ABSTRACT
Background
Adequate availability of long-chain polyunsaturated fatty acids (LC-PUFAs) is important for human health from pregnancy to adulthood. Previous studies on fatty acid desaturase (FADS) gene single-nucleotide polymorphisms (SNPs) have been performed predominantly in Western populations and showed that FADS SNPs had a marked impact on LC-PUFA composition in blood and tissues.
Objectives
We aimed to investigate the influence of fetal FADS genotypes on LC-PUFA composition in umbilical artery plasma lipids in Indonesian infants.
Design
We performed a cross-sectional study to assess for these associations.
Results
A total of 12 cord plasma n–6 (ω-6) and n–3 (ω-3) fatty acids were analyzed for associations with 18 FADS gene cluster SNPs from 390 women with single parturition from the Indonesian Prospective Study of Atopic Dermatitis in Infants (ISADI). Fetal FADS genotypes influenced cord plasma LC-PUFA composition, but, in contrast to previous studies from Western populations, the quantitatively predominant SNPs were associated with lower LC-PUFA content.
Conclusion
To our knowledge, this study was the first in South East Asia on FADS genotypes and arterial cord blood fatty acids to show an association between fetal LC-PUFA composition and fetal FADS SNPs. The FADS genotype distribution differs markedly between different geographical populations. This trial was registered at clinicaltrials.gov as NCT02401178.
Keywords: FADS1, FADS2, FADS3, polymorphisms, fetus, LC-PUFA composition, Indonesia
INTRODUCTION
Adequate availability of essential PUFAs and the long-chain PUFA (LC-PUFA) metabolites of both the n–6 and n–3 (families is important in every stage of human life from pregnancy to adulthood, because these fatty acids modulate motor and cognitive function, mental health, cardiovascular function, and inflammatory responses, such as allergies and eczema (1, 2). However, studies on the effects and the magnitude of these effects remain inconclusive (3–5). For example, the authors of a meta-analysis that explored the effects of LC-PUFA supply in infant formula on vision and neurodevelopment concluded that although some studies showed significant benefits, overall, no significant effect was detectable (4). With regards to allergies, limited evidence supports a benefit of maternal n–3 LC-PUFA intake during pregnancy or lactation for reducing allergic disease in children (5). Caution is needed when interpreting current evidence, as many of the included trials had methodologic limitations such as small sample sizes, high attribution rates, and a lack of intention-to-treat analyses (3).
Studies published in the last decade have demonstrated a marked impact of single-nucleotide polymorphisms (SNPs) in the fatty acid desaturase (FADS) gene clusters on the activity of FADS enzymes, as well as precursor PUFA and LC-PUFA levels in blood and tissue (6–15). Desaturase enzymes convert precursor PUFAs, namely linoleic acid (LA) and α-linolenic acid (ALA), through a desaturation and elongation process, to LC-PUFA metabolites such as arachidonic acid (ARA), EPA, and DHA. In European populations, minor allele carriers of FADS SNPs had increased substrate amounts (LA and ALA) and decreased product amounts (ARA, EPA, and DHA), which are reflective of reduced desaturase enzyme activity, as shown in numerous studies involving a wide range of subjects (69–4457), and genome-wide associations (9–14, 16, 17).
As most previous studies have been conducted in European populations (9–14), we studied variants of the FADS1, FADS2, and FADS3 genes in Indonesian newborns and their relation to LC-PUFA composition in umbilical artery plasma. Our primary endpoint was to characterize the impact of genetic variation in the FADS1–3 gene clusters on plasma LC-PUFA composition in cord blood. The secondary endpoints were the characterization of the frequency of FADS1, FADS2, and FADS3 gene SNPs in the Indonesian population, their relation to the fatty acid composition of umbilical artery plasma lipids, and differences in minor alleles among various populations.
METHODS
Study area and population
We performed a cross-sectional analysis among 390 mother-infant pairs from the Indonesian Prospective Study of Atopic Dermatitis in Infants (ISADI). The primary endpoint of the ISADI research was to assess the role of filaggrin (FLG) gene mutation, FADS1/FADS2 gene polymorphisms, and LC-PUFAs in atopic dermatitis patients (18). Our investigation of FADS1/FADS2 gene polymorphisms and LC-PUFAs in all infants is complete, but genetic exploration of the FLG gene is not yet complete. Based on the available data, we explored the impact of FADS gene cluster SNPs on the LC-PUFA composition of cord blood lipids.
We used a consecutive sampling procedure to collect participants. The number of participants was calculated using the formula of categoric descriptive sample size. The study was conducted at Kemayoran Primary Health Care, central Jakarta, from April 2014 to December 2015. Participants were apparently healthy newborn infants whose parents agreed to participate in the study, and provided written informed consent.
Inclusion criteria were apparently healthy newborns born at term (37–42 wk of gestation) with a birth weight ≥2500 g, to mothers with normal gestational history and who had omnivorous diets.
Exclusion criteria were serious congenital anomalies or diseases in the infant, maternal gestational hypertension, gestational diabetes mellitus, or other severe illness, disorders, or the intake of n–3 and n–6 supplements by the mother during pregnancy and breastfeeding, or by the infant.
Ethics
The present study was conducted according to the International Ethical Guidelines for Biomedical Research Involving Human Subjects. All procedures were approved by the Permanent Medical Research Ethics Committee in Medicine and Health/Faculty of Medicine Universitas Indonesia/Dr Cipto Mangunkusumo Hospital (47/H2.F1/ETIK/2014, extended by letter 148/UN2.F1/ETIK/2015). Written informed consent was obtained from all parents of the study subjects. The study was registered at clinicaltrials.gov as NCT02401178.
Procedures
Sample collection and handling
Umbilical artery blood specimens were collected into EDTA tubes directly after birth, but before detachment of the placenta. Specimens were immediately centrifuged. The plasma and buffy coat were frozen at −80°C, transported on dry ice to LMU, Munich, Germany, and stored at −80°C until further analysis.
LC-PUFA measurements
Plasma glycerophospholipid fatty acids were quantified as described previously (18). Briefly, selective preparation of methyl ester derivatives of glycerophospholipid fatty acids was achieved by coprecipitation of triacylglycerols and cholesterol esters with proteins and base-catalyzed transesterification excluding methyl ester synthesis from nonesterified fatty acids. Aliquots (100 µL) of EDTA plasma were combined with methanol containing dipentadecanoyl phosphatidylcholine as an internal standard. Tubes were centrifuged (3030 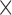 g; 10 min; 4ºC) and the supernatant transferred to another vial. Sodium methoxide solution was added for fatty acid methyl ester (FAME) synthesis from glycerophospholipid fatty acids at room temperature. After 4 min, the reaction was stopped with methanolic HCl, and the FAME was extracted into 1 mL hexane for gas chromatographic analysis. The FAME was quantified by gas chromatography with flame ionization detection (Agilent 7890 GC; Agilent, Waldbronn, Germany), using a 50-m, 0.22-mm inner diameter BPX70 column (SGE, Weiterstadt, Germany). Injection was performed with a programmable temperature vaporizer (Gerstel, Mühlheim, Germany) to avoid preconcentration of hexane extracts before gas chromatography. FAME peaks were identified and calibrated relative to pentadecanoic acid methyl ester (internal standard) by comparison with a FAME standard mixture (GLC-569B; Nu-Check Prep, Inc., Elysian, MN). Agilent Chemstation (revision B.04.03) was used for peak integration. Interassay coefficients of variation were determined by analysis of multiple aliquots of a sample distributed within the analysis of study subjects. Fatty acid results are reported as a percentage of total fatty acids analyzed (% wt/wt) (19). In this analysis, 12 PUFA measurements were included, namely LA (C18:2n–6), γ-linolenic acid (C18:3n–6), eicosadienoic acid (C20:2n–6), dihomo-γ-linolenic acid (DGLA/C20:3n–6), ARA (C20:4n–6), adrenic acid (C22:4n–6), docosapentaenoic acid (DPA, C22:5n–6 and C22:5n–3), ALA (C18:3n–3), eicosatrienoic acid (C20:3n–3), eicosapentaenoic acid (EPA, C20:5n–3), and docosahexaenoic acid (DHA, C22:6n–3).
