Abstract
Tumor necrosis factor receptor-associated factor (TRAF) is an important binding protein of tumor necrosis factor (TNF) superfamily and the toll/IL-1 receptor (TIR) superfamily, which play an important role in innate immunity and ac-quired immunity. TRAFs family have 7 members (TRAF1-7), and TRAF6 has its special facture and biological function. TRAF6 has two special domains: C-terminal domain and N-terminal domain, which could integrate with multiple kinases and regulate signaling pathway function as an E3 ubiquitin ligase. Studies have increasingly found that TRAF6 is closely re-lated to central nervous system diseases, such as stroke, Traumatic brain injury, neurodegenerative diseases and neuropathic pain. Further research on the pathophysiological mechanism may be expected to become the new targets for the treatment of central nervous system diseases
Keywords: TRAF6, E3 ubiquitin ligase, ubiquitination, inflammation, ROS, central nervous system diseases
1. The discovery and structural features of tumor necrosis factor receptor-associated factor 6 (TRAF6)
TRAFs family is a kind of multifunctional intracellular adaptin, which is originally discovered by its direct integration with different tumor necrosis factors in human and rodent cells [1], and it exists in a variety of biological cells. 7 members in TRAFs family of mammals are found, and they are named TRAF1-7 by the sequence of their discovery, but there is a dispute that whether TRAF7 belongs to TRAFs family because it lacks the homeodomain of TRAF family. TRAF protein structure is characterized by its C-terminal TRAF domain, which includes N-terminal coiled region and C-terminal β sandwich structure [2], and this structure can regulate the interaction with different proteins, including the oligomerization of TRAF and the interaction between the upstream regulatory molecular and the downstream effector molecular. Therefore, an important function of TRAF protein is to connect the upstream receptors and the downstream enzymes as kinds of adaptin in the agglomeration process of receptor-associated signal complexes. In addition, TRAF protein contains 1-7 zinc finger motifs and N-terminal ring finger domain except for TRAF1, which participate in the activation of Nuclear Factor-kappa B (NF-κB) and Jun N-terminal Kinase (JNK), but in bone metabolism, the zinc finger motifs mediate the monocyte/macrophages differentiate to osteoclast, and the ring finger domain is correlated to the maturity and activation of osteoclast and process of bone resorption. Besides, TRAF4 locates in the nucleus, all TRAF proteins of mammals are located in the cytoplasm. Containing the core structure of catalytic domain of E3 ubiquitin ligase, the ring finger structure exists in a large number of ubiquitin ligases. Smaller structural differences determine the specificity of the integration between different TRAF and different receptors. TRAF6 has the smallest homology than typical TRAF protein structure and biggest different TRAF-C domain than other TRAFs [3]. There is mounting evidence that TRAF6 can regulate signaling pathways as E3 ubiquitin ligase [2], apart from acting as adaptin. TRAF6 is first found by Ishida [4], and TRAF6 could mediate the expression of interleukin-1 (IL-1) signal [5]. TRAF6 is composed of 530 amino acids, and its relative molecular mass is about 60000. Compared with the domain of typical TRAF protein, TRAF6 has the smallest homology and the biggest difference of C-terminal domain [6]. TRAF6 has been widely distributed as it is abundant in brain, lung, liver, skeletal muscle and kidney, and could be found in heart, spleen, and testis.
2. The role of TRAF6 in signal trans-duction and its physiological function
As a kind of important multifunctional signal molecule in cells, TRAF6 can integrate with ubiquitin through the thioester bond in catalytic reaction and pass the ubiquitin to the relative substrate, thus participating in a series of signal transduction. It has been found that TRAF6 plays an important role in the immune and inflammatory reaction. We have known that TRAF6 is an important adaptin in TRAFs family, it could be activated rapidly and gather together to intracellular areas, then carry out its physiological function mainly through Toll Like Receptor 4 (TLR4) signaling pathway. In this process, TRAF6 plays its role by acting as an intersection of activating NF-κB signaling pathway and Mitogen-Activated Protein Kinase (MAPK) signaling pathway, and it mediates two main signaling pathways: MyD88-dependent pathway and MyD88-independent pathway [7]. Through MyD88-dependent pathway, TLR4 transduces the extracellular stimuli into intracellular signals through integrating with extracellular inflammatory factors, and the intracellular TIR area of TLR4 can combine with C-terminal of MyD88, meanwhile, the N-terminal death domain of MyD88 unites with the N-terminal death domain of IL-1 Receptor Associated Kinase (IRAK), activating IRAK phosphorylation and integrating with TRAF6 and its receptors and transforming growth factor kinase-1(TAK1)-TAB1-TAB2 complex, which contributes to the formation of IRAK-TRAF6-TAK1-TAB1-TAB2 complex and causes a series of cascade reaction that results in the degradation of IκB-α and the activation of MAPKs (ERK,P38 and JNK). In this process, transcription factors NF-κB and AP-1 are activated ultimately that leads to a great number of pathophysiologic process, including cell apoptosis, inflammatory response and proliferation [8]. In MyD88-independent pathway, through recruiting interleukin receptors (TIRs) and the domain of β-interferons (TRIFs), TRAF6 can activate NF-κB and IRFs to mediate the production of inflammatory mediator and interferon, thus participating in the inflammatory response. Using RAW264.7 macrophage system, Jakus PB [9] found that the expression level of TRAF6 increased dramatically in an experiment that LPS induced the generation of inflammatory response, and if the expression of TRAF6 was inhibited, the activity of MAPK (JNK, P38, et al.) decreased accordingly and the inflammatory response relieved obviously. In addition, TRAF6 can mediate the production of intracellular reactive oxygen species (ROS) [10]. The research of West AP [11] has shown that TRAF6 could translocate to mitochondria in the signal transduction of toll-like receptor signaling pathway, and the existence of TRAF6 could increase the generation of ROS that exists in the mitochondria of macrophage, on the contrary, the ability of macrophage that lacks TRAF6 to generate ROS would reduce significantly. Moreover, researches show that the formation of TRAF6-ASK1 complex and TRAF2-ASK1 complex plays an important role in cell death that mediated by oxidative stress [12], and TLR4/MyD88/TRAF6/c-Src/NADPH oxidase pathway could regulate the generation of ROS [13]. Besides, studies have demonstrated that mammalian target of rapamyein (mTOR) can inhibit autophagy signaling pathway [14]. As a kind of atypia serine/threonine protein kinase and a member of phosphatidylinositol 3 kinase (PI3K) protein kinase family, mammalian target of rapamycin (mTOR) can interact with the extracellular signals coming from nutrition, energy and growth factors, thus participating in the physiological process of cell growth and proliferation. mTOR1 and mTOR2 complexes are the two main types of mTOR. Further research found that mTOR could inhibit autophagy through regulating TRAF6 and autophagy-related gene AMBRA1 [15]. In normal organisms, TRAF6 can promote ULK1 ubiquitin and lead to the degradation of the protein, but when the body encounters the stressful situation, such as stroke, brain trauma, the expression of TRAF6 will increase notably and mediate the ULK1 phosphorylation, thus inhibiting autophagy and causing tissue damage. Researchers also showed that TRAF6 can play its role in regulating autophagy acting as the upstream regulatory factors of mTOR, and the lack of TRAF6 could promote the development of autophagy [16].
Table 1.
