Abstract
Mammalian STE20-like kinase-1 (Mst1) is a generally expressed apoptosis-promoting kinase and a key bridge-builder of apoptotic signaling in the etiology of tissue injury. Despite the fact that the biological function of Mst1 and its role in the cell's signalling network have yet to be determined, however, there is a lot of evidence that Mst1 plays an important role in cell death which results from tissue injury. Previous studies have shown that Mst1 is not only a target for some apop-tosis-related molecules such as caspase 3 and P53, but also act as an activator of these proteinases to magnify apoptosis sig-nal pathways. This article reviews the role of Mst1 in the signaling pathways which is related with the neuronal cell apopto-sis or microglia activation following myocardial and brain injury. Therefore, this work contributes to better understanding of the pathological process of myocardial and brain injury
Keywords: Ischemia-reperfusion injury, ischemic stroke, oxidative stress, Mst1, apopotosis, microglia
1. Introduction
Hypoxic-ischemic cardio-cerebrovascular diseases has become the highest morbidity, disability and mortality of the disease [1-3]. The best approach to rescue myocardial and brain ischemia patients is to restore myocardial and brain tissue blood supply timely. But at the same time bringing new damage to myocardial and brain, that is ischemia-reperfusion (I-R) injury [4]. Although significant advances in the technological and medical treatments have been made, many patients still suffer from varying degrees of dysfunction, which directly weakens their quality of life [5]. Therefore, it is extremely important to find effective strategies to improve the functional recovery of stroke patients.
The mechanism of myocardial and cerebral IR injury refers to oxidative stress, mitochondrial dysfunction, cell inflammation and apoptosis, a series of cell and molecular biology events [6, 7]. In the previous studies, Oxidative stress is still regarded as a major contributor to cell death during I-R injury, although different mechanisms of cell death are likely simultaneously activated [8, 9]. Oxidative stress leads to the gene expression not only in innate immunity but programmed cell death and has an effect on cell survival and homeostasis [10, 11]. Although many research results of oxidative stress injury have been reported and it is well established that a number of related regulators in vivo, but remarkably little is known about the mechanisms underlying the biological effects of oxidative stress.
In 1996, Lu et al. indicated that apoptosis of some cell lines like NIH-3T3, HL-60, and human prostatic tumour (LnCap), nduced by an array of apoptotic stimuli was connected with the activation of a phosphorylated myelin basic protein kinase which wieight 36 kDa [12]. Although they postulated that the 36 kDa myelin basic protein kinase is a common component in some signalling pathways of cell apoptosis and that maybe it is a cleavage product of a higher molecular weight enzyme, until a long time later, the protein was identified as a cleavage fragment of Mst1. Actually, this enzyme had only been described by Creasy and Chernoff a year earlier [13, 14]. Mst1 is widely known as a major component of the Hippo signaling pathway to mediate organs size and homeostasis by regulating cell proliferation and differentiation [15-17]. Recently, there are emerging studies that have shown that Mst1 plays an important role in regulating inflammation, stress response and apoptosis in myocardial injury, spinal cord injury and brain injury [18, 19]. Mst1 exerts apoptotic effect by being activated by its activator, autophosphorylation and in turn phosphorylation of downstream targets such as FOXO3 (Forkhead box O3) and Bcl-xL [20, 21]. Recently, Mst1 has been proven to play a crucial role in neuronal cell death which is induced by oxidative stress [8, 22, 23]. In conclusion, there is growing evidence indicating that Mst1 plays an important role in cell death caused by I-R injury.
2. Mst1 and cerebral ischemia-reperfusion injury
During the past decade, Cerebral ischemic stroke has become a major public health issue characterized by high rates of morbidity, mortality and disability [24]. In cerebral I-R-induced injury, even though the neuronal cell death has always been the focus of investigation [25, 26], a growing number of evidences demonstrate that microglial act as the critical regulator of extracellular environment of neurons, its activation plays a paramount role in neuronal cell death caused by I-R injury [8, 11]. In the resting state, microglia are the common immune cells of the central nervous system and have an action of immunologic surveillance [27]. When I-R injury occurs, oxidative stress leads to neuronal cell death and releases damage-associated molecular pattern molecules (DAMPs) and purines (ATP) that activate scavenger receptors and Toll-like receptors (TLRs) in microglia, leading to the accumulation of activated microglia around the injury region [28]. In addition, another similar study demonstrated that in response to infection or brain injury, microglial migrate to the injury region of the central nervous system, where they accumulate and become activated, the accumulation of microglial cells subsequently clear up the dead cells and cell debris [29]. Nevertheless, the inundatory microglial activation may have a detrimental effect on neurons [8, 30], which can be indicative of, besides the decrease of neuronal cell death, a potential therapeutic strategy that may inhibit microglial activation in the therapy of the stroke.
