ABSTRACT
Background
Meat intake is associated with increased risk of type 2 diabetes (T2D). It is not clear if egg intake is associated with T2D risk because purported associations may be due to concurrent consumption of eggs with meat.
Objective
Our aim was to differentiate any associations between meat and egg consumption and the risk of T2D.
Design
In this longitudinal study, 55,851 participants of the Adventist Health Study 2 who were free of diabetes provided demographic, anthropometric, and dietary data at baseline. Meat and egg intakes were assessed with a validated quantitative food-frequency questionnaire. Responses to 2 follow-up questionnaires determined incident T2D cases. Multivariate-adjusted logistic regression was used to determine relations between meat and egg intake and incident T2D.
Results
T2D cases identified during a mean 5.3 y of follow-up totaled 2772. Meat intake of >0 to <25 g/d, ≥25 to <70 g/d, and ≥70 g/d significantly increased the risk of T2D compared with no meat intake (OR: 1.29; 95% CI: 1.16, 1.44; OR: 1.42; 95% CI: 1.25, 1.61; and OR: 1.65; 95% CI: 1.39, 1.96, respectively; P-trend < 0.0001). Egg intake compared with no egg intake was not associated with T2D risk. A significant meat-egg interaction (P = 0.019) showed that within every category of egg intake, there was an incremental rise in T2D risk as meat intake increased. However, within categories of meat intake, increasing egg intake did not increase the risk of T2D except among nonmeat-eaters consuming ≥5 eggs/wk (OR: 1.52; 95% CI: 1.09, 2.12).
Conclusions
Meat consumption, but not egg consumption, is independently associated with T2D risk. Egg intake seems not to increase T2D risk further with meat intake. Our findings suggest that the purported egg-T2D risk relation in US populations may be biased due to failure to investigate egg-meat interactions. Further investigations are needed to ascertain T2D risk among nonmeat-eaters with high egg intakes.
Keywords: western diet, typical American diet, Adventist Health Study, vegetarian, cohort, Adventist
INTRODUCTION
It is important to identify modifiable risk factors for type 2 (T2D) diabetes due to the high prevalence (9.3% of US and 8.5% of international adults) (1) and comorbidities of the disease worldwide (2, 3). It is well established that obesity is a major driver of the diabetes epidemic (4–6), and meat intake, particularly red and processed meat (7), has been associated with increased risk of type 2 diabetes (7–10). Eggs have also been suggested as a possible risk factor (11). At present, the evidence of the effect of egg intake on T2D remains limited and inconclusive, and it is not clear if egg consumption has different effects when eaten with or without meat, or when consumed by overweight compared with normal-weight individuals. Investigations on the relation between egg intake and incident T2D risk in prospective studies reported either a null association (11–17), a reduced risk (18), or an increased risk (19, 20). A meta-analysis of prospective studies done in the United States indicated that greater egg consumption increases T2D risk (21). In systematic reviews of prospective cohort studies from various geographic locations, overall findings show either modest or null associations (12, 22–24); however, association with increased T2D risk is evident in US populations in all these meta-analyses.
Given the observed positive relation between egg consumption and T2D risk in US populations, the established role of meat intake in diabetes risk, and concomitant meat and egg consumption as a US dietary practice, the objective of this study was to disentangle the relations between these variables, and to examine the independent association between egg consumption and the risk of T2D. The Adventist Health Study-2 (AHS-2) cohort presents a wide range of egg and meat intakes, with substantial proportions that do not consume either or both foods, as well as diverse combinations of variability in egg and meat intake across individuals. Thus, this study population offers an ideal opportunity to investigate the effects of egg consumption on T2D, independent of the effect of meats and other foods that often accompany eggs in the American diet.
METHODS
Study population
The AHS-2 is a prospective cohort of >96,000 Seventh-Day Adventist adults recruited from the United States and Canada between February 2002 and May 2007. Designed primarily to examine associations between diet and health outcomes, the study is composed of ∼65% women and ∼27% blacks. Recruitment and data collection methods for the parent cohort have been described previously (25). The cohort is composed of participants with different dietary patterns and a wide variation in egg and meat intake, ranging from nonconsumption to daily consumption (26). Approval for the AHS-2 study was obtained from the Loma Linda University Human Subjects Committee Institutional Review Board, and written informed consent was acquired from all AHS-2 participants upon enrollment.
From the initial 96,203 AHS-2 participants, 65,353 completed the Hospital History Questionnaire (HHQ) version 3 or 5. We excluded those with missing gender, age <30 y, improbable questionnaire response patterns (e.g., identical responses to all questions on a page), or extremes of BMI (kg/m2; <16 or >60) or estimated energy intake (<500 or >4500 kcal/d) (n = 4477); existing cases of type 1 or type 2 diabetes at baseline (n = 4758); and inconsistent reports of T2D between HHQ version 3 and 5 (n = 267). Our analytic sample size was 55,851.
Incident type 2 diabetes: follow-up and case ascertainment
Every 2 y, a brief follow-up HHQ is mailed to participants. In HHQ versions 3 and 5, participants were asked about the first time they were diagnosed with T2D (among other conditions). Approximately 68% of participants returned the HHQ version 3 (diagnoses from 2002 to 2008) and 49% returned HHQ version 5 (diagnoses through year 2013); 45% of the study population returned both HHQ3 and HHQ5. HHQ3 provides 3 options for year of diagnosis, along with a question pertaining to treatment of the indicated condition in the last 12 mo (“No” or “Yes”), and HHQ5 offers 8 options for year of diagnosis, as well as a question regarding prescriptions for the indicated condition.
Incident cases of T2D were identified based on consistency of information given on both HHQ3 and HHQ5 for those who returned both HHQs, or information given in 1 of the HHQs if only 1 HHQ was returned. Those with discrepant responses in the 2 HHQs were excluded. Self-reported diabetes had been previously validated where diagnosis of T2D was confirmed by medical record review in 98.4% of cases (27); a similar method has been used in other cohort studies (28).
