Abstract
In this study, antioxidant, chemical, microbiological, and sensory attributes changes taking place during the production of probiotic yoghurt using pulp of soursop (Annona muricata), sweetsop (Annona squamosa), and custard apple (Annona reticulata) were evaluated. The products were stored at 4 °C for 28 d, during which time physicochemical properties and viability of probiotic bacteria and yoghurt starter cultures were evaluated weekly. Yoghurts prepared with fruit pulps displayed higher antioxidant activities on the first day of storage compared to the control. During the storage, the addition of fruit pulps influenced (p < 0.05) pH, titratable acidity, syneresis and counts of B. animalis ssp. lactis BB-12 of yoghurts, whereas counts of Streptococcus thermophiles and Lactobacillus delbrueckii ssp. bulgaricus were found to be insignificant. Sensory evaluation results showed that yoghurt containing soursop fruit pulp had better sensory scores than other treatments. Therefore, these results proved that soursop can be used to produce probiotic yoghurt with enhanced physicochemical, microbiological and sensory properties.
Keywords: Food science, Microbiology
1. Introduction
At present, health-conscious consumers have become more aware of the relationship between wellbeing and healthy food. Hence, consumers tend to prefer food products that bring a simple but clear health benefits. The term functional food is used to indicate a food that contains one or more health-promoting component(s) beyond traditional nutrients. Major functionality claims are for gut health (especially in Japan and Europe), heart health (especially in the USA and Europe), promoting natural defenses, and boosting energy levels (Weststrate et al., 2002).
Yoghurt is a fermented dairy product, that is fermented and acidified by addition of a starter culture containing fermenting bacteria such as Streptococcus thermophiles and Lactobacillus delbrueckii ssp. bulgaricus. Yoghurt has gained widespread consumer acceptance as a healthy food providing health benefits such as improved lactose tolerance, promote weight management, strengthen the immune system, cancer prevention, prevention of osteoporosis, and a variety of health attributes associated with probiotic bacteria since yoghurt is one of the major carrier foods for probiotic cells (Pradeep Prasanna and Charalampopoulos, 2018; Ranadheera et al., 2012a).
Fruits are considered as an excellent source of antioxidants and prebiotic fibers and polyphenols (Fernandez and Marette, 2017). Consumption of fruits and yoghurt in combination has a potential to provide extra nutritional-physiological value that involve in synergetic effect on health. Probiotics are living microorganisms having beneficial effect on host health. Generally, 1–100 million cfu/g should have to be present in probiotic product to transfer the probiotic effect to consumers (Rybka and Kailasapathy, 1995). Probiotics are responsible for the balance of host intestinal micro flora and inhibition of carcinogens. They can increase lactose tolerance for dairy products and reduce serum cholesterol (Pereira and Gibson, 2002). A prebiotic is defined as 'a non-digestible food ingredient that beneficially affects the host by selectively stimulating the growth and/or activity of one or a limited number of bacteria in the colon, and thus improves host health' (Gibson and Roberfroid, 1995). Often, they are non-digestible carbohydrates provide significant health benefits for adults, including reduction of the risk of coronary heart disease and type-2 diabetes and maintenance of a healthy body weight (Nicklas et al., 2011).
Antioxidant compounds in foods play a significant role as a health-protecting factor. They are capable of deactivating free radicals which can cause cells and tissue damages. These damages cause malfunctioning of cells or cell death. Epidemiological studies have shown that antioxidants can prevent development of degenerative diseases such as cancer, coronary heart diseases, obesity, type 2 diabetes, hypertension, premature aging and inflammatory diseases (Rafieian-Kopaei et al., 2013).
Annona muricata, Annona squamosa and Annona reticulate are members of family annonaceae and they have known medicinal benefits. A. muricata, is also known as soursop, graviola and guanabana whereas, A. squamosa is called as sweetsop and sugar apple. Custard apple is the common name for A. reticulate. Antioxidant properties of A. muricata (Almeida et al., 2011), A. squamosa (Kothari and Seshadri, 2010) and A. reticulate (Dua and Srivastav, 2013) have been reported. They contain high amount of dietary fiber which may have a role as prebiotics. A. muricata has been used in various food applications such as yoghurt, wine and drinks (Lutchmedial et al., 2004). However, Annona squamosa and Annona reticulate have been barely studied and there are limited reliable and recorded data on application of these two fruits in food systems. In addition, these fruits may have prebiotic effect leading for higher viable count of probiotics in yoghurt like products during the refrigerated storage. Therefore, the present study was conducted to evaluate the addition of pulp of A. muricata, A. squamosa and A. reticulate on physicochemical and microbiological properties of probiotic yoghurts containing B. animalis ssp. lactis BB-12 during refrigerated storage for 28 d. Furthermore, the effects of Annona fruit pulp (AFP)s on antioxidant and sensory properties of yoghurts were also examined.
2. Materials and methods
2.1. Extraction of AFP and pasteurization
The fully ripened fruits of soursop, sweetsop, and custard apple were rinsed with 45 °C warm water, peeled and manually de-seeded. The pulps were extracted separately using a kitchen blender. The extracted pulps were pasteurized at 79 °C for 69 s as previously described by Umme et al. (1997).
2.2. Preparation of yoghurt inoculum
Working cultures of yoghurt bacteria and B. animalis ssp. lactis BB-12 were prepared. In the production of the working thermophilic yoghurt culture, sterilised milk was inoculated with thermophilic yoghurt cultures (YC-X11 YoFlex, Chr. Hansen, Hoersholm, Denmark), consisting Streptococcus thermophiles and Lactobacillus delbrueckii ssp. bulgaricus at a rate of 1% (w/v) and incubated at the 42 °C for ∼4 h. In the case of bifidobacteria, the sterilised milk was supplemented with 0.5% filter sterilised yeast extract (0.5%, v/v) and inoculated with B. animalis ssp. lactis BB-12 (Chr. Hansen, Hoersholm, Denmark) at a rate of 1% (w/v). The mixture was incubated at 37 °C under anaerobic conditions for 18 h.