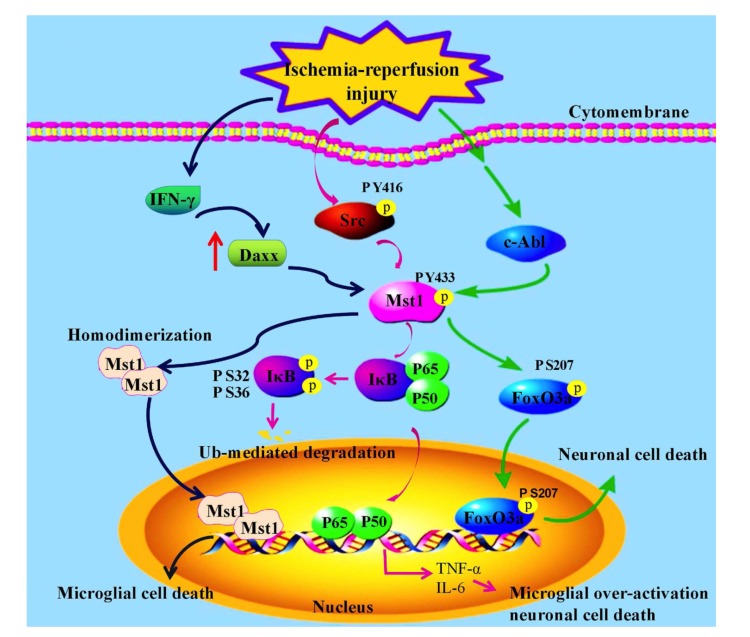
Mst1 is well known as a Pro-apoptotic molecule and is commonly expressed in mammalian cells. In cerebral IR injury, Tyrosine kinase c-Abl phosphorylates Mst1 at Y-433 and activates Mst1, in turn Mst1 phosphorylates forkhead box O1/3 (FoxO1/3) transcription factors at serine 207 [8, 31], thereby promoting nuclear translocation of FoxO1/3 and activates the Mst1-FoxO1/3 signaling pathway and may directly activate the neuronal cell death program by promoting BIM expression [11]. Recently, studies confirmed that post-translational modification of FoxO3 distinctly and differentially regulate microglial functions according to a time-dependent manner of oxygen-glucose deprivation (OGD), an in vitro model of ischemia [11, 32]. FoxO3a stimulates the activation and proliferation of microglia at the early stages of OGD, however, at later phase after OGD, induces apoptosis of microglial [11] (Fig. 1). In conclusion, these findings demonstrate that c-Abl/Mst1/FoxO3 signaling pathway plays a considerable role in regulating different microglial functions.
Fig. (1).
The roles of Mst1 in cerebral ischemia-reperfusion injury.
Furthermore, in OGD-BV-2 cells model, microglia were activated and divided into two phenotypes, which are termed as M1 microglia and M2 microglia [33, 34]. Although M1 microglia plays an important role in clearing injured tissue fragments and pathogens, meanwhile, these microglia could also evoke new injury to surrounding neurons because M1 microglia can release some kinds of inflammatory cytokines. On the contrary, M2 microglia are essential in reducing inflammatory response and tissue protection because of its function of release neuroprotective factors. Therefore, inducing activated microglia toward M2 state will contribute to reduced neuronal damage, evoked by cerebral ischemic injury. Recently, there is evidence showing that Mst1 participates in the activation of microglia and its transition to M2 state in OGD-BV-2 cells model [35]. In this model, the expression level of p-MST1-Y433 was significantly increased following OGD. Besides, Malibatol A could promote the expression of M2 microglia makers instead of M1 state by inhibiting the expression of p-MST1(Y433) induced by OGD in BV-2 cells, thereby reducing BV-2 cells apoptosis.
In addition, recent studies have shown that in microglia, specific absence of Mst1 reduces stroke-induced brain injury [15]. Mst1 regulates the stroke-induced activation of microglia by direct phosphorylation of IκBa at serine residues S32 and S36. Further study reveal that Src kinase directly phosphorylates Mst1 at Y433 and activates Mst1, so Src kinase plays a key role in the upstream of the Mst1–IκB signal during microglia activation [36] (Fig. 1). The phosphorylation and degradation of IκB is a pointer of NF-κB activation and an inhibitor of NF-κB during ischemic stroke [37]. In particular, the activation of NF-κB has an effect on delaying inflammation and neurotoxicity [38]. Consequently, Src-Mst1-IκB signaling pathway plays a significant role in stroke-induced microglial activation.
The above studies show that phosphorylated Mst1 promotes the activation of microglia. Interestingly, homologous dimerization of Mst1 inhibits microglial activation in inflammatory stimuli or tissue injury. As an example, Hee Jae Yun et al. [30] have shown that in answer to tissue injury or inflammatory stimulimulation, microglia is activated and releases both neurotrophic and neurotoxic substances subsequently [39]. Consistent activation of microglia for a long time may trigger non-reversible damage to neurons and lead to neurological diseases [40-43]. Daxx and Mst1 regulate apoptosis which is caused by interferon-γ(IFN-γ) in microglia [30]. The expression level of Daxx is upregulated by IFN-γ [44], then the high level of Daxx can lead to the homodimerization of Mst1 and activates Mst1 [30]. Subsequently, activated Mst1 dimer nuclear translocation and promote induced microglia death. Knockdown Daxx also weakens IFN-γ causing cell death in rat microglia. Additionally, deficiency of Mst1, the death of microglia induced by IFN-γ was meaningfully reduced compared with wild-type mice [30] (Fig. 1). Consequently, dimerized Mst1 is a pivotal mediator of microglia death caused by the inflammatory factor.
In summary, phosphorylated Mst1 promotes the activation of microglia, leading to neuronal cell death in cerebral I-R injury or stroke, whereas dimerization of Mst1 inhibits the activation of microglia caused by tissue injury or inflammatory reactionfile://localhost/F/:%25E8%25BD%25AF%25E4%25BB%25B6%25E5%25AE%2589%25E8%25A3%2585:Dict:6.3.69.8341:resultui:frame:javascript/void(0)%3B.
4. Mst1 and myocardial ischemia reperfusion injury
In recent several years, there has been an increasing awareness of Ischemic heart disease which is a significant component of cardiovascular disease and a primary cause of death worldwide [45, 46]. Although many approaches in understanding the causes of ischemic heart disease have been discovered, the mechanism of myocardial I-R injury has not yet been enough elucidated. Consequently, the treatment method of myocardial I-R injury is immature at present.
