]
- 25.Teresi LM, Lufkin RB, Reicher MA, et al. Asymptomatic degenerative disk disease and spondylosis of the cervical spine: MR imaging. Radiology. 1987;164(1):83–88. doi: 10.1148/radiology.164.1.3588931. [DOI] [PubMed] [Google Scholar]
- 26.Roh JS, Teng AL, Yoo JU, Davis J, Furey C, Bohlman HH. Degenerative disorders of the lumbar and cervical spine. Orthop Clin North Am. 2005;36(3):255–262. doi: 10.1016/j.ocl.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 27.Shim JH, Park CK, Lee JH, et al. A comparison of angled sagittal MRI and conventional MRI in the diagnosis of herniated disc and stenosis in the cervical foramen. Eur Spine J. 2009;18(8):1109–1116. doi: 10.1007/s00586-009-0932-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bogduk N, Mercer S. Biomechanics of the cervical spine. I: Normal kinematics. Clin Biomech (Bristol, Avon) 2000;15(9):633–648. doi: 10.1016/s0268-0033(00)00034-6. [DOI] [PubMed] [Google Scholar]