The main developmental history of TRAF6.
| Time | Incident | Refs. |
|---|---|---|
| 1996 | The discovery of TRAF6, and it is the only one member of TRAF family that takes part in the signal transduction of TNFR and IL-1R/ TLR superfamily | (Ishida et al., 1996) (Cao et al., 1996) |
| 2000 | TRAF6 is found relevant to AD | (Akama and Van Eldik, 2000) |
| 2003 | TRAF6 is required for optimal DC maturation and activation | (Kobayashi et al., 2003) |
| 2004 | TRAF6 plays an essential role in NGF-dependent signaling pathway in Schwann cells | (Yeiser et al., 2004) |
| 2010 | the expression level of TRAF6 has been detected in nigral dopaminergic neurons | (Zucchelli et al., 2010) |
| 2011 | TRAF6 was first described in Traumatic brain injury | (Chen et al., 2011) |
| 2012 | The expression of TRAF6 increases after ischemic stroke | (Liu et al., 2012) |
| 2012 | TRAF6 is associated with Ischemia-reperfusion injury,and peaked at 1h after ischemic | (Yuan et al., 2013) |
| 2013 | The characteristic of TRAF6,E3 ubiquitin ligase,is highly related to ischemic stroke | (Wu et al., 2013) |
| 2013 | TRAF6 promotes Akt activation in the process of TBI | (Farook et al., 2013) |
| 2013 | TRAF6 mediated autophagy in AD | (Salminen et al., 2013) |
| 2014 | TRAF6 mediated inflammatory response in AD | (Olajide et al., 2014) |
| 2014 | TRAF6 may aggravate neuropathic pain through JNK/CCL2 pathway | (Lu et al., 2014) |
| 2015 | MicroRNA-146a-5p releaves neuropathic pain by inhibiting TRAF6 | (Lu et al., 2015) |
3. TRAF6 in central nervous system (CNS)
3.1. TRAF6 and Stroke
Stroke is a kind of acute cerebrovascular disease characterized by the focal lack of neurological function, including ischemic stroke and hemorrhagic stroke. Ischemic stroke, the majority of stroke, is a kind of cerebral infarction, caused by cerebral circulation insufficiency that owing to cerebral thrombosis, cerebral vasospasm, or other cautions, which is the leading cause of disability and the second leading cause of death in China [17, 18]. A large number of clinical studies and animal experiments have shown that ischemic cerebral injury and ischemia-reperfusion injury are complex physiopathologic process, involving energy metabolism abnormality, the toxic effects of excitatory amino acids, inflammatory reaction, oxidative stress, the generation of free radical, brain edema, neuron apoptosis/die, and with the progress of the disease, the brain tissues can show reconstitution, including neuron regeneration, neovascularization and gliocyte proliferation [19-21]. There is a growing body evidence that inflammatory response plays an important role in ischemic cerebral injury [22]. Some studies show that the development of inflammatory response could be observed within only a few hours after ischemic cerebral injury, which involves the accumulating process of multiple leukocyte (neutrophils, monocytes, et al.) [23, 24]. Previous studies have demonstrated that TLR signaling pathway is the most important pathway in the peripheral blood of stroke patients [25], and it could be activated by various matters, such as the production of protein degradation, damaged DNA, fibrinogen, and heat shock protein after ischemic cerebral injury. Subsequently, a great number of proinflammatory cytokine that mainly comes from the activated innate immunity, generates and promotes the development of inflammatory response. One recent study has confirmed that not TLR2 or TLR3, it is TLR4 that mediates the TLR signaling pathway and increases in the peripheral blood of ischemic stroke patients [26]. TRAF6 could activate the NF-κB and AP-1 to participate in inflammatory response through the MyD88-dependent pathway and MyD88-independent pathway as well. By using middle cerebral artery occlusion model, researchers have found that the expression level TRAF6 increased significantly and brain edema is obvious, however, after application of TRAF6 inhibitor, cerebral infarction area decreased, the degree of brain edema relieved, immunohistochemistry and western blot showed that the expression of TRAF6 decreased obviously as well [27]. Likewise, some clinical trials, selecting the same number of ischemic stroke group and the control group have shown that TRAF6 plays the most important role in cerebral ischemia by acting as the downstream molecular after the activation of TLR4 signaling pathway in the peripheral blood of ischemic stroke patients, and it may have an important effect on the prognosis of ischemic cerebral injury [28]. In addition, the expression of TRAF6 has also a close relationship to Ischemia-reperfusion injury. There is clear evidence confirming that apoptosis is the leading cause of cell death after ischemia-reperfusion [29], and TRAF6 is the only one member of TRAF family that takes part in the signal transduction of the TNF receptor superfamily and the interleukin-1 receptor (IL-1R)/toll-like receptor (TLR) superfamily [30, 31], which participates in regulating cell death, survival and stress response of cells in different signaling pathway. The lack of TRAF6 could cause the defection of signals, the inactivation of NF-κB and the decrease of cytokines production [32]. Furthermore, a great number of studies have shown that TRAF6 participates in all kinds of apoptosis and the occurrence of a variety of biological effects through activating IκB kinase complex and mitogen-activated protein kinases [33]. By establishing the middle cerebral artery occlusion-reperfusion model, researchers could examine the mRNA expression of TRAF6 with the help of RT-PCR, and compared with the sham groups, the mRNA expression level of TRAF6 increased rapidly at the beginning of cerebral ischemia, reached the peak at 1 hour after ischemia, subsequently, it maintained the level after reperfusion, and declined gradually at 12-24 hours after reperfusion. Further, analyze the relationship between TRAF6 and caspase-3, the expression condition of TRAF6 agreed with previous experiments [34]. It is worth mentioning that although the stroke includes ischemic stroke and hemorrhagic stroke, the studies about TRAF6 mainly focus on the aspect of ischemic cerebral injury at present, and the research on the relationship between TRAF6 and cerebral hemorrhage or subarachnoid hemorrhage has been reported rarely at home or abroad. There is a little research finding that TRAF6 takes part in the process of hemorrhagic stroke through its E3 ubiquitin ligase activity, TRAF6 can inhibit autophagy and promote oxidative stress, thus aggravating brain injury, but if the E3 ubiquitin ligase activity is inhibited by related drugs, such as zinc finger protein A20 or TRAF6 mutant that lacks E3 ubiquitin ligase activity, the condition of brain injury will be ameliorated [35, 36]. We need further studies to make clear that the role of TRAF6 in related signaling pathway is neuroprotection or nerve injury, which could provide a new target for the clinical prevention and treatment of stroke.
3.2. TRAF6 and Traumatic Brain Injury
Traumatic brain injury (TBI) is a kind of neurological injury with high morbidity and mortality, which may touch off a series of cellular and molecular events. Typical features of TBI include neurons apoptosis, inflammatory response, the activation of microglia and the proliferation of reactive astrocytes following few hours to few days after the appearance of injury [37-39], and all of these changes mentioned above would further cause dysfunction of histiocytes, thus damaging cognition, emotion and behavior function [40]. According to the degree of injury, TBI could be classified to be mild, moderate and severe, however, no matter how severe the injury is, the most common performance of TBI is cell death [41]. Although the distribution and function of TRAF6 in CNS have not been fully understood, a large number of studies have confirmed that TRAF6 is necessary for the development of CNS and apoptosis. Depending on its characterized C-terminal TRAF domain and N-terminal ring finger domain, TRAF6 can directly integrate with the death domain of caspase-8, thus activating and regulating apoptosis. Some western blot analysis showed that the expression level of TRAF6 increased after 7 days of brain trauma and then declined gradually. Further study also found that, compared with contralateral brain tissues, TRAF6 manifested accumulation by using immunohistochemistry and immunofluorescence technology [42]. In addition, there are studies showing that the cell death caused by traumatic brain injury is connected to the activation of Akt [43, 44]. We have known that the important step of the activation of Akt is that it must translocate to cytomembrane from cytoplasm, then it can be phosphorylated and activated through interacting with the growth factors existing on the cell surface [45]. In this progress, with the help of E3 ubiquitin ligase activity, TRAF6 could make Akt ubiquitination and translocate to the position of phosphorylation, thus indirectly regulating apoptosis. Once TRAF6 has started lacking, the whole progress will be damaged [46]. Here, it is necessary to say that the realization of TRAF6 ubiquitination depends on the role of K63-associated ubiquitination, but not K48-associated ubiquitination, so the stability of Akt will not be affected [46]. Furthermore, it has been known that NF-κB signaling pathway plays an important role in CNS, involving apoptosis, inflammatory response, traumatic brain injury, viral infection, cell proliferation and tumorigenesis, and TRAF6 activates NF-κB and JNK, thus playing its role in the physiopathological mechanism of CNS diseases [42].
