Abstract
The leucine-rich repeat kinase 2 (LRRK2) gene and α-synuclein gene (SNCA) are the key influencing factors of Parkinson’s disease (PD). It is reported that dysfunction of LRRK2 may influence the accumulation of α-synuclein and its pathology to alter cellular functions and signaling pathways by the kinase activation of LRRK2. The accumulation of α-synuclein is one of the main stimulants of microglial activation. Microglia are macrophages that reside in the brain, and acti-vation of microglia is believed to contribute to neuroinflammation and neuronal death in PD. Therefore, clarifying the com-plex relationship among LRRK2, α-synuclein and microglials could offer targeted clinical therapies for PD. Here, we provide an updated review focused on the discussion of the evidence supporting some of the key mechanisms that are important for LRRK2-dependent neurodegeneration in PD
Keywords: Leucine-rich repeat kinase 2, MAPK, α-synuclein, neurodegeneration, neuroinflammation, microglia, Parkinson’s disease
1. INTRODUCTION
Parkinson disease (PD), characterized by tremor, rigidity, bradykinesia and postural instability, is the second most common neurodegenerative disorder among the elderly population [1-4]. Decreased pigmentation in the substantia nigra pars compacta (SNpc) due to the loss of dopaminergic neurons is the most common pathological finding of PD [5]. Most PD cases occur in a sporadic manner while 5% to 10% of cases are inherited with mutations identified in several genes in families [6]. Although familial PD is relatively scarce, comprehending the function of mutated gene products will provide a precious opportunity to clarify the molecular pathogenic mechanisms and pathways underlying neuronal degeneration and to develop disease-modifying or neuroprotective therapies.
At least 20 genes are associated with familial PD, while >20 genetic risk loci have been reported from PD genome-wide association studies (GWAS) [5]. The most noticed genes are those encoding α-synuclein (SNCA), glucocerebrosidase (GBA), parkin (PARK2), Pten-induced kinase 1 (PINK1), microtubule-associated protein tau (MAPT) and leucine-rich repeat kinase 2 (LRRK2) [5]. Mutations in the LRRK2 gene have been recognized as genetic risk factors for both familial and sporadic forms of PD [7]. The structure of LRRK2 contains a combination of guanosine triphosphatase (GTPase), kinase, and scaffolding domains [8], and the pathological functions of LRRK2 have mainly been associated with aberrant kinase activity. In general, high kinase activity of LRRK2 pathogenic mutants has been associated with pathological features of PD, such as dopaminergic neuronal cell death, impaired dopamine neurotransmission, defects in protein synthesis and degradation, inflammatory responses, and oxidative damage [7, 9].
In-vitro studies indicated that LRRK2 induced Ser/Thr phosphorylation but not Tyr phosphorylation and G2019S mutation augmented LRRK2 kinase activity, which results in overphosphorylation of downstream mitogen-activated protein kinase (MAPK) kinase (MKK), and eventually leads to activation of neuronal death signal pathway [10]. In addition, LRRK2 is reported to be able to up-regulate SNCA transcription via specific activation of the extracellular signal-regulated kinases (ERK) cascade [11]. Lewy bodies (LBs) are the pathological hallmark of PD. α-synuclein, the major protein component of LBs, is one of the key molecules involved in familial and sporadic PD [12]. Aberrant accumulation of α-synuclein can promote both neuronal dysfunction and neuroinflammation by activating microglia abnormally, the resident immune cells of the brain [13]. Microglial activation is a normal biological process in which microglia respond to changes in their local environment, dynamically modifying their contributions to central nervous system function accordingly [14-16]. In other words, LRRK2 could mediate α-synuclein-induced microglial activation based on either a commonality of receptor pathways responding to α-synuclein, or a potential involvement in multiple microglial signaling cascades [17, 18]. This indicates that these PD-associated genes may impulse the progression of inflammation-mediated neurodegeneration via participating in abnormal immune responses [19]. Thus, in the present review, we will provide valuable insight into the MAPK kinase of LRRK2 and its emerging function in the regulation of SNCA and neuroinflammation in the molecular mechanisms underlying the pathogenesis of PD.
1.1. The Structure and Localization of LRRK2
LRRK2 is a large (2527 amino acids) protein consisting of several domains with different functions. The central portion of LRRK2 contains a Ras of Complex (Roc) GTPase and a C-terminus of Roc (COR) domain, followed by serine-threonine kinase domains. The ROC-COR bidomain and kinase region together constitute the catalytic core of LRRK2, which therefore encompasses two enzymatic activities [20, 21]. In addition, there are several protein–protein interaction domains including ankyrin and leucine-rich repeat motifs at the N-terminus, and WD40 repeats at the C-terminus [22].
LRRK2 is widely expressed in many tissues such as brain, heart, kidney and lungs [23]. It is also reported that in biofluids such as urine, cerebrospinal fluid (CSF) and blood, with LRRK2 found in peripheral blood mononuclear cells (PBMCs), including lymphocytes and monocytes [24]. In the mammalian brain, both mRNA and protein of LRRK2 have been detected highly expressed in dopamine-innervated areas such as cerebral cortex, striatum as well as in the cerebellum and hippocampus, while at low levels in dopaminergic neurons of the substantia nigra and ventral tegmental area [25-27]. In cells, LRRK2 is mainly found throughout the cytoplasm associated with various intracellular membranes and vesicular structures, such as lipid raft, early endosomes, lysosomes, plasma membrane and synaptic vesicles, as well as in the endoplasmic reticulum, Golgi complex and outer mitochondrial membrane [28, 29].
1.2. LRRK2 and Its Mutations in PD
Mutations in LRRK2 are account for 5–13% of familial PD and 1–5% of sporadic PD [20]. Seven of the reported missense LRRK2 mutations have been identified as pathogenic, including R1441G, R1441C, R1441H, Y1699C, G2019S, R1628P, G2385R and I2020T, which are located in different functional domains of LRRK2 [22, 30]. Interestingly, variants in LRRK2 are appeared to be population-specific. For example, the G2019S mutation (substitution of glycine 2019 with a serine) which leads to constitutive activation of the kinase is the most prevalent [31], and it contributes to ~36% of familial and sporadic PD in North Africa Arabs [32], ~30% of familial PD in Ashkenazi Jewish populations [33], up to 6% of familial cases in Europe and 3% of apparently sporadic PD in Europe and North America [34], but it is absent in Asian populations [35]. Several other LRRK2 mutations, including G2385R, R1628P, S1647T, R1398H and N551K, have been reported to be associated with PD in specific Asian populations [36-39]. Independent studies from Singapore, Taiwan and mainland China have demonstrated that the G2385R or R1628P variant increases the risk of PD in Chinese populations [36, 37, 40-43]. In addition, the G2385R variant has been indicated to increase the risk of PD in Japanese and Korean populations [44, 45]. Moreover, these variants have not been found in Indians and other Caucasians [46]. There is no difference in clinical feature and diversification of neurochemicals between familial PD due to LRRK2 mutations and idiopathic PD, including profound dopaminergic neuronal degeneration and gliosis in the SNpc, decreased levels of dopamine in the caudate putamen, and the appearance of α-synuclein-positive LB pathology in the brainstem [47, 48], which suggests that understanding LRRK2 function has implications for all forms of PD.
1.3. Possible Mechanisms of LRRK2 in Parkinson Disease
1.3.1. MAPK Kinase Activity of LRRK2
Phosphorylation of protein plays an important role in MAPK-associated signal transduction pathways in both normal and pathologic states [49]. The c-Jun N-terminal kinases (JNKs), p38 MAPKs and the extracellular signal-regulated kinases (ERKs) are three primary branches of the MAPK superfamily of serine/threonine protein kinases [49, 50]. JNKs, which include three isoforms (JNK1, JNK2 and JNK3), are activated by a number of environmental stresses implicated in PD including, toxins, inflammatory agonists and misfolded protein-induced ER stress [51]. The p38 kinases with four isoforms (p38α, p38β, p38γ, andp38δ) are mentioned more restricted to inflammatory agonists, while the two ERK isoforms (ERK1 and ERK2) are activated principally in response to mitogens, although a high level of cross-talk exists between the different MAPK branches [52].
Kinase domain of LRRK2 closely resembles that of mixed lineage kinases (MLKs), which mediate cellular stress responses by activating the p38 MAPKs and JNKs via their upstream kinases, the MKKs [53]. Of the MKKs, highly homologous kinases MKK3 and MKK6 act upstream of the p38 MAPKs, while MKK4 and MKK7 act upstream of the JNKs [53]. It is reported that LRRK2 binds to these MKKs through the COR and kinase domains, and phosphorylates the MKKs. Binding of LRRK2 to MKK6 is correlated with increased levels of both proteins in the plasma membrane and cytoplasm, and this change is dependent on the activity of MKK6 [51, 54]. Furthermore, the G2019S mutation of LRRK2 was found to exhibit a gain-of-function and enhances effect on LRRK2 kinase activity, and G2019S mutation is able to induce over-phosphorylation of MKK4Ser257, abnormal activation of MKK4-mediated cell death pathway and degeneration of SNpc dopaminergic cells in G2019S transgenic mice, which means that G2019S mutation could cause the death of SNpc dopaminergic neurons by activating JNK-c-Jun signaling pathway [10]. It has been suggested that over-expression of wild-type (wt)LRRK2 in human embryonic kidney HEK293 cells could selectively activate the ERKs [11]. In addition, neuroprotective effects of (wt)LRRK2 against hydrogen peroxide stress seemed to be mediated through ERK1/2 signaling in HEK293 and SH-SY5Y cells [55]. PD-associated mutants G2019S and R1441C could induce ERK phosphorylation to the same extent as (wt)LRRK2, but could not be found with kinase-dead LRRK2, which indicates that this effect is kinase-dependent [11]. In addition, induction of the ERK module by LRRK2 was associated with a diminutive but significant induction of SNCA, which was suppressed by treatment with the selective MAPK/ERK kinase inhibitor U0126, illustrating that LRRK2 could up-regulate SNCA transcription in HEK293 cells in a kinase-dependent manner by specific activation of the MAPK/ERK cascade [11, 56]. Thus, there is no difficulty in finding that pathological functions of LRRK2 have mainly been associated with its aberrant kinase activity, and delineating how the kinase activity of LRRK2 influence the progression of PD will be contributed to pharmaceutically efficient therapies of PD.