Dietary data and covariates
Usual dietary intake during the previous year was assessed at baseline by a self-administered quantitative food-frequency questionnaire (FFQ) of >200 food items (25). The AHS-2 FFQ had been validated for foods and nutrients (29, 30) against 6 repeated 24-h dietary recalls. Energy-adjusted validity correlations for eggs were 0.64 (95% CI: 0.52, 0.76) for nonblacks and 0.52 (95% CI: 0.36, 0.65) for blacks, and for all meats were 0.86 (95% CI: 0.82, 0.90) for nonblacks and 0.85 (95% CI: 0.79, 0.89) for blacks (29). Frequency of egg intake was categorized as 0 egg (i.e., never), 1–3 eggs/mo, 1–4 eggs/wk or ≥5 eggs/wk based on a single item in the FFQ for eggs (fried, boiled, scrambled, deviled, omelet, or egg salad, but not Egg-Beaters) including use in mixed dishes. Energy-adjusted (using the residual method) meat intake (beef, lamb, pork, chicken, or turkey, including use in mixed dishes) was categorized as never (0 g/d), low (>0 to <25 g/d), medium (≥25 to <70 g/d), or high (≥70 g/d).
Information on demographics, education, prevalent disease, smoking, and physical activity was also obtained from the baseline questionnaire. Participants were asked to report weight and height, and these values were used to calculate BMI. Self-reported anthropometrics have been previously validated in the AHS-2 cohort (31). BMIs were classified as normal/low (16–24.9), overweight (25–29.9), or obese (≥30). Race was self-identified and those reporting black/African-American, West Indian/Caribbean, African, or other black were considered as black, and all others as nonblack for this analysis.
Covariates, all measured at baseline and selected on an a priori basis as likely confounders based on prior studies and suspected relations, were as follows: age (continuous), gender (male or female), energy intake (continuous), moderate-to-vigorous physical activity (<20, 21–60, 61–150, or ≥151 min/wk), television hours (<1, 1–2, and >2 h/d), sleep hours (<7, 7, or >7 h/d), smoking (never or past/current), alcohol (never-drinker or past/current drinker), and energy-adjusted intake of refined grains, vegetables, coffee, dairy, soy beans, nuts/seeds, fruits, and fish (continuous in grams per day) (see footnote for Table 2). Subjects missing any values for dietary variables were excluded from the analytic sample, as their estimated energy intake was missing as a result.
TABLE 2.
Adjusted ORs for T2D according to egg and meat consumption and BMI1
| OR (95% CI) | |||
|---|---|---|---|
| Unadjusted2 | Adjusted3 | Fully adjusted4 | |
| BMI | |||
| Normal | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Overweight | 2.71 (2.42, 3.05) | 2.55 (2.26, 2.88) | 2.42 (2.14, 2.73) |
| Obese | 7.68 (6.87, 8.59) | 6.82 (6.06, 7.69) | 6.16 (5.46, 6.96) |
| P-trend | <0.0001 | <0.0001 | <0.0001 |
| Meat intake, g/d | |||
| 0 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| >0 to <25 | 1.76 (1.60, 1.93) | 1.60 (1.45, 1.77) | 1.29 (1.16, 1.44) |
| ≥25 to <70 | 2.30 (2.07, 2.55) | 1.96 (1.75, 2.19) | 1.42 (1.25, 1.61) |
| ≥70 | 3.17 (2.74, 3.66) | 2.60 (2.23, 3.02) | 1.65 (1.39, 1.96) |
| P-trend | <0.0001 | <0.0001 | <0.0001 |
| Egg intake | |||
| 0 eggs | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| 1–3 eggs/mo | 1.42 (1.26, 1.59) | 1.30 (1.15, 1.46) | 0.98 (0.86, 1.11) |
| 1–4 eggs/wk | 1.71 (1.54, 1.89) | 1.48 (1.33, 1.65) | 0.96 (0.85, 1.11) |
| ≥5 eggs/wk | 2.49 (2.12, 2.93) | 1.98 (1.67, 2.35) | 1.06 (0.88, 1.28) |
| P-trend | <0.0001 | <0.0001 | 0.95 |
1ORs were computed using logistic regression; the 2-way interactions egg intake-BMI, egg-meat intake, and meat-BMI were tested. Marginal trend P-values were obtained from stratified analysis. T2D, type 2 diabetes.
2Adjusted for age, race, gender. n = 55,477 (cases = 2775).
3Additionally adjusted for energy intake, television hours, sleep hours, smoking, exercise. n = 52,718 (cases = 2594).
4Additionally adjusted for refined grains, vegetables, coffee, dairy, soy, nuts/seeds, fruits, and fish, with further adjustment for egg intake, meat intake, and BMI where applicable. BMI was also entered as cubic B-spline terms with 4 knots based on equal percentiles. n = 52,718 (cases = 2594).
Statistical analysis
Baseline means and percentages of demographic characteristics, lifestyle variables, and dietary intake were compared between diabetes cases and noncases and across categories of egg and meat consumption. To reduce the impact of dietary measurement error often associated with underreporting of dietary intake (32, 33), energy adjustment using the residual method was done for intake of meat and other foods. Logistic regression analyses were used to calculate ORs for risk of T2D according to BMIs, egg consumption, and meat consumption, separately. We also tested for a 2-way interaction between egg intake and BMI, egg and meat intake, and meat intake and BMI. We computed ORs for T2D for meat and egg intake across categories of BMI. Additionally, we computed ORs for different categories of egg intake across categories of meat consumption. Covariates were adjusted, first for age, gender, and race, then further for energy intake, television hours, sleep hours, smoking, and exercise. The fully adjusted model controlled for these aforementioned covariates plus dietary variables (refined grains, vegetables, coffee, dairy, soy, nuts/seeds, fruits, and fish) as well as all combinations of egg intake, meat intake, and BMI, depending on the main exposure variable under consideration. BMI was also included in the fully adjusted models as cubic B-spline terms with 4 knots based on equal percentiles unless when treated as categoric. All continuous dietary variables were untransformed, as transformations had no important effect on estimated regression coefficients of interest.