3.3. TRAF6 and Neurodegenerative Diseases
Neurodegenerative disease is a kind of morbid state caused by the loss of related neurons and/or myelin sheath, as time goes on, the disease will deteriorate and cause dysfunction of tissues and organs. This disease can be divided into two classes: acute and chronic neurodegenerative diseases, and the former includes cerebral ischemia, brain injury, epilepsy, the latter includes Alzheimer’s disease, Parkinson’ disease, Huntington’ disease, amyotrophic lateral sclerosis and different types of spinocerebellar ataxias. Here, we mainly introduce Alzheimer’s disease (AD), Parkinson’ disease (PD) and Multiple Sclerosis (MS). Alzheimer’s disease is the most common type of neurodegenerative diseases and the main cause of dementia. There is no agreement about its pathogenesis, which involves the theory of cholinergic, the theory of β-amyloid cascade, the theory of oxidative stress and other multiple fronts [47]. There are mounting evidence that autophagy plays an important role in AD, which may be inhibited in the course of disease [48-50], and Beclin-1 plays a significant role in the process of autophagy by acting as adaptin as well [51]. Researchers have revealed that in the process of AD, TLR4 is activated firstly, then interacts with Beclin-1, and further activates TRAF6 through MyD88 and TRIF, which could make Beclin-1 ubiquitination and inhibit oligomerization and autophagy, thus resulting in nerve injury [52, 53]. In addition, there is also evidence showing that TRAF6 can mediate the inflammatory response through TLR4/TRAF6/IKK/NF-κB signaling pathway [54]. Moreover, in Alzheimer’s disease, a large number of activated astrocytes and microglia can be found aroundβ-amyloid plaques, which induce the production of inflammatory cytokines such as IL-1B and TNF-α, and TRAF6 is indispensable in this process [55]. Parkinson’ disease is a kind of progressive neurodegenerative disease characterized by the selective loss of dopaminergic neurons in the nigrostriatal pathway and the occurrence of eosinophilic inclusions called Lewy bodies in the remaining neuronal cytoplasm. Although most of the PD patients are sporadic, the gene mutation of PARK7/DJ-1 is connected to autosomal recessive early-onset PD, while the missense mutation of aSYN (PARK1, PARK4) is associated with autosomal dominant inheritance of PD. We have known that the expression level of TRAF6 has been detected in nigral dopaminergic neurons [56], and further studies revealed that TRAF6 has closed links with DJ-1 and aSYN, all of which can be used as the substrate of E3 ubiquitin ligase activity of TRAF6. On the one hand, TRAF6 can promote the polyubiquitinated mutational DJ-1 enter into the cytoplasmic gather, on the other hand, in the post-mortem brain tissues of PD patients, researchers found that TRAF6 and aSYN co-exist in Lewy bodies [56, 57], these results show that TRAF6 may have something to do with Parkinson’ disease, but the concrete mechanism is still not clear, which needs further in-depth study. MS is a progressive, inflammatory and demyelinating disease of CNS, at present, the etiology MS is still unknown, but research has found that immune cells, especially monocytes and T cells, play an important role in the development of MS [58-60]. However, the report about TRAF6 and MS is little, research has only found that, in MS, TRAF6 interacts with CD40, ultimately monocytes recruitment and macrophage activation, and this result may be a potential direction for the therapy of MS [61].
3.4. TRAF6 and Neuropathic Pain
Neuropathic pain is caused by the primary or secondary damage or dysfunction of peripheral of CNS, which is always a problem besetting medical profession [62]. At present, the pathogenesis of neuropathic pain is not clear, but in recent years, there are mounting studies showing that glial cells, including astrocytes and microglia in the CNS, play an important role in the occurrence and development of neuropathic pain [63-65]. As neuropathic pain occurs, the activation of glial cells causes the expression of TLR4 receptor [66, 67], further activates the cellular kinases, such as MAPKs [68-70], and upregulates the expression of inflammatory mediators, for instance, TNF-α and IL-1B [71, 72]. Subsequently, TRAF6 is activated, which could latterly activate the JNK in the glial cells and mediated the production of chemokine CCL2 [73, 74], and TNF-α, IL-1B and CCL2 have been proven to relieve the neuropathic pain [75-77]. In addition, after spinal nerve ligation(SNL), the expression level of TRAF6 increases significantly, while the expression of TRAF6 is inhibited, the neuropathic pain caused by SNL can be alleviated effectively [73]. Further experiments show that TRAF6 can be found in both astrocytes and microglia at 3 days following neuropathic pain, and mainly exists in astrocytes at 10 days [73]. All these results reveal that TRAF6 may play an important role in the pathogenesis of neuropathic pain, but the specific mechanism still needs to be elucidated clearly.
3.5. TRAF6 and CNS Tumors
CNS tumors, especially malignant tumors, are always threatening to human health, despite efforts being made to improve therapeutic strategies constantly, the average survival of malignant tumors patients have only been slightly improved, and people are still trying to search the approach to improve the prognosis of patients. In this respect, little has been reported about the relationship between TRFA6 and CNS tumors. miR-146b-5p, a kind of miRNA, can inhibit glioma cells proliferation and induce apoptosis, and TRFA6 is a direct functional target of miR-146b-5p in gliomas, silencing of TRAF6 can achieve anti-tumor effects [78]. In addition, some researchers report that, based on its E3 ubiquitin ligase activity, TRAF6 mediates the stimulation of AKT, thus promotes tumorigenesis through EGFR/AKT pathway [79]. These findings suggest that TRAF6 can be a potential therapeutic target for CNS tumors, though the study about it is not deep.
