Abstract
As a result of ischemia or hemorrhage, blood supply to neurons is disrupted which subsequently promotes a cas-cade of pathophysiological responses resulting in cell loss. Many mechanisms are involved solely or in combination in this disorder including excitotoxicity, mitochondrial death pathways, and the release of free radicals, protein misfolding, apopto-sis, necrosis, autophagy and inflammation. Besides neuronal cell loss, damage to and loss of astrocytes as well as injury to white matter contributes also to cerebral injury. The core problem in stroke is the loss of neuronal cells which makes recov-ery difficult or even not possible in the late states. Acute treatment options that can be applied for stroke are mainly targeting re-establishment of blood flow and hence, their use is limited due to the effective time window of thrombolytic agents. How-ever, if the acute time window is exceeded, neuronal loss starts due to the activation of cell death pathways. This review will explore the most updated cellular death mechanisms leading to neuronal loss in stroke. Ischemic and hemorrhagic stroke as well as subarachnoid hemorrhage will be debated in the light of cell death mechanisms and possible novel molecular and cel-lular treatment options will be discussed.
Keywords: Ischemic stroke, hemorrhagic stroke, apoptosis, necrosis, autophagy, pyroptosis, neuroprotective therapies
1. Introduction
Stroke is one of the leading causes of death worldwide and affects nearly 17 million individuals per year [1]. It is divided into mainly two types, namely occlusive' with 'ischemia (80-85% of total cerebral strokes [2]) and hemorrhagic strokes. Brain physiology and blood flow are two processes that play an important role in stroke. Ischemic strokes result from obstructed cerebral arteries and are caused via thrombus formation or emboli or atherosclerosis. Hemorrhagic stroke can be divided as intracerebral hemorrhage hemorrhage (ICH) and subarachnoid hemorrhage (SAH). ICH is mostly due to long lasting increased blood pressure (hypertension). The current treatment for ischemic stroke in the acute time window is reperfusion with recombinant tissue plasminogen activator (rtPA) via i.v. administration within 4.5 hours of onset or intravascular cloth retrieval with devices [3]. However, only 5% of ischemic stroke patients are eligible for this treatment [4]. Altogether, stroke leads to brain damage which causes long-term/lifelong disabilities and or even death. Current research seek for long- term therapeutics primarily to restore post-ischemic neuronal damage. But in order to establish novel treatment options, it is crucial to understand involved cell death mechanisms. In this review we attempt to emphasize post-stroke inflammation and the most updated cell death mechanisms in stroke and discuss several molecular and cellular mechanisms that are potential candidates for novel treatment options.
2. Post-stroke injury propagated by inflammation
Ischemic tissue follows a series of secondary events including vascular, cellular and molecular alterations. The vascular response to ischemia activates endothelial cells and upregulates circulating leukocytes [5] and adhesion molecules including E- (endothelial surface) and P- (platelet surface) and L- (leukocyte surface) selectins, ICAM-1 and integrins. Leukocytes can travel across endothelial cells to the brain by interacting via these adhesion molecules and secrete pro-inflammatory cytokines into the brain. The acute inflammatory response after stroke therefore leads to the interactions between platelets, leukocytes, lymphocytes and endothelial cells that are thereupon responsible for blood-brain barrier (BBB) injury and infiltration of immune cells into the brain parenchyma [6]. The injured BBB can further exacerbate leakage into the brain causing edema and worsen tissue injury. In physiological conditions, injured regions attract inflammatory cascades with an attempt to recover the damaged site. In stroke injury this is also the case, although, with respect to the severity of the injury, the infarct size and area at stake, the harmful cascades may weight more than the recovery processes which disturb the balance of the cellular microenvironment leading to the activation of deleterious pathways including different cell death mechanisms. The inflammatory response to the injured site is therefore not always beneficial but on the contrary can have a catalytic effect on the ongoing post-ischemic injury. Most importantly, inflammation in the brain initiates the release of cytokines and free radicals which lead to cellular injury. Next to these processes, as a secondary event of inflammatory responses, the damaged tissue is removed by the defending immune system and synaptic remodeling is established.
3. Post-stroke cell death exacerbated by many overlaying mechanisms
Next to the role of inflammation, also other cells and factors serve to cerebral injury after stroke. Glial cells play an important role in promoting the regulation of the BBB, angiogenesis and synaptogenesis in physiological conditions but during stroke they may cause a glial scar at the site of damage and thereby prevent further plasticity [7]. Furthermore, the role of calcium, mitochondrial integrity and its response, the release of free radicals and oxidative stress, the role of stressed endoplasmic reticulum (ER) on protein misfolding, white matter injury, glial and astrocytic response and disrupted BBB integrity during inflammation are of high importance in the progress of cell death during post-ischemic stroke [8]. Hence, many of these mechanisms overlap intrinsic pathways and may co-exist in post-stroke injury [9]. The dual role of inflammation as well as the fine crossroad of the activation of different cell death pathways is highly dependent on the individual’s physiological condition and the extent of injury. In fact, this fine tuning of signal transduction both beneficial as deleterious, is complex and may need to be addressed on many levels simultaneously, hence that renders treatment therapies very difficult.
4. Cell death mechanisms in stroke
Several pathways are involved in post-stroke injury which are dependent on the delicate balance between restoration and deleterious pathways. If more damage is accomplished than restored, cell death mechanism may be initiated, these include apoptosis, necrosis, autophagocytosis, necroptosis and pyroptosis. Many of these pathways are extensively discussed in literature, see Table 1. Next, we will highlight the most crucial mechanisms involved in cellular death in stroke.
Table 1.
Post-stroke events leading to inflammation, neurodegeneration stress and/or cell death.
| Factors | Affected Cell/Organelle | Involved Pathways | Outcome |
|---|---|---|---|
| ATP depletion | Neurons and glial cells [20, 21] | Dysfunctional Ca2+ extruders | Apoptosis |
| Excitotoxicity | Apoptosis, necrosis | ||
| Cytoskeletal degradation | Necrosis | ||
| ER [22-26] | Failure of SERCA | ER Ca2+ depletion, Stress | |
| IRE1 oligomerization/downstream kinases | Apoptosis | ||
| Misfolded protein accumulation | Necrosis, apoptosis, autophagy | ||
| Misfolded proteins | ER [20, 22-24, 27-29] | PERK/eIF2α kinase phosphorylation | Autophagy |
| Increased chop expression | Apoptosis | ||
| Protein synthesis inhibition | Ribophagy | ||
| GRP78 chaperone capacity exceeds | Apoptosis | ||
| Excessive glutamate release | Apoptosis, autophagocytosis, necrosis | ||
| Calcium influx | Mitochondria [30-32] | mtPTP opening | Apoptosis |
| ROS production | Oxidative stress, apoptosis, necrosis | ||
| Free radical release | Oxidative stress | ||
| Neurons [12, 33-35] | Phospholipases and esterases activation | Necrosis | |
| Excitotoxicity | Hyperexcitation, dysfunction, apoptosis, necrosis | ||
| T253D-αCaMKII | Excitotoxic cell death | ||
| O-GlcNAcylation of nNOS | Apoptosis | ||
| Astrocytes [36] | Reactive astrocytes | Glial scar formation | |
| Pro-inflammatory cytokine release | Inflammation | ||
| Microglia [37, 38] | Dysfunction | Demyelination and white matter loss, neuronal death | |
| Oligodendrocytes [39] | NOS/NO/ONOO-/excessive PARP-1 | Mitochondrial stress, apoptosis, inflammation | |
| Free radicals | Neurons and glial cells [40] | NADPH oxidase | ROS formation |
| PI3-kinase/Akt pathway activation | Necrosis | ||
| NF-kB upregulation | Inflammation | ||
| TRPM7 channel activation | Increased Ca2+ influx | ||
| Parkin | Mitophagy | ||
| ROS | Apoptosis | ||
| Inflammatory responses | Microglia [41] | TNF-α and IL-1β release | Increase inflammation and CAM |
| Astrocytes [36, 42] | MMPs and MPO release | BBB breakdown | |
| IL-15 | Lymphocyte toxicity | ||
| Dendritic cells [43] | Cytokine release | Inflammation | |
| Endothelial cells [44] | ICAM-1, P-selectin, E-selectin | Attract leukocytes to ischemic area, inflammation | |
| Factors | Affected Cell/Organelle | Involved Pathways | Outcome |
| Inflammatory responses | Lymphocytes [45, 46] | Pro-inflammatory cytokines | Inflammation |
| Macrophages [47-49] | MMP-3 and MMP-9 release | BBB breakdown and degradation of extracellular matrix | |
| TLR 2 and TLR4/IL-23 | Neural cell death | ||
| Neutrophils [50, 51] | iNOS release | Toxic levels of NO | |
| Reactive astrocytes [52] | P2X7 receptor activation and MMP-9 release | Ca2+ overload and mitochondrial depolarization, BBB breakdown | |
| Oligodendrocytes [53] | IL-6 | Inflammation | |
| Brain resident cells and astrocytes [54, 55] |
MCP-1/CCL2 | Migration of leukocytes, monocytes/macrophages/microglia to the ischemic core | |
| Neurons [56-58] | Bax/AIF | Pro-apoptotic | |
| Drp1/mitochondrial dysfunction | Apoptotic cell death | ||
| α-Syn aggregation | Neurons [59-62] | LRRK2 | Apoptotic cell death |
| GSK-3β/Tau hyperphosphorylation/downregulation of Nrf2 | Apoptotic cell death | ||
| Nrf2/NDP52 | Autophagy |
Abbreviations: ER, Endoplasmic reticulum; SERCA, Sarco/endoplasmic reticulum Ca2+-ATPase; IRE1, Inositol-requiring enzyme 1; PERK, PKR-like ER kinase; eIF2α, Eukaryotic translation initiation factor 2A; mtPTP, Mitochondrial permeability transition pore; ROS, Reactive oxygen species; T253D-αCaMKII, Phosphomimic form of calcium/calmodulin-dependent protein kinase type II subunit alpha; O-GlcNAcylation of nNOS, Ser(Thr)-O-linked β-N-acetylglucosamine glycosylation of neuronal nitric oxide synthase; NOS, Nitric oxide synthase; NO, Nitric oxide; ONOO-, Peroxynitrate; PARP-1, Poly [ADP-ribose] polymerase 1; NADPH, Nicotinamide adenine dinucleotide phosphate-oxidase; PI3-kinase, Phosphoinositide-3 kinase; Akt, Protein kinase B; NF-kB, Nuclear factor-kB; TRPM7, Transient receptor potential cation channel, subfamily M, member 7; TNF-α, Tumor necrosis factor alpha; IL, Interleukin; CAM, Cell adhesion molecule; MMP, Matrix metalloproteinase; MPO, Myeloperoxidase; ICAM-1, Intercellular adhesion molecule 1; TLR, Toll-like receptor; iNOS, Inducible nitric oxide synthase; P2X7, P2X purinoceptor 7; CCL2, Chemokine (C-C motif) ligand 2; Bax, Bcl-2 associated X protein; AIF, Apoptosis-inducing factor; Drp1, Dynamin related protein 1; LRRK2, Leucine-rich repeat kinase 2; GSK-3β, Glycogen synthase kinase 3 beta; Nrf2, Nuclear factor erythroid 2-related factor 2; NDP52, Nuclear Domain 10 Protein 52.
5. Excitotoxic cell death
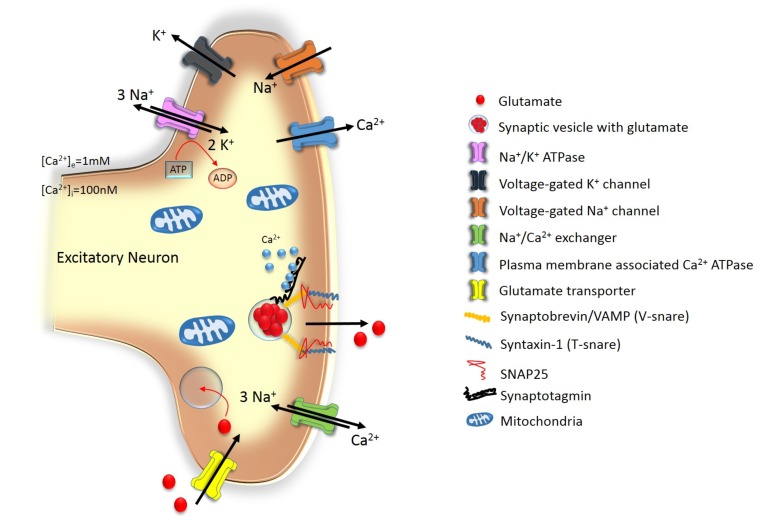
Cell death activated by excitotoxicity can be initiated at several moments of post-injury processes [10]. First of all, when neurons get less blood supply due to an ischemic event especially in the core region of ischemia, the significantly decreased oxygen supply leads to hypoxia hence decreases ATP production. A decreased ATP production leads to failure of the Na+/K+ pump and the plasma membrane Ca2+/ATP-pump. Normally, Ca2+ is always leaking in a low amount into the cell, however if ATP production is decreased, calcium extruders (plasma-membrane associated Ca2+/ATPase and Na+/Ca2+ exchanger) stop working and the intracellular calcium concentration, [Ca2+]i, increases. Membrane transporters involved in calcium homeostasis at the presynaptic neuronal membrane are illustrated in Fig. (1). Secondly, increased and unstoppable intracellular calcium binds to synaptotagmin, located at the vesicular membranes floating in the axon terminal, which in turn causes the vesicles to fuse with the membrane and releases glutamate into the synaptic cleft. Excessive available glutamate leads to hyper excitation of the glutamate receptors namely NMDA, AMPAR and kainate receptors on the post-synaptic membrane. Their hyperexcitaton increases intracellular calcium load and hence, events leading to cell death start. These cascade of events lead to postsynaptic cell death mediated by mainly glutamatergic pathways. Thirdly, continuous glutamate presence leads to continuous depolarization and therefore continuous Ca2+ influx. In normal resting state, the NMDAR contains a Mg2+ in its core that pops out when glutamate binds to the non-GluN1 subunits. Excessive influx of Ca2+ via the NMDAR activates calpein [11, 12] in the post-synaptic neuron which in turn inhibits the Na+/Ca2+X3 receptor. To this end, calcium concentrations get even higher in the neuron. Moreover, massive calcium influx causes activation of calpein which causes proteolysis of cytosolic substrates and therefore leads to cell death. Calpastatin is a useful drug that can decrease neuronal death on this step [13]. Calpastatin is a naturally occurring protein which upon specific binding to activated calpein, can inhibit calpein activity. This calpastatin-calpain interaction is the most relevant mechanism responsible for the regulation of Ca2+-induced proteolysis [14-16].