All analyses were conducted using SAS software version 9.4 (SAS Institute, Cary, NC). A P value of <0.05 was considered to indicate statistical significance, and all tests were 2-sided.
RESULTS
The analytic population of 55,851 was 35% male and had a mean ± SD age at enrollment of 57.8 ± 13.5 y. During a mean 5.3 y of follow-up, 2774 participants were diagnosed with T2D. Table 1 presents the baseline characteristics of the study cohort stratified by T2D status, meat intake, and frequency of egg consumption. Approximately 28% never consumed eggs, 67% consumed eggs occasionally or weekly, and 5% consumed eggs on a daily basis. Approximately 53% ate 0 g/d, 26% ate 0.1 to <25 g/d, 15% ate 25 to <70 g/d, and 5% consumed ≥70 g/d of meat. Diabetes cases were more likely to be older and have a higher BMI than noncases. Subjects with more-frequent egg consumption tended to have higher meat and total energy intake. Descriptive characteristics of participants according to BMI levels are displayed in Supplemental Table 1 (see Supplemental Materials).
TABLE 1.
Baseline characteristics of the participants and intake of select foods according to diabetes status, meat intake, and frequency of egg intake1
| Diabetes incidence | Meat intake, g/d | Egg intake | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Characteristics | Noncase | Case | 0 | >0 to <25 | ≥25 to <70 | ≥70 | 0 | 1–3 eggs/mo | 1–4 eggs/wk | ≥5 eggs/wk |
| n (%) | 53,077 (95.0) | 2774 (5.0) | 29,704 (53.2) | 14,750 (26.4) | 8530 (15.3) | 2867 (5.1) | 15,524 (27.8) | 14,292 (25.6) | 23,213 (41.6) | 2822 (5.0) |
| Age at baseline, y | 57.6 ± 13.6 | 60.5 ± 11.4 | 58.8 ± 13.9 | 58.9 ± 13.5 | 56.1 ± 12.8 | 53.4 ± 11.9 | 58.9 ± 13.6 | 57.4 ± 13.3 | 57.3 ± 13.6 | 57.5 ± 13.0 |
| Male sex, % | 35.2 | 35.6 | 36.4 | 33.2 | 34.8 | 35.8 | 35.2 | 32.9 | 36.3 | 38.1 |
| Race, % | ||||||||||
| Non-black | 83.8 | 78.9 | 89.7 | 83.0 | 76.8 | 71.6 | 81.4 | 81.1 | 86.0 | 88.2 |
| Black | 16.2 | 21.1 | 10.3 | 17.0 | 23.2 | 28.4 | 18.6 | 18.9 | 14.0 | 11.8 |
| Educational level, % | ||||||||||
| High school or less | 15.8 | 19.0 | 13.2 | 17.9 | 18.6 | 18.0 | 15.7 | 16.5 | 15.6 | 18.4 |
| Some college | 37.9 | 42.6 | 35.1 | 39.1 | 41.8 | 41.0 | 37.2 | 38.3 | 38.3 | 40.9 |
| College or higher | 46.3 | 38.4 | 51.7 | 43.0 | 39.6 | 41.0 | 47.2 | 45.2 | 46.1 | 40.7 |
| BMI, kg/m2 | 26.2 ± 5.2 | 31.0 ± 6.4 | 25.2 ± 4.8 | 26.6 ± 5.2 | 27.8 ± 5.6 | 28.9 ± 6.1 | 25.0 ± 4.84 | 26.6 ± 5.3 | 27.1 ± 5.4 | 28.5 ± 6.1 |
| Normal, % | 46.7 | 15.6 | 55.8 | 42.9 | 34.0 | 27.0 | 58.1 | 44.1 | 38.9 | 30.9 |
| Overweight, % | 34.7 | 34.0 | 30.9 | 36.6 | 38.4 | 38.8 | 29.0 | 35.9 | 37.6 | 35.0 |
| Obese, % | 18.6 | 50.4 | 13.3 | 20.5 | 27.7 | 34.2 | 12.9 | 20.0 | 23.5 | 34.1 |
| Exercise, % | ||||||||||
| <1/wk | 32.2 | 43.6 | 30.8 | 33.0 | 35.4 | 35.8 | 29.9 | 34.5 | 33.2 | 36.4 |
| 1–2/wk | 20.6 | 18.4 | 19.8 | 20.3 | 21.6 | 21.9 | 18.5 | 20.4 | 22.0 | 19.9 |
| ≥3/wk | 47.2 | 38.0 | 49.4 | 46.7 | 43.0 | 42.3 | 51.6 | 45.2 | 44.8 | 43.8 |
| Television viewing, % | ||||||||||
| <1 h/d | 29.6 | 17.0 | 39.2 | 25.0 | 18.7 | 15.4 | 39.3 | 27.4 | 24.1 | 21.6 |
| 1–2 h/d | 48.0 | 46.8 | 44.4 | 51.3 | 50.7 | 49.9 | 43.3 | 48.5 | 50.8 | 48.0 |
| ≥2 h/d | 22.3 | 36.2 | 16.4 | 23.7 | 30.6 | 34.7 | 17.4 | 24.2 | 25.2 | 30.5 |
| Sleep time, % | ||||||||||
| ≤6 h/d | 28.2 | 35.3 | 23.7 | 30.0 | 33.7 | 35.7 | 27.2 | 30.9 | 27.8 | 31.2 |
| 7 h/d | 38.8 | 35.0 | 40.3 | 38.2 | 37.0 | 35.5 | 38.4 | 37.9 | 39.6 | 35.1 |
| ≥8 h/d | 33.0 | 29.7 | 36.0 | 31.7 | 29.4 | 28.7 | 34.4 | 31.2 | 32.6 | 33.8 |
| Smoking history, % | ||||||||||
| Never | 83.3 | 78.2 | 88.2 | 82.9 | 76.9 | 72.9 | 84.9 | 83.0 | 82.6 | 76.3 |
| Current or former | 16.7 | 21.8 | 11.8 | 17.1 | 23.1 | 27.1 | 15.1 | 17.0 | 17.4 | 23.7 |