Conclusion
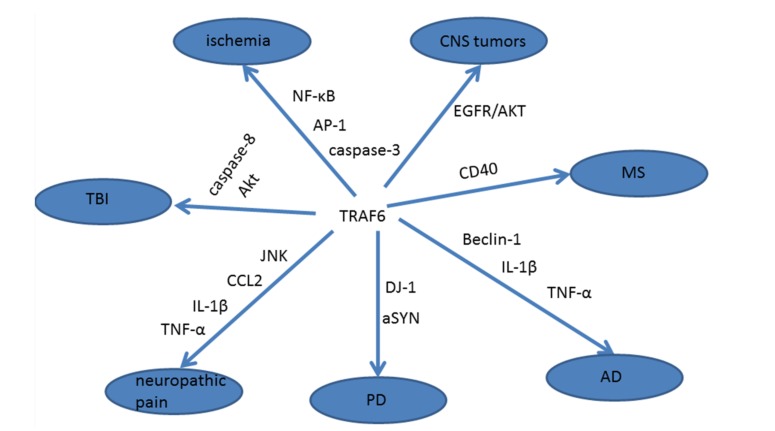
Schematic of the involvement of TRAF6 in nervous system diseases was shown in Fig. (1), and the progress in TRAF6 research was shown in Table 1. In summary, as the important and multifunctional intracellular adaptin, TRAF6 plays an important role in CNS diseases, such as stroke, traumatic brain injury, neurodegenerative diseases, neuropathic pain and CNS tumors, and the effective regulation of TRAF6 may contribute to the prevention and treatment of the CNS diseases, but it must be realized that TRAF6 is an important multifunctional signal molecule in cells, the research about the role of TRAF6 in various signaling pathways and the interaction of TRAF6 with other signaling molecule is not enough, the understanding and clinical application of TRAF6 are especially scanty. We have known that TRAF6 has characteristic E3 ubiquitin ligase activity, it may become a breakthrough point for the research of TRAF6, and contribute to the intensive study of TRAF6 with related diseases.
Fig. (1).
The relationship between TRAF6 and some central nervous system diseases.
Acknowledgements
This work was supported by supported by the Project of Jiangsu Provincial Medical Innovation Team (CXTDA2017003), Jiangsu Provincial Medical Youth Talent (QNRC2016728), Suzhou Key Medical Center (Szzx201501), grant from the National Natural Science Foundation of China (NO. 81571121), the Natural Science Foundation of Jiangsu Province (No. BK20170363), Scientific Department of Jiangsu Province (No. BL2014045), Suzhou Government (No. SYS201608, and LCZX201601), Jiangsu Province (No. 16KJB320008).
List of Abbreviations
- AD
Alzheimer’s Disease
- CNS
Central Nervous System
- IL-1
Interleukin-1
- IRAK
IL-1 Receptor Associated Kinase
- JNK
Jun N-Terminal Kinase
- MAPK
Mitogen Activated Protein Kinase
- MS
Multiple Sclerosis
- mTOR
Mammalian Target of Rapamycin
- NF-κB
Nuclear Factor-kappa B
- PD
Parkinson’ Disease
- PI3K
Phosphatidyl Inositol 3 Kinase
- ROS
Reactive Oxygen Species
- SNL
Spinal Nerve Ligation
- TBI
Traumatic Brain Injury
- TIR
Toll/IL-1 Receptor
- TLR4
Loll Like Receptor 4
- TNF
Tumor Necrosis Factor
- TRAF
Tumor Necrosis Factor Receptor-associated Factor
Consent for Publication
Not applicable.
Conflict of Interest
The authors declare no conflict of interest, financial or otherwise.
References
- 1.Zapata J.M., Martínez-García V., Lefebvre S. Phylogeny of the TRAF/MATH domain. Adv. Exp. Med. Biol. 2007;597:1–24. doi: 10.1007/978-0-387-70630-6_1. [http://dx.doi.org/10.1007/978-0-387-70630-6_1]. [PMID: 17633013]. [DOI] [PubMed] [Google Scholar]
- 2.Ha H., Han D., Choi Y. TRAF-mediated TNFR-family signaling. 2009. [DOI] [PubMed] [Google Scholar]
- 3.Lee N.K., Lee S.Y. Modulation of life and death by the tumor necrosis factor receptor-associated factors (TRAFs). J. Biochem. Mol. Biol. 2002;35(1):61–66. doi: 10.5483/bmbrep.2002.35.1.061. [PMID: 16248971]. [DOI] [PubMed] [Google Scholar]
- 4.Ishida T., Mizushima Si., Azuma S., Kobayashi N., Tojo T., Suzuki K., Aizawa S., Watanabe T., Mosialos G., Kieff E., Yamamoto T., Inoue J. Identification of TRAF6, a novel tumor necrosis factor receptor-associated factor protein that mediates signaling from an amino-terminal domain of the CD40 cytoplasmic region. J. Biol. Chem. 1996;271(46):28745–28748. doi: 10.1074/jbc.271.46.28745. [http://dx.doi. org/10.1074/jbc.271.46.28745]. [PMID: 8910514]. [DOI] [PubMed] [Google Scholar]
- 5.Cao Z., Xiong J., Takeuchi M., Kurama T., Goeddel D.V. TRAF6 is a signal transducer for interleukin-1. Nature. 1996;383(6599):443–446. doi: 10.1038/383443a0. [http://dx.doi.org/10.1038/383443a0]. [PMID: 8837778]. [DOI] [PubMed] [Google Scholar]
- 6.Lee N.K., Lee S.Y. Modulation of life and death by the tumor necrosis factor receptor-associated factors (TRAFs). J. Biochem. Mol. Biol. 2002;35(1):61–66. doi: 10.5483/bmbrep.2002.35.1.061. [PMID: 16248971]. [DOI] [PubMed] [Google Scholar]
- 7.Vallabhapurapu S., Karin M. Regulation and function of NF-kappaB transcription factors in the immune system. Annu. Rev. Immunol. 2009;27:693–733. doi: 10.1146/annurev.immunol.021908.132641. [http://dx.doi.org/10.1146/annurev. immunol.021908.132641]. [PMID: 19302050]. [DOI] [PubMed] [Google Scholar]
- 8.Cabal-Hierro L., Lazo P.S. Signal transduction by tumor necrosis factor receptors. Cell. Signal. 2012;24(6):1297–1305. doi: 10.1016/j.cellsig.2012.02.006. [http://dx. doi.org/10.1016/j.cellsig.2012.02.006]. [PMID: 22374304]. [DOI] [PubMed] [Google Scholar]
- 9.Jakus P.B., Kalman N., Antus C., Radnai B., Tucsek Z., Gallyas F., Jr, Sumegi B., Veres B. TRAF6 is functional in inhibition of TLR4-mediated NF-κB activation by resveratrol. J. Nutr. Biochem. 2013;24(5):819–823. doi: 10.1016/j.jnutbio.2012.04.017. [http://dx.doi.org/10.1016/j.jnutbio. 2012.04.017]. [PMID: 22925919]. [DOI] [PubMed] [Google Scholar]
- 10.Chandel N.S., Schumacker P.T., Arch R.H. Reactive oxygen species are downstream products of TRAF-mediated signal transduction. J. Biol. Chem. 2001;276(46):42728–42736. doi: 10.1074/jbc.M103074200. [http://dx. doi.org/10.1074/jbc.M103074200]. [PMID: 11559697]. [DOI] [PubMed] [Google Scholar]
- 11.West A.P., Brodsky I.E., Rahner C., Woo D.K., Erdjument-Bromage H., Tempst P., Walsh M.C., Choi Y., Shadel G.S., Ghosh S. TLR signalling augments macrophage bactericidal activity through mitochondrial ROS. Nature. 2011;472(7344):476–480. doi: 10.1038/nature09973. [http://dx.doi.org/10.1038/nature09973]. [PMID: 21525932]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Noguchi T., Takeda K., Matsuzawa A., Saegusa K., Nakano H., Gohda J., Inoue J., Ichijo H. Recruitment of tumor necrosis factor receptor-associated factor family proteins to apoptosis signal-regulating kinase 1 signalosome is essential for oxidative stress-induced cell death. J. Biol. Chem. 2005;280(44):37033–37040. doi: 10.1074/jbc.M506771200. [http://dx.doi.org/10.1074/jbc.M506771200]. [PMID: 16129676]. [DOI] [PubMed] [Google Scholar]