Fig. (1).
The axon terminal of a neuron with membrane transporters and intracellular components which are prone to excitotoxicity.
Besides the activation of glutamate vesicles, the excessive [Ca2+]i causing mitochondrial membrane depolarization leads to disturbed mitochondrial functions which in turn activate the release of free radicals, enzymes and proteases that also lead to cellular death pathways activation in the neurons of ischemic tissue [10, 17]. Other less prominent routes include possible involvement of dysfunctional glutamate receptors on the pre-synaptic membrane or absent and/or dysfunction of glutamate degrading enzymes in the synaptic cleft which normally maintain physiological concentrations of free glutamate. Also, dysfunctional astrocytes and glial cell that normally mediate in restoring extracellular calcium levels play a role in neuronal cell loss after stroke injury.
One way or another, the most crucial molecular event that mediates the progressive activation of cell death pathways after stroke injury is the excessive intracellular calcium amount that in physiological conditions is constantly being maintained in low levels in the environment either by the neuron or by surrounding supportive astrocytes/glial cells. However, destroyed Ca2+ homeostasis activates either programmed cell death (apoptosis) or collapses due to lost control of cell mechanisms (necrosis), and in some areas of ischemia (especially in penumbral region) both mechanisms of cell death pathways are activated depending on the energy status of the cell. The downstream effects of increased Ca2+ influx is accompanied by the activation of Ca2+-dependent catabolic processes such as proteases, phospholipases, Poly [ADP-ribose] polymerase 1 (PARP-1) and nucleases which lead to cellular toxicity lasting in neuronal death [18, 19].
6. Apoptosis
Excessive [Ca2+]I, is normally removed by mitochondria but in ischemic tissue excessive accumulation of calcium in these mitochondria leads to its membrane depolarization and dysfunction. Dysfunctional mitochondria releases cytochrome C via the mitochondrial permeability transition pore (mtPTP). Cytochrome C, then activates caspase that leads to DNA fragmentation and eventually cell death. Caspases are the main drivers of apoptosis. Moreover, caspases are also involved in the cross-talk between apoptosis and autophagy [63]. Apoptosis is a process that needs energy and hence is prominently present in neurons in the ischemic penumbral region where there is energy production although its amount is decreased significantly [64].
Mitochondrial apoptosis is mainly regulated by B-cell lymphoma-2 (Bcl-2) family proteins which are either pro-apoptotic (e.g. Bax, Bok) or anti-apoptotic (e.g. Bid, Bcl-2) [65]. As a response to danger signals or stress, the expression and activation levels of Bcl-2 family members determine whether programmed cell death will be initiated or restrained [66, 67]. This family of protein members control neuronal apoptosis by acting on the integrity of the mitochondrial outer membrane and energetics as well as regulate Ca2+ homeostasis in mitochondria and ER. Via use of gene targeting, several studies have demonstrated that a single deletion of the pro-apoptotic Bax gene is sufficient to be neuroprotective [68, 69]. After pro-apoptotic proteins are released into the cytoplasm, they initiate the activation of a cascade of caspase enzyme family. Caspase-3 is the last and executive enzyme in the cascade for apoptosis. This enzyme cuts proteins, enzymes and nucleotides leading to dysfunction in the cell homeostasis and hence cell death. In apoptosis, the cell membrane structure is preserved until the cell eventually dies. In contrast to apoptosis, necrosis starts with cell swelling and cell membrane disintegration. Next to mitochondria, also stressed ER induces activation of caspase proteins leading to neuronal apoptosis [27].
Interestingly, several chronic neurodegeneration-related proteins such as α-Synuclein, Parkin, PTEN-induced putative kinase 1 (PINK 1), Parkinson disease protein 7 (DJ-1) and leucine-rich repeat kinase 2 (LRRK2) are also involved in neuronal cell death after ischemic stroke [70]. The post-stroke condition with inflammation, oxidative stress and ER stress altogether provides an optimal environment for α-Synuclein aggregation that promotes inflammation. Especially reactive oxygen species (ROS) enhance α-Synuclein expression and aggregation [71]. Knockdown of α-Synuclein leads to attenuated ischemic markers such as mitochondrial dysfunction, oxidative stress, apoptosis and autophagy [72]. Several studies have shown that increased presence of DJ-1 expression after focal ischemia is neuroprotective by preventing ischemic neuronal death by suppressing ROS production [73-77]. Furthermore, LRRK2-mediated apoptosis in cerebral ischemia is propagated by the modulation of tau phosphorylation in the presence of α-Synuclein. Parkin and PINK 1 are known for their neuroprotective functions after stress-induced mitochondrial injury [78-80]. However, inactivation of these factors due to oxidative and nitrosative stress after stroke injury depletes this protective pathway signaling which leads to neurodegeneration and apoptotic cell death.
7. Necrosis
In ischemic core area, blood flow decreases significantly (below 20% of baseline values). This decrease leads to significant reduction in the amount of ATP. ATP is essential in the maintenance of neuronal membrane potential via Na+/K+-ATPase pump. In ischemia, this pump fails and the regulation of the concentrations of Na+ and K+ inside and outside of the neuronal cell membrane is lost. To this end, Na+ accumulates inside the cell leading to the formation of cellular edema. If this event continuous, the cell membrane will start to rupture and release its content to the extracellular environment and finally the nuclei will degrade. Degradation and release of cellular components to the extracellular space leads to inflammatory reaction around the dying cell. Ca2+ overload, excessive ROS and reactive nitrogen species (RNS) formation also lead to mitochondrial swelling and thereby contribute to neuronal death. Necrosis is recognized by morphological changes such as vacuolation of the cytoplasm, disrupted cell membrane integrity and loss of cell content, presence and release of pro-inflammatory molecules and inflammation in and around the dying cell [81]. The neurons in the ischemic core are prominently subjected to necrosis [64]. On the other hand, astrocytes die by delayed necrosis as demonstrated by Gürer et al. [82]. At penumbral area, astrocytes (although swelled at the beginning) resisted to ischemic conditions better than neurons and showed less apoptotic signaling than necrosis.
8. Necroptosis
At early times of stroke studies, necrosis was thought to be distinct from apoptosis. In recent years, however, it was shown that necrotic cell death can also be driven by defined molecular pathways. Necroptosis, a type of necrotic cell death [83] dependent on receptor-interacting protein kinase-1 (RIPK1) has been recognized and demonstrated in stroke [84].
In apoptosis, especially caspase-3 and 8 are activated. On the other hand, necroptosis requires that the function of caspase-8 is inhibited or disrupted. The downstream mediators in the necroptosis pathway are currently incompletely understood, but the rapid swelling of necroptotic cells that results in plasma membrane rupture are resembling necrosis. In accordance with this knowledge, previously it has been shown by Kilinc et al. and Unal-Cevik et al. that ischemic neurons showed both necrotic and apoptotic features [85, 86]. The authors found lysosomal rupture, lysosomal enzymes spilling into the cytoplasm immediately after ischemia, suggesting that lysosomal membrane integrity was rapidly lost as occurs in necrosis. The same neurons also exhibited caspase-3 and Bid cleavage and cytochrome-C release as markers of apoptosis.
In general, death receptors mediate apoptosis, however these death receptors can also activate necrotic cell death [87]. Increased expression of ligands for death receptors in the ischemic penumbra are Fas ligand (FasL) and tumor necrosis factor alpha (TNF-α) which in apoptosis-eligible cells activate downstream kinases. However, in apoptosis deficient cells, these ligands are not capable of activating caspases but instead activate RIP1 kinase and necroptosis [88]. While, RIP1 is a death-domain containing kinase, its kinase activity is not essential in the propagation of apoptosis. Studies have shown that necrostatins, inhibitors of necroptosis [89], have no effect on apoptosis. How the activation of RIP1 kinase leads to programmed necrosis is not yet revealed, however it seems that Bcl-2 modifying protein (Bmf), one of the Bcl-2 family protein members, plays a key role in mediating necroptosis [90] by inducing necrotic mitochondrial damage. Besides RIP1, also PARP1, nicotinamide adenine dinucleotide phosphate (NADPH) oxidases and calpains have been assigned a role in mitochondrial mediated necroptosis [91].
Recently, Zille et al. demonstrated that cell death mechanisms in cultured neurons exposed to hemoglobin or hemin showed simultaneous features of both necroptosis and ferroptosis (an iron- and ROS-dependent form of regulated cell death [92]) in their ICH model [93]. The authors also recommended future treatment approaches to decrease the signaling of both pathways to below their threshold in order to achieve cell survival.
9. Autophagy in stroke: good and bad
Autophagy is another programmed cell death pathway in mammalian cells that regulates the bulk degradation of aggregated macromolecules and damaged organelles (associated with several stress environments) in the cytosol via the lysosomal system [94]. This pathway is interestingly essential for healthy cells on long term [95]. Autophagy is divided into three pathways including chaperone-mediated autophagy, microautophagy and macroautophagy [96] from which macroautophagy is the most demonstrated pathway in mammalian systems such as ischemic stroke [97], ICH [98] and SAH [99]. Several evidence has proven that autophagy is involved in ischemic brain tissue [100, 101] and hemorrhagic stroke [98, 102]. Some experiments have located autophagy in the ischemic penumbra [103]. Although some studies propose protective involvement of autophagy in cerebral ischemia [104], other increasing evidence shows increased neuronal cell death in models of ischemic brain injury [95]. Also, interactions between autophagy and apoptosis appear to decide the fate of the cells. The signaling pathway of autophagy is driven on mechanistic target of rapamycin (mTOR) and AMP-activated protein kinase (AMPK) pathways, which leads to autophagy inhibition [105] and initiation respectively [106]. Autophagy basically starts with the formation of autophagosomes (bilayer membrane vesicles) which engulf damaged organelles from the cytosol, then fuse with lysozomes and form autolysozomes which degrade the engulfed content. Furthermore, the selective removal of damaged mitochondria from the injured cell through autophagosome is called ‘mitophagy’ and this unique mechanism plays an important role in cerebral ischemia [107] as well as in several neurodegenerative diseases. An increased accumulation of autophagosomes in the cytosol is mainly observed together with upregulated LC3-II (autophagosomal membrane component). The extent to which autophagy is present in a system is prominently based on the LC3-II/I ratio. It is proposed that autophagy could only be beneficial if the autophagy stream is intact, and that detrimental effects could be caused if the autophagy flux was impaired mainly leading to cell death. Moreover, a moderate activation of autophagy seems beneficial in post-stroke condition, however, decreased or increased autophagy proteins suggest a pro-death role of autophagy. Also, ischemic excitotoxicity participates in the autophagy stream.
Following ischemic and hemorrhagic stroke, an overload of ferritin causes brain damage and cell death which are mediated by a process called ‘ferritinophagy’ [108]. This is also a form of autophagy, only the selective turnover of ferritin is accomplished by the autophagosomes. The nuclear receptor coactivator 4 (NCOA4) is the targeted receptor in this pathway [109].
Post-stroke brain damage and neuronal cell death is likely for a major part caused by loss of supporting astrocytes. Recently, it was revealed that autophagy also contributes to cell death of ischemic astrocytes [110]. Both in neurons and non-neural cells, lysosomal proteases are involved in mediating Cyt-c release and caspase activation leading to apoptosis. Although the exact mechanism of autophagy in stroke is unclear, yet it was found that inhibition of autophagy causes lysosomal membrane stabilization that retains the signaling of lysosomal proteases such as cathepsins which subsequently inhibit the truncated BH3 interacting domain death agonist (tBid)-mitochondrial apoptosis pathway in ischemic astrocytes [111].
Furthermore, autophagy is also involved in post-stroke inflammatory response where microglia regulate cytokine production [112]. Together with apoptosis, autophagy also effects p53-mediated cell death pathway after ischemic stroke/reperfusion insult [113].
Weis et al. showed lately that autophagy in the brain of neonates is dependent on gender and location [114]. In their study, autophagy and apoptosis were studied in the brain following hypoxia-ischemia induction in rats and it was found that the lysosome activity decreased whereas caspase-3 activity increased in the cortex of female rats compared to male rats [114]. Also, the researchers found that autophagy occurred at specific locations only, namely the cortex and hippocampus [114].
In general, it is believed that autophagy is time-dependent and has dual roles with turnovers from being beneficially anti-apoptotic enhanced at basal levels to deterioration into pro-apoptotic favor under ischemic or hypoxic conditions. However, discrepancies have arisen about whether autophagy should be enhanced or diminished for treatment approaches.
10. Pyroptosis: an inflammatory cell death mechanism
Defined as host cell death and inflammation, pyroptosis is morphologically and mechanistically distinct from other types of cell death. One of the features of pyroptosis is the dependence on caspase 1 [115]. Cells that undergo pyroptosis show morphological caspase-1 dependent features such as rapid plasma-membrane rupture and release of pro-inflammatory intracellular contents, DNA damage and cell lysis, destruction of actin cytoskeleton and eventually cell death by an yet unknown mechanism [116, 117].
Damage associated molecular patterns (DAMPs) released by dying cells and some pro-inflammatory cytokines such as interleukin (IL)-1α released by necrotic cells [118], initiate the inflammatory response. As a response to tissue injury, toll-like receptors (TLRs) initiate a signaling cascade that leads to the cellular activation and production of inflammatory cytokines (TNF, IL-6, IL-8 and IFNs). Furthermore, the nucleotide-binding oligomerization domain-containing 1 (NOD1) and NOD2 of nod-like receptors (NLRs) also trigger signaling cascades leading to inflammatory cytokine production. Both TLRs and NOD1 and NOD2 mediate cells to undergo caspase 1 activation and produce cytokine IL-1β.
DAMPs activate intracellular pattern recognition receptors (PRRs) which lead to the formation of multiprotein complexes called inflammasomes such as NLR Pyrin domain containing 1 (NLRP1) and NLRP3 [119]. Moreover, it is suggested that plasma membrane PRRs on neurons and glial cells can play an important role in activating nuclear factor kappa B (NFκB) and mitogen activated protein kinase (MAPK) pathways [120].
