ABSTRACT
Background
High dietary intake or blood concentrations (as biomarkers of dietary intake) of vitamin C, carotenoids, and vitamin E have been associated with reduced risk of cardiovascular disease, cancer, and mortality, but these associations have not been systematically assessed.
Objective
We conducted a systematic review and meta-analysis of prospective studies of dietary intake and blood concentrations of vitamin C, carotenoids, and vitamin E in relation to these outcomes.
Design
We searched PubMed and Embase up to 14 February 2018. Summary RRs and 95% CIs were calculated with the use of random-effects models.
Results
Sixty-nine prospective studies (99 publications) were included. The summary RR per 100-mg/d increment of dietary vitamin C intake was 0.88 (95% CI: 0.79, 0.98, I2 = 65%, n = 11) for coronary heart disease, 0.92 (95% CI: 0.87, 0.98, I2 = 68%, n = 12) for stroke, 0.89 (95% CI: 0.85, 0.94, I2 = 27%, n = 10) for cardiovascular disease, 0.93 (95% CI: 0.87, 0.99, I2 = 46%, n = 8) for total cancer, and 0.89 (95% CI: 0.85, 0.94, I2 = 80%, n = 14) for all-cause mortality. Corresponding RRs per 50-μmol/L increase in blood concentrations of vitamin C were 0.74 (95% CI: 0.65, 0.83, I2 = 0%, n = 4), 0.70 (95% CI: 0.61, 0.81, I2 = 0%, n = 4), 0.76 (95% CI: 0.65, 0.87, I2 = 56%, n = 6), 0.74 (95% CI: 0.66, 0.82, I2 = 0%, n = 5), and 0.72 (95% CI: 0.66, 0.79, I2 = 0%, n = 8). Dietary intake and/or blood concentrations of carotenoids (total, β-carotene, α-carotene, β-cryptoxanthin, lycopene) and α-tocopherol, but not dietary vitamin E, were similarly inversely associated with coronary heart disease, stroke, cardiovascular disease, cancer, and/or all-cause mortality.
Conclusions
Higher dietary intake and/or blood concentrations of vitamin C, carotenoids, and α-tocopherol (as markers of fruit and vegetable intake) were associated with reduced risk of cardiovascular disease, total cancer, and all-cause mortality. These results support recommendations to increase fruit and vegetable intake, but not antioxidant supplement use, for chronic disease prevention.
Keywords: vitamin C, carotenoids, beta-carotene, vitamin E, coronary heart disease, stroke, cardiovascular disease, cancer, mortality, meta-analysis
INTRODUCTION
Worldwide cardiovascular disease and cancer accounted for 25.5 million deaths in 2013 (1). A high intake of fruits, vegetables, and nuts has been associated with reduced risk of cardiovascular disease, cancer, and mortality (2–11) and these findings provide a basis for dietary recommendations to prevent chronic diseases and premature mortality (9).
Fruit, vegetables, berries, herbs, nuts, and legumes contain a number of nutrients that have been hypothesized to contribute to their purported beneficial effects including fiber, vitamin C, vitamin E, carotenoids, flavonoids, potassium, and a large number of other components (12). Dietary intake as well as blood concentrations of some of these nutrients including vitamin C, vitamin E, and carotenoids have been related to risk of cardiovascular disease, cancer, and all-cause mortality (13–28). Blood concentrations of vitamin C and carotenoids are considered biomarkers of fruit and vegetable intake (29–31) and studies that use biomarkers of fruit and vegetable intake complement questionnaire-based studies by providing an integrated measure of both intake and absorption (32). Many prospective studies have investigated the association between dietary antioxidants including vitamin C, vitamin E, and carotenoids and coronary heart disease (13, 14, 33–43), stroke (13, 41, 44–52), cardiovascular disease overall (41, 53–56), total cancer (14, 20, 36, 53, 56, 57), and all-cause mortality (14, 19, 36, 38, 53, 54, 56, 58–60), but studies are not entirely consistent with some showing an inverse association (14, 36, 38, 39, 41, 43, 47, 48, 54, 55, 57, 58) and others reporting no significant association (19, 33–35, 37, 40, 42, 44–46, 49–53, 56, 59–61). Some of the previous studies may have been too small and therefore underpowered to find a significant association (20, 33, 35, 44, 49, 50, 52, 53, 59). In addition, some degree of measurement error and regression dilution bias is inevitable in dietary studies and may at least to some degree have attenuated some findings in previous studies. Aune et al. (62) recently reported stronger inverse associations between blood concentrations of carotenoids when compared with dietary intake as measured by food frequency questionnaires in relation to breast cancer risk. There was only a 5–6% reduction in the RR for the highest compared with the lowest dietary intake of carotenoids; however, when blood concentrations of carotenoids were analyzed there was a 25–30% reduction in the RR for those with the highest compared with the lowest concentrations (62) and similar results have been found in pooled analyses (63, 64). One meta-analysis also suggested an inverse association between dietary intake and blood concentrations of vitamin C and risk of stroke (65). However, whether or not blood concentrations of different types of antioxidants are associated with risk of cardiovascular disease, total cancer, and mortality is not clear (13–25, 28) and has not previously been comprehensively evaluated across multiple exposures and outcomes in a meta-analysis. For these reasons we conducted a meta-analysis of dietary intake and blood concentrations of vitamin C (ascorbic acid), vitamin E (tocopherol), and carotenoids (total carotenoids, α-carotene, β-carotene, lycopene, β-cryptoxanthin) and the risk of coronary heart disease, stroke, cardiovascular disease, total cancer, and all-cause mortality, and specifically aimed to clarify the strength and the shape of the dose-response relation.
METHODS
Search strategy
We searched the PubMed and Embase databases from their inception to 31 May 2014 using a comprehensive list of search terms shown in Supplemental Table 1 and the searches were later updated to 14 February 2018. There was no protocol for the current review. We included prospective cohort studies and nested case-control studies that reported adjusted RR estimates (ORs, HRs, incidence rate ratios, or risk ratios) of the association between vitamin C, vitamin E, and carotenoids in the diet or measured in blood and the risk of coronary heart disease, stroke, cardiovascular disease, total cancer, and all-cause mortality. Studies that only assessed supplemental intake of these antioxidants were excluded. For the dose-response analysis a quantitative measure of intake or the blood concentrations for ≥3 categories had to be available. A list of the excluded studies and reasons for exclusion is found in Supplemental Table 2. Standard criteria for reporting meta-analyses were followed (66).
Data extraction
We extracted the following data from each study: first author's last name, publication year, country where the study was conducted, study name, follow-up period, sample size, sex, age, number of cases or deaths, dietary assessment method (type, number of items, and whether it was validated), laboratory method for analysis of antioxidants in blood, exposure, exposure dose, RRs and 95% CIs, and variables adjusted for in the analysis. DA conducted the data extraction and NK and LTF checked the extractions for accuracy.
Statistical methods
To take into account heterogeneity between studies, we used a random-effects model to calculate summary RRs and 95% CIs for the highest compared with the lowest level of antioxidant exposure and for the dose-response analysis (67). The average of the natural logarithm of the RRs was estimated, and the RR from each study was weighted with the use of random-effects weighting. A 2-tailed P < 0.05 was considered statistically significant.
Details of the methods for the dose-response analysis have been published elsewhere (62). Briefly, we used the method described by Greenland and Longnecker (68) to compute linear trends and 95% CIs from the natural logs of the RRs and CIs across categories of antioxidant exposure. We used mean or median values when provided in the papers, but used the midpoint of the upper and lower boundaries when the exposure was provided as a range. When the highest and lowest categories were open-ended or had extreme upper or lower values we used the width of the adjacent interval to estimate the upper and lower boundaries for the category. For studies that did not report the distribution of cases and person-years or noncases we estimated the distribution by dividing the total number of cases or person-years by the number of categories. For studies that reported plasma or serum concentrations of carotenoids, α-carotene, β-carotene, or lycopene in µmol/L we converted the data to µg/dL by dividing the concentration in µmol/L by 0.01863 (69), whereas for studies of β-cryptoxanthin we divided the concentration in µmol/L by 0.01809. For studies of vitamin E, data in µmol/L were divided by 232.2 to convert the data to µg/dL (70). For the linear dose-response analysis we used an increment with the size of the approximate mean difference between the highest and lowest level of exposure across studies.
A potential nonlinear dose-response relation between antioxidant exposures and cardiovascular disease, cancer, and mortality was examined with the use of restricted cubic splines with 3 knots at 10th, 50th, and 90th percentiles of the distribution, which was combined with the use of multivariate meta-analysis (71, 72). A likelihood ratio test was used to assess the difference between the nonlinear and linear models to test for nonlinearity (73).
Heterogeneity between studies was assessed through the use of Q and I2 statistics (74). We conducted subgroup analyses by study characteristics including duration of follow-up, sex, geographic location, number of cases, study quality, exclusion of prevalent cases of diseases at baseline, and adjustment for confounders, as well as influence analyses to investigate the robustness of the results when there were ≥6 studies in the dose-response analysis. Study quality was assessed with the use of the Newcastle-Ottawa Scale, which awards 0–9 stars based on the selection, comparability, and outcome assessment (75). Small-study effects, such as publication bias, were assessed with the use of a funnel plot and Egger's test, with results considered to indicate potential small-study bias when P < 0.10 (76). Stata version 13.0 software (StataCorp) was used for the statistical analyses.
RESULTS
A total of 69 prospective studies (99 publications) (13–28, 33–61, 70, 77–127) were included in the analyses of dietary (includes total intake and dietary intake only) antioxidant intake and blood concentrations of antioxidants and coronary heart disease, stroke, cardiovascular disease, cancer, and mortality (Supplemental Tables 3–12, Figure 1). Thirty-six of the studies were from Europe, 24 from America, and 9 studies were from Asia (Supplemental Tables 3–12). One publication included combined results from two studies (53) and another publication included results from two studies (81). Because some studies only reported a high against low comparison or a continuous estimate, the number of studies included for each exposure and outcome comparison in the high against low and dose-response analyses may differ slightly from the total number of studies included in the analysis of each exposure and outcome. The number of studies, cases or deaths, and participants and references for studies included in each analysis are provided in Table 1. Supplemental Tables 3–12 show a summary of the study characteristics of the included studies. Supplemental Tables 13–24 show the results of the nonlinear dose-response analyses and Supplemental Tables 25–34 show the results of the subgroup analyses. Figure 1 shows a flow chart of the study selection. Figures 2–14 show the results for the dose-response analyses of vitamin C and β-carotene in relation to all outcomes and carotenoids and vitamin E in relation to all-cause mortality. Supplemental Figures 1–95 show the results of the high against low analysis as well as dose-response results for the remaining antioxidant exposures and outcomes. Supplemental Figures 96–107 show the funnel plots from the tests for publication bias and Supplementary Figures 108–125 shows the results from the influence analyses excluding one study at a time.
FIGURE 1.
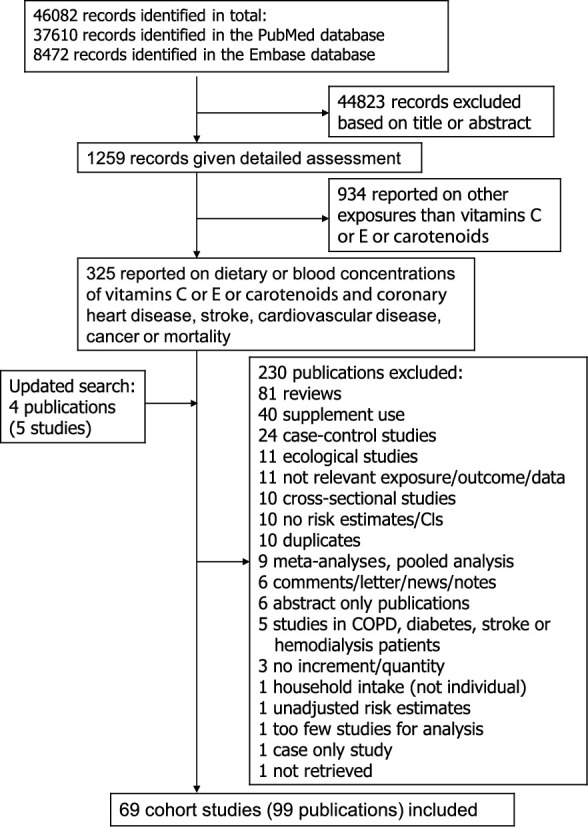
Flow chart of study selection. COPD, chronic obstructive pulmonary disease.
TABLE 1.
