Abstract
It has been confirmed that apoptosis, autophagy and necrosis are the three major modes of cell death. For a long time, necrosis is regarded as a deranged or accidental cell demise. In recent years, there is evidence showing that necrotic cell death can be a well regulated and orchestrated event, which is also known as programmed cell death or “necroptosis”. Necroptosis can be triggered by a variety of external stimuli and regulated by a caspase-independent pathway. It plays a key role in the pathogenesis of some diseases including neurological diseases. In the past two decades, a variety of studies have revealed that the necroptosis related pathway is activated in stroke, and plays a crucial role in the pathogenesis of stroke. Moreover, necroptosis may serve as a potential target in the therapy of stroke because genetic or pharmacological inhibition of necroptosis has been shown to be neuroprotective in stroke in vitro and in vivo. In this review, we briefly summarize re-cent advances in necroptosis, introduce the mechanism and strategies targeting necroptosis in stroke, and finally propose some issues in the treatment of stroke by targeting necroptosis
Keywords: Programmed cell death, necroptosis, stroke, RIP1, RIP3, MLKL, Nec-1
1. Introduction
Stroke is caused by a poor blood flow to the brain, and can be divided into two main types: ischemic stroke and hemorrhagic stroke. Although the incidence of stroke is still increasing, the mortality of stroke tends to reduce in recent years due to the reinforcement of patients’ education on stroke prevention [1], early diagnosis and therapy [2], establishment of stroke unit [3], timely thrombolysis [4], and improvement of quality of care [5]. However, stroke is still the second most common cause of death following ischemic heart disease, and accounts for 9% of all deaths world wide [6]. In addition, it has high disability and recurrence rate, increasing the economic burdens to the family and the society. Currently, the available therapeutic strategies for stroke include thrombolytic therapy, anticoagulants, anti-platelet drugs, and neuroprotective agents such as reactive oxygen species (ROS) scavenger [7], endothelial nitric oxide synthase activator [8], mammalian target of rapamycin activator or inhibitor [9], and tumor necrosis factor receptor-associated factor 1 inhibitor [10]. However, thrombolytic therapy is limited due to the short time window and the efficacy of other therapies has not been confirmed in clinical trials so far. Thus, more effort is needed to develop new therapies for stroke on the basis of further understanding of the pathogenesis of stroke.
Stroke may trigger a variety of biochemical and molecular mechanisms leading to the neurological dysfunction including the activation of excitotoxic glutamatergic signaling [11], ionic imbalance [12], and excess production of ROS [7, 13]. The scathing mechanisms may proceed via non-programmed cell death (necrosis) or by programmed cell death (apoptosis or necroptosis) [14]. It has been confirmed that apoptosis, autophagy and necrosis are the three major modes of cell death [15]. Studies have revealed that cerebral ischemia may cause both necrosis and apoptosis of cells in the brain. At the infarct zone, neurons are mainly necrotic, while the main death form of neurons is apoptosis in the ischemic penumbra or the perifocal tissues during the first few hours after a stroke [16-18]. Autophagy takes an important role in adapting to the nutritional deprivation and eliminating aggregated proteins, but inappropriate activation of autophagy also in turn causes cell death in the ischemic brain injury. Thus, autophagy has been regarded as a double-edged sword with therapeutic potential in cerebral ischemia [19].
In the early 1970s, Kerr et al. put forward the term “apoptosis”, a regulated cell death process, which is morphologically distinct from necrosis [20]. For a long period, necrosis is regarded as a deranged or accidental cell demise that is a passive process caused by overwhelming stress. Since the 1990s, the purely unregulated nature of necrosis was challenged that necrotic cell death, at least in part, can be a well regulated and orchestrated event as apoptosis [21, 22]. The accumulated knowledge has paved the way for the term of “programmed necrosis”, which has not been introduced by Chan et al. until 2003 [23]. In 2005, Degterev et al. found that necrostatin-1 (Nec-1) could inhibit necrotic cell death mediated by downstream regulator receptor-interacting protein 1 (RIP1) of Fas/tumor necrosis factor receptor 1 (TNFR1), rather than apoptosis, in the condition of caspase-8 inhibition in a model of ischemic brain injury. Furthermore, Nec-1 has a longer-time window in contrast of Z-VAD-fmk, an inhibitor of apoptosis [24]. These results show that this type of necrotic cell death can be controlled and occur later than apoptosis, and is termed “necroptosis” later [25]. With our understanding of the mechanism of necroptosis in ischemic neurons, it is possible to offer neuroprotection by interfering with necrosis. Caspase-8 activity is partially dependent on the level of ATP, which is insufficient for the maintenance of caspase-8 activity in the acute stage of cerebral ischemia because of a serious shortage of ATP production. Therefore, necroptosis is probably the major route of death of neurons in the acute stage of cerebral ischemia [26]. Moreover, therapy targeting necrosis has a longer-time window, which is clinically important. Thus, the elucidation of mechanisms underlying the necroptosis of neurons and other cells in the brain is helpful to develop new therapeutic targets and effective strategies for the treatment of stroke.
Recently, studies have implicated necroptosis in a variety of disease states, and it has a central pathophysiological relevance in some diseases including stroke [27], myocardial ischemia/reperfusion (I/R) injury [28], atherosclerosis [29], renal I/R injury [30], pancreatitis [31], inflammatory bowel disease [32] and other disorders. In this review, we briefly summarize recent advances in the necroptosis and introduce the mechanisms of necroptosis and clinical strategies targeting necroptosis in the therapy of stroke.
2. Characteristics of necroptosis
Necroptosis has some distinctive features compared to other modes of cell death, especially apoptosis (Table 1). Morphologically, necroptosis is characterized by morphology resembling unregulated necrosis: early loss of plasma membrane integrity, gain in cell volume and swelling organelles. In contrast, apoptosis is characterized by cell shrinkage, plasma membrane blebbing, and nuclear and organelle condensation and fragmentation. Biochemically, significant depletion of cellular ATP and leakage of intracellular contents are present during necroptosis, suggesting an energy-consuming process. Molecularly, necroptosis has no involvement of caspase and its signals are dependent on RIP1, receptor-interacting protein 3 (RIP3) and mixed lineage kinase domain-like protein (MLKL), while caspase activation is indispensible for the apoptosis. During the necroptosis, the permeabilization of plasma membrane, a major characteristic of necroptosis, may cause the release of damage associated molecular patterns (DAMPs) including mitochondrial DNA and high-mobility group box 1 protein, which may induce immune response and inflammation [33].
Table 1.
Comparisons between necroptosis and apoptosis.
| Necroptosis | Apoptosis |
|---|---|
| Morphological markers | |
| Plasma membrane swelling and rupture; Cytoplasm swelling and vacuolization; No nuclear fragmentation; Mitochondria and organelle swelling |
Plasma membrane blebbing; Cytoplasm fragmentation; apoptotic body formation; Nuclear condensation and fragmentation; Organelle fragmentation |
| Biochemical markers | |
| ATP depletion; ROS production; Calcium and sodium influx | ATP increase; ROS production; Mitochondria outer membrane permeabilization |
| Molecular markers | |
| Signaling by RIP1, RIP3, and MLKL; Regulation by death receptors; Inhibition by caspases; Random DNA degradation; Extracellular release of DAMPs, such as mitochondrial DNA and HMGB1 | Signaling by the Bcl-2 family proteins; Regulation by death receptors; Caspase activation; DNA digestion by endonucleases; Cytosolic release of mitochondrial cytochrome c, SMAC and AIF |
Although there are distinctive features between necroptosis and other cell death modes, there is still complicated interrelationship between necroptosis and other cell death pathways, and necroptosis has been viewed as a back-up process when apoptosis is abrogated [34] (Fig. 1).
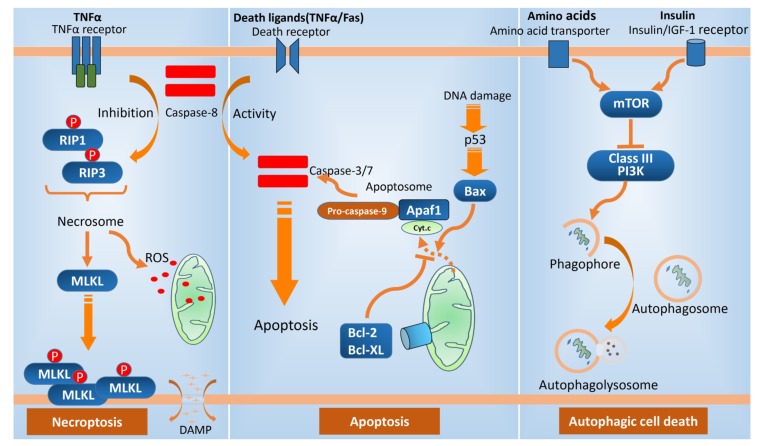
Fig. (1).
Three modes of programmed cell death. Apoptosis, a programmed form of nonlytic cell death, can induce the activation of effector caspases via a variety of upstream signals. In the so-called “intrinsic cell death pathway,” the P53 expression mediated by DNA damage triggers the production of Bax, a kind of pro-apoptotic protein, which can act to permeabilize the outer membranes of the mitochondria, releasing cytochrome c, which results in the oligomerization of an adapter protein, APAF1. APAF1 then binds to and activates the initiator caspase, caspase-9, and apoptosome forms. Bcl-2 protein family has anti-apoptotic activity. In the extrinsic cell death pathway, normally, caspase 8 can trigger apoptosis by classical caspase cascade. In the presence of caspase 8 inhibition, necroptosis is activated by the phosphorylation of RIP1 and RIP3, and then necrosome forms, which may activate the executioner of necroptosis, MLKL, leading to necroptosis. Autophagy can be induced in response to environmental signals including amino acids, hormones, and energy consumption. The best-characterized regulatory protein, mTOR, can act to inhibit autophagy. The Class III PI3K complex contributes to membrane expansion, resulting in the formation of a closed double-membrane structure, the autophagosome, which fuses with a lysosome to form an autolysosome.
3. Mechanisms and regulation of necroptosis (Fig. 2)
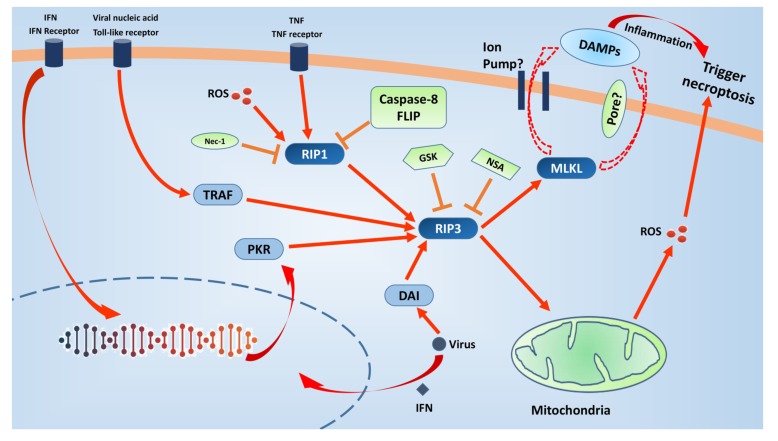
Fig. (2).
Necroptosis-related signalling pathways and it’s therapeutic targets. Necroptosis can be induced by multiple triggers,such as tumor necrosis factor (TNF), interferons (IFNs), viral nucleic acid, reactive oxygen species (ROS). Caspase-8 and cellular inhibitor of apoptosis proteins (cIAPs) prevent either RIP1 or RIP3 activation. When the caspase-8 activity is inhibited, necroptosis is induced. The binding between RIP1 and RIP3 via their homotypic interaction motif (RHIM) domains may form “necrosome”, which then induces the auto-phosphorylation of RIP3 and subsequent execution of necrosis. There are other two RHIM-containing proteins, Toll/IL-1 receptor domain-containing adaptor inducing IFN-β (TRIF) and DNA-dependent activator of interferon regulatory factors (DAI), can activate RIP3 independently of RIP1 within the necrosome. Furthermore, IFNs also trigger necroptosis independently of RIP1 via the interferon-induced protein kinase PKR. ROS can activates RIP1 autophosphorylation on serine residue 161 (S161), and subsequently enables RIP1 to recruit RIP3. Pseudokinase MLKL is a substrate of RIP3 and may serve as a major executioner of necroptosis. At present, two available non-exclusive models are proposed for the executioner mechanism of MLKL. However, RIP3 also activates, independently of MLKL, ROS-driven necroptosis. Necroptosis may cause rapid plasma membrane permeabilization and the subsequent release of cell contents and exposure of damage associated molecular patterns (DAMPs), which in turn trigger necroptosis. In conclusion, necroptosis fuels the vicious circle via amplifying inflammatory response or mitochondrial ROS. Necrostatin-1 (Nec-1) was shown to inhibit the kinase activity of RIP1. Furthermore, there are three RIP3 kinase inhibitors, GSK’840, GSK’843 and GSK’872. Necrosulfonamide (NSA) inhibits MLKL and is able to protect human cells from necroptotic stimuli. These might be attractive therapeutic targets.