- 13.Lin C.C., Lee I.T., Yang Y.L., Lee C.W., Kou Y.R., Yang C.M. Induction of COX-2/PGE(2)/IL-6 is crucial for cigarette smoke extract-induced airway inflammation: Role of TLR4-dependent NADPH oxidase activation. Free Radic. Biol. Med. 2010;48(2):240–254. doi: 10.1016/j.freeradbiomed.2009.10.047. [http://dx.doi.org/10.1016/j.freeradbiomed. 2009.10.047]. [PMID: 19892012]. [DOI] [PubMed] [Google Scholar]
- 14.Jung C.H., Ro S.H., Cao J., Otto N.M., Kim D.H. mTOR regulation of autophagy. FEBS Lett. 2010;584(7):1287–1295. doi: 10.1016/j.febslet.2010.01.017. [http:// dx.doi.org/10.1016/j.febslet.2010.01.017]. [PMID: 20083114]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nazio F., Strappazzon F., Antonioli M., Bielli P., Cianfanelli V., Bordi M., Gretzmeier C., Dengjel J., Piacentini M., Fimia G.M., Cecconi F. mTOR inhibits autophagy by controlling ULK1 ubiquitylation, self-association and function through AMBRA1 and TRAF6. Nat. Cell Biol. 2013;15(4):406–416. doi: 10.1038/ncb2708. [http://dx.doi.org/ 10.1038/ncb2708]. [PMID: 23524951]. [DOI] [PubMed] [Google Scholar]
- 16.Linares J.F., Duran A., Yajima T., Pasparakis M., Moscat J., Diaz-Meco M.T. K63 polyubiquitination and activation of mTOR by the p62-TRAF6 complex in nutrient-activated cells. Mol. Cell. 2013;51(3):283–296. doi: 10.1016/j.molcel.2013.06.020. [http://dx.doi.org/10.1016/j.molcel.2013.06. 020]. [PMID: 23911927]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Koh S.H., Park H.H. Neurogenesis in stroke recovery. Transl. Stroke Res. 2017;8(1):3–13. doi: 10.1007/s12975-016-0460-z. [http://dx.doi.org/10.1007/s12975-016-0460-z]. [PMID: 26987852]. [DOI] [PubMed] [Google Scholar]
- 18.Alhadidi Q., Bin S.M.S., Shah Z.A. Cofilin as a Promising therapeutic target for ischemic and hemorrhagic stroke. Transl. Stroke Res. 2016;7(1):33–41. doi: 10.1007/s12975-015-0438-2. [http://dx.doi.org/10.1007/s12975-015-0438-2]. [PMID: 26670926]. [DOI] [PubMed] [Google Scholar]
- 19.Zhu W., Casper A., Libal N.L., Murphy S.J., Bodhankar S., Offner H., Alkayed N.J. Preclinical evaluation of recombinant T cell receptor ligand RTL1000 as a therapeutic agent in ischemic stroke. Transl. Stroke Res. 2015;6(1):60–68. doi: 10.1007/s12975-014-0373-7. [http://dx.doi.org/10. 1007/s12975-014-0373-7]. [PMID: 25270354]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu X., Zhao S., Liu F., Kang J., Xiao A., Li F., Zhang C., Yan F., Zhao H., Luo M., Luo Y., Ji X. Remote ischemic postconditioning alleviates cerebral ischemic injury by attenuating endoplasmic reticulum stress-mediated apoptosis. Transl. Stroke Res. 2014;5(6):692–700. doi: 10.1007/s12975-014-0359-5. [http://dx.doi.org/10.1007/s12975-014-0359-5]. [PMID: 25043802]. [DOI] [PubMed] [Google Scholar]
- 21.Seifert H.A., Pennypacker K.R. Molecular and cellular immune responses to ischemic brain injury. Transl. Stroke Res. 2014;5(5):543–553. doi: 10.1007/s12975-014-0349-7. [http://dx.doi.org/10.1007/s12975-014-0349-7]. [PMID: 24895236]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.O’Collins V.E., Macleod M.R., Donnan G.A., Horky L.L., van der Worp B.H., Howells D.W. 1,026 experimental treatments in acute stroke. Ann. Neurol. 2006;59(3):467–477. doi: 10.1002/ana.20741. [http://dx.doi.org/ 10.1002/ana.20741]. [PMID: 16453316]. [DOI] [PubMed] [Google Scholar]
- 23.Ross A.M., Hurn P., Perrin N., Wood L., Carlini W., Potempa K. Evidence of the peripheral inflammatory response in patients with transient ischemic attack. J. Stroke Cerebrovasc. Dis. 2007;16(5):203–207. doi: 10.1016/j.jstrokecerebrovasdis.2007.05.002. [http://dx.doi.org/10.1016/j.jstrokecerebrovasdis. 2007.05.002]. [PMID: 17845917]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huang J., Upadhyay U.M., Tamargo R.J. Inflammation in stroke and focal cerebral ischemia. Surg. Neurol. 2006;66(3):232–245. doi: 10.1016/j.surneu.2005.12.028. [http://dx.doi.org/10.1016/j.surneu.2005.12.028]. [PMID: 16935624]. [DOI] [PubMed] [Google Scholar]
- 25.Barr T.L., Conley Y., Ding J., Dillman A., Warach S., Singleton A., Matarin M. Genomic biomarkers and cellular pathways of ischemic stroke by RNA gene expression profiling. Neurology. 2010;75(11):1009–1014. doi: 10.1212/WNL.0b013e3181f2b37f. [http://dx.doi.org/10.1212/WNL.0b013 e3181f2b37f]. [PMID: 20837969]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wu D., Sheu J.S., Liu H.C., Yuan R.Y., Yu J.M., Sheu J.J., Hung C.H., Hu C.J. Increase of toll-like receptor 4 but decrease of interleukin-8 mRNA expression among ischemic stroke patients under aspirin treatment. Clin. Biochem. 2012;45(16-17):1316–1319. doi: 10.1016/j.clinbiochem.2012.04.022. [http://dx.doi.org/10.1016/j.clinbiochem.2012.04.022]. [PMID: 22580394]. [DOI] [PubMed] [Google Scholar]
- 27.Liu Z., He D., Zhang X., Li Y., Zhu C., Dong L., Zhang X., Xing Y., Wang C., Qiao H., Chen L. Neuroprotective effect of early and short-time applying sophoridine in pMCAO rat brain: down-regulated TRAF6 and up-regulated p-ERK1/2 expression, ameliorated brain infaction and edema. Brain Res. Bull. 2012;88(4):379–384. doi: 10.1016/j.brainresbull.2012.04.003. [http://dx.doi.org/10.1016/j.brainresbull.2012.04.003]. [PMID: 22521762]. [DOI] [PubMed] [Google Scholar]
- 28.Wu D., Lee Y.G., Liu H.C., Yuan R.Y., Chiou H.Y., Hung C.H., Hu C.J. Identification of TLR downstream pathways in stroke patients. Clin. Biochem. 2013;46(12):1058–1064. doi: 10.1016/j.clinbiochem.2013.05.059. [http:// dx.doi.org/10.1016/j.clinbiochem.2013.05.059]. [PMID: 23726813]. [DOI] [PubMed] [Google Scholar]
- 29.Nakka V.P., Gusain A., Mehta S.L., Raghubir R. Molecular mechanisms of apoptosis in cerebral ischemia: multiple neuroprotective opportunities. Mol. Neurobiol. 2008;37(1):7–38. doi: 10.1007/s12035-007-8013-9. [http://dx. doi.org/10.1007/s12035-007-8013-9]. [PMID: 18066503]. [DOI] [PubMed] [Google Scholar]