1.3.2. Relation of LRRK2 and α-synuclein
The SNCA gene encoding the presynaptic protein α-synuclein, located in the long arm of human chromosome 4, is one of the key molecules involved in familial and sporadic PD [12, 57, 58]. Three described SNCA pathogenic mutations A53T, A30P and E46K have been linked to an early-onset familial parkinsonism that presents similar to the sporadic form of PD [59-62]. α-synuclein comprises 140 amino acids, which form an amphipathic region, a NAC domain, and an acidic tail [63]. Due to the hydrophobicity of the NAC domain, α-synuclein easily forms toxic fibrillar structures and an excess amount of α-synuclein, which induces cell death, eventually leading to PD [64, 65]. α-synuclein is expressed in neurons and localized at presynaptic terminals. It is associated with the synaptic vesicles trafficking/recycling pool, and defects in vesicle exocytosis/neurotransmitter release, which observed in response to overexpression or knockdown of α-synuclein together [66, 67]. These findinds suggest that α-synuclein plays a crucial role in the regulation of synaptic function, neurotransmission, and plasticity in PD [67].
LRRK2 has been revealed to co-localize with early stages of aggregating α-synuclein in lower brainstem of PD and dementia with LBs patients [68], suggesting that LRRK2 dysfunction might contribute to the early formation of LBs. Some neuropathological studies have reported that the LRRK2-correlated neuropathology is strikingly heterogeneous, can additionally present with α-synuclein and tau pathologies [68-70]. Since the pathological reciprocity between LRRK2 and α-synuclein at the protein level is hard to understand, several studies established LRRK2 and α-synuclein variants mice models to research whether LRRK2 and α-synuclein act synergistically in the pathogenesis of PD. One independent study characterized a range of double-mutant mice overexpressing PD-related A53T α-synuclein mutation with various forms of LRRK2 in the mice forebrain [71]. The results demonstrated that LRRK2 could strength α-synuclein-mediated cytotoxicity and suggested that inhibition of LRRK2 expression may be used as a potential therapeutic choice for moderating α-synuclein-induced neurodegeneration. However, the results have been challenged by Daher, J.P., who revealed that overexpression or deletion of human G2019S LRRK2 failed to influence the premature lethality of A53T-α-synuclein transgenic mice, and LRRK2 deletion had no impact on presymptomatic behavioral deficits in these mice by adjusting LRRK2 overexpression mainly in the hind-brain of a well-established human A53T α-synuclein transgenic mouse model [72]. But the study failed to provide support for co-expression of LRRK2 and α-synuclein in similar neuronal populations. Subsequently, this model was further improved. Herzig et al. [73] generated double co-expressing high levels of α-synuclein and LRRK2 variants in both forebrain and brainstem neurons of a large population of transgenic mice, which demonstrated that high LRRK2 levels did not alter the levels of endogenous α-synuclein and tau and did not change α-synucleinopathy in mice, whereas high LRRK2 levels improved motor skills in the presence and absence of α-synuclein transgene-induced disease in some specific lines. With the G2019S-LRRK2 rats (G2019S+) and littermate non-transgenic controls (G2019S-) unilaterally injected with recombinant adeno-associated viral(rAAV)2/1-α-synuclein virus model, Daher, J.P. demonstrated that G2019S-LRRK2 expression exacerbates neuroinflammation and dopaminergic neurodegeneration caused by α-synuclein overexpression in comparison to wild-type rats, and these effects can be moderated by the chronic administration of an effective LRRK2 kinase inhibitor [74]. Moreover, the effect of LRRK2 on α-synuclein has been found to be age-dependent in one recent research. It is demonstrated that the density of striatal dopaminergic terminals, nigral cell counts, tyrosine hydroxylase protein levels and exocytotic dopamine release measured in striatal synaptosomes, or striatal extracellular dopamine levels monitored by in vivo microdialysis were similar between ≥12-month-old G2019S knock-in mice and wild-type controls [75]. Although the western blot analysis showed no genotype difference in striatal levels of endogenous α-synuclein or α-synuclein bound to 3,4-dihydroxyphenylacetaldehyde (a toxic metabolite of dopamine), an increase in dopamine transporter levels and activity and a higher Serine129-phosphorylated α-synuclein levels in the striatum of 12-month-old G2019S KI mice were detected, which were not found in 3-month-old mice [75]. These results of the research revealed that the G2019S mutation causes progressive dysfunctions of dopamine transporters at striatal dopaminergic terminals, along with Serine129-phosphorylated α-synuclein overload, which are not associated with dopamine homeostasis dysregulation or neuron loss but might contribute to intrinsic dopaminergic terminal vulnerability [75].
Therefore, the inconsistent results of these studies indicate that LRRK2-mediated exacerbation of α-synuclein neuropathology might be greatly dependent on different cell type, brain region and the age of different patients, further researches are required to understand the specific function(s) of LRRK2 on α-synuclein.
1.3.3. LRRK2 and α-synuclein in Neuroinflammation
Neuroinflammation involves the activation of microglia and astrocytes to release inflammatory mediators within the brain, and the subsequent recruitment of peripheral immune cells [76-79]. A lot of studies reported that chronic inflammation can contribute to the degeneration of dopaminergic neurons and the progression of PD [77, 78, 80]. The levels of inflammatory cytokines, especially tumour necrosis factor-α (TNF-α), interleukin-1β (IL-1β), IL-2, IL-6, IL-8, and interferon-γ (IFN-γ), have been detected elevated in the cerebrospinal fluid, blood, striatum and SNpc of experimental animal models and PD patients [81, 82]. Microglia, which are macrophages resident in the CNS, represent the first line of defense of innate immune system by conducting a suite of homoeostatic functions, and participate in many brain diseases like cerebrovascular disease [14, 83-86], glioma [87, 88], and Alzheimer's disease [89]. Microglia are responsible for homeostatic and trophic support of the nervous system, phagocytosis of extracellular debris, and the initiation of inflammation [90]. In PD, microglia act as the scavenger of α-synuclein to exert their protective roles, and on the other side, increasing studies provided evidence that chronically activated microglia and astrocytes may play a certain role in promoting the progression of degeneration in PD [91].
A lot of experimental findings pointed out that LRRK2 highly expressed in immune cells, such as B cells, microglia, macrophages, and monocytes, with lower levels in T cells [92], which suggests that LRRK2 might play a predominant modifying role to the innate immune system and inflammation in PD. Higher expression of LRRK2 in both B cells and monocytes as compared to T cells [18, 93] suggests a certain role in the innate immune system, or the first line of defense against infection. It is observed that after inflammation induced by LPS, a robust induction of LRRK2 protein in microglia cells of mouse SNpc or striatum occurred [94]. In addition, in vitro studies reported the similar results, which the expression of LRRK2 protein in microglia cell cultures would be increased after an inflammatory stimulus induced by LPS or IFN-γ [95], but not after HIV-1 Tat protein-induced inflammation [96]. Studies in microglia reported that the expression and phosphorylation of LRRK2 were increased after induced by Toll-like receptor2 (TLR2) or TLR4 stimulation [94, 97, 98], and both of these cell surface receptors have been implicated in the SYN-induced activation of microglia [99, 100]. As PD-associated mutants G2019S and R1441G of LRRK2, it is observed that the expression of LRRK2 G2019S could strengthen the mobilization of myeloid cells in response to a series of pro-inflammatory stimulant [94]. Furthermore, it is reported that compared with wild-type control microglia cells, microglia cells activated by LPS from LRRK2 R1441G transgene mice exerted increased expression and secretion of pro-inflammatory cytokines and reduced expression of anti-inflammatory cytokines [95]. And added conditioned medium from LPS-stimulated LRRK2 R1441G microglia to elementary cortical neurons could cause the increase of neuronal death compared with medium from wild-type LRRK2 microglia. Therefore, these results further indicate that LRRK2 is involved in the cellular pathways induced by inflammation, and that LRRK2 R1441G mutation might force microglia toward a pro-inflammatory state, which in turn leads to exacerbated inflammation and consequent neurodegeneration in PD patients.