g; 10 min; 4ºC) and the supernatant transferred to another vial. Sodium methoxide solution was added for fatty acid methyl ester (FAME) synthesis from glycerophospholipid fatty acids at room temperature. After 4 min, the reaction was stopped with methanolic HCl, and the FAME was extracted into 1 mL hexane for gas chromatographic analysis. The FAME was quantified by gas chromatography with flame ionization detection (Agilent 7890 GC; Agilent, Waldbronn, Germany), using a 50-m, 0.22-mm inner diameter BPX70 column (SGE, Weiterstadt, Germany). Injection was performed with a programmable temperature vaporizer (Gerstel, Mühlheim, Germany) to avoid preconcentration of hexane extracts before gas chromatography. FAME peaks were identified and calibrated relative to pentadecanoic acid methyl ester (internal standard) by comparison with a FAME standard mixture (GLC-569B; Nu-Check Prep, Inc., Elysian, MN). Agilent Chemstation (revision B.04.03) was used for peak integration. Interassay coefficients of variation were determined by analysis of multiple aliquots of a sample distributed within the analysis of study subjects. Fatty acid results are reported as a percentage of total fatty acids analyzed (% wt/wt) (19). In this analysis, 12 PUFA measurements were included, namely LA (C18:2n–6), γ-linolenic acid (C18:3n–6), eicosadienoic acid (C20:2n–6), dihomo-γ-linolenic acid (DGLA/C20:3n–6), ARA (C20:4n–6), adrenic acid (C22:4n–6), docosapentaenoic acid (DPA, C22:5n–6 and C22:5n–3), ALA (C18:3n–3), eicosatrienoic acid (C20:3n–3), eicosapentaenoic acid (EPA, C20:5n–3), and docosahexaenoic acid (DHA, C22:6n–3).
Genotyping
FADS1, FADS2, and FADS3 SNP analysis was performed at the Research Unit of Molecular Epidemiology at Helmholtz Zentrum Munich, Germany, as previously described (20). DNA was extracted from the buffy coat of umbilical artery blood by the Puregene DNA isolation kit (Gentra Systems). Genotyping was performed using iPLEX Gold Chemistry (Sequenom) and matrix-assisted laser desorption ionization-time of flight mass spectrometry, with methods to detect allelic differences. In brief, locations containing certain SNPs were amplified by polymerase chain reaction, using specific primers. After deactivation by alkaline phosphatase, single-base elongation was performed in accordance to the print order. After salt ion removal by ion switch and elongation reaction, the specimen was transferred to a silicone chip and covered with 3-hydroxypicolinic acid. The differences from specific alleles were measured by matrix-assisted laser desorption ionization-time of flight mass spectrometry. Allele recognition from SNPs was performed by Mass ARRAY Typer version 4.0.5 (Sequenom). SNPs for FADS genes were selected based on 3 criteria: 1) the SNP has been studied in previous publications; 2) the SNP candidates being considered are SNPs that have already been shown to be associated with LC-PUFA status; and 3) minor allele frequency (MAF) >10%.
Statistical analysis
All data processing was performed with SAS version 9.4 (SAS Institute Inc, NC), or R 3.2.4 (http://www/r-project.org). Allele frequency, Hardy-Weinberg equilibrium (HWE), and linkage disequilibrium were assessed with R-package Genetics (https://cran.r-project.org/web/packages/genetics/index.html). The linkage disequilibrium test for each pair of SNP loci was tested with Lewontin's D′. Normal distribution of fatty acid was assessed by the Kolmogorov-Smirnov test. Regression analysis was performed to explore the associations between the genetic variation of FADS1 to FADS3 genes and LC-PUFA composition (21). Reported P values in tables are uncorrected for multiple testing, with *** indicating significance after Bonferroni correction to account for multiple testing (critical corrected P value is P < 1.84 × 10–4, i.e., usual α of 0.05 divided by the number of tests = 17 SNPs × 16 fatty acid outcomes).
RESULTS
A total of 390 healthy infants, 190 girls and 200 boys, who met the inclusion criteria were enrolled in this study. The mean ± SD birth weights were 3022 ± 337.2 g for girls and 3132.1 ± 399 g for boys. The mean Apgar scores were 9 at 1 min, and 10 at 5 min. The average maternal age of delivery was 27.5 y. Most parents came from low socioeconomic backgrounds with senior high school education.
No statistical differences in the fatty acids were found between boys and girls for any of the n–6 and n–3 fatty acids, except for ALA and the ratio of EPA to ALA (Table 1). The ratios of ARA to LA, as well as EPA and DHA to ALA, were calculated as markers of the activity of fatty acid desaturation mediated by the enzymes D5D and D6D, respectively. The ratio of DGLA to LA is used to indicate the D6D activity, whereas D5D activity is reflected by the ratio of ARA to DGLA.
TABLE 1.
Fatty acid content (wt%) of cord plasma glycerophospholipids1
| Girls | Boys | Total | ||
|---|---|---|---|---|
| Fatty acid | (n = 190) | (n = 200) | P | (n = 390) |
| n–6 | ||||
| 18:2 (LA) | 8.62 ± 1.15 | 8.74 ± 1.17 | 0.297 | 8.68 ± 1.16 |
| 18:3 (GLA) | 0.16 ± 0.03 | 0.16 ± 0.04 | 0.678 | 0.16 ± 0.04 |
| 20:2 (EDA) | 0.49 ± 0.11 | 0.50 ± 0.13 | 0.262 | 0.49 ± 0.12 |
| 20:3 (DGLA) | 5.63 ± 0.91 | 5.60 ± 0.80 | 0.727 | 5.62 ± 0.85 |
| 20:4 (ARA) | 15.71 ± 1.56 | 15.44 ± 1.71 | 0.101 | 15.57 ± 1.65 |
| 22:4 (DTA) | 0.65 ± 0.12 | 0.64 ± 0.11 | 0.576 | 0.64 ± 0.12 |
| 22:5 (DPA) | 1.27 ± 0.34 | 1.29 ± 0.33 | 0.448 | 1.28 ± 0.33 |
| 20:3/18:2 | 0.66 ± 0.12 | 0.65 ± 0.13 | 0.445 | 0.66 ± 0.12 |
| 20:4/20:3 | 2.89 ± 0.71 | 2.84 ± 0.68 | 0.458 | 2.87 ± 0.69 |
| 20:4/18:2 | 1.86 ± 0.35 | 1.81 ± 0.38 | 0.144 | 1.83 ± 0.37 |
| n–3 | ||||
| 18:3 (ALA) | 0.05 ± 0.03 | 0.06 ± 0.02 | 0.023* | 0.05 ± 0.03 |
| 20:3 (ERA) | 0.13 ± 0.02 | 0.14 ± 0.02 | 0.156 | 0.14 ± 0.02 |
| 20:5 (EPA) | 0.12 ± 0.07 | 0.11 ± 0.05 | 0.162 | 0.11 ± 0.06 |
| 22:5 (DPA) | 0.24 ± 0.10 | 0.22 ± 0.08 | 0.180 | 0.23 ± 0.09 |
| 22:6 (DHA) | 5.17 ± 1.24 | 5.06 ± 1.02 | 0.342 | 5.12 ± 1.14 |
| 20:5/18:3 | 2.49 ± 1.51 | 2.05 ± 1.08 | 0.001* | 2.26 ± 1.32 |
1Values of the wt% are means ± SDs. Pvalue is from t test for the difference in measurements between girls and boys. *Significant differences, P < 0.05. Enzyme activity of D5D was determined by the ratio of ARA/DGLA. Enzyme activity of D6D was determined by the ratio of DGLA/LA. ALA, α-linolenic acid; ARA, arachidonic acid; DGLA, dihomo-γ-linolenic acid; DPA, docosapentaenoic acid; DTA, docosatetraenoic acid; EDA, eicosadienoic acid; ERA, eicosatrienoic acid; GLA, γ-linolenic acid; LA, linoleic acid.