- 30.Kobayashi T., Walsh M.C., Choi Y. The role of TRAF6 in signal transduction and the immune response. Microbes Infect. 2004;6(14):1333–1338. doi: 10.1016/j.micinf.2004.09.001. [http://dx.doi.org/10.1016/j.micinf.2004.09.001]. [PMID: 15555541]. [DOI] [PubMed] [Google Scholar]
- 31.Wu H., Arron J.R. TRAF6, a molecular bridge spanning adaptive immunity, innate immunity and osteoimmunology. BioEssays. 2003;25(11):1096–1105. doi: 10.1002/bies.10352. [http://dx.doi.org/10.1002/bies.10352]. [PMID: 14579250]. [DOI] [PubMed] [Google Scholar]
- 32.Gohda J., Matsumura T., Inoue J. Cutting edge: TNFR-associated factor (TRAF) 6 is essential for MyD88-dependent pathway but not toll/IL-1 receptor domain-containing adaptor-inducing IFN-beta (TRIF)-dependent pathway in TLR signaling. J. Immunol. 2004;173(5):2913–2917. doi: 10.4049/jimmunol.173.5.2913. [http://dx.doi.org/10.4049/jimmunol.173.5.2913]. [PMID: 15322147]. [DOI] [PubMed] [Google Scholar]
- 33.Loniewski K.J., Patial S., Parameswaran N. Sensitivity of TLR4- and -7-induced NF kappa B1 p105-TPL2-ERK pathway to TNF-receptor-associated-factor-6 revealed by RNAi in mouse macrophages. Mol. Immunol. 2007;44(15):3715–3723. doi: 10.1016/j.molimm.2007.04.002. [http://dx.doi. org/10.1016/j.molimm.2007.04.002]. [PMID: 17507094]. [DOI] [PubMed] [Google Scholar]
- 34.Yuan P., Liu Z., Liu M., Huang J., Li X., Zhou X. Up-regulated tumor necrosis factor-associated factor 6 level is correlated with apoptosis in the rat cerebral ischemia and reperfusion. Neurol. Sci. 2013;34(7):1133–1138. doi: 10.1007/s10072-012-1199-2. [http://dx.doi.org/10.1007/s10072-012-1199-2]. [PMID: 23001490]. [DOI] [PubMed] [Google Scholar]
- 35.Dou Y., Shen H., Feng D., Li H., Tian X., Zhang J., Wang Z., Chen G. Tumor necrosis factor receptor-associated factor 6 participates in early brain injury after subarachnoid hemorrhage in rats through inhibiting autophagy and promoting oxidative stress. J. Neurochem. 2017;142(3):478–492. doi: 10.1111/jnc.14075. [http://dx.doi.org/10.1111/jnc. 14075]. [PMID: 28543180]. [DOI] [PubMed] [Google Scholar]
- 36.Meng Z., Zhao T., Zhou K., Zhong Q., Wang Y., Xiong X., Wang F., Yang Y., Zhu W., Liu J., Liao M., Wu L., Duan C., Li J., Gong Q., Liu L., Xiong A., Yang M., Wang J., Yang Q. A20 ameliorates intracerebral hemorrhage-induced inflammatory Injury by Regulating TRAF6 polyubiquitination. J. Immunol. 2017;198(2):820–831. doi: 10.4049/jimmunol.1600334. [http://dx.doi.org/10.4049/jimmunol.1600334]. [PMID: 27986908]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yu T.S., Zhang G., Liebl D.J., Kernie S.G. Traumatic brain injury-induced hippocampal neurogenesis requires activation of early nestin-expressing progenitors. J. Neurosci. 2008;28(48):12901–12912. doi: 10.1523/JNEUROSCI.4629-08.2008. [http://dx.doi.org/10.1523/JNEUROSCI.4629-08. 2008]. [PMID: 19036984]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Di Giovanni S., Movsesyan V., Ahmed F., Cernak I., Schinelli S., Stoica B., Faden A.I. Cell cycle inhibition provides neuroprotection and reduces glial proliferation and scar formation after traumatic brain injury. Proc. Natl. Acad. Sci. USA. 2005;102(23):8333–8338. doi: 10.1073/pnas.0500989102. [http://dx.doi.org/10.1073/pnas.0500989102]. [PMID: 15923260]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fawcett J.W., Asher R.A. The glial scar and central nervous system repair. Brain Res. Bull. 1999;49(6):377–391. doi: 10.1016/s0361-9230(99)00072-6. [http://dx.doi. org/10.1016/S0361-9230(99)00072-6]. [PMID: 10483914]. [DOI] [PubMed] [Google Scholar]
- 40.Zhang Y.P., Cai J., Shields L.B., Liu N., Xu X.M., Shields C.B. Traumatic brain injury using mouse models. Transl. Stroke Res. 2014;5(4):454–471. doi: 10.1007/s12975-014-0327-0. [http://dx.doi.org/10.1007/s12975-014-0327-0]. [PMID: 24493632]. [DOI] [PubMed] [Google Scholar]
- 41.Raghupathi R., Graham D.I., McIntosh T.K. Apoptosis after traumatic brain injury. J. Neurotrauma. 2000;17(10):927–938. doi: 10.1089/neu.2000.17.927. [http://dx.doi.org/10.1089/neu.2000.17.927]. [PMID: 11063058]. [DOI] [PubMed] [Google Scholar]
- 42.Chen J., Wu X., Shao B., Zhao W., Shi W., Zhang S., Ni L., Shen A. Increased expression of TNF receptor-associated factor 6 after rat traumatic brain injury. Cell. Mol. Neurobiol. 2011;31(2):269–275. doi: 10.1007/s10571-010-9617-6. [http://dx.doi.org/10.1007/s10571-010-9617-6]. [PMID: 21072581]. [DOI] [PubMed] [Google Scholar]
- 43.Farook J.M., Shields J., Tawfik A., Markand S., Sen T., Smith S.B., Brann D., Dhandapani K.M., Sen N. GADD34 induces cell death through inactivation of Akt following traumatic brain injury. Cell Death Dis. 2013;4:e754. doi: 10.1038/cddis.2013.280. [http://dx.doi.org/10.1038/cddis. 2013.280]. [PMID: 23907468]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Noshita N., Lewén A., Sugawara T., Chan P.H. Akt phosphorylation and neuronal survival after traumatic brain injury in mice. Neurobiol. Dis. 2002;9(3):294–304. doi: 10.1006/nbdi.2002.0482. [http://dx.doi.org/10.1006/ nbdi.2002.0482]. [PMID: 11950275]. [DOI] [PubMed] [Google Scholar]
- 45.Filippa N., Sable C.L., Hemmings B.A., Van Obberghen E. Effect of phosphoinositide-dependent kinase 1 on protein kinase B translocation and its subsequent activation. Mol. Cell. Biol. 2000;20(15):5712–5721. doi: 10.1128/mcb.20.15.5712-5721.2000. [http://dx.doi.org/10.1128/MCB.20.15.5712-5721.2000]. [PMID: 10891507]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yang W.L., Wang J., Chan C.H., Lee S.W., Campos A.D., Lamothe B., Hur L., Grabiner B.C., Lin X., Darnay B.G., Lin H.K. The E3 ligase TRAF6 regulates Akt ubiquitination and activation. Science. 2009;325(5944):1134–1138. doi: 10.1126/science.1175065. [http://dx.doi.org/ 10.1126/science.1175065]. [PMID: 19713527]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cupino T.L., Zabel M.K. Alzheimer’s silent partner: cerebral amyloid angiopathy. Transl. Stroke Res. 2014;5(3):330–337. doi: 10.1007/s12975-013-0309-7. [http://dx.doi.org/10.1007/s12975-013-0309-7]. [PMID: 24323728]. [DOI] [PubMed] [Google Scholar]