2.3. Preparation of AFP incorporated yoghurt
Milk was standardized to 15% (w/v) total solids with skimmed milk powder. The mixture was pasteurized at 85 °C for 30 min and cooled to 43 °C and inoculated with the standard working thermophilic yoghurt culture and the working culture of B. animalis ssp. lactis BB-12 at the level of 1% (v/v). The mixture was incubated at 42 ± 2 °C until the pH reached 4.5. After the fermentation, the samples were transferred into a refrigerator at 4 °C until further used. For the preparation of fruit incorporated yoghurt, 10 % (w/v) concentrated pasteurized AFP was added to the milk before adding skim milk powder during the standardization. The samples were collected from each yoghurt on 1, 7, 14, 21 and 28 d of storage for analysis.
2.4. Analysis of antioxidants
2.4.1. Sample (pulp extract) preparation
The AFP was extracted as described by Chavan et al. (2013). In brief, the pulp was extracted using 80% methanol, keeping a solvent to AFP ratio of 10:1. AFP (5 g) was mixed with 50 ml of the solvent and ground for 3 min in a mechanical grinder. Extractions were kept at room temperature for 24 hrs and followed by filtration through Whatman No. 1 filter paper. Filtrates were stored at –20 °C until further used.
2.4.2. Yoghurt water extract preparation
Yoghurt water extract of the control and AFP yoghurts were carried out as explained by Amirdivani and Baba (2011). For this purpose, the yoghurt sample (10 g) was mixed with 2.5 ml of distilled water and pH of the sample was adjusted to 4.0 using 1 M HCl. The mixture was then incubated at 45 °C for 10 min followed by centrifugation (10000 rpm, 20 min, at 4 °C). The supernatant was harvested and the pH was adjusted to 7.0 using NaOH (0.1 M). The neutralized supernatant was centrifuged (10000 rpm, 20 min, at 4 °C) and the supernatant was stored at -20 °C until further used.
2.4.3. Total phenolic content, DPPH radical scavenging ability, and FRAP of AFP and yoghurt extracts
The total phenolic content (TPC) of AFP extract and yoghurt extracts was determined according to the method described by Singleton and Rossi (1965). DPPH (2, 2-diphenyl-l-picrylhydrazyl) radical scavenging ability of AFP extracts and yoghurt extracts were evaluated according to the procedure described by Brand-Williams et al. (1995). FRAP (Ferrous reducing antioxidant power) was performed according to the method described by Benzie and Strain (1996).
2.5. Viability of yoghurt starters and probiotic bacteria
Viability of yoghurt starter cultures and bifidobacteria of yoghurts during the storage was determined using the medium and conditions as previously described by Turgut and Cakmakci (2018).
2.6. Physicochemical analysis
The yoghurt samples were analysed for different parameters during the storage on 1, 7, 14, 21, and 28 d. The pH of the yoghurt samples during storage was measured using a pH meter (Mettler Toledo, UK) at room temperature. For the determination of titratable acidity (TA), 10 g of yoghurt sample suspended in 20 ml of deionized water was titrated against 0.1 N NaOH using phenolphthalein as the indicator as explained by Amirdivani and Baba (2011). The water holding capacity (WHC) of yoghurt samples were determined using the method by Sodini et al. (2005). The syneresis of yoghurt samples was determined using the technique reported by Prasanna et al. (2013).
2.7. Sensory evaluation
Three types yoghurts and the control yoghurt were subjected into a sensory evaluation with 30 untrained panellists. The sensory evaluation was carried out using a five-point hedonic scale (5- like very much to 1- dislike very much) based on acceptance to the product in order to evaluate the degree of likeliness for selected quality attributes i.e. flavour, colour, texture, aroma, and overall acceptability.
2.8. Statistical analysis
Results of antioxidant level, cell count, pH, syneresis, water holding capacity and titratable acidity of samples were analysed using one-way ANOVA procedure of SAS, version 9.2 (SAS Institute Inc., Cary NC, USA) and the mean separation was achieved using Tukey's procedure. Results of sensory analysis were analysed with Kruskal-Wallis non-parametric analysis (SPSS v. 15.0).
3. Results and discussion
3.1. Antioxidant analysis for raw and pasteurized AFP
Phenolic compounds appeared to be more sensitive to thermal processing as after pasteurization where soursop and sweetsop AFP extracts showed significant (p < 0.05) reduction in TPC from 116.72 ± 9.91 to 93.17 ± 9.71 and 412.28 ± 5.31 to 269.83 ± 9.67 mg GAE/100 g of AFP respectively (Table 1). However, custard apple showed no significant (p < 0.05) difference. The IC50 value of pasteurized AFP extract and the IC50 values of raw AFP extract was not significantly (p < 0.05) different. Therefore, the radical scavenging effect of extracts on the DPPH radicals act as the same for three types of AFP extracts.
Table 1.
Antioxidant content of raw and pasteurized AFP.
| Total phenolic content (mg GAE/100 g) |
IC50 value (mg/ml) |
Total antioxidant capacity (mM Fe (II)/100 g) |
||||
|---|---|---|---|---|---|---|
| Raw | Pasteurized | Raw | Pasteurized | Raw | Pasteurized | |
| Soursop | 116.72 ± 9.91Ba | 93.17 ± 9.71Cb | 8.93 ± 2.87Aa | 6.23 ± 1.17Aa | 0.82 ± 0.09Ba | 3.03 ± 0.24Bb |
| Sweetsop | 412.28 ± 5.31Aa | 269.83 ± 9.67Bb | 5.69 ± 2.78Aa | 3.84 ± 0.02Aa | 5.37 ± 0.17Aa | 9.11 ± 0.37Ab |
| Custard apple | 361.22 ± 20.21Aa | 368.82 ± 10.67Aa | 7.20 ± 3.85Aa | 4.74 ± 1.71Aa | 9.23 ± 1.53Aa | 11.56 ± 2.31Ab |
ABMeans with different uppercase are significantly different (p < 0.05) of TPC, DPPH, FRAP between each type of AFP. abcde Means with different lowercase are significantly different (p < 0.05) of TPC, DPPH, FRAP between raw and pasteurized AFP. mg GAE: mg gallic acid equivalent.