Given that chronic neuroinflammation is recognized to contribute to PD pathogenesis, understanding the complex relation between LRRK2 and microglia cells may disclose novel pathways for therapeutic intervention. Increasing studies proved that the kinase activity of LRRK2 may play an important role in this mechanism. On the one hand, LRRK2 was reported to negatively regulate the clearance of α-synuclein accompanied by down-regulation of the endocytosis pathway [56]. The study found that α-synuclein was taken up in larger amounts and cleared from the supernatant more effectively in LRRK2-knockout microglia than for microglia isolated from wild-type (WT) mice [56]. Besides, as mentioned above, LRRK2 could up-regulate SNCA transcription by specific activation of the MAPK/ERK cascade [11]. Accumulation of toxic levels of α-synuclein could lead to the activation of both microglia [101] and astroglia [102]. After that, the TLRs including TLR4 and TLR2 in activated microglia, which promoted by a-synuclein and its oligomer, could initiate signaling pathways that promote expression of inflammatory mediators, such as nuclear factor kappa-B (NF-κB), which described as a “master switch” for gene expression of various inflammatory mediators [103]. The activated NF-κB subsequently increases the expression of various proinflammatory molecules [104]. Release of these proinflammatory molecules from activated microglia into the local milieu enhances the oxidative stress of the SNpc, which results in degeneration of dopaminergic neurons [103]. Consistently, loss of LRRK2 or inhibition of its kinase activity has been reported to result in increased phosphorylation of NF-κB inhibitory subunit p50 at S337, a protein kinase A (PKA)-specific phosphorylation site, with consequent accumulation of p50 in the nucleus. This abnormally higher proportion of nuclear P-p50 might hamper p65:p50, functional heterodimers of NF-κB, to efficiently bind to DNA and activate genes transcription upon LPS or α-syn-mediated inflammation [105]. These findings indicate a role of LRRK2 in microglia activation and sustainment of neuroinflammation and in controlling of NF-κB p50 inhibitory signaling. It was also showed that protein level of active phospho-JNKThr183/Tyr185 and active phospho-c-JunSer63 was significantly upregulated in the SN of G2019S LRRK2 mice, and phospho-c-JunSer63 caused the activation of caspase-9, caspase-8, and caspase-3 [10]. In addition, previous studies have reported that LRRK2 exhibited the ability to phosphorylate p53 at T304 and T377 of threonine-X-arginine (TXR) motif in neurons [106]. As is known to all, the caspase family and p53 play a crucial role in apoptosis of cells.
Overall, the results above strongly implicated that LRRK2 plays an important role as a regulator of neuroinflammation in PD by shifting the balance between neuroprotection and neurotoxicity of microglia and might be involved in more than one cellular process due to its complex architecture with different functional domains.
CONCLUSION AND FUTURE PROSPECTIVE
PD is a fatal neurodegenerative disease that will increase in frequency based on current demographic trends. Microglia may serve as vigilant protectors in PD by internalizing and degrading pathologic α-synuclein and attenuating propagation of synucleinopathy, and this homeostatic process may be altered by inherited LRRK2 mutations. Here, we describe multiple studies for the association between LRRK2 with α-synuclein protein as well as how these interactions may contribute to neuroinflammation mediated by microglia, which may be helpful to understand the function of LRRK2 on the pathogenesis and disease progression in PD. However, many cellular pathways and processes have not been discussed here that may be regulated by mutant LRRK2, such as autophagy, Golgi complex integrity, and the endolysosomal pathway, which may contribute to neurodegeneration.
Identification of mutations in LRRK2 that cause autosomal-dominant parkinsonism turns a new chapter for PD research. There is no doubt that further structure function studies of LRRK2 are required, and a complete understanding of LRRK2 function will offer a number of opportunities for the identification of novel molecular targets for attenuating LRRK2-dependent neurodegeneration in PD.
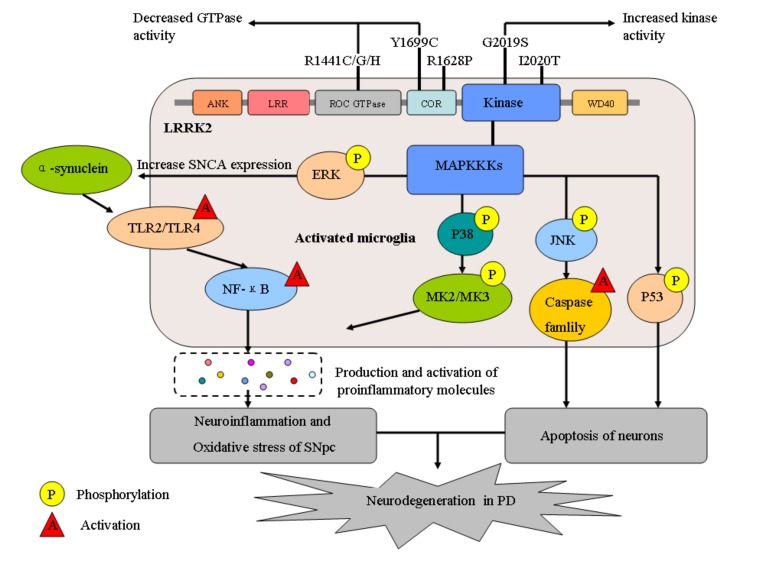
Fig. (1).
LRRK2 domain struture and its possible mechanisms of neurodegeneration in PD. GTPase: guanosine triphosphatase; LRRK2: leucine-rich repeat kinase; ERK: extracellular signal-regulated kinase; MAPKKs: mitogen-activated protein kinase kinases; JNK: c-Jun N-terminal kinase; NF-κB: nuclear factor kappa-B; TLR2/4: Toll-like receptor 2/4; MK2/3: MAPKAPK2/3; SNpc: substantia nigra pars compacta; PD: Parkinson disease.
Table 1. Published studies on LRRK2 kinase activity and the interaction between LRRK2 and α-synuclein in neurodegeneration.
| Group | Study | Model | Conclusion | Refs. |
|---|---|---|---|---|
| MAPK kinase of LRRK2 | Liou, A.K.F. et al., 2008 |
In vitro: HEK293 and SH-SY5Y cells | LRRK2 wild-type but not its mutants has the capacity to attenuate H2O2-induced cell death via activation of the ERK1/2 pathway. | [55] |
| Hsu, C.H. et al., 2010 | in vitro: HEK293 cells in vivo: C. elegans | LRRK2 is able to phosphorylate MKK3, 6 and 7 and G2019S, R1441C and I2020T, enhance binding of LRRK2 to MKK6. | [54] | |
| Chen, C.Y. et al., 2012 |
In vivo: G2019S LRRK2 transgenic mice | Mutant (G2019S) LRRK2 activates MKK4-JNK-c-Jun pathway in the SNpc and causes the resulting degeneration of SNpc dopaminergic neurons in PD transgenic mice. | [10] | |
| Relation of LRRK2 and SNCA | Lin, X. et al., 2009 |
In vivo: LRRK2 transgenic mice |
Over-expression of LRRK2 enhances α-synuclein-mediated cytotoxicity and inhibition of LRRK2 expression could act as a potential therapeutic option for ameliorating α-synuclein-induced neurodegeneration. | [71] |
| Carballo-Carbajal, I. et al., 2010 | In vitro: HEK293 cells | LRRK2 can specifically modify SNCA biology via activation of the ERK/MAPK cascade that ultimately leads to SNCA transcriptional up-regulation. | [11] | |
| Daher, J.P. et al., 2012 | In vivo: A53T-α-synuclein transgenic mice | The overexpression of human G2019S LRRK2 or LRRK2 deletion failed to influence the premature lethality of A53T-α-synuclein transgenic mice. | [72] | |
| Herzig, M.C. et al., 2012 |
In vivo: LRRK2 transgenic mice |
High LRRK2 levels are well tolerated and not sufficient to drive or exacerbate neuronal α-synucleinopathy. | [73] | |
| Daher, J.P. et al., 2015 | In vivo: G2019S-LRRK2 transgenic mice | Chronic inhibition of LRRK2 kinase activity is well tolerated in rats and provides neuroprotection from α-synuclein overexpression. | [74] | |
| Maekawa, T. et al., 2016 |
In vivo: LRRK2-KO mice |
LRRK2 negatively regulates the clearance of α-synuclein accompanied by down-regulation of the endocytosis pathway. | [56] | |
| Longo, F. et al., 2017 |
In vivo: G2019S LRRK2-KI mice |
G2019S mutation causes progressive dysfunctions of dopamine transporters, along with Serine129-phosphorylated α-synuclein overload, which are not associated with dopamine homeostasis dysregulation or neuron loss but might contribute to intrinsic dopaminergic terminal vulnerability. | [75] | |
| LRRK2 in neuroinflammation | Gardet, A. et al., 2010 | Clinical blood samples | LRRK2 is a target gene of IFN-γ, and it might be involved in signaling pathways relevant to Crohn's disease pathogenesis. | [18] |
| Thévenet, J. et al., 2011 |
Clinical blood samples | Expression of LRRK2 protein but not mRNA in activated CD14+CD16+ monocytes. | [93] | |
| Moehle, M.S. et al., 2012 |
In vivo: LRRK2 WT/KO mice | LRRK2 is involved in regulating responses in immune cells of the brain and further implicate microglial involvement in late-onset PD. | [94] | |
| Gillardon, F. et al., 2012 |
In vivo: LRRK2 transgenic mice |
Enhanced neuroinflammation may contribute to neurodegeneration in Parkinson's disease patients carrying LRRK2 mutations. | [95] | |
| Russo, I. et al., 2015 |
In vitro: Lrrk2−/− primary microglia cells | The role of LRRK2 in microglia activation and sustainment of neuroinflammation acted by controlling of NF-κB p50 inhibitory signaling |
[105] | |
| Ho, D. H. et al., 2017 |
In vitro: microglia model BV2 cells | LRRK2 kinase activity in microglia can contribute to neuroinflammation in PD via phosphorylating p53 at T304 and T377 site. | [106] |
MAPK: mitogen-activated protein kinase; LRRK2: leucine-rich repeat kinase; SNCA: α-synuclein gene; ERK: extracellular signal-regulated kinase; MKK: MAPK kinase; JNK: c-Jun N-terminal kinase; SNpc: substantia nigra pars compacta; IFN: interferon; PD: Parkinson disease; NF-κB: nuclear factor kappa-B.