- 48.Nixon R.A. Autophagy, amyloidogenesis and Alzheimer disease. J. Cell Sci. 2007;120(Pt 23):4081–4091. doi: 10.1242/jcs.019265. [http://dx.doi.org/10. 1242/jcs.019265]. [PMID: 18032783]. [DOI] [PubMed] [Google Scholar]
- 49.Nixon R.A., Yang D.S. Autophagy failure in Alzheimer’s disease--locating the primary defect. Neurobiol. Dis. 2011;43(1):38–45. doi: 10.1016/j.nbd.2011.01.021. [http://dx.doi.org/10.1016/j.nbd.2011.01.021]. [PMID: 21296668]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.García-Arencibia M., Hochfeld W.E., Toh P.P., Rubinsztein D.C. Autophagy, a guardian against neurodegeneration. Semin. Cell Dev. Biol. 2010;21(7):691–698. doi: 10.1016/j.semcdb.2010.02.008. [http://dx.doi.org/10.1016/ j.semcdb.2010.02.008]. [PMID: 20188203]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Salminen A., Kaarniranta K., Kauppinen A., Ojala J., Haapasalo A., Soininen H., Hiltunen M. Impaired autophagy and APP processing in Alzheimer’s disease: The potential role of Beclin 1 interactome. Prog. Neurobiol. 2013;106-107:33–54. doi: 10.1016/j.pneurobio.2013.06.002. [http://dx.doi.org/ 10.1016/j.pneurobio.2013.06.002]. [PMID: 23827971]. [DOI] [PubMed] [Google Scholar]
- 52.Shi C.S., Kehrl J.H. MyD88 and Trif target Beclin 1 to trigger autophagy in macrophages. J. Biol. Chem. 2008;283(48):33175–33182. doi: 10.1074/jbc.M804478200. [http://dx.doi.org/10.1074/jbc.M804478200]. [PMID: 18772134]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shi C.S., Kehrl J.H. TRAF6 and A20 regulate lysine 63-linked ubiquitination of Beclin-1 to control TLR4-induced autophagy. Sci. Signal. 2010;3(123):ra42. doi: 10.1126/scisignal.2000751. [http://dx.doi.org/10.1126/scisignal. 2000751]. [PMID: 20501938]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Olajide O.A., Kumar A., Velagapudi R., Okorji U.P., Fiebich B.L. Punicalagin inhibits neuroinflammation in LPS-activated rat primary microglia. Mol. Nutr. Food Res. 2014;58(9):1843–1851. doi: 10.1002/mnfr.201400163. [http://dx.doi.org/10.1002/mnfr.201400163]. [PMID: 25066095]. [DOI] [PubMed] [Google Scholar]
- 55.Akama K.T., Van Eldik L.J. Beta-amyloid stimulation of inducible nitric-oxide synthase in astrocytes is interleukin-1beta- and tumor necrosis factor-alpha (TNFalpha)-dependent, and involves a TNFalpha receptor-associated factor- and NFkappaB-inducing kinase-dependent signaling mechanism. J. Biol. Chem. 2000;275(11):7918–7924. doi: 10.1074/jbc.275.11.7918. [http://dx.doi.org/10.1074/jbc.275.11.7918]. [PMID: 10713108]. [DOI] [PubMed] [Google Scholar]
- 56.Zucchelli S., Codrich M., Marcuzzi F., Pinto M., Vilotti S., Biagioli M., Ferrer I., Gustincich S. TRAF6 promotes atypical ubiquitination of mutant DJ-1 and alpha-synuclein and is localized to Lewy bodies in sporadic Parkinson’s disease brains. Hum. Mol. Genet. 2010;19(19):3759–3770. doi: 10.1093/hmg/ddq290. [http://dx.doi.org/10.1093/hmg/ ddq290]. [PMID: 20634198]. [DOI] [PubMed] [Google Scholar]
- 57.Vilotti S., Codrich M., Dal Ferro M., Pinto M., Ferrer I., Collavin L., Gustincich S., Zucchelli S. Parkinson’s disease DJ-1 L166P alters rRNA biogenesis by exclusion of TTRAP from the nucleolus and sequestration into cytoplasmic aggregates via TRAF6. PLoS One. 2012;7(4):e35051. doi: 10.1371/journal.pone.0035051. [http://dx.doi.org/10.1371/ journal.pone.0035051]. [PMID: 22532838]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Compston A., Coles A. Multiple sclerosis. Lancet. 2008;372(9648):1502–1517. doi: 10.1016/S0140-6736(08)61620-7. [http://dx.doi.org/10.1016/S0140-6736(08) 61620-7]. [PMID: 18970977]. [DOI] [PubMed] [Google Scholar]
- 59.De Jager P.L., Hafler D.A. New therapeutic approaches for multiple sclerosis. Annu. Rev. Med. 2007;58:417–432. doi: 10.1146/annurev.med.58.071105.111552. [http://dx.doi. org/10.1146/annurev.med.58.071105.111552]. [PMID: 17217332]. [DOI] [PubMed] [Google Scholar]
- 60.Stys P.K., Zamponi G.W., van Minnen J., Geurts J.J. Will the real multiple sclerosis please stand up? Nat. Rev. Neurosci. 2012;13(7):507–514. doi: 10.1038/nrn3275. [http://dx.doi.org/10.1038/nrn3275]. [PMID: 22714021]. [DOI] [PubMed] [Google Scholar]
- 61.Aarts S.A.B.M., Seijkens T.T.P., Kusters P.J.H., van der Pol S.M.A., Zarzycka B., Heijnen P.D.A.M., Beckers L., den Toom M., Gijbels M.J.J., Boon L., Weber C., de Vries H.E., Nicolaes G.A.F., Dijkstra C.D., Kooij G., Lutgens E. Inhibition of CD40-TRAF6 interactions by the small molecule inhibitor 6877002 reduces neuroinflammation. J. Neuroinflammation. 2017;14(1):105. doi: 10.1186/s12974-017-0875-9. [http://dx.doi.org/10.1186/s12974-017-0875-9]. [PMID: 28494768]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dworkin R.H., Backonja M., Rowbotham M.C., Allen R.R., Argoff C.R., Bennett G.J., Bushnell M.C., Farrar J.T., Galer B.S., Haythornthwaite J.A., Hewitt D.J., Loeser J.D., Max M.B., Saltarelli M., Schmader K.E., Stein C., Thompson D., Turk D.C., Wallace M.S., Watkins L.R., Weinstein S.M. Advances in neuropathic pain: diagnosis, mechanisms, and treatment recommendations. Arch. Neurol. 2003;60(11):1524–1534. doi: 10.1001/archneur.60.11.1524. [http:// dx.doi.org/10.1001/archneur.60.11.1524]. [PMID: 14623723]. [DOI] [PubMed] [Google Scholar]
- 63.Ji R.R., Berta T., Nedergaard M. Glia and pain: is chronic pain a gliopathy? Pain. 2013;154(Suppl. 1):S10–S28. doi: 10.1016/j.pain.2013.06.022. [http://dx.doi. org/10.1016/j.pain.2013.06.022]. [PMID: 23792284]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Moalem G., Tracey D.J. Immune and inflammatory mechanisms in neuropathic pain. Brain Res. Brain Res. Rev. 2006;51(2):240–264. doi: 10.1016/j.brainresrev.2005.11.004. [http://dx.doi.org/10.1016/j.brainresrev.2005.11.004]. [PMID: 16388853]. [DOI] [PubMed] [Google Scholar]