Inflammasomes, expressed abundantly in the brain and immune cells, may play important roles in detecting cellular damage and mediating inflammatory responses to aseptic tissue injury during ischemic stroke [119]. Signaling through NLRP1 and NLRP3 inflammasomes produces cleaved caspase-1 [121, 122], which in turn both initiates the formation and regulates the release of the pro-inflammatory cytokines IL-1β and IL-18 that are released into the extracellular environment [123-125]. The evidence for the role of inflammasomes and pyroptosis in stroke pathology is well defined in several studies [126-128]. This pro-inflammatory programmed cell death pathway is facilitated by caspases (caspase 1 or caspase 4/5/11) [129-131]. Caspase 1 deficiency, or pharmacological inhibition, provides protection against inflammation, cell death and organ dysfunction that are associated with these diseases, making caspase 1 an attractive therapeutic target against pyroptosis in post-stroke injury [132-134].
11. Microglia an active player in stroke
Brain resident cells, microglia, are the first active responders to ischemia. While these cells produce and release many pro-inflammatory factors by producing reactive species, attracting immune cells, phagocytosis, and producing inflammatory mediators such as IL-1β, TNF-α, IL-6 and matrix metalloproteinases (MMPs) etc., these microglia also generate anti-inflammatory mediators such as IL-4, IL-10, and transforming growth factor beta (TGF-β) during the restoration phase of the inflammation [135, 136]. The ischemic brain triggers microglia activation which has been linked to worsen stroke outcomes. Other brain cells and brain entered leukocytes are in close relationship with microglia involved in the infarct region. Hence, the influence of activated microglia on post-stroke inflammation and brain damage is not fully understood. Similar to activated microglia, activated astrocytes also produce pro- and anti-inflammatory cytokines. Therefore both, microglia ad astrocytes are prominent regulators of neuronal cell death after ischemic brain injury and are potential target candidates for stroke treatment [128, 137-141].
One of the mediators of acute brain injury is the pro-inflammatory cytokine IL-1. Together with activated microglia and other inflammatory cells (neutrophils, macrophages, lymphocytes) the post-stroke inflammatory response further propagates [142] and covers the area around the ischemic core, so called ‘peri-infarct area’ or ‘inflammatory penumbra’. After initiating a cascade of immune cells, post-stroke inflammation is ultimately enhancing either recovery or cell death. Inflammatory cytokines, produced by microglia, such as IL-6, TNF-α and adhesion molecules are involved in the early stages of neurological impairment and ischemic infarct volume [143]. Moreover, it has been revealed that IL-6 predicts both the severity of the stroke lesions and functional outcome of patients [144, 145]. The plasma IL-6 concentrations associated with the severity of acute ischemic stroke in humans could therefore serve as a prognostic marker [53].
12. The biphasic response of neuro-vascular unit injury and protection
Whereas excitotoxicity and oxidative stress is present during the first hours till days after stroke injury, inflammation can last for months. The members of the neurovascular unit (endothelia, neurons, astrocytes, and pericytes) together with microglia, oligodendrocytes, neutrophils, monocytes and lymphocytes are involved in post-stroke injury [146]. The neurovascular unit cells are the first main responders (minutes to hours) to the vascular occlusion while immune cells initiate a robust inflammatory response that will be needed later in the delayed recovery phase (days to months). In the acute phase after stroke onset, lost cell-cell crosstalk between the cells of the neurovascular unit mediates both acute injury and delayed recovery. Moreover, the neurovascular unit mediators (NMDA, MMPs, HMGB1, VEGF, ROS, TNFα etc.) are deleterious in the acute phase after stroke onset causing immune cell recruitment, BBB breakdown and cell death mainly in the core area after stroke injury, however are on the contrary involved beneficially in the recovery phase by mediating angiogenesis, axonal sprouting and neurogenesis in the preserved peri-infarct area [146-148]. For example, the vascular endothelial growth factor (VEGF) increases BBB permeability in the acute phase, and facilitates angiogenesis and neurogenesis in the delayed stroke phase [149, 150]. Stroke pathophysiology therefore shows a biphasic phenomenon [146-148] which makes it rather difficult for stroke treatment.
13. Epigenetics role in cell death and inflammation after stroke
Epigenetic processes such as DNA methylation, post-translational modifications of histone proteins and microRNAs (miRNAs) which regulate gene expression without directly changing the sequence of DNA have recently emerged as important regulators of increased plasticity during the recovery phase of stroke [151-153]. Global DNA methylation levels change dramatically after ischemic injury and contribute to cell death by silencing the neuroprotective genes [154-156]. On the other hand, it is also shown that DNA methylation is involved in restorative processes such as adult neurogenesis and synaptic plasticity [157]. Upon this, several DNA methylation and methyltransferase inhibitors such as Zebularine and Resveratrol have been suggested as therapeutic epigenetic targets against cerebral ischemia [156, 158].
Another epigenetic mechanism, histone deacetylase enzyme, deacetylates specific lysine residues in histone tails which leads to chromatin condensation and gene repression. The extent of histone deacetylase expression after stroke injury is dependent on cell type and location [159]. There are several types of histone deacetylases enzymes, from which stroke causes a global reduction in acetylation levels of histones H3 [160, 161] and H4 [162] in the ischemic brain. Histone deacetylase starts within hours of stroke onset and can last for two weeks [163]. Histone deacetylase inhibitors can specifically change gene expression and ameliorate ischemic damage by increasing the expression of neuroprotective proteins such as Bcl-2 and Hsp70 and thereby become potential therapeutic targets for ischemic stroke therapy [164].
Furthermore, ischemic stroke injury dramatically alters the expression of miRNAs that are involved in neurogenesis and brain repair [165]. One of the miRNAs that is downregulated after stroke is miR-124a, which normally acts to reduce the proliferation of neural progenitor cells in the subventricular zone of adult animals [166]. Downregulated miR-124a leads to increased cellular proliferation and neurogenesis [166] and is therefore a beneficial endogenous brain repair mechanism after stroke injury.
The ability for dynamic and reversible epigenetic modifications in neurons makes this approach very suitable for stroke treatment. While preliminary studies show modulation of neural cell regeneration and promoted brain repair and functional recovery after cerebral ischemia, yet these epigenetic strategies are far from clinical level and need more investigations.
14. Novel molecular and cellular treat-ment strategies
Treatment in the acute phase of stroke, is prominently based on quick recovery of the blood flow or by neuroprotective agents aiming to disrupt the progression of the deleterious pathways [167]. On long-term, current approaches seek to achieve both to minimize the damage to the peri-infarct area and simultaneously maximize the restoration potential of lost neuronal cells. Post-stroke restoration occurs mainly by increased spine formation, axonal spouting and plasticity when the brain reorganizes after the injury [168, 169]. However, this is generally not enough to restore back to physiologically functional level. Also, neuronal circuitry can be imbalanced for a long period after the stroke accident. Cortical stimulation therapies can reorganize this inhibition-excitation balance and restore the neuronal circuitry [170, 171]. With regard to the post-stroke inflammation, in order to inhibit components of the inflammation cascade after stroke, some potential molecular and cellular treatment modalities could include ER stress inhibitors, microglia derived cytokines, free radical scavengers, cyclooxygenase-2 (COX-2) inhibitors and inhibitors of cell death pathways. Also, immunotherapy targeting potential receptors, cytokines, and adhesion molecules as well as epigenetic mechanisms have lately been gaining more scientific attention. A list of several potential targets for stroke recovery is provided in Table 2.
Table 2.
Potential neuroprotective targets for post-stroke brain damage and neuronal loss.
| Therapeutic Candidate | Targeted Cell | Outcome | Study Model | |
|---|---|---|---|---|
| Endogenous/Drug Therapies | ||||
| Uric acid | Neuronal mitochondria | Reduces excessive intracellular calcium, reduces excitotoxicity | In vitro rat hippocampal neurons [175] | |
| GRP78 | Neuronal ER | Ca2+ homeostasis, protects against excitotoxicity and apoptosis | In vitro rat hippocampal neurons [176] | |
| Arundic Acid (ONO-2506) | Astrocytes | Diminishes activation of astrocytes, reduces toxic levels of S-100β |
In vitro astrocytes [177], permanent focal ischemia or transient focal ischemia models in rodents [178], patients with acute ischemic stroke [179] |
|
| Resveratrol | Neurons | Prevents oxidative stress, attenuates neuronal death | Rats subjected to global cerebral ischemia [180] | |
| Coumestrol | Neurons | Neuroprotective, prevents long-term neuronal death | Rat model of global ischemia [181, 182] | |
| TNFα and TNFβ | Neurons | Maintenance of calcium homeostasis | In vitro embryonic rat hippocampal, septal, and cortical neurons [183] | |
| GDNF | Neurons | Protects against NMDA-induced cell death | In vitro mouse cortical neurons and astrocytes and glial cells [184] | |
| TAT-GDNF | Stroke volume | Transports GDNF across the BBB, reduces caspase 3 activity, increases viable neurons | Mouse model of focal cerebral ischemia [185] | |
| IgG-GDNF and IgG-TNFR combination therapy | Stroke volume | Transports GDNF and TNFR across the BBB, reduces stroke volume in acute ischemic stroke | Mouse model of focal cerebral ischemia [186] | |
| BDNF-MAb | Stroke volume | Transports BDNF across the BBB, reduction in stroke volume and an improvement in functional outcomes | Rat model of permanent MCAO [187] | |
| IGF-1 | Microglia and neural stem cells | Axonal growth and neurogenesis, tissue repair, cell proliferation, migration | In vitro neural progenitor cells, primary microglial culture [188] | |
| Fumarate | Monocytes | Increases the level of neuroprotective IL-10, improves functional outcome, decreases edema volume after stroke | Mouse model of permanent MCAO [189] | |
| TGF-β1 | Microglia | Microglial phenotype changes from pro-inflammatory to anti-inflammatory phenotype, functional recovery | Murine model of ICH and plasma TGF-β1 levels of patients after ICH [190] | |
| Absence of Collagen XV | Endothelial cells | Neuroprotective | Mouse model of ischemic stroke [191] | |
| Humanized monoclonal anti-E/P-selectin | Endothelial cells | Neurovascular protective | Primate model of cerebral ischemia [192] | |
| P-selectin | Endothelial cells | Reduces BBB breakdown | P-selectin deficient mice, transient ischemic stroke [193] | |
| β2-integrin blocking | Neutrophils | Reduces neutrophil recruitment and decreases neutrophil mediated inflammation | Mouse model of renal ischemia-reperfusion [194] | |
| GCSF | Monocytes | Reduces monocyte recruitment, reduces integrin expression on monocytes |
Mouse model of focal brain ischemia [195] | |
| VEGF-A | Neurons | Pro-angiogenesis, neurogenesis, neuroprotective | Rat model of MCAO [196] | |
| HSPG and CSPG | Reactive astrocytes |
Reduces glial scar formation | Rat model of chronic stroke [197, 198] | |
| Perlecan domain V | Endothelial cells | Neuroprotective, improves stroke-affected motor function, and increases the post-stroke angiogenic response | Mouse and rat model of stroke [199] | |
| Therapeutic Candidate | Targeted Cell | Outcome | Study Model | |
| Endogenous/Drug Therapies | ||||
| Inhibition of caspase-1 or caspase-11 | Neurons, astrocytes and microglia | Decreases apoptosis and pro-inflammatory cytokines, promotes cell survival | Rat model of permanent MCAO [132], organotypic rat slices [133], mouse model of MCAO [134] | |
| MFGE8 | Neurons and glia | Inhibits inflammasome-induced IL-1β | Mouse model of permanent focal cerebral ischemia [200] | |
| IL-1β antagonist | Neurons | Neuroprotective | Rat model of transient cerebral ischemia [123] | |
| IVIg | Neurons | Suppresses NLRP1 and NLRP3 inflammasome mediated neuronal death | Rat model of ischemic stroke [125] | |
| Beclin, Parkin | Neuronal ER and mitochondria | Mitophagy, cell survival | In vitro primary neuron culture and mouse model of transient MCAO [26], rat model of MCAO [201] | |
| Necrostatin-1 | Neurons | Inhibits necroptosis, suppresses apoptosis and autophagy, reduces brain edema and blood–brain barrier disruption, improves neurological outcome | Mouse model of MCAO, primary mouse cortical neuron culture [202], mouse ICH model [203], rat ICH model [204] | |
| mtPGAM5 | Neurons | Mitophagic protection against necroptosis | In vitro MEF cells and mouse model of focal middle carotid artery occlusion/reperfusion [205] | |
| TRPM7 gene silencing | Neurons | Neuroprotective, neuronal survival | In vitro hippocampal and cortical neurons [206], rat model of ischemia [207], primary neuronal culture and neonatal mice model of hypoxia–ischemia [208] | |
| Genetic inhibition of RIPK1 | Neurons | Reduces acute neuronal death and improves functional outcome | Mice models of ICH [209] | |
| PSD-95 inhibitor | Neurons | Inhibits PSD-95/nNOS/NMDA receptor-induced excitotoxicity [210], reduces infarct volumes, improves neurobehavioral outcome | Rat model of pial vessel occlusion or transient or permanent MCAO [211], neonatal mice pups with hypoxic-ischemia model [212] | |
| miR-181a antagomiR | Astrocytes | Enhances estrogen receptor-α mediated stroke protection in females, reduces infarction size and improves behavioral outcome | In vitro cortical male and female astrocyte culture and female mice model of MCAO [213], mice model of MCAO [214] | |
| miR-365 antagomiR | Reactive astrocytes | Modulation of PAX6-mediated astrocyte-to-neuron conversion, reduces neurological deficits and cerebral infarct volume | Rat model of MCAO [215] | |
| miR-3473b antagomiR | Microglia | Reduces infarct damage, reduces microglia-mediated neuroinflammation | Mouse model of MCAO [216] | |
| miR-15a/16-1 cluster antagomiR | Stroke volume | Reduces pro-inflammatory responses, upregulates anti-apoptotic proteins, improves neurobehavioral performance | Mouse model of MCAO [217] | |
| miR-30d-5p antagomiR | Neurons | Increases autophagy and decreases apoptosis, decreases infarct volume, improves neurological performance | Rat model of cerebral hypoxic-ischemia [218] | |
| Cell Therapies | ||||
| IPS cells | Neurons | Replace lost neurons | Mouse and rat models of stroke [219] | |
| Exogenous MSCs | Glial cells | Reduce microglia/macrophages, enhance gliogenesis, reduce scar thickness | Retired breeder rats subjected to MCAO [220, 221] | |
| Therapeutic Candidate | Targeted Cell | Outcome | Study Model | |
| Cell Therapies | ||||
| Autologous MSCs | Stroke volume | Less prominent atrophy, improved functional outcome | Patients with cerebral infarct at the middle cerebral arterial territory and with severe neurological deficits [222] | |
| Exogenous MSCs | Proliferating cells and oligodendrocytes | Facilitate axonal sprouting and remyelination | Adult rat model of MCAO [223] | |
| NT2N, ReNeurons | Exogenous neural cells | Neuroprotective, restoration of damaged areas | Rat model of stroke [224], rodent models of stroke model and human patients after stroke injury [225] | |
| Autologous CD34+ cells | Stroke volume | Improved clinical functional outcomes, reduced lesion volumes, promotion of angiogenesis and neurogenesis | Patients with acute ischemic stroke [226] | |
Abbreviations: GRP78, 78-kDa glucose-regulated protein; S-100β, Glial specific protein; TNF, Tumor necrosis factor; MCAO, Middle cerebral artery occlusion; GDNF, Glial cell line-derived neurotrophic factor; TAT, 86-amino acid protein; IgG, Immunoglobulin G; NMDA, N-methyl-D-aspartate receptor; BDNF, Brain-derived neurotrophic factor; IGF-1, Insulin-like Growth Factor 1; IL, Interleukin; TGF-β, Transforming growth factor beta; GCSF, Granulocyte colony stimulating factor; VEGF-A, Vascular endothelial growth factor A; CSPG, Chondroitin sulfate proteoglycan; HSPG, Heparan sulfate proteoglycans; MFGE8, milk fat globule-epidermal growth factor 8; IVIg, Intravenous immunoglobulin; mtPGAM5, Mitochondrial protein PGAM5; MEF, Mouse embryonic fibroblast; TRPM7, Transient receptor potential cation channel, subfamily M, member 7; RIPK1, Receptor-interacting protein kinase-1; PSD-95, Postsynaptic density protein 95; IPS, Induced pluripotent stem cell; MSC, Mesenchymal stem cell; NT2N, Teratocarcinoma-derived Ntera2/D1 neuron-like cells; CD34+, Hematopoietic progenitor cell antigen.