3.1. Initiation of Necroptosis
Necroptosis can be induced by multiple triggers, including the tumor necrosis factor (TNF) family of cytokines, such as TNF, TNF-related apoptosis-inducing ligand and FasL [27, 35]; ROS [36, 37]; the activated Toll-like receptors (TLRs), such as TLR3 and TLR4 [38]; type I interferons (IFNs) [39] and certain pathogens [40-42].
The most extensively studied pathway leading to necroptosis is triggered by TNF-α/TNFR-induced pathway [43]. After the binding of TNF-α to TNFR1, the activated receptor may interact with RIP1 via the death domains (DD), which may recruit cellular inhibitor of apoptosis proteins (cIAPs) (such as cIAP1 and cIAP2) and subsequently form the plasma membrane associated complex, leading to the activation of nuclear factor κB and mitogen-activated protein kinases (MAPKs). During this process, RIP1 can be polyubiquitinated by cIAPs and other E3 ubiquitin ligases [44]. The auto-ubiquitination and subsequent degradation of cIAPs induce the RIP1 deubiquitination, which is able to dissociate RIP1 from the cell membrane, causing its conversion from a pro-survival protein into a pro-death protein [45]. The binding of cytoplasmic RIP1 to FAS-associated death domain (FADD) may recruit procaspase-8, leading to the caspase-8 activation. The activated caspase-8 is able to inhibit the necroptosis through cleaving the key regulators of necroptosis (such as RIP1 and RIP3). When the caspase-8 activity is inhibited, cell apoptosis is inhibited and the necroptosis is induced [46]. The binding between RIP1 and RIP3 via their homotypic interaction motif (RHIM) domains may form a functional amyloid signaling complex, also known as “necrosome”, which then induces the auto-phosphorylation of RIP3 and the subsequent execution of necrosis [47]. In addition to RIP1, it has been reported that there are other two RHIM-containing proteins, Toll/IL-1 receptor domain-containing adaptor inducing IFN-β (TRIF) and DNA-dependent activator of interferon regulatory factors (DAI), can activate RIP3 within the necrosome [47]. For example, TLR3 as a biological stimulus exert necroptotic effects via the adapter protein TRIF [38]. Certain pathogens can also trigger necroptosis depending on the DNA-dependent activator of DAI [42, 48]. Furthermore, IFNs trigger necroptosis independently of RIP1 via the IFN-induced protein kinase PKR [39]. Previous studies have shown that ROS may also regulate Smac mimetic/TNF-α-induced necroptotic signaling [36]. Recently, Han et al. found that RIP1 autophosphorylation on serine residue 161 (S161) is promoted by mitochondrial ROS, which is indispensable for the recruitment of RIP3 into the necrosome. This suggests that the ROS can act as a new biological trigger, exerting necroptotic effects [37].
3.2. Execution of Necroptosis
Pseudokinase MLKL is a substrate of RIP3 and may serve as a major executioner of necroptosis [49, 50]. There is evidence showing that RHIM dependent oligomerization and intramolecular autophosphorylation of RIP3 may recruit and phosphorylate MLKL, which induces a conformational change in the pseudokinase domain and the subsequent exposure of the 4-helical bundle domain [51, 52]. At present, two available non-exclusive models are proposed for the executioner mechanism of MLKL: (1) the plasma membrane serves as a platform for the recruitment of Ca2+ or Na+ ion channel [53]; (2) the binding of MLKL to negatively charged phosphatidylinositol phosphates forms a direct pore-forming complex [54].
However, RIP3 also activates, independent of MLKL, ROS-driven necroptosis and FADD-mediated apoptosis [55, 56]. RIP3 binds to Ca2+-calmodulin–dependent protein kinase (CaMKII) and, via phosphorylation, oxidation or both, activates CaMKII, which accounts for the opening of mitochondrial permeability transition pore and leads to myocyte necroptosis [57]. Thus, mitochondrial ROS not only trigger necroptosis but also act as an executive of necrosis, which means a positive feedback loop. It is worth mentioning that necroptosis may cause rapid plasma membrane permeabilization and the subsequent release of cell contents and exposure of DAMPs [58]; the pattern recognition receptor may recognize these DAMPs to activate immune response and induce the subsequent clearance of necrotic cells [59]. It has been found that defective clearance of necrotic cells may cause the persistence of inflammation and excessive tissue injury, which serves as a mechanism underlying the pathogenesis of some diseases [60]. In conclusion, necroptosis fuels the vicious circle via amplifying inflammatory response or mitochondrial ROS.
3.3. Disposal of Necrotic Cells
The macrophages may internalize necrotic cells by forming spacious macropinosomes [61], which is accompanied by macrophage ruffling and has involvement in the sorting of fluid-phase macromolecules [62]. Apoptotic cells may emit a series of well-defined ‘find-me’ [63, 64] and ‘eat-me’ signals. As in apoptosis, under some conditions, cells experiencing necrosis may also externalize phosphatidylserine before the plasma membrane permeabilization [65], which facilitates their recognition and internalization by phagocytes [66, 67].
3.4. Inhibitors of Necropotisis
The majority of the knowledge of necroptosis is gained using necroptosis inhibitors or genetic modification technique. To date, some inhibitors of necroptosis have been developed for the study of necroptosis.
3.4.1. RIP1 Inhibitors
The first inhibitor of necroptosis (Nec-1) was identified in 2005 in a phenotypic screen for small molecules that are able to inhibit the TNF induced necrosis in human monocytic U937 cells [24]. Nec-1 was subsequently shown to inhibit the kinase activity of RIP1 [EC50= 494 nM)] [27]. However, Nec-1 is identical to the indoleamine-2,3-dioxygenase (IDO) enzyme inhibitor methyl-thiohydantoin-tryptophan. In late studies, investigators developed a new inhibitor of necroptosis, Nec-1s (also called 7-Cl-O-Nec-1; EC50=206 nM). Nec-1s lacks IDO inhibitory activity, but has increased plasma stability and increased specificity for RIP over a broad range of kinases as well as no toxic effect described for Nec-1 [68]. Several structurally distinct necrostatins (Nec-1, Nec-3, Nec-4, Nec-5 and Nec-7) are also developed, but Nec-7 (EC50 = 10.6 μM) does not inhibit RIP1 kinase [69].
3.4.2. RIP3 Inhibitors
There are three RIP3 kinase inhibitors, GSKʹ840, GSKʹ843 and GSKʹ872. Of them, GSKʹ843 and GSKʹ872 at higher concentrations may promote TNF-induced RIP1 dependent apoptosis and caspase 8 activation [70], while GSKʹ840 exhibits the most specific profile. In addition, GSKʹ840 is particularly interesting because it does not induce RIP1 kinase activity-dependent apoptosis at higher concentrations, which is in contrast to GSKʹ843 and GSKʹ872. Currently, however, GSKʹ840 can not inhibit murine RIP3, which prevents its assessment in murine models.
3.4.3. MLKL Inhibitors
(E)-N-(4-(N-(4,6-dimethylpyrimidin-2-yl) sulfamoyl)phenyl)-3-(5- nitrothiophene - 2- yl) acrylamide (known as necrosulfonamide [NSA]) was the first compound reported to inhibit MLKL [49]. In fact, NSA was used to identify MLKL as a downstream target of RIP3. Of note, NSA is able to protect human cells from necroptotic stimuli, which is not found in murine cells because NSA alkylates the Cys86 of human MLKL and Cys86 is absent in murine MLKL. Thus, it is not suitable in murine preclinical models. In recent years, a new class of MLKL inhibitors based on ‘compound 1’ (also known as GW806742X or SYN-1215) is described to target the pseudokinase domain of MLKL [51].
4. Necroptosis associated pathologies
Over the past two decades, a variety of studies have shown that necroptosis is closely related to many pathologies, including I/R injury, inflammatory diseases, infection, autoimmune diseases and other disorders [71].
4.1. I/R Injury
Ischemia is caused by the obstruction of blood flow to a tissue, resulting in the insufficient supply of oxygen and nutrients, and if severe, in cell death. Reperfusion following the restoration of blood flow may cause a burst of ROS, also leading to cell death. The renal I/R was to be attenuated in RIP3-deficient mice [72], and RIP3 deficiency also decreased cardiac hypertrophy and inflammation after myocardial infarction [73]. In addition, RIP1 inhibitor Nec-1 is also able to attenuate myocardial I/R [74], which is ascribed to the inhibition of oxidative stress, necrosis and inflammation.
4.2. Neurological Diseases
Recent studies have shown that necroptosis is involved in the pathogenesis of some neurological diseases including stroke, traumatic brain injury (TBI), spinal cord injury (SCI) and neurodegenerative diseases. You et al. found administration of Nec-1 before or 15 min after controlled cortical impact decreased brain tissue damage (days 14 and 35) and improved motor performance and spatial memory [75]. An lncRNA microarray analysis showed the expression of some lncRNAs related to necroptosis changed in rat hippocampus after TBI [76]. In a mouse TBI model, Nec-1 pretreatment was able to attenuate brain injury via inhibiting apoptosis and autophagy simultaneously [77]. In a SCI rat model, Wang et al. showed Nec-1 pre-treatment protected the spinal cord against injury via inhibiting both necroptosis and apoptosis [78]. In R6/2transgenic mouse model of Huntington’s disease, Nec-1 was found to delay the disease onset, and the increased expression of RIP1 was observed at the onset of disease symptoms [79]. In primary newborn mouse cortical cells treated with aluminum, Nec-1 significantly improved the cell viability in a concentration dependent manner; Nec-1 also improves the learning and memory retention of mice treated with aluminum [80].
4.3. Retinal Injury
In retinitis pigmentosa mice with interphotoreceptor retinoid-binding protein (IRBP) deficiency (Irbp-/-), the retina had increased expression of both RIP1 and RIP3, but inhibition of RIP1 kinase activity significantly prevented cone and rod photoreceptor degeneration [81]. In addition, RIP3-deficiency resulted in the protection of cone cells against necroptosis in a mouse model of retinitis pigmentosa (rd10 mice) [82]. In an experimental model of retinal detachment, more than 10-fold increase in RIP3 expression was observed after retinal detachment, and Nec-1 or RIP3 deficiency significantly alleviated necrotic changes and inhibited oxidative stress and mitochondrial release of AIF [83]. Nec-1 or RIP3 silencing also inhibited the retinal pigment epithelial cell death secondary to oxidative stress [84] and attenuated retinal I/R [85].
4.4. Inflammation
In TNF-induced systemic inflammatory response syndrome (SIRS), Duprez et al. found deletion of RIP3 or pretreatment with Nec-1 completely protected against lethal SIRS and reduced the amounts of circulating DAMPs [86]. RIP3-deficient mice could also be markedly protected from TNF-α-mediated shock, while blocking necroptosis by Nec-1 did not protect from TNF-α/zVAD-mediated shock, and further accelerated time to death [87]. RIP3 expression was found to correlate with the sensitivity of pancreatic acinar cells to necroptosis, and in a cerulein-induced pancreatitis mouse model, RIP3 or MLKL deficiency protected mice from acute pancreatitis [31, 88], which suggests the involvement of necroptosis in the pathogenesis of pancreatitis. In ConA-induced acute hepatitis, increased RIP3 expression was observed in the liver, hepatocyte-specific caspase-8 deletion increased necrotic damage and Nec-1 decreased hepatocyte necrotic cell death [89, 90]. In acetaminophen-induced acute liver failure mice, hepatic RIP1 and RIP3 were activated, and either pretreatment or post-treatment with Nec-1 significantly attenuated APAP-induced acute liver failure and improved the survival by preventing APAP-induced necroptosis [91]. In mice after chronic ethanol feeding, increased RIP3 expression was detected in the liver [92], and a similar finding was also observed in patients with alcoholic liver disease [93], and RIP3-deficiency protected mice against both ethanol-induced liver injury and inflammation [93]. RIP3 expression also increased in the terminal ileum of patients with Crohn’s disease, and the TNF induced cell death was associated with increased RIP3 expression, but prevented by Nec-1 in cultured intestinal organoids [94].