The results of FRAP analysis showed that raw AFP extract of custard apple and sweetsop differ significantly (p < 0.05) from soursop AFP extract. Custard apple and sweetsop AFP extract showed the highest total antioxidant capacity. After thermal process, all three AFP extracts reported significantly (p < 0.05) high antioxidant capacity. The interaction among two factors i.e. AFP extract type and pulp extract status (raw or pasteurized) of this experiment was significant (p < 0.05). This significance indicated that soursop, sweetsop and custard apple responded differently to heat treatment. The results of the present study are in line with the findings of a similar study carried out to determine effect of thermal and high hydrostatic pressure processing on antioxidant activity and colour of fruit smoothies (Keenan et al., 2010). They reported that increasing antioxidant activity alongside decreasing total phenolic content followed by the thermal process. In addition, it has been emphasized that food processing as cooking, grinding are able to improve the extractability of antioxidant compounds by breaking down cell walls (Gärtner et al., 1997).
3.2. Antioxidant analysis of fruit yoghurts
3.2.1. TPC of fruit yoghurts
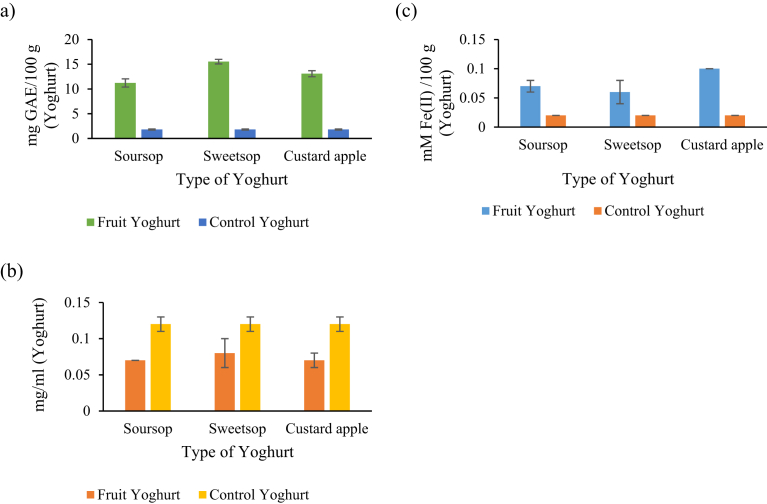
In the case of the FY, all type of yoghurts showed significantly (p < 0.05) high TPC compared to control yoghurt [Fig. 1 (a)]. The TPC respective to the control yoghurt is probably due to the presence of polyphenols in milk, that mostly derived from feed (Besle et al., 2010) and the protein and reducing compounds (Chouchouli et al., 2013). The highest phenolic content was observed in SWY with 15.53 ± 0.46 mg GAE/100 g of yoghurt followed by CAY (13.08 ± 0.61 mg GAE/100 g) and SOY (11.22 ± 0.83 mg GAE/100 g of yoghurt). Similarly, higher TPC value was reported by Karaaslan et al. (2011) for yoghurt supplemented with grape and callus extracts. In another study, Chouchouli et al. (2013) observed higher TPC value for yoghurt containing grape seed extracts. In addition, addition of strawberry pulp was shown to enhance phenolic content and antioxidant properties of yoghurt (Oliveira et al., 2015). Furthermore, green tea supplementation increased in TPC value of probiotic yoghurt during the refrigerated storage (Muniandy et al., 2016). In a recent study, a higher TPC value was observed in yoghurt supplemented with osmo-air-dried mulberry compared to the control (Sigdel et al., 2018).
Fig. 1.
Antioxidant activities of yoghurts. (a) TPC of yoghurts. (b). IC50 value for yoghurts. (c) Antioxidant properties of yoghurts using FRAP assay. Vertical lines represent standard deviations.
3.2.2. Average IC50 (mg/ml) values for DPPH assay of yoghurts
The DPHH assay is considered as a simple method which gives information on the radical scavenging activity of the antioxidant substances exist in a sample (Najgebauer-Lejko et al., 2011). IC50 value (mg/ml) is the concentration of sample required to scavenge 50% DPPH free radical and is calculated from a calibration curve by a linear regression. The results of IC50 of yoghurts are shown in Fig. 1 (b). There were significant differences (p < 0.05) in the DPPH scavenging activity among the four yoghurt types. All the AFPs containing yoghurts showed scavenging abilities in the descending order the control < SWY < CAY < SOY where the lowest IC50 values were observed in SOY (0.07 ± 0.00 mg/ml). Similarly, addition of sour cherry pulp (Şengül et al., 2012), grape seed extract (Chouchouli et al., 2013) and grape and callus extract (Karaaslan et al., 2011) to yoghurt was shown to have higher antioxidant activity than control yoghurt using DPPH scavenging assay. In another study, aqueous extract of Matricaria recutita was shown to increase DPPH activity in yoghurt (Caleja et al., 2016). Ramos et al. (2017) observed higher DPPH activity in fermented milk supplemented with herbal extract mixture composed of Ilex paraguariensis, Syzygium aromaticum, and Cymbopogon citratus.
3.2.3. Antioxidant properties of yoghurts determined using FRAP assay
This assay has been reported to be suitable to measure antioxidant activity of substances having half-reaction redox potential below 0.7 V. This measures only non-protein antioxidant capacity. Milk component such as urate, ascorbate, ∝-tocopherol and bilirubin have been characterised to have ferric reducing ability (Najgebauer-Lejko et al., 2011). The ferric reducing capacity of each yoghurt type is shown in Fig. 1 (c). The total antioxidant of tested yoghurts ranged from 0.02 ± 0.00 to 0.1 ± 0.01 mM Fe (II)/100 g of yoghurt. Among the four yoghurt types CAY yoghurt showed the highest in total antioxidant capacity followed by SOY and SWY. The control yoghurt showed significantly (p < 0.05) lowest antioxidant capacity. Similarly, yoghurt containing pomegranate juice was shown to have a higher ferric reducing capacity compared to control yoghurt (Trigueros et al., 2014).