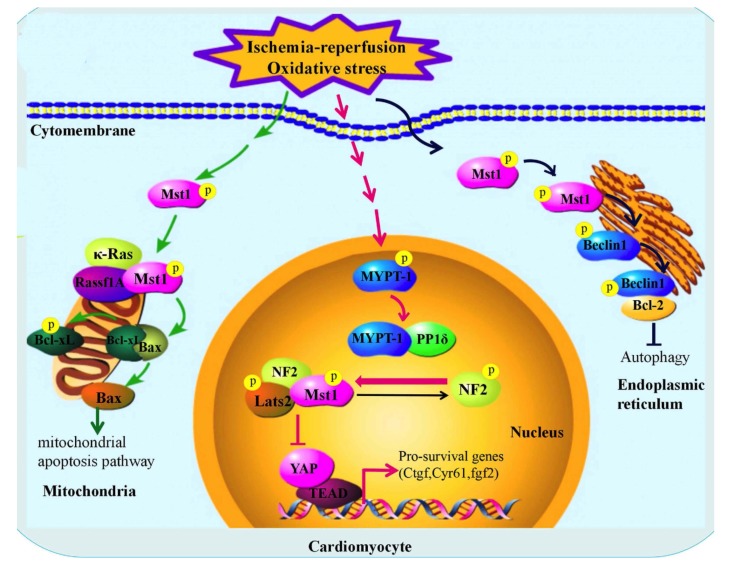
In response to a variety of stresses, including myocardial I-R, volume overload and pressure, Mst1 is activated in myocardial cells, through many different kinds of molecular mechanisms involving mechanical stress, cytokines and oxidative stress [47, 48]. Mst1 can be translocated to the nucleus, the ER and mitochondria, although is localized primarily in the cytoplasm and activated by scaffolding proteins, like NF2 and Rassf1A, by different mechanisms [49, 50]. Particularly, Under the stimulation of myocardial I-R injury Mst1moved to the mitochondria in cardiomyocytes, and activated by Rassf1A and K-Ras-dependent regulatory mechanisms. In turn, the activated Mst1 phosphorylates Bcl-xL at Ser14 [47, 51]. On account of the phosphorylation at the Ser14 of Bcl-xL which resulted in the dissociation of Bcl-xL from B cell leukemia/lymphoma 2–associated (Bcl-2–associated) protein x (Bax), accordingly activating Bax and inducing mitochondrial apoptotic pathways [51, 52]. In addition, in Mst1 knockout mice, deficiency of Ser14 phosphorylation of Bcl-xL obviously weakens I-R injury and the development of cardiomyopathy, because the phosphorylation of Bcl-xL at Ser14 has completely disappeared due to the lack of Mst1 [45]. Therefore, the function of Mst1 in response to myocardial I-R is particularly mediated by Ser14 phosphorylation of Bcl-xL. In knockin (KI) mice, Ser14 of Bcl-xL is replaced by Ala, so Mst1 cannot phosphorylate Bcl-xL and unsuccessfully activates mitochondrial apoptosis pathway [47]. The in vivo data further indicate that significant protection represents that the phosphorylation of Bcl-xL at Ser14 is the master mechanism mediating the pro-apoptotic function of Mst1 during myocardial I-R injury (Fig. 2). Consequently, inhibition of Mst1 is able to reduce the I-R injury and forestalls cardiac remodeling/dysfunction after chronic myocardial infarction, suggesting that Mst1 is a promising target of cardiac therapy for ischemic heart injury.
Fig. (2).
The roles of Mst1 in myocardial ischemia-reperfusion injury.
Similarly, another work has demonstrated that in response to oxidative stress caused by myocardial I-R injury, Mst1 is activated both in mouse myocardium in vivo and cultured cardiomyocytes [49]. In order to simulate oxidative stress during I-R, treatment of neonatal rat ventricular myocytes (NRVMs) was conducted with Hydrogen Peroxide and induced activation of MYPT-1, showing a lack of Ser696 phosphorylation of MYPT-1 indicative of its activation [53]. Previous work identified that PP1-MYPT-1 was identified as NF2 activator by dephosphorylation of Ser518 in Drosophila and in mammalian cells [54]. So, MYPT-1 is activated by oxidative stress, mediated NF2 dephosphorylation and activation in cardiomyocytes. In NRVMs, overexpression of NF2 promoted Mst1 activation [50]. Mst1 is known to promote cell death [20, 55], therefore, through evaluated cardiomyocyte apoptosis in the case of increased NF2 expression, it was found that increased NF2 expression prompted a significant increase in TUNEL-positive cardiomyocytes, and this reaction is greatly weakened through inhibition of Mst1. Similarly, NF2 activates caspase-3, which was meaningfully attenuated by inhibition of Mst1 [56]. On the other hand, reducing the level of endogenous NF2 using siRNA weakens both the activation of Mst1 and cardiomyocyte death induced by myocardial I-R injury [10, 49]. Mst1 is known as an important part of the Hippo pathway, and NF2 can promote cardiomyocyte death through engaging Hippo signaling at the level of Mst1. Furthermore, NF2 forms a complex with Mst1 and Lats2 which are the main components of Hippo pathway, then the complex inhibits nuclear transferation of YAP which is another important component of hippo pathway [57, 58] (Fig. 2), therefore, YAP cannot initiate the transcription of the pro-survival genes such as ctgf, cyr61, finally, loss of cytoprotective effect.
Upregulation of autophagy during myocardial I-R injury [2, 59]. Autophagy within the normal physiological range is usually beneficial to clear protein aggregates and damaged mitochondria. However, Mst1 suppresses autophagy and promotes apoptosis by phosphorylating Beclin1 and inducing homodimerization of Beclin1 [60]. The conclusion reiterates that Mst1 is a promising target of myocardial I-R injury.
In summary, the above research proves that Ser14 sites of endogenous Bcl-xL is a critical target of Mst1 and that it plays an essential role in mediating the effect of Mst1 in I-R injury. Oxidative stress stimulates dephosphorylation of NF2 via MYPT-1-PP1 thereby promoting an active conformation of NF2. Activated NF2 forms a complex with Mst1 and Lats2 in the cardiomyocyte nucleus, promotes Mst1 activation, and negatively regulates YAP target gene expression, leading to YAP which cannot initiate the transcription of the pro-survival genes such as ctgf and cyr61, eventually leading to cell death. In addition, Mst1 also inhibits autophagy during myocardial I-R injury.