4.5. Infection
Vaccinia virus (VV) encodes the caspase inhibitor B13R/Spi2, which blocks apoptosis upon infection, but sensitizes cells to RIP1/RIP3-dependent necroptosis. VV infection induced the formation of pro-necrotic RIP1-RIP3 complex in the liver, accompanied by the induction of TNF expression and inflammation [95], but RIP3-deficient mice and RIP1 D138N/D138N mice showed attenuated necrotic tissue injury and inflammation [95, 96]. Murine cytomegalovirus also encodes a necrotic inhibitor, vIRA, which can prevent premature host cell death upon infection. Studies have shown that vIRA may inhibit necrotic cell death after TNF treatment by disrupting the pronecrotic RIP1-RIP3 complex [97]. Necrotic cell death has also been observed after infection by other viruses, such as human immunodeficiency virus type-1, herpes simplex virus type-1 and T3D, and Nec-1 pre-treatment is able to attenuate the cell death induced by these viruses [98-100].
5. Necroptosis and stroke
Some studies have revealed that necroptosis is closely related to the pathogenesis of stroke as Nec-1 (Nec-1s) or genetic modulation of necroptosis related proteins may improve brain injury and neurological function after stroke (Table 2).
Table 2.
Animal studies on the treatment of stroke by targeting necroptosis.
| Authors | Interventions | Animal Models | Results | |||
|---|---|---|---|---|---|---|
| Zille et al. 2017 | Chemical inhibitors implicated in all known cell death pathways | Cultured neurons exposed to hemoglobin or hemin | Experimental intracerebral hemorrhage shares features of ferroptotic and necroptotic, but not caspase-dependent apoptosis or autophagy | |||
| Shen et al. 2017 | Nec-1 Mutation of serine kinase phosphorylation site of RIP1 |
ICH in rats | Inhibition of necroptosis | |||
| Qu et al. 2017 | Inhibition of the RIP3-MLKL or RIP3-CaMKIIdelta interaction | OGD/zVAD in oligodendrocytes HI in postnatal day 6 (P6) rats |
Disrupted development of myelin was attenuated | |||
| Xu et al. 2017 | Necroptosis, autophagy, and apoptosis inhibitor | Global cerebral I/R injury model in rats | Mitochondria are involved in the execution of programmed necrosis, and AIF is the mediating molecule | |||
| Qu et al. 2016 | Inhibition of MLKL | OGD/zVAD in cortical neurons HI in rats |
Attenuated neuronal death induced by OGD/zVAD and brain damage induced by HI | |||
| LaRocca et al. 2016 | nec-1s Treatment with high levels of glucose |
HI in mice | Resulted in increased infarct size, which can be prevented by nec-1s | |||
| Yin et al. 2015 | Nec-1 pretreatment | Global cerebral I/R injury | Nec-1 pretreatment prevented hippocampal CA1 neuronal death and I/R induced changes in RIP3 | |||
| Xuan et al. 2015 | A Water-Soluble Extract from the Culture Medium of Ganoderma lucidum Mycelia | HI in type 2 diabetic KKAy mice | Reduced H/I-induced neurological deficits and brain infarction volume and suppressed superoxide production, neuronal cell death, and vacuolation in the ischemic penumbra | |||
| Wang et al. 2015 | extracellular protons | MCAO in mice | Acid stimulation recruits RIP1 to the ASIC1a C-terminus, causing RIP1 phosphorylation and subsequent neuronal death | |||
| Su et al. 2015 | Nec-1 pretreatment | ICH in mice | Nec-1 pretreatment improved neurological function, attenuated brain edema, reduced RIP1-RIP3 interaction and PI positive cell death and inhibited microglia activation | |||
| Askalan et al. 2015 | For 90min in the mild-moderate HI or 180min in the severe HI. | HI in rats | Necroptosis was significantly higher in the peri-infarct of the severe HI lesion compared to the moderate HI lesion. In males, but not in females, apoptosis was higher in moderate compared to severe HI | |||
| Vieira et al. 2014 | RIP3 KD or overexpression | OGD in primary cultures of hippocampal neurons | RIP3 KD abrogated the component of OGD-induced necrotic neuronal death while RIP3 overexpression exacerbated neuronal death following OGD | |||
| Liu et al. 2014 | RIP3 deficiency in mice KD of CYLD or RIP1 or RIP3 or MLKL in HT-22 cells |
Intracerebroventricular injection of TNF-alpha in mice TNF-alpha-induced toxicity of hippocampal neurons in HT-22 cells |
RIP3 deficiency attenuates TNF-alpha-initiated loss of hippocampal neurons The cell death is suppressed by KD of CYLD or RIP1 or RIP3 or MLKL |
|||
| King et al. 2014 | Nec-1 | ICH in mice | Nec-1 significantly reduced hematoma volume and BBB opening, attenuated edema development, and improved neurobehavioral outcomes | |||
| Chang et al. 2014 | Nec-1 | ICH in mice | Nec-1 suppressed apoptosis, autophagy, and necroptosis to exert these neuroprotective effects after ICH | |||
| Dai et al. 2013 | Curcumin | Iron induced neurotoxicity in primary cortical neurons | Curcumin attenuated necroptosis in a dose-dependent manner and decreased expression of receptor interacting protein 1 in a dose- and time-dependent manner | |||
| Zhu et al. 2012 | RIP3 deficiency | ICH in mice | Mice deficient in RIP3 had 50% less PI+ cells at 24 h. Permeable cells remained in the brain for at least 24 h with <10% spontaneous resealing | |||
| Chen et al. 2012 | GA | OGD/zVAD in cultured primary neurons |
GA protected against neuronal injury and decreased RIP1 protein level in a time- and concentration-dependent manner | |||
| Authors | Interventions | Animal Models | Results | |||
| Chavez-Valdez et al. 2012 | Nec-1 immediately after HI | Neonatal HI in mice | Nec-1 immediately after HI, is strongly mitoprotective and prevents secondary energy failure by blocking early NO* accumulation, glutathione oxidation and attenuating mitochondrial dysfunction | |||
| Northington et al. 2011 | Nec-1 | Neonatal HI in mice | Necrostatin treatment attenuated necrotic cell death, HI-induced oxidative damage and markers of inflammation | |||
| Xu et al. 2010 | Nec-1 HNG |
OGD in cultured mouse primary cortical neurons MCAO in mice |
Nec-1 or HNG alone had protective effects on OGD-induced cell death. Combined treatment with Nec-1 and HNG resulted in more neuroprotection than Nec-1 or HNG alone | |||
| Xu et al. 2010 | PJ34 Nec-1 |
Glutamate-induced necroptosis in HT-22 cells |
Nec-1 is not a direct PARP inhibitor and that its signaling target is located upstream of PARP | |||
| Li et al. 2008 | Nec-1 | NMDA-induced excitotoxicity in rat's cultured cortical neurons | Nec-1 inhibited NMDA-induced decrease of cell viability, attenuated NMDA-induced leakage of LDH and suppressed NMDA-induced elevation of intracellular Ca2+ | |||
| Degterev et al. 2005 | Nec-1 | Delayed mouse ischemic brain injury | A specific and potent small-molecule inhibitor of necroptosis, necrostatin-1, blocks a critical step in necroptosis | |||
Abbreviations: RIP1: Receptor-interacting protein 1; RIP3: Receptor-interacting protein 3; MLKL: Mixed lineage kinase domain-like; Nec-1: Necrostatin-1, RIP1 inhibitor; Nec-1s: Nec-1 derivatives; KD: knock-down; I/R: Ischemia/reperfusion; HNG: Gly(14)-humanin, apoptosis inhibitor; GA: Geldanamycin; PJ34: a potent and specific inhibitor of poly(ADP-ribose)-polymerase (PARP); PI: Propidium iodide; BBB: Blood-brain barrier; ICH: Collagenase-induced intracerebral hemorrhage; OGD/zVAD: Oxygen-glucose deprivation plus caspase inhibitor zVAD treatment; OGD: Oxygen-glucose deprivation; HI: Hypoxia-ischemia; MCAO: Middle cerebral artery occlusion; ASIC1a: Acid-sensing ion channel 1; ICH: Intracerebral hemorrhage; LDH: Lactate dehydrogenase; NMDA: N-methyl-D-aspartic acid.
Liu et al. found necroptosis mediated TNF-induced toxicity of HT-22 hippocampal neuronal cells because these cells were sensitive to TNF-α only upon caspase blockage and subsequently underwent necrosis and the cell death was suppressed by knockdown of CYLD, RIP1, RIP3 or MLKL [101]. In cultured neurons exposed to hemoglobin or hemin (an in vitro model of intracerebral hemorrhage [ICH]), features of ferroptotic and necroptotic cell death, but not caspase-dependent apoptosis or autophagy, were observed, and chemical inhibitors of ferroptosis and necroptosis protected against hemoglobin- and hemin-induced toxicity (abrogating death by >80%) [102]. Qu et al. investigated the functions and mechanisms of MLKL in mediating neuronal damage in developing brain after hypoxia-ischemia (H/I). In neurons treated with oxygen-glucose deprivation (OGD) plus caspase inhibitor zVAD treatment (OGD/zVAD), RIP1 and RIP3 expressions were up-regulated and the interaction of RIP1-RIP3 with MLKL increased; siRNA mediated inhibition of MLKL attenuated neuronal death induced by OGD/zVAD [103]. In addition, induction of RIP1 protein instability was able to protect the primary neurons against OGD/zVAD induced necroptosis [104]. In an in vitro model of global ischemia (hippocampal neurons exposed to OGD), RIP1 and RIP3 expression increased and both formed necrosomes, but caspase-8 mRNA expression was transiently decreased following OGD; RIP3 knock-down (KD) abrogated OGD-induced necrotic neuronal death while RIP3 over-expression exacerbated neuronal death following OGD [105]. Necroptosis was shown to be an early mechanism of cell death occurring within the first 24 h post-ischemic injury [106] and inversely linked to the caspase-dependent cell death [107].
Degterev et al. for the first time found that necroptosis contributes to mouse middle cerebral artery occlusion (MCAO) brain injury in vivo and the mechanism was distinct from that of apoptosis [24]. Moreover, they identified a specific and potent small-molecule inhibitor of necroptosis, Nec-1, which could block a critical step in the necroptosis (but not apoptosis) and exerted neurological protection in this animal model. Furthermore, the protective effect of 7-Cl-Nec-1 was also detectable even when it was administered 6 h after the onset of injury, and they speculated the extended time window of neuroprotection by 7-Cl-Nec-1 in vivo might reflect a delayed induction of necroptosis during stroke. Xu et al. investigated the neuroprotection of Nec-1 in vivo and in vitro [108]. They found Nec-1 could protect the mouse brain against I/R injury, and combined use of an apoptosis inhibitor Gly(14)-humanin and Nec-1 conferred synergistic neuroprotection in vivo and in vitro. In a global cerebral I/R injury model, neuronal death and rat mortality were greatly inhibited by Nec-1 and 3-MA (an autophagy inhibitor) pre-treatment, but not by Ac-DMQD-CHO (caspase-3 inhibitor) [109] and there was accompanied nuclear translocation and co-localization of RIP3 and AIF as well as their interaction after I/R injury. Moreover, they also evaluated the cell specificity of necroptosis. Results showed caspase-8 was expressed richly in glial fibrillary acidic protein (GFAP) - positive astrocytes and Iba-1-positive microglia, but not in NeuN-positive neurons. Thus, they concluded that caspase-3 and caspase-8 are not involved in regulating hippocampal CA1 neuronal death induced by I/R injury. In a global cerebral ischemia induced by the four-vessel occlusion method, Nec-1 induced inhibition of RIP3 up-regulation and nuclear translocation was also found to be related to its neuroprotection [110].
In a collagenase-induced ICH mouse model, Zhu et al. found the ultrastructural features of necrosis at 24 h after ICH by electron microscopy, and less necrotic cells were also found in RIP3-deficient mice after ICH [111]. In the same model, King et al. found Nec-1 was able to significantly reduce hematoma volume, limit cell death, reduce blood-brain barrier opening, attenuate edema development to sham level, and improve neurobehavioral outcome at 72 h after ICH [112]. Similar findings were found in the study of Su et al. [113]. In another in vivo ICH model established by the injection of autologous blood, inhibition of RIP1 by a specific chemical inhibitor or genetic knockdown attenuated brain edema and improved neurological score, which was also confirmed in an in vitro model using OxyHb-treated neurons [114]. Chang et al. also found the specific inhibitor Nec-1 also suppressed apoptosis and autophagy to exert neuroprotective effects after ICH [115]. In an endovascular perforation induced subarachnoid hemorrhage (SAH) rat model, SAH was found to up-regulate RIP1, RIP3, phosphorylated DRP1 and NLRP3 inflammasome, and Nec-1 treatment reduced RIP1, RIP3, phosphorylated DRP1 and NLRP3 inflammasome, subsequently alleviating brain injury secondary to SAH [116].
