Abstract
The multiple endocrine neoplasia type 1 (MEN1) syndrome is caused by germline mutations in the MEN1 gene encoding menin, with tissue-specific tumors of the parathyroids, anterior pituitary, and enteropancreatic endocrine tissues. Also, 30–40% of sporadic pancreatic endocrine tumors show somatic MEN1 gene inactivation. Although menin is expressed in all cell types of the pancreas, mouse models with loss of menin in either pancreatic α-cells, or β-cells, or total pancreas develop β-cell-specific endocrine tumors (insulinomas). Loss of widely expressed tumor suppressor genes may produce tissue-specific tumors by reactivating one or more embryonic-specific differentiation factors. Therefore, we determined the effect of menin overexpression or knockdown on the expression of β-cell differentiation factors in a mouse β-cell line (MIN6). We show that the β-cell differentiation factor Hlxb9 is posttranscriptionally upregulated upon menin knockdown, and it interacts with menin. Hlxb9 reduces cell proliferation and causes apoptosis in the presence of menin, and it regulates genes that modulate insulin level. Thus, upon menin loss or from other causes, dysregulation of Hlxb9 predicts a possible combined mechanism for β-cell proliferation and insulin production in insulinomas. These observations help to understand how a ubiquitously expressed protein such as menin might control tissue-specific tumorigenesis. Also, our findings identify Hlxb9 as an important factor for β-cell proliferation andinsulin regulation.
Keywords: multiple endocrine neoplasias, gene regulation, gene expression, islet cells
Introduction
In patients with the multiple endocrine neoplasia type 1 (MEN1) syndrome, germline inactivating mutation in one copy of the MEN1 gene encoding menin predisposes to endocrine tumors of the parathyroids, anterior pituitary, and enteropancreatic endocrine tissues and hormone non-secreting non-endocrine tumors such as facial angiofibroma, truncal collagenoma, and lipoma (Thakker 2010). These tumors show somatic inactivation of the normal MEN1 allele by mutation or deletion, resulting in the complete loss of menin function. Studies on mouse models have demonstrated that Men1 homozygous null mice are embryonic lethal at E11.5–E13.5, and Men1 heterozygous mice show endocrine tumors similar to those found in the human MEN1 syndrome (Balasubramanian & Scacheri 2009). However, the role of menin in early development and how menin loss initiates tumors in specific endocrine and non-endocrine tissues are not completely understood.
Somatic inactivation of one or both copies of the MEN1 gene is observed in 30–40% of commonly occurring sporadic pancreatic endocrine tumors, particularly in gastrinoma and insulinoma (β-cell tumor; Jensen et al. 2008, Jiao et al. 2011). Menin is a 610 amino acid (aa), predominantly nuclear protein expressed in all tissues including in all cell types of the pancreas. Mouse models with loss of menin in either pancreatic α-cells, or pancreatic β-cells, or total pancreas develop β-cell-specific endocrine tumors (Balasubramanian & Scacheri 2009, Shen et al. 2009, 2010, Lu et al. 2010). Also, interestingly, mice with liver-specific menin loss did not develop tumors in the liver (Balasubramanian & Scacheri 2009). The molecular basis for the pathogenesis of the cell-type-specific tumors from the loss of ubiquitously expressed menin needs to be determined.
Loss of widely expressed tumor suppressor genes may produce tissue-specific tumors by reactivating one or more tissue-specific factors associated with embryonic development and differentiation (Sherr 2004, Briegel 2006). Also, in some cancers, tissue-specific dysregulation of developmental factors through mutation or abnormal expression or altered subcellular localization has been shown to contribute to neoplasia (Guo et al. 2004, Kimura 2011). We investigated this paradigm in the pathogenesis of β-cell tumors. A cascade of known transcription factors has been shown to direct progenitor cells to develop into the pancreatic cell lineage, endocrine cell lineage, and finally into the different islet cell types of the endocrine pancreas. These islet cells – α, β, δ, ε, and PP cells – secrete various hormones specific for each cell type – glucagon, insulin, somatostatin, ghrelin, and pancreatic polypeptide respectively. Mouse models have shown that the loss of any particular factor leads to specific loss of only certain islet cell types, confirming the cell-type-specific actions of these transcription factors (Melloul 2004). HLXB9 is a homeobox containing transcription factor that is essential in the early stages of pancreatic development; it is also expressed in mature β-cells, brain, testis, and in the lymphoid lineage, and it is an important regulator of motor neuron identity in both Drosophila and vertebrates (Harrison et al. 1999). Mice deficient in Hlxb9 generate defective motor neurons (Thaler et al. 1999). Knockout of Hlxb9 in mice also leads to the selective agenesis of the dorsal pancreas, with disorganized islet structure and reduced number of β-cells in the ventral pancreas (Harrison et al. 1999, Li et al. 1999). In mice, temporally extended Hlxb9 expression under the control of the Pdx1 promoter leads to a complete impairment of pancreas development causing pancreatic cells to adopt intestinal fates (Li & Edlund 2001). Therefore, tight temporal regulation of Hlxb9 expression is critical for proper pancreas development and function. Mutations in some factors associated with β-cell differentiation – PDX1, HNF1α, HNF1β, HNF4α, MAFA, and NEUROD1 – have been observed in maturity-onset diabetes of the young (MODY); however, no such mutations were found in HLXB9 (MNX1) (Garin et al. 2009, Naylor & Philipson 2011). Hereditary mutations of HLXB9 occur in Currarino syndrome characterized by sacral agenesis and anorectal malformations (Ross et al. 1998). Further studies investigating the causes and consequences of HLXB9 dysregulation will be important to understand the role of HLXB9 in normal β-cell physiology and in disease.
Pancreatic islet β-cell differentiation factors could be important candidates for aberrant reactivation upon menin loss resulting in the increased proliferation and hormonal abnormality of β-cell tumors. Therefore, in order to gain insight into the islet β-cell-specific proliferation and hormone hypersecretion upon menin loss, we undertook a targeted approach to test the possible role of menin in the regulation of islet β-cell differentiation factors and identified Hlxb9 as an important factor for β-cell proliferation and insulin regulation.
Materials and methods
Antibodies and siRNA/shRNA
The antibodies against β-cell differentiation factors and other antibodies are listed in Supplementary Table 1, see section on supplementary data given at the end of this article. The following siRNA/shRNA were used: mouse Men1 siRNA (Qiagen, Valencia, CA, USA; SI01303715, SI01303722, SI01303736, and SI05382258), mouse Hlxb9 siRNA (Dharmacon, Waltham, MA, USA; L-049859–01), mouse visinin-like protein-1 (Vsnl1) siRNA (Dharmacon, L-060595–01), negative control siRNA (Qiagen, 1027280), mouse Men1 shRNA construct, and control (Ang et al. 2011).
Mammalian cell culture and transfection
Human embryonic kidney 293 (HEK293) cells (ATCC, Manassas, VA, USA), alpha-TC1 (ATCC), 266–6 (ATCC), and Men1-null or Men1-nullCMen1 mouse embryonic fibroblasts (MEFs) (Schnepp et al. 2004) were grown in DMEM high glucose (Invitrogen) supplemented with 10% FCS (Gemini Bioproducts, Sacramento, CA, USA) and antibiotic/antimycotic (Invitrogen). MIN6 cells (Miyazaki et al. 1990) were grown in DMEM low glucose (Invitrogen) supplemented with 15% FCS and antibiotic/antimycotic.