3.3. Viability of B. animalis ssp. lactis BB-12 and yoghurt bacteria during refrigerated storage
Table 2 shows survival of probiotic and yoghurt bacteria during the refrigerated storage for 28 d.
Table 2.
Changes in the viable counts of yoghurt starter bacteria and bifidobacteria of different yoghurts during refrigerated storage (4 °C) for 28 days.
| Yoghurt Type | Period of storage (Days) |
||||
|---|---|---|---|---|---|
| 1 | 7 | 14 | 21 | 28 | |
|
Viability of probiotic B. animalis ssp. lactis BB-12 | |||||
| Soursop | 8.42 ± 0.09 Aa | 8.40 ± 0.07 Aa | 8.39 ± 0.25 Aa | 8.16 ± 0.09 Aab | 7.93 ± 0.05 Ab |
| Sweetsop | 8.29 ± 0.13 Aa | 8.28 ± 0.04 Aa | 8.26 ± 0.06 Aa | 8.15 ± 0.08 Aab | 7.90 ± 0.08 Ac |
| Custard apple | 8.53 ± 0.08 Aa | 8.51 ± 0.46 Aa | 8.48 ± 0.22 Aa | 8.23 ± 0.14 Aa | 7.98 ± 0.24 Aa |
| Control | 8.28 ± 0.28 Aa | 7.94 ± 0.05 Aa | 8.29 ± 0.21 Aa | 8.10 ± 0.15 Aab | 7.62 ± 0.15 Bc |
|
Viability of yoghurt bacteria Streptococcus thermophilus | |||||
| Soursop | 8.46 ± 0.07 Aa | 8.39 ± 0.12 Aa | 8.41 ± 0.15 Aa | 8.30 ± 0.11 Aab | 8.17 ± 0.01 Ab |
| Sweetsop | 8.41 ± 0.06 Aa | 8.43 ± 0.16 Aa | 8.45 ± 0.16 Aa | 8.30 ± 0.07 Aa | 8.23 ± 0.04 Aa |
| Custard apple | 8.38 ± 0.20 Aab | 8.45 ± 0.08 Aa | 8.48 ± 0.13 Aa | 8.25 ± 0.05 Aab | 8.23 ± 0.07 Aab |
| Control | 8.49 ± 0.14 Aab | 8.54 ± 0.19 Aa | 8.62 ± 0.27 Aa | 8.30 ± 0.14 Aabc | 8.23 ± 0.03 Abc |
| L. delbrueckii subsp. bulgaricus | |||||
| Soursop | 8.49 ± 0.31 Aa | 8.19 ± 0.14 Ab | 7.98 ± 0.14 Ab | 7.75 ± 0.20 Ac | 7.62 ± 0.16 Ac |
| Sweetsop | 8.15 ± 0.10 Aa | 7.84 ± 0.10 Ab | 7.60 ± 0.13 Ac | 7.64 ± 0.11 Abc | 7.63 ± 0.14 Abc |
| Custard apple | 8.15 ± 0.17 Aa | 7.97 ± 0.12 Aa | 7.82 ± 0.19 Aab | 7.79 ± 0.22 Ab | 7.67 ± 0.05 Ac |
| Control | 8.58 ± 0.22 Aa | 8.23 ± 0.12 Ab | 7.92 ± 0.09 Ac | 7.76 ± 0.10 Abc | 7.75 ± 0.11 Ac |
AB Means in the same column for each type of microorganism without common letter differ significantly (p < 0.05) for a particular day of storage. Data are expressed as mean ± standard deviation. abcde Means in the same row without common letter differ significantly (p < 0.05) for each type of yoghurt.
All the three types of FYs alone with the control were able to maintain significantly higher (p < 0.05) level of steady probiotic count than the recommended therapeutic minimum of 106 cfu/ml over 28 days of shelf life, which is refer to the minimum number of viable cells that need to be consumed to obtain the probiotic effect (Donkor et al., 2006). There was no significant difference in viable count of B. animalis ssp. lactis BB-12 of all the yoghurt samples from day 1 to day 21. However, on day 28, significantly (p < 0.05) low viable count in control yoghurt was observed compared to that of FYs. There is no reliable information on survival of the same strain of probiotic in fruit yoghurt containing sweetsop, soursop and custard apple. However, Kailasapathy et al. (2008) did not observe any difference between viability of yoghurts with or without fruit including mango, mixed berry, passion fruit and strawberry. In general, it has been reported that addition of fruit based material either as juice or pulp may negatively effect on growth and viability of probiotic bacteria in food products due to acidity and the presence of antimicrobial compounds (Buriti et al., 2007). However, in this study, B. animalis ssp. lactis BB-12 could maintain a higher viability in the presence of all the AFPs which indicate the suitability of these AFP to be in cooperated in the yoghurt containing probiotic such as B. animalis ssp. lactis BB-12. In addition, addition of dietary fibre is considered to improve probiotic viability in food products during the storage (do Espírito Santo et al., 2012a). Similarly, the results of the present study showed that there was a higher viability of B. animalis ssp. lactis BB-12 in yoghurt containing sweetsop, soursop and custard apple compared to that of the control yoghurt during the latter part of the storage. This may be due to the ability of the probiotics to metabolize fibre and other nutrients of these AFPs. In contrast to the present results, Ranadheera et al. (2012b) did not observe any positive effect of addition of commercial fruit juice consisted of apple juice, orange juice, banana puree, pineapple juice, mango puree and passionfruit fruit juice to goat milk yoghurt on the viability of B. animalis ssp. lactis BB-12. Fruits are considered to be good sources of phenolic compounds which have been shown to affect viability of probiotics. In addition, proteins and dietary fiber of foods may protect probiotics from acidic environment (Patel, 2017). Furthermore, prebiotic properties of fruit containing yogurt have been reported (Barat and Ozcan, 2018; Ranadheera et al., 2012b).