The distribution of all 18 FADS1, FADS2, and FADS3 SNPs genotyped in this study population is presented in Table 2. Genotyping was done along a 34-kb portion from position 618,393,876 (rs174548) to position 61,888,645 (rs174455). The positions in base pairs were defined according to genome build 108, assembly GRCH38.p2 (https://www.ncbi.nlm.nih.gov/snp). This lists major and minor FADS alleles, MAF, and P values of the HWE tests. One of the SNPs (rs968567) was monomorphic in the study and, therefore, could not be analyzed further. The other 17 SNPs of the ISADI study were consistent with HWE. The SNPs rs174548, rs174556, and rs174561 had an almost identical MAF of 27% and were highly collinear (the linkage disequilibrium calculation gave a D′ value of 0.99999). Consequently, 14 SNPs were included in further analyses to avoid informational repetition.
TABLE 2.
Characteristics of SNPs analyzed in the FADS1–FADS3 gene clusters in Indonesian infants1
| Gene | dbSNP build 108 | Position, bp | Major/minor alleles | MAF, % | HWE P value |
|---|---|---|---|---|---|
| FADS1 | rs174548 | 61,803,876 | C/G | 27 | 0.696 |
| rs174556 | 61,813,163 | A/G | 27 | 0.696 | |
| rs174561 | 61,815,236 | G/A | 27 | 0.696 | |
| Intergenic | rs3834458 | 61,827,449 | del/T | 22 | 0.456 |
| rs968567 | 61,828,092 | C/T | NA | NA | |
| FADS2 | rs174570 | 61,829,740 | T/C | 23 | 0.477 |
| rs174574 | 61,832,870 | A/C | 22 | 0.548 | |
| rs2727271 | 61,835,886 | A/T | 25 | 0.685 | |
| rs174576 | 61,836,038 | A/C | 22 | 0.368 | |
| rs174578 | 61,838,027 | A/T | 22 | 0.368 | |
| rs174579 | 61,838,141 | C/T | 28 | 1.000 | |
| rs174602 | 61,856,942 | C/T | 41 | 0.465 | |
| rs498793 | 61,857,233 | C/T | 15 | 0.421 | |
| rs526126 | 61,857,413 | C/G | 22 | 0.056 | |
| rs174575 | 61,834,531 | C/G | 30 | 1.000 | |
| Intergenic | rs174448 | 61,872,101 | T/C | 48 | 0.760 |
| rs174449 | 61,872,907 | C/T | 47 | 0.186 | |
| FADS3 | rs174455 | 61,888,645 | C/T | 45 | 0.474 |
1dbSNP, database single-nucleotide polymorphism; del., deleted; FADS, fatty acid desaturase; HWE, Hardy-Weinberg equilibrium; MAF, minor allele frequency; NA, not available.
Table 3 compares the MAF of the FADS genes among different populations in Indonesia, the Mexican POSGRAD (Prenatal Omega-3 Supplementation on Child Growth and Development) Study (22), and Europe; data from the latter come from the HELENA (Healthy Lifestyle in Europe by Nutrition in Adolescence) Study, which included 9 countries (23), the British ALSPAC (Avon Longitudinal Study of Parents and Children) Study (20), the German LISA (Influences of Lifestyle-related Factors on the Immune System and the Development of Allergies in Childhood) Study, and the KOALA (“Kind, Ouders en gezondheid: Aandacht voor Leefstijl en Aanleg”) Birth Cohort Study in the Netherlands (8).
TABLE 3.
Comparison of common single-nucleotide polymorphisms among different populations1
| Gene | dbSNP build 108 | ISADI | MAF, % | POSGRAD | MAF, % | ALSPAC | MAF, % | KOALA | MAF, % | LISA | MAF, % | HELENA | MAF, % |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| FADS1 | rs174545 | — | — | — | — | — | — | G | 32.9 | G | 31.1 | — | — |
| rs174546 | — | — | — | — | — | — | T | 32.9 | T | 31 | T | 31 | |
| rs174548 | G | 27 | G | 22 | C | 30.1 | — | — | — | — | — | — | |
| rs174556 | G | 27 | G | 24 | A | 29.6 | T | 30.7 | T | 28.5 | — | — | |
| rs174561 | A | 27 | A | 24 | G | 29.6 | C | 30.7 | C | 28.5 | — | — | |
| Intergenic | rs3834458 | T | 22 | NA | NA | del | 33.0 | del | 33.1 | del | 31 | — | — |
| rs968567 | T | NA | NA | NA | T | 16.9 | — | — | — | — | T | 16 | |
| FADS2 | rs174570 | C | 23 | C | 27 | T | 12.6 | — | — | — | — | T | 11 |
| rs174574 | C | 22 | C | 20 | A | 34.0 | — | — | — | — | — | — | |
| rs2727271 | T | 25 | T | 40 | T | 11.6 | — | — | — | — | — | — | |
| rs174576 | C | 22 | C | 21 | A | 34.0 | — | — | — | — | — | — | |
| rs174578 | T | 22 | T | 21 | A | 34.0 | — | — | — | — | — | — | |
| rs174579 | T | 28 | T | 38 | T | 21.4 | — | — | — | — | — | — | |
| rs174602 | T | 41 | T | 37 | G | 20.4 | — | — | — | — | C | 22 | |
| rs498793 | T | 15 | T | 35 | T | 39.9 | — | — | — | — | C | 40 | |
| rs526126 | G | 22 | NA | NA | G | 18.1 | — | — | — | — | G | 20 | |
| rs174575 | G | 30 | G | 40 | NA | NA | — | — | — | — | — | — | |
| Intergenic | rs174448 | C | 48 | T | 29 | C | 35.9 | — | — | — | — | — | — |
| rs174449 | T | 47 | T | 28 | C | 35.1 | — | — | — | — | — | — | |
| FADS3 | rs174455 | T | 45 | T | 22 | C | 34.8 | — | — | — | — | — | — |
1ALSPAC, Avon Longitudinal Study of Parents and Children; dbSNP, database single-nucleotide polymorphism; del., deleted; FADS, fatty acid desaturase; HELENA, Healthy Lifestyle in Europe by Nutrition in Adolescence; ISADI, Indonesian Study of Atopic Dermatitis of Indonesian Infants; KOALA, “Kind, Ouders en gezondheid: Aandacht voor Leefstijl en Aanleg” Birth Cohort Study; LISA, Influences of Lifestyle-related Factors on the Immune System and the Development of Allergies in Childhood Study; MAF, minor allele frequency; POSGRAD, Prenatal Omega-3 Supplementation on Child Growth and Development.