Summary RRs for the high vs. low and dose-response analyses of antioxidants and risk of coronary heart disease, stroke, cardiovascular disease, total cancer and mortality
| Outcome | Comparison | No. of studies | Cases, n | Participants, n | RR (95% CI) | I 2 | P heterogeneity | P (Egger' test) | References |
|---|---|---|---|---|---|---|---|---|---|
| Dietary vitamin C | |||||||||
| Coronary heart disease | High vs. low | 12 | 4297 | 241,579 | 0.83 (0.71, 0.98) | 50.7 | 0.02 | 0.97 | (13, 14, 33–41, 49) |
| Per 100 mg/d | 11 | 4167 | 240,824 | 0.88 (0.79, 0.98) | 65.1 | 0.001 | 0.007 | (13, 14, 33–41) | |
| Stroke | High vs. low | 13 | 7294 | 298,066 | 0.84 (0.77, 0.91) | 6.2 | 0.39 | 0.93 | (41, 44–52, 58, 61, 123) |
| Per 100 mg/d | 12 | 7049 | 296,596 | 0.92 (0.87, 0.98) | 68.0 | <0.0001 | 0.02 | (41, 44–52, 58, 123) | |
| Cardiovascular disease | High vs. low | 9 | 7986 | 246,711 | 0.84 (0.77, 0.91) | 0 | 0.53 | 0.71 | (19, 26, 41, 53, 55, 56, 80, 81) |
| Per 100 mg/d | 10 | 8175 | 247,765 | 0.89 (0.85, 0.94) | 27.2 | 0.19 | 0.81 | (19, 20, 26, 41, 53, 55, 56, 80, 81) | |
| Total cancer | High vs. low | 7 | 7068 | 181,318 | 0.87 (0.78, 0.95) | 17.7 | 0.30 | 0.91 | (14, 36, 53, 56, 57, 81) |
| Per 100 mg/d | 8 | 7208 | 181,318 | 0.93 (0.87, 0.99) | 45.5 | 0.08 | 0.28 | (14, 20, 36, 53, 56, 57, 81) | |
| Mortality | High vs. low | 16 | 38,079 | 315,214 | 0.86 (0.80, 0.92) | 68.5 | <0.0001 | 0.001 | (14, 19, 36, 38, 53, 54, 56, 58–60, 81–84, 127) |
| Per 100 mg/d | 14 | 36,404 | 295,152 | 0.89 (0.85, 0.94) | 80.2 | <0.0001 | <0.0001 | (14, 19, 20, 36, 38, 53, 54, 56, 58–60, 81, 84) | |
| Vitamin C in blood | |||||||||
| Coronary heart disease | High vs. low | 4 | 1368 | 6992 | 0.71 (0.59, 0.86) | 0.4 | 0.39 | 0.90 | (13, 14, 87, 92) |
| Per 50 µmol/L | 4 | 1420 | 7514 | 0.74 (0.65, 0.83) | 0 | 0.71 | 0.49 | (13–15, 92) | |
| Stroke | High vs. low | 5 | 957 | 27,843 | 0.60 (0.49, 0.73) | 0 | 0.86 | 0.70 | (16, 17, 58, 87, 98) |
| Per 50 µmol/L | 4 | 926 | 24,869 | 0.70 (0.61, 0.81) | 0 | 0.41 | 0.36 | (16, 17, 58, 98) | |
| Cardiovascular disease | High vs. low | 5 | 2792 | 45,273 | 0.61 (0.45, 0.83) | 55.6 | 0.06 | 0.22 | (18, 19, 21, 22, 103) |
| Per 50 µmol/L | 6 | 2981 | 46,327 | 0.76 (0.65, 0.87) | 55.7 | 0.05 | 0.31 | (18–22, 103) | |
| Total cancer | High vs. low | 5 | 1831 | 47,678 | 0.68 (0.57, 0.80) | 0 | 0.51 | 0.65 | (14, 18, 21, 22, 116) |
| Per 50 µmol/L | 5 | 1681 | 45,758 | 0.74 (0.66, 0.82) | 0 | 0.49 | 0.82 | (14, 18, 20–22) | |
| Mortality | High vs. low | 8 | 7528 | 47,238 | 0.68 (0.60, 0.77) | 43.1 | 0.09 | 0.07 | (14, 18, 19, 21–23, 58, 126) |
| Per 50 µmol/L | 8 | 8179 | 48,060 | 0.72 (0.66, 0.79) | 48.2 | 0.06 | 0.13 | (14, 18–23, 58) | |
| Dietary total carotenoids | |||||||||
| Coronary heart disease | High vs. low | 5 | 1835 | 91,838 | 0.78 (0.67, 0.90) | 0 | 0.54 | 0.59 | (14, 34, 35, 37, 42) |
| Per 5000 µg/d | 5 | 1835 | 91,838 | 0.85 (0.77, 0.93) | 37.0 | 0.18 | 0.22 | (14, 34, 35, 37, 42) | |
| Stroke | High vs. low | 0 | — | — | — | — | — | — | — |
| Per 5000 µg/d | 0 | — | — | — | — | — | — | — | |
| Cardiovascular disease | High vs. low | 2 | 3198 | 134,358 | 0.87 (0.74, 1.01) | 0 | 0.51 | — | (81) |
| Per 5000 µg/d | 4 | 3584 | 135,971 | 0.80 (0.70, 0.90) | 0 | 0.76 | 0.93 | (20, 55, 81) | |
| Total cancer | High vs. low | 3 | 4441 | 135,038 | 0.93 (0.82, 1.06) | 0 | 0.59 | 0.42 | (14, 81) |
| Per 5000 µg/d | 4 | 4581 | 136,092 | 0.93 (0.82, 1.05) | 4.4 | 0.37 | 0.93 | (14, 20, 81) | |
| Mortality | High vs. low | 5 | 11,431 | 188,025 | 0.87 (0.80, 0.94) | 11.6 | 0.34 | 0.45 | (14, 42, 54, 81) |
| Per 5000 µg/d | 6 | 12,148 | 189,079 | 0.88 (0.83, 0.93) | 2.2 | 0.40 | 0.51 | (14, 20, 42, 54, 81) | |
| Carotenoids in blood | |||||||||
| Coronary heart disease | High vs. low | 4 | 634 | 6014 | 0.67 (0.53, 0.85) | 0 | 0.88 | 0.20 | (14, 87, 88, 90) |
| Per 100 µg/dL | 3 | 502 | 3040 | 0.83 (0.72, 0.95) | 0 | 0.56 | 0.69 | (14, 88, 90) | |
| Stroke | High vs. low | 1 | 31 | 2974 | 0.48 (0.18, 1.28) | — | — | — | — |
| Per 100 µg/dL | 0 | — | — | — | — | — | — | — | |
| Cardiovascular disease | High vs. low | 2 | 1386 | 14,324 | 0.81 (0.64, 1.03) | 0 | 0.34 | — | (108, 109) |
| Per 100 µg/dL | 3 | 1534 | 15,492 | 0.61 (0.33, 1.10) | 53.8 | 0.12 | — | (107, 109) | |
| Total cancer | High vs. low | 5 | 1178 | 20,231 | 0.74 (0.60, 0.90) | 0 | 0.99 | 0.39 | (14, 108, 110, 116, 118) |
| Per 100 µg/dL | 3 | 741 | 14,976 | 0.61 (0.36, 1.03) | 72.5 | 0.03 | 0.49 | (14, 107, 108) | |
| Mortality | High vs. low | 6 | 1578 | 17,391 | 0.75 (0.64, 0.88) | 0 | 0.80 | 0.65 | (14, 24, 25, 108, 121, 125) |
| Per 100 µg/dL | 7 | 1966 | 18,559 | 0.74 (0.62, 0.88) | 50.9 | 0.06 | 0.14 | (14, 24, 25, 107, 108, 121, 125) | |
| Dietary β-Carotene | |||||||||
| Coronary heart disease | High vs. low | 4 | 2104 | 99,345 | 0.73 (0.63, 0.85) | 0 | 0.46 | 0.29 | (36, 38, 39, 43) |
| Per 5000 µg/d | 4 | 2104 | 99,345 | 0.82 (0.68, 0.98) | 45.0 | 0.14 | 0.20 | (36, 38, 39, 43) | |
| Stroke | High vs. low | 7 | 5468 | 201,587 | 0.84 (0.75, 0.94) | 21.9 | 0.26 | 0.25 | (44, 45, 47, 48, 51, 52, 123) |
| Per 5000 µg/d | 7 | 5468 | 201,587 | 0.81 (0.66, 0.98) | 59.4 | 0.02 | 0.07 | (44, 45, 47, 48, 51, 52, 123) | |
| Cardiovascular disease | High vs. low | 4 | 1767 | 39,643 | 0.98 (0.84, 1.15) | 10.4 | 0.34 | 0.21 | (19, 53, 56, 80) |
| Per 5000 µg/d | 4 | 1851 | 38,988 | 0.91 (0.74, 1.11) | 36.6 | 0.19 | 0.47 | (53, 55, 56, 80) | |
| Total cancer | High vs. low | 4 | 2797 | 46,280 | 0.90 (0.81, 1.00) | 0 | 0.55 | 0.02 | (36, 53, 56, 57) |
| Per 5000 µg/d | 4 | 2797 | 46,280 | 0.96 (0.90, 1.02) | 24.9 | 0.26 | 0.006 | (36, 53, 56, 57) | |
| Mortality | High vs. low | 8 | 11,729 | 142,798 | 0.82 (0.78, 0.87) | 0 | 0.51 | 0.57 | (19, 36, 38, 53, 54, 56, 60, 127) |
| Per 5000 µg/d | 6 | 11,120 | 143,140 | 0.92 (0.85, 0.98) | 66.2 | 0.01 | 0.008 | (36, 38, 53, 54, 56, 60) | |
| β-Carotene in blood | |||||||||
| Coronary heart disease | High vs. low | 4 | 1128 | 3179 | 0.73 (0.57, 0.94) | 0 | 0.61 | 0.11 | (89, 91, 93, 95) |
| Per 25 µg/dL | 3 | 1005 | 2933 | 0.80 (0.66, 0.97) | 10.4 | 0.33 | 0.90 | (91, 93, 95) | |
| Stroke | High vs. low | 3 | 1548 | 30,144 | 0.85 (0.71, 1.01) | 0 | 0.66 | 0.52 | (97, 99, 100) |
| Per 25 µg/dL | 3 | 1548 | 30,144 | 0.85 (0.74, 0.97) | 0 | 0.50 | 0.27 | (97, 99, 100) | |
| Cardiovascular disease | High vs. low | 7 | 3232 | 34,090 | 0.71 (0.57, 0.88) | 17.7 | 0.30 | 0.13 | (19, 21, 27, 103, 105, 106, 109) |
| Per 25 µg/dL | 8 | 3451 | 24,428 | 0.86 (0.78, 0.96) | 3.1 | 0.41 | 0.38 | (19–21, 27, 102, 103, 106, 109) | |
| Total cancer | High vs. low | 6 | 2654 | 66,892 | 0.76 (0.65, 0.89) | 0 | 0.80 | 0.22 | (21, 27, 28, 105, 115, 118) |
| Per 25 µg/dL | 8 | 2519 | 56,773 | 0.76 (0.68, 0.85) | 0 | 0.74 | 0.17 | (20, 21, 27, 28, 102, 115, 117, 120) | |
| Mortality | High vs. low | 7 | 5659 | 23,141 | 0.68 (0.55, 0.83) | 43.5 | 0.10 | 0.15 | (19, 21, 27, 117, 121, 122, 124) |
| Per 25 µg/dL | 7 | 5659 | 23,141 | 0.81 (0.72, 0.90) | 46.5 | 0.08 | 0.20 | (19–21, 27, 102, 117, 121, 124) | |
| α-Carotene in blood | |||||||||
| Coronary heart disease | High vs. low | 4 | 1963 | 18,251 | 0.88 (0.71, 1.10) | 0 | 0.63 | 0.64 | (91, 93–95) |
| Per 10 µg/dL | 4 | 1963 | 18,251 | 0.87 (0.71, 1.07) | 0 | 0.89 | 0.60 | (91, 93–95) | |
| Stroke | High vs. low | 3 | 784 | 16,943 | 0.74 (0.48, 1.14) | 0 | 0.73 | 0.58 | (94, 99, 100) |
| Per 10 µg/dL | 3 | 784 | 16,943 | 0.80 (0.55, 1.18) | 0 | 0.80 | 0.39 | (94, 99, 100) | |
| Cardiovascular disease | High vs. low | 4 | 3556 | 30,640 | 0.80 (0.58, 1.09) | 68.2 | 0.02 | 0.25 | (94, 106, 108, 109) |
| Per 10 µg/dL | 5 | 3745 | 31,694 | 0.83 (0.62, 1.10) | 68.2 | 0.01 | 0.81 | (20, 94, 106, 108, 109) | |
| Total cancer | High vs. low | 2 | 878 | 17,671 | 0.62 (0.40, 0.96) | 0 | 0.56 | — | (94, 118) |
| Per 10 µg/dL | 4 | 1177 | 19,043 | 0.58 (0.45, 0.74) | 0 | 0.66 | 0.95 | (20, 94, 117, 120) | |
| Mortality | High vs. low | 4 | 4285 | 18,928 | 0.76 (0.59, 0.98) | 49.9 | 0.12 | 0.08 | (24, 94, 117, 121) |
| Per 10 µg/dL | 5 | 5002 | 19,982 | 0.71 (0.64, 0.79) | 0 | 0.86 | 0.07 | (20, 24, 94, 117, 121) | |
| β-Cryptoxanthin in blood | |||||||||
| Coronary heart disease | High vs. low | 2 | 811 | 1902 | 1.01 (0.43, 2.37) | 70.8 | 0.06 | — | (91, 93) |
| Per 15 µg/dL | 2 | 811 | 1902 | 1.12 (0.33, 3.79) | 67.3 | 0.08 | — | (91, 93) | |
| Stroke | High vs. low | 0 | — | — | — | — | — | — | — |
| Per 15 µg/dL | 0 | — | — | — | — | — | — | — | |
| Cardiovascular disease | High vs. low | 3 | 2246 | 42,636 | 0.83 (0.67, 1.03) | 0 | 0.77 | 0.38 | (104, 106, 108) |
| Per 15 µg/dL | 4 | 2435 | 43,690 | 0.90 (0.76, 1.06) | 0 | 0.70 | 0.79 | (20, 104, 106, 108) | |
| Total cancer | High vs. low | 2 | 777 | 16,421 | 0.83 (0.60, 1.15) | 0 | 0.56 | — | (108, 118) |
| Per 15 µg/dL | 3 | 944 | 14,665 | 0.74 (0.60, 0.91) | 0 | 0.99 | 0.16 | (20, 108, 120) | |
| Mortality | High vs. low | 2 | 3104 | 13,931 | 0.81 (0.64, 1.03) | 27.0 | 0.24 | — | (108, 121) |
| Per 15 µg/dL | 3 | 3821 | 14,985 | 0.84 (0.76, 0.94) | 0 | 0.99 | 0.84 | (20, 108, 121) | |
| Dietary lycopene | |||||||||
| Coronary heart disease | High vs. low | 2 | 1199 | 111,731 | 0.88 (0.71, 1.10) | 11.1 | 0.29 | — | (43, 78) |
| Per 12,000 µg/d | 2 | 1199 | 111,731 | 0.91 (0.76, 1.08) | 19.9 | 0.26 | — | (43, 78) | |
| Stroke | High vs. low | 3 | 1371 | 108,776 | 0.80 (0.63, 1.01) | 35.1 | 0.21 | 0.36 | (45, 47, 78) |
| Per 12,000 µg/d | 3 | 1371 | 108,776 | 0.72 (0.39, 1.34) | 83.7 | 0.002 | 0.31 | (45, 47, 78) | |
| Cardiovascular disease | High vs. low | 1 | 719 | 39,876 | 0.90 (0.69, 1.17) | — | — | — | (78) |
| Per 12,000 µg/d | 2 | 916 | 40,435 | 0.94 (0.79, 1.12) | 0 | 0.32 | — | (55, 78) | |
| Total cancer | High vs. low | 0 | — | — | — | — | — | — | — |
| Per 12,000 µg/d | 0 | — | — | — | — | — | — | — | |
| Mortality | High vs. low | 2 | 881 | 48,805 | 0.74 (0.54, 1.02) | 48.1 | 0.17 | — | (54, 127) |
| Per 12,000 µg/d | 0 | — | — | — | — | — | — | — | |
| Lycopene in blood | |||||||||
| Coronary heart disease | High vs. low | 4 | 1128 | 3179 | 0.90 (0.62, 1.29) | 52.9 | 0.10 | 0.47 | (89, 91, 93, 95) |
| Per 25 µg/dL | 3 | 1005 | 2933 | 1.11 (0.32, 3.85) | 72.7 | 0.03 | 0.15 | (91, 93, 95) | |
| Stroke | High vs. low | 2 | 491 | 1625 | 0.59 (0.36, 0.96) | 0 | 0.35 | — | (99, 100) |
| Per 25 µg/dL | 2 | 491 | 1625 | 0.49 (0.19, 1.25) | 59.7 | 0.12 | — | (99, 100) | |
| Cardiovascular disease | High vs. low | 4 | 1749 | 43,667 | 0.88 (0.70, 1.10) | 8.0 | 0.35 | 0.24 | (104, 106, 108, 109) |
| Per 25 µg/dL | 5 | 1938 | 44,721 | 0.81 (0.63, 1.06) | 34.8 | 0.19 | 0.15 | (20, 104, 106, 108, 109) | |
| Total cancer | High vs. low | 3 | 918 | 17,418 | 0.81 (0.54, 1.21) | 65.5 | 0.06 | 0.13 | (108, 118, 119) |
| Per 25 µg/dL | 5 | 1129 | 18,015 | 0.95 (0.68, 1.33) | 65.9 | 0.02 | 0.13 | (20, 108, 117, 119, 120) | |
| Mortality | High vs. low | 4 | 3233 | 16,438 | 0.91 (0.77, 1.07) | 0 | 0.60 | 0.08 | (108, 117, 121, 124) |
| Per 25 µg/dL | 5 | 3950 | 17,492 | 0.87 (0.74, 1.02) | 25.5 | 0.25 | 0.07 | (20, 108, 117, 121, 124) | |
| Dietary vitamin E | |||||||||
| Coronary heart disease | High vs. low | 9 | 3010 | 239,610 | 0.86 (0.65, 1.05) | 68.2 | 0.001 | 0.81 | (14, 34, 35, 37–39, 41, 49, 77) |
| Per 5 µg/d | 8 | 2880 | 238,855 | 0.94 (0.87, 1.02) | 67.6 | 0.003 | 0.18 | (14, 34, 35, 37–39, 41, 77) | |
| Stroke | High vs. low | 10 | 7003 | 311,965 | 0.89 (0.78, 1.02) | 34.4 | 0.13 | 0.58 | (41, 44–49, 51, 61, 123) |
| Per 5 µg/d | 8 | 6688 | 292,966 | 0.97 (0.93, 1.02) | 54.4 | 0.03 | 0.18 | (41, 44–48, 51, 123) | |
| Cardiovascular disease | High vs. low | 8 | 7852 | 233,310 | 0.90 (0.78, 1.03) | 55.5 | 0.03 | 0.05 | (19, 41, 53, 55, 56, 80, 81) |
| Per 5 µg/d | 8 | 7928 | 233,130 | 0.99 (0.96, 1.01) | 41.6 | 0.10 | 0.06 | (20, 41, 53, 55, 56, 80, 81) | |
| Total cancer | High vs. low | 5 | 5578 | 168,182 | 1.01 (0.92, 1.10) | 0 | 0.69 | 0.72 | (14, 53, 56, 81) |
| Per 5 µg/d | 6 | 5718 | 169,236 | 0.97 (0.93, 1.02) | 61.1 | 0.03 | 0.07 | (14, 20, 53, 56, 81) | |
| Mortality | High vs. low | 9 | 15,321 | 229,830 | 0.98 (0.93, 1.04) | 2.9 | 0.41 | 0.92 | (14, 19, 38, 53, 54, 56, 81, 127) |
| Per 5 µg/d | 8 | 15,429 | 222,223 | 1.00 (0.99, 1.01) | 0 | 0.59 | 0.07 | (14, 20, 38, 53, 54, 56, 81) | |
| α-Tocopherol in blood | |||||||||
| Coronary heart disease | High vs. low | 8 | 1407 | 47,374 | 1.22 (0.96, 1.57) | 0 | 0.80 | 0.18 | (14, 85, 86, 89–91, 95, 96) |
| Per 500 µg/dL | 5 | 1182 | 42,882 | 1.05 (0.95, 1.15) | 18.6 | 0.30 | 0.51 | (14, 90, 91, 95, 96) | |
| Stroke | High vs. low | 5 | 1966 | 69,569 | 0.71 (0.58, 0.85) | 0 | 0.70 | 0.61 | (85, 96, 97, 99, 100) |
| Per 500 µg/dL | 4 | 1951 | 69,386 | 0.90 (0.86, 0.95) | 0 | 0.43 | 0.61 | (96, 97, 99, 100) | |
| Cardiovascular disease | High vs. low | 6 | 8053 | 47,012 | 0.79 (0.56, 1.10) | 68.8 | 0.007 | 0.69 | (19, 21, 70, 79, 85, 103) |
| Per 500 µg/dL | 8 | 8607 | 51,283 | 0.92 (0.82, 1.03) | 69.9 | 0.002 | 0.97 | (19–21, 70, 79, 102, 103, 107) | |
| Total cancer | High vs. low | 10 | 7201 | 56,258 | 0.80 (0.74, 0.87) | 0 | 0.73 | 0.34 | (14, 21, 70, 110–114, 116, 118) |
| Per 500 µg/dL | 10 | 6919 | 51,210 | 0.91 (0.83, 0.99) | 70.1 | <0.0001 | 0.77 | (14, 20, 21, 70, 102, 107, 111–114) | |
| Mortality | High vs. low | 6 | 18,316 | 47,853 | 0.89 (0.72, 1.08) | 59.6 | 0.03 | 0.79 | (14, 19, 21, 70, 121, 124) |
| Per 500 µg/dL | 9 | 20,051 | 52,376 | 0.94 (0.89,0.99) | 56.3 | 0.02 | 0.97 | (14, 19–21, 70, 102, 107, 121, 124) | |
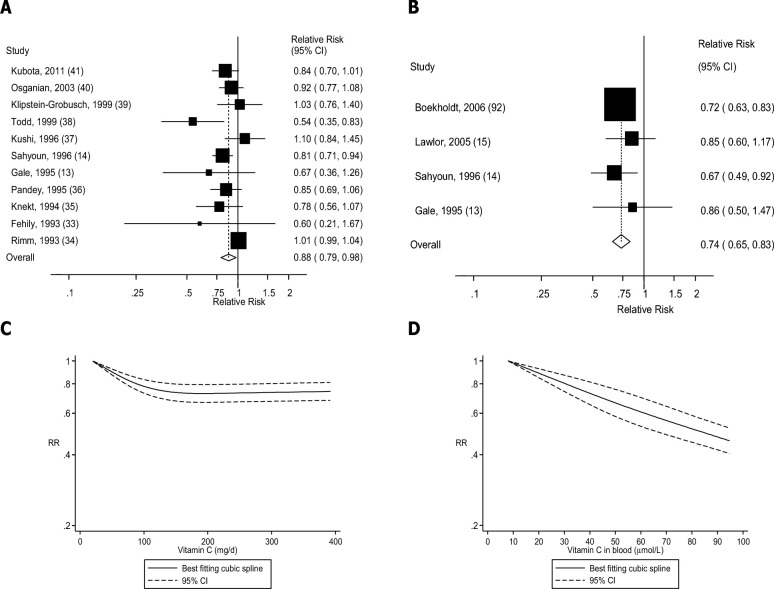
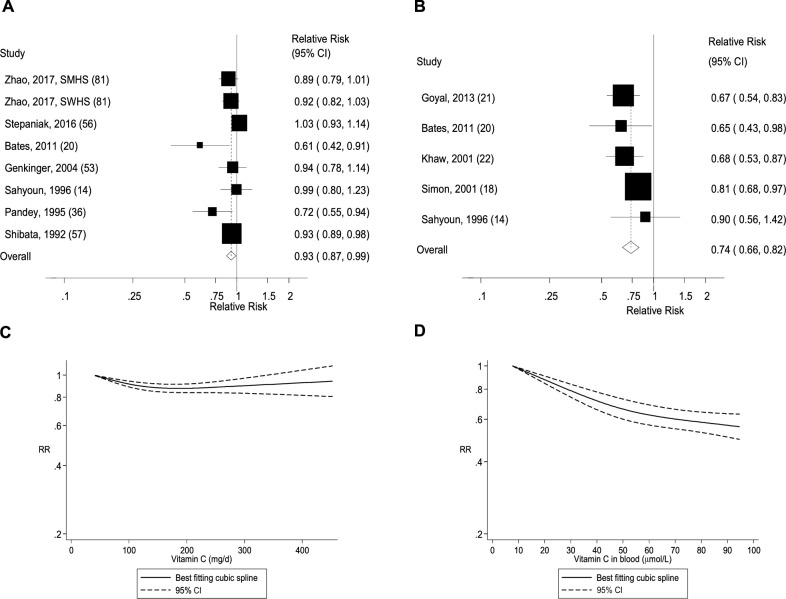
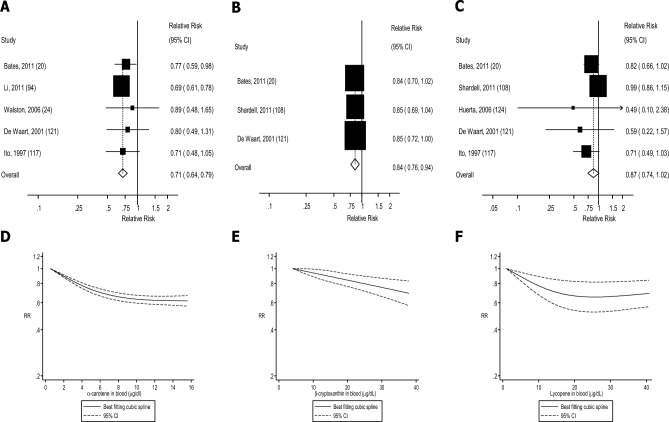
FIGURE 2.