In neonatal brain H/I model, blockade of RIP-1 kinase after H/I afforded neuroprotection [106] and this protection was possibly by interrupting RIP1-RIP3-driven oxidative injury and inflammation [117]. In the study of Qu et al., results showed siRNA mediated inhibition of MLKL was also able to reduce infarct area and improve neurological score in an experimental neonatal rat H/I model [103]. In a mouse model of brain H/I injury, the increased infarct size in mice with hyperglycemia was prevented by treatment with Nec-1s, suggesting that increased necroptosis accounts for the exacerbation of brain injury in conditions of hyperglycemia [118], and prevention of necroptosis was also found to attenuate H/I-induced injury of type 2 diabetic mouse brain [119]. In the moderately ischemic brain, necroptosis was predominantly found in the core of the lesion, the peri-infarct area of the severely ischemic brain had significantly more necroptosis than the peri-infarct area of the moderately injured brain, and necroptotic cell death was as active in the peri-infarct area as it was in the core of the lesion [120].
In oligodendrocytes treated by OGD/zVAD in vitro, the expression of RIP3 was up-regulated, and the interaction of RIP3 with RIP1, MLKL, and CaMKIIδ increased; inhibition of the RIP3-MLKL or RIP3-CaMKIIδ interaction attenuated OGD/zVAD induced oligodendrocyte death, which was not found after inhibition of RIP3-RIP1 interaction. These protective mechanisms have involvement of the translocation of MLKL to the oligodendrocyte membrane and the phosphorylation of CaMKIIδ. In neonatal H/I rats, inhibition of RIP3-MLKL or RIP3-CaMKIIδ interaction attenuated the disrupted development of myelin [121]. Acidotoxicity is common among neurological disorders, such as ischemic stroke. Wang et al. investigated the relationship between acidosis and neuronal necroptosis [122]. They found acid stimulation could recruit RIP1 to the acid-sensing ion channel 1a (ASIC1a) C-terminus, causing RIP1 phosphorylation and subsequent neuronal death; in a mouse MCAO model, ASIC1a-RIP1 interaction and RIP1 phosphorylation were found in affected brain areas, and the Asic1a deficiency significantly prevented RIP1 phosphorylation and brain damage, which indicates ASIC1a-mediated RIP1 activation is involved in ischemic neuronal injury. Overload of iron, which is released from hemoglobin, also contributes to brain injury after ICH. Dai et al. found iron overload promoted the necroptosis of primary cortical neurons but curcumin was able to attenuate ferrous chloride induced necroptosis [123]. Excitotoxicity is a key mechanism of tissue destruction in cerebral ischemia (stroke). Investigators found PARP activation was involved in the glutamate-induced necroptosis of HT-22 cells [124], and necroptosis was related to the N-Methyl-D-aspartate-induced excitotoxicity in rat cortical neurons [125].
Conclusion and Prospective
6.1. Although some studies have confirmed that necroptosis is involved in the pathogensis of stroke, and treatment targeting necroptosis related key proteins have been confirmed to be neuroprotective in different animal models and different cell types, few studies are undertaken to investigate the clinical use of necroptosis inhibitors or gene modification of necroptosis. As shown above, RIP1, RIP3 and MLKL are the three proteins specific to necroptosis. Thus, clinical strategies may target RIP1, RIP3, MLKL, or RIP1/RIP3 interaction, but inhibition of TNFR seems to be infeasible because TNFR related signaling pathways are related to some cell processes and not unique to necroptosis. Of note, NSA is able to inhibit MLKL in human cells, but may not affect the necroptosis in animal models. As shown in a lot of drugs, favorable results are usually obtained from pre-clinical studies, but less effectiveness is observed in clinical practice. Thus, more studies are needed to investigate the role of necroptosis in stroke in clinical settings.
6.2. As mentioned above, Nec-1 is identical to the IDO enzyme inhibitor methyl-thiohydantoin-tryptophan, and thus it might also inhibit the IDO enzyme, but Nec-1 has been used in a majority of studies, and more specific inhibitor 7-Cl-Nec-1 is seldom used. Whether concomitant inhibition of IDO may bias the results is still unclear. Other inhibitors of necroptosis have never been used in stroke models.
6.3. Although a variety of studies investigated the therapeutic effects of necroptosis inhibitors in different disease models, few studies explored the potential toxicity of these inhibitors. Degterev et al. investigated the effects of Nec-1 on cell adhesion, actin and microtubule cytoskeletons, the morphology of various cellular compartments and others, and no detrimental effects were observed [24]. What are the half maximal inhibitory concentration and median lethal dose is needed to confirm in animal and cell models, and more studies are warranted to investigate the toxicity of these inhibitors of necroptosis, which is crucial for its clinical application. Moreover, the pharmacokinetics of these inhibitors (bioavailability, site where they are metabolized and the enzymes responsible for their metabolism, way in which they are excreted, their permeability to blood brain barrier and so on) are also needed to elucidate in animal models before its clinical use.
6.4. The dose-response curve is needed to be delineated to confirm the optimal dose of inhibitors. The optimal routes by which these drugs are used are also needed to investigate. Necroptosis inhibitors are usually administered intraperitoneally or intracerebroventricularly, and whether intravenous administration achieves similar effectiveness and which is the better route for administration are also needed to confirm.
6.5. As studied by Degterev et al., the new inhibitor 7-Cl-Nec-1 was able to exert neuroprotective effects even administered at 6 h after stroke. Thus, what is the optimal time point at which these inhibitors still achieve neuroprotection is warranted to determine. This may be based on the dynamic change in cell necroptosis of the injured brain.
6.6. It has been confirmed that there is cross talk among different death modes. In the available studies, several also revealed that combined inhibition of necroptosis and apoptosis exerted synergistic neuroprotection in animal models. Whether this is clinically applicable is also required to confirm in more animal models.
6.7. As mentioned above, the role of necroptosis in the stroke has been investigated in oligodendrocytes, neurons and HT-22 cells. However, not only oligodendrocytes and neurons are involved in the pathogenesis of stroke, but also astrocytes, neural progenitor cell, vascular epithelial cells and other cell types in the brain are also closely related to the occurrence of stroke. Thus, what is the role of necroptosis of other cell types and whether there is an interaction between the necroptosis of two or more cell types are still needed to be studied in detail.
Acknowledgements
This study was supported by the National Natural Science Foundation of China (No. 81371316; 81772015). We thank Hu Qin in School of Medicine, Shanghai Jiaotong University for her constructive advice on this paper.
List of Abbreviations
- ASIC1a
Acid-sensing ion channel 1
- cIAPs
Cellular inhibitor of apoptosis proteins
- CaMKII
Ca2+-calmodulin–dependent protein kinase
- DAMP
Damage associated molecular pattern
- DD
Death domain
- DAI
DNA-dependent activator of interferon regulatory factors
- FADD
FAS-associated death domain
- GA
Geldanamycin
- GFAP
Glial fibrillary acidic protein
- H/I
Hypoxia-ischemia
- ICH
Intracerebral hemorrhage
- IDO
Indoleamine-2,3-dioxygenase
- I/R
Ischemia/reperfusion
- KD
Knock-down
- LDH
Lactate dehydrogenase
- LC3II
microtubule-associated protein light chain-3
- MCAO
Middle cerebral artery occlusion
- MLKL
Mixed lineage kinase domain-like
- Nec-1
Necrostatin-1, RIP1 inhibitor
- Nec-1s
Nec-1 derivatives
- OGD
Oxygen-glucose deprivation
- RIP1
Receptor-interacting protein 1
- RIP3
Receptor-interacting protein 3
- RHIM
RIP homotypic interaction motif
- ROS
Reactive oxygen species
- SAH
Subarachnoid hemorrhage
- TLR
Toll like receptor
- TNF
Tumor necrosis factor
- TNFR
Tumor necrosis factor receptor
Consent for Publication
Not applicable.
Conflict of Interest
The authors declare no conflict of interest, financial or otherwise.
References
- 1.Daviet J.C., Bonan I., Caire J.M., Colle F., Damamme L., Froger J., Leblond C., Leger A., Muller F., Simon O., Thiebaut M., Yelnik A. Therapeutic patient education for stroke survivors: Non-pharmacological management. A literature review. Ann. Phys. Rehabil. Med. 2012;55(9-10):641–656. doi: 10.1016/j.rehab.2012.08.011. [http://dx.doi.org/10.1016/j.rehab. 2012.08.011]. [PMID: 23000090]. [DOI] [PubMed] [Google Scholar]
- 2.Christerson S. Also children and adolescents can suffer from stroke. Early diagnosis can improve prognosis. New guidelines for diagnosis and therapy. Lakartidningen. 2013;110(41):1799–1802. [PMID: 24187893]. [PubMed] [Google Scholar]
- 3.Mehrpour M., Taghipour S., Abdollahi S., Oliaee F., Goran A., Motamed M., Ashayeri R. Positive impact of stroke unit establishment on patient recovery in Firoozgar hospital. Med. J. Islam. Repub. Iran. 2016;30:446. [PMID: 28210611]. [PMC free article] [PubMed] [Google Scholar]
- 4.Emberson J., Lees K.R., Lyden P., Blackwell L., Albers G., Bluhmki E., Brott T., Cohen G., Davis S., Donnan G., Grotta J., Howard G., Kaste M., Koga M., von Kummer R., Lansberg M., Lindley R.I., Murray G., Olivot J.M., Parsons M., Tilley B., Toni D., Toyoda K., Wahlgren N., Wardlaw J., Whiteley W., del Zoppo G.J., Baigent C., Sandercock P., Hacke W., Stroke Thrombolysis Trialists’ Collaborative Group Effect of treatment delay, age, and stroke severity on the effects of intravenous thrombolysis with alteplase for acute ischaemic stroke: a meta-analysis of individual patient data from randomised trials. Lancet. 2014;384(9958):1929–1935. doi: 10.1016/S0140-6736(14)60584-5. [http://dx.doi.org/10.1016/S0140-6736(14) 60584-5]. [PMID: 25106063]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.O’Brien E.C., Wu J., Zhao X., et al. Healthcare resource Availability, quality of care, and acute ischemic stroke outcomes. J. Am. Heart Assoc. 2017;6:e003813. doi: 10.1161/JAHA.116.003813. [http://dx.doi.org/10.1161/JAHA.116. 003813]. [PMID: 28159820]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim A.S., Johnston S.C. Temporal and geographic trends in the global stroke epidemic. Stroke. 2013;44(6) Suppl. 1:S123–S125. doi: 10.1161/STROKEAHA.111.000067. [http://dx.doi. org/10.1161/STROKEAHA.111.000067]. [PMID: 23709707]. [DOI] [PubMed] [Google Scholar]