Tables 4 and 5 list the regression coefficients (β coefficients), related P values, and explained variance (R2) of the regression of each fatty acid level, from n–6 and n–3 (% wt/wt), respectively, for each of the 14 analyzed SNPs of the FADS gene clusters. The composition of the n–6 fatty acids and ratios of DGLA to LA, ARA to DGLA, and ARA to LA were significantly associated with almost all SNPs of the FADS1 and most of FADS2 gene clusters, even after Bonferroni correction of P values for multiple testing. However, genetic variants of rs498793, rs526126, and rs174575 of the FADS2 gene; rs174448 and rs174449 of the intergenic FADS2 and FADS3 gene; and rs174455 of the FADS3 gene, showed no (or only rarely) statistically significant relations with any of the n–6 fatty acids, after correction for multiple testing.
TABLE 4.
Regression of n–6 levels (wt% of cord plasma glycerophospholipids) on each of 14 SNPs of the FADS1–3 regions1
| Fatty acid | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C18:2n–6 | ln(C18:3n–6) | C20:2n–6 | C20:3n–6 | C20:4n–6 | ln(C22:4n–6) | C22:5n–6 | C20:3n–6/C18:2n–6 | ln(C20:4n–6/C20:3n–6) | ln(C20:4n–6/C18:2n–6) | |||
| FADS1 | rs174548 | β coefficient | −0.562 | 0.062 | −0.046 | −0.835 | 1.186 | 0.055 | 0.080 | −0.053 | 0.233 | 0.144 |
| P value | 6.97 × 10–10*** | 5.28 × 10-04 | 1.89 × 10-06*** | 4.51 × 10-42*** | 3.71 × 10-21*** | 8.72 × 10-05*** | 2.42 ×10-03 | 6.44 × 10-08*** | 1.58 × 10-45*** | 3.97 × 10-22*** | ||
| R 2, % | 9.2 | 2.8 | 5.5 | 37.9 | 20.4 | 3.7 | 2.1 | 7.1 | 40.4 | 21.3 | ||
| Intergenic | rs3834458 | β coefficient | −0.659 | 0.059 | −0.053 | −0.877 | 1.298 | 0.060 | 0.101 | −0.050 | 0.249 | 0.163 |
| P value | 7.39 × 10-12*** | 1.66 ×10-03 | 2.1 × 10-07*** | 4.72 × 10-41*** | 1.33 × 10-22*** | 5.38 × 10-05*** | 3.21 × 10-04 | 1.71 × 10-06*** | 2.49 × 10-46*** | 1.97 × 10-25*** | ||
| R 2, % | 11.2 | 2.3 | 6.5 | 37.2 | 21.8 | 3.9 | 3.1 | 5.5 | 41.0 | 24.4 | ||
| FADS2 | rs174570 | β coefficient | −0.650 | 0.059 | −0.053 | −0.795 | 1.249 | 0.051 | 0.084 | −0.042 | 0.230 | 0.158 |
| P value | 5.8 × 10-12*** | 1.35 ×10-03 | 1.06 × 10-07*** | 6.23 × 10-34*** | 1.2 × 10-21*** | 5.05 × 10-04 | 2.37 × 10-03 | 6.06 × 10-05*** | 5.29 × 10-40*** | 5.7 × 10-25*** | ||
| R 2, % | 11.3 | 2.4 | 6.8 | 31.6 | 20.9 | 2.8 | 2.1 | 3.8 | 36.4 | 23.9 | ||
| rs174574 | β coefficient | −0.674 | 0.058 | −0.052 | −0.870 | 1.297 | 0.056 | 0.099 | −0.048 | 0.247 | 0.165 | |
| P value | 3.44 × 10-12*** | 2.27 ×10-03 | 3.9 × 10-07*** | 2.06 × 10-39*** | 3.45 × 10-22*** | 1.74 × 10-04*** | 4.79 × 10-04 | 5.39 × 10-06*** | 1.26 × 10-44* | 1.33 × 10-25*** | ||
| R 2, % | 11.6 | 2.1 | 6.2 | 35.9 | 21.4 | 3.3 | 2.9 | 5.0 | 39.8 | 24.5 | ||
| rs2727271 | β coefficient | 0.355 | −0.022 | 0.021 | 0.403 | −0.760 | −0.016 | −0.045 | 0.018 | −0.124 | −0.093 | |
| P value | 1.34 × 10-05*** | 1.6 ×10-01 | 1.38 × 10-02 | 9.54 × 10-12*** | 2.79 × 10-11*** | 1.96 × 10-01 | 5.37 × 10-02 | 4.52 × 10-02 | 3.79 × 10-15*** | 5.9 × 10-12*** | ||
| R 2, % | 4.6 | 0.3 | 1.3 | 11.1 | 10.6 | 0.2 | 0.7 | 0.8 | 14.6 | 11.3 | ||
| rs174576 | β coefficient | −0.669 | 0.056 | −0.051 | −0.851 | 1.251 | 0.056 | 0.101 | −0.046 | 0.241 | 0.161 | |
| P value | 3.48 × 10-12*** | 2.86 ×10-03 | 6.32 ×10-07*** | 4.3 × 10-38*** | 7.09 × 10-21*** | 1.83 × 10-04*** | 3.15 × 10-04 | 1.11 × 10-05*** | 1.47 × 10-42*** | 7.55 × 10-25*** | ||
| R 2, % | 11.6 | 2.0 | 6.0 | 34.9 | 20.2 | 3.3 | 3.1 | 4.6 | 38.3 | 23.8 | ||
| rs174579 | β coefficient | 0.130 | −0.020 | 0.018 | 0.233 | −0.140 | −0.026 | −0.028 | 0.018 | −0.053 | −0.023 | |
| P value | 1.63 × 10-01 | 2.66 × 10-01 | 5.54 × 10-02 | 6.2 × 10-04 | 2.92 × 10-01 | 6.02 × 10-02 | 2.97 × 10-01 | 6.54× 10-02 | 3.82× 10-03 | 1.42 × 10-01 | ||
| R 2, % | 0.2% | 0.1% | 0.7% | 2.7% | 0.0% | 0.7% | 0.0% | 0.6% | 1.9% | 0.3% | ||
| rs174602 | β coefficient | −0.220 | 0.021 | −0.016 | −0.375 | 0.527 | 0.027 | 0.047 | −0.028 | 0.105 | 0.059 | |
| P value | 7.99 × 10-03 | 1.85× 10-01 | 6.04 × 10-02 | 3.71 × 10-10*** | 6.56 × 10-06*** | 2.96 × 10-02 | 4.73 × 10-02 | 1.64 × 10-03 | 8.12 × 10-11*** | 2.26 × 10-05*** | ||
| R 2, % | 1.6% | 0.2% | 0.7% | 9.4% | 4.9% | 1.0% | 0.8% | 2.3% | 10.1% | 4.3% | ||
| rs498793 | β coefficient | 0.111 | −0.004 | 0.028 | 0.174 | −0.311 | −0.012 | −0.029 | 0.013 | −0.052 | −0.031 | |
| P value | 3.6× 10-01 | 8.67 × 10-01 | 2.35 × 10-02 | 4.99 × 10-02 | 6.96 X 10-02 | 5.14 × 10-01 | 3.97 × 10-01 | 3.07 × 10-01 | 3.15 × 10-02 | 1.27 × 10-01 | ||
| R 2, % | 0.0 | −0.3 | 1.1 | 0.7 | 0.6 | −0.1 | −0.1 | 0.0 | 0.9 | 0.3 | ||
| rs526126 | β coefficient | 0.056 | −0.001 | 0.008 | 0.135 | −0.424 | 0.009 | 0.022 | 0.012 | −0.053 | −0.033 | |
| P value | 5.55 × 10-01 | 9.67 × 10-01 | 4. × 10-01 | 5.53 × 10-02 | 1.68 × 10-03 | 5.23 × 10-01 | 4.21 × 10-01 | 2.45 × 10-01 | 5.02 × 10-03 | 3.8 × 10-02 | ||
| R 2, % | −0.2 | −0.3 | −0.1 | 0.7 | 2.3 | −0.2 | −0.1 | 0.1 | 1.8 | 0.9 | ||
| rs174575 | β coefficient | 0.138 | −0.019 | 0.015 | 0.264 | −0.136 | −0.031 | −0.040 | 0.021 | −0.059 | −0.024 | |
| P value | 1.3× 10-01 | 2.7 × 10-01 | 1.21 × 10-01 | 7.5 × 10-05*** | 2.96 x 10-01 | 2.42 × 10-02 | 1.26 x 10-01 | 2.98 × 10-02 | 1.21 × 10-03 | 1.23 × 10-01 | ||
| R 2, % | 0.3 | 0.1 | 0.4 | 3.7 | 0.0 | 1.1 | 0.3 | 1.0 | 2.4 | 0.4 | ||
| Intergenic | rs174448 | β coefficient | 0.129 | −0.009 | 0.009 | 0.133 | −0.103 | −0.023 | −0.065 | 0.004 | −0.032 | −0.023 |