Acknowledgements
Declared none.
Consent for Publication
Not applicable.
Conflict of Interest
The authors declare no conflict of interest, financial or otherwise.
REFERENCEs
- 1.Dauer W., Przedborski S. Parkinson’s disease: mechanisms and models. Neuron. 2003;39(6):889–909. doi: 10.1016/s0896-6273(03)00568-3. [http://dx.doi.org/10.1016/ S0896-6273(03)00568-3]. [PMID: 12971891]. [DOI] [PubMed] [Google Scholar]
- 2.Lees A.J., Hardy J., Revesz T. Parkinson’s disease. Lancet. 2009;373(9680):2055–2066. doi: 10.1016/S0140-6736(09)60492-X. [http://dx.doi.org/10.1016/S0140-6736(09) 60492-X]. [PMID: 19524782]. [DOI] [PubMed] [Google Scholar]
- 3.Ito M., Hirayama M., Yamai K., Goto S., Ito M., Ichihara M., Ohno K. Drinking hydrogen water and intermittent hydrogen gas exposure, but not lactulose or continuous hydrogen gas exposure, prevent 6-hydorxydopamine-induced Parkinson’s disease in rats. Med. Gas Res. 2012;2(1):15. doi: 10.1186/2045-9912-2-15. [http://dx.doi.org/10.1186/2045-9912-2-15]. [PMID: 22608009]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu C.L., Zhang K., Chen G. Hydrogen therapy: from mechanism to cerebral diseases. Med. Gas Res. 2016;6(1):48–54. doi: 10.4103/2045-9912.179346. [http://dx. doi.org/10.4103/2045-9912.179346]. [PMID: 27826423]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Houlden H., Singleton A.B. The genetics and neuropathology of Parkinson’s disease. Acta Neuropathol. 2012;124(3):325–338. doi: 10.1007/s00401-012-1013-5. [http://dx.doi.org/10.1007/s00401-012-1013-5]. [PMID: 22806825]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gasser T. Mendelian forms of Parkinson’s disease. Biochim. Biophys. Acta. 2009;1792(7):587–596. doi: 10.1016/j.bbadis.2008.12.007. [http://dx.doi.org/10.1016/ j.bbadis.2008.12.007]. [PMID: 19168133]. [DOI] [PubMed] [Google Scholar]
- 7.Mata I.F., Wedemeyer W.J., Farrer M.J., Taylor J.P., Gallo K.A. LRRK2 in Parkinson’s disease: protein domains and functional insights. Trends Neurosci. 2006;29(5):286–293. doi: 10.1016/j.tins.2006.03.006. [http://dx. doi.org/10.1016/j.tins.2006.03.006]. [PMID: 16616379]. [DOI] [PubMed] [Google Scholar]
- 8.Jaleel M., Nichols R.J., Deak M., Campbell D.G., Gillardon F., Knebel A., Alessi D.R. LRRK2 phosphorylates moesin at threonine-558: characterization of how Parkinson’s disease mutants affect kinase activity. Biochem. J. 2007;405(2):307–317. doi: 10.1042/BJ20070209. [http:// dx.doi.org/10.1042/BJ20070209]. [PMID: 17447891]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tsika E., Moore D.J. Mechanisms of LRRK2-mediated neuro-degeneration. Curr. Neurol. Neurosci. Rep. 2012;12(3):251–260. doi: 10.1007/s11910-012-0265-8. [http://dx.doi.org/10.1007/s11910-012-0265-8]. [PMID: 22441981]. [DOI] [PubMed] [Google Scholar]
- 10.Chen C.Y., Weng Y.H., Chien K.Y., Lin K.J., Yeh T.H., Cheng Y.P., Lu C.S., Wang H.L. (G2019S) LRRK2 activates MKK4-JNK pathway and causes degeneration of SN dopaminergic neurons in a transgenic mouse model of PD. Cell Death Differ. 2012;19(10):1623–1633. doi: 10.1038/cdd.2012.42. [http://dx.doi.org/10.1038/cdd.2012.42]. [PMID: 22539006]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carballo-Carbajal I., Weber-Endress S., Rovelli G., Chan D., Wolozin B., Klein C.L., Patenge N., Gasser T., Kahle P.J. Leucine-rich repeat kinase 2 induces α-synuclein expression via the extracellular signal-regulated kinase pathway. Cell. Signal. 2010;22(5):821–827. doi: 10.1016/j.cellsig.2010.01.006. [http://dx.doi.org/10.1016/j.cellsig.2010.01.006]. [PMID: 20074637]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bendor J.T., Logan T.P., Edwards R.H. The function of α-synuclein. Neuron. 2013;79(6):1044–1066. doi: 10.1016/j.neuron.2013.09.004. [http://dx.doi.org/10. 1016/j.neuron.2013.09.004]. [PMID: 24050397]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Daher J.P., Volpicelli-Daley L.A., Blackburn J.P., Moehle M.S., West A.B. Abrogation of α-synuclein-mediated dopaminergic neurodegeneration in LRRK2-deficient rats. Proc. Natl. Acad. Sci. USA. 2014;111(25):9289–9294. doi: 10.1073/pnas.1403215111. [http://dx.doi.org/10.1073/pnas. 1403215111]. [PMID: 24927544]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhao H., Garton T., Keep R.F., Hua Y., Xi G. Microglia/macrophage polarization after experimental intracerebral Hemorrhage. Transl. Stroke Res. 2015;6(6):407–409. doi: 10.1007/s12975-015-0428-4. [http://dx. doi.org/10.1007/s12975-015-0428-4]. [PMID: 26446073]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Becker K.J. Strain-related differences in the immune response: relevance to human stroke. Transl. Stroke Res. 2016;7(4):303–312. doi: 10.1007/s12975-016-0455-9. [http://dx.doi.org/10.1007/s12975-016-0455-9]. [PMID: 26860504]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xiong X.Y., Yang Q.W. Rethinking the roles of inflammation in the intracerebral hemorrhage. Transl. Stroke Res. 2015;6(5):339–341. doi: 10.1007/s12975-015-0402-1. [http://dx.doi.org/10.1007/s12975-015-0402-1]. [PMID: 25940771]. [DOI] [PubMed] [Google Scholar]
- 17.Austin S.A., Floden A.M., Murphy E.J., Combs C.K. Alpha-synuclein expression modulates microglial activation phenotype. J. Neurosci. 2006;26(41):10558–10563. doi: 10.1523/JNEUROSCI.1799-06.2006. [http://dx.doi.org/10.1523/ JNEUROSCI.1799-06.2006]. [PMID: 17035541]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gardet A., Benita Y., Li C., Sands B.E., Ballester I., Stevens C., Korzenik J.R., Rioux J.D., Daly M.J., Xavier R.J., Podolsky D.K. LRRK2 is involved in the IFN-gamma response and host response to pathogens. J. Immunol. 2010;185(9):5577–5585. doi: 10.4049/jimmunol.1000548. [http:// dx.doi.org/10.4049/jimmunol.1000548]. [PMID: 20921534]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schapansky J., Nardozzi J.D., LaVoie M.J. The complex relationships between microglia, alpha-synuclein, and LRRK2 in Parkinson’s disease. Neuroscience. 2015;302:74–88. doi: 10.1016/j.neuroscience.2014.09.049. [http://dx.doi. org/10.1016/j.neuroscience.2014.09.049]. [PMID: 25284317]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Drolet R.E., Sanders J.M., Kern J.T. Leucine-rich repeat kinase 2 (LRRK2) cellular biology: a review of recent advances in identifying physiological substrates and cellular functions. J. Neurogenet. 2011;25(4):140–151. doi: 10.3109/01677063.2011.627072. [http://dx.doi.org/10.3109/01677063.2011. 627072]. [PMID: 22077787]. [DOI] [PubMed] [Google Scholar]
- 21.Rideout H.J. Leucine-Rich Repeat Kinase 2 (LRRK2). Springer; 2017. [http://dx.doi.org/10.1007/978-3-319-49969-7] [Google Scholar]
- 22.Mills R.D., Mulhern T.D., Cheng H.C., Culvenor J.G. Analysis of LRRK2 accessory repeat domains: prediction of repeat length, number and sites of Parkinson’s disease mutations. Biochem. Soc. Trans. 2012;40(5):1086–1089. doi: 10.1042/BST20120088. [http://dx.doi.org/10.1042/ BST20120088]. [PMID: 22988870]. [DOI] [PubMed] [Google Scholar]
- 23.Westerlund M., Belin A.C., Anvret A., Bickford P., Olson L., Galter D. Developmental regulation of leucine-rich repeat kinase 1 and 2 expression in the brain and other rodent and human organs: Implications for Parkinson’s disease. Neuroscience. 2008;152(2):429–436. doi: 10.1016/j.neuroscience.2007.10.062. [http://dx.doi.org/10.1016/j.neuroscience.2007.10.062]. [PMID: 18272292]. [DOI] [PubMed] [Google Scholar]