Dietary intake and blood concentrations of vitamin C and coronary heart disease: dose-response analyses. (A) Dietary vitamin C and coronary heart disease: linear dose-response analysis. The summary RR per 100 mg/d was 0.88 (95% CI: 0.79, 0.98, I2 = 65%, Pheterogeneity = 0.001, n = 11). (B) Vitamin C in blood and coronary heart disease: linear dose-response analysis. The summary RR per 50 µmol/L was 0.74 (95% CI: 0.65, 0.83, I2 = 0%, Pheterogeneity = 0.71, n = 4). (C) Dietary vitamin C and coronary heart disease: nonlinear dose-response analysis. There was evidence of nonlinearity between dietary vitamin C and coronary heart disease (Pnonlinearity < 0.0001). (D) Vitamin C in blood and coronary heart disease: nonlinear dose-response analysis. There was no evidence of nonlinearity for vitamin C in blood and coronary heart disease (Pnonlinearity = 0.49). Summary RRs and 95% CIs were calculated with the use of random-effects models, and the nonlinear dose-response analyses were conducted with the use of restricted cubic splines.
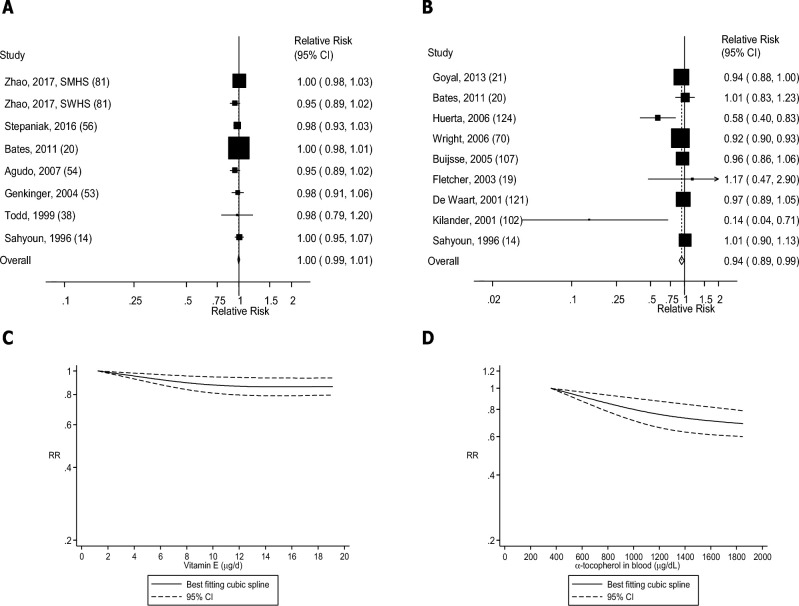
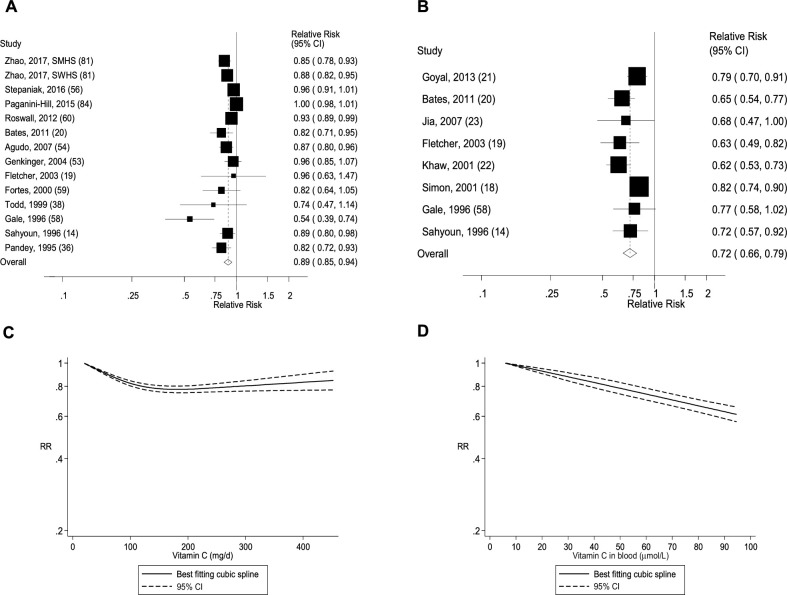
FIGURE 14.
(A) Dietary intake of vitamin E and mortality: linear dose-response analysis. The summary RR per 5 µg/d was 0.99 (95% CI: 0.96, 1.01, I2 = 42%, Pheterogeneity = 0.10, n = 8). (B) Blood concentrations of α-tocopherol and mortality: linear dose-response analysis. The summary RR per 500 µg/dL was 0.94 (95% CI: 0.90, 0.98, I2 = 43%, Pheterogeneity = 0.09, n = 8). (C) Dietary intake of vitamin E and mortality: nonlinear dose-response analysis. There was evidence of nonlinearity between dietary vitamin E and mortality (Pnonlinearity < 0.0001). (D) Blood concentrations of α-tocopherol and mortality: nonlinear dose-response analysis. There was indication of nonlinearity for α-tocopherol in blood and mortality (Pnonlinearity = 0.05). Summary RRs and 95% CIs were calculated with the use of random-effects models, and the nonlinear dose-response analyses were conducted with the use of restricted cubic splines. SMHS, Shanghai Men's Health Study; SWHS, Shanghai Women's Health Study.
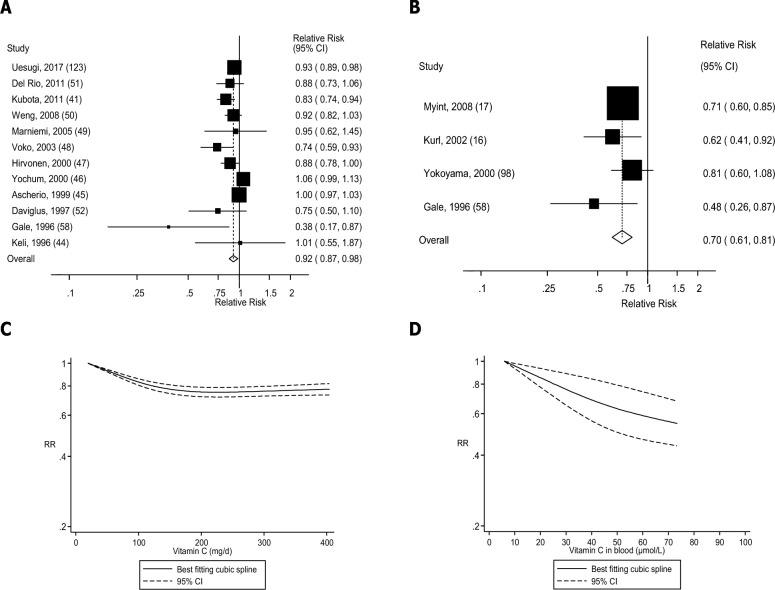
FIGURE 3.
Dietary intake and blood concentrations of vitamin C and stroke: dose-response analyses. (A) Dietary vitamin C and stroke: linear dose-response analysis. The summary RR per 100 mg/d was 0.92 (95% CI: 0.87, 0.98, I2 = 68%, Pheterogeneity < 0.0001, n = 12). (B) Vitamin C in blood and stroke: linear dose-response analysis. The summary RR per 50 µmol/L was 0.70 (95% CI: 0.61, 0.81, I2 = 0%, Pheterogeneity = 0.41, n = 4). (C) Dietary vitamin C and stroke: nonlinear dose-response analysis. There was evidence of nonlinearity between dietary vitamin C and stroke (Pnonlinearity < 0.0001). (D) Vitamin C in blood and stroke: nonlinear dose-response analysis. There was no evidence of nonlinearity for vitamin C in blood and stroke (Pnonlinearity = 0.16). Summary RRs and 95% CIs were calculated with the use of random-effects models, and the nonlinear dose-response analyses were conducted with the use of restricted cubic splines.
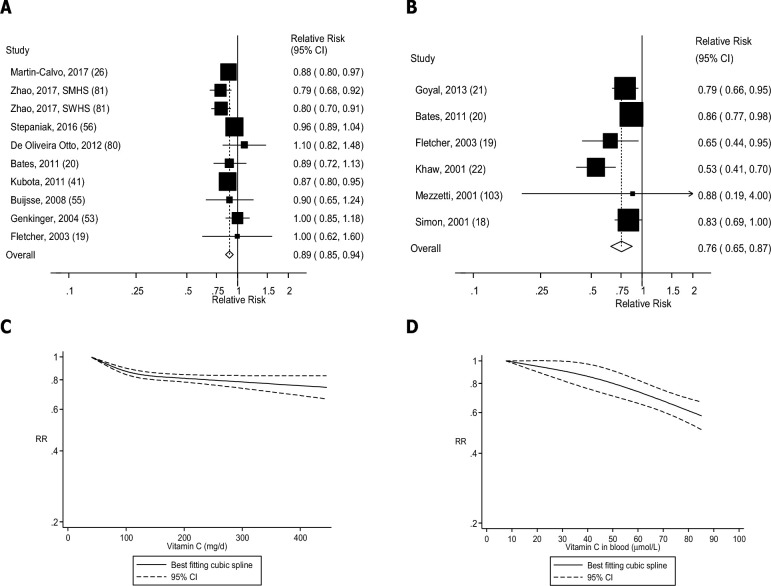
FIGURE 4.
Dietary intake and blood concentrations of vitamin C and cardiovascular disease: dose-response analyses. (A) Dietary vitamin C and cardiovascular disease: linear dose-response analysis. The summary RR per 100 mg/d was 0.89 (95% CI: 0.85, 0.94, I2 = 27%, Pheterogeneity = 0.19, n = 10). (B) Vitamin C in blood and cardiovascular disease: linear dose-response analysis. The summary RR per 50 µmol/L was 0.76 (95% CI: 0.65, 0.87, I2 = 56%, Pheterogeneity = 0.05, n = 6). (C) Dietary vitamin C and cardiovascular disease: nonlinear dose-response analysis. There was evidence of nonlinearity between dietary vitamin C and cardiovascular disease (Pnonlinearity < 0.0001). (D) Vitamin C in blood and cardiovascular disease: nonlinear dose-response analysis. There was no evidence of nonlinearity for vitamin C in blood and cardiovascular disease (Pnonlinearity = 0.26). Summary RRs and 95% CIs were calculated with the use of random-effects models, and the nonlinear dose-response analyses were conducted with the use of restricted cubic splines. SMHS, Shanghai Men's Health Study; SWHS, Shanghai Women's Health Study.
FIGURE 5.
Dietary intake and blood concentrations of vitamin C and total cancer: dose-response analyses. (A) Dietary vitamin C and total cancer: linear dose-response analysis. The summary RR per 100 mg/d was 0.93 (95% CI: 0.87, 0.99, I2 = 46%, Pheterogeneity = 0.08, n = 8). (B) Vitamin C in blood and total cancer: linear dose-response analysis. The summary RR per 50 µmol/L was 0.74 (95% CI: 0.66, 0.82, I2 = 0%, Pheterogeneity = 0.49, n = 5). (C) Dietary vitamin C and total cancer: nonlinear dose-response analysis. There was evidence of nonlinearity between dietary vitamin C and total cancer (Pnonlinearity = 0.007). (D) Vitamin C in blood and total cancer: nonlinear dose-response analysis. There was evidence of nonlinearity for vitamin C in blood and total cancer (Pnonlinearity = 0.006). Summary RRs and 95% CIs were calculated with the use of random-effects models, and the nonlinear dose-response analyses were conducted with the use of restricted cubic splines. SMHS, Shanghai Men's Health Study; SWHS, Shanghai Women's Health Study.