| P value | 1.18 × 10-01 | 5.79 × 10-01 | 2.95 × 10-01 | 2.94 × 10-02 | 3.8 × 10-01 | 6.93 × 10-02 | 5.73 × 10-03 | 6.44 × 10-01 | 5.02 × 10-02 | 1. × 10-01 | ||
| R 2, % | 0.4 | −0.2 | 0.0 | 1.0 | −0.1 | 0.6 | 1.7 | −0.2 | 0.7 | 0.4 | ||
| Intergenic | rs174449 | β coefficient | −0.158 | 0.015 | −0.015 | −0.206 | 0.183 | 0.021 | 0.065 | −0.009 | 0.051 | 0.032 |
| P value | 4.99 × 10-02 | 3.44 × 10-01 | 7.25 × 10-02 | 4.85 × 10-04 | 1.11 × 10-01 | 8.95 × 10-02 | 4.72 × 10-03 | 2.85 × 10-01 | 1.5 × 10-03 | 1.74 × 10-02 | ||
| R 2, % | 0.7 | 0.0 | 0.6 | 2.9 | 0.4 | 0.5 | 1.8 | 0.0 | 2.3 | 1.2 | ||
| FADS3 | rs174455 | β coefficient | −0.173 | 0.007 | −0.007 | −0.162 | 0.130 | 0.018 | 0.066 | −0.004 | 0.039 | 0.030 |
| P value | 3.5 × 10-02 | 6.45 × 10-01 | 4.29 × 10-01 | 7.36 × 10-03 | 2.68 × 10-01 | 1.51 × 10-01 | 4.5 × 10-03 | 6.66 × 10-01 | 1.75 × 10-02 | 2.97 × 10-02 | ||
| R 2, % | 0.9 | −0.2 | −0.1 | 1.6 | 0.1 | 0.3 | 1.8 | −0.2 | 1.2 | 1.0 | ||
1Outcome is respective fatty acid wt% of cord plasma glycerophospholipids; ln indicates whether the outcome was log transformed to account for severe right skewness of the fatty acid distribution, or entered into the regression equation on the original scale. The β coefficient is the estimated regression coefficient from unadjusted linear regression of each fatty acid on each genetic variant of the FADS gene regions. The P values are uncorrected for multiple testing, with *** indicating significance after Bonferroni correction for multiple testing (critical P value is 1.84 × 10–4).
TABLE 5.
Regression of n–3 levels (wt% of cord plasma glycerophospholipids) on each of 14 SNPs of FADS1–3 regions1
| Fatty acid | ||||||||
|---|---|---|---|---|---|---|---|---|
| ln(C18:3n–3) | ln(C20:3n–3) | ln(C20:5n–3) | ln(C22:5n–3) | C22:6n–3 | ln(C20:5n–3/C18:3n–3) | |||
| FADS1 | rs174548 | β coefficient | −0.076 | 0.015 | 0.012 | 0.013 | 0.0260 | 0.088 |
| P value | 1.22 × 10–2 | 2.21 × 10−01 | 7.22 × 10−01 | 6.75 × 10−01 | 4.34 × 10−03 | 3.31 × 10−02 | ||
| R 2, % | 1.4 | 0.1 | −0.2 | −0.2 | 1.8 | 0.9 | ||
| Intergenic | rs3834458 | β coefficient | −0.096 | 0.014 | −0.012 | −0.003 | 0.318 | 0.083 |
| P value | 2.83 × 10−03 | 3.11 × 10−01 | 7.31 × 10−01 | 9.36 × 10−01 | 9.65 × 10−04 | 5.79 × 10−02 | ||
| R 2, % | 2.0 | 0.0 | −0.2 | −0.3 | 2.5 | 0.7 | ||
| FADS2 | rs174570 | β coefficient | −0.091 | 0.012 | −0.001 | −0.004 | 0.302 | 0.090 |
| P value | 3.95 × 10−03 | 3.43 × 10−01 | 9.8 × 10−01 | 8.93 × 10−01 | 1.45 × 10−03 | 3.69 × 10−02 | ||
| R 2, % | 1.9 | 0.0 | −0.3 | −0.3 | 2.3 | 0.9 | ||
| rs174574 | β coefficient | −0.100 | 0.012 | −0.007 | −0.010 | 0.299 | 0.093 | |
| P value | 1.89 × 10−03 | 3.89 × 10−01 | 8.38 × 10−01 | 7.63 × 10−01 | 2.15 × 10−03 | 3.55 × 10−02 | ||
| R 2, % | 2.2 | −0.1% | −0.2 | −0.2 | 2.2 | 0.9 | ||
| rs2727271 | β coefficient | 0.070 | −0.016 | 0.000 | 0.005 | −0.178 | −0.071 | |
| P value | 8.34 × 10−03 | 1.55 × 10−01 | 9.97 × 10−01 | 8.43 × 10−01 | 2.73 × 10−02 | 5.32 × 10−02 | ||
| R 2, % | 1.5 | 0.3 | −0.3 | −0.2 | 1.0 | 0.7 | ||
| rs174576 | β coefficient | −0.101 | 0.010 | −0.009 | −0.008 | 0.296 | 0.093 | |
| P value | 1.53 × 10−03 | 4.43 × 10−01 | 8.14 × 10−01 | 7.98 × 10−01 | 2.23 × 10−03 | 3.4 × 10−02 | ||
| R 2, % | 2.3 | −0.1 | −0.2 | −0.2 | 2.1 | 0.9 | ||
| rs174579 | β coefficient | −0.001 | 0.11 | 0.004 | 0.001 | −0.033 | 0.005 | |
| P value | 9.76 × 10−01 | 3.66 × 10−01 | 9.09 × 10−01 | 9.86 × 10−01 | 7.2 × 10−01 | 9.08 × 10−01 | ||
| R 2, % | −0.3 | 0.0 | −0.3 | −0.3 | −0.2 | −0.3 | ||
| rs174602 | β coefficient | −0.046 | −0.012 | −0.012 | −0.028 | 0.076 | 0.034 | |
| P value | 8.7 × 10−02 | 2.76 × 10−01 | 6.85 × 10−01 | 2.9 × 10−01 | 3.53 × 10−01 | 3.58 × 10−01 | ||
| R 2, % | 0.5 | 0.0 | −0.2 | 0.0 | 0.0 | 0.0 | ||
| rs498793 | β coefficient | 0.034 | 0.009 | 0.036 | 0.018 | 0.043 | 0.002 | |
| P value | 3.92 × 10−01 | 5.65 × 10−01 | 4.19 × 10−01 | 6.45 × 10−01 | 7.17 × 10−01 | 9.69 × 10−01 | ||
| R 2, % | −0.1 | −0.2 | −0.1 | −0.2 | −0.2 | −0.3 | ||
| rs526126 | β coefficient | 0.071 | 0.020 | −0.049 | −0.005 | −0.137 | −0.120 | |
| P value | 2.22 × 10−02 | 1.15 × 10−01 | 1.59 × 10−01 | 8.84 × 10−01 | 1.43 × 10−01 | 4.41 × 10−03 | ||
| R 2, % | 1.1 | 0.4 | 0.3 | −0.3 | 0.3 | 1.8 | ||
| rs174575 | β coefficient | −0.001 | 0.010 | 0.015 | 0.008 | −0.016 | 0.015 | |
| P value | 9.85 × 10−01 | 4.25 × 10−01 | 6.55 × 10−01 | 7.87 × 10−01 | 8.61 × 10−01 | 7.02 × 10−01 | ||
| R 2, % | −0.3 | −0.1 | −0.2 | −0.2 | −0.3 | −0.2 | ||
| Intergenic | rs174448 | β coefficient | 0.129 | −0.009 | 0.009 | 0.133 | −0.103 | −0.023 |
| P value | 1.18 × 10−01 | 5.79 × 10−01 | 2.95 × 10−01 | 2.94 × 10−02 | 3.8 × 10−01 | 6.93 × 10−02 | ||
| R 2, % | 0.4 | −0.2 | 0.0 | 1.0 | −0.1 | 0.6 | ||
| Intergenic | rs174449 | β coefficient | −0.020 | 0.007 | −0.044 | −0.028 | 0.006 | −0.023 |
| P value | 4.38 × 10−01 | 5.02 × 10−01 | 1.37 × 10−01 | 2.91 × 10−01 | 9.38 × 10−01 | 5.12 × 10−01 | ||
| R 2, % | −0.1 | −0.1 | 0.3 | 0.0 | −0.3 | −0.1 | ||
| FADS3 | rs174455 | β coefficient | −0.024 | −0.002 | −0.053 | −0.036 | −0.007 | −0.029 |
| P value | 3.77 × 10−01 | 8.34 × 10−01 | 7.75 × 10−02 | 1.71 × 10−01 | 9.34 × 10−01 | 4.2 × 10−01 | ||
| R 2, % | −0.1 | −0.2 | 0.5 | 0.2 | −0.3 | −0.1 | ||