During the storage period, the counts of S. thermophiles were stable for the four yoghurts throughout. Nevertheless, count of L. delbrueckii subsp. bulgaricus of each yoghurt type was slightly decreased throughout the storage which indicate that it is less robust compared to that of S. thermophiles in all yoghurt types. This finding is in accordance with previous observation on viability of yoghurt starter culture, in particular with bifidobacteria, as acidity of yoghurt is increased with the storage period (Prasanna et al., 2013). Overall results indicate that the prebiotic effect in FYs due to incorporated AFP selectively stimulate the growth and activity of specific bacteria. More specifically, FYs showed significantly (p < 0.05) high viable cell count only for B. bifidum neither S. thermophilus nor L. delbrueckii subsp. bulgaricus. Similarly, Kaplan and Hutkins (2000) reported that some fructooligosaccharides increase the growth of probiotic bacteria as L. acidophilus and Bifidobacterium spp., and does not stimulate bacteria as the starter cultures S. thermophilus and L. delbrueckii subsp. bulgaricus.
3.4. Physicochemical analysis of yoghurts
3.4.1. Changes in pH and titratable acidity of yoghurts during storage
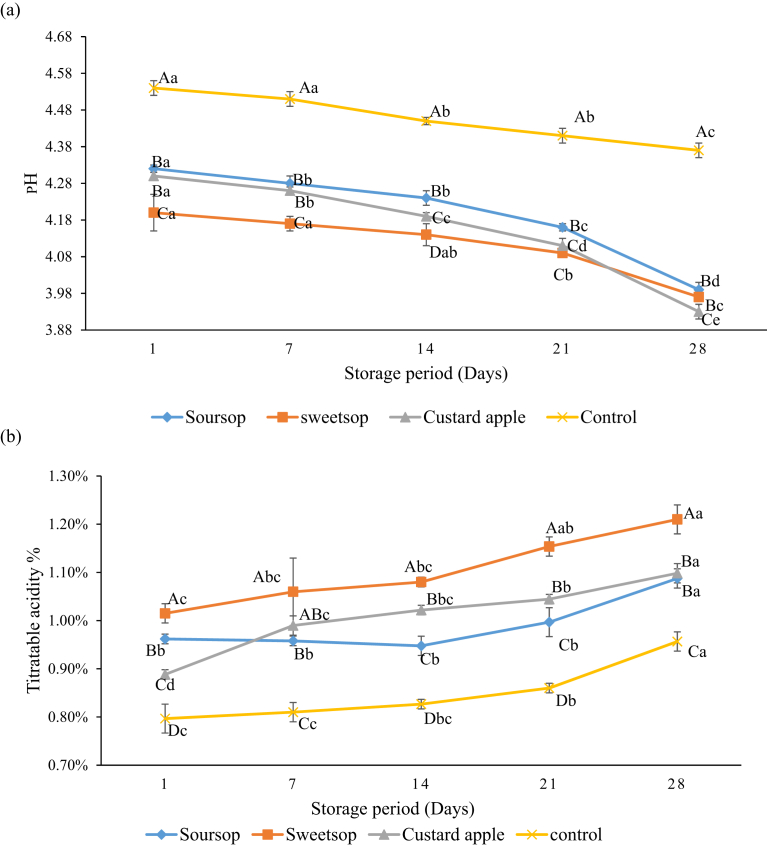
There was a decrease in pH and increase in titratable acidity (TA) during storage for all the treatments and the results are shown in Fig. 2. However, the drop in pH and increase in TA were higher for the samples containing AFP compared to those of the control yoghurt and this is due to continued residual fermentation at the refrigerated storage. The higher decrease in pH and concurrent increase in acidity observed in the AFP containing treatments are due to higher availability of carbohydrate sources from fruits to the metabolic activity of both yoghurt starter cultures (S. thermophilus nor L. delbrueckii subsp. bulgaricus) and B. animalis ssp. lactis BB-12 resulting higher level of organic acids. Sah et al. (2016) reported similar pH changes for the control yoghurt and yoghurt containing pineapple peel powder. In addition, do Espírito Santo et al. (2012b) also reported that TA in yoghurt with passion fruit peel powder was higher than in their respective control yoghurts.
Fig. 2.
(a) Changes of pH during storage at 4 °C. (b) Changes of titratable acidity during storage at 4 °C. Vertical lines represent standard deviations. ABC Means with different uppercase are significantly (p < 0.05) different between each type of yoghurt, for a particular day of storage; abcde Means with different lowercase are significantly (p < 0.05) different between each day, for each type of yoghurt during the storage.
3.4.2. Syneresis of yoghurt during storage
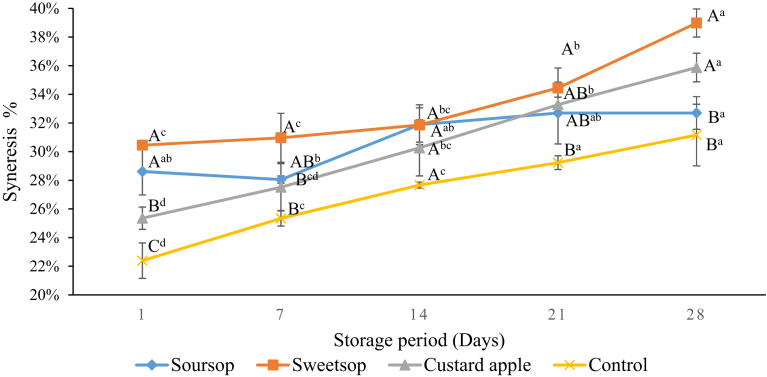
Syneresis is a major visible issue in commercial yoghurt manufacturing which leads accumulation of whey on the surface of the gel, which can lead to poor consumer acceptance of the product (Ghasempour et al., 2012). The values of syneresis of the different yoghurts are shown in Fig. 3. The control yoghurt showed a lower syneresis values compared to the yoghurts produced with AFPs throughout the storage. The higher syneresis level observed in the yoghurt with AFP is in agreement with the findings of other studies with yoghurt produced with pineapple peel powder (Sah et al., 2016) and kiwi fruit marmalade (Tarakci, 2010). This could be due to thermodynamic incompatibility between polysaccharides of AFPs and milk proteins. In addition, increase in syneresis of AFP containing yoghurts during the storage is due to continued reduction of pH of yoghurts observed in this study leading for contraction of the casein network and higher level of syneresis as explained by Sah et al. (2016) and Ranadheera et al. (2012b).