Potential drugs that can be used along with thrombolytic treatments are agents that may provide inhibition on cell death pathways like caspase-3, calpein, and cathepsin inhibitors [172] and agents balancing profound autophagy. Although there are a number of potential drugs, unfortunately they can’t penetrate to brain tissue due to the BBB. For example, caspase-3 inhibitors provide significant protection if given via intracerebral application. But using targeted drug delivery strategies can overcome the BBB issue. Karatas et al. clearly demonstrated that intravenously applied nanoparticles loaded with z-DEVD-FMK (a caspase inhibitor) provided significant neuroprotection after stroke and associated also with clinical improvement [173].
Both autophagy and apoptosis in neurons in the penumbral area after ischemic stroke can be evaluated for LC3-II and cleaved caspase-3 expression, respectively. While numerous studies have revealed that apoptosis and autophagy are co-occurring simultaneously in the penumbral area, Deng et al. showed recently that during the first 5 hours both autophagy and apoptosis are upregulated while after 72 hours of permanent ischemic stroke model, autophagy is still upregulated but apoptosis downregulated [174]. With regard to the latter, the study underlined that therapeutic strategies should rely on the dynamic activation status of both pathways.
Dhungana et al. showed that the lack of endothelial collagen XV is neuroprotective after ischemic stroke in collagen XV (member of the multiplexin family) knock-out mice and that this was correlative with rtPA treatment mechanism causing increased unbound collagen XV in plasma of wild-type mice [191]. Also, the study showed that the protective behavior of the absence of collagen XV may be mediated by the increased type A VEGF production after stroke onset [191]. VEGF-A is particularly of interest due to its abilities to mediate neuroprotection, angiogenesis, neurogenesis, the migration and survival of neurons and axon guidance [196].
In a recent study, Wei-Na et al. revealed that microglia depletion aggravates brain inflammation and brain injury after ischemia. In their middle cerebral artery occlusion (MCAO) model of mice, they used a colony-stimulating factor I receptor (CSF1R) inhibitor that inhibits microglia survival and proposed that microglia have neuroprotective effects after stroke by inhibiting the response from astrocytes in the post-ischemic inflammatory condition [227].
Interestingly, necroptosis inhibitors can reduce programmed necrotic cell death by selectively targeting the inactive form of the RIP1 kinase [89]. This necrostatin-1 was shown neuroprotective against ischemic brain damage. Moreover, recently researchers also demonstrated that intracerebroventricular injection of necrostatin-1 can improve neurologic outcomes in mice following ICH [228].
The cell death mechanism, pyroptosis, mainly mediated by NACHT, LRR and PYD domains-containing protein 3
(NLRP3) inflammasome contributes to ischemic brain injury. Regulation of the activation of this NLRP3 inflammasome at the molecular level may lead to potential novel therapeutic approaches [229]. Inhibitors of this inflammasome have revealed neuroprotection, however, many aspects of this approach need to be solved before it can be translated to the clinical level.
Next to targeting cellular factors, also focus can be led on the restoration of the neurovascular unit, especially the role of pericytes are crucial in ischemic injury. Namely, pericytes contribute to rapid and localized proteolytic degradation of the BBB during cerebral ischemia [230], and therefore can be suitable targets for the recovery of the capillary flow by antioxidative/antinitrative treatments.
Selectins can be a therapeutic target for neurovascular protection since they have important roles in cell death mechanisms of stroke [192, 231-233]. For example, P-selectin mediates the recruitment of leukocytes in the early phase after stroke and is a potential target for reducing BBB breakdown [193]. Furthermore, a humanized monoclonal anti-E/P-selectin antibody investigated in nonhuman primate stroke model was demonstrated to be safe and a potential effective clinical treatment for human stroke [192]. The study showed that, immediately after the onset of 1 hour of temporary ischemia, trends showed reduced polymorphonuclear leukocyte infiltration into ischemic cortex, reduced infarct volumes (by 41%), improved neurological score (by 35%), and improved ability to self-care (by 39%) [192].
Due to the infiltration of monocytes into the brain that correlates with brain edema and stroke outcome, recently, Weise et al. investigated the response of monocytes to high-dose of granulocyte colony stimulating factor (GCSF) and found out that this cytokine/hormone reduces monocyte infiltration into the ischemic brain [195]. The study further showed that GCSF decreases integrin expression on circulating inflammatory monocytes after stroke in an IL-10 dependent way. Thus, reduced presence of adhesion molecules leads to reduced infiltration of monocytes into the brain and can be neuroprotective after stroke injury.
Specific miRNAs are also emerging as new molecular targets in both prevention of stroke as well as in recovering post-stroke injury. miRNAs are endogenously expressed noncoding short single-stranded RNAs that play a role in the regulation of gene expression at the post-transcriptional level, via degradation or translational inhibition of their target mRNAs [234]. Since stroke treatment has not succeeded with targeting single genes due to complex overlapping pathways, miRNAs are especially useful because of their ability to simultaneously regulate many target genes. miRNAs which are silencing their target mRNA are called antagomiRs. During stroke prevention, the use of synthetic antagomiRs may reduce focal cerebral damage [235]. Additionally, even in post-stroke injury antagomiRs have shown promising results in vivo [214, 215]. One of these antagomiRs, miR-181, administered 2 hours after MCAO to mice reduced the infarct volume and improved long-term recovery. Besides, post-stroke inflammation was reduced and anti-apoptotic BCL2 and apoptosis inhibitor XIAP were increased, indicating a neuroprotective outcome. In another study, it was found that the overexpression of miR-365 in ischemic brain and hypoxic cultured astrocytes caused a decreased PAX6 expression leading to reduced astrocyte-to-neuron conversion. Following this result, the miR-365 antagomiR was investigated and the modulation of astrocyte-to-neuron conversion was targeted by knockdown of miR-365 in a rat model of ischemic injury. The inhibition of miR-365 caused an increase in PAX6 expression and enhanced the conversion of astrocytes into mature neurons in rat brain after MCAO. Overall, the use of antagomiRs may be quite promising in enhancing post-stroke neurogenesis and brain repair.
15. Cell therapies
Stem cell therapy is a potential therapy for stroke, however, many issues are addressed within this area including the therapeutic dose, type of cell therapy, and thorough understanding of the molecular and cellular mechanism [236].
Cell therapies for post-stroke recovery have different mechanism as target. In fact, endogenous neural stem cells, located in the subventricular zone and the dentate gyrus, can be targeted for increased proliferation and/or migration to the damaged site to compensate for and/or replace the lost neurons (Table 2). This approach can be established by using factors such as glial cell line-derived neurotrophic factor (GDNF), brain-derived neurotrophic factor (BDNF), GCSF, insulin-like Growth Factor 1 (IGF-1), drugs such as indomethacin, non-coding RNA and hormones such as erythropoietin for neural stem cell proliferation, and stromal derived factor 1, integrin β1, and extracellular matrix manipulations for neural stem cell migration (8). Exogenous cell replacement therapies aim to deliver, teratocarcinoma-derived Ntera2/D1 neuron-like cells (NT2N) [225], ReNeuron and neural progenitor cells to the damaged areas in the brain. Stem cells can act as immunoregulators, promote angiogenesis and help restore the BBB. They further achieve synapse formation, dendritic branching and axonal plasticity. Currently, induced pluripotent stem (IPS) cells are very promising in reprogramming cells to the desired neural cells which may be potential candidates in replacing the lost tissue in post-ischemic injury. But these cell therapies [237] are on their baby steps yet and much effort and enormous studies are necessary in order to provide translational approaches for stroke treatment.
Conclusion
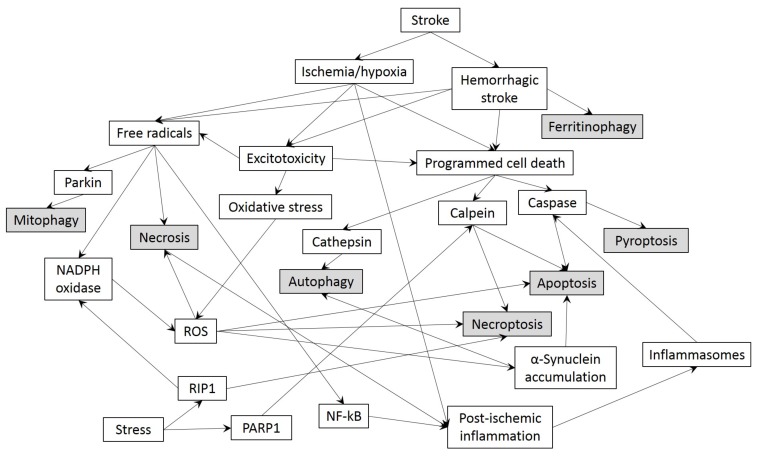
Many novel cellular treatment strategies lead to failures in human strokes and this can result from several factors. One of these factors is related to the translation of bench-to-bed data. Novel treatment modalities are tested in very well structured animal models with controlled physiological parameters including temperature, O2 and CO2 levels. Additionally, all human strokes and time to hospital admissions are different from each another showing high inter-individual variability. Besides, in many preclinical stroke models treatments are given at a certain time point after injury onset, however patients admitted to hospital after stroke may not be suitable for application of novel drugs due to late admission and results are not as homogenous as pre-clinical studies. These abovementioned variations may alter the therapeutic response in human strokes. Also, preclinical studies usually utilize minimum numbers of animals and conditions that may vary between laboratories. To this end, the Stroke Treatment Academic Industry Roundtable (STAIR) [238] recommends consolidation of data from different laboratories into a mega merged databank to advance both preclinical as well as clinical experimental design and outcome. Finally, it is also recommended to establish targeting multiple mechanisms simultaneously instead of meeting a solo pathway after stroke injury. Namely, crosstalk between different cell death pathways determines the fate of an injured cell after ischemic or hemorrhagic stroke. There is increasing evidence that multiple cell death pathways are simultaneously present in the ischemic core and penumbral area. Moreover, these pathways are fine-tuned and have either beneficial, deleterious or dual roles in the progression of post-stroke brain damage. The final death or survival state of a stroke-injured cell is likely dependent on the threshold of incoming and outgoing signaling cascades. Many treatment modalities incorporate the interruption of solo signaling cascades leading to suppression of the activation of cell death mechanisms. However, the cell death pathways are overlapping each other in several steps of the cascades and share common features which may end up in opposite cell fates when activated or inhibited, rendering a rather difficult system to regulate (Fig. 2). If the signaling mechanism of these cell death pathways could be enlightened for the interfering parts, or possible combination therapies may be developed for targeting multiple cascades at the same time, it might actually bring us a step closer to a promising approach for stroke treatment.
Fig. (2).
Schematic overview of different and complex cell death pathways involved in stroke.
Acknowledgements
Declared none.
List of Abbreviations
- AMPK
AMP-Activated Protein Kinase
- BBB
Blood-Brain Barrier
- Bcl-2
B-Cell Lymphoma-2
- BDNF
Brain-Derived Neurotrophic Factor
- Bmf
Bcl-2 Modifying Protein
- COX-2
Cyclooxygenase-2
- CSF1R
Colony-Stimulating Factor 1 Receptor
- DAMP
Damage-Associated Molecular Patterns
- DJ-1
Parkinson Disease Protein 7
- ER
Endoplasmic Reticulum
- FasL
Fas Ligand
- GABA
Gamma-Aminobutyric Acid
- GCSF
Granulocyte Colony-Stimulating Factor
- GDNF
Glial Cell Line-Derived Neurotrophic Factor
- ICH
Intracerebral Hemorrhage
- IGF-1
Insulin-like Growth Factor 1
- IL
Interleukin
- IPS
Induced Pluripotent Stem Cells
- LRRK2
Leucine-Rich Repeat Kinase 2
- MAPK
Mitogen Activated Protein Kinase
- MCAO
Middle Cerebral Artery Occlusion
- miRNA
microRNA
- MMP
Matrix Metalloproteinase
- mTOR
Mechanistic Target of Rapamycin
- mtPTP
Mitochondrial Permeability Transition Pore
- NADPH
Nicotinamide Adenine Dinucleotide Phosphate-Oxidase
- NCOA4
Nuclear Receptor Coactivator 4
- NFκB
Nuclear Factor Kappa B
- NLR
Nod Like Receptors
- NLRP1
NACHT, LRR and PYD Domains-Containing Protein 1
- NLRP3
NACHT, LRR and PYD Domains-Containing Protein 3
- NOD
Nucleotide-Binding Oligomerization Domain
- NT2N
Teratocarcinoma-Derived Ntera2/D1 Neuron-Like Cells
- PARP-1
Poly [ADP-Ribose] Polymerase 1
- PINK 1
PTEN-Induced Putative Kinase 1
- PRR
Pattern Recognition Receptors
- RIPK1
Receptor-Interacting Protein Kinase-1
- RNS
Reactive Nitrogen Species
- ROS
Reactive Oxygen Species
- rtPA
Recombinant Tissue Plasminogen Activator
- SAH
Subarachnoid Hemorrhage
- tBid
Truncated BH3 Interacting Domain Death Agonist
- TGF-β
Transforming Growth Factor Beta
- TLR
Toll Like Receptor
- TNF-α
Tumor Necrosis Factor Alpha
- VEGF
Vascular Endothelial Growth Factor A
Consent for Publication
Not applicable.