For menin knockdown, cells were electroporated in solution T (Amaxa/Lonza, Rochester, NY, USA), and for Hlxb9 and Vsnl1 knockdown, cells were first electroporated in solution T followed after 48 h by a second round of transfection with RNAiMAX (Invitrogen). Cells were processed for protein and/or RNA isolation after total 72-h post-transfection. For overexpression, plasmids were electroporated into MIN6, HEK293, or MEFs cells in solution T or transfected with Lipofectamine 2000 (Invitrogen). After 48 h, cells were harvested for protein and/or RNA.
Western blot
Whole cell extract (WCE) was prepared in CIP buffer: 100 mM NaCl, 50 mM Tris–Cl (pH 8.0), 10 mM MgCl2, 0.5% Igepal, and EDTA-free protease inhibitor cocktail t (Roche, Indianapolis, IN, USA), and western blots were performed as per standard protocols.
RNA isolation and quantitative real-time RT-PCR
RNA was isolated using the RNeasy Mini Kit (Qiagen) and treated with DNaseI (Ambion, Grand Island, NY, USA). Oligo(dT)-primed first-strand cDNA was used for quantitative real-time RT-PCR (QRT-PCR) with the Brilliant SYBR Green QPCR Master Mix and the Mx3000p cycler (Stratagene, Santa Clara, CA, USA). QRT-PCR was normalized to β-actin or to Gapdh. The 2−ΔΔCt method was used to quantitate the relative change in gene expression. The data were plotted as fold change over their corresponding controls. All primer sequences are listed in Supplementary Table 2, see section on supplementary data given at the end of this article.
GST pull-down assay
The coding region of Hlxb9 was amplified using MIN6 RNA and cloned into pcDNA3.1(−)-myc-his-A (mh-Vector) (Invitrogen) in-frame with a downstream myc-his-tag (mh-Hlxb9). The full-length coding region of Hlxb9 and Hlxb9 N- or C-terminal aa 1–240 and 241–404 were amplified from the mh-Hlxb9 plasmid and cloned into the GST-fusion vector pGEX-5X-1 (Amersham/Pharmacia, GE Healthcare, Piscataway, NJ, USA). Hlxb9 internal deletions of aa 121–134, 121–167, and 121–174 were generated by site-directed mutagenesis of the GST-Hlxb9 plasmid. Full-length human menin (flag-Menin) or menin regions aa 41–610, 178–610, 324–610, 1–323, 1–448, 1–476, and 1–502 were generated by PCR using the FLAG-MAC-menin plasmid (Knapp et al. 2000) as a template and cloned into pflag-CMV4 (Sigma).
GST or GST-fused proteins (GST-Menin, GST-Hlxb9, and GST-Hlxb9 deletions) were expressed in bacteria in the Escherichia coli BL21-PRIL strain (Stratagene) and purified on glutathione sepharose beads (GE Healthcare) as described (Agarwal et al. 1999). WCE or 35S-labeled in vitro translated (IVT) protein was precleared at 4 °C for 30 min using glutathione sepharose beads. Equal amounts of GST alone, or GST-fused proteins coupled to glutathione sepharose beads, were incubated overnight at 4 °C with precleared WCE or IVT proteins. The beads were washed thoroughly five times with CIP buffer. The bound proteins were detected by western blot with appropriate antibodies or by autoradiography (for IVT proteins).
Co-immunoprecipitation assay
WCE from MIN6 cells was prepared in CIP buffer. WCE (400 mg) was used for immunoprecipitation (IP) as described (Agarwal et al. 1999) with 4 mg anti-Hlxb9. Menin and Hlxb9 were detected by western blot. Because Hlxb9 co-migrates with the IgG heavy chain, the reciprocal IP with anti-menin to detect Hlxb9 was unsuccessful. For co-IP of menin missense mutants (H139D, A176P, A242V, A309P, T344R, and W436R) with mh-Hlxb9, twice the amount of plasmid was transfected for the flag-tagged menin missense mutants due to the previously known reduced expression of menin missense mutants (Agarwal et al. 1999, Canaff et al. 2012). Total DNA amount transfected was maintained constant by including empty vector.
Mammalian one-hybrid assay
MIN6 cells were transfected with Gal4-UAS luciferase reporter plasmid pFR (Stratagene) alone or together with a plasmid containing Gal4 DNA-binding domain (Gal4DBD) fused Hlxb9 (pM-Hlxb9 or pCMVBD-Hlxb9). Gal4DBD-JunD (pM-JunD; Agarwal et al. 1999) served as a positive control. The pCDNA3.1 plasmid was used to equalize the total amount of transfected DNA. Cells were harvested 48 h post-transfection, and luciferase activity was measured by using the Dual-Luciferase Assay Kit (Promega, Madison, WI, USA).
Gene expression microarray
Total RNA was isolated using RNeasy (Qiagen) from three independent transient transfections of MIN6 cells with control siRNA or Hlxb9 siRNA. Gene expression analysis was performed at the NIDDK microarray core facility using Affymetrix Genechip mouse genome 430, 2.0 arrays (Affymetrix, Santa Clara, CA, USA). Microarray data were normalized and analyzed using Affymetrix Genechip Software Microarray Analysis Suite 5.0. The microarray data have been submitted to Gene Expression Omnibus (GEO, identifier #GSE32653).
Chromatin IP assay
MIN6 cells were cross-linked at room temperature with 1% formaldehyde for 10 min. Cells (107) were lysed in 0.6 ml lysis buffer supplied in the Chromatin IP (ChIP) Kit (Upstate/Millipore, Billerica, MA, USA) and processed for ChIP as per the manufacturer. After overnight reverse cross-link at 65 °C, DNA fragments were purified using the QIAquick PCR Purification Kit (Qiagen). The purified DNA fragments were analyzed using primer pairs designed from the evolutionary conserved promoter regions of mouse Vsnl1 and phospholipase D1 (Pld1; Supplementary Table 2). PCR products were assessed on agarose gels stained with ethidium bromide.
Cell proliferation and cell cycle analysis
Equal number of MIN6 cells (106) were transfected with the indicated plasmid DNA, and the cell number was determined 96 h post-transfection using a Cellometer auto T4 plus cell counter (Nexcelom Biosciences, Lawrence, MA, USA). The same cells (2.5×105) were analyzed in Vindelov’s propidium iodide buffer for distribution of cell cycle phases (FACSCalibur, BD Biosciences, San Jose, CA, USA; Cellquest and Modfit, NHLBI flow cytometry core).
Apoptosis assays
The level of apoptosis was determined using the cellular DNA fragmentation ELISA kit (Roche). ApoAlert Annexin V–FITC Apoptosis kit (Clontech, Mountain View, CA, USA) was used for apoptosis detection by immunofluorescence. Microscopy and photomicrography were performed with AxioObserver Z1 (Zeiss, Thornwood, NY, USA).
Immunohistochemistry
Formalin-fixed paraffin-embedded (FFPE) pancreatic tissue sections from 18-month-old Men+/− mice (Wang et al. 2011) were processed for immunohistochemistry (IHC) with anti-HLXB9 (Histoserv, Germantown, MD, USA). IHC with the same HLXB9 antibody did not work in FFPE human pancreatic tissue sections.
Statistical analysis
Data were presented as mean±S.E.M. of at least three independent experiments. Differences between groups were compared by unpaired two-tailed Student’s t-tests. Significant difference was considered if P values were <0.05 (*) or <0.01 (**).