Dietary vitamin C intake
Twelve (13, 14, 33–41, 49), thirteen (41, 44–52, 58, 61, 123), eleven (9 publications) (19, 26, 41, 53–56, 80, 81), nine (7 publications) (14, 20, 36, 53, 56, 57, 81), and seventeen (16 publications) (14, 19, 20, 36, 38, 53, 54, 56, 58–60, 81–84, 127) studies were included in the analysis of dietary vitamin C intake and risk of coronary heart disease, stroke, cardiovascular disease, total cancer, and all-cause mortality, respectively. A statistically significant 12%, 8%, 11%, 7%, and 11% reduction in the RR was observed for coronary heart disease, stroke, cardiovascular disease, total cancer, and mortality (Figures 2A–6A, Table 1, Supplemental Figures 1–5), respectively. There was evidence of nonlinearity for coronary heart disease (Pnonlinearity < 0.0001), stroke (Pnonlinearity < 0.0001), cardiovascular disease (Pnonlinearity < 0.0001), total cancer (Pnonlinearity = 0.006), and mortality (Pnonlinearity < 0.0001) with stronger reductions in risk at lower amounts of intake in all analyses (Figures 2C–6C, Supplemental Table 13).
FIGURE 6.
Dietary intake and blood concentrations of vitamin C and mortality: dose-response analyses. (A) Dietary vitamin C and mortality: linear dose-response analysis. The summary RR per 100 mg/d was 0.89 (95% CI: 0.85, 0.94, I2 = 80%, Pheterogeneity < 0.0001, n = 14). (B) Vitamin C in blood and mortality: linear dose-response analysis. The summary RR per 50 µmol/L was 0.72 (95% CI: 0.66, 0.79, I2 = 48%, Pheterogeneity = 0.06, n = 8). (C) Dietary vitamin C and mortality: nonlinear dose-response analysis. There was evidence of nonlinearity between dietary vitamin C and mortality (Pnonlinearity < 0.0001). (D) Vitamin C in blood and mortality: nonlinear dose-response analysis. There was no evidence of nonlinearity for vitamin C in blood and mortality (Pnonlinearity = 0.90). Summary RRs and 95% CIs were calculated with the use of random-effects models, and the nonlinear dose-response analyses were conducted with the use of restricted cubic splines. SMHS, Shanghai Men's Health Study; SWHS, Shanghai Women's Health Study.
Vitamin C in blood
Four (13–15, 92), five (16, 17, 58, 87, 98), six (18–22, 103), six (14, 18, 20–22, 116), and eight (9 publications) (14, 18–23, 58, 126) studies were included in the analysis of blood concentrations of vitamin C and risk of coronary heart disease, stroke, cardiovascular disease, total cancer, and mortality, respectively. Inverse associations were observed for all outcomes, with 24–30% reductions in the RR (Figures 2B–6B, Supplemental Figures 6–10). There was evidence of a nonlinear association between vitamin C in blood and total cancer (Pnonlinearity = 0.006), with stronger inverse associations at lower vitamin C concentrations but not for coronary heart disease (P = 0.49), stroke (Pnonlinearity = 0.16), cardiovascular disease (Pnonlinearity = 0.26), or mortality (Pnonlinearity = 0.90) (Figures 2D–6D, Supplemental Table 14).
Total dietary carotenoid intake
Five (14, 34, 35, 37, 42), four (3 publications) (20, 55, 81), four (3 publications) (14, 20, 81), and six (5 publications) (14, 20, 42, 54, 81) studies were included in the analysis of total dietary carotenoid intake and risk of coronary heart disease, cardiovascular disease, total cancer, and mortality, respectively. A statistically significant 15%, 20%, and 12% reduction in the RR was observed for coronary heart disease, cardiovascular disease, and mortality (Figure 7A, Table 1, Supplemental Figures 11A, 12, 13, 14A, and 17), respectively, but no association was observed for total cancer (Table 1, Supplemental Figures 15 and 16A). There was evidence for a nonlinear association between total carotenoid intake and cardiovascular disease (Pnonlinearity = 0.002), total cancer (Pnonlinearity = 0.01), and mortality (Pnonlinearity < 0.0001), but not for coronary heart disease (Pnonlinearity = 0.95) (Figure 7B, Supplemental Table 15, Supplemental Figures 11B, 14B, and 16B), with most of the reduction in risk observed up to an intake of between 4000 and 6000 µg/d.
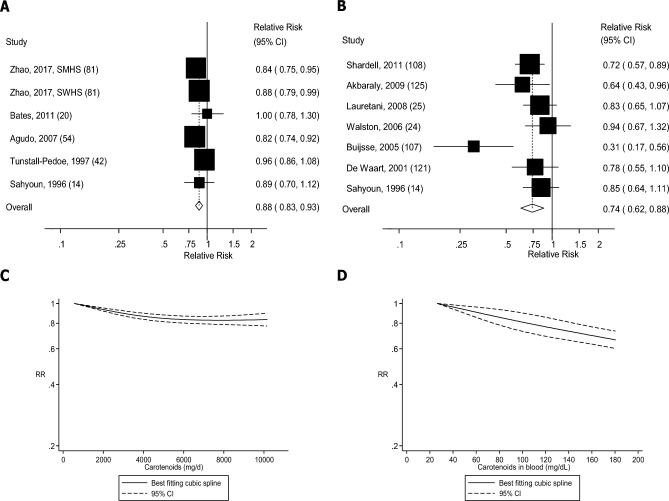
FIGURE 7.
Dietary intake and blood concentrations of carotenoids and mortality: dose-response analyses. (A) Dietary carotenoids and mortality: linear dose-response analysis. The summary RR per 5000 µg/d was 0.88 (95% CI: 0.83, 0.93, I2 = 2%, Pheterogeneity = 0.40, n = 6). (B) Carotenoids in blood and mortality: linear dose-response analysis. The summary RR per 50 µg/dL was 0.69 (95% CI: 0.59, 0.81, I2 = 50%, Pheterogeneity = 0.04, n = 10). (C) Dietary carotenoids and mortality: nonlinear dose-response analysis. There was evidence of nonlinearity between dietary carotenoids and mortality (Pnonlinearity = 0.01). (D) Carotenoids in blood and mortality: nonlinear dose-response analysis. There was no evidence of nonlinearity for vitamin C in blood and mortality (Pnonlinearity = 0.73). Summary RRs and 95% CIs were calculated with the use of random-effects models, and the nonlinear dose-response analyses were conducted with the use of restricted cubic splines. SMHS, Shanghai Men's Health Study; SWHS, Shanghai Women's Health Study.
Total carotenoids in blood
Four (14, 87, 88, 90), two (108, 109), three (14, 108, 110), and seven (14, 24, 25, 107, 108, 121, 125) studies were included in the analyses of blood concentrations of total carotenoids and risk of coronary heart disease, cardiovascular disease, total cancer, and mortality, respectively. A statistically significant 17% and 31% reduction in RR was observed for coronary heart disease and mortality, respectively, and a significant association was observed for total cancer only in the high compared with low analysis and the nonlinear dose-response analysis (Table 1, Figure 7B, Supplemental Figures 18A, 19, and 22A–24), whereas the association for cardiovascular disease only was significant in the nonlinear analysis (Table 1, Supplemental Figures 20 and 21, Supplemental Table 16). There was evidence of a nonlinear association for coronary heart disease (Pnonlinearity = 0.008) and cardiovascular disease (Pnonlinearity = 0.004), but not for total cancer (Pnonlinearity = 0.33) or mortality (Pnonlinearity = 0.73) (Figure 7, Supplemental Figures 18D, 20D, and 22D, Supplemental Table 16).
Dietary β-carotene
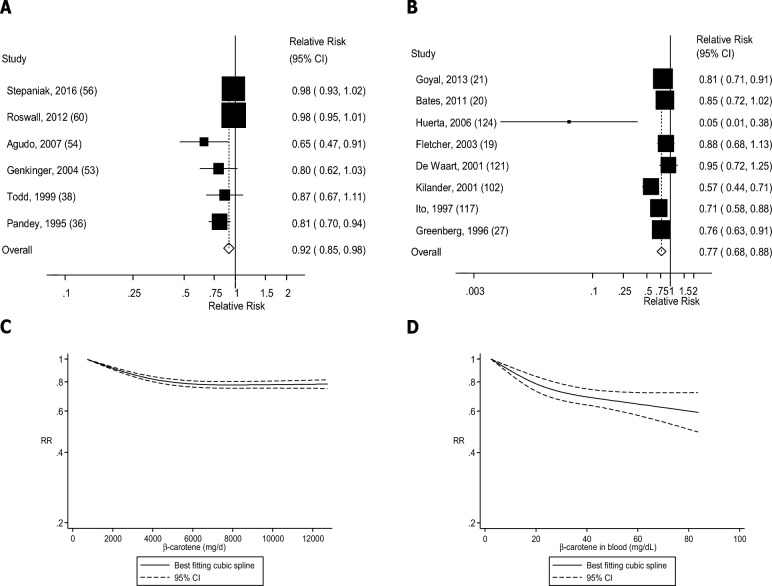
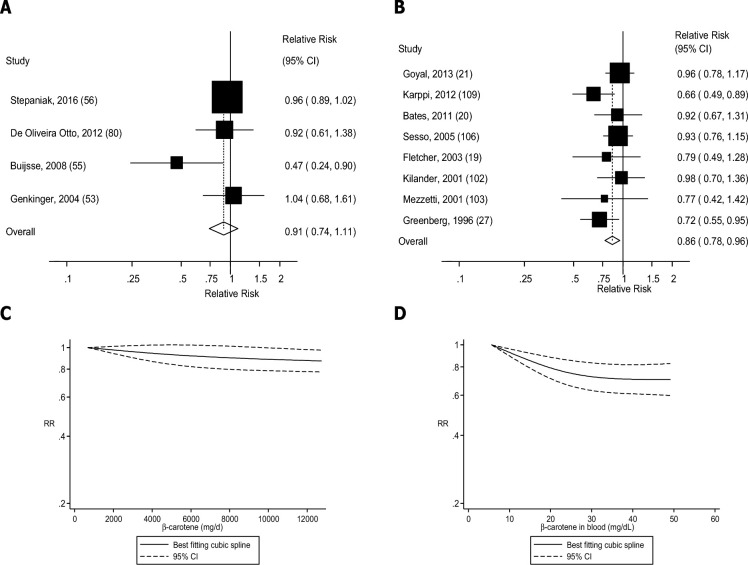
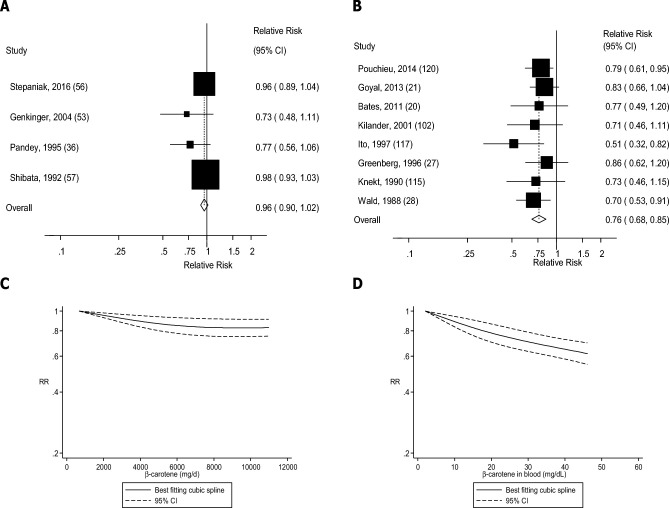
Four (36, 38, 39, 43), seven (44, 45, 47, 48, 51, 52, 123), six (5 publications) (19, 53, 55, 56, 80), five (4 publications) (36, 53, 56, 57), and seven (6 publications) (36, 38, 53, 54, 56, 60) studies were included in the analysis of dietary β-carotene intake and risk of coronary heart disease, stroke, cardiovascular disease, total cancer, and mortality, respectively. Dietary β-carotene was associated with 8–19% reductions in the RRs of coronary heart disease, stroke, and mortality in the dose-response analysis (Table 1, Figures 8A, 9A, and 12A, Supplemental Figures 25, 26, and 29), but there was no association for cardiovascular disease and total cancer (Figures 10A and 11A, Table 1, Supplemental Figures 27 and 28). There was evidence of a nonlinear association between β-carotene intake and coronary heart disease (Pnonlinearity = 0.006), stroke (Pnonlinearity < 0.0001), total cancer (Pnonlinearity = 0.003), and mortality (Pnonlinearity < 0.0001), with a stronger reduction in risk at the lower amounts of intake, but there was no evidence of an association or nonlinearity for cardiovascular disease (Pnonlinearity = 0.51) (Figures 8C–12C, Supplemental Table 17).
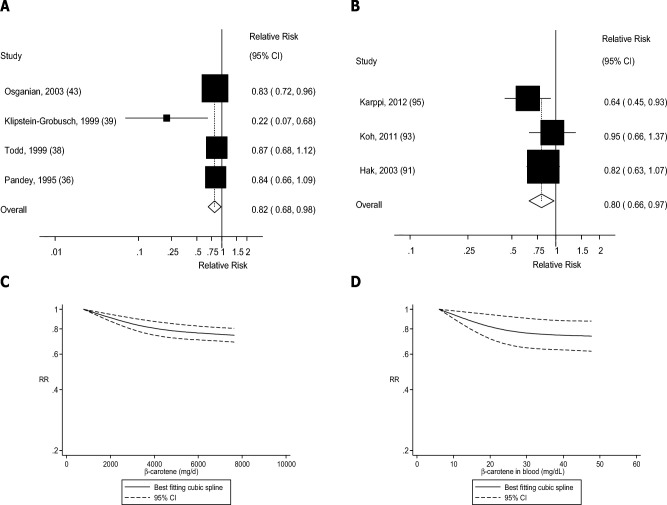
FIGURE 8.
Dietary β-carotene and blood concentrations of β-carotene and coronary heart disease: dose-response analyses. (A) Dietary β-carotene and coronary heart disease: linear dose-response analysis. The summary RR per 5000 µg/d was 0.82 (95% CI: 0.68, 0.98, I2 = 45%, Pheterogeneity = 0.14, n = 4). (B) β-Carotene in blood and coronary heart disease: linear dose-response analysis. The summary RR per 25 µg/dL was 0.76 (95% CI: 0.62, 0.93, I2 = 22%, Pheterogeneity = 0.28, n = 4). (C) Dietary β-carotene and coronary heart disease: nonlinear dose-response analysis. There was evidence of nonlinearity between dietary β-carotene and coronary heart disease (Pnonlinearity = 0.006). (D) β-Carotene in blood and coronary heart disease: nonlinear dose-response analysis. There was evidence of nonlinearity for β-carotene in blood and coronary heart disease (Pnonlinearity = 0.002). Summary RRs and 95% CIs were calculated with the use of random-effects models, and the nonlinear dose-response analyses were conducted with the use of restricted cubic splines.
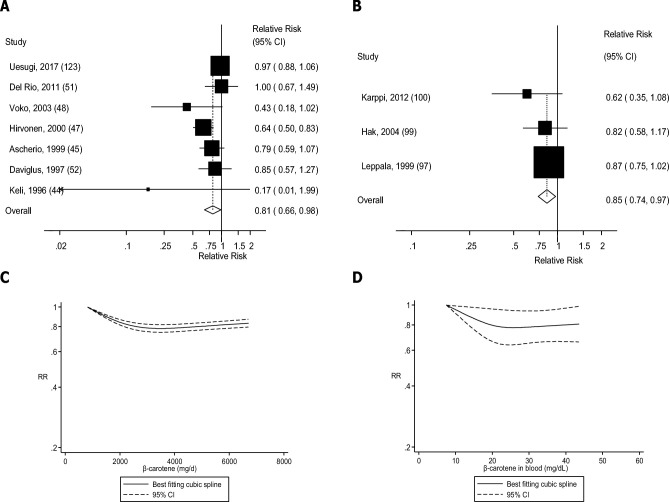
FIGURE 9.
(A) Dietary β-carotene and stroke: linear dose-response analyses. The summary RR per 5000 µg/d was 0.81 (95% CI: 0.66, 0.98, I2 = 59%, Pheterogeneity = 0.02, n = 7). (B) β-Carotene in blood and stroke: linear dose-response analysis. The summary RR per 25 µg/dL was 0.85 (95% CI: 0.74, 0.97, I2 = 0%, Pheterogeneity = 0.50, n = 3). (C) Dietary β-carotene and stroke: nonlinear dose-response analysis. There was evidence of nonlinearity between dietary β-carotene and stroke (Pnonlinearity < 0.0001). (D) β-Carotene in blood and stroke: nonlinear dose-response analysis. There was evidence of nonlinearity for β-carotene in blood and stroke (Pnonlinearity = 0.07). Summary RRs and 95% CIs were calculated with the use of random-effects models, and the nonlinear dose-response analyses were conducted with the use of restricted cubic splines.
FIGURE 12.