Conflict of Interest
The authors declare no conflict of interest, financial or otherwise.
References
- 1.Feigin V.L., Forouzanfar M.H., Krishnamurthi R., Mensah G.A., Connor M., Bennett D.A., Moran A.E., Sacco R.L., Anderson L., Truelsen T., O’Donnell M., Venketasubramanian N., Barker-Collo S., Lawes C.M., Wang W., Shinohara Y., Witt E., Ezzati M., Naghavi M., Murray C. Global and regional burden of stroke during 1990-2010: findings from the Global Burden of Disease Study 2010. Lancet. 2014;383(9913):245–254. doi: 10.1016/s0140-6736(13)61953-4. [http://dx.doi. org/10.1016/S0140-6736(13)61953-4]. [PMID: 24449944]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Feigin V.L., Lawes C.M., Bennett D.A., Anderson C.S. Stroke epidemiology: a review of population-based studies of incidence, prevalence, and case-fatality in the late 20th century. Lancet Neurol. 2003;2(1):43–53. doi: 10.1016/s1474-4422(03)00266-7. [http://dx.doi.org/10.1016/S1474-4422(03)00266-7]. [PMID: 12849300]. [DOI] [PubMed] [Google Scholar]
- 3.Marier J. Tissue plasminogen activator for acute ischemic stroke. N. Engl. J. Med. 1995;333(24):1581–1587. doi: 10.1056/NEJM199512143332401. [http://dx.doi.org/10. 1056/NEJM199512143332401]. [PMID: 7477192]. [DOI] [PubMed] [Google Scholar]
- 4.Hacke W., Lichy C. Thrombolysis for acute stroke under antiplatelet therapy: safe enough to be beneficial? Nat. Clin. Pract. Neurol. 2008;4(9):474–475. doi: 10.1038/ncpneuro0867. [http://dx.doi.org/10.1038/ncpneuro 0867]. [PMID: 18665145]. [DOI] [PubMed] [Google Scholar]
- 5.Huang J., Upadhyay U.M., Tamargo R.J. Inflammation in stroke and focal cerebral ischemia. Surg. Neurol. 2006;66(3):232–245. doi: 10.1016/j.surneu.2005.12.028. [http://dx.doi.org/10.1016/j.surneu.2005.12.028]. [PMID: 16935624]. [DOI] [PubMed] [Google Scholar]
- 6.Zhang R., Chopp M., Zhang Z., Jiang N., Powers C. The expression of P- and E-selectins in three models of middle cerebral artery occlusion. Brain Res. 1998;785(2):207–214. doi: 10.1016/s0006-8993(97)01343-7. [http://dx.doi. org/10.1016/S0006-8993(97)01343-7]. [PMID: 9518615]. [DOI] [PubMed] [Google Scholar]
- 7.Gleichman A.J., Carmichael S.T. Astrocytic therapies for neuronal repair in stroke. Neurosci. Lett. 2014;565:47–52. doi: 10.1016/j.neulet.2013.10.055. [http://dx.doi.org/10.1016/j.neulet.2013.10.055]. [PMID: 24184876]. [DOI] [PubMed] [Google Scholar]
- 8.George P.M., Steinberg G.K. Novel stroke therapeutics: unraveling stroke pathophysiology and its impact on clinical treatments. Neuron. 2015;87(2):297–309. doi: 10.1016/j.neuron.2015.05.041. [http://dx.doi.org/10.1016/j.neuron. 2015.05.041]. [PMID: 26182415]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pagnussat A.S., Faccioni-Heuser M.C., Netto C.A., Achaval M. An ultrastructural study of cell death in the CA1 pyramidal field of the hippocapmus in rats submitted to transient global ischemia followed by reperfusion. J. Anat. 2007;211(5):589–599. doi: 10.1111/j.1469-7580.2007.00802.x. [http://dx. doi.org/10.1111/j.1469-7580.2007.00802.x]. [PMID: 17784936]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Szydlowska K., Tymianski M. Calcium, ischemia and excitotoxicity. Cell Calcium. 2010;47(2):122–129. doi: 10.1016/j.ceca.2010.01.003. [http://dx.doi.org/10.1016/ j.ceca.2010.01.003]. [PMID: 20167368]. [DOI] [PubMed] [Google Scholar]
- 11.Siesjö B.K., Bengtsson F. Calcium fluxes, calcium antagonists, and calcium-related pathology in brain ischemia, hypoglycemia, and spreading depression: a unifying hypothesis. J. Cereb. Blood Flow Metab. 1989;9(2):127–140. doi: 10.1038/jcbfm.1989.20. [http://dx.doi.org/10.1038/ jcbfm.1989.20]. [PMID: 2537841]. [DOI] [PubMed] [Google Scholar]
- 12.Bano D., Nicotera P. Ca2+ signals and neuronal death in brain ischemia. Stroke. 2007;38(2) Suppl.:674–676. doi: 10.1161/01.STR.0000256294.46009.29. [http://dx.doi.org/ 10.1161/01.STR.0000256294.46009.29]. [PMID: 17261713]. [DOI] [PubMed] [Google Scholar]
- 13.Anagli J., Han Y., Stewart L., Yang D., Movsisyan A., Abounit K.A. novel calpastatin-based inhibitor improves postischemic neurological recovery. Biochem. Biophys. Res. Commun. 2009;385(1):94–99. doi: 10.1016/j.bbrc.2009.04.141. [http://dx.doi.org/10.1016/j.bbrc.2009.04.141]. [DOI] [PubMed] [Google Scholar]
- 14.Goll D.E., Thompson V.F., Li H., Wei W., Cong J. The calpain system. Physiol. Rev. 2003;83(3):731–801. doi: 10.1152/physrev.00029.2002. [http://dx.doi.org/10. 1152/physrev.00029.2002]. [PMID: 12843408]. [DOI] [PubMed] [Google Scholar]
- 15.Betts R., Weinsheimer S., Blouse G.E., Anagli J. Structural determinants of the calpain inhibitory activity of calpastatin peptide B27-WT. J. Biol. Chem. 2003;278(10):7800–7809. doi: 10.1074/jbc.M208350200. [http://dx.doi. org/10.1074/jbc.M208350200]. [PMID: 12500971]. [DOI] [PubMed] [Google Scholar]
- 16.Wendt A., Thompson V.F., Goll D.E. Interaction of calpastatin with calpain: a review. Biol. Chem. 2004;385(6):465–472. doi: 10.1515/BC.2004.054. [http:// dx.doi.org/10.1515/BC.2004.054]. [PMID: 15255177]. [DOI] [PubMed] [Google Scholar]
- 17.Rama R., García J.C. 2016. [Google Scholar]
- 18.Berliocchi L., Bano D., Nicotera P. Ca2+ signals and death programmes in neurons. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2005;360(1464):2255–2258. doi: 10.1098/rstb.2005.1765. [http://dx.doi.org/10.1098/rstb.2005. 1765]. [PMID: 16321795]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Friedman L.K. Calcium: a role for neuroprotection and sustained adaptation. Mol. Interv. 2006;6(6):315–329. doi: 10.1124/mi.6.6.5. [http://dx.doi.org/ 10.1124/mi.6.6.5]. [PMID: 17200459]. [DOI] [PubMed] [Google Scholar]
- 20.Xu C., Bailly-Maitre B., Reed J.C. Endoplasmic reticulum stress: cell life and death decisions. J. Clin. Invest. 2005;115(10):2656–2664. doi: 10.1172/JCI26373. [http://dx.doi.org/10.1172/JCI26373]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Urano F., Wang X., Bertolotti A., Zhang Y., Chung P., Harding H.P., Ron D. Coupling of stress in the ER to activation of JNK protein kinases by transmembrane protein kinase IRE1. Science. 2000;287(5453):664–666. doi: 10.1126/science.287.5453.664. [http://dx.doi.org/10.1126/science.287. 5453.664]. [PMID: 10650002]. [DOI] [PubMed] [Google Scholar]
- 22.Bodalia A., Li H., Jackson M.F. Loss of endoplasmic reticulum Ca2+ homeostasis: contribution to neuronal cell death during cerebral ischemia. Acta Pharmacol. Sin. 2013;34(1):49–59. doi: 10.1038/aps.2012.139. [http://dx. doi.org/10.1038/aps.2012.139]. [PMID: 23103622]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Su Y., Li F. Endoplasmic reticulum stress in brain ischemia. 2016. [DOI] [PubMed]
- 24.Rashid H-O., Yadav R.K., Kim H-R, Chae H-J. E.R. 2015.
- 25.Cai Y., Arikkath J., Yang L., Guo M-L., Periyasamy P., Buch S. Interplay of endoplasmic reticulum stress and autophagy in neurodegenerative disorders. 2016. [DOI] [PMC free article] [PubMed]
- 26.Zhang X., Yuan Y., Jiang L., Zhang J., Gao J., Shen Z. Endoplasmic reticulum stress induced by tunicamycin and thapsigargin protects against transient ischemic brain injury. 2014. [DOI] [PMC free article] [PubMed]
- 27.Sheng R., Liu X-Q., Zhang L-S., Gao B., Han R., Wu Y-Q., Zhang X.Y., Qin Z.H. Autophagy regulates endoplasmic reticulum stress in ischemic preconditioning. Autophagy. 2012;8(3):310–325. doi: 10.4161/auto.18673. [http://dx.doi.org/10.4161/auto.18673]. [PMID: 22361585]. [DOI] [PubMed] [Google Scholar]
- 28.Kouroku Y., Fujita E., Tanida I., Ueno T., Isoai A., Kumagai H., Ogawa S., Kaufman R.J., Kominami E., Momoi T. ER stress (PERK/eIF2α phosphorylation) mediates the polyglutamine-induced LC3 conversion, an essential step for autophagy formation. Cell Death Differ. 2007;14(2):230–239. doi: 10.1038/sj.cdd.4401984. [http://dx.doi.org/10. 1038/sj.cdd.4401984]. [PMID: 16794605]. [DOI] [PubMed] [Google Scholar]
- 29.Tajiri S., Oyadomari S., Yano S., Morioka M., Gotoh T., Hamada J.I., Ushio Y., Mori M. Ischemia-induced neuronal cell death is mediated by the endoplasmic reticulum stress pathway involving CHOP. Cell Death Differ. 2004;11(4):403–415. doi: 10.1038/sj.cdd.4401365. [http:// dx.doi.org/10.1038/sj.cdd.4401365]. [PMID: 14752508]. [DOI] [PubMed] [Google Scholar]
- 30.Friberg H., Wieloch T. Mitochondrial permeability transition in acute neurodegeneration. Biochimie. 2001;84(2–3):241–250. doi: 10.1016/s0300-9084(02)01381-0. [http://dx.doi.org/10.1016/S0300-9084(02)01381-0]. [DOI] [PubMed] [Google Scholar]
- 31.Kalogeris T., Bao Y., Korthuis R.J. Mitochondrial reactive oxygen species: A double edged sword in ischemia/reperfusion vs preconditioning. Redox Biol. 2014;2:702–714. doi: 10.1016/j.redox.2014.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Manzanero S., Santro T., Arumugam T.V. Neuronal oxidative stress in acute ischemic stroke: Sources and contribution to cell injury. Neurochem. Int. 2013;62(5):712–718. doi: 10.1016/j.neuint.2012.11.009. [DOI] [PubMed] [Google Scholar]
- 33.Yamashima T. Ca2+-dependent proteases in ischemic neuronal death: a conserved ‘calpain-cathepsin cascade’ from nematodes to primates. Cell Calcium. 2004;36(3-4):285–293. doi: 10.1016/j.ceca.2004.03.001. [http://dx.doi.org/ 10.1016/j.ceca.2004.03.001]. [PMID: 15261484]. [DOI] [PubMed] [Google Scholar]
- 34.Rostas J.A.P., Hoffman A., Murtha L.A., Pepperall D., McLeod D.D., Dickson P.W. Ischaemia- and excitotoxicity-induced CaMKII-mediated neuronal cell death: The relative roles of CaMKII auto-phosphorylation at T286 and T253. Neurochem. Int. 2017;104:6–10. doi: 10.1016/j.neuint.2017.01.002. [DOI] [PubMed] [Google Scholar]
- 35.Chen R., Gong P., Tao T., Gao Y., Shen J., Yan Y., Duan C., Wang J., Liu X. O-GlcNAc glycosylation of nNOS promotes neuronal apoptosis following glutamate excitotoxicity. Cell. Mol. Neurobiol. 2017;37(8):1465–1475. doi: 10.1007/s10571-017-0477-1. [http://dx.doi.org/10.1007/s10571-017-0477-1]. [PMID: 28238085]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Roy-O’Reilly M., McCullough L.D. Astrocytes fuel the fire of lymphocyte toxicity after stroke. Proc. Natl. Acad. Sci. USA. 2017;114(3):425–427. doi: 10.1073/pnas.1619813114. [http://dx.doi.org/10.1073/pnas.1619813114]. [PMID: 28062689]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kaushal V., Schlichter L.C. Mechanisms of microglia-mediated neurotoxicity in a new model of the stroke penumbra. J. Neurosci. 2008;28(9):2221–2230. doi: 10.1523/JNEUROSCI.5643-07.2008. [http://dx.doi.org/10.1523/JNEUROSCI. 5643-07.2008]. [PMID: 18305255]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stys P.K. White matter injury Mechanisms. Curr. Mol. Med. 2004;4(2):113–130. doi: 10.2174/1566524043479220. [http://dx.doi.org/10.2174/1566524043479220]. [DOI] [PubMed] [Google Scholar]
- 39.Baxter P., Chen Y., Xu Y., Swanson R.A. Mitochondrial dysfunction induced by nuclear poly(ADP-ribose) polymerase-1: a treatable cause of cell death in stroke. Transl. Stroke Res. 2014;5(1):136–144. doi: 10.1007/s12975-013-0283-0. [http://dx.doi.org/10.1007/s12975-013-0283-0]. [PMID: 24323707]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.van der Burgh R., Boes M. Mitochondria in autoinflammation: cause, mediator or bystander? Trends Endocrinol. Metab. 2015;26(5):263–271. doi: 10.1016/j.tem.2015.03.004. [http://dx.doi.org/10.1016/j.tem.2015.03.004]. [PMID: 25850613]. [DOI] [PubMed] [Google Scholar]
- 41.Iadecola C., Anrather J. The immunology of stroke: from mechanisms to translation. Nat. Med. 2011;17(7):796–808. doi: 10.1038/nm.2399. [http://dx. doi.org/10.1038/nm.2399]. [PMID: 21738161]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li M., Li Z., Yao Y., Jin W-N., Wood K., Liu Q., Shi F.D., Hao J. Astrocyte-derived interleukin-15 exacerbates ischemic brain injury via propagation of cellular immunity. Proc. Natl. Acad. Sci. USA. 2017;114(3):E396–E405. doi: 10.1073/pnas.1612930114. [http://dx.doi.org/10.1073/ pnas.1612930114]. [PMID: 27994144]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Felger J.C., Abe T., Kaunzner U.W., Gottfried-Blackmore A., Gal-Toth J., McEwen B.S. Brain dendritic cells in ischemic stroke: Time course, activation state, and origin. Brain Behav. Immun. 2010;24(5):724–737. doi: 10.1016/j.bbi.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yilmaz G., Granger D.N. Cell adhesion molecules and ischemic stroke. Neurol. Res. 2008;30(8):783–793. doi: 10.1179/174313208X341085. [http://dx.doi.org/ 10.1179/174313208X341085]. [PMID: 18826804]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Arumugam T.V., Granger D.N., Mattson M.P. Stroke and T-cells. Neuromolecular Med. 2005;7(3):229–242. doi: 10.1385/NMM:7:3:229. [http://dx.doi. org/10.1385/NMM:7:3:229]. [PMID: 16247183]. [DOI] [PubMed] [Google Scholar]
- 46.Hurn P.D., Subramanian S., Parker S.M., Afentoulis M.E., Kaler L.J., Vandenbark A.A., Offner H. T- and B-cell-deficient mice with experimental stroke have reduced lesion size and inflammation. J. Cereb. Blood Flow Metab. 2007;27(11):1798–1805. doi: 10.1038/sj.jcbfm.9600482. [http://dx.doi.org/10.1038/sj.jcbfm.9600482]. [PMID: 17392692]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ponomarev E.D., Shriver L.P., Maresz K., Dittel B.N. Microglial cell activation and proliferation precedes the onset of CNS autoimmunity. J. Neurosci. Res. 2005;81(3):374–389. doi: 10.1002/jnr.20488. [http://dx. doi.org/10.1002/jnr.20488]. [PMID: 15959904]. [DOI] [PubMed] [Google Scholar]
- 48.Suzuki Y., Hattori K., Hamanaka J., Murase T., Egashira Y., Mishiro K., Ishiguro M., Tsuruma K., Hirose Y., Tanaka H., Yoshimura S., Shimazawa M., Inagaki N., Nagasawa H., Iwama T., Hara H. Pharmacological inhibition of TLR4-NOX4 signal protects against neuronal death in transient focal ischemia. Sci. Rep. 2012;2:896. doi: 10.1038/srep00896. [http://dx.doi.org/10.1038/srep00896]. [PMID: 23193438]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shichita T., Hasegawa E., Kimura A., Morita R., Sakaguchi R., Takada I., Sekiya T., Ooboshi H., Kitazono T., Yanagawa T., Ishii T., Takahashi H., Mori S., Nishibori M., Kuroda K., Akira S., Miyake K., Yoshimura A. Peroxiredoxin family proteins are key initiators of post-ischemic inflammation in the brain. Nat. Med. 2012;18(6):911–917. doi: 10.1038/nm.2749. [http://dx.doi.org/10.1038/nm.2749]. [PMID: 22610280]. [DOI] [PubMed] [Google Scholar]
- 50.Ruhnau J., Schulze J., Dressel A., Vogelgesang A. Thrombosis, neuroinflammation, and poststroke infection: The multifaceted role of neutrophils in stroke. J. Immunol. Res. 2017;2017:5140679. doi: 10.1155/2017/5140679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ruhnau J., Schulze J., Dressel A., Vogelgesang A. Neutrophils as a therapeutic target in stroke. 2017.