Conclusion and Prospective
Research in recent years has discovered several interacting proteins and downstream signal pathways of Mst1 in IR injury or stroke, cerebral infraction and myocardial infraction following reperfusion, which are both typical I-R injury. This review summarizes a novel role of Mst1 in microglia activation, apoptosis and neuronal cell death during I-R injury or stroke. Mst1 activation appears to be linked to different molecules activation such as Src, c-Abl and Daxx under the conditions of oxidative stress induced by I-R injury or stroke. The main contents of this review can be summarized as follows: (1) Phosphorylated Mst1 at Y433 has an activating effect on microglia and promotes neuron death, whereas dimerization of Mst1 from the cytoplasm into the nucleus, inhibits microglia activation and promotes its death during brain I-R injury. (2) Under the stimulation of oxidative stress, phosphorylated Mst1 enters mitochondria and interacts with molecules such as Bax and Rassf1A to induce mitochondrial apoptosis, and phosphorylated Mst1 can also enter the nucleus, and form a complex with Lats2 and NF2 in the regulation of MYPT-1, thereby blocking the regulation of YAP on pro-survival genes transcription, leading to apoptosis. In addition, Mst1 also promotes cell death by inhibiting autophagy during myocardial I-R injury. Hence, these studies prove that Mst1 plays a central role in microglial activation, aoptosis induced by ischemic stroke or I-R injury, indicating that Mst1 is an effective target for the treatment of brain and myocardial I-R injury.
Although the studies of the mechanism of IR injury is deep and developed, the pivot point of the mechanism is not yet clear and needs further study. In cerebral I-R injury, Mst1 not only participates in the activation of microglia through the Src–Mst1–IκB signaling pathway, but is also involve in the death of primary rat microglia through the IFN-γ-Daxx-Mst1 signaling pathway. It is well known that M1 microglial can release toxic factors what would be detrimental for the survival of the surrounding neurons. Therefore, blocking Src-Mst1-IκB signaling pathway and meanwhile promoting IFN-γ-Daxx-Mst1 signaling pathway may be an effective way to treat activated microglia-induced neuronal apoptosis after I-R brain injury. In myocardial I-R injury, Mst1 not only participates in the regulation of transcription of some apoptosis-related molecules, but also involves in cell death through activation of mitochondrial apoptotic pathway and autophagy pathway. Therefore, affecting targeted silencing Mst1 or inhibiting its phosphorylation and blocking the related pro-apoptotic pathway may be a new direction for the effective treatment of I-R injury.
In addition, the drugs of heart and cerebral I-R injury which are obtained from animal experiment are not able to achieve the desired effect in clinical trials, this may be related to complicated mechanism of I-R injury, single-drug approach is not sufficient to combat the pathophysiological changes of cardiovascular and cerebrovascular disease caused by reperfusion. So the physiological, pharmacological and thrombolytic therapy should be combined with the the clinical treatment trends of heart and cerebral I-R injury. How to fully and effectively use the known mechanism of I-R injury to prevent and treat ischemic cardio-cerebrovascular diseases requires further clinical practice.
Consent for Publication
Not applicable.
Acknowledgements
Declared none.
Conflict of Interest
The authors declare no conflict of interest, financial or otherwise.
References
- 1.Alhadidi Q., Bin Sayeed M.S., Shah Z.A. Cofilin as a promising therapeutic target for ischemic and hemorrhagic stroke. Transl. Stroke Res. 2016;7(1):33–41. doi: 10.1007/s12975-015-0438-2. [http://dx.doi.org/10.1007/s12975-015-0438-2]. [PMID: 26670926]. [DOI] [PubMed] [Google Scholar]
- 2.Wei H., Li Y., Han S., Liu S., Zhang N., Zhao L., Li S., Li J. cPKCγ-modulated autophagy in neurons alleviates ischemic injury in brain of mice with ischemic stroke through Akt-mTOR pathway. Transl. Stroke Res. 2016;7(6):497–511. doi: 10.1007/s12975-016-0484-4. [http://dx.doi.org/10. 1007/s12975-016-0484-4]. [PMID: 27510769]. [DOI] [PubMed] [Google Scholar]
- 3.Lapchak P.A. Critical early thrombolytic and endovascular reperfusion therapy for acute ischemic stroke victims: a call for adjunct neuroprotection. Transl. Stroke Res. 2015;6(5):345–354. doi: 10.1007/s12975-015-0419-5. [http:// dx.doi.org/10.1007/s12975-015-0419-5]. [PMID: 26314402]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nakata M., Nakagomi T., Maeda M., Nakano-Doi A., Momota Y., Matsuyama T. Induction of perivascular neural stem cells and possible contribution to neurogenesis following transient brain ischemia/reperfusion injury. Transl. Stroke Res. 2017;8(2):131–143. doi: 10.1007/s12975-016-0479-1. [http://dx.doi.org/10.1007/s12975-016-0479-1]. [PMID: 27352866]. [DOI] [PubMed] [Google Scholar]