Fig. 3.
Changes in syneresis of yoghurts during storage at 4 °C. Vertical lines represent standard deviations. AB Means with different uppercase are significantly (p < 0.05) different between each type of yoghurt, for a particular day of storage; abcde Means with different lowercase are significantly (p < 0.05) different between each day, for each type of yoghurt during the storage.
3.5. Sensory evaluation of yoghurts
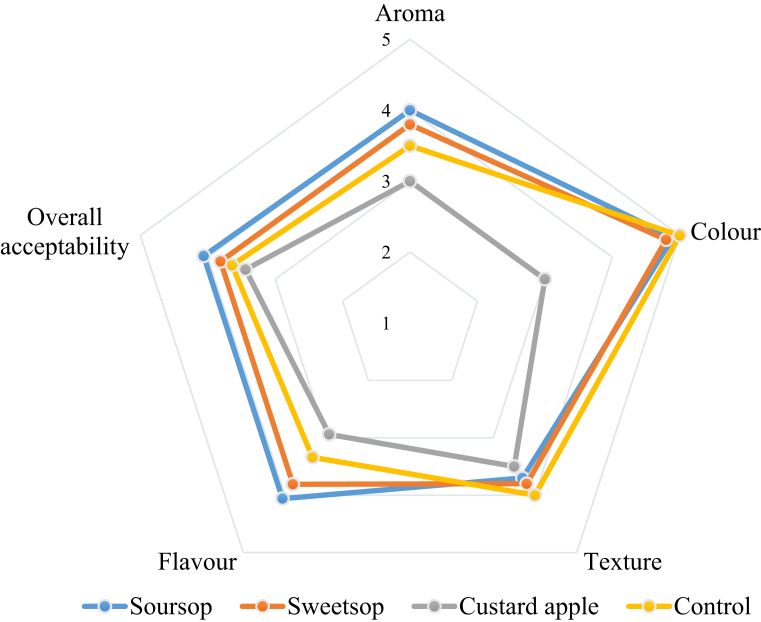
The results of the sensory evaluation of the yoghurts are shown in Fig. 4. In general, among fruit yoghurts, SOY received the highest scores for all sensory attributes while addition of custard apple resulted yoghurt with the lowest scores for the sensory attributes. SOY was scored higher on average by the panellists compared to the control yoghurt in terms of flavour, aroma and overall acceptability, which may be due to combined effects from flavour compounds from fruit juice and improved growth performances of B. animalis ssp. lactis BB-12 leading for higher production of flavour compounds. These compounds may include esters, 3-methylthiohexan-1-ol, 2-methyl-4-propyl-1, 3-oxathione enantiomers and edulans I and II (Espírito-Santo et al., 2013). In addition, Ranadheera et al. (2012b) reported that addition of fruit based materials could lead for higher natural sugar content of fruit yoghurt leading for higher consumer acceptability. These finding are supported by Şengül et al. (2012) who observed the highest overall acceptability score to the yoghurt containing sour cheery fruit pulp. However, the control yoghurt recorded the highest score for the texture compared to AFP yoghurts which were recorded as having higher syneresis and graininess by some of the panellists. Though, SOY showed higher acidity level compared to control yoghurt, there was a higher consumer acceptability than control yoghurt in the present study, which may be due to the natural sugars of the added fruit pulps.
Fig. 4.
Pattern of variation of sensory properties of yoghurts.
4. Conclusions
Soursop (Annona muricata), sweetsop (Annona squamosa), and custard apple (Annona reticulata) were successfully used to produce probiotic fruit yoghurts with B. animalis ssp. lactis BB-12. Addition of fruit pulp significantly increased antioxidant activity of yoghurts. Fruit pulp incorporation resulted significantly lower pH and higher titratable acidity of yoghurt during storage. Yoghurt containing fruit pulp showed higher syneresis compared to control during the storage study. Addition of fruit pulp supported the growth and viability of B. animalis ssp. lactis BB-12 in yoghurt during the storage while it did not have a significant effect on the performances of yoghurt bacteria. The panellists recorded the highest scores for flavour, aroma, colour and overall acceptability to the yoghurt containing soursop fruit pulp. Therefore, it can be concluded that soursop can be used in manufacturing a yoghurt with good antioxidant properties, sensory attributes, and probiotic count during the storage.