Dietary intake and blood concentrations of β-carotene and mortality: dose-response analyses. (A) Dietary β-carotene and mortality: linear dose-response analysis. The summary RR per 5000 µg/d was 0.92 (95% CI: 0.85, 0.98, I2 = 66%, Pheterogeneity = 0.01, n = 6). (B) β-Carotene in blood and mortality: linear dose-response analysis. The summary RR per 25 µg/dL was 0.81 (95% CI: 0.72, 0.90, I2 = 47%, Pheterogeneity = 0.08, n = 7). (C) Dietary β-carotene and mortality: nonlinear dose-response analysis. There was evidence of nonlinearity between dietary β-carotene and mortality (Pnonlinearity ≤ 0.0001). (D) β-Carotene in blood and mortality: nonlinear dose-response analysis. There was evidence of nonlinearity for β-carotene in blood and mortality (Pnonlinearity = 0.005). Summary RRs and 95% CIs were calculated with the use of random-effects models, and the nonlinear dose-response analyses were conducted with the use of restricted cubic splines.
FIGURE 10.
Dietary β-carotene and blood concentrations of β-carotene and cardiovascular disease: dose-response analyses. (A) Dietary β-carotene and cardiovascular disease: linear dose-response analysis. The summary RR per 5000 µg/d was 0.87 (95% CI: 0.63, 1.20, I2 = 58%, Pheterogeneity = 0.10, n = 3). (B) β-Carotene in blood and cardiovascular disease: linear dose-response analysis. The summary RR per 25 µg/dL was 0.85 (95% CI: 0.76, 0.95, I2 = 9%, Pheterogeneity = 0.36, n = 7). (C) Dietary β-carotene and cardiovascular disease: nonlinear dose-response analysis. There was no evidence of nonlinearity between dietary β-carotene and cardiovascular disease (Pnonlinearity = 0.51). (D) β-Carotene in blood and cardiovascular disease: nonlinear dose-response analysis. There was evidence of nonlinearity for β-carotene in blood and cardiovascular disease (Pnonlinearity = 0.006). Summary RRs and 95% CIs were calculated with the use of random-effects models, and the nonlinear dose-response analyses were conducted with the use of restricted cubic splines.
FIGURE 11.
Dietary β-carotene and blood concentrations of β-carotene and total cancer: dose-response analyses. (A) Dietary β-carotene and total cancer: linear dose-response analysis. The summary RR per 5000 µg/d was 0.96 (95% CI: 0.90, 1.02, I2 = 25%, Pheterogeneity = 0.26, n = 4). (B) β-Carotene in blood and total cancer: linear dose-response analysis. The summary RR per 25 µg/dL was 0.77 (95% CI: 0.68, 0.86, I2 = 0%, Pheterogeneity = 0.64, n = 7). (C) Dietary β-carotene and total cancer: nonlinear dose-response analysis. There was evidence of nonlinearity between dietary β-carotene and total cancer (Pnonlinearity = 0.003). (D) β-Carotene in blood and total cancer: nonlinear dose-response analysis. There was evidence of nonlinearity for β-carotene in blood and total cancer (Pnonlinearity = 0.60). Summary RRs and 95% CIs were calculated with the use of random-effects models, and the nonlinear dose-response analyses were conducted with the use of restricted cubic splines.
β-Carotene in blood
Four (89, 91, 93, 95), three (97, 99, 100), seven (19–21, 27, 103, 106, 109), seven (20, 21, 27, 28, 115, 117, 120), and seven (19–21, 27, 117, 121, 124) studies were included in the analysis of blood concentrations of β-carotene and risk of coronary heart disease, stroke, cardiovascular disease, total cancer, and mortality, respectively. A 15–24% reduction in RR was observed for blood concentrations of β-carotene in relation to all outcomes (Table 1, Figures 8B–12B, Supplemental Figures 30–34). There was indication of a nonlinear association for coronary heart disease (Pnonlinearity = 0.002), stroke (Pnonlinearity = 0.07), cardiovascular disease (Pnonlinearity = 0.006), and mortality (Pnonlinearity = 0.005), with stronger associations at the lower β-carotene concentrations, but not for total cancer (Pnonlinearity = 0.60) (Figures 8D–12D, Supplemental Table 18).
Dietary α-carotene
Two (45, 123) and two (54, 123) studies were included in the analysis of dietary α-carotene and risk of stroke and mortality, respectively. There was a 22% reduction in the RR of mortality for high compared with low intake of α-carotene, but no association was observed for stroke (Supplemental Figures 35–37).
α-Carotene in blood
Four (91, 93–95), three (94, 99, 100), five (20, 94, 106, 108, 109), four (5 publications) (20, 94, 117, 118, 120), and five (20, 24, 94, 117, 121) studies were included in the analysis of blood concentrations of α-carotene and risk of coronary heart disease, stroke, cardiovascular disease, total cancer, and mortality, respectively. A 42% and 29% reduction in RR for total cancer and mortality was observed for α-carotene in blood, but no association was observed for coronary heart disease, stroke, or cardiovascular disease (Figure 13A, Table 1, Supplemental Figures 38–46). There was evidence of nonlinear associations for coronary heart disease (Pnonlinearity = 0.01), cardiovascular disease (Pnonlinearity <0.0001), total cancer (Pnonlinearity < 0.0001), and mortality (Pnonlinearity < 0.0001), with stronger inverse associations at the lower blood concentrations than at higher blood concentrations, but not for stroke (Pnonlinearity = 0.87) (Figure 13D, Supplemental Figures 38B, 40B, 42B, and 44B, Supplemental Table 19).
FIGURE 13.
Blood concentrations of α-carotene, β-cryptoxanthin, and lycopene and mortality: dose-response analyses. (A) Blood concentrations of α-carotene and mortality: linear dose-response analysis. The summary RR per 10 µg/dL was 0.71 (95% CI: 0.64, 0.79, I2 = 0%, Pheterogeneity = 0.86, n = 5). (B) Blood concentrations of β-cryptoxanthin and mortality: linear dose-response analysis. The summary RR per 15 µg/dL was 0.84 (95% CI: 0.76, 0.94, I2 = 0%, Pheterogeneity = 0.99, n = 3). (C) Blood concentrations of lycopene and mortality: linear dose-response analysis. The summary RR per 25 µg/dL was 0.87 (95% CI: 0.74, 1.02, I2 = 26%, Pheterogeneity = 0.25, n = 5). (D) Blood concentrations of α-carotene and mortality, nonlinear dose-response analysis. There was evidence of nonlinearity between α-carotene in blood and mortality (Pnonlinearity ≤ 0.0001). (E) Blood concentrations of β-cryptoxanthin and mortality: nonlinear dose-response analysis. There was no evidence of nonlinearity for β-cryptoxanthin in blood and mortality (Pnonlinearity = 0.98). (F) Blood concentrations of lycopene and mortality: nonlinear dose-response analysis. There was evidence of nonlinearity for lycopene in blood and mortality (Pnonlinearity = 0.001). Summary RRs and 95% CIs were calculated with the use of random-effects models, and the nonlinear dose-response analyses were conducted using restricted cubic splines.
Dietary β-cryptoxanthin
Two studies (54, 123) were included in the high compared with low analysis of dietary β-cryptoxanthin and mortality. There was a 26% reduction in the RR for high compared with low intake (Supplemental Figure 47).
β-Cryptoxanthin in blood
Two (91, 93), four (20, 104, 106, 108), three (20, 108, 120), and three (20, 108, 121) studies were included in the analyses of blood concentrations of β-cryptoxanthin and risk of coronary heart disease, cardiovascular disease, total cancer, and mortality, respectively. A 26% and 16% reduction in the RR of total cancer and mortality was observed, respectively, but no significant association was observed for coronary heart disease and cardiovascular disease (Figure 13B, Table 1, Supplemental Figures 48–54). There was evidence of a nonlinear association between β-cryptoxanthin in blood and coronary heart disease (Pnonlinearity = 0.01), but not for cardiovascular disease (Pnonlinearity = 0.20) or mortality (Pnonlinearity = 0.98) (Figure 13E, Supplemental Figures 48B and 50B, Supplemental Table 20).
Dietary lutein
Two (54, 127) studies were included in the high compared with low analysis of dietary lutein and mortality and there was no significant association (Supplemental Figure 55).
Lutein in blood
Three (89, 91, 93) studies were included in the high compared with low analysis of lutein in blood and risk of coronary heart disease and there was no significant association (Supplemental Figure 56).
Dietary zeaxanthin
Two (89, 93) and two (54, 127) studies were included in the high compared with low analysis of dietary zeaxanthin and risk of coronary heart disease and mortality, respectively, and there was no significant association in both analyses (Supplemental Figures 57 and 58).
Lutein and zeaxanthin in blood
Four (20, 104, 106, 108), three (20, 108, 118), and two (20, 108) studies were included in the analyses of lutein and zeaxanthin in blood and risk of cardiovascular disease, total cancer, and mortality, respectively. No significant association was observed in all analyses (Supplemental Figures 59–62).
Dietary lycopene
Two (43, 78), three (45, 47, 78), and two (55, 78) studies were included in the analysis of dietary lycopene and risk of coronary heart disease, stroke, and cardiovascular disease. No studies were identified for total cancer, and only 2 were identified for mortality (54, 127), but dose-response analyses were not possible. There was no association between dietary lycopene and coronary heart disease, stroke, cardiovascular disease, or all-cause mortality (Table 1, Supplemental Figures 63–68). There was no evidence of a nonlinear association between lycopene intake and coronary heart disease (Pnonlinearity = 0.97) or stroke (Pnonlinearity = 0.81) (Supplemental Figures 63B and 65B, Supplemental Table 21).
Lycopene in blood
Three (91, 93, 95), two (93, 95), five (20, 95, 104, 106, 108), five (20, 108, 117, 119, 120), and five (20, 108, 117, 121, 124) studies were included in the analysis of blood concentrations of lycopene and risk of coronary heart disease, stroke, cardiovascular disease, total cancer, and mortality, respectively. There was no significant association between lycopene in blood and any of the outcomes in the linear dose-response analyses or high and low analyses (Figure 13C, Table 1, Supplemental Figures 69–77). Although the test for nonlinearity was not significant for coronary heart disease (Pnonlinearity = 0.30), there was indication of a nonlinear association between lycopene in blood and stroke (Pnonlinearity < 0.0001), cardiovascular disease (Pnonlinearity = 0.005), total cancer (Pnonlinearity < 0.0001), and mortality (Pnonlinearity = 0.001), and most of the reduction in risk was observed at the lower range of lycopene concentrations (Figure 13F, Supplemental Figures 69B, 71B, 73B, and 75B, Supplemental Table 22).
Dietary vitamin E
Nine (14, 34, 35, 37–39, 41, 49, 77), ten (41, 44–48, 49, 51, 61, 123), nine (7 publications) (20, 41, 53, 55, 56, 80, 81), seven (5 publications) (14, 20, 53, 56, 81), and eleven (9 publications) (14, 19, 20, 38, 53, 54, 56, 81, 127) studies were included in the analysis of dietary vitamin E (including both diet and total vitamin E) and risk of coronary heart disease, stroke, cardiovascular disease, total cancer, and mortality, respectively. Dietary vitamin E was not significantly associated with any of the outcomes in the linear dose-response analysis (Table 1, Figure 14A, Supplemental Figures 78–86). However, there was indication of nonlinearity between vitamin E and coronary heart disease (Pnonlinearity < 0.0001), stroke (Pnonlinearity < 0.0001), cardiovascular disease (Pnonlinearity < 0.0001), total cancer (Pnonlinearity = 0.003), and mortality (Pnonlinearity < 0.0001) (Figure 14, Supplemental Figures 78C, 80C, 82C, and 84C, Supplemental Table 23), with stronger reductions in risk at lower amounts of intake.
α-Tocopherol in blood
Five (14, 90, 91, 95, 96), four (96, 97, 99, 100), seven (19–21, 70, 79, 103, 107), eight (9 publications) (14, 20, 21, 70, 107, 110, 112–114), and eight (14, 19–21, 70, 107, 121, 124) studies were included in the analysis of α-tocopherol in blood and risk of coronary heart disease, stroke, cardiovascular disease, total cancer, and mortality, respectively. There was a 10%, 9%, and 6% reduction in the risk of stroke, total cancer, and mortality, respectively, for each 500-μg/dL increase in blood concentrations of α-tocopherol, but no significant association was observed for coronary heart disease or cardiovascular disease overall (Figure 14B, Supplemental Figures 78B, 80B, 82B, 84B, and 87–91). The test for nonlinearity was significant for coronary heart disease (Pnonlinearity = 0.02) and mortality (Pnonlinearity = 0.05), but not for stroke (Pnonlinearity = 0.79), cardiovascular disease (Pnonlinearity = 0.32), or total cancer (Pnonlinearity = 0.59) (Figure 14, Supplemental Figures 78D, 80D, 82D, and 84D, Supplemental Table 24).
γ-Tocopherol in blood
Three (90, 91, 96) and two (91, 96) studies were included in the analyses of γ-tocopherol in blood and coronary heart disease and stroke, respectively. No significant association was observed in both analyses (Supplemental Figures 92–95).
Publication bias, subgroup and sensitivity analyses, influence analyses, study quality.
There was evidence of publication bias in the following analyses: dietary vitamin C and coronary heart disease, stroke, and all-cause mortality; carotenoids in blood and all-cause mortality; dietary β-carotene and stroke, total cancer, and all-cause mortality; α-carotene in blood and all-cause mortality; lycopene in blood and all-cause mortality; and dietary vitamin E and cardiovascular disease, total cancer, and all-cause mortality (Supplemental Figures 96–107).
In stratified analyses there was little evidence of heterogeneity between subgroups of studies that investigated vitamin C intake and coronary heart disease, stroke, cardiovascular disease, total cancer, or mortality (Supplemental Tables 25–26). In the analysis of blood concentrations of vitamin C and cardiovascular disease there was evidence of heterogeneity among subgroups of studies that excluded subjects with prevalent disease at baseline (P = 0.04), with a stronger association among studies with such exclusions compared with those without (Supplemental Table 27). In the analysis of blood concentrations of vitamin C and mortality, there was heterogeneity by duration of follow-up (P = 0.02), with a weaker association among studies with longer follow-up than among studies with shorter follow-up (Supplemental Table 27), and by geographic location (P = 0.02), with a stronger association among European studies than among American studies. There was also heterogeneity between subgroups which adjusted for education, smoking, alcohol, BMI, physical activity, hypertension, and serum cholesterol (Pheterogeneity = 0.02 or 0.03 for all) (Supplemental Table 27), with weaker associations among studies with such adjustment; however, the inverse associations persisted among studies with such adjustment. There was no between-subgroup heterogeneity for carotenoids in blood and mortality (Supplemental Table 29), dietary β-carotene and stroke (Supplemental Table 30), and β-carotene in blood and cardiovascular disease, total cancer, and mortality (Supplemental Table 31), and little evidence of heterogeneity among studies of vitamin E and coronary heart disease, stroke, cardiovascular disease, total cancer, and all-cause mortality (Supplemental Tables 32–33), and among studies of α-tocopherol in blood and cardiovascular disease, total cancer, and mortality (Supplemental Table 34).
In further influence analyses excluding 1 study at a time, most of the associations were robust to the influence of individual studies (Supplemental Figures 108–125).
The study quality of the included studies was, in general, high as the vast majority of the included studies were in the subgroup with 7–9 points (Supplemental Tables 25–34).
DISCUSSION
This meta-analysis showed an inverse association between dietary intake and blood concentrations of vitamin C and risk of coronary heart disease, stroke, cardiovascular disease overall, total cancer, and all-cause mortality. Vitamin C is found abundantly in fruits, vegetables, especially berries, citrus fruits and juices, kiwi, broccoli, and peppers and some legumes (green beans), as well as dietary supplements. Plasma ascorbic acid correlates with intake of fruit and vegetables (22, 98, 128), especially citrus fruit and citrus fruit juices (14, 129), and epidemiological studies have found a reduced risk of cardiovascular disease, total cancer, and/or all-cause mortality with a high intake of these foods (11).
Dietary carotenoid intake as well as intake of specific carotenoids (β-carotene, lycopene) were inversely associated with coronary heart disease, stroke, and mortality, whereas blood concentrations of carotenoids (total, β-carotene, α-carotene, lycopene, β-cryptoxanthin) were inversely associated with cardiovascular disease, total cancer, and/or all-cause mortality.
Carotenoids are mainly found in green and yellow fruits and vegetables as well as dietary supplements. Intakes of fruits and vegetables, raw vegetables, green or green and yellow vegetables, and carrots are correlated with blood concentrations of total carotenoids, α-carotene, and β-carotene (14, 91, 130, 131), and studies have reported a reduced risk of coronary heart disease, stroke, cardiovascular disease, total cancer, and/or all-cause mortality with a high intake of these food sources of carotenoids (11). Blood concentrations of β-cryptoxanthin correlate well with total fruit and citrus fruit intake (91, 130, 131), whereas blood concentrations of lycopene correlate with tomato and tomato juice intake (91, 131–133). Intake of tomatoes has been inversely associated with coronary heart disease, although associations with other outcomes are less clear (11).