- 5.Ahnstedt H., Sweet J., Cruden P., Bishop N., Cipolla M.J. Effects of early post-ischemic reperfusion and tPA on cerebrovascular function and nitrosative stress in female ratS. Transl. Stroke Res. 2016;7(3):228–238. doi: 10.1007/s12975-016-0468-4. [http://dx.doi.org/10.1007/s12975-016-0468-4]. [PMID: 27125535]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhai M., Li B., Duan W., Jing L., Zhang B., Zhang M., Yu L., Liu Z., Yu B., Ren K., Gao E., Yang Y., Liang H., Jin Z., Yu S. Melatonin ameliorates myocardial ischemia reperfusion injury through SIRT3-dependent regulation of oxidative stress and apoptosis. J. Pineal Res. 2017;63(2) doi: 10.1111/jpi.12419. [http://dx.doi.org/10.1111/jpi. 12419]. [PMID: 28500761]. [DOI] [PubMed] [Google Scholar]
- 7.Bellanti F. Hypoxia-inducible factor-1 in myocardial ischaemia/ reperfusion injury. Acta Physiol. (Oxf.) 2017;221(2):93–94. doi: 10.1111/apha.12903. [http://dx. doi.org/10.1111/apha.12903]. [PMID: 28581154]. [DOI] [PubMed] [Google Scholar]
- 8.Lee S.J., Seo B.R., Choi E.J., Koh J.Y. The role of reciprocal activation of cAbl and Mst1 in the oxidative death of cultured astrocytes. Glia. 2014;62(4):639–648. doi: 10.1002/glia.22631. [http://dx.doi.org/10.1002/ glia.22631]. [PMID: 24464935]. [DOI] [PubMed] [Google Scholar]
- 9.Wakayama K., Shimamura M., Suzuki J.I., Watanabe R., Koriyama H., Akazawa H., Nakagami H., Mochizuki H., Isobe M., Morishita R., Angiotensin I.I. Angiotensin II peptide vaccine protects ischemic brain through reducing oxidative stress. Stroke. 2017;48(5):1362–1368. doi: 10.1161/STROKEAHA.116.016269. [http://dx.doi.org/10.1161/STROKEAHA. 116.016269]. [PMID: 28364024]. [DOI] [PubMed] [Google Scholar]
- 10.Zhang R., Xu M., Wang Y., Xie F., Zhang G., Qin X. Nrf2-a promising therapeutic target for defensing against oxidative stress in stroke. Mol. Neurobiol. 2016 doi: 10.1007/s12035-016-0111-0. [PMID: 27696223]. [DOI] [PubMed] [Google Scholar]
- 11.Weng L., Wu Z., Zheng W., Meng H., Han L., Wang S., Yuan Z., Xu Y. Malibatol A enhances alternative activation of microglia by inhibiting phosphorylation of Mammalian Ste20-like kinase1 in OGD-BV-2 cells. Neurol. Res. 2016;38(4):342–348. doi: 10.1080/01616412.2016.1174423. [http://dx. doi.org/10.1080/01616412.2016.1174423]. [PMID: 27098434]. [DOI] [PubMed] [Google Scholar]
- 12.Lu M.L., Sato M., Cao B., Richie J.P. UV irradiation-induced apoptosis leads to activation of a 36-kDa myelin basic protein kinase in HL-60 cells. Proc. Natl. Acad. Sci. USA. 1996;93(17):8977–8982. doi: 10.1073/pnas.93.17.8977. [http://dx.doi.org/10.1073/pnas.93.17.8977]. [PMID: 8799139]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Creasy C.L., Chernoff J. Cloning and characterization of a human protein kinase with homology to Ste20. J. Biol. Chem. 1995;270(37):21695–21700. doi: 10.1074/jbc.270.37.21695. [http://dx.doi.org/10.1074/jbc.270.37.21695]. [PMID: 7665586]. [DOI] [PubMed] [Google Scholar]
- 14.Creasy C.L., Chernoff J. Cloning and characterization of a member of the MST subfamily of Ste20-like kinases. Gene. 1995;167(1-2):303–306. doi: 10.1016/0378-1119(95)00653-2. [http://dx.doi.org/10.1016/0378-1119(95)00653-2]. [PMID: 8566796]. [DOI] [PubMed] [Google Scholar]
- 15.Fallahi E., O’Driscoll N.A., Matallanas D. The MST/Hippo Pathway and Cell Death: A Non-Canonical Affair. Genes (Basel) 2016;7(6):E28. doi: 10.3390/genes7060028. [http://dx.doi.org/10.3390/genes7060028]. [PMID: 27322327]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.de Souza P.M., Lindsay M.A. Mammalian Sterile20-like kinase 1 and the regulation of apoptosis. Biochem. Soc. Trans. 2004;32(Pt3):485–488. doi: 10.1042/BST0320485. [http://dx.doi.org/10.1042/bst0320485]. [PMID: 15157167]. [DOI] [PubMed] [Google Scholar]
- 17.Poon C.L., Mitchell K.A., Kondo S., Cheng L.Y., Harvey K.F. The hippo pathway regulates neuroblasts and brain size in Drosophila melanogaster. Curr. Biol. 2016;26(8):1034–1042. doi: 10.1016/j.cub.2016.02.009. [http://dx.doi.org/10.1016/j.cub.2016.02.009]. [PMID: 26996505]. [DOI] [PubMed] [Google Scholar]
- 18.Sciarretta S., Zhai P., Maejima Y., Del Re D.P., Nagarajan N., Yee D., Liu T., Magnuson M.A., Volpe M., Frati G., Li H., Sadoshima J. mTORC2 regulates cardiac response to stress by inhibiting MST1. Cell Reports. 2015;11(1):125–136. doi: 10.1016/j.celrep.2015.03.010. [http://dx.doi.org/ 10.1016/j.celrep.2015.03.010]. [PMID: 25843706]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang P.F., Xu D.Y., Zhang Y., Liu X.B., Xia Y., Zhou P.Y., Fu Q.G., Xu S.G. Deletion of mammalian sterile 20-like kinase 1 attenuates neuronal loss and improves locomotor function in a mouse model of spinal cord trauma. Mol. Cell. Biochem. 2017;431(1-2):11–20. doi: 10.1007/s11010-017-2969-1. [http://dx.doi.org/10.1007/s11010-017-2969-1]. [PMID: 28210902]. [DOI] [PubMed] [Google Scholar]