- 7.Davis S.M., Pennypacker K.R. Targeting antioxidant enzyme expression as a therapeutic strategy for ischemic stroke. Neurochem. Int. 2016 doi: 10.1016/j.neuint.2016.12.007. [PMID: 28043837]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhu J., Song W., Li L., Fan X. Endothelial nitric oxide synthase: a potential therapeutic target for cerebrovascular diseases. Mol. Brain. 2016;9:30. doi: 10.1186/s13041-016-0211-9. [http://dx.doi.org/10.1186/s13041-016-0211-9]. [PMID: 27000187]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maiese K. Cutting through the complexities of mTOR for the treatment of stroke. Curr. Neurovasc. Res. 2014;11(2):177–186. doi: 10.2174/1567202611666140408104831. [http:// dx.doi.org/10.2174/1567202611666140408104831]. [PMID: 24712647]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lu Y.Y., Li Z.Z., Jiang D.S., Wang L., Zhang Y., Chen K., Zhang X.F., Liu Y., Fan G.C., Chen Y., Yang Q., Zhou Y., Zhang X.D., Liu D.P., Li H. TRAF1 is a critical regulator of cerebral ischaemia-reperfusion injury and neuronal death. Nat. Commun. 2013;4:2852. doi: 10.1038/ncomms3852. [http://dx.doi.org/10.1038/ncomms3852]. [PMID: 24284943]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Martin H.G., Wang Y.T. Blocking the deadly effects of the NMDA receptor in stroke. Cell. 2010;140(2):174–176. doi: 10.1016/j.cell.2010.01.014. [http://dx.doi.org/ 10.1016/j.cell.2010.01.014]. [PMID: 20141829]. [DOI] [PubMed] [Google Scholar]
- 12.Besancon E., Guo S., Lok J., Tymianski M., Lo E.H. Beyond NMDA and AMPA glutamate receptors: emerging mechanisms for ionic imbalance and cell death in stroke. Trends Pharmacol. Sci. 2008;29(5):268–275. doi: 10.1016/j.tips.2008.02.003. [http://dx.doi.org/10.1016/j.tips.2008.02.003]. [PMID: 18384889]. [DOI] [PubMed] [Google Scholar]
- 13.Shirley R., Ord E.N., Work L.M. Oxidative stress and the use of antioxidants in stroke. Antioxidants. 2014;3(3):472–501. doi: 10.3390/antiox3030472. [http://dx.doi.org/10.3390/antiox3030472]. [PMID: 26785066]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Puyal J., Ginet V., Clarke P.G. Multiple interacting cell death mechanisms in the mediation of excitotoxicity and ischemic brain damage: a challenge for neuroprotection. Prog. Neurobiol. 2013;105:24–48. doi: 10.1016/j.pneurobio.2013.03.002. [http://dx.doi.org/10.1016/j.pneurobio.2013.03.002]. [PMID: 23567504]. [DOI] [PubMed] [Google Scholar]
- 15.Hotchkiss R.S., Strasser A., McDunn J.E., Swanson P.E. Cell death. N. Engl. J. Med. 2009;361(16):1570–1583. doi: 10.1056/NEJMra0901217. [http://dx.doi.org/ 10.1056/NEJMra0901217]. [PMID: 19828534]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Linnik M.D., Zobrist R.H., Hatfield M.D. Evidence supporting a role for programmed cell death in focal cerebral ischemia in rats. 1993. [DOI] [PubMed]
- 17.Charriaut-Marlangue C., Margaill I., Borrega F., Plotkine M., Ben-Ari Y. NG-nitro-L-arginine methyl ester reduces necrotic but not apoptotic cell death induced by reversible focal ischemia in rat. Eur. J. Pharmacol. 1996;310(2-3):137–140. doi: 10.1016/0014-2999(96)00385-8. [http://dx.doi.org/10.1016/ 0014-2999(96)00385-8]. [PMID: 8884209]. [DOI] [PubMed] [Google Scholar]
- 18.Guégan C., Ceballos-Picot I., Nicole A., Kato H., Onténiente B., Sola B. Recruitment of several neuroprotective pathways after permanent focal ischemia in mice. Exp. Neurol. 1998;154(2):371–380. doi: 10.1006/exnr.1998.6913. [http://dx.doi.org/10.1006/exnr.1998.6913]. [PMID: 9878175]. [DOI] [PubMed] [Google Scholar]
- 19.Wei K., Wang P., Miao C.Y. A double-edged sword with therapeutic potential: an updated role of autophagy in ischemic cerebral injury. CNS Neurosci. Ther. 2012;18(11):879–886. doi: 10.1111/cns.12005. [http://dx.doi.org/ 10.1111/cns.12005]. [PMID: 22998350]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kerr J.F., Wyllie A.H., Currie A.R. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br. J. Cancer. 1972;26(4):239–257. doi: 10.1038/bjc.1972.33. [http://dx.doi.org/10.1038/bjc.1972.33]. [PMID: 4561027]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vercammen D., Vandenabeele P., Beyaert R., Declercq W., Fiers W. Tumour necrosis factor-induced necrosis versus anti-Fas-induced apoptosis in L929 cells. Cytokine. 1997;9(11):801–808. doi: 10.1006/cyto.1997.0252. [http://dx. doi.org/10.1006/cyto.1997.0252]. [PMID: 9367540]. [DOI] [PubMed] [Google Scholar]
- 22.Kawahara A., Ohsawa Y., Matsumura H., Uchiyama Y., Nagata S. Caspase-independent cell killing by Fas-associated protein with death domain. J. Cell Biol. 1998;143(5):1353–1360. doi: 10.1083/jcb.143.5.1353. [http://dx.doi. org/10.1083/jcb.143.5.1353]. [PMID: 9832562]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chan F.K., Shisler J., Bixby J.G., Felices M., Zheng L., Appel M., Orenstein J., Moss B., Lenardo M.J. A role for tumor necrosis factor receptor-2 and receptor-interacting protein in programmed necrosis and antiviral responses. J. Biol. Chem. 2003;278(51):51613–51621. doi: 10.1074/jbc.M305633200. [http://dx.doi.org/10.1074/jbc.M305633200]. [PMID: 14532286]. [DOI] [PubMed] [Google Scholar]
- 24.Degterev A., Huang Z., Boyce M., Li Y., Jagtap P., Mizushima N., Cuny G.D., Mitchison T.J., Moskowitz M.A., Yuan J. Chemical inhibitor of nonapoptotic cell death with therapeutic potential for ischemic brain injury. Nat. Chem. Biol. 2005;1(2):112–119. doi: 10.1038/nchembio711. [http://dx. doi.org/10.1038/nchembio711]. [PMID: 16408008]. [DOI] [PubMed] [Google Scholar]
- 25.Linkermann A., Green D.R. Necroptosis. N. Engl. J. Med. 2014;370(5):455–465. doi: 10.1056/NEJMra1310050. [http://dx.doi.org/10.1056/NEJMra1310050]. [PMID: 24476434]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vanlangenakker N., Vanden B.T., Krysko D.V., Festjens N., Vandenabeele P. Molecular mechanisms and pathophysiology of necrotic cell death. Curr. Mol. Med. 2008;8(3):207–220. doi: 10.2174/156652408784221306. [http://dx. doi.org/10.2174/156652408784221306]. [PMID: 18473820]. [DOI] [PubMed] [Google Scholar]
- 27.Degterev A., Hitomi J., Germscheid M., Ch’en I.L., Korkina O., Teng X., Abbott D., Cuny G.D., Yuan C., Wagner G., Hedrick S.M. Gerbe,r S. A.; Lugovskoy, A.; Yuan, J. Identification of RIP1 kinase as a specific cellular target of necrostatins. Nat. Chem. Biol. 2008;4(5):313–321. doi: 10.1038/nchembio.83. [http://dx.doi.org/10.1038/nchembio.83]. [PMID: 18408713]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Oerlemans M.I., Liu J., Arslan F., den Ouden K., van Middelaar B.J., Doevendans P.A., Sluijter J.P. Inhibition of RIP1-dependent necrosis prevents adverse cardiac remodeling after myocardial ischemia-reperfusion in vivo. Basic Res. Cardiol. 2012;107(4):270. doi: 10.1007/s00395-012-0270-8. [http://dx.doi.org/10.1007/s00395-012-0270-8]. [PMID: 22553001]. [DOI] [PubMed] [Google Scholar]
- 29.Lin J., Li H., Yang M., Ren J., Huang Z., Han F., Huang J., Ma J., Zhang D., Zhang Z., Wu J., Huang D., Qipao M., Jin G., Wu Q., Huang Y., Du J., Han J. A role of RIP3-mediated macrophage necrosis in atherosclerosis development. Cell Reports. 2013;3(1):200–210. doi: 10.1016/j.celrep.2012.12.012. [http://dx.doi.org/10.1016/j.celrep.2012.12.012]. [PMID: 23333278]. [DOI] [PubMed] [Google Scholar]
- 30.Linkermann A., Bräsen J.H., Himmerkus N., Liu S., Huber T.B., Kunzendorf U., Krautwald S. Rip1 (receptor-interacting protein kinase 1) mediates necroptosis and contributes to renal ischemia/ reperfusion injury. Kidney Int. 2012;81(8):751–761. doi: 10.1038/ki.2011.450. [http://dx.doi. org/10.1038/ki.2011.450]. [PMID: 22237751]. [DOI] [PubMed] [Google Scholar]
- 31.He S., Wang L., Miao L., Wang T., Du F., Zhao L., Wang X. Receptor interacting protein kinase-3 determines cellular necrotic response to TNF-alpha. Cell. 2009;137(6):1100–1111. doi: 10.1016/j.cell.2009.05.021. [http://dx.doi.org/ 10.1016/j.cell.2009.05.021]. [PMID: 19524512]. [DOI] [PubMed] [Google Scholar]
- 32.Welz P.S., Wullaert A., Vlantis K., Kondylis V., Fernández-Majada V., Ermolaeva M., Kirsch P., Sterner-Kock A., van Loo G., Pasparakis M. FADD prevents RIP3-mediated epithelial cell necrosis and chronic intestinal inflammation. Nature. 2011;477(7364):330–334. doi: 10.1038/nature10273. [http://dx.doi.org/10.1038/nature10273]. [PMID: 21804564]. [DOI] [PubMed] [Google Scholar]
- 33.de Almagro M.C., Vucic D. Necroptosis: Pathway diversity and characteristics. Semin. Cell Dev. Biol. 2015;39:56–62. doi: 10.1016/j.semcdb.2015.02.002. [http://dx. doi.org/10.1016/j.semcdb.2015.02.002]. [PMID: 25683283]. [DOI] [PubMed] [Google Scholar]
- 34.Vanden B.T., Kaiser W.J., Bertrand M.J., Vandenabeele P. Molecular crosstalk between apoptosis, necroptosis, and survival signaling. Mol. Cell. Oncol. 2015;2(4):e975093. doi: 10.4161/23723556.2014.975093. [http://dx.doi.org/ 10.4161/23723556.2014.975093]. [PMID: 27308513]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Holler N., Zaru R., Micheau O., Thome M., Attinger A., Valitutti S., Bodmer J.L., Schneider P., Seed B., Tschopp J. Fas triggers an alternative, caspase-8-independent cell death pathway using the kinase RIP as effector molecule. Nat. Immunol. 2000;1(6):489–495. doi: 10.1038/82732. [http://dx.doi.org/10.1038/82732]. [PMID: 11101870]. [DOI] [PubMed] [Google Scholar]
- 36.Schenk B., Fulda S. Reactive oxygen species regulate Smac mimetic/TNFα-induced necroptotic signaling and cell death. Oncogene. 2015;34(47):5796–5806. doi: 10.1038/onc.2015.35. [http://dx.doi.org/10.1038/onc.2015.35]. [PMID: 25867066]. [DOI] [PubMed] [Google Scholar]
- 37.Zhang Y., Su S.S., Zhao S., Yang Z., Zhong C.Q., Chen X., Cai Q., Yang Z.H., Huang D., Wu R., Han J. RIP1 autophosphorylation is promoted by mitochondrial ROS and is essential for RIP3 recruitment into necrosome. Nat. Commun. 2017;8:14329. doi: 10.1038/ncomms14329. [http://dx. doi.org/10.1038/ncomms14329]. [PMID: 28176780]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kaiser W.J., Sridharan H.J., Huang C.J., Mandal P.J., Upton J.W.J., Gough P.J., Sehon C.A., Marquis R.W.J., Bertin J., Mocarski E.S. Toll-like receptor 3-mediated necrosis via TRIF, RIP3, and MLKL. J. Biol. Chem. 2013;288(43):31268–31279. doi: 10.1074/jbc.M113.462341. [http://dx.doi.org/10.1074/ jbc.M113.462341]. [PMID: 24019532]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Thapa R.J., Nogusa S., Chen P., Maki J.L., Lerro A., Andrake M., Rall G.F., Degterev A., Balachandran S. Interferon-induced RIP1/RIP3-mediated necrosis requires PKR and is licensed by FADD and caspases. Proc. Natl. Acad. Sci. USA. 2013;110(33):E3109–E3118. doi: 10.1073/pnas.1301218110. [http://dx.doi.org/10.1073/pnas.1301218110]. [PMID: 23898178]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Guo H., Kaiser W.J., Mocarski E.S. Manipulation of apoptosis and necroptosis signaling by herpesviruses. Med. Microbiol. Immunol. (Berl.) 2015;204(3):439–448. doi: 10.1007/s00430-015-0410-5. [http://dx.doi.org/10.1007/s00430-015-0410-5]. [PMID: 25828583]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Upton J.W., Kaiser W.J., Mocarski E.S. Virus inhibition of RIP3-dependent necrosis. Cell Host Microbe. 2010;7(4):302–313. doi: 10.1016/j.chom.2010.03.006. [http://dx.doi.org/10.1016/j.chom.2010.03.006]. [PMID: 20413098]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Huang Z., Wu S.Q., Liang Y., Zhou X., Chen W., Li L., Wu J., Zhuang Q., Chen C., Li J., Zhong C.Q., Xia W., Zhou R., Zheng C., Han J. RIP1/RIP3 binding to HSV-1 ICP6 initiates necroptosis to restrict virus propagation in mice. Cell Host Microbe. 2015;17(2):229–242. doi: 10.1016/j.chom.2015.01.002. [http://dx.doi.org/10.1016/j.chom.2015.01.002]. [PMID: 25674982]. [DOI] [PubMed] [Google Scholar]