- 52.Swanson R.A., Ying W., Kauppinen T.M. Astrocyte Influences on Ischemic Neuronal Death. Curr. Mol. Med. 2004;4(2):193–205. doi: 10.2174/1566524043479185. [http://dx.doi.org/10.2174/1566524043479185]. [DOI] [PubMed] [Google Scholar]
- 53.Shaafi S., Sharifipour E., Rahmanifar R., Hejazi S., Andalib S., Nikanfar M., Baradarn B., Mehdizadeh R. Interleukin-6, a reliable prognostic factor for ischemic stroke. Iran. J. Neurol. 2014;13(2):70–76. [PMID: 25295149]. [PMC free article] [PubMed] [Google Scholar]
- 54.Chen Y., Hallenbeck J.M., Ruetzler C., Bol D., Thomas K., Berman N.E.J., Vogel S.N. Overexpression of monocyte chemoattractant protein 1 in the brain exacerbates ischemic brain injury and is associated with recruitment of inflammatory cells. J. Cereb. Blood Flow Metab. 2003;23(6):748–755. doi: 10.1097/01.WCB.0000071885.63724.20. [http://dx.doi.org/ 10.1097/01.WCB.0000071885.63724.20]. [PMID: 12796723]. [DOI] [PubMed] [Google Scholar]
- 55.Mojsilovic-Petrovic J., Callaghan D., Cui H., Dean C., Stanimirovic D.B., Zhang W. Hypoxia-inducible factor-1 (HIF-1) is involved in the regulation of hypoxia-stimulated expression of monocyte chemoattractant protein-1 (MCP-1/CCL2) and MCP-5 (Ccl12) in astrocytes. J. Neuroinflammation. 2007;4(1):12. doi: 10.1186/1742-2094-4-12. [http://dx.doi.org/ 10.1186/1742-2094-4-12]. [PMID: 17474992]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cheung E.C.C., Melanson-Drapeau L., Cregan S.P., Vanderluit J.L., Ferguson K.L., McIntosh W.C., Park D.S., Bennett S.A., Slack R.S. Apoptosis-inducing factor is a key factor in neuronal cell death propagated by BAX-dependent and BAX-independent mechanisms. J. Neurosci. 2005;25(6):1324–1334. doi: 10.1523/JNEUROSCI.4261-04.2005. [http://dx. doi.org/10.1523/JNEUROSCI.4261-04.2005]. [PMID: 15703386]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zuo W., Yang P.F., Chen J., Zhang Z., Chen N.H. Drp-1, a potential therapeutic target for brain ischaemic stroke. Br. J. Pharmacol. 2016;173(10):1665–1677. doi: 10.1111/bph.13468. [http://dx.doi.org/10.1111/bph. 13468]. [PMID: 26915692]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pradeep H., Sharma B., Rajanikant G.K. Drp1 in Ischemic neuronal death: An unusual suspect. Curr. Med. Chem. 2014;21(19):2183–2189. doi: 10.2174/0929867321666131228203513. [DOI] [PubMed] [Google Scholar]
- 59.Song B., Ao Q., Wang Z., Liu W., Niu Y., Shen Q., Zuo H., Zhang X., Gong Y. Phosphorylation of tau protein over time in rats subjected to transient brain ischemia. Neural Regen. Res. 2013;8(34):3173–3182. doi: 10.3969/j.issn.1673-5374.2013.34.001. [PMID: 25206638]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wen Y., Yang S., Liu R., Simpkins J.W. Transient cerebral ischemia induces site-specific hyperphosphorylation of tau protein. Brain Res. 2004;1022(1-2):30–38. doi: 10.1016/j.brainres.2004.05.106. [http://dx.doi.org/10.1016/ j.brainres.2004.05.106]. [PMID: 15353210]. [DOI] [PubMed] [Google Scholar]
- 61.Chen X., Liu Y., Zhu J., Lei S., Dong Y., Li L. GSK-3β downregulates Nrf2 in cultured cortical neurons and in a rat model of cerebral ischemia-reperfusion. Sci. Rep. 2016;6:20196. doi: 10.1038/srep20196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jo C., Gundemir S., Pritchard S., Jin Y.N., Rahman I., Johnson G.V. Nrf2 reduces levels of phosphorylated tau protein by inducing autophagy adaptor protein NDP52. Nat. Commun. 2014;5:3496. doi: 10.1038/ncomms4496. [http://dx.doi.org/10.1038/ncomms4496]. [PMID: 24667209]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wu H., Che X., Zheng Q., Wu A., Pan K., Shao A., Wu Q., Zhang J., Hong Y. Caspases: a molecular switch node in the crosstalk between autophagy and apoptosis. Int. J. Biol. Sci. 2014;10(9):1072–1083. doi: 10.7150/ijbs.9719. [http://dx.doi.org/10.7150/ijbs.9719]. [PMID: 25285039]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sabri M., Lass E. Macdonald, RL Early brain injury: a common mechanism in subarachnoid hemorrhage and global cerebral ischemia. Stroke Res. Treat. 2013;2013:394036. doi: 10.1155/2013/394036. [http://dx. doi.org/10.1155/2013/394036]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.D’Orsi B., Mateyka J., Prehn J.H.M. Control of mitochondrial physiology and cell death by the Bcl-2 family proteins Bax and Bok. Neurochem. Int. 2017;109:162–170. doi: 10.1016/j.neuint.2017.03.010. [http://dx.doi.org/ 10.1016/j.neuint.2017.03.010]. [PMID: 28315370]. [DOI] [PubMed] [Google Scholar]
- 66.Danial N.N., Korsmeyer S.J. Cell death: critical control points. Cell. 2004;116(2):205–219. doi: 10.1016/s0092-8674(04)00046-7. [http://dx.doi.org/10.1016/S0092-8674(04)00046-7]. [PMID: 14744432]. [DOI] [PubMed] [Google Scholar]
- 67.Youle R.J., Strasser A. The BCL-2 protein family: opposing activities that mediate cell death. Nat. Rev. Mol. Cell Biol. 2008;9(1):47–59. doi: 10.1038/nrm2308. [http://dx.doi.org/10.1038/nrm2308]. [PMID: 18097445]. [DOI] [PubMed] [Google Scholar]
- 68.D’Orsi B., Kilbride S.M., Chen G., Perez Alvarez S., Bonner H.P., Pfeiffer S., Plesnila N., Engel T., Henshall D.C., Düssmann H., Prehn J.H. Bax regulates neuronal Ca2+ homeostasis. J. Neurosci. 2015;35(4):1706–1722. doi: 10.1523/JNEUROSCI.2453-14.2015. [http://dx.doi.org/10.1523/ JNEUROSCI.2453-14.2015]. [PMID: 25632145]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.D’Orsi B., Engel T., Pfeiffer S., Nandi S., Kaufmann T., Henshall D.C., Prehn J.H. Bok is not pro-apoptotic but suppresses poly ADP-ribose polymerase-dependent cell death pathways and protects against excitotoxic and seizure-induced neuronal injury. J. Neurosci. 2016;36(16):4564–4578. doi: 10.1523/JNEUROSCI.3780-15.2016. [http://dx.doi.org/10.1523/ JNEUROSCI.3780-15.2016]. [PMID: 27098698]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kim T., Vemuganti R. Mechanisms of Parkinson’s disease-related proteins in mediating secondary brain damage after cerebral ischemia. J. Cereb. Blood Flow Metab. 2017;37(6):1910–1926. doi: 10.1177/0271678X17694186. [http://dx.doi.org/10.1177/0271678X17694186]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yoon D-K., Hwang I.K., Yoo K-Y., Lee Y-B., Lee J-J., Kim J-H., Kang T.C., Lee B.H., Sohn H.S., Won M.H. Comparison of α-synuclein immunoreactivity and protein levels in ischemic hippocampal CA1 region between adult and aged gerbils and correlation with Cu,Zn-superoxide dismutase. Neurosci. Res. 2006;55(4):434–441. doi: 10.1016/j.neures.2006.04.014. [http://dx.doi.org/10.1016/j.neures.2006.04.014]. [PMID: 16759729]. [DOI] [PubMed] [Google Scholar]
- 72.Kim T., Mehta S.L., Kaimal B., Lyons K., Dempsey R.J., Vemuganti R. Poststroke induction of α-synuclein mediates ischemic brain damage. J. Neurosci. 2016;36(26):7055–7065. doi: 10.1523/JNEUROSCI.1241-16.2016. [http://dx. doi.org/10.1523/JNEUROSCI.1241-16.2016]. [PMID: 27358461]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhou M., Xia Z.Y., Lei S.Q., Leng Y., Xue R. Role of mitophagy regulated by Parkin/DJ-1 in remote ischemic postconditioning-induced mitigation of focal cerebral ischemia-reperfusion. Eur. Rev. Med. Pharmacol. Sci. 2015;19(24):4866–4871. [PMID: 26744879]. [PubMed] [Google Scholar]
- 74.Yanagisawa D., Kitamura Y., Inden M., Takata K., Taniguchi T., Morikawa S., Morita M., Inubushi T., Tooyama I., Taira T., Iguchi-Ariga S.M., Akaike A., Ariga H. DJ-1 protects against neurodegeneration caused by focal cerebral ischemia and reperfusion in rats. J. Cereb. Blood Flow Metab. 2008;28(3):563–578. doi: 10.1038/sj.jcbfm.9600553. [http://dx.doi.org/10.1038/sj.jcbfm.9600553]. [PMID: 17882163]. [DOI] [PubMed] [Google Scholar]
- 75.Kitamura Y., Watanabe S., Taguchi M., Takagi K., Kawata T., Takahashi-Niki K., Yasui H., Maita H., Iguchi-Ariga S.M., Ariga H. Neuroprotective effect of a new DJ-1-binding compound against neurodegeneration in Parkinson’s disease and stroke model rats. Mol. Neurodegener. 2011;6(1):48. doi: 10.1186/1750-1326-6-48. [http://dx.doi.org/ 10.1186/1750-1326-6-48]. [PMID: 21740546]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yu H.H., Xu Q., Chen H.P., Wang S., Huang X.S., Huang Q.R., He M. Stable overexpression of DJ-1 protects H9c2 cells against oxidative stress under a hypoxia condition. Cell Biochem. Funct. 2013;31(8):643–651. doi: 10.1002/cbf.2949. [http://dx.doi.org/10.1002/cbf.2949]. [PMID: 23281015]. [DOI] [PubMed] [Google Scholar]
- 77.Aleyasin H., Rousseaux M.W., Phillips M., Kim R.H., Bland R.J., Callaghan S., Slack R.S., During M.J., Mak T.W., Park D.S. The Parkinson’s disease gene DJ-1 is also a key regulator of stroke-induced damage. Proc. Natl. Acad. Sci. USA. 2007;104(47):18748–18753. doi: 10.1073/pnas.0709379104. [http://dx.doi.org/10.1073/pnas.0709379104]. [PMID: 18003894]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhao Y., Chen F., Chen S., Liu X., Cui M., Dong Q. The Parkinson’s disease-associated gene PINK1 protects neurons from ischemic damage by decreasing mitochondrial translocation of the fission promoter Drp1. J. Neurochem. 2013;127(5):711–722. doi: 10.1111/jnc.12340. [http://dx.doi.org/10.1111/jnc.12340]. [PMID: 23772688]. [DOI] [PubMed] [Google Scholar]
- 79.Huang Y., Chen H., Zhu J., Zhao F., Qu Y., Mu D. Effects of PINK1 gene on cell apoptosis and cell autophagy in neonatal mice with hypoxic-ischemic brain damage Zhongguo dang dai er ke za zhi. Chinese J. Contemp. Pediatr. 2016;18(3):263–269. doi: 10.7499/j.issn.1008-8830.2016.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Chen S-D., Lin T-K., Yang D-I., Lee S-Y., Shaw F-Z., Liou C-W., Chuang Y.C. Roles of PTEN-induced putative kinase 1 and dynamin-related protein 1 in transient global ischemia-induced hippocampal neuronal injury. Biochem. Biophys. Res. Commun. 2015;460(2):397–403. doi: 10.1016/j.bbrc.2015.03.045. [http://dx.doi.org/10.1016/j.bbrc.2015.03. 045]. [PMID: 25791474]. [DOI] [PubMed] [Google Scholar]
- 81.Edinger A.L., Thompson C.B. Death by design: apoptosis, necrosis and autophagy. Curr. Opin. Cell Biol. 2004;16(6):663–669. doi: 10.1016/j.ceb.2004.09.011. [http://dx.doi.org/10.1016/j.ceb.2004.09.011]. [PMID: 15530778]. [DOI] [PubMed] [Google Scholar]
- 82.Gürer G., Gursoy-Ozdemir Y., Erdemli E., Can A., Dalkara T. Astrocytes are more resistant to focal cerebral ischemia than neurons and die by a delayed necrosis. Brain Pathol. 2009;19(4):630–641. doi: 10.1111/j.1750-3639.2008.00226.x. [http://dx.doi.org/10.1111/j.1750-3639.2008.00226.x]. [PMID: 18947334]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zhou W., Yuan J., editors. Necroptosis in health and diseases. Semin. Cell. Develop. Biol. Elsevier; 2014. [DOI] [PubMed] [Google Scholar]