- 24.Biskup S., Moore D.J., Rea A., Lorenz-Deperieux B., Coombes C.E., Dawson V.L., Dawson T.M., West A.B. Dynamic and redundant regulation of LRRK2 and LRRK1 expression. BMC Neurosci. 2007;8:102. doi: 10.1186/1471-2202-8-102. [http://dx.doi.org/10.1186/1471-2202-8-102]. [PMID: 18045479]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Galter D., Westerlund M., Carmine A., Lindqvist E., Sydow O., Olson L. LRRK2 expression linked to dopamine-innervated areas. Ann. Neurol. 2006;59(4):714–719. doi: 10.1002/ana.20808. [http://dx.doi.org/10.1002/ana. 20808]. [PMID: 16532471]. [DOI] [PubMed] [Google Scholar]
- 26.Higashi S., Biskup S., West A.B., Trinkaus D., Dawson V.L., Faull R.L., Waldvogel H.J., Arai H., Dawson T.M., Moore D.J., Emson P.C. Localization of Parkinson’s disease-associated LRRK2 in normal and pathological human brain. Brain Res. 2007;1155:208–219. doi: 10.1016/j.brainres.2007.04.034. [http://dx.doi.org/10.1016/j.brainres.2007.04.034]. [PMID: 17512502]. [DOI] [PubMed] [Google Scholar]
- 27.Biskup S., Moore D.J., Celsi F., Higashi S., West A.B., Andrabi S.A., Kurkinen K., Yu S.W., Savitt J.M., Waldvogel H.J., Faull R.L., Emson P.C., Torp R., Ottersen O.P., Dawson T.M., Dawson V.L. Localization of LRRK2 to membranous and vesicular structures in mammalian brain. Ann. Neurol. 2006;60(5):557–569. doi: 10.1002/ana.21019. [http://dx.doi.org/10.1002/ana.21019]. [PMID: 17120249]. [DOI] [PubMed] [Google Scholar]
- 28.Alegre-Abarrategui J., Christian H., Lufino M.M., Mutihac R., Venda L.L., Ansorge O., Wade-Martins R. LRRK2 regulates autophagic activity and localizes to specific membrane microdomains in a novel human genomic reporter cellular model. Hum. Mol. Genet. 2009;18(21):4022–4034. doi: 10.1093/hmg/ddp346. [http://dx.doi.org/10.1093/ hmg/ddp346]. [PMID: 19640926]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hatano T., Kubo S., Imai S., Maeda M., Ishikawa K., Mizuno Y., Hattori N. Leucine-rich repeat kinase 2 associates with lipid rafts. Hum. Mol. Genet. 2007;16(6):678–690. doi: 10.1093/hmg/ddm013. [http://dx.doi.org/ 10.1093/hmg/ddm013]. [PMID: 17341485]. [DOI] [PubMed] [Google Scholar]
- 30.Cookson M.R. The role of leucine-rich repeat kinase 2 (LRRK2) in Parkinson’s disease. Nat. Rev. Neurosci. 2010;11(12):791–797. doi: 10.1038/nrn2935. [http://dx.doi.org/10.1038/nrn2935]. [PMID: 21088684]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Correia Guedes L., Ferreira J.J., Rosa M.M., Coelho M., Bonifati V., Sampaio C. Worldwide frequency of G2019S LRRK2 mutation in Parkinson’s disease: a systematic review. Parkinsonism Relat. Disord. 2010;16(4):237–242. doi: 10.1016/j.parkreldis.2009.11.004. [http://dx.doi.org/10.1016/ j.parkreldis.2009.11.004]. [PMID: 19945904]. [DOI] [PubMed] [Google Scholar]
- 32.Lesage S., Dürr A., Tazir M., Lohmann E., Leutenegger A.L., Janin S., Pollak P., Brice A. LRRK2 G2019S as a cause of Parkinson’s disease in North African Arabs. N. Engl. J. Med. 2006;354(4):422–423. doi: 10.1056/NEJMc055540. [http://dx.doi.org/10.1056/NEJMc055540]. [PMID: 16436781]. [DOI] [PubMed] [Google Scholar]
- 33.Ozelius L.J., Senthil G., Saunders-Pullman R., Ohmann E., Deligtisch A., Tagliati M., Hunt A.L., Klein C., Henick B., Hailpern S.M., Lipton R.B., Soto-Valencia J., Risch N., Bressman S.B. LRRK2 G2019S as a cause of Parkinson’s disease in Ashkenazi Jews. N. Engl. J. Med. 2006;354(4):424–425. doi: 10.1056/NEJMc055509. [http:// dx.doi.org/10.1056/NEJMc055509]. [PMID: 16436782]. [DOI] [PubMed] [Google Scholar]
- 34.Lesage S., Leutenegger A.L., Ibanez P., Janin S., Lohmann E., Dürr A., Brice A. LRRK2 haplotype analyses in European and North African families with Parkinson disease: a common founder for the G2019S mutation dating from the 13th century. Am. J. Hum. Genet. 2005;77(2):330–332. doi: 10.1086/432422. [http://dx.doi.org/10.1086/432422]. [PMID: 16145815]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hu Z.X., Peng D.T., Cai M., Pu J.L., Lei X.G., Yin X.Z., Ou-Yang Z.Y., Luo W., Zhang B.R. A study of six point mutation analysis of LRRK2 gene in Chinese mainland patients with Parkinson’s disease. Neurol. Sci. 2011;32(4):741–742. doi: 10.1007/s10072-010-0453-8. [http://dx. doi.org/10.1007/s10072-010-0453-8]. [PMID: 21234781]. [DOI] [PubMed] [Google Scholar]
- 36.Tan E.K., Zhao Y., Skipper L., Tan M.G., Di Fonzo A., Sun L., Fook-Chong S., Tang S., Chua E., Yuen Y., Tan L., Pavanni R., Wong M.C., Kolatkar P., Lu C.S., Bonifati V., Liu J.J. The LRRK2 Gly2385Arg variant is associated with Parkinson’s disease: genetic and functional evidence. Hum. Genet. 2007;120(6):857–863. doi: 10.1007/s00439-006-0268-0. [http://dx.doi.org/10.1007/s00439-006-0268-0]. [PMID: 17019612]. [DOI] [PubMed] [Google Scholar]
- 37.Ross O.A., Wu Y.R., Lee M.C., Funayama M., Chen M.L., Soto A.I., Mata I.F., Lee-Chen G.J., Chen C.M., Tang M., Zhao Y., Hattori N., Farrer M.J., Tan E.K., Wu R.M. Analysis of Lrrk2 R1628P as a risk factor for Parkinson’s disease. Ann. Neurol. 2008;64(1):88–92. doi: 10.1002/ana.21405. [http://dx.doi.org/10.1002/ana.21405]. [PMID: 18412265]. [DOI] [PubMed] [Google Scholar]
- 38.Zheng Y., Liu Y., Wu Q., Hong H., Zhou H., Chen J., Wang H., Xian W., Li J., Liu Z., Pei Z., Chen L. Confirmation of LRRK2 S1647T variant as a risk factor for Parkinson’s disease in southern China. Eur. J. Neurol. 2011;18(3):538–540. doi: 10.1111/j.1468-1331.2010.03164.x. [http:// dx.doi.org/10.1111/j.1468-1331.2010.03164.x]. [PMID: 20629711]. [DOI] [PubMed] [Google Scholar]
- 39.Chen L., Zhang S., Liu Y., Hong H., Wang H., Zheng Y., Zhou H., Chen J., Xian W., He Y., Li J., Liu Z., Pei Z., Zeng J. LRRK2 R1398H polymorphism is associated with decreased risk of Parkinson’s disease in a Han Chinese population. Parkinsonism Relat. Disord. 2011;17(4):291–292. doi: 10.1016/j.parkreldis.2010.11.012. [http://dx.doi.org/10.1016/ j.parkreldis.2010.11.012]. [PMID: 21159540]. [DOI] [PubMed] [Google Scholar]
- 40.An X.K., Peng R., Li T., Burgunder J.M., Wu Y., Chen W.J., Zhang J.H., Wang Y.C., Xu Y.M., Gou Y.R., Yuan G.G., Zhang Z.J. LRRK2 Gly2385Arg variant is a risk factor of Parkinson’s disease among Han-Chinese from mainland China. Eur. J. Neurol. 2008;15(3):301–305. doi: 10.1111/j.1468-1331.2007.02052.x. [http://dx.doi.org/10.1111/j.1468-1331.2007.02052.x]. [PMID: 18201193]. [DOI] [PubMed] [Google Scholar]
- 41.Li C., Ting Z., Qin X., Ying W., Li B., Qiang G. L.; Jian Fang, M.; Jing, Z.; Jian Qing, D.; Sheng Di, C. The prevalence of LRRK2 Gly2385Arg variant in Chinese Han population with Parkinson’s disease. Mov. Disord. 2007;22(16):2439–2443. doi: 10.1002/mds.21763. [http://dx.doi.org/10.1002/mds.21763]. [PMID: 17960808]. [DOI] [PubMed] [Google Scholar]
- 42.Zhou Y., Luo X., Li F., Tian X., Zhu L., Yang Y., Ren Y., Pang H. Association of Parkinson’s disease with six single nucleotide polymorphisms located in four PARK genes in the northern Han Chinese population. J. Clin. Neurosci. 2012;19(7):1011–1015. doi: 10.1016/j.jocn.2011.09.028. [http://dx.doi.org/10.1016/j.jocn.2011.09.028]. [PMID: 22575062]. [DOI] [PubMed] [Google Scholar]
- 43.Yu L., Hu F., Zou X., Jiang Y., Liu Y., He X., Xi J., Liu L., Liu Z., He L., Xu Y. LRRK2 R1628P contributes to Parkinson’s disease susceptibility in Chinese Han populations from mainland China. Brain Res. 2009;1296:113–116. doi: 10.1016/j.brainres.2009.08.047. [http://dx.doi.org/10. 1016/j.brainres.2009.08.047]. [PMID: 19699188]. [DOI] [PubMed] [Google Scholar]