Results
Menin regulates the β-cell differentiation factor Hlxb9
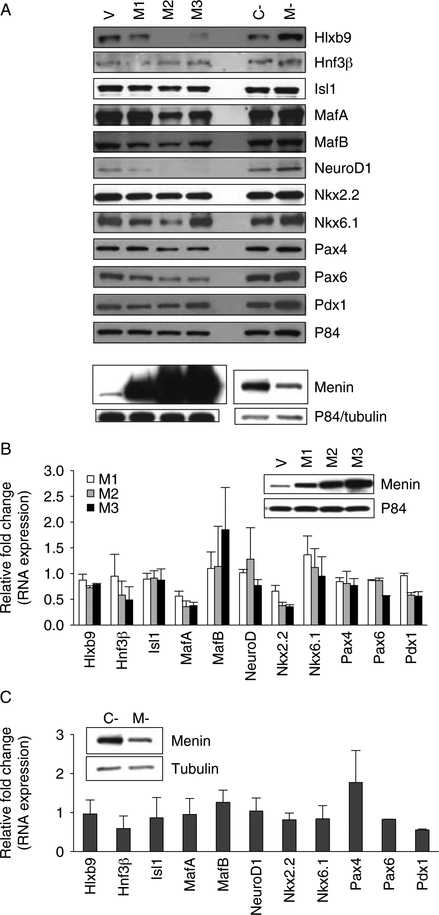
The mouse pancreatic β-cell line MIN6 was used to study the effect of menin on RNA and protein expression of 12 transcription factors known to participate in the differentiation of the β-cell lineage: Hlxb9, Hnf3β, Isl1, MafA, MafB, NeuroD1, Ngn3, Nkx2.2, Nkx6.1, Pax4, Pax6, and Pdx1 (Melloul 2004). Stable overexpression of menin in this cell line decreased cell proliferation and delayed cell cycle progression from G0/G1 into S phase (Supplementary Figure 1, see section on supplementary data given at the end of this article). Also, endogenous expression of all factors, except Ngn3, was easily detectable in MIN6 cells (Fig. 1A). These observations show that in MIN6 cells, menin’s function to suppress growth is intact and the MIN6 cell culture system can be easily and effectively used to study the possible menin-mediated regulation of β-cell differentiation factors.
Figure 1.
Effect of menin on β-cell differentiation factors in MIN6 cells. (A) Protein expression of transcription factors associated with β-cell differentiation upon menin overexpression or menin knockdown. Representative western blots of whole cell extract (WCE) of cells transfected as indicated. V indicates vector-transfected sample (1.5 μg plasmid), M1, M2, and M3 indicate menin overexpressing samples transfected with 0.6, 0.9, and 1.5 μg plasmid respectively; C- and M- indicate control siRNA and Men1 siRNA-transfected samples respectively. Ngn3 was not detectable in MIN6 cells. Anti-P84 or anti-tubulin was used to assess protein loading. (B and C) RNA expression of transcription factors associated with β-cell differentiation upon menin overexpression (B, n=3) or menin knockdown (C, n=3). QRT-PCR data are shown as fold change of expression of each gene in RNA samples of cells transfected as in ‘A’ compared with vector transfected cells (vector transfectedZ1; B) or fold change of expression of each gene in Men1 siRNA-transfected cells compared with control siRNA-transfected cells (control siRNA transfected= 1; C). None of the transcription factors showed significantly altered RNA expression. Inset: western blots showing level of menin overexpression or menin knockdown. P84 was used to assess protein loading.
Western blot analysis showed that the expression of some factors, such as Hlxb9, Isl1, Nkx6.1, MafA, NeuroD1, Pax4, and Pax6, was decreased upon transient menin overexpression and was increased upon transient menin knockdown (Fig. 1A). However, none of the transcription factors showed significantly altered RNA expression upon menin overexpression or upon menin knockdown (Fig. 1B and C). Further analysis of the factors potentially affected by menin (Hlxb9, Isl1, Nkx6.1, MafA, NeuroD1, Pax4, and Pax6) revealed that only Hlxb9 protein (also known as HB9, Mnx1, or Mnr2) was consistently altered upon increased or decreased menin levels. Therefore, menin-mediated regulation of the homeobox transcription factor Hlxb9 was further investigated.
Menin interacts with Hlxb9
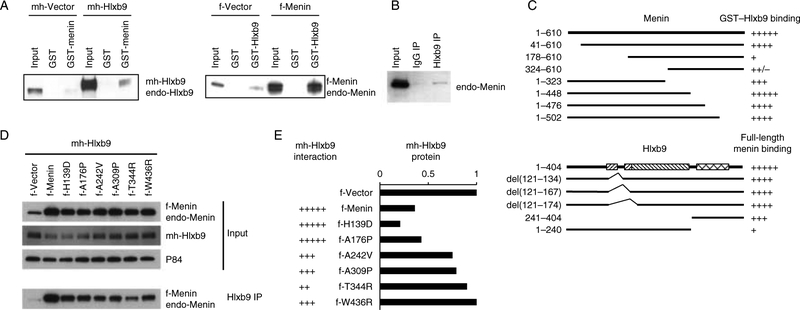
Because menin did not alter Hlxb9 RNA level, posttranscriptional regulatory mechanisms were explored. Upon menin overexpression or menin knockdown, Hlxb9 protein half-life or protein stability was unaffected (Supplementary Figure 2A and B, see section on supplementary data given at the end of this article), the distribution of Hlxb9 in nuclear and cytoplasmic compartments was unaffected (Supplementary Figure 2C), and the phosphorylated form of Hlxb9 was unaffected (Supplementary Figure 2D). Also, Hlxb9 3’-UTR luciferase assay showed that menin did not activate any microRNA to regulate Hlxb9 expression (Supplementary Figure 2E). However, GST pull-down assays showed that GST-menin could bind to endogenous Hlxb9 and transfected Hlxb9 from MIN6 cells (Fig. 2A). In reciprocal experiments, GST-Hlxb9 bound endogenous and transfected menin (Fig. 2A). Furthermore, anti-Hlxb9 immunoprecipitates contained menin (Fig. 2B). Hlxb9 could not be detected in anti-menin immunoprecipitates because the band corresponding to Hlxb9 co-migrates with the IgG heavy chain. In vitro-translated (IVT) menin or Hlxb9 did not bind to GST-Hlxb9 or GST-menin respectively, suggesting that the interaction was not direct or it required secondary modification of these proteins (data not shown). Therefore, the above assays indicated that menin could potentially regulate Hlxb9 through protein–protein interaction.
Figure 2.
Hlxb9 interacts with menin. (A) GST pull-down assay. WCE from MIN6 cells transfected with mychis-Vector (mh-Vector) or with mychis-Hlxb9 (mh-Hlxb9) plasmid (left panel) was incubated with GST or GST-menin beads. Bound Hlxb9 was detected by western blot. Input (1/20th amount of WCE incubated with beads) was analyzed side-by-side. WCEs of cells transfected with flag-Vector (f-Vector) or flag-Menin (f-Menin) plasmid (right panel) were incubated with GST or GST-Hlxb9 beads. Bound menin was detected by western blot. Myc-Hlxb9 and endo-Hlxb9 indicate transfected mychis-Hlxb9 protein and endogenous-Hlxb9 respectively. F-Menin and endo-Menin indicate transfected flag-menin protein and endogenous menin respectively. (B) Co-immunoprecipitation assay. WCE immunoprecipitated with IgG or anti-Hlxb9 followed by western blot with anti-menin. Input lane shows 1/20th amount of WCE used for immuno-precipitation. (C) Menin:Hlxb9 interaction regions. Top panel shows a schematic diagram of full-length menin (aa 1–610) and menin deletion constructs containing the indicated amino acids from the N- or C-terminal region. Bottom panel shows a diagram of GST-fused full-length Hlxb9(aa 1–404) and GST-fused Hlxb9 constructs with the indicated internal deletions or containing the indicated amino acids from the N- or C-terminal region. In Hlxb9 (bottom panel), the left slanting hatched boxes indicate poly-alanine containing regions; right slanting hatched box indicates conserved domain, and crosshatched box indicates the homeodomain. The binding ability of menin constructs to GST-Hlxb9 or to GST-Hlxb9 deletions is represented by ‘+’ sign, ‘+’ is lowest binding, ‘+++++’ is highest binding. Western blot images from GST-Hlxb9 pull-down assays are shown in Supplementary Figure 3B and C. (D) Hlxb9 interaction with menin missense mutants. WCEs of MIN6 cells expressing mh-Hlxb9 together with f-Menin or indicated f-Menin missense mutants were assessedfor Hlxb9–menin interaction by immunoprecipitation with anti-Hlxb9 followed by western blot with anti-menin. Menin (normal and missense mutants) and Hlxb9 levels are shown in the western blots in the upper panels (input). P84 was used to assess protein loading. f-Menin and endo-Menin corresponds to transfected flag-Menin and endogenous menin respectively. (E) Hlxb9 interaction and expression. Data corresponding to Hlxb9 interaction with menin and Hlxb9 expression from the western blots in (D) are shown. The interaction ability of Hlxb9 is represented by ‘+’ sign, ‘+’ is lowest binding, ‘+++++’ is highest binding.