- 20.Ling P., Lu T.J., Yuan C.J., Lai M.D. Biosignaling of mammalian Ste20-related kinases. Cell. Signal. 2008;20(7):1237–1247. doi: 10.1016/j.cellsig.2007.12.019. [http://dx.doi.org/10.1016/j.cellsig.2007.12.019]. [PMID: 18255267]. [DOI] [PubMed] [Google Scholar]
- 21.Jang S.W., Yang S.J., Srinivasan S., Ye K. Akt phosphorylates MstI and prevents its proteolytic activation, blocking FOXO3 phosphorylation and nuclear translocation. J. Biol. Chem. 2007;282(42):30836–30844. doi: 10.1074/jbc.M704542200. [http://dx.doi.org/10.1074/jbc.M704542200]. [PMID: 17726016]. [DOI] [PubMed] [Google Scholar]
- 22.Xiao L., Chen D., Hu P., Wu J., Liu W., Zhao Y., Cao M., Fang Y., Bi W., Zheng Z., Ren J., Ji G., Wang Y., Yuan Z. The c-Abl-MST1 signaling pathway mediates oxidative stress-induced neuronal cell death. J. Neurosci. 2011;31(26):9611–9619. doi: 10.1523/JNEUROSCI.0035-11.2011. [http://dx.doi.org/10.1523/JNEUROSCI.0035-11.2011]. [PMID: 21715626]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lehtinen M.K., Yuan Z., Boag P.R., Yang Y., Villén J., Becker E.B., DiBacco S., de la Iglesia N., Gygi S., Blackwell T.K., Bonni A. A conserved MST-FOXO signaling pathway mediates oxidative-stress responses and extends life span. Cell. 2006;125(5):987–1001. doi: 10.1016/j.cell.2006.03.046. [http://dx.doi.org/10.1016/j.cell.2006.03.046]. [PMID: 16751106]. [DOI] [PubMed] [Google Scholar]
- 24.Zhu L., He D., Han L., Cao H. Stroke research in China over the past decade: analysis of NSFC funding. Transl. Stroke Res. 2015;6(4):253–256. doi: 10.1007/s12975-015-0404-z. [http://dx.doi.org/10.1007/s12975-015-0404-z]. [PMID: 26040422]. [DOI] [PubMed] [Google Scholar]
- 25.Madineni A., Alhadidi Q., Shah Z.A. Cofilin inhibition restores neuronal cell death in oxygen-glucose deprivation model of ischemia. Mol. Neurobiol. 2016;53(2):867–878. doi: 10.1007/s12035-014-9056-3. [http://dx.doi.org/ 10.1007/s12035-014-9056-3]. [PMID: 25526862]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Peng S., Zhao S., Yan F., Cheng J., Huang L., Chen H., Liu Q., Ji X., Yuan Z. HDAC2 selectively regulates FOXO3a-mediated gene transcription during oxidative stress-induced neuronal cell death. J. Neurosci. 2015;35(3):1250–1259. doi: 10.1523/JNEUROSCI.2444-14.2015. [http://dx. doi.org/10.1523/JNEUROSCI.2444-14.2015]. [PMID: 25609639]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu B., Hong J.S. Role of microglia in inflammation-mediated neurodegenerative diseases: mechanisms and strategies for therapeutic intervention. J. Pharmacol. Exp. Ther. 2003;304(1):1–7. doi: 10.1124/jpet.102.035048. [http://dx.doi.org/10.1124/jpet.102.035048]. [PMID: 12490568]. [DOI] [PubMed] [Google Scholar]
- 28.Jin X., Yamashita T. Microglia in central nervous system repair after injury. J. Biochem. 2016;159(5):491–496. doi: 10.1093/jb/mvw009. [http://dx.doi.org/ 10.1093/jb/mvw009]. [PMID: 26861995]. [DOI] [PubMed] [Google Scholar]
- 29.Schilling M., Besselmann M., Müller M., Strecker J.K., Ringelstein E.B., Kiefer R. Predominant phagocytic activity of resident microglia over hematogenous macrophages following transient focal cerebral ischemia: an investigation using green fluorescent protein transgenic bone marrow chimeric mice. Exp. Neurol. 2005;196(2):290–297. doi: 10.1016/j.expneurol.2005.08.004. [http://dx.doi.org/10.1016/j.expneurol.2005.08.004]. [PMID: 16153641]. [DOI] [PubMed] [Google Scholar]
- 30.Yun H.J., Yoon J.H., Lee J.K., Noh K.T., Yoon K.W., Oh S.P., Oh H.J., Chae J.S., Hwang S.G., Kim E.H., Maul G.G. ; Lim D.S., Choi E.J. Daxx mediates activation-induced cell death in microglia by triggering MST1 signalling. EMBO J. 2011;30(12):2465–2476. doi: 10.1038/emboj.2011.152. [http://dx.doi.org/10.1038/emboj.2011.152]. [PMID: 21572393]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yuan Z., Lehtinen M.K., Merlo P., Villén J., Gygi S., Bonni A. Regulation of neuronal cell death by MST1-FOXO1 signaling. J. Biol. Chem. 2009;284(17):11285–11292. doi: 10.1074/jbc.M900461200. [http://dx.doi.org/10. 1074/jbc.M900461200]. [PMID: 19221179]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Qin X., Sun Z.Q., Dai X.J., Mao S.S., Zhang J.L., Jia M.X., Zhang Y.M. Toll-like receptor 4 signaling is involved in PACAP-induced neuroprotection in BV2 microglial cells under OGD/reoxygenation. Neurol. Res. 2012;34(4):379–389. doi: 10.1179/1743132812Y.0000000028. [http://dx.doi.org/10.1179/1743132812Y.0000000028]. [PMID: 22643083]. [DOI] [PubMed] [Google Scholar]