- 44.Zabetian C.P., Yamamoto M., Lopez A.N., Ujike H., Mata I.F., Izumi Y., Kaji R., Maruyama H., Morino H., Oda M., Hutter C.M., Edwards K.L., Schellenberg G.D., Tsuang D.W., Yearout D., Larson E.B., Kawakami H. LRRK2 mutations and risk variants in Japanese patients with Parkinson’s disease. Mov. Disord. 2009;24(7):1034–1041. doi: 10.1002/mds.22514. [http://dx.doi.org/10.1002/mds.22514]. [PMID: 19343804]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kim J.M., Lee J.Y., Kim H.J., Kim J.S., Shin E.S., Cho J.H., Park S.S., Jeon B.S. The LRRK2 G2385R variant is a risk factor for sporadic Parkinson’s disease in the Korean population. Parkinsonism Relat. Disord. 2010;16(2):85–88. doi: 10.1016/j.parkreldis.2009.10.004. [http://dx.doi.org/10. 1016/j.parkreldis.2009.10.004]. [PMID: 19854095]. [DOI] [PubMed] [Google Scholar]
- 46.Papapetropoulos S., Adi N., Shehadeh L., Bishopric N., Singer C., Argyriou A.A., Chroni E. Is the G2019S LRRK2 mutation common in all southern European populations? J. Clin. Neurosci. 2008;15(9):1027–1030. doi: 10.1016/j.jocn.2007.08.013. [http://dx.doi.org/10.1016/j.jocn.2007.08. 013]. [PMID: 18617409]. [DOI] [PubMed] [Google Scholar]
- 47.Martin I., Kim J.W., Dawson V.L., Dawson T.M. LRRK2 pathobiology in Parkinson’s disease. J. Neurochem. 2014;131(5):554–565. doi: 10.1111/jnc.12949. [http://dx.doi.org/10.1111/jnc.12949]. [PMID: 25251388]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Di Fonzo A., Rohé C.F., Ferreira J., Chien H.F., Vacca L., Stocchi F., Guedes L., Fabrizio E., Manfredi M., Vanacore N., Goldwurm S., Breedveld G., Sampaio C., Meco G., Barbosa E., Oostra B.A., Bonifati V. A frequent LRRK2 gene mutation associated with autosomal dominant Parkinson’s disease. Lancet. 2005;365(9457):412–415. doi: 10.1016/S0140-6736(05)17829-5. [http://dx.doi.org/10.1016/S0140-6736(05) 17829-5]. [PMID: 15680456]. [DOI] [PubMed] [Google Scholar]
- 49.Kyosseva S.V. Mitogen-activated protein kinase signaling. Int. Rev. Neurobiol. 2004;59:201–220. doi: 10.1016/S0074-7742(04)59008-6. [http://dx.doi.org/10.1016/ S0074-7742(04)59008-6]. [PMID: 15006489]. [DOI] [PubMed] [Google Scholar]
- 50.Dérijard B., Raingeaud J., Barrett T., Wu I.H., Han J., Ulevitch R.J., Davis R.J. Independent human MAP-kinase signal transduction pathways defined by MEK and MKK isoforms. Science. 1995;267(5198):682–685. doi: 10.1126/science.7839144. [http://dx.doi.org/10.1126/science.7839144]. [PMID: 7839144]. [DOI] [PubMed] [Google Scholar]
- 51.Hsu C.H., Chan D., Wolozin B. LRRK2 and the stress response: interaction with MKKs and JNK-interacting proteins. Neurodegener. Dis. 2010;7(1-3):68–75. doi: 10.1159/000285509. [http://dx.doi.org/10.1159/000285509]. [PMID: 20173330]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Takekawa M., Tatebayashi K., Saito H. Conserved docking site is essential for activation of mammalian MAP kinase kinases by specific MAP kinase kinase kinases. Mol. Cell. 2005;18(3):295–306. doi: 10.1016/j.molcel.2005.04.001. [http://dx.doi.org/10.1016/j.molcel.2005.04.001]. [PMID: 15866172]. [DOI] [PubMed] [Google Scholar]
- 53.Gallo K.A., Johnson G.L. Mixed-lineage kinase control of JNK and p38 MAPK pathways. Nat. Rev. Mol. Cell Biol. 2002;3(9):663–672. doi: 10.1038/nrm906. [http://dx.doi.org/10.1038/nrm906]. [PMID: 12209126]. [DOI] [PubMed] [Google Scholar]
- 54.Hsu C.H., Chan D., Greggio E., Saha S., Guillily M.D., Ferree A., Raghavan K., Shen G.C., Segal L., Ryu H., Cookson M.R., Wolozin B. MKK6 binds and regulates expression of Parkinson’s disease-related protein LRRK2. J. Neurochem. 2010;112(6):1593–1604. doi: 10.1111/j.1471-4159.2010.06568.x. [http://dx.doi.org/10.1111/j.1471-4159.2010.06568.x]. [PMID: 20067578]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Liou A.K.F., Leak R.K., Li L., Zigmond M.J. Wild-type LRRK2 but not its mutant attenuates stress-induced cell death via ERK pathway. Neurobiol. Dis. 2008;32(1):116–124. doi: 10.1016/j.nbd.2008.06.016. [http://dx.doi.org/ 10.1016/j.nbd.2008.06.016]. [PMID: 18675914]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Maekawa T., Sasaoka T., Azuma S., Ichikawa T., Melrose H.L., Farrer M.J., Obata F. Leucine-rich repeat kinase 2 (LRRK2) regulates α-synuclein clearance in microglia. BMC Neurosci. 2016;17(1):77. doi: 10.1186/s12868-016-0315-2. [http://dx.doi.org/10.1186/s12868-016-0315-2]. [PMID: 27903237]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mullin S., Schapira A. α-Synuclein and mitochondrial dysfunction in Parkinson’s disease. Mol. Neurobiol. 2013;47(2):587–597. doi: 10.1007/s12035-013-8394-x. [http://dx.doi.org/10.1007/s12035-013-8394-x]. [PMID: 23361255]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Olanow C.W., Brundin P. Parkinson’s disease and alpha synuclein: is Parkinson’s disease a prion-like disorder? Mov. Disord. 2013;28(1):31–40. doi: 10.1002/mds.25373. [http://dx.doi.org/10.1002/mds.25373]. [PMID: 23390095]. [DOI] [PubMed] [Google Scholar]
- 59.Polymeropoulos M.H., Lavedan C., Leroy E., Ide S.E., Dehejia A., Dutra A., Pike B., Root H., Rubenstein J., Boyer R., Stenroos E.S., Chandrasekharappa S., Athanassiadou A., Papapetropoulos T., Johnson W.G., Lazzarini A.M., Duvoisin R.C., Di Iorio G., Golbe L.I., Nussbaum R.L. Mutation in the alpha-synuclein gene identified in families with Parkinson’s disease. Science. 1997;276(5321):2045–2047. doi: 10.1126/science.276.5321.2045. [http://dx.doi.org/10.1126/ science.276.5321.2045]. [PMID: 9197268]. [DOI] [PubMed] [Google Scholar]
- 60.Krüger R., Kuhn W., Müller T., Woitalla D., Graeber M., Kösel S., Przuntek H., Epplen J.T., Schöls L., Riess O. Ala30Pro mutation in the gene encoding alpha-synuclein in Parkinson’s disease. Nat. Genet. 1998;18(2):106–108. doi: 10.1038/ng0298-106. [http://dx.doi. org/10.1038/ng0298-106]. [PMID: 9462735]. [DOI] [PubMed] [Google Scholar]
- 61.Zarranz J.J., Alegre J., Gómez-Esteban J.C., Lezcano E., Ros R., Ampuero I., Vidal L., Hoenicka J., Rodriguez O., Atarés B., Llorens V., Gomez Tortosa E., del Ser T., Muñoz D.G., de Yebenes J.G. The new mutation, E46K, of alpha-synuclein causes Parkinson and Lewy body dementia. Ann. Neurol. 2004;55(2):164–173. doi: 10.1002/ana.10795. [http://dx.doi.org/10.1002/ana.10795]. [PMID: 14755719]. [DOI] [PubMed] [Google Scholar]
- 62.Chartier-Harlin M.C., Kachergus J., Roumier C., Mouroux V., Douay X., Lincoln S., Levecque C., Larvor L., Andrieux J., Hulihan M., Waucquier N., Defebvre L., Amouyel P., Farrer M., Destée A. Alpha-synuclein locus duplication as a cause of familial Parkinson’s disease. Lancet. 2004;364(9440):1167–1169. doi: 10.1016/S0140-6736(04)17103-1. [http:// dx.doi.org/10.1016/S0140-6736(04)17103-1]. [PMID: 15451224]. [DOI] [PubMed] [Google Scholar]
- 63.Jo E., McLaurin J., Yip C.M., St George-Hyslop P., Fraser P.E. alpha-Synuclein membrane interactions and lipid specificity. J. Biol. Chem. 2000;275(44):34328–34334. doi: 10.1074/jbc.M004345200. [http://dx.doi.org/10. 1074/jbc.M004345200]. [PMID: 10915790]. [DOI] [PubMed] [Google Scholar]
- 64.Uversky V.N., Eliezer D. Biophysics of Parkinson’s disease: structure and aggregation of alpha-synuclein. Curr. Protein Pept. Sci. 2009;10(5):483–499. doi: 10.2174/138920309789351921. [http://dx.doi.org/10.2174/ 138920309789351921]. [PMID: 19538146]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Saha A.R., Ninkina N.N., Hanger D.P., Anderton B.H., Davies A.M., Buchman V.L. Induction of neuronal death by alpha-synuclein. Eur. J. Neurosci. 2000;12(8):3073–3077. doi: 10.1046/j.1460-9568.2000.00210.x. [http://dx.doi. org/10.1046/j.1460-9568.2000.00210.x]. [PMID: 10971650]. [DOI] [PubMed] [Google Scholar]