To determine whether the menin–Hlxb9 interaction could occur in other cell types, GST-Hlxb9 pull-down assays were performed using WCEs of HEK293 cells and MEFs (Supplementary Figure 3A, see section on supplementary data given at the end of this article). Similar to the observation in MIN6 cells, menin could bind to Hlxb9 efficiently in HEK293 cells; however, in MEFs, the binding was weak suggesting a cell-type specificity of menin–Hlxb9 interaction.
In interaction domain mapping assays, all four C-terminal truncations of menin interacted with Hlxb9, whereas N-terminal deletions of aa 1–177 or 1–323 showed lowest binding to Hlxb9 (Fig. 2C and Supplementary Figure 3B). Deletion of the N-terminal 40 aa of menin did not significantly change binding to Hlxb9. This indicated that the central region of menin closer to the N-terminus was critical for Hlxb9 binding. Disease-associated missense mutants of menin located in this central region were impaired for Hlxb9 interaction (Fig. 2D and E). Also, menin missense mutants that showed impaired Hlxb9 interaction did not reduce Hlxb9 protein level (Fig. 2D and E); thus, indicating that Hlxb9 protein level was regulated by menin interaction. Hlxb9 has four distinct domains (UniprotKB identification no., Q9QZW9): two poly-alanine regions (aa 121–134 and 168–174), central conserved domain (aa 168–241), and C-terminal homeodomain-containing region (aa 241–300). Deletion of the poly-alanine regions did not affect menin binding. However, C-terminal deletion of Hlxb9 (aa 241–404) substantially decreased binding to menin (Fig. 2C and Supplementary Figure 3C). This indicated that the Hlxb9 homeodomain region was crucial for menin interaction.
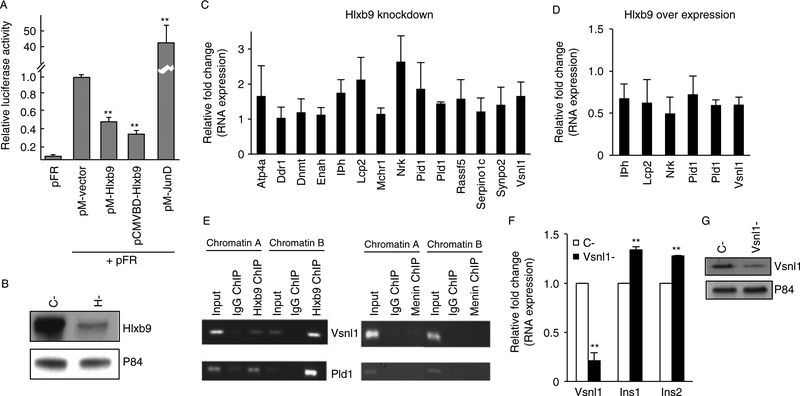
Hlxb9 target genes
Downstream target genes of Hlxb9 have not been discovered. Hlxb9 has been shown to act as a transcriptional repressor (William et al. 2003), and this was also observed in MIN6 cells (Fig. 3A) Therefore, identification of de-repressed genes would easily reveal Hlxb9-regulated genes. RNA samples from three independent transfections of MIN6 cells were used to assess the difference in gene expression upon control siRNA transfection and Hlxb9 siRNA transfection (Fig. 3B). The microarray data analysis showed that genes upregulated or downregulated upon Hlxb9 knockdown had relatively low fold changes (maximum 2.18-fold; Supplementary Table 3, see section on supplementary data given at the end of this article). Sixteen genes of most significance (P value ≤0.002) were selected for validation by QRT-PCR (12 upregulated genes and 4 downregulated genes). Eleven out of the 12 upregulated genes were subsequently validated (Palld did not yield a PCR product; Fig. 3C). Furthermore, QRTPCR analysis showed that genes that were upregulated upon Hlxb9 knockdown were downregulated upon Hlxb9 overexpression (Fig. 3D). Therefore, microarray analysis revealed several Hlxb9 target genes in β-cells (MIN6 cells).
Figure 3.
Hlxb9 target genes in MIN6 cells. (A) Hlxb9 represses transcription. Two different Gal4-DBD fused Hlxb9 constructs (pM-HB9 and pCMVDBDHB9) were assessed for transcriptional activity on a Gal4-UAS-luciferase reporter (pFR). Hlxb9 repressed transcription (luciferase expression) by1.54-fold (pM-HB9) or 2.78-fold (pCMVDBD-HB9). pM-JunD served as a positive control for activating transcription. **Significant effect (P<0.01).(B) Hlxb9 knockdown for gene expression microarray analysis. Three independent transient transfections were performed with control siRNA (C-) or Hlxb9 siRNA (H-). A representative western blot from one such transfection is shown. (C) Validation of Hlxb9 target genes identified by microarray analysis. RNA samples processed for microarray analysis (n=3) and one additional RNA sample of C- and H- were used for QRT-PCR. Of 16 target genes, Palld and Krt4 did not yield a PCR product. QRT-PCR data were plotted as fold change in expression over C- RNA samples (equal to 1). Eleven out of the 12 target genes were significantly upregulated (P<0.05).(D) Hlxb9 target genes upon Hlxb9 overexpression. RNA samples (n=3) from cells transfected with mychis-vector (mh-Vector) or mychis-Hlxb9 (mh-Hlxb9) were assessed for the expression of six target genes, which showed highest significant fold decrease in H-. Data were plotted as fold change in expression over mh-Vector-transfected RNA sample (equals to 1). All six target genes were significantly downregulated (P<0.05). (E) Hlxb9 occupies the promoters of Vsnl1 and Pld1. Primer pairs corresponding to evolutionarily conserved promoter regions of Vsnl1 and Pld1 were used for PCR from anti-Hlxb9 (left panel) or anti-menin (right panel) ChIPs. ChIP with mouse or rabbit IgG served as negative controls for Hlxb9 ChIP and menin ChIP respectively. Shown are ethidium bromide-stained agarose gels of the indicated PCR products from two different ChIP experiments (chromatin A and chromatin B). Input corresponds to 1/50th of chromatin that was used for ChIP. (F and G) Vsnl1 knockdown increases insulin mRNA in MIN6 cells. RNA samples from control siRNA (C-) or Vsnl1 siRNA (Vsnl1-) transfected cells (n=3) were analyzed by QRT-PCR using primer pairs specific for Vsnl1, insulin 1 (Ins1), and insulin 2 (Ins2). Data are shown as fold change in expression compared to control siRNA transfectedcells (equal to 1), **P<0.01. Vsnl1 knockdown was confirmed by western blot (G).