- 33.Pérez-de Puig I., Miró F., Salas-Perdomo A., Bonfill-Teixidor E., Ferrer-Ferrer M., Márquez-Kisinousky L., Planas A.M. IL-10 deficiency exacerbates the brain inflammatory response to permanent ischemia without preventing resolution of the lesion. J. Cereb. Blood Flow Metab. 2013;33(12):1955–1966. doi: 10.1038/jcbfm.2013.155. [http://dx.doi.org/10. 1038/jcbfm.2013.155]. [PMID: 24022622]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lalancette-Hébert M., Swarup V., Beaulieu J.M., Bohacek I., Abdelhamid E., Weng Y.C., Sato S., Kriz J. Galectin-3 is required for resident microglia activation and proliferation in response to ischemic injury. J. Neurosci. 2012;32(30):10383–10395. doi: 10.1523/JNEUROSCI.1498-12.2012. [http://dx.doi.org/10.1523/JNEUROSCI.1498-12.2012]. [PMID: 22836271]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Weng L., Wu Z., Zheng W., Meng H., Han L., Wang S., Yuan Z., Xu Y. Malibatol A enhances alternative activation of microglia by inhibiting phosphorylation of Mammalian Ste20-like kinase1 in OGD-BV-2 cells. Neurol. Res. 2016;38(4):342–348. doi: 10.1080/01616412.2016.1174423. [http://dx. doi.org/10.1080/01616412.2016.1174423]. [PMID: 27098434]. [DOI] [PubMed] [Google Scholar]
- 36.Zhao S., Yin J., Zhou L., Yan F., He Q., Huang L., Peng S., Jia J., Cheng J., Chen H., Tao W., Ji X., Xu Y., Yuan Z. Hippo/MST1 signaling mediates microglial activation following acute cerebral ischemia-reperfusion injury. Brain Behav. Immun. 2016;55:236–248. doi: 10.1016/j.bbi.2015.12.016. [http://dx.doi.org/10.1016/j.bbi.2015.12.016]. [PMID: 26721416]. [DOI] [PubMed] [Google Scholar]
- 37.Herrmann O., Baumann B., de Lorenzi R., Muhammad S., Zhang W., Kleesiek J., Malfertheiner M., Köhrmann M., Potrovita I., Maegele I., Beyer C., Burke J.R., Hasan M.T., Bujard H., Wirth T., Pasparakis M., Schwaninger M. IKK mediates ischemia-induced neuronal death. Nat. Med. 2005;11(12):1322–1329. doi: 10.1038/nm1323. [http://dx.doi.org/10.1038/nm1323]. [PMID: 16286924]. [DOI] [PubMed] [Google Scholar]
- 38.Block M.L., Zecca L., Hong J.S. Microglia-mediated neurotoxicity: uncovering the molecular mechanisms. Nat. Rev. Neurosci. 2007;8(1):57–69. doi: 10.1038/nrn2038. [http://dx.doi.org/10.1038/nrn2038]. [PMID: 17180163]. [DOI] [PubMed] [Google Scholar]
- 39.Tang Z., Gan Y., Liu Q., Yin J.X., Liu Q., Shi J., Shi F.D. CX3CR1 deficiency suppresses activation and neurotoxicity of microglia/macrophage in experimental ischemic stroke. J. Neuroinflammation. 2014;11:26. doi: 10.1186/1742-2094-11-26. [http://dx.doi.org/10.1186/1742-2094-11-26]. [PMID: 24490760]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Guo S., Liu Y., Ma R., Li J., Su B. Neuroprotective effect of endogenous cannabinoids on ischemic brain injury induced by the excess microglia-mediated inflammation. Am. J. Transl. Res. 2016;8(6):2631–2640. [PMID: 27398146]. [PMC free article] [PubMed] [Google Scholar]
- 41.Hellström Erkenstam N., Smith P.L., Fleiss B., Nair S., Svedin P., Wang W., Boström M., Gressens P., Hagberg H., Brown K.L., Sävman K., Mallard C. Temporal characterization of microglia/macrophage phenotypes in a mouse model of neonatal hypoxic-Ischemic brain injury. Front. Cell. Neurosci. 2016;10:286. doi: 10.3389/fncel.2016.00286. [http://dx.doi.org/10.3389/fncel.2016.00286]. [PMID: 28018179]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang H., Guo W., Liu H., Zeng R., Lu M., Chen Z., Xiao Q. Inhibition of inflammatory mediator release from microglia can treat ischemic/hypoxic brain injury. Neural Regen. Res. 2013;8(13):1157–1168. doi: 10.3969/j.issn.1673-5374.2013.13.001. [PMID: 25206410]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kaur C., Ling E.A. Microglia--a therapeutic target in neurological diseases and disorders. CNS Neurol. Disord. Drug Targets. 2013;12(6):719. doi: 10.2174/18715273113126660179. [http://dx.doi.org/10.2174/18715273113126660179]. [PMID: 24047518]. [DOI] [PubMed] [Google Scholar]
- 44.Muromoto R., Ishida M., Sugiyama K., Sekine Y., Oritani K., Shimoda K., Matsuda T. Sumoylation of Daxx regulates IFN-induced growth suppression of B lymphocytes and the hormone receptor-mediated transactivation. J. Immunol. 2006;177(2):1160–1170. doi: 10.4049/jimmunol.177.2.1160. [http://dx.doi.org/10.4049/jimmunol.177.2.1160]. [PMID: 16818774]. [DOI] [PubMed] [Google Scholar]
- 45.Russo I., Penna C., Musso T., Popara J., Alloatti G., Cavalot F., Pagliaro P. Platelets, diabetes and myocardial ischemia/ reperfusion injury. Cardiovasc. Diabetol. 2017;16(1):71. doi: 10.1186/s12933-017-0550-6. [http:// dx.doi.org/10.1186/s12933-017-0550-6]. [PMID: 28569217]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hausenloy D.J., Yellon D.M. Myocardial ischemia-reperfusion injury: a neglected therapeutic target. J. Clin. Invest. 2013;123(1):92–100. doi: 10.1172/JCI62874. [http://dx.doi.org/10.1172/JCI62874]. [PMID: 23281415]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nakamura M., Zhai P., Del Re D.P., Maejima Y., Sadoshima J. Mst1-mediated phosphorylation of Bcl-xL is required for myocardial reperfusion injury. JCI Insight. 