| Total energy, kcal/d | 1893.8 ± 723.8 | 1952.4 ± 787.2 | 1901.2 ± 700.9 | 1897.8 ± 734.4 | 1853.6 ± 745.5 | 1963.1 ± 775.5 | 1845.1 ± 719.0 | 1742.5 ± 697.7 | 1977.5 ± 710.1 | 2297.5 ± 811.3 |
| Egg intake,2 % | ||||||||||
| 0 eggs | 28.1 | 19.8 | 46.4 | 17.0 | 10.2 | 8.7 | — | — | — | — |
| 1–3 eggs/mo | 25.6 | 25.2 | 23.4 | 30.2 | 26.7 | 21.4 | — | — | — | — |
| 1–4 eggs/wk | 41.4 | 46.9 | 27.9 | 48.0 | 55.6 | 57.9 | — | — | — | — |
| ≥5 eggs/wk | 4.8 | 8.1 | 2.4 | 4.8 | 7.5 | 12.0 | — | — | — | — |
| Mean consumption of foods,3 g/d | ||||||||||
| Meat | 22.1 ± 34.1 | 33.6 ± 40.8 | 0.0 ± 0.0 | 12.5 ± 6.5 | 43.8 ± 12.9 | 103.9 ± 37.5 | 8.6 ± 23.4 | 21.6 ± 32.3 | 30.0 ± 36.3 | 44.9 ± 49.1 |
| Refined grains | 91.9 ± 72.0 | 99.0 ± 74.7 | 81.4 ± 64.4 | 92.9 ± 73.4 | 106.4 ± 78.3 | 108.5 ± 79.6 | 77.3 ± 70.1 | 95.2 ± 73.0 | 100.3 ± 71.8 | 93.0 ± 70.7 |
| Vegetables | 300.3 ± 172.7 | 291.2 ± 169.2 | 315.3 ± 175.6 | 299.1 ± 173.7 | 280.4 ± 162.2 | 274.4 ± 169.2 | 337.6 ± 198.7 | 294.0 ± 169.2 | 281.8 ± 150.6 | 271.2 ± 169.7 |
| Fruits | 309.3 ± 217.7 | 274.8 ± 213.1 | 340.6 ± 218.8 | 318.6 ± 224.6 | 267.9 ± 203.7 | 224.9 ± 184.9 | 381.8 ± 247.1 | 311.5 ± 218.3 | 265.6 ± 182.7 | 224.1 ± 182.2 |
| Coffee | 68.2 ± 175.3 | 74.7 ± 179.9 | 23.8 ± 100.4 | 72.1 ± 167.1 | 117.8 ± 219.2 | 153.4 ± 268.6 | 28.4 ± 121.2 | 64.0 ± 168.1 | 89.6 ± 193.0 | 138.2 ± 248.9 |
| Dairy | 132.5 ± 181.3 | 151.9 ± 187.6 | 85.8 ± 152.9 | 152.7 ± 193.1 | 186.7 ± 196.1 | 182.8 ± 183.9 | 54.4 ± 132.6 | 139.3 ± 190.0 | 175.6 ± 185.7 | 191.9 ± 193.6 |
| Soy | 17.0 ± 31.0 | 11.9 ± 25.8 | 23.8 ± 35.2 | 15.1 ± 29.5 | 9.1 ± 22.5 | 6.1 ± 18.4 | 27.5 ± 38.8 | 14.4 ± 28.5 | 11.9 ± 24.3 | 10.2 ± 25.0 |
| Nuts/seeds | 22.6 ± 19.2 | 19.8 ± 19.8 | 26.7 ± 20.0 | 21.9 ± 19.0 | 17.8 ± 17.0 | 15.0 ± 15.2 | 27.3 ± 21.4 | 21.9 ± 19.0 | 20.0 ± 17.1 | 19.0 ± 18.2 |
1Values are means ± SDs unless otherwise indicated. Race was self-reported.
2The standard portion of egg is 1 large egg.
3Consumption of eggs, meat, refined grains, vegetables, fruits, coffee, dairy, soy, and nuts/seeds were adjusted for total energy intake using the residual method.
The odds of T2D in relation to BMI levels and meat and egg intake categories are presented in Table 2. ORs were attenuated after adjustment for dietary and lifestyle covariates. In the fully adjusted model, the OR of developing T2D was 2.42 (95% CI: 2.14, 2.73) in overweight and 6.16 (95% CI: 5.46, 6.96) in obese compared with normal-weight participants. ORs incrementally increased across categories of meat intake (P-trend < 0.0001). For those consuming ≥70 g meat/d, the OR of T2D was 1.65 (95% CI: 1.39, 1.96) compared with nonmeat-consumers. The OR of T2D did not change across categories of egg intake, ranging from never to ≥5 eggs/wk (P-trend = 0.95) in the fully adjusted model. Because adjustment for various dietary factors as well as meat intake and BMI attenuated the associations between egg intake and T2D risk to nonsignificance, further analyses were done to determine which of the covariates were mainly responsible for the attenuation. From Model 2, dietary covariates and BMI were added and yielded ORs of 1.12, 1.18, and 1.37 for the 3 egg intake groups, respectively. BMI was removed and replaced with meat intake and the computed ORs for the 3 egg groups were then 1.11, 1.16, and 1.43, respectively. When adjustment was done only for BMI and meat intake but not for the other dietary variables, the ORs were 1.01, 1.01, and 1.12, respectively. These ORs were closer to the adjusted ORs in the fully adjusted model of Table 2, which indicates that both meat intake and BMI were mainly responsible for the attenuation of the egg intake-T2D risk associations.