Hlxb9 occupies the promoter of genes associated with the regulation of insulin level
Among the Hlxb9 target genes identified, there were two genes that have been shown to affect cellular insulin level: Vsnl1 or Vilip1 (Dai et al. 2006, Fu et al. 2009) and Pld1 (Hughes et al. 2004, Ma et al. 2010). ChIP assays revealed that Hlxb9 occupied the Vsnl1 and Pld1 promoter; menin did not co-occupy these regions (Fig. 3E). Therefore, Vsnl1 and Pld1 were direct targets of Hlxb9 in β-cells. Vsnl1 knockdown caused a low but consistently observed increase in insulin mRNA (Fig. 3F and G). These data suggest a novel mechanism of increased insulin by Hlxb9 upregulation, from genes repressed by Hlxb9 (which are negative regulators of insulin).
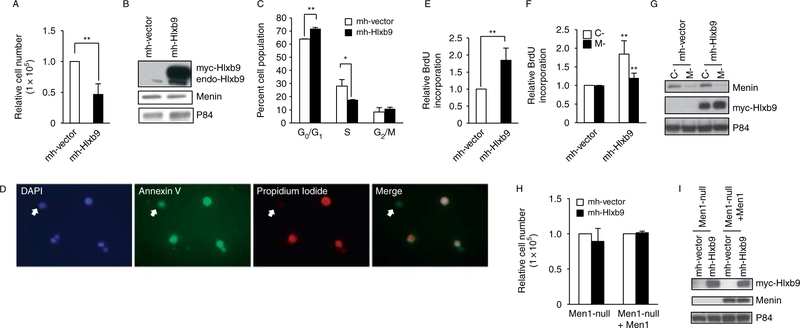
Hlxb9 overexpression reduces cell proliferation and causes apoptosis
Hlxb9 has been shown to cause apoptosis in Drosophila pioneer neuron cells (Miguel-Aliaga et al. 2008). However, the role of Hlxb9 on β-cell growth and proliferation is not known. Therefore, the consequence of Hlxb9 overexpression was examined in β-cells (MIN6 cells). Overexpression of Hlxb9 caused a 50% decrease in cell number (Fig. 4A and B), with slightly more cells (1.12-fold) in G0/G1 phase and significantly less cells (0.63-fold) in S phase (Fig. 4C). The reduced cell number observed upon Hlxb9 overexpression was due to increased apoptosis as shown by Annexin V–FITC and propidium iodide–rhodamine staining of Hlxb9 overexpressing cells (Fig. 4D). Moreover, apoptosis detection assay (DNA fragmentation) showed that Hlxb9 overexpression resulted in a 1.85-fold increase in DNA fragmentation (Fig. 4E). These data showed that in the presence of endogenous menin, Hlxb9 overexpression could cause β-cell apoptosis. In experiments where combined menin knockdown and Hlxb9 overexpression was performed, menin knockdown could partially rescue the apoptosis from Hlxb9 overexpression (Fig. 4F and G). Hlxb9 overexpression together with or without menin in Men1-null MEFs did not decrease cell number and did not even cause apoptosis (Fig. 4H and I). This could be due to a weak menin–Hlxb9 interaction observed in MEFs (Supplementary Figure 3A) compared with MIN6 or HEK293 cells, suggesting a possible cell-type-specific activity of Hlxb9.
Figure 4.
Hlxb9 induces apoptosis in MIN6 cells. (A and B) Cell proliferation assay. Equal number of cells transfected with mychis-vector (mh-Vector) or mychis-Hlxb9 (mh-Hlxb9) plasmids were analyzed 96 h post-transfection, and cell number was assessed by counting. Data are shown as fold change in cell number over mh-Vector transfected cells (equal to 1). **P<0.01. Hlxb9 overexpression was confirmed by western blot (B). (C) Flow cytometry. Cells from ‘A’ were assessed for distribution of cell cycle phases after propidium iodide staining. Percentage of cells in G0/G1, S, and G2/M phases are shown. **P<0.01 and *P<0.05. (D) Annexin V/propidium iodide (PI) staining. Cells from ‘A’ were stained with annexin V and PI and mounted in DAPI-containing anti-fade mounting medium. Arrows show a cell with early apoptosis (positive for green annexin V staining but negative for red PI staining). The other three cells in the images show late apoptosis (positive for both annexin V and PI staining). Hlxb9 co-expression could not be assessed in the same preparation (it would require another color).(E) DNA fragmentation assay. Cells transfected as in ‘A’ were treated with BrdU for 6 h, and DNA fragments in the cytoplasmic lysate was assessed by ELISA. Data are shown as fold change of BrdU incorporation over mh-Vector transfected cells (equal to 1). **P<0.01. (F and G) Apoptosis assay in cells with combined menin knockdown and Hlxb9 overexpression. MIN6 cells transfected with mh-Vector or mh-Hlxb9 together with control (C-) or Menin shRNA (M-) were assessed for apoptosis by DNA fragmentation assays as in (E). Western blots show the expression of menin and Hlxb9(G). Anti-P84 was used to assess protein loading. (H and I) Cell proliferation assay in MEFs. Equal number of Men1−/−–MEFs (Men1-null) or menin reconstituted Men1−/−–MEFs (Men1-nullCMen1) were transfected with mh-Vector or with mh-Hlxb9 plasmids. Cell number was assessed 72 h post-transfection by counting. Data are shown as fold change in cell number over mh-Vector transfected cells (equal to 1). Western blot shows Hlxb9 overexpression (I).
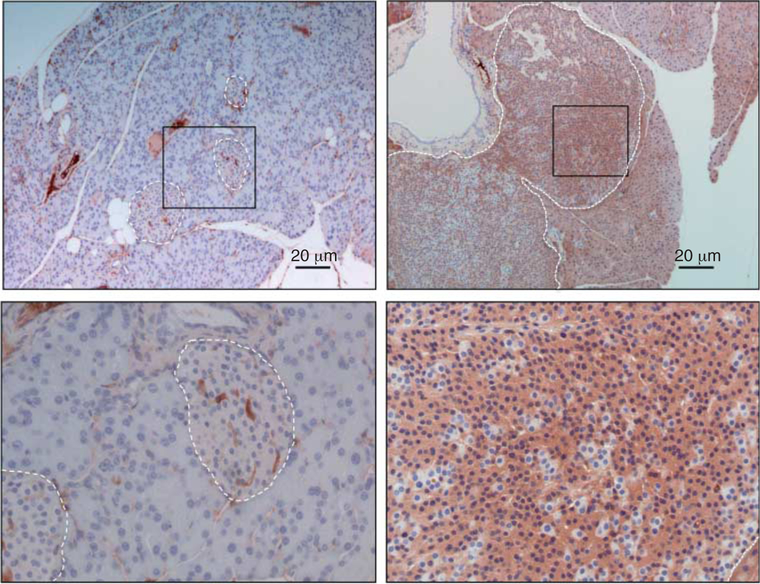
Hlxb9 expression is increased in mouse islet tumor
To extend our findings from MIN6 cells into pancreatic islet tumors upon menin loss, pancreas sections from Men1+/− mice (Wang et al. 2011) were used to detect Hlxb9 by immunohistochemistry (IHC). Hlxb9 staining was higher in islet tumors compared with wild-type islets (Fig. 5). Serial sections from the same tumor were previously analyzed for loss of menin expression and increased insulin (Wang et al. 2011). This observation demonstrates that menin loss in islet tumor coincides with increased Hlxb9 level.