2016;1(5):e86217. doi: 10.1172/jci.insight.86217. [http://dx. doi.org/10.1172/jci.insight.86217]. [PMID: 27218122]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Del Re D.P., Matsuda T., Zhai P., Gao S., Clark G.J., Van Der Weyden L., Sadoshima J. Proapoptotic Rassf1A/Mst1 signaling in cardiac fibroblasts is protective against pressure overload in mice. J. Clin. Invest. 2010;120(10):3555–3567. doi: 10.1172/JCI43569. [http://dx.doi.org/10. 1172/JCI43569]. [PMID: 20890045]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Matsuda T., Zhai P., Sciarretta S., Zhang Y., Jeong J.I., Ikeda S., Park J., Hsu C.P., Tian B., Pan D., Sadoshima J., Del Re D.P. NF2 activates hippo signaling and promotes ischemia/reperfusion injury in the heart. Circ. Res. 2016;119(5):596–606. doi: 10.1161/CIRCRESAHA.116.308586. [http://dx.doi.org/10.1161/CIRCRESAHA.116.308586]. [PMID: 27402866]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mia M.M., Chelakkot-Govindalayathil A.L., Singh M.K. Targeting NF2-Hippo/Yap signaling pathway for cardioprotection after ischemia/reperfusion injury. Ann. Transl. Med. 2016;4(24):545. doi: 10.21037/atm.2016.11.85. [http://dx.doi.org/10.21037/atm.2016.11.85]. [PMID: 28149906]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Del Re D.P., Matsuda T., Zhai P., Maejima Y., Jain M.R., Liu T., Li H., Hsu C.P., Sadoshima J. Mst1 promotes cardiac myocyte apoptosis through phosphorylation and inhibition of Bcl-xL. Mol. Cell. 2014;54(4):639–650. doi: 10.1016/j.molcel.2014.04.007. [http://dx.doi.org/10.1016/ j.molcel.2014.04.007]. [PMID: 24813943]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Finucane D.M., Bossy-Wetzel E., Waterhouse N.J., Cotter T.G., Green D.R. Bax-induced caspase activation and apoptosis via cytochrome c release from mitochondria is inhibitable by Bcl-xL. J. Biol. Chem. 1999;274(4):2225–2233. doi: 10.1074/jbc.274.4.2225. [http://dx.doi.org/10.1074/ jbc.274.4.2225]. [PMID: 9890985]. [DOI] [PubMed] [Google Scholar]
- 53.Alp Y.F.I., Kaleli D. D.; Aypar, E.; Ark, M.; Özdemir, O.; Uydeş Doğan, B.S. Atorvastatin acutely reduces the reactivity to spasmogens in rat aorta: implication of the inhibition of geranylgeranylation and MYPT-1 phosphorylation. Fundam. Clin. Pharmacol. 2016;30(2):96–106. doi: 10.1111/fcp.12173. [http://dx.doi.org/10.1111/fcp.12173]. [PMID: 26610064]. [DOI] [PubMed] [Google Scholar]
- 54.Rong R., Surace E.I., Haipek C.A., Gutmann D.H., Ye K. Serine 518 phosphorylation modulates merlin intramolecular association and binding to critical effectors important for NF2 growth suppression. Oncogene. 2004;23(52):8447–8454. doi: 10.1038/sj.onc.1207794. [http://dx.doi.org/ 10.1038/sj.onc.1207794]. [PMID: 15378014]. [DOI] [PubMed] [Google Scholar]
- 55.Shi L., Al-Baadani A., Zhou K., Shao A., Xu S., Chen S., Zhang J. PCMT1 ameliorates neuronal apoptosis by inhibiting the activation of MST1 after subarachnoid hemorrhage in rats. Transl. Stroke Res. 2017 doi: 10.1007/s12975-017-0540-8. [Epub ahead of print]. [http://dx.doi.org/10.1007/ s12975-017-0540-8]. [PMID: 28534197]. [DOI] [PubMed] [Google Scholar]
- 56.Odashima M., Usui S., Takagi H., Hong C., Liu J., Yokota M., Sadoshima J. Inhibition of endogenous Mst1 prevents apoptosis and cardiac dysfunction without affecting cardiac hypertrophy after myocardial infarction. Circ. Res. 2007;100(9):1344–1352. doi: 10.1161/01.RES.0000265846.23485.7a. [http://dx.doi.org/10.1161/01.RES.0000265846.23485.7a]. [PMID: 17395874]. [DOI] [PubMed] [Google Scholar]
- 57.Heallen T., Morikawa Y., Leach J., Tao G., Willerson J.T., Johnson R.L., Martin J.F. Hippo signaling impedes adult heart regeneration. Development. 2013;140(23):4683–4690. doi: 10.1242/dev.102798. [http://dx. doi.org/10.1242/dev.102798]. [PMID: 24255096]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Xin M., Kim Y., Sutherland L.B., Murakami M., Qi X., McAnally J., Porrello E.R., Mahmoud A.I., Tan W., Shelton J.M., Richardson J.A., Sadek H.A., Bassel-Duby R., Olson E.N. Hippo pathway effector Yap promotes cardiac regeneration. Proc. Natl. Acad. Sci. USA. 2013;110(34):13839–13844. doi: 10.1073/pnas.1313192110. [http://dx.doi. org/10.1073/pnas.1313192110]. [PMID: 23918388]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Qi Z., Dong W., Shi W., Wang R., Zhang C., Zhao Y., Ji X., Liu K.J., Luo Y. Bcl-2 phosphorylation triggers autophagy switch and reduces mitochondrial damage in limb remote ischemic conditioned rats after ischemic stroke. Transl. Stroke Res. 2015;6(3):198–206. doi: 10.1007/s12975-015-0393-y. [http://dx.doi.org/10.1007/s12975-015-0393-y]. [PMID: 25744447]. [DOI] [PubMed] [Google Scholar]
- 60.Maejima Y., Kyoi S., Zhai P., Liu T., Li H., Ivessa A., Sciarretta S., Del Re D.P., Zablocki D.K., Hsu C.P., Lim D.S., Isobe M., Sadoshima J. Mst1 inhibits autophagy by promoting the interaction between Beclin1 and Bcl-2. Nat. Med. 2013;19(11):1478–1488. doi: 10.1038/nm.3322. [http://dx.doi.org/10.1038/nm.3322]. [PMID: 24141421]. [DOI] [PMC free article] [PubMed] [Google Scholar]