1Outcome is respective fatty acid (wt% of cord plasma glycerophospholipids); ln indicates whether the outcome was log transformed to account for severe right skewness of the fatty acid distribution, or entered into the regression equation on the original scale. β Coefficient is the estimated regression coefficient from unadjusted linear regression of each fatty acid on each genetic variant of the FADS gene regions. The P values are uncorrected for multiple testing (critical P value is 1.84 × 10–4). FADS, fatty acid desaturase; SNP, single-nucleotide polymorphism.
The variance (R2) of fatty acid levels explained by SNPs was highest for DGLA, with 37.9% for genetic variants in the FADS1gene, 37.2% for rs3834458 (intergenic FADS1/2), and 35.9% for rs174574 in the FADS2 gene. The next highest R2 was 21.8% for regression of ARA in rs3834458 (intergenic FADS1/2), followed by ∼20% for FADS1 and some FADS2 genetic variants.
The explained variance (R2) for the ARA:DGLA ratio was mainly determined by genetic variants of FADS1 or intergenic FADS1/2 and some FADS2 genetic variants, accounting for 41.0% (rs3834458) of variation of the ratio. This finding supports the conclusion that the FADS1 gene product is involved in D5-desaturation from DGLA to ARA. In addition, the FADS2 gene product D6D is involved.
The high explained variance (R2) for the ARA:LA ratio for some FADS2-related SNPs of 25.5% (rs174574), and the FADS1/2 intergenic SNP rs3834458 with 24.4%, provides further evidence that the FADS2 gene product is involved in D6-desaturation from LA to ARA. In contrast, only small amounts of variance ranging from <1% to 2.5% of n–3 fatty acids were explained by FADS SNPs in this Indonesian infant population. The highest R2 of 2.5% was found for the FADS1/2 intergenic SNP rs3834458 with regards to DHA level, followed by R2 >2.0% for some SNPs of the FADS2 gene (rs174570 rs174574, rs174576, and rs174578). This finding provides further support that the FADS2 gene product is involved in D6-desaturation. Interestingly, the explained variance for the EPA:ALA ratio was <1% for all SNPs, except for rs526126 (FADS2), with an R2 of 1.8%.
DISCUSSION
Our study demonstrated a significant association of cord blood composition of precursor PUFAs and LC-PUFAs with common variants in the FADS gene clusters. In this population-based study, we found a higher content of ARA (15.57% ± 1.65%) and DHA (5.12% ± 1.14%) than other PUFAs in umbilical artery plasma glycerophospholipids. This finding is in agreement with those of the LISA Study (24), and reflects the active and preferential maternal-fetal transfer of LC-PUFAs across the human placenta (25, 26).
The extent to which the fetus can produce LC-PUFAs from precursors, i.e., from n–6 LA and n–3 ALA, is unknown. LC-PUFA formation is facilitated by both elongases encoded by ELOVL genes and by desaturases encoded by FADS genes (27). In our study, we found a close association between FADS SNPs and indicators of the activity of FADS1- and FADS2-encoded enzymes, i.e., blood levels of n–6 LC-PUFAs. As much as 20.4% of the ARA level variation was determined by the rs174548 SNP. Effects of similar size were also found for other SNPs. In contrast, Lattka et al. (20) showed that variations of FADS genes had a relativity low impact on LC-PUFA composition in cord venous plasma fatty acid, with a coefficient of variation of 2.99% determined by FADS SNPs. Umbilical artery samples may better reflect the effects of FADS SNP variation because the umbilical artery transports blood from the fetus back to the placenta.
We assessed 18 SNPs from the FADS1–3 gene clusters, but only 14 SNPs were included in further analyses, in order to avoid informational repetition. HWE in this study population had a value of >5%, which indicated that the population is in a constant state and not influenced by mutations, natural selection, nonrandom mating, genetic drift, or gene flow.
Indeed, we noted different FADS1 and FADS2 SNPs distributions in our Indonesian population compared with European populations. We then explored minor alleles in different populations. The major alleles of European populations (7, 8, 20, 23) were the minor alleles of the ISADI population. Comparing the minor SNP differences between the British ALSPAC Study (20) and the ISADI Study, we found a 70% difference for all SNPs, except for rs968567, rs2727271, rs174579, rs498793, and rs526126. On the other hand, 14 out of 15 minor alleles examined were the same among participants of the Indonesian ISADI study and the Mexican POSGRAD Study (22) (namely, rs174548, rs174556, rs174561, rs174570, rs174574, rs2727271, rs174576, rs174578, rs174579, rs174602, rs498793, rs174575, rs174449, and rs174455).
In addition, we found that minor alleles in different populations appeared to have different functional effects. In our study, the minor alleles in Indonesian infants were associated with lower levels of substrates (LA and ALA) and higher levels of products (DGLA and ARA) and ratios of DGLA/LA, ARA/DGLA, and ARA/LA, indicating higher desaturase activity. In contrast, the minor alleles of European populations were associated with higher substrate and lower LC-PUFA levels, indicating lower desaturase activity (9, 13–17, 20, 23). The difference in the activity of FADS genes of different populations might be the source of heterogeneity in the meta-analysis, as it was not accounted for in almost all of the past trials (3).