Figure 5.
Expression of Hlxb9 in normal islets or islet tumor from Men1+/− mice. Pancreas sections from 18-month-old Men1+/− mice were assessed for Hlxb9 expression by immunohistochemistry staining with anti-Hlxb9. White dashed line indicates the outline of pancreatic islet. Lower panel is the high-power magnification of the boxed area from the upper panel. Brown color indicates positive Hlxb9 staining (brown in nucleus and cytoplasm). Islet tumor (upper right and lower right panel) shows increased Hlxb9 staining compared with normal islet (upper left and lower left panel). Blue staining in nuclei is from the hematoxylin counterstain. Scale bar is shown.
Discussion
We have investigated the molecular basis for the tissue-specific tumorigenesis from menin loss, particularly for the pathogenesis of tumors of the pancreatic islet β-cells (insulinoma). We propose a possible mechanism of how dysregulation of an embryonic differentiation factor, the homeobox transcription factor Hlxb9, can account for the increased insulin level and probably for the increased cell number found in β-cell tumors upon menin loss.
The β-cell differentiation factor Hlxb9 is downstream of menin
Among the 12 different β-cell differentiation factors examined in MIN6 cells (Hlxb9, Hnf3β, Isl1, MafA, MafB, NeuroD1, Ngn3, Nkx2.2, Nkx6.1, Pax4, Pax6, and Pdx1), the expression of Hlxb9 protein decreased upon menin overexpression, and conversely, Hlxb9 protein level increased upon menin knockdown. Interestingly, Hlxb9 RNA was previously observed as moderately upregulated in the hyperplastic islets of the mouse model of MEN1 (Scacheri et al. 2006). In MIN6 cells, we did not observe any change in the amount of Hlxb9 RNA from variation in menin level, indicating that menin-mediated regulation of Hlxb9 protein was also posttranscriptional.
Among several assays performed to study various posttranscriptional regulatory mechanisms, we found that menin could interact with the Hlxb9 C-terminal homeodomain region. Homeodomain is important for DNA binding, which is known to facilitate protein stability. Menin loss would increase the efficiency of Hlxb9 DNA-binding perhaps resulting in stabilization and accumulation of Hlxb9, a possibility that requires further investigation. An interesting feature of menin’s crystal structure is the large central cavity spanning aa 78–381 that is highly conserved between Neomatostella and human/mouse menin, a predicted binding site for interacting proteins (Murai et al. 2011, Huang et al. 2012). Our domain-mapping results suggest that Hlxb9 binding also occurs within this large cavity.
Although many menin-interacting proteins have been discovered, several of which are transcriptional regulators (Balogh et al. 2010), no such interacting proteins have been reported for Hlxb9. Our finding of Hlxb9 interaction with menin not only adds another important interaction partner for menin but also reveals an interaction of menin with a tissue-specific factor in β-cells, thus explaining tissue specificity of tumors upon menin loss in mice and in humans. A study investigating the role of the α-cell factor MafB in β-cell proliferation (Lu et al. 2011) and our study demonstrate the importance of islet differentiation factors as promising candidates involved in the pathogenesis of β-cell tumors, although MafB expression was not affected by menin in MIN6 cells (current data). However, the role of Hlxb9–menin interaction in the context of other menin partners or other β-cell-specific factors remains to be determined.
Some Hlxb9 target genes regulate cellular insulin content and insulin secretion
Hlxb9 is a homeobox transcription factor, but its consensus DNA-binding sequence and its target genes are unknown. Gal4DBD-fused Hlxb9 has been shown to repress transcription in motor neuron cells (William et al. 2003). Our data show that Gal4DBD-Hlxb9 also repressed transcription in β-cells. Among the Hlxb9 target genes in MIN6 cells, we found two genes that are associated with insulin expression and/or secretion, Vsnl1 and Pld1. Hlxb9 occupied the promoter region of both Vsnl1 and Pld1, indicating direct transcriptional repression by Hlxb9. Menin loss would predict increased binding of Hlxb9 to these promoters, thereby increasing the repression of the negative regulators of insulin. Vsnl1 is a member of the neuronal Ca2+ sensor protein family, and its expression was found to be downregulated in several different types of human cancers (Braunewell & Klein-Szanto 2009). Our observation of a similar downregulation of Vsnl1 expression by increased Hlxb9 suggests that direct dysregulation of Hlxb9 or dysregulation of Hlxb9 from menin loss could be tumorigenic. Vsnl1 knockdown caused increased insulin mRNA (Fig. 3F; Dai et al. 2006) and significantly enhanced glucose-stimulated insulin secretion (Dai et al. 2006). The ubiquitously expressed enzyme Pld1 is essential for regulated insulin secretion from β-cells, through the mTOR pathway (Hughes et al. 2004, Ma et al. 2010). Blockade of Pld1 activity inhibited insulin secretion in both MIN6 cells and in isolated pancreatic islets (Hughes et al. 2004). These observations identify a new role for Hlxb9 in β-cells from its target genes in the regulation of cellular insulin content and insulin secretion.
Role of Hlxb9 in β-cell proliferation and tumorigenesis
Apoptosis from Hlxb9 has been reported in Drosophila motor neurons (Miguel-Aliaga et al. 2008). Similarly, transient overexpression of Hlxb9 in MIN6 cells blocked the increase in cell number over time due to a concomitant increase in apoptosis. Furthermore, Hlxb9 overexpression when coupled with transient menin knockdown could partially rescue the apoptosis caused by Hlxb9 overexpression. However, unlike in MIN6 cells, in Men1-null MEFs (the only menin-null cell line currently available) or in menin reconstituted Men1-null MEFs, Hlxb9 overexpression did not affect cell number and did not even induce apoptosis, although this could also be due to the inability of menin and Hlxb9 to interact in MEFs compared to MIN6 cells and HEK293 cells. This result suggested a tissue/cell-type-specific physiological interaction of Hlxb9 and menin in pancreatic β-cells. Our observation of apoptosis from Hlxb9 overexpression is another important new role of Hlxb9 in β-cells. Evasion of apoptosis is a hallmark of cancer. Hlxb9 dysregulation and blockade of its pro-apoptotic properties could result in pathological consequences in mature β-cells. These observations need further analysis to test the possibility of the tumorigenic aspects of Hlxb9.
Although in humans menin loss is associated with tumors of other islet cell types, such as the glucagon-secreting α-cells (Thakker 2010), in mouse models of α-cell-specific loss of menin, insulinomas are observed (Shen et al. 2010). Also, in the Pdx1-Cre-Men1 mouse model where menin loss occurs in the entire pancreas, tumorigenesis is restricted to the β-cells (Shen et al. 2009). Menin’s interaction with a β-cell-specific factor such as Hlxb9 could account for such cell-type selective tumorigenesis in the pancreas of these mice. Hlxb9 expression has not been observed in the islet α-cells or in the exocrine pancreas (Harrison et al. 1999). Transient menin knockdown in an islet α-cell line (alpha-TC1), or in a pancreatic exocrine cell line (266–6), did not induce Hlxb9 expression (data not shown). Further investigations with Hlxb9 in Men1 mouse models will be informative to determine the contribution of Hlxb9 in the tumorigenesis of the different cells types in the pancreas.