Declarations
Author contribution statement
S.S. Senadeera, Pradeep Prasanna Prasanna Herathge, N.W.I.A. Jayawardana, Dimuthu Chanaka Samarasinghe Gunasekara, P. Senadeera, Ananda Chandrasekar: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- Almeida M.M.B., de Sousa P.H.M., Arriaga Â.M.C., do Prado G.M., de Carvalho Magalhães C.E., Maia G.A., de Lemos T.L.G. Bioactive compounds and antioxidant activity of fresh exotic fruits from northeastern Brazil. Food Res. Int. 2011;44(7):2155–2159. [Google Scholar]
- Amirdivani S., Baba A.S. Changes in yogurt fermentation characteristics, and antioxidant potential and in vitro inhibition of angiotensin-1 converting enzyme upon the inclusion of peppermint, dill and basil. LWT - Food Sci. Technol. 2011;44(6):1458–1464. [Google Scholar]
- Barat A., Ozcan T. Growth of probiotic bacteria and characteristics of fermented milk containing fruit matrices. Int. J. Dairy Technol. 2018;71:120–129. [Google Scholar]
- Benzie I.F., Strain J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Anal. Biochem. 1996;239(1):70–76. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- Besle J., Viala D., Martin B., Pradel P., Meunier B., Berdagué J., Fraisse D., Lamaison J., Coulon J. Ultraviolet-absorbing compounds in milk are related to forage polyphenols. J. Dairy Sci. 2010;93(7):2846–2856. doi: 10.3168/jds.2009-2939. [DOI] [PubMed] [Google Scholar]
- Brand-Williams W., Cuvelier M.-E., Berset C. Use of a free radical method to evaluate antioxidant activity. LWT - Food Sci. Technol. 1995;28(1):25–30. [Google Scholar]
- Buriti F.C., Komatsu T.R., Saad S.M. Activity of passion fruit (Passiflora edulis) and guava (Psidium guajava) pulps on Lactobacillus acidophilus in refrigerated mousses. Braz. J. Microbiol. 2007;38(2):315–317. [Google Scholar]
- Caleja C., Barros L., Antonio A.L., Carocho M., Oliveira M.B.P.P., Ferreira I.C.F.R. Fortification of yogurts with different antioxidant preservatives: a comparative study between natural and synthetic additives. Food Chem. 2016;210:262–268. doi: 10.1016/j.foodchem.2016.04.114. [DOI] [PubMed] [Google Scholar]
- Chavan J., Jagtap U., Gaikwad N., Dixit G., Bapat V. Total phenolics, flavonoids and antioxidant activity of Saptarangi (Salacia chinensis L.) fruit pulp. J. Plant Biochem. Biotechnol. 2013;22(4):409–413. [Google Scholar]
- Chouchouli V., Kalogeropoulos N., Konteles S.J., Karvela E., Makris D.P., Karathanos V.T. Fortification of yoghurts with grape (Vitis vinifera) seed extracts. LWT - Food Sci. Technol. 2013;53(2):522–529. [Google Scholar]
- do Espírito Santo A.P., Cartolano N.S., Silva T.F., Soares F.A.S.M., Gioielli L.A., Perego P., Converti A., Oliveira M.N. Fibers from fruit by-products enhance probiotic viability and fatty acid profile and increase CLA content in yoghurts. Int. J. Food Microbiol. 2012;154(3):135–144. doi: 10.1016/j.ijfoodmicro.2011.12.025. [DOI] [PubMed] [Google Scholar]
- do Espírito Santo A.P., Perego P., Converti A., Oliveira M.N. Influence of milk type and addition of passion fruit peel powder on fermentation kinetics, texture profile and bacterial viability in probiotic yoghurts. LWT - Food Sci. Technol. (Lebensmittel-Wissenschaft -Technol.) 2012;47(2):393–399. [Google Scholar]
- Donkor O., Henriksson A., Vasiljevic T., Shah N. Effect of acidification on the activity of probiotics in yoghurt during cold storage. Int. Dairy J. 2006;16(10):1181–1189. [Google Scholar]
- Dua D., Srivastav N.S. Anti-cancerous and antioxidant potential of aqueous extracts of Annona reticulata, Podophyllum peltatum, Psidium guajava, Ananas comosus, Carissa carandas on MCF-7 cancer cell line. Int. J. Integr. Sci. Innovat. Technol. 2013;2(4):15–19. https://webzoom.freewebs.com/ijiit/documents/130204A03.pdf [Google Scholar]
- Espírito-Santo A.P., Lagazzo A., Sousa A.L.O.P., Perego P., Converti A., Oliveira M.N. Rheology, spontaneous whey separation, microstructure and sensorial characteristics of probiotic yoghurts enriched with passion fruit fiber. Food Res. Int. 2013;50(1):224–231. [Google Scholar]
- Fernandez M.A., Marette A. Potential health benefits of combining yogurt and fruits based on their probiotic and prebiotic properties. Adv. Nutr. Int. Rev. J. 2017;8(1):155S–164S. doi: 10.3945/an.115.011114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gärtner C., Stahl W., Sies H. Lycopene is more bioavailable from tomato paste than from fresh tomatoes. Am. J. Clin. Nutr. 1997;66(1):116–122. doi: 10.1093/ajcn/66.1.116. [DOI] [PubMed] [Google Scholar]
- Ghasempour Z., Alizadeh M., Bari M.R. Optimisation of probiotic yoghurt production containing Zedo gum. Int. J. Dairy Technol. 2012;65(1):118–125. [Google Scholar]
- Gibson G.R., Roberfroid M.B. Dietary modulation of the human colonic microbiota: introducing the concept of prebiotics. J. Nutr. 1995;125(6):1401–1412. doi: 10.1093/jn/125.6.1401. [DOI] [PubMed] [Google Scholar]
- Kailasapathy K., Harmstorf I., Phillips M. Survival of Lactobacillus acidophilus and Bifidobacterium animalis ssp. lactis in stirred fruit yogurts. LWT - Food Sci. Technol. (Lebensmittel-Wissenschaft -Technol.) 2008;41(7):1317–1322. [Google Scholar]
- Kaplan H., Hutkins R.W. Fermentation of fructooligosaccharides by lactic acid bacteria and bifidobacteria. Appl. Environ. Microbiol. 2000;66(6):2682–2684. doi: 10.1128/aem.66.6.2682-2684.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karaaslan M., Ozden M., Vardin H., Turkoglu H. Phenolic fortification of yogurt using grape and callus extracts. LWT - Food Sci. Technol. 2011;44(4):1065–1072. [Google Scholar]
- Keenan D.F., Brunton N.P., Gormley T.R., Butler F., Tiwari B.K., Patras A. Effect of thermal and high hydrostatic pressure processing on antioxidant activity and colour of fruit smoothies. Innovat. Food Sci. Emerg. Technol. 2010;11(4):551–556. [Google Scholar]