- 66.Zhou W., Hurlbert M.S., Schaack J., Prasad K.N., Freed C.R. Overexpression of human alpha-synuclein causes dopamine neuron death in rat primary culture and immortalized mesencephalon-derived cells. Brain Res. 2000;866(1-2):33–43. doi: 10.1016/s0006-8993(00)02215-0. [http://dx.doi.org/ 10.1016/S0006-8993(00)02215-0]. [PMID: 10825478]. [DOI] [PubMed] [Google Scholar]
- 67.Snead D., Eliezer D. Alpha-synuclein function and dysfunction on cellular membranes. Exp. Neurobiol. 2014;23(4):292–313. doi: 10.5607/en.2014.23.4.292. [http://dx.doi.org/10.5607/en.2014.23.4.292]. [PMID: 25548530]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zimprich A., Biskup S., Leitner P., Lichtner P., Farrer M., Lincoln S., Kachergus J., Hulihan M., Uitti R.J., Calne D.B., Stoessl A.J., Pfeiffer R.F., Patenge N., Carbajal I.C., Vieregge P., Asmus F., Müller-Myhsok B., Dickson D.W., Meitinger T., Strom T.M., Wszolek Z.K., Gasser T. Mutations in LRRK2 cause autosomal-dominant parkinsonism with pleomorphic pathology. Neuron. 2004;44(4):601–607. doi: 10.1016/j.neuron.2004.11.005. [http://dx.doi.org/10.1016/j.neuron. 2004.11.005]. [PMID: 15541309]. [DOI] [PubMed] [Google Scholar]
- 69.Ruffmann C., Giaccone G., Canesi M., Bramerio M., Goldwurm S., Gambacorta M., Rossi G., Tagliavini F., Pezzoli G. Atypical tauopathy in a patient with LRRK2-G2019S mutation and tremor-dominant Parkinsonism. Neuropathol. Appl. Neurobiol. 2012;38(4):382–386. doi: 10.1111/j.1365-2990.2011.01216.x. [http://dx.doi.org/10.1111/j.1365-2990.2011. 01216.x]. [PMID: 21883375]. [DOI] [PubMed] [Google Scholar]
- 70.Wider C., Dickson D.W., Wszolek Z.K. Leucine-rich repeat kinase 2 gene-associated disease: redefining genotype-phenotype correlation. Neurodegener. Dis. 2010;7(1-3):175–179. doi: 10.1159/000289232. [http:// dx.doi.org/10.1159/000289232]. [PMID: 20197701]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lin X., Parisiadou L., Gu X.L., Wang L., Shim H., Sun L., Xie C., Long C.X., Yang W.J., Ding J., Chen Z.Z., Gallant P.E., Tao-Cheng J.H., Rudow G., Troncoso J.C., Liu Z., Li Z., Cai H. Leucine-rich repeat kinase 2 regulates the progression of neuropathology induced by Parkinson’s-disease-related mutant alpha-synuclein. Neuron. 2009;64(6):807–827. doi: 10.1016/j.neuron.2009.11.006. [http://dx.doi.org/10. 1016/j.neuron.2009.11.006]. [PMID: 20064389]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Daher J.P., Pletnikova O., Biskup S., Musso A., Gellhaar S., Galter D., Troncoso J.C., Lee M.K., Dawson T.M., Dawson V.L., Moore D.J. Neurodegenerative phenotypes in an A53T α-synuclein transgenic mouse model are independent of LRRK2. Hum. Mol. Genet. 2012;21(11):2420–2431. doi: 10.1093/hmg/dds057. [http://dx.doi.org/ 10.1093/hmg/dds057]. [PMID: 22357653]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Herzig M.C., Bidinosti M., Schweizer T., Hafner T., Stemmelen C., Weiss A., Danner S., Vidotto N., Stauffer D., Barske C., Mayer F., Schmid P., Rovelli G., van der Putten P.H., Shimshek D.R. High LRRK2 levels fail to induce or exacerbate neuronal alpha-synucleinopathy in mouse brain. PLoS One. 2012;7(5):e36581. doi: 10.1371/journal.pone.0036581. [http://dx.doi.org/10.1371/journal.pone.0036581]. [PMID: 22615783]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Daher J.P., Abdelmotilib H.A., Hu X., Volpicelli-Daley L.A., Moehle M.S., Fraser K.B., Needle E., Chen Y., Steyn S.J., Galatsis P., Hirst W.D., West A.B. Leucine-rich Repeat Kinase 2 (LRRK2) Pharmacological Inhibition Abates α-Synuclein Gene-induced Neurodegeneration. J. Biol. Chem. 2015;290(32):19433–19444. doi: 10.1074/jbc.M115.660001. [http://dx.doi.org/10.1074/jbc.M115.660001]. [PMID: 26078453]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Longo F., Mercatelli D., Novello S., Arcuri L., Brugnoli A., Vincenzi F., Russo I., Berti G., Mabrouk O.S., Kennedy R.T., Shimshek D.R., Varani K., Bubacco L., Greggio E., Morari M. Age-dependent dopamine transporter dysfunction and Serine129 phospho-α-synuclein overload in G2019S LRRK2 mice. Acta Neuropathol. Commun. 2017;5(1):22. doi: 10.1186/s40478-017-0426-8. [http://dx.doi.org/10.1186/ s40478-017-0426-8]. [PMID: 28292328]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Montaner J., Salat D., García-Berrocoso T., Molina C.A., Chacón P., Ribó M., Alvarez-Sabín J., Rosell A. Reperfusion therapy for acute stroke improves outcome by decreasing neuroinflammation. Transl. Stroke Res. 2010;1(4):261–267. doi: 10.1007/s12975-010-0038-0. [http:// dx.doi.org/10.1007/s12975-010-0038-0]. [PMID: 24323553]. [DOI] [PubMed] [Google Scholar]
- 77.Schain M., Kreisl W.C. Neuroinflammation in Neurodegenerative Disorders-a Review. Curr. Neurol. Neurosci. Rep. 2017;17(3):25. doi: 10.1007/s11910-017-0733-2. [http://dx.doi.org/10.1007/s11910-017-0733-2]. [PMID: 28283959]. [DOI] [PubMed] [Google Scholar]
- 78.Spielman L.J., Little J.P., Klegeris A. Physical activity and exercise attenuate neuroinflammation in neurological diseases. Brain Res. Bull. 2016;125:19–29. doi: 10.1016/j.brainresbull.2016.03.012. [http://dx.doi.org/10.1016/j. brainresbull.2016.03.012]. [PMID: 27021169]. [DOI] [PubMed] [Google Scholar]
- 79.Sävman K., Heyes M.P., Svedin P., Karlsson A. Microglia/macrophage-derived inflammatory mediators galectin-3 and quinolinic acid are elevated in cerebrospinal fluid from newborn infants after birth asphyxia. Transl. Stroke Res. 2013;4(2):228–235. doi: 10.1007/s12975-012-0216-3. [http://dx.doi.org/10.1007/s12975-012-0216-3]. [PMID: 23807898]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gao H.M., Hong J.S. Why neurodegenerative diseases are progressive: uncontrolled inflammation drives disease progression. Trends Immunol. 2008;29(8):357–365. doi: 10.1016/j.it.2008.05.002. [http://dx.doi.org/10.1016/ j.it.2008.05.002]. [PMID: 18599350]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mogi M., Harada M., Riederer P., Narabayashi H., Fujita K., Nagatsu T. Tumor necrosis factor-alpha (TNF-alpha) increases both in the brain and in the cerebrospinal fluid from parkinsonian patients. Neurosci. Lett. 1994;165(1-2):208–210. doi: 10.1016/0304-3940(94)90746-3. [http://dx. doi.org/10.1016/0304-3940(94)90746-3]. [PMID: 8015728]. [DOI] [PubMed] [Google Scholar]
- 82.Mogi M., Harada M., Narabayashi H., Inagaki H., Minami M., Nagatsu T. Interleukin (IL)-1 beta, IL-2, IL-4, IL-6 and transforming growth factor-alpha levels are elevated in ventricular cerebrospinal fluid in juvenile Parkinsonism and Parkinson’s disease. Neurosci. Lett. 1996;211(1):13–16. doi: 10.1016/0304-3940(96)12706-3. [http://dx.doi.org/10.1016/0304-3940(96)12706-3]. [PMID: 8809836]. [DOI] [PubMed] [Google Scholar]
- 83.Wu L.J. Microglial voltage-gated proton channel Hv1 in ischemic stroke. Transl. Stroke Res. 2014;5(1):99–108. doi: 10.1007/s12975-013-0289-7. [http://dx.doi. org/10.1007/s12975-013-0289-7]. [PMID: 24323712]. [DOI] [PubMed] [Google Scholar]