Our findings begged the question as to the reason for great variations in the FADS gene clusters between populations. Our observations support the theories of natural selection (28–30) and human migration effects (31–33). Indonesia has the world's second-longest coastline. The coastline of the country is ∼54,720 km long, hence, fish and other marine mammals are common food sources. This result was similar to the study of Fumagalli et al. (29) in an Inuit population. They found higher amounts of substrate n–3 fatty acids and a decrease in the amounts of EPA and DPA, with no significant effect on DHA. The similar changes could, therefore, be an effect of selection driven by environmental conditions, i.e., a habitual diet rich in LC-PUFAs that did not provide an advantage to individuals with a genetically rapid LC-PUFA synthesis (29). Agricultural diets, on the other hand, would have led to a higher consumption of grains and other plant-derived foods. Alleles that increase the rate of conversion of short-chain PUFAs to LC-PUFAs would, therefore, have been favored (30).
The 93% resemblance between Indonesian and Mexican FADS SNPs, and the 70% difference in the ALSPAC Study population, might support the theory of human migration known as “out of Africa” that divides ancestor migration from Africa, either heading west to Europe or heading east to Asia and onwards to Latin America (31). Karafet et al. (32) found that the Y chromosome of the western Indonesian population was related to haplogroups that may have entered Indonesia during the Paleolithic era from mainland Asia, whereas a study by Moreno-Estrada et al. (33) stated that some Mexican ancestors originated from Eastern Asia.
In addition to the the association of FADS1 and FADS2 SNPs with LC-PUFA composition, we also assessed for a possible association between rs174455 from the FADS3 gene and LC-PUFA composition. No significant association was revealed, but there was a trend in the same direction as FADS1 and FADS2. This finding was similar to a study by Koletzko et al. (14), who found a weak association between rs174455 and changes in substrates and products. Two special SNPs, rs2727271 and rs498793, showed effects in the opposite direction compared to other SNPs; they decreased products, but increased substrates. Findings for these 2 SNPs were also reported by Lattka et al. (20): with only 1 minor allele from rs2727271, there was a 0.76% decrease in ARA level. In addition, the 1 minor allele from rs498793 led to a 0.31% decrease in ARA level.
We found no significant associations between FADS 1–3 SNPs and n–3 LC-PUFA composition. It appears that maternal dietary EPA and DHA have a greater effect on the cord blood levels of LC-PUFAs than endogenous synthesis.
Sufficient availability of LC-PUFAs is considered essential for early development. In this study, polymorphisms in the FADS gene cluster were shown to modify the fatty acid composition of glycerophospholipid plasma of umbilical arterial blood. We can conclude that polymorphisms in fetal FADS genes are associated with fetal n–6 LC-PUFA synthesis, in addition to effects of placental transfer of preformed LC-PUFAs. When comparing SNPs of different populations, the similarity of the FADS1–3 SNP distribution patterns in this Indonesian and a previously studied Mexican population (22) are noteworthy, as both differed from European populations. Both the Indonesian and the Mexican populations showed the minor alleles to be associated with high levels of LC-PUFA synthesis, whereas the major genotypes were associated with low levels of LC-PUFA synthesis. Therefore, the supply of preformed LC-PUFA may be particularly important in these populations to achieve similar plasma and tissue concentrations, as well as related biological effects.
As the major alleles of these populations differ and each SNP differs in outcome, direct extrapolation from observational and intervention studies in European populations to Indonesian populations appears questionable, due to their genetically determined differences in PUFA metabolism. Hence, intervention studies in infant populations in Indonesia or other populations with similar genotype distributions seem necessary.
ACKNOWLEDGEMENTS
The authors’ contributions were as follows—CT, BK, and DRS: conceived and designed the experiments; CT, PR, MM, HS, SRH, BK, and DRS: analyzed the data; ER: performed the genotyping; HD: examined the PUFAs; CT, PR, BK, DRS, SRH, MM, ZM, HS, SI, and RI: wrote the article; and all authors: read and approved the final manuscript. All the authors declare no conflict of interest regarding this manuscript.
Notes
Supported by the Mead Johnson Pediatric Nutrition Institute (grant 8670 to CT) and by the Commission of the European Communities with the European Research Council Advanced Grant META-GROWTH (ERC-2012-AdG 322605). The funders had no influence on the design, implementation, analysis and interpretation of the data.
Abbreviations used: A, adrenic acid; ALA, α-linolenic acid; ALSPAC study, Avon Longitudinal Study of Parents and Children; ARA, arachidonic acid; DGLA, dihomo-γ-linolenic acid; FADS, fatty acid desaturase; FAME, fatty acid methyl ester; HWE, Hardy-Weinberg equilibrium; ISADI, Indonesian Prospective Study of Atopic Dermatitis of Infants; LA, linoleic acid; LC-PUFA, long-chain PUFA; MAF, minor allele frequency; SNP, single-nucleotide polymorphism.
REFERENCES
- 1. Lattka E, Illig T, Heinrich J, Koletzko B. Do FADS genotypes enhance our knowledge about fatty acid related phenotypes?. Clin Nutr. 2010;29:277–87. [DOI] [PubMed] [Google Scholar]
- 2. Lattka E, Illig T, Heinrich J, Koletzko B. FADS gene cluster polymorphisms: important modulators of fatty acid levels and their impact on atopic diseases. J Nutrigenet Nutrigenomics. 2009;2:119–28. [DOI] [PubMed] [Google Scholar]
- 3. Koletzko B. Human milk lipids. Ann Nutr Metab. 2016;69:28–40. [DOI] [PubMed] [Google Scholar]
- 4. Jasani B, Simmer K, Patole SK, Rao SC. Long chain polyunsaturated fatty acid supplementation in infants born at term. Cochrane Database Syst Rev. 2017;3:CD000376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gunaratne AW, Makrides M, Collins CT. Maternal prenatal and/or postnatal n-3 long chain polyunsaturated fatty acids (LCPUFA) supplementation for preventing allergies in early childhood. Cochrane Database Syst Rev. 2015;7:CD010085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cho HP, Nakamura MT, Clarke SD. Cloning, expression and nutritional regulation of the mammalian delta-6 desaturase. J Biol Chem. 1999;274:471–7. [DOI] [PubMed] [Google Scholar]
- 7. Rzehak P, Heinrich J, Klopp N, Schaeffer L, Hoff S, Wolfram G, Illig T, Linselsen J. Evidence for an association between genetic variants of the fatty acid desaturase 1 fatty acid desaturase 2 (FADS1 FADS2) gene cluster and the fatty acid composition of erythrocyte membranes. Br J Nutr. 2009;101:20–6. [DOI] [PubMed] [Google Scholar]
- 8. Rzehak P, Thijs C, Standl M, Mommers M, Glaser C, Jansen E, Klopp N, Kopperman GH, Singmann P, Postma DS et al.. Variants of the FADS1 FADS2 gene cluster, blood levels of polyunsaturated fatty acids and eczema in children within the first 2 years of life. PLoS One. 2010;5:e13261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Koletzko B, Lien E, Agostoni C, Bohles H, Campoy C, Cetin I, Decsi T, Dudenhausen JW, Dupont C, Forsyth S et al.. The roles of long-chain polyunsaturated fatty acids in pregnancy, lactation and infancy: review of current knowledge and consensus recommendation. J Perinat Med. 2008;36:5–14. [DOI] [PubMed] [Google Scholar]