- 43.Pasparakis M., Vandenabeele P. Necroptosis and its role in inflammation. Nature. 2015;517(7534):311–320. doi: 10.1038/nature14191. [http://dx.doi.org/10.1038/ nature14191]. [PMID: 25592536]. [DOI] [PubMed] [Google Scholar]
- 44.Wertz I.E., Dixit V.M. Ubiquitin-mediated regulation of TNFR1 signaling. Cytokine Growth Factor Rev. 2008;19(3-4):313–324. doi: 10.1016/j.cytogfr.2008.04.014. [http://dx.doi.org/10.1016/j.cytogfr.2008.04.014]. [PMID: 18515172]. [DOI] [PubMed] [Google Scholar]
- 45.Ofengeim D., Yuan J. Regulation of RIP1 kinase signalling at the crossroads of inflammation and cell death. Nat. Rev. Mol. Cell Biol. 2013;14(11):727–736. doi: 10.1038/nrm3683. [http://dx.doi.org/10.1038/nrm3683]. [PMID: 24129419]. [DOI] [PubMed] [Google Scholar]
- 46.Wallach D., Kang T.B., Yang S.H., Kovalenko A. The in vivo significance of necroptosis: lessons from exploration of caspase-8 function. Cytokine Growth Factor Rev. 2014;25(2):157–165. doi: 10.1016/j.cytogfr.2013.12.001. [http://dx.doi.org/10.1016/j.cytogfr.2013.12.001]. [PMID: 24411566]. [DOI] [PubMed] [Google Scholar]
- 47.Vanden B.T., Hassannia B., Vandenabeele P. An outline of necrosome triggers. Cell. Mol. Life Sci. 2016;73(11-12):2137–2152. doi: 10.1007/s00018-016-2189-y. [http://dx.doi.org/10.1007/s00018-016-2189-y]. [PMID: 27052312]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang X., Li Y., Liu S., Yu X., Li L., Shi C., He W., Li J., Xu L., Hu Z., Yu L., Yang Z., Chen Q., Ge L., Zhang Z., Zhou B., Jiang X., Chen S., He S. Direct activation of RIP3/MLKL-dependent necrosis by herpes simplex virus 1 (HSV-1) protein ICP6 triggers host antiviral defense. Proc. Natl. Acad. Sci. USA. 2014;111(43):15438–15443. doi: 10.1073/pnas.1412767111. [http://dx.doi.org/10.1073/pnas.1412767111]. [PMID: 25316792]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sun L., Wang H., Wang Z., He S., Chen S., Liao D., Wang L., Yan J., Liu W., Lei X., Wang X. Mixed lineage kinase domain-like protein mediates necrosis signaling downstream of RIP3 kinase. Cell. 2012;148(1-2):213–227. doi: 10.1016/j.cell.2011.11.031. [http://dx.doi.org/10.1016/j.cell.2011.11.031]. [PMID: 22265413]. [DOI] [PubMed] [Google Scholar]
- 50.Zhao J., Jitkaew S., Cai Z., Choksi S., Li Q., Luo J., Liu Z.G. Mixed lineage kinase domain-like is a key receptor interacting protein 3 downstream component of TNF-induced necrosis. Proc. Natl. Acad. Sci. USA. 2012;109(14):5322–5327. doi: 10.1073/pnas.1200012109. [http://dx.doi.org/10.1073/ pnas.1200012109]. [PMID: 22421439]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hildebrand J.M., Tanzer M.C., Lucet I.S., Young S.N., Spall S.K., Sharma P., Pierotti C., Garnier J.M., Dobson R.C. Webb AI1, Tripaydonis, A.; Babon, J.J.; Mulcair, M. D.; Scanlon, M.J.; Alexander, W.S.; Wilks, A.F.; Czabotar, P. E.; Lessene, G.; Murphy, J.M.; Silke, J. Activation of the pseudokinase MLKL unleashes the four-helix bundle domain to induce membrane localization and necroptotic cell death. Proc. Natl. Acad. Sci. USA. 2014;111(42):15072–15077. doi: 10.1073/pnas.1408987111. [http:// dx.doi.org/10.1073/pnas.1408987111]. [PMID: 25288762]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Murphy J.M., Czabotar P.E., Hildebrand J.M., Lucet I.S., Zhang J.G., Alvarez-Diaz S., Lewis R., Lalaoui N., Metcalf D., Webb A.I., Young S.N., Varghese L.N., Tannahill G.M., Hatchell E.C., Majewski I.J., Okamoto T., Dobson R.C., Hilton D.J., Babon J.J., Nicola N.A., Strasser A., Silke J., Alexander W.S. The pseudokinase MLKL mediates necroptosis via a molecular switch mechanism. Immunity. 2013;39(3):443–453. doi: 10.1016/j.immuni.2013.06.018. [http://dx.doi.org/10.1016/j. immuni.2013.06.018]. [PMID: 24012422]. [DOI] [PubMed] [Google Scholar]
- 53.Chen X., Li W., Ren J., Huang D., He W.T., Song Y., Yang C., Li W., Zheng X., Chen P., Han J. Translocation of mixed lineage kinase domain-like protein to plasma membrane leads to necrotic cell death. Cell Res. 2014;24(1):105–121. doi: 10.1038/cr.2013.171. [http://dx.doi.org/10.1038/cr. 2013.171]. [PMID: 24366341]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang H., Sun L., Su L., Rizo J., Liu L., Wang L.F., Wang F.S., Wang X. Mixed lineage kinase domain-like protein MLKL causes necrotic membrane disruption upon phosphorylation by RIP3. Mol. Cell. 2014;54(1):133–146. doi: 10.1016/j.molcel.2014.03.003. [http://dx.doi.org/10.1016/j.molcel.2014. 03.003]. [PMID: 24703947]. [DOI] [PubMed] [Google Scholar]
- 55.Nogusa S., Thapa R.J., Dillon C.P., Liedmann S., Oguin T.H., III, Ingram J.P., Rodriguez D.A., Kosoff R., Sharma S., Sturm O., Verbist K., Gough P.J., Bertin J., Hartmann B.M., Sealfon S.C., Kaiser W.J., Mocarski E.S., López C.B., Thomas P.G., Oberst A., Green D.R., Balachandran S. RIPK3 activates parallel pathways of MLKL-Driven necrop-tosis and FADD-mediated Apoptosis to protect against influ-enza A virus. Cell Host Microbe. 2016;20(1):13–24. doi: 10.1016/j.chom.2016.05.011. [http://dx.doi.org/10.1016/j.chom.2016.05.011]. [PMID: 27321907]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhang D.W., Shao J., Lin J., Zhang N., Lu B.J., Lin S.C., Dong M.Q., Han J. RIP3, an energy metabolism regulator that switches TNF-induced cell death from apoptosis to necrosis. Science. 2009;325(5938):332–336. doi: 10.1126/science.1172308. [http://dx.doi.org/10.1126/science.1172308]. [PMID: 19498109]. [DOI] [PubMed] [Google Scholar]
- 57.Zhang T., Zhang Y., Cui M., Jin L., Wang Y., Lv F., Liu Y., Zheng W. Shang, H, Zhang, J.; Zhang, M.; Wu, H.; Guo, J.; Zhang, X.; Hu, X.; Cao, C.M.; Xiao, R.P. CaMKII is a RIP3 substrate mediating ischemia- and oxidative stress-induced myocardial necroptosis. Nat. Med. 2016;22(2):175–182. doi: 10.1038/nm.4017. [http://dx.doi.org/10.1038/nm. 4017]. [PMID: 26726877]. [DOI] [PubMed] [Google Scholar]
- 58.Kaczmarek A., Vandenabeele P., Krysko D.V. Necroptosis: the release of damage-associated molecular patterns and its physiological relevance. Immunity. 2013;38(2):209–223. doi: 10.1016/j.immuni.2013.02.003. [http://dx.doi.org/10.1016/ j.immuni.2013.02.003]. [PMID: 23438821]. [DOI] [PubMed] [Google Scholar]
- 59.Tsai S.Y. Segovia. J.A.; Chang, T.H.; Morris, I.R.; Berton, M.T.; Tessier, P.A.; Tardif, M.R.; Cesaro, A.; Bose, S. DAMP molecule S100A9 acts as a molecular pattern to enhance inflammation during influenza A virus infection: role of DDX21-TRIF-TLR4-MyD88 pathway. PLoS Pathog. 2014;10(1):e1003848. doi: 10.1371/journal.ppat.1003848. [http://dx.doi.org/ 10.1371/journal.ppat.1003848]. [PMID: 24391503]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Vandenabeele P., Galluzzi L., Vanden B.T., Kroemer G. Molecular mechanisms of necroptosis: an ordered cellular explosion. Nat. Rev. Mol. Cell Biol. 2010;11(10):700–714. doi: 10.1038/nrm2970. [http://dx.doi.org/10. 1038/nrm2970]. [PMID: 20823910]. [DOI] [PubMed] [Google Scholar]
- 61.Krysko D.V., Brouckaert G., Kalai M., Vandenabeele P., D’Herde K. Mechanisms of internalization of apoptotic and necrotic L929 cells by a macrophage cell line studied by electron microscopy. J. Morphol. 2003;258(3):336–345. doi: 10.1002/jmor.10161. [http://dx.doi.org/10.1002/jmor. 10161]. [PMID: 14584035]. [DOI] [PubMed] [Google Scholar]
- 62.Krysko D.V., Denecker G., Festjens N., Gabriels S., Parthoens E., D’Herde K., Vandenabeele P. Macrophages use different internalization mechanisms to clear apoptotic and necrotic cells. Cell Death Differ. 2006;13(12):2011–2022. doi: 10.1038/sj.cdd.4401900. [http://dx.doi.org/10.1038/sj.cdd. 4401900]. [PMID: 16628234]. [DOI] [PubMed] [Google Scholar]
- 63.Lauber K., Bohn E., Kröber S.M., Xiao Y.J., Blumenthal S.G., Lindemann R.K., Marini P., Wiedig C., Zobywalski A., Baksh S., Xu Y., Autenrieth I.B., Schulze-Osthoff K., Belka C., Stuhler G., Wesselborg S. Apoptotic cells induce migration of phagocytes via caspase-3-mediated release of a lipid attraction signal. Cell. 2003;113(6):717–730. doi: 10.1016/s0092-8674(03)00422-7. [http://dx.doi.org/10.1016/S0092-8674(03)00422-7]. [PMID: 12809603]. [DOI] [PubMed] [Google Scholar]
- 64.Elliott M.R., Chekenim F.B., Trampontm P.C., Lazarowskim E.R., Kadl A., Walk S.F., Park D., Woodson R.I., Ostankovich M., Sharmam P., Lysiak J.J., Harden T.K., Leitinger N., Ravichandran K.S. Nucleotides released by apoptotic cells act as a find-me signal to promote phagocytic clearance. Nature. 2009;461(7261):282–286. doi: 10.1038/nature08296. [http://dx.doi.org/10.1038/nature08296]. [PMID: 19741708]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kryskom O., De Ridder L., Cornelissenm M. Phosphatidylserine exposure during early primary necrosis (oncosis) in JB6 cells as evidenced by immunogold labeling technique. Apoptosis. 2004;9(4):495–500. doi: 10.1023/B:APPT.0000031452.75162.75. [http://dx.doi.org/10.1023/B:APPT.0000031452.75162.75]. [PMID: 15192332]. [DOI] [PubMed] [Google Scholar]
- 66.Brouckaert G., Kalai M., Krysko D.V., et al. Phagocytosis of necrotic cells by macrophages is phosphatidylserine dependent and does not induce inflammatory cytokine production. Mol. Biol. Cell. 2004;15(3):1089–1100. doi: 10.1091/mbc.E03-09-0668. [http://dx.doi.org/10.1091/mbc.e03-09-0668]. [PMID: 14668480]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hirt U.A., Leist M. Rapid, noninflammatory and PS-dependent phagocytic clearance of necrotic cells. Cell Death Differ. 2003;10(10):1156–1164. doi: 10.1038/sj.cdd.4401286. [http://dx.doi.org/10.1038/sj.cdd.4401286]. [PMID: 14502239]. [DOI] [PubMed] [Google Scholar]