- 84.Wan S., Cheng Y., Jin H., Guo D., Hua Y., Keep R.F., Xi G. Microglia activation and polarization after intracerebral hemorrhage in mice: the role of protease-activated receptor-1. Transl. Stroke Res. 2016;7(6):478–487. doi: 10.1007/s12975-016-0472-8. [http://dx.doi.org/10.1007/ s12975-016-0472-8]. [PMID: 27206851]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Fahlenkamp A.V., Coburn M., de Prada A., Gereitzig N., Beyer C., Haase H., Rossaint R., Gempt J., Ryang Y.M. Expression analysis following argon treatment in an in vivo model of transient middle cerebral artery occlusion in rats. Med. Gas Res. 2014;4:11. doi: 10.1186/2045-9912-4-11. [http://dx.doi.org/10.1186/2045-9912-4-11]. [PMID: 25671080]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Chen S., Yang Q., Chen G., Zhang J.H. An update on inflammation in the acute phase of intracerebral hemorrhage. Transl. Stroke Res. 2015;6(1):4–8. doi: 10.1007/s12975-014-0384-4. [http://dx.doi.org/10.1007/s12975-014-0384-4]. [PMID: 25533878]. [DOI] [PubMed] [Google Scholar]
- 87.Dijksterhuis J.P., Arthofer E., Marinescu V.D., Nelander S., Uhlén M., Pontén F., Mulder J., Schulte G. High levels of WNT-5A in human glioma correlate with increased presence of tumor-associated microglia/monocytes. Exp. Cell Res. 2015;339(2):280–288. doi: 10.1016/j.yexcr.2015.10.022. [http://dx.doi.org/10.1016/j.yexcr.2015.10.022]. [PMID: 26511503]. [DOI] [PubMed] [Google Scholar]
- 88.Rolón-Reyes K., Kucheryavykh Y.V., Cubano L.A., Inyushin M., Skatchkov S.N., Eaton M.J., Harrison J.K., Kucheryavykh L.Y. Microglia activate migration of glioma cells through a Pyk2 intracellular pathway. PLoS One. 2015;10(6):e0131059. doi: 10.1371/journal.pone.0131059. [http:// dx.doi.org/10.1371/journal.pone.0131059]. [PMID: 26098895]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Grubman A., Kanninen K.M., Malm T. Multitasking microglia and Alzheimer’s disease: diversity, tools and therapeutic targets. J. Mol. Neurosci. 2016;60(3):390–404. doi: 10.1007/s12031-016-0825-5. [http://dx.doi.org/10.1007/ s12031-016-0825-5]. [PMID: 27660215]. [DOI] [PubMed] [Google Scholar]
- 90.Suzuki H. What is early brain injury? Transl. Stroke Res. 2015;6(1):1–3. doi: 10.1007/s12975-014-0380-8. [http://dx.doi.org/10.1007/s12975-014-0380-8]. [PMID: 25502277]. [DOI] [PubMed] [Google Scholar]
- 91.Whitton P.S. Inflammation as a causative factor in the aetiology of Parkinson’s disease. Br. J. Pharmacol. 2007;150(8):963–976. doi: 10.1038/sj.bjp.0707167. [http://dx.doi.org/10.1038/sj.bjp.0707167]. [PMID: 17339843]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hakimi M., Selvanantham T., Swinton E., Padmore R.F., Tong Y., Kabbach G., Venderova K., Girardin S.E., Bulman D.E., Scherzer C.R., LaVoie M.J., Gris D., Park D.S., Angel J.B., Shen J., Philpott D.J., Schlossmacher M.G. Parkinson’s disease-linked LRRK2 is expressed in circulating and tissue immune cells and upregulated following recognition of microbial structures. J. Neural Transm. (Vienna) 2011;118(5):795–808. doi: 10.1007/s00702-011-0653-2. [http://dx. doi.org/10.1007/s00702-011-0653-2]. [PMID: 21552986]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Thévenet J., Pescini Gobert R., Hooft van Huijsduijnen R. ; Wiessner C., Sagot Y.J. Regulation of LRRK2 expression points to a functional role in human monocyte maturation. PLoS One. 2011;6(6):e21519. doi: 10.1371/journal.pone.0021519. [http://dx.doi.org/10.1371/journal.pone.0021519]. [PMID: 21738687]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Moehle M.S., Webber P.J., Tse T., Sukar N., Standaert D.G., DeSilva T.M., Cowell R.M., West A.B. LRRK2 inhibition attenuates microglial inflammatory responses. J. Neurosci. 2012;32(5):1602–1611. doi: 10.1523/JNEUROSCI.5601-11.2012. [http://dx.doi.org/10.1523/JNEUROSCI.5601-11.2012]. [PMID: 22302802]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Gillardon F., Schmid R., Draheim H. Parkinson’s disease-linked leucine-rich repeat kinase 2(R1441G) mutation increases proinflammatory cytokine release from activated primary microglial cells and resultant neurotoxicity. Neuroscience. 2012;208:41–48. doi: 10.1016/j.neuroscience.2012.02.001. [http://dx.doi.org/10.1016/j.neuroscience.2012.02.001]. [PMID: 22342962]. [DOI] [PubMed] [Google Scholar]
- 96.Marker D.F., Puccini J.M., Mockus T.E., Barbieri J., Lu S.M., Gelbard H.A. LRRK2 kinase inhibition prevents pathological microglial phagocytosis in response to HIV-1 Tat protein. J. Neuroinflammation. 2012;9:261. doi: 10.1186/1742-2094-9-261. [http://dx.doi.org/10.1186/1742-2094-9-261]. [PMID: 23190742]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Greggio E., Civiero L., Bisaglia M., Bubacco L. Parkinson’s disease and immune system: is the culprit LRRKing in the periphery? J. Neuroinflammation. 2012;9:94. doi: 10.1186/1742-2094-9-94. [http://dx.doi.org/10.1186/ 1742-2094-9-94]. [PMID: 22594666]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Schapansky J., Nardozzi J.D., Felizia F., LaVoie M.J. Membrane recruitment of endogenous LRRK2 precedes its potent regulation of autophagy. Hum. Mol. Genet. 2014;23(16):4201–4214. doi: 10.1093/hmg/ddu138. [http:// dx.doi.org/10.1093/hmg/ddu138]. [PMID: 24682598]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Fellner L., Irschick R., Schanda K., Reindl M., Klimaschewski L., Poewe W., Wenning G.K., Stefanova N. Toll-like receptor 4 is required for α-synuclein dependent activation of microglia and astroglia. Glia. 2013;61(3):349–360. doi: 10.1002/glia.22437. [http://dx.doi.org/10.1002/ glia.22437]. [PMID: 23108585]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kim C., Ho D.H., Suk J.E., You S., Michael S., Kang J., Joong Lee S., Masliah E., Hwang D., Lee H.J., Lee S.J. Neuron-released oligomeric α-synuclein is an endogenous agonist of TLR2 for paracrine activation of microglia. Nat. Commun. 2013;4:1562. doi: 10.1038/ncomms2534. [http://dx.doi.org/10.1038/ncomms2534]. [PMID: 23463005]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ahn T.B., Langston J.W., Aachi V.R., Dickson D.W. Relationship of neighboring tissue and gliosis to α-synuclein pathology in a fetal transplant for Parkinson’s disease. Am. J. Neurodegener. Dis. 2012;1(1):49–59. [PMID: 23383381]. [PMC free article] [PubMed] [Google Scholar]
- 102.Fellner L., Stefanova N. The role of glia in α-synucleinopathies. Mol. Neurobiol. 2013;47(2):575–586. doi: 10.1007/s12035-012-8340-3. [http://dx.doi.org/10.1007/ s12035-012-8340-3]. [PMID: 22941028]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Tiwari P.C., Pal R. The potential role of neuroinflammation and transcription factors in Parkinson disease. Dialogues Clin. Neurosci. 2017;19(1):71–80. doi: 10.31887/DCNS.2017.19.1/rpal. [PMID: 28566949]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Yamamoto M., Sato S., Hemmi H., Hoshino K., Kaisho T., Sanjo H., Takeuchi O., Sugiyama M., Okabe M., Takeda K., Akira S. Role of adaptor TRIF in the MyD88-independent toll-like receptor signaling pathway. Science. 2003;301(5633):640–643. doi: 10.1126/science.1087262. [http://dx.doi.org/10.1126/science.1087262]. [PMID: 12855817]. [DOI] [PubMed] [Google Scholar]
- 105.Russo I., Berti G., Plotegher N., Bernardo G., Filograna R., Bubacco L., Greggio E. Leucine-rich repeat kinase 2 positively regulates inflammation and down-regulates NF-κB p50 signaling in cultured microglia cells. J. Neuroinflammation. 2015;12:230. doi: 10.1186/s12974-015-0449-7. [http://dx.doi.org/10.1186/s12974-015-0449-7]. [PMID: 26646749]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ho D.H., Seol W., Eun J.H., Son I.H. Phosphorylation of p53 by LRRK2 induces microglial tumor necrosis factor α-mediated neurotoxicity. Biochem. Biophys. Res. Commun. 2017;482(4):1088–1094. doi: 10.1016/j.bbrc.2016.11.163. [http://dx.doi.org/10.1016/j.bbrc.2016.11.163]. [PMID: 27914807]. [DOI] [PubMed] [Google Scholar]