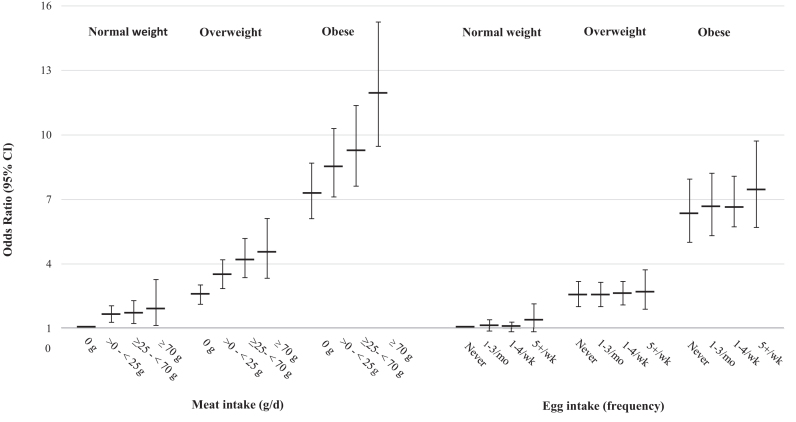
Figure 1 presents the multivariate odds of T2D at different levels of meat or egg consumption for each BMI category. At any level of BMI, there was an incremental rise in the risk of T2D for increasing categories of meat intake; however, no significant difference in the risk of T2D was observed across increasing categories of egg intake. The interaction between meat intake and BMI was not significant (P = 0.18), nor was that between egg intake and BMI (P = 0.98). It seems from these data that the risk of T2D attributable to obesity is far more important than that from meat or egg intake. A tabular presentation of Figure 1 with ORs (95% CIs) is available in the Online Supporting Material (Supplemental Table 2).
FIGURE 1.
ORs (95% CIs) of T2D by meat or egg intake levels at different BMI categories. ORs were estimated, relative to the reference category (normal BMI and 0 g/d for meat intake; normal BMI and “never” for egg intake), using logistic regression models including BMI, either meat or egg intake and its interaction with BMI, while adjusting for age, gender, energy, television hours, sleep hours, smoking, exercise, intake of refined grains, vegetables, soy, fruits, dairy, coffee, nuts/seeds, fish, and eggs or meat where applicable. Meat-BMI and egg-BMI interactions were not significant. These data are also available in tabulated form in Supplemental Table 2 of the Online Supporting Material. T2D, type 2 diabetes.
There was a significant interaction between meat and egg intake (P = 0.019), and we therefore estimated the ORs from a model that includes the interaction term. Table 3 presents the ORs for T2D across categories of concurrent egg and meat intake using no-meat-no-egg intake as the reference. Within every category of egg intake, there was an increment in ORs for T2D across increasing meat intake categories, and these trends were statistically significant, except for the category ≥5 eggs/wk. Contrariwise, within categories of meat intake, there were no significant increments on risk of T2D across increasing frequency of egg intake. However, among nonmeat-eaters, daily egg consumers had an OR of 1.52 (95% CI: 1.09, 2.12) compared with those who never consumed eggs.
TABLE 3.
Estimated risk of T2D [ORs (95% CI)] by combined egg and meat consumption1
| Egg intake | ||||||
|---|---|---|---|---|---|---|
| Meat intake, g/d | Cases/total2 | 0 eggs | 1–3 eggs/mo | 1–4 eggs/wk | ≥5 eggs/wk | P-trend3 |
| 0 | 1.00 (reference) | 1.04 (0.88, 1.24) | 1.05 (0.89, 1.23) | 1.52 (1.09, 2.12)* | 0.745 | |
| 517/14,619 | (349/12,039) | (84/1687) | (63/691) | (21/202) | ||
| >0 to <25 | 1.26 (0.98, 1.62) | 1.43 (1.20, 1.71)* | 1.37 (1.16, 1.61)* | 1.39 (1.02, 1.91)* | 0.438 | |
| 653/13,475 | (238/6856) | (251/4138) | (115/1906) | (49/575) | ||
| ≥25 to <70 | 2.12 (1.57, 2.87)* | 1.33 (1.05, 1.68)* | 1.55 (1.29, 1.86)* | 1.65 (1.22, 2.22)* | 0.597 | |
| 1213/21,981 | (317/8481) | (421/7236) | (337/4707) | (138/1557) | ||
| ≥70 | 2.22 (1.35, 3.65)* | 1.83 (1.30, 2.58)* | 1.75 (1.38, 2.21)* | 1.77 (1.22, 2.57)* | 0.598 | |
| 211/2643 | (47/717) | (55/791) | (68/755) | (41/380) | ||
| P-trend3 | <0.0001 | 0.007 | <0.0001 | 0.396 | ||
1ORs were estimated using logistic regression model including meat and egg intakes and their interaction, adjusting for age, gender, energy, television hours, sleep hours, smoking, exercise, BMI, and intakes of refined grains, vegetables, soy, fruits, dairy, coffee, fish, and nuts/seeds. *P ≤ 0.01; interaction between egg and meat intake was significant (P = 0.019). T2D, type 2 diabetes.
2 n = 52,718 (cases = 2594).
3Marginal trend P values obtained from stratified analysis.
DISCUSSION
The results of the present study demonstrate that for every category of BMI, increased meat intake corresponds to increased T2D risk estimates, but increased egg intake is not associated with T2D risk. Additionally, meat-egg interaction is significant, with incremental rise in T2D risk as meat intake increased within every egg intake category. Conversely, egg intake is not associated with T2D risk within different meat intake categories except when consumption was ≥5 eggs/wk among nonconsumers of meat.