- 68.Takahashi N., Duprez L., Grootjans S., Cauwels A., Nerinckx W., DuHadaway J.B., Goossens V., Roelandt R., Van Hauwermeiren F., Libert C., Declercq W., Callewaert N., Prendergast G.C., Degterev A., Yuan J., Vandenabeele P. Necrostatin-1 analogues: critical issues on the specificity, activity and in vivo use in experimental disease models. Cell Death Dis. 2012;3:e437. doi: 10.1038/cddis.2012.176. [http:// dx.doi.org/10.1038/cddis.2012.176]. [PMID: 23190609]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zheng W., Degterev A., Hsu E., Yuan J., Yuan C. Structure-activity relationship study of a novel necroptosis inhibitor, necrostatin-7. Bioorg. Med. Chem. Lett. 2008;18(18):4932–4935. doi: 10.1016/j.bmcl.2008.08.058. [http://dx. doi.org/10.1016/j.bmcl.2008.08.058]. [PMID: 18768316]. [DOI] [PubMed] [Google Scholar]
- 70.Mandal P., Berger S.B., Pillay S., Moriwaki K., Huang C., Guo H., Lich J.D., Finger J., Kasparcova V., Votta B. Ouellette M5, King B.W.; Winooski, D.; Lakdawala, A.S.; DeMartino, M.P.; Casillas, L.N.; Haile, P.A.; Sehon, C.A.; Marquis, R.W.; Upton, J, Daley-Bauer, L.P.; Roback, L.; Ramia, N.; Dovey, C.M.; Carette J.E.; Chan, F.K.; Bertin, J.; Gough, P.J.; Mocarski, E.S.; Kaiser, W.J. RIP3 induces apoptosis independent of pronecrotic kinase activity. Mol. Cell. 2014;56(4):481–495. doi: 10.1016/j.molcel.2014.10.021. [http://dx.doi.org/10.1016/j.molcel.2014.10.021]. [PMID: 25459880]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jouan-Lanhouet S., Riquet F., Duprez L., Vanden B.T., Takahashi N., Vandenabeele P. Necroptosis, in vivo detection in experimental disease models. Semin. Cell Dev. Biol. 2014;35:2–13. doi: 10.1016/j.semcdb.2014.08.010. [http://dx.doi.org/10.1016/j.semcdb.2014.08.010]. [PMID: 25160988]. [DOI] [PubMed] [Google Scholar]
- 72.Linkermann A., Bräsen J.H., Darding M., Jin M.K., Sanz A.B., Heller J.O., De Zen F., Weinlich R., Ortiz A., Walczak H., Weinberg J.M., Green D.R., Kunzendorf U., Krautwald S. Two independent pathways of regulated necrosis mediate ischemia-reperfusion injury. Proc. Natl. Acad. Sci. USA. 2013;110(29):12024–12029. doi: 10.1073/pnas.1305538110. [http:// dx.doi.org/10.1073/pnas.1305538110]. [PMID: 23818611]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Luedde M., Lutz M., Carter N., Sosna J., Jacoby C., Vucur M., Gautheron J., Roderburg C., Borg N., Reisinger F., Hippe H.J., Linkermann A., Wolf M.J., Rose-John S., Lüllmann-Rauch R., Adam D., Flögel U., Heikenwalder M., Luedde T. Fre,y N. RIP3, a kinase promoting necroptotic cell death, mediates adverse remodelling after myocardial infarction. Cardiovasc. Res. 2014;103(2):206–216. doi: 10.1093/cvr/cvu146. [http://dx.doi.org/10.1093/cvr/cvu146]. [PMID: 24920296]. [DOI] [PubMed] [Google Scholar]
- 74.Lim S.Y., Davidson S.M., Mocanu M.M., Yellon D.M., Smith C.C. The cardioprotective effect of necrostatin requires the cyclophilin-D component of the mitochondrial permeability transition pore. Cardiovasc. Drugs Ther. 2007;21(6):467–469. doi: 10.1007/s10557-007-6067-6. [http://dx.doi.org/ 10.1007/s10557-007-6067-6]. [PMID: 17965927]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.You Z., Savitz S.I., Yang J., Degterev A., Yuan J., Cuny G.D., Moskowitz M.A., Whalen M.J. Necrostatin-1 reduces histopathology and improves functional outcome after controlled cortical impact in mice. J. Cereb. Blood Flow Metab. 2008;28(9):1564–1573. doi: 10.1038/jcbfm.2008.44. [http://dx.doi.org/10.1038/jcbfm.2008.44]. [PMID: 18493258]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wang C.F., Zhao C.C., Weng W.J., Lei J., Lin Y., Mao Q., Gao G.Y., Feng J.F., Jiang J.Y. Alteration in long non-coding RNA expression after traumatic brain injury in rats. J. Neurotrauma. 2017;34(13):2100–2108. doi: 10.1089/neu.2016.4642. [http://dx.doi.org/10.1089/neu.2016.4642]. [PMID: 28145813]. [DOI] [PubMed] [Google Scholar]
- 77.Wang Y.Q., Wang L., Zhang M.Y., Wang T., Bao H.J., Liu W.L., Dai D.K., Zhang L., Chang P., Dong W.W., Chen X.P., Tao L.Y. Necrostatin-1 suppresses autophagy and apoptosis in mice traumatic brain injury model. Neurochem. Res. 2012;37(9):1849–1858. doi: 10.1007/s11064-012-0791-4. [http://dx.doi.org/10.1007/s11064-012-0791-4]. [PMID: 22736198]. [DOI] [PubMed] [Google Scholar]
- 78.Wang Y., Wang H., Tao Y., Zhang S., Wang J., Feng X. Necroptosis inhibitor necrostatin-1 promotes cell protection and physiological function in traumatic spinal cord injury. Neuroscience. 2014;266:91–101. doi: 10.1016/j.neuroscience.2014.02.007. [http://dx.doi.org/10.1016/j.neuroscience.2014.02.007]. [PMID: 24561219]. [DOI] [PubMed] [Google Scholar]
- 79.Zhu S., Zhang Y., Bai G., Li H. Necrostatin-1 ameliorates symptoms in R6/2 transgenic mouse model of Huntington’s disease. Cell Death Dis. 2011;2:e115. doi: 10.1038/cddis.2010.94. [http://dx.doi.org/10.1038/cddis.2010.94]. [PMID: 21359116]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Qinli Z., Meiqing L., Xia J., Li X., Weili G., Xiuliang J., Junwei J., Hailan Y., Ce Z., Qiao N. Necrostatin-1 inhibits the degeneration of neural cells induced by aluminum exposure. Restor. Neurol. Neurosci. 2013;31(5):543–555. doi: 10.3233/RNN-120304. [PMID: 23735313]. [DOI] [PubMed] [Google Scholar]
- 81.Sato K., Li S., Gordon W.C., He J., Liou G.I., Hill J.M., Travis G.H., Bazan N.G., Jin M. Receptor interacting protein kinase-mediated necrosis contributes to cone and rod photoreceptor degeneration in the retina lacking interphotoreceptor retinoid-binding protein. J. Neurosci. 2013;33(44):17458–17468. doi: 10.1523/JNEUROSCI.1380-13.2013. [http://dx.doi.org/10.1523/ JNEUROSCI.1380-13.2013]. [PMID: 24174679]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Murakami Y., Matsumoto H., Roh M., Suzuki J., Hisatomi T., Ikeda Y., Miller J.W., Vavvas D.G. Receptor interacting protein kinase mediates necrotic cone but not rod cell death in a mouse model of inherited degeneration. Proc. Natl. Acad. Sci. USA. 2012;109(36):14598–14603. doi: 10.1073/pnas.1206937109. [http://dx.doi.org/10.1073/pnas.1206937109]. [PMID: 22908283]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Trichonas G., Murakami Y., Thanos A., Morizane Y., Kayama M., Debouck C.M., Hisatomi T., Miller J.W., Vavvas D.G. Receptor interacting protein kinases mediate retinal detachment-induced photoreceptor necrosis and compensate for inhibition of apoptosis. Proc. Natl. Acad. Sci. USA. 2010;107(50):21695–21700. doi: 10.1073/pnas.1009179107. [http://dx.doi.org/ 10.1073/pnas.1009179107]. [PMID: 21098270]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hanus J., Zhang H., Wang Z., Liu Q., Zhou Q., Wang S. Induction of necrotic cell death by oxidative stress in retinal pigment epithelial cells. Cell Death Dis. 2013;4:e965. doi: 10.1038/cddis.2013.478. [http://dx.doi.org/ 10.1038/cddis.2013.478]. [PMID: 24336085]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Rosenbaum D.M., Degterev A., David J., Rosenbaum P.S., Roth S., Grotta J.C., Cuny G.D., Yuan J., Savitz S.I. Necroptosis, a novel form of caspase-independent cell death, contributes to neuronal damage in a retinal ischemia-reperfusion injury model. J. Neurosci. Res. 2010;88(7):1569–1576. doi: 10.1002/jnr.22314. [PMID: 20025059]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Duprez L., Takahashi N., Van Hauwermeiren F., et al. RIP kinase-dependent necrosis drives lethal systemic inflammatory response syndrome. Immunity. 2011;35(6):908–918. doi: 10.1016/j.immuni.2011.09.020. [http://dx.doi.org/10. 1016/j.immuni.2011.09.020]. [PMID: 22195746]. [DOI] [PubMed] [Google Scholar]
- 87.Linkermann A., Bräsen J.H., De Zen F., Weinlich R., Schwendener R.A., Green D.R., Kunzendorf U., Krautwald S. Dichotomy between RIP1- and RIP3-mediated necroptosis in tumor necrosis factor-α-induced shock. Mol. Med. 2012;18:577–586. doi: 10.2119/molmed.2011.00423. [http:// dx.doi.org/10.2119/molmed.2011.00423]. [PMID: 22371307]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wu J., Huang Z., Ren J., Zhang Z., He P., Li Y., Ma J., Chen W., Zhang Y., Zhou X., Yang Z., Wu S.Q., Chen L., Han J. Mlkl knockout mice demonstrate the indispensable role of Mlkl in necroptosis. Cell Res. 2013;23(8):994–1006. doi: 10.1038/cr.2013.91. [http://dx.doi.org/10.1038/ cr.2013.91]. [PMID: 23835476]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Jouan-Lanhouet S., Arshad M.I., Piquet-Pellorce C., Martin-Chouly C., Le Moigne-Muller G., Van Herreweghe F., Takahashi N., Sergent O., Lagadic-Gossmann D., Vandenabeele P., Samson M., Dimanche-Boitrel M.T. TRAIL induces necroptosis involving RIPK1/RIPK3-dependent PARP-1 activation. Cell Death Differ. 2012;19(12):2003–2014. doi: 10.1038/cdd.2012.90. [http://dx.doi.org/10.1038/cdd.2012.90]. [PMID: 22814620]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Liedtke C., Bangen J.M., Freimuth J., Beraza N., Lambertz D., Cubero F.J., Hatting M., Karlmark K.R., Streetz K.L., Krombach G.A., Tacke F., Gassler N., Riethmacher D., Trautwein C. Loss of caspase-8 protects mice against inflammation-related hepatocarcinogenesis but induces non-apoptotic liver injury. Gastroenterology. 2011;141(6):2176–2187. doi: 10.1053/j.gastro.2011.08.037. [http://dx.doi.org/10.1053/j.gastro.2011.08. 037]. [PMID: 21878202]. [DOI] [PubMed] [Google Scholar]
- 91.Zhang Y.F., He W., Zhang C., Liu X.J., Lu Y., Wang H., Zhang Z.H., Chen X., Xu D.X. Role of receptor interacting protein (RIP)1 on apoptosis-inducing factor-mediated necroptosis during acetaminophen-evoked acute liver failure in mice. Toxicol. Lett. 2014;225(3):445–453. doi: 10.1016/j.toxlet.2014.01.005. [http://dx.doi.org/10.1016/j.toxlet.2014.01.005]. [PMID: 24440347]. [DOI] [PubMed] [Google Scholar]
- 92.Roychowdhury S., Chiang D.J., Mandal P., McMullen M.R., Liu X., Cohen J.I., Pollard J., Feldstein A.E., Nagy L.E. Inhibition of apoptosis protects mice from ethanol-mediated acceleration of early markers of CCl4 -induced fibrosis but not steatosis or inflammation. Alcohol. Clin. Exp. Res. 2012;36(7):1139–1147. doi: 10.1111/j.1530-0277.2011.01720.x. [http://dx.doi.org/ 10.1111/j.1530-0277.2011.01720.x]. [PMID: 22273278]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Roychowdhury S., McMullen M.R., Pisano S.G., Liu X., Nagy L.E. Absence of receptor interacting protein kinase 3 prevents ethanol-induced liver injury. Hepatology. 2013;57(5):1773–1783. doi: 10.1002/hep.26200. [http:// dx.doi.org/10.1002/hep.26200]. [PMID: 23319235]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Günther C., Martini E., Wittkopf N., Amann K., Weigmann B., Neumann H., Waldner M.J., Hedrick S.M., Tenzer S., Neurath M.F., Becker C. Caspase-8 regulates TNF-α-induced epithelial necroptosis and terminal ileitis. Nature. 2011;477(7364):335–339. doi: 10.1038/nature10400. [http://dx.doi.org/10.1038/nature10400]. [PMID: 21921917]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Cho Y.S., Challa S., Moquin D., Genga R., Ray T.D., Guildford M., Chan F.K. Phosphorylation-driven assembly of the RIP1-RIP3 complex regulates programmed necrosis and virus-induced inflammation. Cell. 2009;137(6):1112–1123. doi: 10.1016/j.cell.2009.05.037. [http://dx.doi.org/10.1016/ j.cell.2009.05.037]. [PMID: 19524513]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Polykratis A., Hermance N., Zelic M., Roderick J., Kim C., Van T.M., Lee T.H., Chan F.K.M., Pasparakis M., Kelliher M.A. Cutting edge: RIPK1 Kinase inactive mice are viable and protected from TNF-induced necroptosis in vivo. J. Immunol. 2014;193(4):1539–1543. doi: 10.4049/jimmunol.1400590. [http://dx.doi.org/10.4049/jimmunol.1400590]. [PMID: 25015821]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Mack C., Sickmann A., Lembo D., Brune W. Inhibition of proinflammatory and innate immune signaling pathways by a cytomegalovirus RIP1-interacting protein. Proc. Natl. Acad. Sci. USA. 2008;105(8):3094–3099. doi: 10.1073/pnas.0800168105. [http://dx.doi.org/10.1073/pnas.0800168105]. [PMID: 18287053]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Peri P., Nuutila K., Vuorinen T., Saukko P., Hukkanen V. Cathepsins are involved in virus-induced cell death in ICP4 and Us3 deletion mutant herpes simplex virus type 1-infected monocytic cells. J. Gen. Virol. 2011;92(Pt 1):173–180. doi: 10.1099/vir.0.025080-0. [http://dx.doi.org/10.1099/vir.0. 025080-0]. [PMID: 20881085]. [DOI] [PubMed] [Google Scholar]