Dietary vitamin E was not significantly associated with any of the outcomes in the linear dose-response analysis; however, inverse associations were observed in the nonlinear dose-response analysis, which might suggest that the nonlinear analysis fit the data better. Blood concentrations of α-tocopherol were inversely associated with risk of stroke, total cancer, and all-cause mortality. Vitamin E is found mainly in vegetable oils, nuts, seeds, and some fruits and vegetables, as well as dietary supplements, and blood concentrations of α-tocopherol are correlated with intake of fresh fruits and juices and vegetables (134) and vitamin E from diet and supplements (134, 135). Vitamin E intake may be less specific to the intake of fruits and vegetables than vitamin C and carotenoids, possibly accounting for the lack of association between dietary vitamin E and cardiovascular disease, cancer, and mortality, although nut intake has been inversely associated with these outcomes (10).
The inverse associations between blood concentrations of vitamin C, vitamin E, total carotenoids, and β-carotene with disease and mortality endpoints were slightly stronger than for dietary intake. However, the dose-response relation appeared to be more often linear or nearly linear for the blood concentrations of vitamin C, carotenoids, β-carotene, and β-cryptoxanthin and mortality, than for both dietary antioxidant intake in the current analysis and fruit and vegetable intake in our recent meta-analysis (11), where most of the reduction in risk was observed at the lower range of intakes. This could reflect a biological saturation effect, an artifact due to measurement errors with attenuation of the association at higher dietary intakes, or other methodological issues. Vitamin C intake is most strongly associated with plasma vitamin C concentrations at lower intakes, and plasma vitamin C is less responsive at intakes >100–200 mg/d (136), which might explain the nonlinearity in the dietary analyses.
Our results have some limitations. Confounding in both dietary and biomarker studies by physical activity, less obesity, less smoking, and lower intakes of red and processed meat is possible. However, 1 study that used identical covariates for the analysis of dietary intake and plasma concentrations of vitamin C in relation to coronary heart disease risk suggested a stronger association between plasma vitamin C and vitamin C intake estimated from food-records compared with vitamin C intake estimated from food frequency questionnaires (137). Reverse causation is also a possibility in studies of biomarkers of antioxidants as antioxidants may be depleted due to oxidative stress or inflammation during the disease process (138). However, this explanation seems less likely as we found that the associations persisted in subgroup analyses of studies that excluded prevalent disease at baseline. In addition, there may have been some inconsistencies between studies in the laboratory methods used for the analyses of blood concentrations of antioxidants, which may have contributed to heterogeneity between studies or could potentially contribute to some of the nonlinear associations observed. There was significant heterogeneity in several of the analyses. Many of the inverse associations persisted in subgroup analyses stratified by sex, location, number of cases, and adjustment for confounding factors, but only in some of the analyses was the heterogeneity explained. Studies of lycopene, α-carotene, β-cryptoxanthin, and dietary carotenoids were small, which limited the statistical power to detect an association and the possibility to conduct subgroup analyses. Evidence of small-study bias or publication bias in some of the analyses suggested that the strength of the associations may have been slightly overestimated in a few analyses, but this is unlikely to substantially alter the overall findings of the study. One last limitation is that there was no protocol for the current review.
Dietary and biomarker studies have different strengths and limitations. Dietary assessments of antioxidant vitamins and carotenoids are prone to recall mismeasurement and do not reflect bioavailability, but could be more time-integrated than a single biomarker measure. Biomarkers reflect absorption being influenced by the degree of processing or cooking of foods, the lipid content of the diet, the degree of fermentation in the colon, menstrual cycle and hormonal factors, and genetic factors (32, 139). Further, metabolism of antioxidants and vitamins, as well as smoking and high alcohol consumption, may reduce blood concentrations of antioxidants (98, 140), which could induce a bias not inherent in dietary studies, although fruit and vegetable intakes explain more variability in plasma carotenoid concentrations than do smoking and alcohol (141).
Strengths of our analysis include the prospective design and high quality of the included studies, minimizing recall and selection bias; the large number of endpoints, which provide great statistical power to detect associations; robustness of results from numerous subgroup and sensitivity analyses; and the detailed dose-response analyses, which clarified the strength and shape of the dose-response relation. Finally, we analyzed both dietary intake and biomarkers of vitamin C, vitamin E, and carotenoids, and with the exception of vitamin E (for which the inverse associations were limited to the nonlinear dose-response analysis), we found that both dietary intake and blood concentrations were inversely associated with risk of cardiovascular disease, cancer, and mortality.
It is likely that the inverse associations between dietary intake and blood concentrations of antioxidants we observed are not due to the effect of single antioxidants but due to multiple antioxidants and food components found in fruit and vegetables. Randomized clinical trials have consistently shown no clear benefit of antioxidant supplements on chronic disease risk and even potential harms of supplementation of vitamin E and β-carotene on mortality and lung cancer risk, respectively (142–144). The randomized trials have used supplements with single or a few antioxidants, whereas the observational studies have assessed these nutrients based on blood concentrations or the dietary intake of foods rich in these nutrients. The dietary sources of these antioxidants (fruits, vegetables, berries, etc.) also contain a myriad of other correlated bioactive compounds that may have synergistic bioactivities. The results from the observational studies could reflect a limited number of bioactive compounds that have not been evaluated in randomized trials or complex, interactive effects of multiple correlated beneficial food components in plant foods. The current meta-analysis therefore addresses a different question than the randomized trials and is therefore not directly comparable with these. In line with synergistic effects of multiple components is a recent randomized intervention study that supplemented diets with kiwi fruit and by using gene expression profiles as proxy outcome, the study indicated that the phytochemicals work in concert rather than individually (145).
Although a combined total antioxidant score may be more strongly correlated with fruit and vegetable intake than individual antioxidants, to date only a few studies have used a total dietary antioxidant score (51, 54, 127, 146, 147) and the results are not substantially different than for individual antioxidants (51, 54, 127, 146, 147). We did not identify any studies that used a total antioxidant biomarker. In addition, other, more specific biomarkers (e.g., metabolomics) may enable distinguishing between types of fruits and vegetables in the future (148, 149). Intervention studies have shown increases in serum concentrations of vitamin C and carotenoids with higher intakes of fruits and vegetables (150). However, the size of the increase in blood concentrations was relatively moderate compared with the amount of fruits and vegetables eaten and the range of the blood concentrations of antioxidants observed in the current meta-analysis. Thus, we cannot exclude the possibility that other factors may contribute, at least partly, to the beneficial effect observed of high serum concentrations of antioxidants. The different vitamin C and carotenoid contents of individual fruits and vegetables make it difficult to quantify the amount of fruit and vegetables required to increase the blood concentration of vitamin C or carotenoids by a certain amount.
Together with largely null findings of randomized clinical trials on dietary antioxidant supplements and chronic disease prevention, the current findings have importance for public health because they support dietary recommendations to increase intake of fruit and vegetables (11), but not supplements, to reduce the risk of chronic diseases and premature mortality. Any further studies should investigate associations between less investigated antioxidants or overall antioxidant scores in both diet and blood and these outcomes as well as less common disease outcomes that have been less investigated to date and might benefit from incorporating repeated measures of diet and blood samples.
In conclusion, higher dietary intake and/or blood concentrations of vitamin C, carotenoids, and α-tocopherol (as markers of fruit, vegetable, and nut intake) were associated with reduced risk of cardiovascular disease, total cancer, and all-cause mortality. It is likely that the associations observed are not due to the individual antioxidants themselves, but rather the combination of multiple beneficial components in fruits and vegetables. These results support the notion that a high intake of fruits and vegetables, especially those high in vitamin C and carotenoids, reduces the risk of cardiovascular disease, cancer, and premature mortality.
Supplementary Material
ACKNOWLEDGEMENTS
The authors' responsibilities were as follows—DA and TN: study concept and design; DA, NK, EG, LTF, PB, DCG, ST, ER, LJV, and TN: acquisition, analysis, or interpretation of data; LTF: checking of data extractions for accuracy; DA, NK, EG, PB, LTF, DCG, ER, LJV, ST, and TN: critical revision of the manuscript for important intellectual content: DA and DCG: statistical analysis; DA, LJV, ER, and ST: obtained funding; TN: study supervision; DA: drafted the manuscript and had full access to all of the data and takes responsibility for the integrity of the data and the accuracy of the data analysis; and all authors: read and approved the final manuscript. None of the authors had any conflicts of interest with regard to this manuscript.
Notes
Supported by Olav og Gerd Meidel Raagholt's Stiftelse for Medisinsk forskning, the Liaison Committee between the Central Norway Regional Health Authority, the Norwegian University of Science and Technology, the Imperial College National Institute of Health Research Biomedical Research Centre, and the South-East Regional Health Authorities of Norway.
The funding sources had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; or decision to submit the manuscript for publication.
Supplemental Tables 1–34 and Supplemental Figures 1–125 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/ajcn.
REFERENCES
- 1. GBD 2013 Mortality and Causes of Death Collaborators Global, regional, and national age–sex specific all-cause and cause-specific mortality for 240 causes of death, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 2015;385:117–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Leenders M, Sluijs I, Ros MM, Boshuizen HC, Siersema PD, Ferrari P, Weikert C, Tjonneland A, Olsen A, Boutron-Ruault MC et al.. Fruit and vegetable consumption and mortality: European Prospective Investigation into Cancer and Nutrition. Am J Epidemiol 2013;178:590–602. [DOI] [PubMed] [Google Scholar]
- 3. Boffetta P, Couto E, Wichmann J, Ferrari P, Trichopoulos D, Bueno-de-Mesquita HB, van Duijnhoven FJ, Buchner FL, Key T, Boeing H et al.. Fruit and vegetable intake and overall cancer risk in the European Prospective Investigation into Cancer and Nutrition (EPIC). J Natl Cancer Inst 2010;102:529–37. [DOI] [PubMed] [Google Scholar]
- 4. Aune D, Lau R, Chan DS, Vieira R, Greenwood DC, Kampman E, Norat T. Nonlinear reduction in risk for colorectal cancer by fruit and vegetable intake based on meta-analysis of prospective studies. Gastroenterology 2011;141:106–18. [DOI] [PubMed] [Google Scholar]
- 5. Aune D, Chan DS, Vieira AR, Rosenblatt DA, Vieira R, Greenwood DC, Norat T. Fruits, vegetables and breast cancer risk: a systematic review and meta-analysis of prospective studies. Breast Cancer Res Treat 2012;134:479–93. [DOI] [PubMed] [Google Scholar]
- 6. Vieira AR, Vingeliene S, Chan DS, Aune D, Abar L, Navarro RD, Greenwood DC, Norat T. Fruits, vegetables, and bladder cancer risk: a systematic review and meta-analysis. Cancer Med 2015;4:136–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Vieira AR, Abar L, Vingeliene S, Chan DS, Aune D, Navarro-Rosenblatt D, Stevens C, Greenwood D, Norat T. Fruits, vegetables and lung cancer risk: a systematic review and meta-analysis. Ann Oncol 2016;27:81–96. [DOI] [PubMed] [Google Scholar]
- 8. Vingeliene S, Chan DS, Aune D, Vieira AR, Polemiti E, Stevens C, Abar L, Rosenblatt DN, Greenwood DC, Norat T. An update of the WCRF/AICR systematic literature review on esophageal and gastric cancers and citrus fruits intake. Cancer Causes Control 2016;27:837–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. World Cancer Research Fund/American Institute for Cancer Research Food, nutrition, physical activity and the prevention of cancer: a global perspective. Washington (DC): AICR, 2007. [Google Scholar]
- 10. Aune D, Keum N, Giovannucci E, Fadnes LT, Boffetta P, Greenwood DC, Tonstad S, Vatten LJ, Riboli E, Norat T. Nut consumption and risk of cardiovascular disease, total cancer, all-cause and cause-specific mortality: a systematic review and dose-response meta-analysis of prospective studies. BMC Med 2016;14:207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Aune D, Giovannucci E, Boffetta P, Fadnes L, Keum N, Norat T, Greenwood D, Riboli E, Vatten L, Tonstad S. Fruit and vegetable intake and the risk of cardiovascular disease, total cancer and all-cause mortality—a systematic review and dose-response meta-analysis of prospective studies. Int J Epidemiol 2017;46:1029–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Steinmetz KA, Potter JD. Vegetables, fruit, and cancer. II. Mechanisms. Cancer Causes Control 1991;2(6):427–42. [DOI] [PubMed] [Google Scholar]
- 13. Gale CR, Martyn CN, Winter PD, Cooper C. Vitamin C and risk of death from stroke and coronary heart disease in cohort of elderly people. BMJ 1995;310:1563–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sahyoun NR, Jacques PF, Russell RM. Carotenoids, vitamins C and E, and mortality in an elderly population. Am J Epidemiol 1996;144:501–11. [DOI] [PubMed] [Google Scholar]
- 15. Lawlor DA, Ebrahim S, Kundu D, Bruckdorfer KR, Whincup PH, Smith GD. Vitamin C is not associated with coronary heart disease risk once life course socioeconomic position is taken into account: prospective findings from the British Women's Heart and Health Study. Heart 2005;91:1086–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kurl S, Tuomainen TP, Laukkanen JA, Nyyssonen K, Lakka T, Sivenius J, Salonen JT. Plasma vitamin C modifies the association between hypertension and risk of stroke. Stroke 2002;33:1568–73. [DOI] [PubMed] [Google Scholar]
- 17. Myint PK, Luben RN, Welch AA, Bingham SA, Wareham NJ, Khaw KT. Plasma vitamin C concentrations predict risk of incident stroke over 10 y in 20 649 participants of the European Prospective Investigation into Cancer Norfolk prospective population study. Am J Clin Nutr 2008;87:64–9. [DOI] [PubMed] [Google Scholar]
- 18. Simon JA, Hudes ES, Tice JA. Relation of serum ascorbic acid to mortality among US adults. J Am Coll Nutr 2001;20:255–63. [DOI] [PubMed] [Google Scholar]
- 19. Fletcher AE, Breeze E, Shetty PS. Antioxidant vitamins and mortality in older persons: findings from the nutrition add-on study to the Medical Research Council Trial of Assessment and Management of Older People in the Community. Am J Clin Nutr 2003;78:999–1010. [DOI] [PubMed] [Google Scholar]