Our findings are consistent with 2 prior reports from the AHS-2 cohort that vegetarian diets are associated with lower prevalent (34) and incident (35) T2D. Vegans (consume no meat or animal-derived foods) have both the lowest prevalence and incidence of T2D followed by lacto-ovo-vegetarians (consume dairy and eggs but no meat) compared with omnivores (34, 35). The increased T2D risk among nonmeat-eaters with an intake of ≥5 eggs/wk was unexpected because this pattern was not seen at the other meat intake levels. Although we controlled for all measured lifestyle and dietary confounders, we cannot discount residual confounding. Also, the small number of nonconsumers of meat may have resulted in an unstable estimate. Further investigation to ascertain if high egg intake is indeed associated with T2D risk would be needed in other nonmeat-eating (vegetarian) groups.
Previous studies investigating the association between egg consumption and T2D risk have shown inconsistent results. Knowledge of the relation of meat intake and BMI with T2D risk and the typical dietary practice of eating eggs with meat has prompted prior studies to adjust for BMI and meat consumption in multivariable models (12, 14, 18, 24). Similar to our results (see Table 2), prior studies adjusting for BMI and meat consumption did not find egg intake-T2D risk associations. However, to our knowledge, no previous study has measured the interactions of meat and egg intake or BMI and egg intake when assessing the relation between egg consumption and T2D risk. Data from our large cohort with diverse dietary patterns ranging from never to daily intakes of meat and eggs provided an excellent opportunity to evaluate whether egg intake was independently associated with T2D incidence.
Our findings on meat intake and incident T2D have shown similar trends as those of prior studies. A European case-cohort study (10) reported an 8% risk increase for every additional 50 g of total meat consumption. After adjustment for covariates, a pooled analysis of 3 prospective studies among US adults found a 14% increase per additional 1 serving/d in total meat consumption (9). A meta-analysis (n = 442,101 participants and 28,288 T2D cases) confirmed these findings (RR per 50 g = 1.51 processed and 1.19 unprocessed meat) (7). Because our reference group in this analysis was the nonmeat consumers, we had the ability to detect T2D risk even with low meat consumption (>0 to <25 g/d). This has not been previously reported.
Prospective studies in Sweden (12) and France (14) showed no association between egg consumption and T2D risk, and a cohort of Finnish men showed that higher egg (18) as well as egg-protein intake was associated with decreased T2D risk (36). Conversely, a cohort study of African Americans (11) suggested a positive association with prevalent but not incident T2D. A more recent meta-analysis (23) of 12 international cohorts illustrated a small increase in T2D risk when comparing the highest to the lowest category of egg intake. Interestingly, when stratified by geographic area, only US cohorts exhibit a 39% greater T2D risk comparing the highest to lowest category of egg intake; conversely, in studies of non-US populations, T2D risk is reduced by 11% (23). Another meta-analysis (24) included the 12 cohorts reviewed in Djoussé et al. (23) and prospective cohorts from Sweden (17) and Finland (20); results also indicated a modest association between egg intake and incident T2D, with a stronger association shown for US studies than for studies elsewhere (24). A third meta-analysis comprised of the cohorts from Tamez et al. (24) and the cohort of Swedish men found an overall null association between egg consumption and T2D risk (12). In accordance with other meta-analyses, stratification by country revealed a higher association in US studies (RR = 1.18; 95% CI: 1.13, 1.24) compared with non-US studies (RR = 0.97; 95% CI: 0.90, 1.05) (12). The observed dissimilarities may be attributed to the finding that egg consumption was associated with smoking, lower physical activity, and increased intakes of red and processed meat, sweets and desserts, and refined grains (16, 19, 23) in US cohorts, whereas this was not the case among non-US cohorts (13, 15, 18). Consistent with these reviews, a cross-sectional study (37) reported that adjusting for dietary cholesterol and BMI attenuated any association between egg intake and insulin resistance, suggesting that egg intake may be linked to dietary behaviors that increase BMI, thereby adversely influencing insulin sensitivity. Additionally, other experimental studies have shown that inclusion of eggs in the diet does not have detrimental effects on glucose metabolism or lipid profiles (38, 39), and may actually improve inflammatory markers in people with existing diabetes (40).
Limitations
Foremost in the limitations of this study is the low response rate to the follow-up hospitalization questionnaires that might have led to the underestimation of T2D incidence and thus, potential weakening of the risk estimates if the nonresponse was related to factors we were not able to control for. Another limitation is possible measurement errors, and hence residual confounding, and incomplete adjustment for other unknown confounders and for adiposity by only using BMI. The inability to perform time-to-event analysis and the self-report of dietary intake and occurrence of T2D are other limitations, although self-report of T2D diagnosis had been ascertained in a previous report (35). In addition, the contribution of different types of meat (e.g., red meats, processed meats, or poultry) was not examined separately, in part, because the study population has a low intake of processed meat. Finally, despite the diverse nature of this cohort in terms of race, sex, geography, socioeconomic status, and dietary intake patterns, generalizability of our findings is limited by the low egg and meat intakes of our cohort relative to the general US population. However, our null findings for egg intake-T2D associations are consistent with studies that used ≥5 eggs/wk as their highest intake category (11–12, 14, 15). The contrast is with US studies that reported higher egg intakes, i.e., ≥7 eggs/wk, except for the modest elevation of T2D risk among nonmeat-consumers with intake of ≥5 eggs/wk.
Conclusions
Our findings indicate that T2D risk is independently associated with meat intake. Egg consumption within the limits of intake in our population is not associated with T2D risk, except the possible elevated risk among nonmeat-eaters who consume ≥5 eggs/wk. Although caution should be exercised when extrapolating our findings to populations with higher intakes of eggs or meat, the results of this study suggest that the primary target of diabetes prevention should be weight management followed by restricting meat intake.
Failure to investigate egg-meat interactions may have biased the purported egg-T2D risk relation in US populations. Thus, by evaluating habitual egg consumption independent from meat intake, our findings may help explain the observed discrepancy in findings regarding T2D risk attributed to egg intake in US compared to non-US studies.