- 99.Berger A.K., Danthi P. Reovirus activates a caspase-independent cell death pathway. MBio. 2013;4(3):e00178–e13. doi: 10.1128/mBio.00178-13. [http://dx. doi.org/10.1128/mBio.00178-13]. [PMID: 23674612]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Pan T., Wu S., He X., Luo H., Zhang Y., Fan M., Geng G., Ruiz V.C., Zhang J., Mills L., Bai C., Zhang H. Necroptosis takes place in human immunodeficiency virus type-1 (HIV-1)-infected CD4+ T lymphocytes. PLoS One. 2014;9(4):e93944. doi: 10.1371/journal.pone.0093944. [http://dx.doi.org/ 10.1371/journal.pone.0093944]. [PMID: 24714696]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Liu S., Wang X., Li Y., Xu L., Yu X., Ge L., Li J., Zhu Y., He S. Necroptosis mediates TNF-induced toxicity of hippocampal neurons. BioMed Res. Int. 2014;2014:290182. doi: 10.1155/2014/290182. [PMID: 25093162]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zille M., Karuppagounder S.S., Chen Y., Gough P.J., Ber-tin J., Finger J., Milner T.A., Jonas E.A., Ratan R.R. Neu-ronal death after hemorrhagic stroke In Vitro and In Vivo shares features of ferroptosis and necroptosis. Stroke. 2017;48(4):1033–1043. doi: 10.1161/STROKEAHA.116.015609. [http:// dx.doi.org/10.1161/STROKEAHA.116.015609]. [PMID: 28250197]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Qu Y., Shi J., Tang Y., Zhao F., Li S., Meng J., Tang J., Lin X., Peng X., Mu D. MLKL inhibition attenuates hypoxia-ischemia induced neuronal damage in developing brain. Exp. Neurol. 2016;279:223–231. doi: 10.1016/j.expneurol.2016.03.011. [http://dx.doi.org/10.1016/j.expneurol.2016.03.011]. [PMID: 26980487]. [DOI] [PubMed] [Google Scholar]
- 104.Chen W.W., Yu H., Fan H.B., Zhang C.C., Zhang M., Zhang C., Cheng Y., Kong J., Liu C.F., Geng D., Xu X. RIP1 mediates the protection of geldanamycin on neuronal injury induced by oxygen-glucose deprivation combined with zVAD in primary cortical neurons. J. Neurochem. 2012;120(1):70–77. doi: 10.1111/j.1471-4159.2011.07526.x. [http://dx.doi.org/10.1111/ j.1471-4159.2011.07526.x]. [PMID: 21985437]. [DOI] [PubMed] [Google Scholar]
- 105.Vieira M., Fernandes J., Carreto L., Anuncibay-Soto B., Santos M., Han J., Fernández-López A., Duarte C.B., Carvalho A.L., Santos A.E. Ischemic insults induce necroptotic cell death in hippocampal neurons through the up-regulation of endogenous RIP3. Neurobiol. Dis. 2014;68:26–36. doi: 10.1016/j.nbd.2014.04.002. [http://dx.doi.org/10.1016/j.nbd.2014. 04.002]. [PMID: 24746856]. [DOI] [PubMed] [Google Scholar]
- 106.Chavez-Valdez R., Martin L.J., Flock D.L., Northington F.J. Necrostatin-1 attenuates mitochondrial dysfunction in neurons and astrocytes following neonatal hypoxia-ischemia. Neuroscience. 2012;219:192–203. doi: 10.1016/j.neuroscience.2012.05.002. [http://dx.doi.org/10.1016/j.neuroscience.2012.05.002]. [PMID: 22579794]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Northington F.J., Chavez-Valdez R., Martin L.J. Neuronal cell death in neonatal hypoxia-ischemia. Ann. Neurol. 2011;69(5):743–758. doi: 10.1002/ana.22419. [http://dx.doi.org/10.1002/ana.22419]. [PMID: 21520238]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Xu X., Chua K.W., Chua C.C., Liu C.F., Hamdy R.C., Chua B.H. Synergistic protective effects of humanin and necrostatin-1 on hypoxia and ischemia/reperfusion injury. Brain Res. 2010;1355:189–194. doi: 10.1016/j.brainres.2010.07.080. [http://dx.doi.org/10.1016/j.brainres.2010.07.080]. [PMID: 20682300]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Xu Y., Wang J., Song X., Qu L., Wei R., He F., Wang K., Luo B. RIP3 induces ischemic neuronal DNA degradation and programmed necrosis in rat via AIF. Sci. Rep. 2016;6:29362. doi: 10.1038/srep29362. [http://dx.doi.org/10.1038/srep29362]. [PMID: 27377128]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Yin B., Xu Y., Wei R.L., He F., Luo B.Y., Wang J.Y. Inhibition of receptor-interacting protein 3 upregulation and nuclear translocation involved in Necrostatin-1 protection against hippocampal neuronal programmed necrosis induced by ischemia/reperfusion injury. Brain Res. 2015;1609:63–71. doi: 10.1016/j.brainres.2015.03.024. [http://dx.doi.org/10.1016/j.brainres. 2015.03.024]. [PMID: 25801119]. [DOI] [PubMed] [Google Scholar]
- 111.Zhu X., Tao L., Tejima-Mandeville E., Qiu J., Park J., Garber K., Ericsson M., Lo E.H., Whalen M.J. Plasmalemma permeability and necrotic cell death phenotypes after intracerebral hemorrhage in mice. Stroke. 2012;43(2):524–531. doi: 10.1161/STROKEAHA.111.635672. [http://dx.doi.org/10.1161/ STROKEAHA.111.635672]. [PMID: 22076006]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.King M.D., Whitaker-Lea W.A., Campbell J.M., Alleyne C.H., Jr, Dhandapani K.M. Necrostatin-1 reduces neurovascular injury after intracerebral hemorrhage. Int. J. Cell Biol. 2014;2014:495817. doi: 10.1155/2014/495817. [http://dx.doi.org/10.1155/2014/495817]. [PMID: 24729786]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Su X., Wang H., Kang D., Zhu J., Sunm Q., Li T., Dingm K. Necrostatin-1 ameliorates intracerebral hemorrhage-induced brain injury in mice through inhibiting RIP1/RIP3 pathway. Neurochem. Res. 2015;40(4):643–650. doi: 10.1007/s11064-014-1510-0. [http://dx.doi.org/10.1007/s11064-014-1510-0]. [PMID: 25576092]. [DOI] [PubMed] [Google Scholar]
- 114.Shen H., Liu C., Zhang D., Yao X., Zhang K., Li H., Chen G. Role for RIP1 in mediating necroptosis in experimental intracerebral hemorrhage model both in vivo and in vitro. Cell Death Dis. 2017;8(3):e2641. doi: 10.1038/cddis.2017.58. [http://dx.doi.org/10.1038/cddis.2017.58]. [PMID: 28252651]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Chang P., Dong W., Zhang M., Wang Z., Wang Y., Wang T., Gao Y., Meng H., Luo B., Luo C., Chen X., Tao L. Anti-necroptosis chemical necrostatin-1 can also suppress apoptotic and autophagic pathway to exert neuroprotective effect in mice intracerebral hemorrhage model. J. Mol. Neurosci. 2014;52(2):242–249. doi: 10.1007/s12031-013-0132-3. [http://dx.doi.org/10.1007/s12031-013-0132-3]. [PMID: 24122153]. [DOI] [PubMed] [Google Scholar]
- 116.Zhou K., Shi L., Wang Z., Zhou J., Manaenko A., Reis C., Chen S., Zhang J. RIP1-RIP3-DRP1 pathway regulates NLRP3 inflammasome activation following subarachnoid hemorrhage. Exp. Neurol. 2017;295:116–124. doi: 10.1016/j.expneurol.2017.06.003. [http://dx.doi.org/10.1016/j.expneurol.2017.06. 003]. [PMID: 28579326]. [DOI] [PubMed] [Google Scholar]
- 117.Northington F.J., Chavez-Valdez R., Graham E.M., Razdan S., Gaudam E.B., Martin L.J. Necrostatin decreases oxidative damage, inflammation, and injury after neonatal HI. J. Cereb. Blood Flow Metab. 2011;31(1):178–189. doi: 10.1038/jcbfm.2010.72. [http://dx.doi.org/10.1038/jcbfm.2010.72]. [PMID: 20571523]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.LaRocca T.J., Sosunov S.A., Shakerley N.L., Ten V.S., Ratner A.J. hyperglycemic conditions prime cells for RIP1-dependent necroptosis. J. Biol. Chem. 2016;291(26):13753–13761. doi: 10.1074/jbc.M116.716027. [http://dx.doi.org/ 10.1074/jbc.M116.716027]. [PMID: 27129772]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Xuan M., Okazaki M., Iwata N., et al. Chronic treatment with a water-soluble extract from the culture medium of ganoderma lucidum Mycelia prevents apoptosis and necroptosis in hypoxia/ischemia-induced injury of Type 2 Diabetic mouse brain. Evid. Based Complement. Alternat. Med. 2015;2015:865986. doi: 10.1155/2015/865986. [http://dx.doi.org/10. 1155/2015/865986]. [PMID: 25945116]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Askalan R., Gabarin N., Armstrong E.A., Fang L.Y., Couchman D., Yager J.Y. Mechanisms of neurodegeneration after severe hypoxic-ischemic injury in the neonatal rat brain. Brain Res. 2015;1629:94–103. doi: 10.1016/j.brainres.2015.10.020. [http://dx.doi.org/10.1016/j.brainres.2015.10.020]. [PMID: 26485031]. [DOI] [PubMed] [Google Scholar]
- 121.Qu Y., Tang J. Wang, H.;Li, S.; Zhao, F.; Zhang, L.; Richard, L. Q.; Mu, D. RIPK3 interactions with MLKL and CaMKII mediate oligodendrocytes death in the developing brain. Cell Death Dis. 2017;8(2):e2629. doi: 10.1038/cddis.2017.54. [http://dx.doi.org/10.1038/cddis.2017.54]. [PMID: 28230861]. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 122.Wang Y.Z., Wang J.J., Huangm Y., Liu F., Zeng W.Z., Li Y., Xiong Z.G., Zhu M.X., Xu T.L. Tissue acidosis induces neuronal necroptosis via ASIC1a channel independent of its ionic conduction. eLife. 2015;4:4. doi: 10.7554/eLife.05682. [http://dx.doi.org/10.7554/eLife.05682]. [PMID: 26523449]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Dai M.C., Zhong Z.H., Sun Y.H., Sun Q.F., Wang Y.T., Yang G.Y., Bian L.G. Curcumin protects against iron induced neurotoxicity in primary cortical neurons by attenuating necroptosis. Neurosci. Lett. 2013;536:41–46. doi: 10.1016/j.neulet.2013.01.007. [http://dx.doi.org/10.1016/j.neulet.2013.01. 007]. [PMID: 23328441]. [DOI] [PubMed] [Google Scholar]
- 124.Xu X., Chua C.C., Zhang M., Geng D., Liu C.F., Hamdy R.C., Chua B.H. The role of PARP activation in glutamate-induced necroptosis in HT-22 cells. Brain Res. 2010;1343:206–212. doi: 10.1016/j.brainres.2010.04.080. [http://dx. doi.org/10.1016/j.brainres.2010.04.080]. [PMID: 20451505]. [DOI] [PubMed] [Google Scholar]
- 125.Li Y., Yang X., Ma C., Qiao J., Zhang C. Necroptosis contributes to the NMDA-induced excitotoxicity in rat’s cultured cortical neurons. Neurosci. Lett. 2008;447(2-3):120–123. doi: 10.1016/j.neulet.2008.08.037. [http://dx.doi.org/10. 1016/j.neulet.2008.08.037]. [PMID: 18723075]. [DOI] [PubMed] [Google Scholar]