Ectopic expression of Hlxb9 from chromosomal translocations in infant acute myeloid leukemia (AML) suggests an oncogenic role of Hlxb9 in leukemogenesis (Nagel et al. 2005, Wildenhain et al. 2010). Given menin’s pro-oncogenic role in acute myeloid and lymphoid leukemia from the mixed lineage leukemia (MLL) gene-associated leukemogenesis (Yokoyama et al. 2005, Smith et al. 2011), it will be interesting to find out what role, if any, the Hlxb9–menin interaction plays in MLL-positive childhood leukemia.
Hlxb9–menin interaction provides insight into the tissue-specific effects in the pancreas upon menin loss. Our study reveals that the β-cell differentiation factor Hlxb9 is important for modulating insulin levels, and for β-cell proliferation, thus elucidating a possible molecular mechanism of β-cell tumorigenesis through Hlxb9 dysregulation. Although we have not directly shown that dysregulated Hlxb9 is tumorigenic, investigation of mechanisms that inactivate the pro-apoptotic function of Hlxb9 (such as menin loss), and their contribution to the pathogenesis of β-cell tumors, will be of interest.
Supplementary Material
Acknowledgements
The monoclonal antibodies against Hlxb9, Isl1, Nkx2.2, Nkx6.1, and Ngn3 developed by Thomas M Jessell and Susan Brenner-Morton and by Ole D Madsen were obtained from the Developmental Studies Hybridoma Bank developed under the auspices of the NICHD and maintained by The University of Iowa, Department of Biology, Iowa City, IA 52242. The authors thank Dr Weiping Chen (NIDDK microarray core facility) for microarray bioinformatics support, Dr Philip McCoy (NHLBI flow cytometry core facility) for cell cycle analysis, Dr Stephen Marx (NIDDK), Drs Gregory Germino and Tanchun Wang (NIDDK) for use of their microscopes, Dr Xianxin Hua (University of Pennsylvania) for Men1−/− and menin-reconstituted Men1−/− MEFs, Dr Yan Wang (NIDDK) for pancreas sections of Men1+/− mice, and Dr Yen-Sin Ang (Mount Sinai School of Medicine) for the menin shRNA construct. The authors are grateful to Dr Xuefeng Yuan (University of Maryland) for helping with figures and statistics.
Funding
This research was funded by the Division of Intramural Research of the National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, Maryland, USA.
Footnotes
Supplementary data
This is linked to the online version of the paper at http://dx.doi.org/10.1530/ERC-12-0077.
Declaration on interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
References
- Agarwal SK, Guru SC, Heppner C, Erdos MR, Collins RM, Park SY, Saggar S,Chandrasekharappa SC, Collins FS, Spiegel AM et al. 1999. Menin interacts with the AP1 transcription factor JunD and represses JunD-activated transcription. Cell 96 143–152. (doi:10.1016/S0092-8674(00)80967-8) [DOI] [PubMed] [Google Scholar]
- Ang YS, Tsai SY, Lee DF, Monk J, Su J, Ratnakumar K, Ding J, Ge Y, Darr H, Chang B et al. 2011. Wdr5 mediates self-renewal and reprogramming via the embryonic stem cell core transcriptional network. Cell 145 183–197. (doi:10.1016/j.cell.2011.03.003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balasubramanian D & Scacheri PC 2009. Functional studies of menin through genetic manipulation of the Men1 homolog in mice. Advances in Experimental Medicine and Biology 668 105–115. (doi:10.1002/gene.1072) [DOI] [PubMed] [Google Scholar]
- Balogh K, Patocs A, Hunyady L & Racz K 2010. Menin dynamics and functional insight: take your partners. Molecular and Cellular Endocrinology 326 80–84. (doi:10.1016/j.mce.2010.04.011) [DOI] [PubMed] [Google Scholar]
- Braunewell KH & Klein-Szanto AJ 2009. Visinin-like proteins (VSNLs): interaction partners and emerging functions in signal transduction of a subfamily of neuronal Ca2+-sensor proteins. Cell and Tissue Research 335 301–316. (doi:10.1007/s00441-008-0716-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briegel KJ 2006. Embryonic transcription factors in human breast cancer.IUBMB Life 58 123–132. (doi:10.1080/15216540600686870) [DOI] [PubMed] [Google Scholar]
- Canaff L, Vanbellinghen JF, Kanazawa I, Kwak H, Garfield N, Vautour L & Hendy GN 2012. Menin missense mutants encoded by the MEN1 gene that are targeted to the proteasome: restoration of expression and activity by CHIP siRNA. Journal of Clinical Endocrinology and Metabolism 97 E282–E291. (doi:10.1210/jc.2011-0241) [DOI] [PubMed] [Google Scholar]
- Dai FF, Zhang Y, Kang Y, Wang Q, Gaisano HY, Braunewell KH, Chan CB & Wheeler MB 2006. The neuronal Ca2+ sensor protein visinin-like protein-1 is expressed in pancreatic islets and regulates insulin secretion. Journal of Biological Chemistry 281 21942–21953. (doi:10.1074/jbc.M512924200) [DOI] [PubMed] [Google Scholar]
- Fu J, Zhang J, Jin F, Patchefsky J, Braunewell KH & Klein-Szanto AJ 2009. Promoter regulation of the visinin-like subfamily of neuronal calcium sensor proteins by nuclear respiratory factor-1. Journal of Biological Chemistry 284 27577–27586. (doi:10.1074/jbc.M109.049361) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garin I, Martinez R, de las Heras J, Perez-Nanclares G, Castano L & Perez deNanclares G 2009. Mutations in MAFA and IAPP are not a common cause of monogenic diabetes. Diabetic Medicine 26 746–748. (doi:10.1111/j.1464-5491.2009.02758.x) [DOI] [PubMed] [Google Scholar]
- Guo RJ, Suh ER & Lynch JP 2004. The role of Cdx proteins in intestinal development and cancer. Cancer Biology & Therapy 3 593–601. (doi:10.4161/cbt.3.7.913) [DOI] [PubMed] [Google Scholar]
- Harrison KA, Thaler J, Pfaff SL, Gu H & Kehrl JH 1999. Pancreas dorsal lobe agenesis and abnormal islets of Langerhans in Hlxb9-deficient mice. Nature Genetics 23 71–75. (doi:10.1038/12674) [DOI] [PubMed] [Google Scholar]
- Huang J, Gurung B, Wan B, Matkar S, Veniaminova NA, Wan K, Merchant JL, Hua X & Lei M 2012. The same pocket in menin binds both MLL and JUND but has opposite effects on transcription. Nature 482 542–546. (doi:10.1038/nature10806) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes WE, Elgundi Z, Huang P, Frohman MA & Biden TJ 2004. Phospholipase D1 regulates secretagogue-stimulated insulin release in pancreatic β-cells. Journal of Biological Chemistry 279 27534–27541.(doi:10.1074/jbc.M403012200) [DOI] [PubMed] [Google Scholar]
- Jensen RT, Berna MJ, Bingham DB & Norton JA 2008. Inherited pancreatic endocrine tumor syndromes: advances in molecular pathogenesis, diagnosis, management, and controversies. Cancer 113 1807–1843. (doi:10.1002/cncr.23648) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiao Y, Shi C, Edil BH, de Wilde RF, Klimstra DS, Maitra A, Schulick RD, Tang LH, Wolfgang CL, Choti MA et al. 2011. DAXX/ATRX, MEN1, and mTOR pathway genes are frequently altered in pancreatic neuroendocrine tumors. Science 331 1199–1203. (doi:10.1126/science.1200609) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura S 2011. Thyroid-specific transcription factors and their roles in thyroid cancer. Journal of Thyroid Research 2011 710213. (doi:10.4061/2011/710213) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knapp JI, Heppner C, Hickman AB, Burns AL, Chandrasekharappa SC,Collins FS, Marx SJ, Spiegel AM & Agarwal SK 2000. Identification and characterization of JunD missense mutants that lack menin binding. Oncogene 19 4706–4712. (doi:10.1038/sj.onc.1203832) [DOI] [PubMed] [Google Scholar]