- Kothari V., Seshadri S. Antioxidant activity of seed extracts of Annona squamosa and Carica papaya. Nutr. Food Sci. 2010;40(4):403–408. [Google Scholar]
- Lutchmedial M., Ramlal R., Badrie N., Chang-Yen I. Nutritional and sensory quality of stirred soursop (Annona muricata L.) yoghurt. Int. J. Food Sci. Nutr. 2004;55(5):407–414. doi: 10.1080/09637480400002800. [DOI] [PubMed] [Google Scholar]
- Muniandy P., Shori A.B., Baba A.S. Influence of green, white and black tea addition on the antioxidant activity of probiotic yogurt during refrigerated storage. Food Packag. Shelf Life. 2016;8:1–8. [Google Scholar]
- Najgebauer-Lejko D., Sady M., Grega T., Walczycka M. The impact of tea supplementation on microflora, pH and antioxidant capacity of yoghurt. Int. Dairy J. 2011;21(8):568–574. [Google Scholar]
- Nicklas T.A., O’Neil C.E., Liska D.J., Almeida N.G., Fulgoni V.L., III Modeling dietary fiber intakes in US adults: implications for public policy. Food Nutr. Sci. 2011;2(09):925–931. [Google Scholar]
- Oliveira A., Alexandre E.M.C., Coelho M., Lopes C., Almeida D.P.F., Pintado M. Incorporation of strawberries preparation in yoghurt: impact on phytochemicals and milk proteins. Food Chem. 2015;171:370–378. doi: 10.1016/j.foodchem.2014.08.107. [DOI] [PubMed] [Google Scholar]
- Patel A.R. Probiotic fruit and vegetable juices- recent advances and future perspective. Int. Food Res. J. 2017;24(5):1850–1857. http://www.ifrj.upm.edu.my/24%20(05)%202017/(2).pdf [Google Scholar]
- Pereira D.I., Gibson G.R. Effects of consumption of probiotics and prebiotics on serum lipid levels in humans. Crit. Rev. Biochem. Mol. Biol. 2002;37(4):259–281. doi: 10.1080/10409230290771519. [DOI] [PubMed] [Google Scholar]
- Prasanna P.H.P., Grandison A.S., Charalampopoulos D. Microbiological, chemical and rheological properties of low fat set yoghurt produced with exopolysaccharide (EPS) producing Bifidobacterium strains. Food Res. Int. 2013;51(1):15–22. [Google Scholar]
- Pradeep Prasanna P.H., Charalampopoulos D. Encapsulation in an alginate–goats’ milk–inulin matrix improves survival of probiotic Bifidobacterium in simulated gastrointestinal conditions and goats’ milk yoghurt. Int. J. Dairy Technol. 2018 [Google Scholar]
- Rafieian-Kopaei M., Baradaran A., Rafieian M. Oxidative stress and the paradoxical effects of antioxidants. J. Res. Med. Sci. 2013;18(7):628. http://jrms.mui.ac.ir/index.php/jrms/article/viewFile/9316/3952 [PMC free article] [PubMed] [Google Scholar]
- Ramos L.R., Santos J.S., Daguer H., Valese A.C., Cruz A.G., Granato D. Analytical optimization of a phenolic-rich herbal extract and supplementation in fermented milk containing sweet potato pulp. Food Chem. 2017;221:950–958. doi: 10.1016/j.foodchem.2016.11.069. [DOI] [PubMed] [Google Scholar]
- Ranadheera C.S., Evans C., Adams M., Baines S. In vitro analysis of gastrointestinal tolerance and intestinal cell adhesion of probiotics in goat's milk ice cream and yogurt. Food Res. Int. 2012;49(2):619–625. [Google Scholar]
- Ranadheera C.S., Evans C.A., Adams M.C., Baines S.K. Probiotic viability and physico-chemical and sensory properties of plain and stirred fruit yogurts made from goat’s milk. Food Chem. 2012;135(3):1411–1418. doi: 10.1016/j.foodchem.2012.06.025. [DOI] [PubMed] [Google Scholar]
- Rybka S., Kailasapathy K. The survival of culture bacteria in fresh and freeze-dried AB yoghurts. Aust. J. Dairy Technol. 1995;50(2):51–57. https://search.proquest.com/openview/4d3110d597aa96deeacee1dcff919e68/1?pq-origsite=gscholar&cbl=36914 [Google Scholar]
- Sah B.N.P., Vasiljevic T., McKechnie S., Donkor O.N. Physicochemical, textural and rheological properties of probiotic yogurt fortified with fibre-rich pineapple peel powder during refrigerated storage. LWT - Food Sci. Technol. (Lebensmittel-Wissenschaft -Technol.) 2016;65:978–986. [Google Scholar]
- Şengül M., Erkaya T., Şengül M., Yildiz H. The effect of adding sour cherry pulp into yoghurt on the physicochemical properties, phenolic content and antioxidant activity during storage. Int. J. Dairy Technol. 2012;65(3):429–436. [Google Scholar]
- Sigdel A., Ojha P., Karki T.B. Phytochemicals and syneresis of osmo-dried mulberry incorporated yoghurt. Food Sci. Nutr. 2018;6(4):1045–1052. doi: 10.1002/fsn3.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singleton V.L., Rossi J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965;16(3):144–158. http://www.ajevonline.org/content/16/3/144.short [Google Scholar]
- Sodini I., Lucas A., Tissier J.P., Corrieu G. Physical properties and microstructure of yoghurts supplemented with milk protein hydrolysates. Int. Dairy J. 2005;15(1):29–35. [Google Scholar]
- Tarakci Z. Influence of kiwi marmalade on the rheology characteristics, color values and sensorial acceptability of fruit yogurt. Kafkas Univ Vet Fak Derg. 2010;16(2):173–178. http://www.vetdergikafkas.org/uploads/pdf/pdf_KVFD_627.pdf [Google Scholar]
- Trigueros L., Wojdyło A., Sendra E. Antioxidant activity and protein-polyphenol interactions in a pomegranate (Punica granatum L.) yogurt. J. Agric. Food Chem. 2014;62(27):6417–6425. doi: 10.1021/jf501503h. [DOI] [PubMed] [Google Scholar]
- Turgut T., Cakmakci S. Probiotic strawberry yogurts: microbiological, chemical and sensory properties. Probiotics Antimicrob. Protein. 2018;10(1):64–70. doi: 10.1007/s12602-017-9278-6. [DOI] [PubMed] [Google Scholar]
- Umme A., Asbi B., Salmah Y., Junainah A., Jamilah B. Characteristics of soursop natural puree and determination of optimum conditions for pasteurization. Food Chem. 1997;58(1–2):119–124. [Google Scholar]
- Weststrate J., Van Poppel G., Verschuren P. Functional foods, trends and future. Br. J. Nutr. 2002;88(S2):S233–S235. doi: 10.1079/BJN2002688. [DOI] [PubMed] [Google Scholar]