- 10. Schaeffer L, Gohlke H, Muller M, Heid IM, Palmer LJ, Kompauer I, Demmelmeir H, Illig T, Koletzko B, Heinrich J. Common genetic variants of the FADS1 FADS2 gene cluster and their reconstructed haplotypes are associated with the fatty acid composition in phospholipids. Hum Mol Genet. 2006;15:1745–56. [DOI] [PubMed] [Google Scholar]
- 11. Xie L, Innis SM. Genetic variants of the FADS1 FADS2 gene cluster are associated with altered (n-6) and (n-3) essential fatty acids in plasma and erythrocyte phospholipids in women during pregnancy and in breast milk during lactation. J Nutr. 2008;138:2222–8. [DOI] [PubMed] [Google Scholar]
- 12. Molto-Puigmarti C, Plat J, Mensink RP, Muller A, Jansen E, Zeegers MP, Thijs C. FADS1 FADS2 gene variants modify the association between fish intake and the docosahexaenoid acid proportion in human milk. Am J Clin Nutr. 2010;91:1368–010. [DOI] [PubMed] [Google Scholar]
- 13. Lattka E, Rzehak P, Szabo E, Jakobik V, Weck M, Weyermann M, Grallert H, Rothebacher D, Heinrich J, Brenner H et al.. Genetic variants in the FADS gene cluster are associated with arachidonic acid concentrations of human breast milk at 1.5 and 6 mo postpartum and influence the course of milk dodecanoic, tetracosenoic, and trans-9-octadecenoic acid concentrations over the duration of lactation. Am J Clin Nutr. 2011;93:382–91. [DOI] [PubMed] [Google Scholar]
- 14. Koletzko B, Lattka E, Zeilinger S, Illig T, Steer C. Genetic variants of the fatty acid desaturase gene cluster predict amounts of red blood cell docosahexaenoic and other polyunsaturated fatty acids in pregnant women: findings from the Avon Longitudinal Study of Parents and Children. Am J Clin Nutr. 2011;93:211–9. [DOI] [PubMed] [Google Scholar]
- 15. Brenna JT, Salem N Jr., Sinclair AJ, Cunnane SC. α-Linolenic acid supplementation and conversion to n-3 long-chain polyunsaturated fatty acids in humans. Prostaglandins Leukot Essent Fatty Acids. 2009;80:85–91. [DOI] [PubMed] [Google Scholar]
- 16. Tanaka T, Shen J, Abecasis GR, Kisialiou A, Ordovas JM, Guralnik JM, Singleton A, Bandinelli S, Cherubini A, Arnett D et al.. Genome-wide associations study of plasma polyunsaturated fatty acids in the InCHIANTI Study. PLoS Genet. 2009;5:e1000338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Illig T, Gieger C, Zhai G, Romisch-Margl W, Wang-Sattler R, Prehn C, Altmaier E, Kastenmuller G, Kato B, Mewes HS et al.. A Genome-wide perspective of genetic variation in human metabolism. Nat Genet. 2010;42:137–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tanjung C, Rzehak P, Mansyur M, Munasir Z, Sudoyo H, Immanuel S, Irawan R, Reischl E, Demmelmair H, Koletzko B et al.. Study protocol to investigate the environmental and genetic aetiology of atopic dermatitis: the Indonesian Prospective Study of Atopic Dermatitis in Infants (ISADI). BMJ Open. 2017;7:e012475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Glaser C, Rzehak P, Demmelmair H, Klopp N, Heinrich J, Koletzko B. Influence of FADS polymorphisms on tracking of serum glycerophospholipid fatty acid concentrations and percentage composition in children. PLoS ONE. 2011;6(7):e21933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lattka E, Koletzko B, Zeilinger S, Hibbeln JR, Klopp N, Ring SM, Steer CD. Umbilical cord PUFA are determined by maternal and child fatty acid desaturase (FADS) genetic variants in the Avon Longitudinal Study of Parents and Children (ALSPAC). Br J Nutr. 2013;109:1196–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hosmer DW, Lemeshow S. Applied logistic regression. New York: John Wiley & Sons; 1989. [Google Scholar]
- 22. Gonzalez-Casanova I, Rzehak P, Stein AD, Feregrino RG, Dommarco JA, Barraza-Villareal A, Demmelmair H, Romieu I, Villalpando S, Martorell R et al.. Maternal single nucleotide polymorphisms in the fatty acid desaturase 1 and 2 coding regions modify the impact of prenatal supplementation with DHA on birth weight. Am J Clin Nutr. 2016;103:1171–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bokor S, Dumont J, Spinneker A, Gonzales-Gross M, Nova E, Widhalm K, Moschonis G, Stehle P, Amouvel P, De Henauw S et al.. Single nucleotide polymorphisms in the FADS gene cluster are associated with delta-5 and delta-6 desaturase activities estimated by serum fatty acid ratios. J Lipid Res. 2010;5:2325–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kohlboeck G, Glaser C, Tiesler C, Demmelmair H, Standl M, Romanos M, Koletzko B, Lehmann I, Heinrich J, LISAplus study Group . Effect of fatty acid status in cord blood serum on children's behavioral difficulties at 10 y of age: results from LISAplus study. Am J Clin Nutr. 2011;94:1592–9. [DOI] [PubMed] [Google Scholar]
- 25. Prieto-Sánchez MT, Ruiz-Palacios M, Blanco-Carnero JE, Pagan A, Hellmuth C, Uhl O, Peissner W, Ruiz-Alcaraz AJ, Parrilla JJ, Koletzko B, Larqué E. Placental MFSD2a transporter is related to decreased DHA in cord blood of women with treated gestational diabetes. Clin Nutr. 2017;36:513–21. [DOI] [PubMed] [Google Scholar]
- 26. Larqué E, Pagán A, Prieto MT, Blanco JE, Gil-Sánchez A, Zornoza-Moreno M, Ruiz-Palacios M, Gázquez A, Demmelmair H, Parrilla JJ, Koletzko B. Placental fatty acid transfer: a key factor in fetal growth. Ann Nutr Metab. 2014;64:247–53. [DOI] [PubMed] [Google Scholar]
- 27. Barman M, Nilsson S, Naluai AT, Sandin A, Wold AE, Sandberg A-S. Single nucleotide polymorphisms in the FADS gene cluster but not the ELOVL2 gene are associated with serum polyunsaturated fatty acid composition and development of allergy (in a Swedish birth cohort). Nutrients. 2015;7:10100–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Nielsen R. Molecular signatures of natural selection. Annu Rev Genet. 2005;39:197–218. [DOI] [PubMed] [Google Scholar]
- 29. Fumagalli M, Moltke I, Grarup N, Racimo F, Bjerregaard P, Jørgensen ME, Korneliussen TS, Gerbault P, Skotte L, Linneberg A et al.. Greenlandic Inuit show genetic signatures of diet and climate adaptation. Science. 2015;349:1343–7. [DOI] [PubMed] [Google Scholar]
- 30. Buckley MT, Racimo F, Allentoft ME, Jensen MK, Jonsson A, Huang H, Hormozdiari F, Sikora M, Marnetto D, Eskin E et al.. Selection in Europeans on fatty acid desaturases associated with dietary changes. Mol Biol Evol. 2017;34:1307–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Klein RG. Darwin and the recent African origin of modern humans. Proc Natl Acad Sci U S A. 2009;106:16007–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Karafet TM, Hallmark B, Cox MP, Sudoyo H, Downey S, Lansing JS, Hammer MF. Major east–west division underlies Y chromosome stratification across Indonesia. Mol Biol Evol. 2010;27:1833–44. [DOI] [PubMed] [Google Scholar]
- 33. Moreno-Estrada A, Gignoux CR, Fernández-López JC, Zakharia F, Sikora M, Contreras AV, Acuna-Alonzo V, Sandoval K, Eng C, Romero-Hidalgo S et al.. The genetics of Mexico recapitulates Native American substructure and affects biomedical traits. Science. 2014;344:1280–5. [DOI] [PMC free article] [PubMed] [Google Scholar]