- Li H & Edlund H 2001. Persistent expression of Hlxb9 in the pancreatic epithelium impairs pancreatic development. Developmental Biology 240 247–253. (doi:10.1006/dbio.2001.0440) [DOI] [PubMed] [Google Scholar]
- Li H, Arber S, Jessell TM & Edlund H 1999. Selective agenesis of the dorsal pancreas in mice lacking homeobox gene Hlxb9. Nature Genetics 23 67–70. (doi:10.1038/12669) [DOI] [PubMed] [Google Scholar]
- Lu J, Herrera PL, Carreira C, Bonnavion R, Seigne C, Calender A, Bertolino P & Zhang CX 2010. α Cell-specific Men1 ablation triggers the transdifferentiation of glucagon-expressing cells and insulinoma development. Gastroenterology 138 1954–1965. (doi:10.1053/j.gastro.2010.01.046) [DOI] [PubMed] [Google Scholar]
- Lu J, Hamze Z, Bonnavion R, Herath N, Pouponnot C, Assade F, Fontaniere S, Bertolino P, Cordier-Bussat M & Zhang CX 2012. Reexpression of oncoprotein MafB in proliferative β-cells and Men1 insulinomas in mouse. Oncogene 31 3647–3654. (doi:10.1038/onc.2011.538) [DOI] [PubMed] [Google Scholar]
- Ma WN, Park SY & Han JS 2010. Role of phospholipase D1 in glucose-induced insulin secretion in pancreatic b cells. Experimental & Molecular Medicine 42 456–464. (doi:10.3858/emm.2010.42.6.047) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melloul D 2004. Transcription factors in islet development and physiology: role of PDX-1 in β-cell function. Annals of the New York Academy of Sciences 1014 28–37. (doi:10.1196/annals.1294.003) [DOI] [PubMed] [Google Scholar]
- Miguel-Aliaga I, Thor S & Gould AP 2008. Postmitotic specification of Drosophila insulinergic neurons from pioneer neurons. PLoS Biology 6 e58. (doi:10.1371/journal.pbio.0060058) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazaki J, Araki K, Yamato E, Ikegami H, Asano T, Shibasaki Y, Oka Y & Yamamura K 1990. Establishment of a pancreatic β cell line that retains glucose-inducible insulin secretion: special reference to expression of glucose transporter isoforms. Endocrinology 127 126–132. (doi:10.1210/endo-127-1-126) [DOI] [PubMed] [Google Scholar]
- Murai MJ, Chruszcz M, Reddy G, Grembecka J & Cierpicki T 2011. Crystal structure of menin reveals binding site for mixed lineage leukemia (MLL) protein. Journal of Biological Chemistry 286 31742–31748. (doi:10.1074/jbc.M111.258186) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagel S, Kaufmann M, Scherr M, Drexler HG & MacLeod RA 2005. Activation of HLXB9 by juxtaposition with MYB via formation of t(6;7)(q23;q36) in an AML-M4 cell line (GDM-1). Genes, Chromosomes & Cancer 42 170–178. (doi:10.1002/gcc.20113) [DOI] [PubMed] [Google Scholar]
- Naylor R & Philipson LH 2011. Who should have genetic testing for maturity-onset diabetes of the young? Clinical Endocrinology 75 422–426. (doi:10.1111/j.1365-2265.2011.04049.x) [DOI] [PubMed] [Google Scholar]
- Ross AJ, Ruiz-Perez V, Wang Y, Hagan DM, Scherer S, Lynch SA, Lindsay S, Custard E, Belloni E, Wilson DI et al. 1998. A homeobox gene, HLXB9, is the major locus for dominantly inherited sacral agenesis. Nature Genetics 20 358–361. (doi:10.1038/3828) [DOI] [PubMed] [Google Scholar]
- Scacheri PC, Davis S, Odom DT, Crawford GE, Perkins S, Halawi MJ, Agarwal SK, Marx SJ, Spiegel AM, Meltzer PS et al. 2006. Genome-wide analysis of menin binding provides insights into MEN1 tumorigenesis. PLoS Genetics 2 e51. (doi:10.1371/journal.pgen.0020051) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnepp RW, Mao H, Sykes SM, Zong WX, Silva A, La P & Hua X 2004. Menin induces apoptosis in murine embryonic fibroblasts. Journal of Biological Chemistry 279 10685–10691. (doi:10.1074/jbc.M308073200) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen HC, He M, Powell A, Adem A, Lorang D, Heller C, Grover AC, Ylaya K, Hewitt SM, Marx SJ et al. 2009. Recapitulation of pancreatic neuroendocrine tumors in human multiple endocrine neoplasia type I syndrome via Pdx1-directed inactivation of Men1. Cancer Research 69 1858–1866. (doi:10.1158/0008-5472.CAN-08-3662) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen HC, Ylaya K, Pechhold K, Wilson A, Adem A, Hewitt SM & Libutti SK 2010. Multiple endocrine neoplasia type 1 deletion in pancreatic α-cells leads to development of insulinomas in mice. Endocrinology 151 4024–4030. (doi:10.1210/en.2009-1251) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherr CJ 2004. Principles of tumor suppression. Cell 116 235–246.(doi:10.1016/S0092-8674(03)01075-4) [DOI] [PubMed] [Google Scholar]
- Smith E, Lin C & Shilatifard A 2011. The super elongation complex (SEC) and MLL in development and disease. Genes and Development 25 661–672. (doi:10.1101/gad.2015411) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thakker RV 2010. Multiple endocrine neoplasia type 1 (MEN1). Best Practice& Research. Clinical Endocrinology & Metabolism 24 355–370. (doi:10.1016/j.beem.2010.07.003) [DOI] [PubMed] [Google Scholar]
- Thaler J, Harrison K, Sharma K, Lettieri K, Kehrl J & Pfaff SL 1999. Active suppression of interneuron programs within developing motor neurons revealed by analysis of homeodomain factor HB9. Neuron 23 675–687. (doi:10.1016/S0896-6273(01)80027-1) [DOI] [PubMed] [Google Scholar]
- Wang Y, Ozawa A, Zaman S, Prasad NB, Chandrasekharappa SC, Agarwal SK & Marx SJ 2011. The tumor suppressor protein menin inhibits AKT activation by regulating its cellular localization. Cancer Research 71 371–382. (doi:10.1158/0008-5472.CAN-10-3221) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wildenhain S, Ruckert C, Röttgers S, Harbott J, Ludwig WD, Schuster FR, Beldjord K, Binder V, Slany R, Hauer J & Borkhardt A 2010. Expression of cell-cell interacting genes distinguishes HLXB9/TEL from MLL-positive childhood acute myeloid leukemia. Leukemia 24 1657–1660. (doi:10.1038/leu.2010.146) [DOI] [PubMed] [Google Scholar]
- William CM, Tanabe Y & Jessell TM 2003. Regulation of motor neuron subtype identity by repressor activity of Mnx class homeodomain proteins. Development 130 1523–1536. (doi:10.1242/dev.00358) [DOI] [PubMed] [Google Scholar]
- Yokoyama A, Somervaille TC, Smith KS, Rozenblatt-Rosen O, Meyerson M & Cleary ML 2005. The menin tumor suppressor protein is an essential oncogenic cofactor for MLL-associated leukemogenesis. Cell 123 207–218. (doi:10.1016/j.cell.2005.09.025) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.