Supplementary Material
ACKNOWLEDGEMENTS
The authors’ contributions were as follows—JS: was responsible for the study concept and design; JS, GEF, MJO, KO, and BW: analyzed and interpreted the data; NMB-C and GS-S: drafted the manuscript; KO and BW: performed the statistical analysis; JS and GEF: obtained funding; NMB-C, BW, and GEF: were responsible for administrative, technical, and material support; JS: supervised the study; and all authors: critically read and approved the final manuscript. The views expressed in this article are those of the authors and do not necessarily represent the views of the NCI or the AEB. None of the authors has any conflict of interest to disclose.
Notes
The original study was funded by National Cancer Institute (NCI) grant 1U01CA152939. The present analysis was funded by the American Egg Board (AEB). The funding organizations had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Supplemental Tables 1 and 2 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/ajcn/.
Abbreviations used:
- AHS-2
Adventist Health Study 2
- FFQ
food-frequency questionnaire
- HHQ
Hospital History Questionnaire
- T2D
type 2 diabetes
REFERENCES
- 1. Centers for Disease Control and Prevention. National Diabetes Statistics Report, 2017. Atlanta, GA: Centers for Disease Control and Prevention, US Department of Health and Human Services; 2017. [Google Scholar]
- 2. Shaw JE, Sicree RA, Zimmet PZ. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res Clin Pract. 2010;87:4–14. [DOI] [PubMed] [Google Scholar]
- 3. Zhang P, Zhang X, Brown J, Vistisen D, Sicree R, Shaw J, Nichols G. Global healthcare expenditure on diabetes for 2010 and 2030. Diabetes Res Clin Pract. 2010;87:293–301. [DOI] [PubMed] [Google Scholar]
- 4. Astrup A. Healthy lifestyles in Europe: prevention of obesity and type II diabetes by diet and physical activity. Public Health Nutr. 2001;4:499–515. [DOI] [PubMed] [Google Scholar]
- 5. Uusitupa M. Gene-diet interaction in relation to the prevention of obesity and type 2 diabetes: evidence from the Finnish Diabetes Prevention Study. Nutr Metab Cardiovasc Dis. 2005;15:225–33. [DOI] [PubMed] [Google Scholar]
- 6. Verma S, Hussain ME. Obesity and diabetes: an update. Diabetes Metab Syndr. 2016; 11:73–79. [DOI] [PubMed] [Google Scholar]
- 7. Micha R, Michas G, Mozaffarian D. Unprocessed red and processed meats and risk of coronary artery disease and type 2 diabetes – an updated review of the evidence. Curr Atheroscler Rep. 2012;14:515–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Becerra-Tomás N, Babio N, Martínez-González MÁ, Corella D, Estruch R, Ros E, Fito M, Serra-Majem L, Salaverria I, Lamuela-Raventos RM et al.. Replacing red meat and processed red meat for white meat, fish, legumes or eggs is associated with lower risk of incidence of metabolic syndrome. Clin Nutr. 2016;35:1442–9. [DOI] [PubMed] [Google Scholar]
- 9. Pan A, Sun Q, Bernstein AM, Schulze MB, Manson JE, Willett WC, Hu FB. Red meat consumption and risk of type 2 diabetes: 3 cohorts of US adults and an updated meta-analysis. Am J Clin Nutr. 2011;94:1088–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. InterAct Consortium. Association between dietary meat consumption and incident type 2 diabetes: the EPIC-InterAct Study. Diabetologia. 2013;56:47–59. [DOI] [PubMed] [Google Scholar]
- 11. Djoussé L, Petrone AB, Hickson DA, Talegawkar SA, Dubbert PM, Taylor H, Tucker KL. Egg consumption and risk of type 2 diabetes among African Americans: the Jackson Heart Study. Clin Nutr. 2015;35:679–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wallin A, Forouhi NG, Wolk A, Larsson SC. Egg consumption and risk of type 2 diabetes: a prospective study and dose–response meta-analysis. Diabetologia. 2016;59:1204–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kurotani K, Nanri A, Goto A, Mizoue T, Noda M, Oba S, Sawada N, Tsugane S, Japan Public Health Center-Based Prospective Study Group . Cholesterol and egg intakes and the risk of type 2 diabetes: the Japan Public Health Center-based Prospective Study. Br J Nutr. 2014;112:1636–43. [DOI] [PubMed] [Google Scholar]
- 14. Lajous M, Bijon A, Fagherazzi G, Balkau B, Boutron-Ruault M-C, Clavel-Chapelon F. Egg and cholesterol intake and incident type 2 diabetes among French women. Br J Nutr. 2015;114:1667–73. [DOI] [PubMed] [Google Scholar]
- 15. Zazpe I, Beunza JJ, Bes-Rastrollo M, Basterra-Gortari FJ, Mari-Sanchis A, Martínez-González MÁ. Egg consumption and risk of type 2 diabetes in a Mediterranean cohort; the Sun Project. Nutr Hosp. 2013;28:105–11. [DOI] [PubMed] [Google Scholar]
- 16. Djoussé L, Kamineni A, Nelson TL, Carnethon M, Mozaffarian D, Siscovick D, Mukamal KJ. Egg consumption and risk of type 2 diabetes in older adults. Am J Clin Nutr. 2010;92:422–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Montonen J, Jarvinen R, Heliovaara M, Reunanen A, Aromaa A, Knekt P. Food consumption and the incidence of type II diabetes mellitus. Eur J Clin Nutr. 2005;59(3):441–8. [DOI] [PubMed] [Google Scholar]
- 18. Virtanen JK, Mursu J, Tuomainen T-P, Virtanen HE, Voutilainen S. Egg consumption and risk of incident type 2 diabetes in men: the Kuopio Ischaemic Heart Disease Risk Factor Study. Am J Clin Nutr. 2015;101:1088–96. [DOI] [PubMed] [Google Scholar]