- 20. Bates CJ, Hamer M, Mishra GD. Redox-modulatory vitamins and minerals that prospectively predict mortality in older British people: the National Diet and Nutrition Survey of people aged 65 years and over. Br J Nutr 2011;105:123–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Goyal A, Terry MB, Siegel AB. Serum antioxidant nutrients, vitamin A, and mortality in U.S. adults. Cancer Epidemiol Biomarkers Prev 2013;22:2202–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Khaw KT, Bingham S, Welch A, Luben R, Wareham N, Oakes S, Day N. Relation between plasma ascorbic acid and mortality in men and women in EPIC-Norfolk prospective study: a prospective population study. European Prospective Investigation into Cancer and Nutrition. Lancet 2001;357:657–63. [DOI] [PubMed] [Google Scholar]
- 23. Jia X, Aucott LS, McNeill G. Nutritional status and subsequent all-cause mortality in men and women aged 75 years or over living in the community. Br J Nutr 2007;98:593–9. [DOI] [PubMed] [Google Scholar]
- 24. Walston J, Xue Q, Semba RD, Ferrucci L, Cappola AR, Ricks M, Guralnik J, Fried LP. Serum antioxidants, inflammation, and total mortality in older women. Am J Epidemiol 2006;163:18–26. [DOI] [PubMed] [Google Scholar]
- 25. Lauretani F, Semba RD, Dayhoff-Brannigan M, Corsi AM, Di Iorio A, Buiatti E, Bandinelli S, Guralnik JM, Ferrucci L. Low total plasma carotenoids are independent predictors of mortality among older persons: the InCHIANTI study. Eur J Nutr 2008;47:335–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Martin-Calvo N, Martinez-Gonzalez MA. Vitamin C intake is inversely associated with cardiovascular mortality in a cohort of Spanish graduates: the SUN Project. Nutrients 2017;9:954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Greenberg ER, Baron JA, Karagas MR, Stukel TA, Nierenberg DW, Stevens MM, Mandel JS, Haile RW. Mortality associated with low plasma concentration of beta carotene and the effect of oral supplementation. JAMA 1996;275:699–703. [DOI] [PubMed] [Google Scholar]
- 28. Wald NJ, Thompson SG, Densem JW, Boreham J, Bailey A. Serum beta-carotene and subsequent risk of cancer: results from the BUPA study. Br J Cancer 1988;57:428–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Smith-Warner SA, Elmer PJ, Tharp TM, Fosdick L, Randall B, Gross M, Wood J, Potter JD. Increasing vegetable and fruit intake: randomized intervention and monitoring in an at-risk population. Cancer Epidemiol Biomarkers Prev 2000;9:307–17. [PubMed] [Google Scholar]
- 30. Macdonald HM, Hardcastle AC, Duthie GG, Duthie SJ, Aucott L, Sandison R, Shearer MJ, Reid DM. Changes in vitamin biomarkers during a 2-year intervention trial involving increased fruit and vegetable consumption by free-living volunteers. Br J Nutr 2009;102:1477–86. [DOI] [PubMed] [Google Scholar]
- 31. Carlsen MH, Karlsen A, Lillegaard IT, Gran JM, Drevon CA, Blomhoff R, Andersen LF. Relative validity of fruit and vegetable intake estimated from an FFQ, using carotenoid and flavonoid biomarkers and the method of triads. Br J Nutr 2011;105:1530–8. [DOI] [PubMed] [Google Scholar]
- 32. Jenab M, Slimani N, Bictash M, Ferrari P, Bingham SA. Biomarkers in nutritional epidemiology: applications, needs and new horizons. Hum Genet 2009;125:507–25. [DOI] [PubMed] [Google Scholar]
- 33. Fehily AM, Yarnell JW, Sweetnam PM, Elwood PC. Diet and incident ischaemic heart disease: the Caerphilly study. Br J Nutr 1993;69:303–14. [DOI] [PubMed] [Google Scholar]
- 34. Rimm EB, Stampfer MJ, Ascherio A, Giovannucci E, Colditz GA, Willett WC. Vitamin E consumption and the risk of coronary heart disease in men. N Engl J Med 1993;328:1450–6. [DOI] [PubMed] [Google Scholar]
- 35. Knekt P, Reunanen A, Jarvinen R, Seppanen R, Heliovaara M, Aromaa A. Antioxidant vitamin intake and coronary mortality in a longitudinal population study. Am J Epidemiol 1994;139:1180–9. [DOI] [PubMed] [Google Scholar]
- 36. Pandey DK, Shekelle R, Selwyn BJ, Tangney C, Stamler J. Dietary vitamin C and beta-carotene and risk of death in middle-aged men. The Western Electric Study. Am J Epidemiol 1995;142:1269–78. [DOI] [PubMed] [Google Scholar]
- 37. Kushi LH, Folsom AR, Prineas RJ, Mink PJ, Wu Y, Bostick RM. Dietary antioxidant vitamins and death from coronary heart disease in postmenopausal women. N Engl J Med 1996;334:1156–62. [DOI] [PubMed] [Google Scholar]
- 38. Todd S, Woodward M, Tunstall-Pedoe H, Bolton-Smith C. Dietary antioxidant vitamins and fiber in the etiology of cardiovascular disease and all-causes mortality: results from the Scottish Heart Health Study. Am J Epidemiol 1999;150:1073–80. [DOI] [PubMed] [Google Scholar]
- 39. Klipstein-Grobusch K, Geleijnse JM, den Breeijen JH, Boeing H, Hofman A, Grobbee DE, Witteman JC. Dietary antioxidants and risk of myocardial infarction in the elderly: the Rotterdam Study. Am J Clin Nutr 1999;69:261–6. [DOI] [PubMed] [Google Scholar]
- 40. Osganian SK, Stampfer MJ, Rimm E, Spiegelman D, Hu FB, Manson JE, Willett WC. Vitamin C and risk of coronary heart disease in women. J Am Coll Cardiol 2003;42:246–52. [DOI] [PubMed] [Google Scholar]
- 41. Kubota Y, Iso H, Date C, Kikuchi S, Watanabe Y, Wada Y, Inaba Y, Tamakoshi A. Dietary intakes of antioxidant vitamins and mortality from cardiovascular disease: the Japan Collaborative Cohort Study (JACC) study. Stroke 2011;42:1665–72. [DOI] [PubMed] [Google Scholar]
- 42. Tunstall-Pedoe H, Woodward M, Tavendale R, A'Brook R, McCluskey MK. Comparison of the prediction by 27 different factors of coronary heart disease and death in men and women of the Scottish Heart Health Study: cohort study. BMJ 1997;315:722–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Osganian SK, Stampfer MJ, Rimm E, Spiegelman D, Manson JE, Willett WC. Dietary carotenoids and risk of coronary artery disease in women. Am J Clin Nutr 2003;77:1390–9. [DOI] [PubMed] [Google Scholar]
- 44. Keli SO, Hertog MG, Feskens EJ, Kromhout D. Dietary flavonoids, antioxidant vitamins, and incidence of stroke: the Zutphen study. Arch Intern Med 1996;156:637–42. [PubMed] [Google Scholar]
- 45. Ascherio A, Rimm EB, Hernan MA, Giovannucci E, Kawachi I, Stampfer MJ, Willett WC. Relation of consumption of vitamin E, vitamin C, and carotenoids to risk for stroke among men in the United States. Ann Intern Med 1999;130:963–70. [DOI] [PubMed] [Google Scholar]
- 46. Yochum LA, Folsom AR, Kushi LH. Intake of antioxidant vitamins and risk of death from stroke in postmenopausal women. Am J Clin Nutr 2000;72:476–83. [DOI] [PubMed] [Google Scholar]
- 47. Hirvonen T, Virtamo J, Korhonen P, Albanes D, Pietinen P. Intake of flavonoids, carotenoids, vitamins C and E, and risk of stroke in male smokers. Stroke 2000;31:2301–6. [DOI] [PubMed] [Google Scholar]
- 48. Voko Z, Hollander M, Hofman A, Koudstaal PJ, Breteler MM. Dietary antioxidants and the risk of ischemic stroke: the Rotterdam Study. Neurology 2003;61:1273–5. [DOI] [PubMed] [Google Scholar]
- 49. Marniemi J, Alanen E, Impivaara O, Seppanen R, Hakala P, Rajala T, Ronnemaa T. Dietary and serum vitamins and minerals as predictors of myocardial infarction and stroke in elderly subjects. Nutr Metab Cardiovasc Dis 2005;15:188–97. [DOI] [PubMed] [Google Scholar]
- 50. Weng LC, Yeh WT, Bai CH, Chen HJ, Chuang SY, Chang HY, Lin BF, Chen KJ, Pan WH. Is ischemic stroke risk related to folate status or other nutrients correlated with folate intake? Stroke 2008;39:3152–8. [DOI] [PubMed] [Google Scholar]
- 51. Del Rio D, Agnoli C, Pellegrini N, Krogh V, Brighenti F, Mazzeo T, Masala G, Bendinelli B, Berrino F, Sieri S et al.. Total antioxidant capacity of the diet is associated with lower risk of ischemic stroke in a large Italian cohort. J Nutr 2011;141:118–23. [DOI] [PubMed] [Google Scholar]
- 52. Daviglus ML, Orencia AJ, Dyer AR, Liu K, Morris DK, Persky V, Chavez N, Goldberg J, Drum M, Shekelle RB et al.. Dietary vitamin C, beta-carotene and 30-year risk of stroke: results from the Western Electric Study. Neuroepidemiology 1997;16:69–77. [DOI] [PubMed] [Google Scholar]
- 53. Genkinger JM, Platz EA, Hoffman SC, Comstock GW, Helzlsouer KJ. Fruit, vegetable, and antioxidant intake and all-cause, cancer, and cardiovascular disease mortality in a community-dwelling population in Washington County, Maryland. Am J Epidemiol 2004;160:1223–33. [DOI] [PubMed] [Google Scholar]
- 54. Agudo A, Cabrera L, Amiano P, Ardanaz E, Barricarte A, Berenguer T, Chirlaque MD, Dorronsoro M, Jakszyn P, Larranaga N et al.. Fruit and vegetable intakes, dietary antioxidant nutrients, and total mortality in Spanish adults: findings from the Spanish cohort of the European Prospective Investigation into Cancer and Nutrition (EPIC-Spain). Am J Clin Nutr 2007;85:1634–42. [DOI] [PubMed] [Google Scholar]
- 55. Buijsse B, Feskens EJ, Kwape L, Kok FJ, Kromhout D. Both alpha- and beta-carotene, but not tocopherols and vitamin C, are inversely related to 15-year cardiovascular mortality in Dutch elderly men. J Nutr 2008;138:344–50. [DOI] [PubMed] [Google Scholar]
- 56. Stepaniak U, Micek A, Grosso G, Stefler D, Topor-Madry R, Kubinova R, Malyutina S, Peasey A, Pikhart H, Nikitin Y et al.. Antioxidant vitamin intake and mortality in three Central and Eastern European urban populations: the HAPIEE study. Eur J Nutr 2016;55:547–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Shibata A, Paganini-Hill A, Ross RK, Henderson BE. Intake of vegetables, fruits, beta-carotene, vitamin C and vitamin supplements and cancer incidence among the elderly: a prospective study. Br J Cancer 1992;66:673–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Gale CR, Martyn CN, Cooper C. Cognitive impairment and mortality in a cohort of elderly people. BMJ 1996;312:608–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Fortes C, Forastiere F, Farchi S, Rapiti E, Pastori G, Perucci CA. Diet and overall survival in a cohort of very elderly people. Epidemiology 2000;11:440–5. [DOI] [PubMed] [Google Scholar]
- 60. Roswall N, Olsen A, Christensen J, Hansen L, Dragsted LO, Overvad K, Tjonneland A. Micronutrient intake in relation to all-cause mortality in a prospective Danish cohort. Food Nutr Res 2012;56:5466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Ross RK, Yuan JM, Henderson BE, Park J, Gao YT, Yu MC. Prospective evaluation of dietary and other predictors of fatal stroke in Shanghai, China. Circulation 1997;96:50–5. [DOI] [PubMed] [Google Scholar]
- 62. Aune D, Chan DS, Vieira AR, Navarro Rosenblatt DA, Vieira R, Greenwood DC, Norat T. Dietary compared with blood concentrations of carotenoids and breast cancer risk: a systematic review and meta-analysis of prospective studies. Am J Clin Nutr 2012;96:356–73. [DOI] [PubMed] [Google Scholar]
- 63. Eliassen AH, Hendrickson SJ, Brinton LA, Buring JE, Campos H, Dai Q, Dorgan JF, Franke AA, Gao YT, Goodman MT et al.. Circulating carotenoids and risk of breast cancer: pooled analysis of eight prospective studies. J Natl Cancer Inst 2012;104:1905–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Zhang X, Spiegelman D, Baglietto L, Bernstein L, Boggs DA, van den Brandt PA, Buring JE, Gapstur SM, Giles GG, Giovannucci E et al.. Carotenoid intakes and risk of breast cancer defined by estrogen receptor and progesterone receptor status: a pooled analysis of 18 prospective cohort studies. Am J Clin Nutr 2012;95:713–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Chen GC, Lu DB, Pang Z, Liu QF. Vitamin C intake, circulating vitamin C and risk of stroke: a meta-analysis of prospective studies. J Am Heart Assoc 2013;2:e000329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 2009;339:b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986;7:177–88. [DOI] [PubMed] [Google Scholar]
- 68. Greenland S, Longnecker MP. Methods for trend estimation from summarized dose-response data, with applications to meta-analysis. Am J Epidemiol 1992;135:1301–9. [DOI] [PubMed] [Google Scholar]
- 69. Switzer BR, Atwood JR, Stark AH, Hatch JW, Travis R, Ullrich F, Lyden ER, Wu X, Chiu Y, Smith JL. Plasma carotenoid and vitamins A and E concentrations in older African American women after wheat bran supplementation: effects of age, body mass and smoking history. J Am Coll Nutr 2005;24:217–26. [DOI] [PubMed] [Google Scholar]
- 70. Wright ME, Lawson KA, Weinstein SJ, Pietinen P, Taylor PR, Virtamo J, Albanes D. Higher baseline serum concentrations of vitamin E are associated with lower total and cause-specific mortality in the Alpha-Tocopherol, Beta-Carotene Cancer Prevention Study. Am J Clin Nutr 2006;84:1200–7. [DOI] [PubMed] [Google Scholar]
- 71. Jackson D, White IR, Thompson SG. Extending DerSimonian and Laird's methodology to perform multivariate random effects meta-analyses. Stat Med 2010;29:1282–97. [DOI] [PubMed] [Google Scholar]
- 72. Orsini N, Li R, Wolk A, Khudyakov P, Spiegelman D. Meta-analysis for linear and nonlinear dose-response relations: examples, an evaluation of approximations, and software. Am J Epidemiol 2012;175:66–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Royston P. A strategy for modelling the effect of a continuous covariate in medicine and epidemiology. Stat Med 2000;19:1831–47. [DOI] [PubMed] [Google Scholar]
- 74. Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med 2002;21:1539–58. [DOI] [PubMed] [Google Scholar]
- 75. Wells G, Shea B, O'Connell D, Peterson J, Welch V, Losos M, Tugwell P. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses [Internet]. [cited 2017 Jul 8]. Available from: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. [Google Scholar]
- 76. Egger M, Davey SG, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Stampfer MJ, Hennekens CH, Manson JE, Colditz GA, Rosner B, Willett WC. Vitamin E consumption and the risk of coronary disease in women. N Engl J Med 1993;328:1444–9. [DOI] [PubMed] [Google Scholar]
- 78. Sesso HD, Liu S, Gaziano JM, Buring JE. Dietary lycopene, tomato-based food products and cardiovascular disease in women. J Nutr 2003;133:2336–41. [DOI] [PubMed] [Google Scholar]
- 79. Marniemi J, Jarvisalo J, Toikka T, Raiha I, Ahotupa M, Sourander L. Blood vitamins, mineral elements and inflammation markers as risk factors of vascular and non-vascular disease mortality in an elderly population. Int J Epidemiol 1998;27:799–807. [DOI] [PubMed] [Google Scholar]
- 80. de Oliveira Otto MC, Alonso A, Lee DH, Delclos GL, Bertoni AG, Jiang R, Lima JA, Symanski E, Jacobs DR Jr., Nettleton JA. Dietary intakes of zinc and heme iron from red meat, but not from other sources, are associated with greater risk of metabolic syndrome and cardiovascular disease. J Nutr 2012;142:526–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Zhao LG, Shu XO, Li HL, Zhang W, Gao J, Sun JW, Zheng W, Xiang YB. Dietary antioxidant vitamins intake and mortality: a report from two cohort studies of Chinese adults in Shanghai. J Epidemiol 2017;27:89–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Enstrom JE, Kanim LE, Breslow L. The relationship between vitamin C intake, general health practices, and mortality in Alameda County, California. Am J Public Health 1986;76:1124–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Enstrom JE, Kanim LE, Klein MA. Vitamin C intake and mortality among a sample of the United States population. Epidemiology 1992;3:194–202. [DOI] [PubMed] [Google Scholar]
- 84. Paganini-Hill A, Kawas CH, Corrada MM. Antioxidant vitamin intake and mortality: the Leisure World Cohort Study. Am J Epidemiol 2015;181:120–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Kok FJ, de Bruijn AM, Vermeeren R, Hofman A, van Laar A, de Bruin M, Hermus RJ, Valkenburg HA. Serum selenium, vitamin antioxidants, and cardiovascular mortality: a 9-year follow-up study in the Netherlands. Am J Clin Nutr 1987;45:462–8. [DOI] [PubMed] [Google Scholar]
- 86. Hense HW, Stender M, Bors W, Keil U. Lack of an association between serum vitamin E and myocardial infarction in a population with high vitamin E levels. Atherosclerosis 1993;103:21–8. [DOI] [PubMed] [Google Scholar]
- 87. Gey KF, Stahelin HB, Eichholzer M. Poor plasma status of carotene and vitamin C is associated with higher mortality from ischemic heart disease and stroke: Basel Prospective Study. Clin Investig 1993;71:3–6. [DOI] [PubMed] [Google Scholar]