- 84.Linkermann A., Green D.R. Necroptosis. N. Engl. J. Med. 2014;370(5):455–465. doi: 10.1056/NEJMra1310050. [http://dx.doi.org/10.1056/NEJMra1310050]. [PMID: 24476434]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kilinc M., Gürsoy-Ozdemir Y., Gürer G., Erdener S.E., Erdemli E., Can A., Dalkara T. Lysosomal rupture, necroapoptotic interactions and potential crosstalk between cysteine proteases in neurons shortly after focal ischemia. Neurobiol. Dis. 2010;40(1):293–302. doi: 10.1016/j.nbd.2010.06.003. [http://dx.doi.org/10.1016/j.nbd.2010.06.003]. [PMID: 20600913]. [DOI] [PubMed] [Google Scholar]
- 86.Ünal-Çevik I., Kilinç M., Can A., Gürsoy-Ozdemir Y., Dalkara T. Apoptotic and necrotic death mechanisms are concomitantly activated in the same cell after cerebral ischemia. Stroke. 2004;35(9):2189–2194. doi: 10.1161/01.STR.0000136149.81831.c5. [http://dx.doi.org/10.1161/01.STR.0000136149.81831. c5]. [PMID: 15256676]. [DOI] [PubMed] [Google Scholar]
- 87.Degterev A., Huang Z., Boyce M., Li Y., Jagtap P., Mizushima N., Cuny G.D., Mitchison T.J., Moskowitz M.A., Yuan J. Chemical inhibitor of nonapoptotic cell death with therapeutic potential for ischemic brain injury. Nat. Chem. Biol. 2005;1(2):112–119. doi: 10.1038/nchembio711. [http://dx.doi.org/10.1038/nchembio711]. [PMID: 16408008]. [DOI] [PubMed] [Google Scholar]
- 88.Holler N., Zaru R., Micheau O., Thome M., Attinger A., Valitutti S., Bodmer J.L., Schneider P., Seed B., Tschopp J. Fas triggers an alternative, caspase-8-independent cell death pathway using the kinase RIP as effector molecule. Nat. Immunol. 2000;1(6):489–495. doi: 10.1038/82732. [http://dx.doi.org/10.1038/82732]. [PMID: 11101870]. [DOI] [PubMed] [Google Scholar]
- 89.Degterev A., Hitomi J., Germscheid M., Ch’en I.L., Korkina O., Teng X., Abbott D., Cuny G.D., Yuan C., Wagner G., Hedrick S.M., Gerber S.A., Lugovskoy A., Yuan J. Identification of RIP1 kinase as a specific cellular target of necrostatins. Nat. Chem. Biol. 2008;4(5):313–321. doi: 10.1038/nchembio.83. [http://dx.doi.org/10.1038/nchembio.83]. [PMID: 18408713]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hitomi J., Christofferson D.E., Ng A., Yao J., Degterev A., Xavier R.J., Yuan J. Identification of a molecular signaling network that regulates a cellular necrotic cell death pathway. Cell. 2008;135(7):1311–1323. doi: 10.1016/j.cell.2008.10.044. [http://dx.doi.org/10.1016/j.cell.2008.10. 044]. [PMID: 19109899]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Baines C.P. Identification of a molecular signaling network that regulates a cellular necrotic cell death pathway. Cell. 2008;135(7):1311–1323. doi: 10.1016/j.cell.2008.10.044. [http://dx.doi.org/10.3389/fphys.2010.00156]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Xie Y., Hou W., Song X., Yu Y., Huang J., Sun X. Ferroptosis: process and function. 2016. [DOI] [PMC free article] [PubMed]
- 93.Zille M., Karuppagounder S.S., Chen Y., Gough P.J., Bertin J., Finger J., Milner T.A., Jonas E.A., Ratan R.R. Neuronal death after hemorrhagic stroke in vitro and in vivo shares features of ferroptosis and necroptosis. Stroke. 2017;48(4):1033–1043. doi: 10.1161/STROKEAHA.116.015609. [http://dx.doi.org/10.1161/STROKEAHA.116.015609]. [PMID: 28250197]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Rubinsztein D.C., Bento C.F., Deretic V. Therapeutic targeting of autophagy in neurodegenerative and infectious diseases. J. Exp. Med. 2015;212(7):979–990. doi: 10.1084/jem.20150956. [http://dx.doi.org/10.1084/jem.20150956]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ginet V., Spiehlmann A., Rummel C., Rudinskiy N., Grishchuk Y., Luthi-Carter R., Clarke P.G., Truttmann A.C., Puyal J. Involvement of autophagy in hypoxic-excitotoxic neuronal death. Autophagy. 2014;10(5):846–860. doi: 10.4161/auto.28264. [http://dx.doi.org/10.4161/auto. 28264]. [PMID: 24674959]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Parzych K.R., Klionsky D.J. An overview of autophagy: morphology, mechanism, and regulation. Antioxid. Redox Signal. 2014;20(3):460–473. doi: 10.1089/ars.2013.5371. [http://dx.doi.org/10.1089/ars.2013.5371]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Huang X.P., Ding H., Lu J.D., Tang Y.H., Deng B.X., Deng C.Q. Autophagy in cerebral ischemia and the effects of traditional Chinese medicine. J. Integr. Med. 2015;13(5):289–296. doi: 10.1016/S2095-4964(15)60187-X. [http://dx. doi.org/10.1016/S2095-4964(15)60187-X]. [PMID: 26343099]. [DOI] [PubMed] [Google Scholar]
- 98.Chang P., Dong W., Zhang M., Wang Z., Wang Y., Wang T., Gao Y., Meng H., Luo B., Luo C., Chen X., Tao L. Anti-necroptosis chemical necrostatin-1 can also suppress apoptotic and autophagic pathway to exert neuroprotective effect in mice intracerebral hemorrhage model. J. Mol. Neurosci. 2014;52(2):242–249. doi: 10.1007/s12031-013-0132-3. [http://dx.doi.org/10.1007/s12031-013-0132-3]. [PMID: 24122153]. [DOI] [PubMed] [Google Scholar]
- 99.Chen S., Wu H., Tang J., Zhang J., Zhang J.H. Neurovascular events after subarachnoid hemorrhage: focusing on subcellular organelles. Neurovascular Events After Subarachnoid Hemorrhage. Springer; 2015. pp. 39–46. [http://dx.doi.org/10.1007/978-3-319-04981-6_7] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Zhang X., Yan H., Yuan Y., Gao J., Shen Z., Cheng Y., Shen Y., Wang R.R., Wang X., Hu W.W., Wang G., Chen Z. Cerebral ischemia-reperfusion-induced autophagy protects against neuronal injury by mitochondrial clearance. Autophagy. 2013;9(9):1321–1333. doi: 10.4161/auto.25132. [http://dx.doi.org/10.4161/auto.25132]. [PMID: 23800795]. [DOI] [PubMed] [Google Scholar]
- 101.Frugier T., Taylor J.M., McLean C., Bye N., Beart P.M., Devenish R.J., Crack P.J. Evidence for the recruitment of autophagic vesicles in human brain after stroke. Neurochem. Int. 2016;96:62–68. doi: 10.1016/j.neuint.2016.02.016. [http://dx.doi.org/10.1016/j.neuint.2016.02.016]. [PMID: 26930584]. [DOI] [PubMed] [Google Scholar]
- 102.Yang Z., Zhao T.Z., Zou Y.J., Zhang J.H., Feng H. Hypoxia Induces autophagic cell death through hypoxia-inducible factor 1α in microglia. PLoS One. 2014;9(5):e96509. doi: 10.1371/journal.pone.0096509. [http://dx.doi.org/ 10.1371/journal.pone.0096509]. [PMID: 24818601]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Pamenter M.E., Perkins G.A., McGinness A.K., Gu X.Q., Ellisman M.H., Haddad G.G. Autophagy and apoptosis are differentially induced in neurons and astrocytes treated with an in vitro mimic of the ischemic penumbra. PLoS One. 2012;7(12):e51469. doi: 10.1371/journal.pone.0051469. [http://dx.doi.org/10.1371/journal.pone.0051469]. [PMID: 23251543]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Carloni S., Buonocore G., Balduini W. Protective role of autophagy in neonatal hypoxia-ischemia induced brain injury. Neurobiol. Dis. 2008;32(3):329–339. doi: 10.1016/j.nbd.2008.07.022. [http://dx.doi.org/10.1016/j.nbd. 2008.07.022]. [PMID: 18760364]. [DOI] [PubMed] [Google Scholar]
- 105.Dunlop E., Tee A. mTOR and autophagy: a dynamic relationship governed by nutrients and energy; Elsevier. Semin. Cell Dev. Biol. 2014;36:121–129. doi: 10.1016/j.semcdb.2014.08.006. [DOI] [PubMed] [Google Scholar]
- 106.Alers S., Löffler A.S., Wesselborg S., Stork B. Role of AMPK-mTOR-Ulk1/2 in the regulation of autophagy: cross talk, shortcuts, and feedbacks. Mol. Cell. Biol. 2012;32(1):2–11. doi: 10.1128/MCB.06159-11. [http://dx.doi. org/10.1128/MCB.06159-11]. [PMID: 22025673]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Tang Y-C., Tian H-X., Yi T., Chen H-B. The critical roles of mitophagy in cerebral ischemia. Protein Cell. 2016;7(10):699–713. doi: 10.1007/s13238-016-0307-0. [http://dx.doi.org/10.1007/s13238-016-0307-0]. [PMID: 27554669]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Bakhshayesh B., Hosseininezhad M., Saadat S.N., Ansar M.M., Ramezani H., Saadat S.M. Iron overload is associated with perihematoma edema growth following intracerebral hemorrhage that may contribute to in-hospital mortality and long-term functional outcome. Curr. Neurovasc. Res. 2014;11(3):248–253. doi: 10.2174/1567202611666140530124855. [http:// dx.doi.org/10.2174/1567202611666140530124855]. [PMID: 24875488]. [DOI] [PubMed] [Google Scholar]
- 109.Mancias J.D., Wang X., Gygi S.P., Harper J.W., Kimmelman A.C. Quantitative proteomics identifies NCOA4 as the cargo receptor mediating ferritinophagy. Nature. 2014;509(7498):105–109. doi: 10.1038/nature13148. [http://dx.doi.org/10.1038/nature13148]. [PMID: 24695223]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Qin A-P., Liu C-F., Qin Y-Y., Hong L-Z., Xu M., Yang L., Liu J., Qin Z.H., Zhang H.L. Autophagy was activated in injured astrocytes and mildly decreased cell survival following glucose and oxygen deprivation and focal cerebral ischemia. Autophagy. 2010;6(6):738–753. doi: 10.4161/auto.6.6.12573. [http://dx.doi.org/10.4161/auto.6.6.12573]. [PMID: 20574158]. [DOI] [PubMed] [Google Scholar]
- 111.Zhou X-Y., Luo Y., Zhu Y-M., Liu Z-H., Kent T.A., Rong J-G., Li W., Qiao S.G., Li M., Ni Y., Ishidoh K., Zhang H.L. Inhibition of autophagy blocks cathepsins-tBid-mitochondrial apoptotic signaling pathway via stabilization of lysosomal membrane in ischemic astrocytes. Cell Death Dis. 2017;8(2):e2618. doi: 10.1038/cddis.2017.34. [http:// dx.doi.org/10.1038/cddis.2017.34]. [PMID: 28206988]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Song J., Oh Y., Lee J.E. miR-Let7A modulates autophagy induction in LPS-activated microglia. Exp. Neurobiol. 2015;24(2):117–125. doi: 10.5607/en.2015.24.2.117. [http://dx.doi.org/10.5607/en.2015.24.2.117]. [PMID: 26113790]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Zhang B., Song C., Feng B., Fan W. Neuroprotection by triptolide against cerebral ischemia/reperfusion injury through the inhibition of NF-κB/PUMA signal in rats. Ther. Clin. Risk Manag. 2016;12:817–824. doi: 10.2147/TCRM.S106012. [http://dx.doi.org/10.2147/TCRM.S106012]. [PMID: 27307742]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Weis S.N., Toniazzo A.P., Ander B.P., Zhan X., Careaga M., Ashwood P. Autophagy in the brain of neonates following hypoxia–ischemia shows sex- and region-specific effects. Neuroscience. 2014;256:201–209. doi: 10.1016/j.neuroscience.2013.10.046. [DOI] [PubMed] [Google Scholar]
- 115.Bergsbaken T., Fink S.L., Cookson B.T. Pyroptosis: host cell death and inflammation. Nat. Rev. Microbiol. 2009;7(2):99–109. doi: 10.1038/nrmicro2070. [http://dx.doi.org/10.1038/nrmicro2070]. [PMID: 19148178]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Fink S.L., Bergsbaken T., Cookson B.T. Anthrax lethal toxin and Salmonella elicit the common cell death pathway of caspase-1-dependent pyroptosis via distinct mechanisms. Proc. Natl. Acad. Sci. USA. 2008;105(11):4312–4317. doi: 10.1073/pnas.0707370105. [http://dx.doi.org/10.1073/ pnas.0707370105]. [PMID: 18337499]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Brennan M.A., Cookson B.T. Salmonella induces macrophage death by caspase-1-dependent necrosis. Mol. Microbiol. 2000;38(1):31–40. doi: 10.1046/j.1365-2958.2000.02103.x. [http://dx.doi.org/10.1046/j.1365-2958.2000.02103.x]. [PMID: 11029688]. [DOI] [PubMed] [Google Scholar]