- 65.Tsuda M., Beggs S., Salter M.W., Inoue K. Microglia and intractable chronic pain. Glia. 2013;61(1):55–61. doi: 10.1002/glia.22379. [http://dx.doi.org/ 10.1002/glia.22379]. [PMID: 22740331]. [DOI] [PubMed] [Google Scholar]
- 66.Tanga F.Y., Raghavendra V., DeLeo J.A. Quantitative real-time RT-PCR assessment of spinal microglial and astrocytic activation markers in a rat model of neuropathic pain. Neurochem. Int. 2004;45(2-3):397–407. doi: 10.1016/j.neuint.2003.06.002. [http://dx.doi.org/10.1016/j.neuint.2003.06.002]. [PMID: 15145554]. [DOI] [PubMed] [Google Scholar]
- 67.Tanga F.Y., Nutile-McMenemy N., DeLeo J.A. The CNS role of Toll-like receptor 4 in innate neuroimmunity and painful neuropathy. Proc. Natl. Acad. Sci. USA. 2005;102(16):5856–5861. doi: 10.1073/pnas.0501634102. [http://dx.doi.org/10.1073/pnas.0501634102]. [PMID: 15809417]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jin S.X., Zhuang Z.Y., Woolf C.J., Ji R.R. p38 mitogen-activated protein kinase is activated after a spinal nerve ligation in spinal cord microglia and dorsal root ganglion neurons and contributes to the generation of neuropathic pain. J. Neurosci. 2003;23(10):4017–4022. doi: 10.1523/JNEUROSCI.23-10-04017.2003. [http://dx.doi.org/10.1523/JNEUROSCI.23-10-04017.2003]. [PMID: 12764087]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhuang Z.Y., Gerner P., Woolf C.J., Ji R.R. ERK is sequentially activated in neurons, microglia, and astrocytes by spinal nerve ligation and contributes to mechanical allodynia in this neuropathic pain model. Pain. 2005;114(1-2):149–159. doi: 10.1016/j.pain.2004.12.022. [http://dx.doi.org/10. 1016/j.pain.2004.12.022]. [PMID: 15733640]. [DOI] [PubMed] [Google Scholar]
- 70.Zhuang Z.Y., Wen Y.R., Zhang D.R., Borsello T., Bonny C., Strichartz G.R., Decosterd I., Ji R.R. A peptide c-Jun N-terminal kinase (JNK) inhibitor blocks mechanical allodynia after spinal nerve ligation: respective roles of JNK activation in primary sensory neurons and spinal astrocytes for neuropathic pain development and maintenance. J. Neurosci. 2006;26(13):3551–3560. doi: 10.1523/JNEUROSCI.5290-05.2006. [http://dx.doi.org/10.1523/JNEUROSCI.5290-05.2006]. [PMID: 16571763]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sweitzer S., Martin D., DeLeo J.A. Intrathecal interleukin-1 receptor antagonist in combination with soluble tumor necrosis factor receptor exhibits an anti-allodynic action in a rat model of neuropathic pain. Neuroscience. 2001;103(2):529–539. doi: 10.1016/s0306-4522(00)00574-1. [http://dx. doi.org/10.1016/S0306-4522(00)00574-1]. [PMID: 11246166]. [DOI] [PubMed] [Google Scholar]
- 72.Zhang Z.J., Cao D.L., Zhang X., Ji R.R., Gao Y.J. Chemokine contribution to neuropathic pain: respective induction of CXCL1 and CXCR2 in spinal cord astrocytes and neurons. Pain. 2013;154(10):2185–2197. doi: 10.1016/j.pain.2013.07.002. [http://dx.doi.org/10.1016/j.pain.2013.07.002]. [PMID: 23831863]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lu Y., Jiang B.C., Cao D.L., Zhang Z.J., Zhang X., Ji R.R., Gao Y.J. TRAF6 upregulation in spinal astrocytes maintains neuropathic pain by integrating TNF-α and IL-1β signaling. Pain. 2014;155(12):2618–2629. doi: 10.1016/j.pain.2014.09.027. [http://dx.doi.org/10.1016/j.pain.2014. 09.027]. [PMID: 25267210]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lu Y., Cao D.L., Jiang B.C., Yang T., Gao Y.J. MicroRNA-146a-5p attenuates neuropathic pain via suppressing TRAF6 signaling in the spinal cord. Brain Behav. Immun. 2015;49:119–129. doi: 10.1016/j.bbi.2015.04.018. [http://dx.doi.org/10.1016/j.bbi.2015.04.018]. [PMID: 25957028]. [DOI] [PubMed] [Google Scholar]
- 75.Gao Y.J., Zhang L., Samad O.A., Suter M.R., Yasuhiko K., Xu Z.Z., Park J.Y., Lind A.L., Ma Q., Ji R.R. JNK-induced MCP-1 production in spinal cord astrocytes contributes to central sensitization and neuropathic pain. J. Neurosci. 2009;29(13):4096–4108. doi: 10.1523/JNEUROSCI.3623-08.2009. [http://dx.doi.org/10.1523/JNEUROSCI.3623-08.2009]. [PMID: 19339605]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Milligan E.D., Twining C., Chacur M., Biedenkapp J., O’Connor K., Poole S., Tracey K., Martin D., Maier S.F., Watkins L.R. Spinal glia and proinflammatory cytokines mediate mirror-image neuropathic pain in rats. J. Neurosci. 2003;23(3):1026–1040. doi: 10.1523/JNEUROSCI.23-03-01026.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wei F., Guo W., Zou S., Ren K., Dubner R. Supraspinal glial-neuronal interactions contribute to descending pain facilitation. J. Neurosci. 2008;28(42):10482–10495. doi: 10.1523/JNEUROSCI.3593-08.2008. [http://dx.doi.org/10.1523/ JNEUROSCI.3593-08.2008]. [PMID: 18923025]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Liu J., Xu J., Li H., Sun C., Yu L., Li Y., Shi C., Zhou X., Bian X., Ping Y., Wen Y., Zhao S., Xu H., Ren L., An T., Wang Q., Yu S. miR-146b-5p functions as a tumor suppressor by targeting TRAF6 and predicts the prognosis of human gliomas. Oncotarget. 2015;6(30):29129–29142. doi: 10.18632/oncotarget.4895. [http://dx.doi.org/10. 18632/oncotarget.4895]. [PMID: 26320176]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Feng H., Lopez G.Y., Kim C.K., Alvarez A., Duncan C.G., Nishikawa R., Nagane M., Su A.J., Auron P.E., Hedberg M.L., Wang L., Raizer J.J., Kessler J.A., Parsa A.T., Gao W.Q., Kim S.H., Minata M., Nakano I., Grandis J.R., McLendon R.E., Bigner D.D., Lin H.K., Furnari F.B., Cavenee W.K., Hu B., Yan H., Cheng S.Y. EGFR phosphorylation of DCBLD2 recruits TRAF6 and stimulates AKT-promoted tumorigenesis. J. Clin. Invest. 2014;124(9):3741–3756. doi: 10.1172/JCI73093. [http://dx.doi.org/10.1172/JCI73093]. [PMID: 25061874]. [DOI] [PMC free article] [PubMed] [Google Scholar]