- 88. Morris DL, Kritchevsky SB, Davis CE. Serum carotenoids and coronary heart disease. The Lipid Research Clinics Coronary Primary Prevention Trial and Follow-up Study. JAMA 1994;272:1439–41. [DOI] [PubMed] [Google Scholar]
- 89. Street DA, Comstock GW, Salkeld RM, Schuep W, Klag MJ. Serum antioxidants and myocardial infarction. Are low levels of carotenoids and alpha-tocopherol risk factors for myocardial infarction? Circulation 1994;90:1154–61. [DOI] [PubMed] [Google Scholar]
- 90. Evans RW, Shaten BJ, Day BW, Kuller LH. Prospective association between lipid soluble antioxidants and coronary heart disease in men. The Multiple Risk Factor Intervention Trial. Am J Epidemiol 1998;147:180–6. [DOI] [PubMed] [Google Scholar]
- 91. Hak AE, Stampfer MJ, Campos H, Sesso HD, Gaziano JM, Willett W, Ma J. Plasma carotenoids and tocopherols and risk of myocardial infarction in a low-risk population of US male physicians. Circulation 2003;108:802–7. [DOI] [PubMed] [Google Scholar]
- 92. Boekholdt SM, Meuwese MC, Day NE, Luben R, Welch A, Wareham NJ, Khaw KT. Plasma concentrations of ascorbic acid and C-reactive protein, and risk of future coronary artery disease, in apparently healthy men and women: the EPIC-Norfolk prospective population study. Br J Nutr 2006;96:516–22. [PubMed] [Google Scholar]
- 93. Koh WP, Yuan JM, Wang R, Lee YP, Lee BL, Yu MC, Ong CN. Plasma carotenoids and risk of acute myocardial infarction in the Singapore Chinese Health Study. Nutr Metab Cardiovasc Dis 2011;21:685–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Li C, Ford ES, Zhao G, Balluz LS, Giles WH, Liu S. Serum alpha-carotene concentrations and risk of death among US adults: the Third National Health and Nutrition Examination Survey Follow-up Study. Arch Intern Med 2011;171:507–15. [DOI] [PubMed] [Google Scholar]
- 95. Karppi J, Laukkanen JA, Makikallio TH, Kurl S. Low serum lycopene and beta-carotene increase risk of acute myocardial infarction in men. Eur J Public Health 2012;22:835–40. [DOI] [PubMed] [Google Scholar]
- 96. Nagao M, Moriyama Y, Yamagishi K, Iso H, Tamakoshi A. Relation of serum alpha- and gamma-tocopherol levels to cardiovascular disease-related mortality among Japanese men and women. J Epidemiol 2012;22:402–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Leppala JM, Virtamo J, Fogelholm R, Albanes D, Heinonen OP. Different risk factors for different stroke subtypes: association of blood pressure, cholesterol, and antioxidants. Stroke 1999;30:2535–40. [DOI] [PubMed] [Google Scholar]
- 98. Yokoyama T, Date C, Kokubo Y, Yoshiike N, Matsumura Y, Tanaka H. Serum vitamin C concentration was inversely associated with subsequent 20-year incidence of stroke in a Japanese rural community. The Shibata study. Stroke 2000;31:2287–94. [DOI] [PubMed] [Google Scholar]
- 99. Hak AE, Ma J, Powell CB, Campos H, Gaziano JM, Willett WC, Stampfer MJ. Prospective study of plasma carotenoids and tocopherols in relation to risk of ischemic stroke. Stroke 2004;35:1584–8. [DOI] [PubMed] [Google Scholar]
- 100. Karppi J, Laukkanen JA, Sivenius J, Ronkainen K, Kurl S. Serum lycopene decreases the risk of stroke in men: a population-based follow-up study. Neurology 2012;79:1540–7. [DOI] [PubMed] [Google Scholar]
- 101. Loria CM, Klag MJ, Caulfield LE, Whelton PK. Vitamin C status and mortality in US adults. Am J Clin Nutr 2000;72:139–45. [DOI] [PubMed] [Google Scholar]
- 102. Kilander L, Berglund L, Boberg M, Vessby B, Lithell H. Education, lifestyle factors and mortality from cardiovascular disease and cancer. A 25-year follow-up of Swedish 50-year-old men. Int J Epidemiol 2001;30:1119–26. [DOI] [PubMed] [Google Scholar]
- 103. Mezzetti A, Zuliani G, Romano F, Costantini F, Pierdomenico SD, Cuccurullo F, Fellin R. Vitamin E and lipid peroxide plasma levels predict the risk of cardiovascular events in a group of healthy very old people. J Am Geriatr Soc 2001;49:533–7. [DOI] [PubMed] [Google Scholar]
- 104. Sesso HD, Buring JE, Norkus EP, Gaziano JM. Plasma lycopene, other carotenoids, and retinol and the risk of cardiovascular disease in women. Am J Clin Nutr 2004;79:47–53. [DOI] [PubMed] [Google Scholar]
- 105. Hercberg S, Galan P, Preziosi P, Bertrais S, Mennen L, Malvy D, Roussel AM, Favier A, Briancon S. The SU.VI.MAX Study: a randomized, placebo-controlled trial of the health effects of antioxidant vitamins and minerals. Arch Intern Med 2004;164:2335–42. [DOI] [PubMed] [Google Scholar]
- 106. Sesso HD, Buring JE, Norkus EP, Gaziano JM. Plasma lycopene, other carotenoids, and retinol and the risk of cardiovascular disease in men. Am J Clin Nutr 2005;81:990–7. [DOI] [PubMed] [Google Scholar]
- 107. Buijsse B, Feskens EJ, Schlettwein-Gsell D, Ferry M, Kok FJ, Kromhout D, de Groot LC. Plasma carotene and alpha-tocopherol in relation to 10-y all-cause and cause-specific mortality in European elderly: the Survey in Europe on Nutrition and the Elderly, a Concerted Action (SENECA). Am J Clin Nutr 2005;82:879–86. [DOI] [PubMed] [Google Scholar]
- 108. Shardell MD, Alley DE, Hicks GE, El-Kamary SS, Miller RR, Semba RD, Ferrucci L. Low-serum carotenoid concentrations and carotenoid interactions predict mortality in US adults: the Third National Health and Nutrition Examination Survey. Nutr Res 2011;31:178–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Karppi J, Laukkanen JA, Makikallio TH, Ronkainen K, Kurl S. Low beta-carotene concentrations increase the risk of cardiovascular disease mortality among Finnish men with risk factors. Nutr Metab Cardiovasc Dis 2012;22:921–8. [DOI] [PubMed] [Google Scholar]
- 110. Willett WC, Polk BF, Underwood BA, Stampfer MJ, Pressel S, Rosner B, Taylor JO, Schneider K, Hames CG. Relation of serum vitamins A and E and carotenoids to the risk of cancer. N Engl J Med 1984;310:430–4. [DOI] [PubMed] [Google Scholar]
- 111. Salonen JT, Salonen R, Lappetelainen R, Maenpaa PH, Alfthan G, Puska P. Risk of cancer in relation to serum concentrations of selenium and vitamins A and E: matched case-control analysis of prospective data. Br Med J (Clin Res Ed) 1985;290:417–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Wald NJ, Thompson SG, Densem JW, Boreham J, Bailey A. Serum vitamin E and subsequent risk of cancer. Br J Cancer 1987;56:69–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Knekt P. Serum vitamin E level and risk of female cancers. Int J Epidemiol 1988;17:281–6. [DOI] [PubMed] [Google Scholar]
- 114. Knekt P, Aromaa A, Maatela J, Aaran RK, Nikkari T, Hakama M, Hakulinen T, Peto R, Saxen E, Teppo L. Serum vitamin E and risk of cancer among Finnish men during a 10-year follow-up. Am J Epidemiol 1988;127:28–41. [DOI] [PubMed] [Google Scholar]
- 115. Knekt P, Aromaa A, Maatela J, Aaran RK, Nikkari T, Hakama M, Hakulinen T, Peto R, Teppo L. Serum vitamin A and subsequent risk of cancer: cancer incidence follow-up of the Finnish Mobile Clinic Health Examination Survey. Am J Epidemiol 1990;132:857–70. [DOI] [PubMed] [Google Scholar]
- 116. Eichholzer M, Stahelin HB, Gey KF, Ludin E, Bernasconi F. Prediction of male cancer mortality by plasma levels of interacting vitamins: 17-year follow-up of the prospective Basel study. Int J Cancer 1996;66:145–50. [DOI] [PubMed] [Google Scholar]
- 117. Ito Y, Suzuki S, Yagyu K, Sasaki R, Suzuki K, Aoki K. Relationship between serum carotenoid levels and cancer death rates in the residents, living in a rural area of Hokkaido, Japan. J Epidemiol 1997;7:1–8. [DOI] [PubMed] [Google Scholar]
- 118. Ito Y, Kurata M, Hioki R, Suzuki K, Ochiai J, Aoki K. Cancer mortality and serum levels of carotenoids, retinol, and tocopherol: a population-based follow-up study of inhabitants of a rural area of Japan. Asian Pac J Cancer Prev 2005;6:10–15. [PubMed] [Google Scholar]
- 119. Karppi J, Kurl S, Nurmi T, Rissanen TH, Pukkala E, Nyyssonen K. Serum lycopene and the risk of cancer: the Kuopio Ischaemic Heart Disease Risk Factor (KIHD) study. Ann Epidemiol 2009;19:512–18. [DOI] [PubMed] [Google Scholar]
- 120. Pouchieu C, Galan P, Ducros V, Latino-Martel P, Hercberg S, Touvier M. Plasma carotenoids and retinol and overall and breast cancer risk: a nested case-control study. Nutr Cancer 2014;66:980–8. [DOI] [PubMed] [Google Scholar]
- 121. De Waart FG, Schouten EG, Stalenhoef AF, Kok FJ. Serum carotenoids, alpha-tocopherol and mortality risk in a prospective study among Dutch elderly. Int J Epidemiol 2001;30:136–43. [DOI] [PubMed] [Google Scholar]
- 122. Hu P, Reuben DB, Crimmins EM, Harris TB, Huang MH, Seeman TE. The effects of serum beta-carotene concentration and burden of inflammation on all-cause mortality risk in high-functioning older persons: MacArthur studies of successful aging. J Gerontol A Biol Sci Med Sci 2004;59:849–54. [DOI] [PubMed] [Google Scholar]
- 123. Uesugi S, Ishihara J, Iso H, Sawada N, Takachi R, Inoue M, Tsugane S. Dietary intake of antioxidant vitamins and risk of stroke: the Japan Public Health Center-based Prospective Study. Eur J Clin Nutr 2017;71:1179–85. [DOI] [PubMed] [Google Scholar]
- 124. Huerta JM, Gonzalez S, Fernandez S, Patterson AM, Lasheras C. Lipid peroxidation, antioxidant status and survival in institutionalised elderly: a five-year longitudinal study. Free Radic Res 2006;40:571–8. [DOI] [PubMed] [Google Scholar]
- 125. Akbaraly TN, Favier A, Berr C. Total plasma carotenoids and mortality in the elderly: results of the Epidemiology of Vascular Ageing (EVA) study. Br J Nutr 2009;101:86–92. [DOI] [PubMed] [Google Scholar]
- 126. Hamer M, Bates CJ, Mishra GD. Depression, physical function, and risk of mortality: National Diet and Nutrition Survey in adults older than 65 years. Am J Geriatr Psychiatry 2011;19:72–8. [DOI] [PubMed] [Google Scholar]
- 127. Henriquez-Sanchez P, Sanchez-Villegas A, Ruano-Rodriguez C, Gea A, Lamuela-Raventos RM, Estruch R, Salas-Salvado J, Covas MI, Corella D, Schroder H et al.. Dietary total antioxidant capacity and mortality in the PREDIMED study. Eur J Nutr 2016;55:227–36. [DOI] [PubMed] [Google Scholar]
- 128. Sanchez-Moreno C, Cano MP, de Ancos B, Plaza L, Olmedilla B, Granado F, Martin A. Mediterranean vegetable soup consumption increases plasma vitamin C and decreases F2-isoprostanes, prostaglandin E2 and monocyte chemotactic protein-1 in healthy humans. J Nutr Biochem 2006;17:183–9. [DOI] [PubMed] [Google Scholar]
- 129. Wrieden WL, Hannah MK, Bolton-Smith C, Tavendale R, Morrison C, Tunstall-Pedoe H. Plasma vitamin C and food choice in the third Glasgow MONICA population survey. J Epidemiol Community Health 2000;54:355–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Maillard V, Kuriki K, Lefebvre B, Boutron-Ruault MC, Lenoir GM, Joulin V, Clavel-Chapelon F, Chajes V. Serum carotenoid, tocopherol and retinol concentrations and breast cancer risk in the E3N-EPIC study. Int J Cancer 2010;127:1188–96. [DOI] [PubMed] [Google Scholar]
- 131. Hendrickson SJ, Willett WC, Rosner BA, Eliassen AH. Food predictors of plasma carotenoids. Nutrients 2013;5:4051–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Tyssandier V, Feillet-Coudray C, Caris-Veyrat C, Guilland JC, Coudray C, Bureau S, Reich M, Amiot-Carlin MJ, Bouteloup-Demange C, Boirie Y et al.. Effect of tomato product consumption on the plasma status of antioxidant microconstituents and on the plasma total antioxidant capacity in healthy subjects. J Am Coll Nutr 2004;23:148–56. [DOI] [PubMed] [Google Scholar]
- 133. Sesso HD, Buring JE, Zhang SM, Norkus EP, Gaziano JM. Dietary and plasma lycopene and the risk of breast cancer. Cancer Epidemiol Biomarkers Prev 2005;14:1074–81. [DOI] [PubMed] [Google Scholar]
- 134. Kang MJ, Lin YC, Yeh WH, Pan WH. Vitamin E status and its dietary determinants in Taiwanese – results of the Nutrition and Health Survey in Taiwan 1993–1996. Eur J Nutr 2004;43:86–92. [DOI] [PubMed] [Google Scholar]
- 135. Sinha R, Patterson BH, Mangels AR, Levander OA, Gibson T, Taylor PR, Block G. Determinants of plasma vitamin E in healthy males. Cancer Epidemiol Biomarkers Prev 1993;2:473–9. [PubMed] [Google Scholar]
- 136. Levine M, Conry-Cantilena C, Wang Y, Welch RW, Washko PW, Dhariwal KR, Park JB, Lazarev A, Graumlich JF, King J et al.. Vitamin C pharmacokinetics in healthy volunteers: evidence for a recommended dietary allowance. Proc Natl Acad Sci USA 1996;93:3704–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Bingham S, Luben R, Welch A, Low YL, Khaw KT, Wareham N, Day N. Associations between dietary methods and biomarkers, and between fruits and vegetables and risk of ischaemic heart disease, in the EPIC Norfolk Cohort Study. Int J Epidemiol 2008;37:978–87. [DOI] [PubMed] [Google Scholar]
- 138. Sanchez-Moreno C, Dashe JF, Scott T, Thaler D, Folstein MF, Martin A. Decreased levels of plasma vitamin C and increased concentrations of inflammatory and oxidative stress markers after stroke. Stroke 2004;35:163–8. [DOI] [PubMed] [Google Scholar]
- 139. Herbeth B, Guilland JC, Rochette L, Siest G, Visvikis-Siest S. Genetic and environmental contributions to serum ascorbic acid concentrations: the Stanislas Family Study. Br J Nutr 2006;96:1013–20. [DOI] [PubMed] [Google Scholar]
- 140. Marangon K, Herbeth B, Lecomte E, Paul-Dauphin A, Grolier P, Chancerelle Y, Artur Y, Siest G. Diet, antioxidant status, and smoking habits in French men. Am J Clin Nutr 1998;67:231–9. [DOI] [PubMed] [Google Scholar]
- 141. Al-Delaimy WK, Ferrari P, Slimani N, Pala V, Johansson I, Nilsson S, Mattisson I, Wirfalt E, Galasso R, Palli D et al.. Plasma carotenoids as biomarkers of intake of fruits and vegetables: individual-level correlations in the European Prospective Investigation into Cancer and Nutrition (EPIC). Eur J Clin Nutr 2005;59:1387–96. [DOI] [PubMed] [Google Scholar]
- 142. The Alpha-Tocopherol Beta-Carotene Cancer Prevention Study Group The effect of vitamin E and beta carotene on the incidence of lung cancer and other cancers in male smokers. N Engl J Med 1994;330:1029–35. [DOI] [PubMed] [Google Scholar]
- 143. Bjelakovic G, Nikolova D, Gluud C. Meta-regression analyses, meta-analyses, and trial sequential analyses of the effects of supplementation with beta-carotene, vitamin A, and vitamin E singly or in different combinations on all-cause mortality: do we have evidence for lack of harm? PLoS One 2013;8:e74558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Bjelakovic G, Nikolova D, Gluud LL, Simonetti RG, Gluud C. Antioxidant supplements for prevention of mortality in healthy participants and patients with various diseases. Cochrane Database Syst Rev 2012;3:CD007176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Bohn SK, Myhrstad MC, Thoresen M, Holden M, Karlsen A, Tunheim SH, Erlund I, Svendsen M, Seljeflot I, Moskaug JO et al.. Blood cell gene expression associated with cellular stress defense is modulated by antioxidant-rich food in a randomised controlled clinical trial of male smokers. BMC Med 2010;8:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Rautiainen S, Levitan EB, Orsini N, Akesson A, Morgenstern R, Mittleman MA, Wolk A. Total antioxidant capacity from diet and risk of myocardial infarction: a prospective cohort of women. Am J Med 2012;125:974–80. [DOI] [PubMed] [Google Scholar]
- 147. Rautiainen S, Larsson S, Virtamo J, Wolk A. Total antioxidant capacity of diet and risk of stroke: a population-based prospective cohort of women. Stroke 2012;43:335–40. [DOI] [PubMed] [Google Scholar]
- 148. Bogers RP, Dagnelie PC, Westerterp KR, Kester AD, van Klaveren JD, Bast A, van den Brandt PA. Using a correction factor to correct for overreporting in a food-frequency questionnaire does not improve biomarker-assessed validity of estimates for fruit and vegetable consumption. J Nutr 2003;133:1213–19. [DOI] [PubMed] [Google Scholar]
- 149. Guertin KA, Moore SC, Sampson JN, Huang WY, Xiao Q, Stolzenberg-Solomon RZ, Sinha R, Cross AJ. Metabolomics in nutritional epidemiology: identifying metabolites associated with diet and quantifying their potential to uncover diet-disease relations in populations. Am J Clin Nutr 2014;100:208–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Souverein OW, de Vries JH, Freese R, Watzl B, Bub A, Miller ER, Castenmiller JJ, Pasman WJ, van Het HK, Chopra M et al.. Prediction of fruit and vegetable intake from biomarkers using individual participant data of diet-controlled intervention studies. Br J Nutr 2015;113:1396–409. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.