- 19. Djoussé L, Gaziano JM, Buring JE, Lee IM. Egg consumption and risk of type 2 diabetes in men and women. Diabetes Care. 2009;32:295–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ericson U, Hellstrand S, Brunkwall L, Schulz CA, Sonestedt E, Wallström P, Gullberg B, Wirfält E, Orho-Melander M. Food sources of fat may clarify the inconsistent role of dietary fat intake for incidence of type 2 diabetes. Am J Clin Nutr. 2015;101(5):1065–80. [DOI] [PubMed] [Google Scholar]
- 21. Shin JY, Xun P, Nakamura Y, He K. Egg consumption in relation to risk of cardiovascular disease and diabetes: a systematic review and meta-analysis. Am J Clin Nutr. 2013;98:146–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Li Y, Zhou C, Zhou X, Li L. Egg consumption and risk of cardiovascular diseases and diabetes: a meta-analysis. Atherosclerosis. 2013;229:524–30. [DOI] [PubMed] [Google Scholar]
- 23. Djoussé L, Khawaja OA, Gaziano JM. Egg consumption and risk of type 2 diabetes: a meta-analysis of prospective studies. Am J Clin Nutr. 2016;103:474–80. [DOI] [PubMed] [Google Scholar]
- 24. Tamez M, Virtanen JK, Lajous M. Egg consumption and risk of incident type 2 diabetes: a dose–response meta-analysis of prospective cohort studies. Br J Nutr. 2016;115:2212–8. [DOI] [PubMed] [Google Scholar]
- 25. Butler TL, Fraser GE, Beeson WL, Knutsen SF, Herring RP, Chan J, Sabate J, Montgomery S, Haddad E, Preston-Martin S et al.. Cohort profile: the Adventist Health Study-2 (AHS-2). Int J Epidemiol. 2008;37:260–5. [DOI] [PubMed] [Google Scholar]
- 26. Orlich M, Singh P, Sabate J, Jaceldo-Siegl K, Fan J, Knutsen S, Beeson WL, Fraser GE. Vegetarian dietary patterns and mortality in Adventist Health Study 2. JAMA Intern Med. 2013;173:1230–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Manson JE, Colditz GA, Stampfer MJ, Willett WC, Krolewski AS, Rosner B, Arky RA, Speizer FE, Hennekens CH. A prospective study of maturity-onset diabetes mellitus and risk of coronary heart disease and stroke in women. Arch Intern Med. 1991;151:1141–7. [PubMed] [Google Scholar]
- 28. Michels KB, Solomon CG, Hu FB, Rosner BA, Hankinson SE, Colditz GA, Manson JE. Type 2 diabetes and subsequent incidence of breast cancer in the Nurses’ Health Study. Diabetes Care. 2003;26:1752–8. [DOI] [PubMed] [Google Scholar]
- 29. Jaceldo-Siegl K, Fan J, Sabate J, Knutsen SF, Haddad E, Beeson WL, Herring RP, Butler TL, Bennett H, Fraser GE. Race-specific validation of food intake obtained from a comprehensive FFQ: the Adventist Health Study-2. Public Health Nutr. 2011;14:1988–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Jaceldo-Siegl K, Knutsen S, Sabate J, Beeson W, Chan J, Herring RP, Butler TL, Haddad E, Bennett H, Montgomery S et al.. Validation of nutrient intake using an FFQ and repeated 24 h recalls in black and white subjects of the Adventist Health Study-2 (AHS-2). Public Health Nutr. 2010;13:812–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bes-Rastrollo M, Sabate J, Jaceldo-Siegl K, Fraser GE. Validation of self-reported anthropometrics in the Adventist Health Study-2. BMC Public Health. 2011;11:1471–2458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. National Institutes of Health, National Cancer Institute. Dietary Assessment Primer, Learn More about Energy Adjustment. (https://dietassessmentprimer.cancer.gov/). Accessed April 9, 2018. [Google Scholar]
- 33. Subar AF, Freedman LS, Tooze JA, Kirkpatrick SI, Boushey C, Neuhouser ML, Thompson FE, Potischman N, Guenther PM, Tarasuk V et al.. Addressing current criticism regarding the value of self-report dietary data. J Nutr. 2015;145(12):2639–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Tonstad S, Butler T, Yan R, Fraser G. Type of vegetarian diet, body weight, and prevalence of type 2 diabetes. Diabetes Care. 2009;32:791–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Tonstad S, Stewart K, Oda K, Batech M, Herring RP, Fraser GE. Vegetarian diets and incidence of diabetes in the Adventist Health Study-2. Nutr Metab Cardiovasc Dis. 2013;23:292–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Virtanen H, Koskinen T, Voutilainen S, Mursu J, Tuomainen T, Kokko P, Virtanen JK. Intake of different dietary proteins and risk of type 2 diabetes in men: the Kuopio Ischaemic Heart Disease Risk Factor Study. Br J Nutr. 2017;11:1–12. [DOI] [PubMed] [Google Scholar]
- 37. Lee C-TC, Liese AD, Lorenzo C, Wagenknecht LE, Haffner SM, Rewers MJ, Hanley AJ. Egg consumption and insulin metabolism in the Insulin Resistance Atherosclerosis Study (IRAS). Public Health Nutr. 2014;17:1595–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ballesteros MN, Valenzuela F, Robles AE, Artalejo E, Aguilar D, Andersen CJ, Valdez H, Fernandez ML. One egg per day improves inflammation when compared to an oatmeal-based breakfast without increasing other cardiometabolic risk factors in diabetic patients. Nutrients. 2015;7:3449–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Fuller NR, Caterson ID, Sainsbury A, Denyer G, Fong M, Gerofi J, Baqleh K, Williams KH, Lau NS, Markovic TP. The effect of a high-egg diet on cardiovascular risk factors in people with type 2 diabetes: the Diabetes and Egg (DIABEGG) study—a 3-mo randomized controlled trial. Am J Clin Nutr. 2015;101:705–13. [DOI] [PubMed] [Google Scholar]
- 40. Blesso CN, Andersen CJ, Barona J, Volek JS, Fernandez ML. Whole egg consumption improves lipoprotein profiles and insulin sensitivity to a greater extent than yolk-free egg substitute in individuals with metabolic syndrome. Metabolism. 2013;62:400–10. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.