- 118.Chen G.Y., Nuñez G. Sterile inflammation: sensing and reacting to damage. Nat. Rev. Immunol. 2010;10(12):826–837. doi: 10.1038/nri2873. [http://dx. doi.org/10.1038/nri2873]. [PMID: 21088683]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Abulafia D.P., de Rivero Vaccari J.P., Lozano J.D., Lotocki G., Keane R.W., Dietrich W.D. Inhibition of the inflammasome complex reduces the inflammatory response after thromboembolic stroke in mice. J. Cereb. Blood Flow Metab. 2009;29(3):534–544. doi: 10.1038/jcbfm.2008.143. [http://dx.doi.org/10.1038/jcbfm.2008.143]. [PMID: 19066616]. [DOI] [PubMed] [Google Scholar]
- 120.Tang S-C., Arumugam T.V., Xu X., Cheng A., Mughal M.R., Jo D.G., Lathia J.D., Siler D.A., Chigurupati S., Ouyang X., Magnus T., Camandola S., Mattson M.P. Pivotal role for neuronal Toll-like receptors in ischemic brain injury and functional deficits. Proc. Natl. Acad. Sci. USA. 2007;104(34):13798–13803. doi: 10.1073/pnas.0702553104. [http://dx.doi.org/10.1073/pnas.0702553104]. [PMID: 17693552]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Schroder K., Tschopp J. The inflammasomes. Cell. 2010;140(6):821–832. doi: 10.1016/j.cell.2010.01.040. [http://dx.doi.org/10.1016/j.cell.2010.01.040]. [PMID: 20303873]. [DOI] [PubMed] [Google Scholar]
- 122.Tschopp J., Schroder K. NLRP3 inflammasome activation: The convergence of multiple signalling pathways on ROS production? Nat. Rev. Immunol. 2010;10(3):210–215. doi: 10.1038/nri2725. [http://dx.doi.org/10. 1038/nri2725]. [PMID: 20168318]. [DOI] [PubMed] [Google Scholar]
- 123.Mulcahy N.J., Ross J., Rothwell N.J., Loddick S.A. Delayed administration of interleukin-1 receptor antagonist protects against transient cerebral ischaemia in the rat. Br. J. Pharmacol. 2003;140(3):471–476. doi: 10.1038/sj.bjp.0705462. [http://dx.doi.org/10.1038/sj.bjp.0705462]. [PMID: 12970087]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Martinon F., Burns K., Tschopp J. The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-β. Mol. Cell. 2002;10(2):417–426. doi: 10.1016/s1097-2765(02)00599-3. [http://dx. doi.org/10.1016/S1097-2765(02)00599-3]. [PMID: 12191486]. [DOI] [PubMed] [Google Scholar]
- 125.Fann D.Y-W., Lee S.Y., Manzanero S., Tang S-C., Gelderblom M., Chunduri P., Bernreuther C., Glatzel M., Cheng Y.L., Thundyil J., Widiapradja A., Lok K.Z., Foo S.L., Wang Y.C., Li Y.I., Drummond G.R., Basta M., Magnus T., Jo D.G., Mattson M.P., Sobey C.G., Arumugam T.V. Intravenous immunoglobulin suppresses NLRP1 and NLRP3 inflammasome-mediated neuronal death in ischemic stroke. Cell Death Dis. 2013;4(9):e790. doi: 10.1038/cddis.2013.326. [http://dx.doi.org/10.1038/cddis.2013.326]. [PMID: 24008734]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Barrington J., Lemarchand E., Allan S.M. A brain in flame; do inflammasomes and pyroptosis influence stroke pathology? Brain Pathol. 2016 doi: 10.1111/bpa.12476. [PMID: 27997059]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Fann DY-W, Lee S-Y, Manzanero S, Chunduri P, Sobey CG, Arumugam TV. Pathogenesis of acute stroke and the role of inflammasomes. 2013. [DOI] [PubMed]
- 128.Pearson V.L., Rothwell N.J., Toulmond S. Excitotoxic brain damage in the rat induces interleukin-1β protein in microglia and astrocytes: correlation with the progression of cell death. Glia. 1999;25(4):311–323. [http://dx.doi.org/10.1002/(SICI)1098-1136 (19990215)25:4<311:AID-GLIA1>3.0.CO;2-E]. [PMID: 10028914]. [PubMed] [Google Scholar]
- 129.Denes A., Lopez-Castejon G., Brough D. Caspase-1: is IL-1 just the tip of the ICEberg? Cell Death Dis. 2012;3(7):e338. doi: 10.1038/cddis.2012.86. [http://dx.doi.org/10.1038/cddis.2012.86]. [PMID: 22764097]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Fink S.L., Cookson B.T. Caspase-1-dependent pore formation during pyroptosis leads to osmotic lysis of infected host macrophages. Cell. Microbiol. 2006;8(11):1812–1825. doi: 10.1111/j.1462-5822.2006.00751.x. [http://dx.doi. org/10.1111/j.1462-5822.2006.00751.x]. [PMID: 16824040]. [DOI] [PubMed] [Google Scholar]
- 131.Miao E.A., Rajan J.V., Aderem A. Caspase-1-induced pyroptotic cell death. Immunol. Rev. 2011;243(1):206–214. doi: 10.1111/j.1600-065X.2011.01044.x. [http://dx.doi. org/10.1111/j.1600-065X.2011.01044.x]. [PMID: 21884178]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Rabuffetti M., Sciorati C., Tarozzo G., Clementi E., Manfredi A.A., Beltramo M. Inhibition of caspase-1-like activity by Ac-Tyr-Val-Ala-Asp-chloromethyl ketone induces long-lasting neuroprotection in cerebral ischemia through apoptosis reduction and decrease of pro inflammatory cytokines. J. Neurosci. 2000;20(12):4398–4404. doi: 10.1523/JNEUROSCI.20-12-04398.2000. [http://dx.doi.org/10.1523/JNEUROSCI.20-12-04398. 2000]. [PMID: 10844008]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Ray A.M., Owen D.E., Evans M.L., Davis J.B., Benham C.D. Caspase inhibitors are functionally neuroprotective against oxygen glucose deprivation induced CA1 death in rat organotypic hippocampal slices. Brain Res. 2000;867(1-2):62–69. doi: 10.1016/s0006-8993(00)02230-7. [http://dx.doi.org/ 10.1016/S0006-8993(00)02230-7]. [PMID: 10837798]. [DOI] [PubMed] [Google Scholar]
- 134.Kang S-J., Wang S., Hara H., Peterson E.P., Namura S., Amin-Hanjani S., Huang Z., Srinivasan A., Tomaselli K.J., Thornberry N.A., Moskowitz M.A., Yuan J. Dual role of caspase-11 in mediating activation of caspase-1 and caspase-3 under pathological conditions. J. Cell Biol. 2000;149(3):613–622. doi: 10.1083/jcb.149.3.613. [http://dx.doi.org/ 10.1083/jcb.149.3.613]. [PMID: 10791975]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Siniscalchi A., Gallelli L., Malferrari G., Pirritano D., Serra R., Santangelo E., De Sarro G. Cerebral stroke injury: the role of cytokines and brain inflammation. J. Basic Clin. Physiol. Pharmacol. 2014;25(2):131–137. doi: 10.1515/jbcpp-2013-0121. [http://dx.doi.org/10.1515/jbcpp-2013-0121]. [PMID: 24515999]. [DOI] [PubMed] [Google Scholar]
- 136.Ekdahl C.T., Kokaia Z., Lindvall O. 2009. [DOI] [PubMed]
- 137.Barreto G.E., White R., Ouyang Y., Xu L. G Giffard R. Astrocytes: targets for neuroprotection in stroke. Central nervous system agents in medicinal chemistry (Formerly Current Medicinal Chemistry-Central Nervous System Agents). CNSAMC. 2011;11(2):164–173. doi: 10.2174/187152411796011303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Wood P.L. Microglia as a unique cellular target in the treatment of stroke: potential neurotoxic mediators produced by activated microglia. Neurol. Res. 1995;17(4):242–248. doi: 10.1080/01616412.1995.11740321. [http://dx.doi.org/10. 1080/01616412.1995.11740321]. [PMID: 7477737]. [DOI] [PubMed] [Google Scholar]
- 139.Patel A.R., Ritzel R., McCullough L.D., Liu F. Microglia and ischemic stroke: a double-edged sword. Int. J. Physiol. Pathophysiol. Pharmacol. 2013;5(2):73–90. [PMID: 23750306]. [PMC free article] [PubMed] [Google Scholar]
- 140.Jin R., Yang G., Li G. Inflammatory mechanisms in ischemic stroke: role of inflammatory cells. J. Leukoc. Biol. 2010;87(5):779–789. doi: 10.1189/jlb.1109766. [http://dx.doi.org/10.1189/jlb.1109766]. [PMID: 20130219]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Giulian D., Vaca K. Inflammatory glia mediate delayed neuronal damage after ischemia in the central nervous system. Stroke. 1993;24(12) Suppl.:I84–I90. [PMID: 8249026]. [PubMed] [Google Scholar]
- 142.Gelderblom M., Leypoldt F., Steinbach K., Behrens D., Choe C-U., Siler D.A., Arumugam T.V., Orthey E., Gerloff C., Tolosa E., Magnus T. Temporal and spatial dynamics of cerebral immune cell accumulation in stroke. Stroke. 2009;40(5):1849–1857. doi: 10.1161/STROKEAHA.108.534503. [http://dx.doi.org/10.1161/STROKEAHA.108.534503]. [PMID: 19265055]. [DOI] [PubMed] [Google Scholar]
- 143.Rodríguez-Yáñez M., Castillo J. Role of inflammatory markers in brain ischemia. Curr. Opin. Neurol. 2008;21(3):353–357. doi: 10.1097/WCO.0b013e3282ffafbf. [http:// dx.doi.org/10.1097/WCO.0b013e3282ffafbf]. [PMID: 18451722]. [DOI] [PubMed] [Google Scholar]
- 144.Nakase T., Yamazaki T., Ogura N., Suzuki A., Nagata K. The impact of inflammation on the pathogenesis and prognosis of ischemic stroke. J. Neurol. Sci. 2008;271(1-2):104–109. doi: 10.1016/j.jns.2008.03.020. [http://dx.doi.org/10.1016/j.jns.2008.03.020]. [PMID: 18479710]. [DOI] [PubMed] [Google Scholar]
- 145.Beridze M., Sanikidze T., Shakarishvili R., Intskirveli N., Bornstein N.M. Selected acute phase CSF factors in ischemic stroke: findings and prognostic value. BMC Neurol. 2011;11(1):41. doi: 10.1186/1471-2377-11-41. [http://dx.doi.org/10.1186/1471-2377-11-41]. [PMID: 21450100]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Maki T, Hayakawa K, Pham L-DD, Xing C, Lo EH. 2013. [DOI] [PMC free article] [PubMed]
- 147.Arai K., Lok J., Guo S., Hayakawa K., Xing C., Lo E.H. Cellular mechanisms of neurovascular damage and repair after stroke. J. Child Neurol. 2011;26(9):1193–1198. doi: 10.1177/0883073811408610. [http://dx.doi.org/10.1177/ 0883073811408610]. [PMID: 21628695]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Xing C., Hayakawa K., Lok J., Arai K., Lo E.H. Injury and repair in the neurovascular unit. Neurol. Res. 2012;34(4):325–330. doi: 10.1179/1743132812Y.0000000019. [http://dx.doi.org/10.1179/1743132812Y.0000000019]. [PMID: 22643075]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Fagan S.C., Hess D.C., Hohnadel E.J., Pollock D.M., Ergul A. Targets for vascular protection after acute ischemic stroke. Stroke. 2004;35(9):2220–2225. doi: 10.1161/01.STR.0000138023.60272.9e. [http://dx.doi.org/10.1161/01.STR. 0000138023.60272.9e]. [PMID: 15284446]. [DOI] [PubMed] [Google Scholar]
- 150.Hansen T.M., Moss A.J., Brindle N.P. Vascular endothelial growth factor and angiopoietins in neurovascular regeneration and protection following stroke. Curr. Neurovasc. Res. 2008;5(4):236–245. doi: 10.2174/156720208786413433. [http://dx.doi.org/10.2174/156720208786413433]. [PMID: 18991658]. [DOI] [PubMed] [Google Scholar]
- 151.Kassis H., Shehadah A., Chopp M., Zhang Z.G. Epigenetics in Stroke Recovery. Genes (Basel) 2017;8(3):89. doi: 10.3390/genes8030089. [http://dx.doi. org/10.3390/genes8030089]. [PMID: 28264471]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Hu Z., Zhong B., Tan J., Chen C., Lei Q., Zeng L. The emerging role of epigenetics in cerebral ischemia. Mol. Neurobiol. 2017;54(3):1887–1905. doi: 10.1007/s12035-016-9788-3. [http://dx.doi.org/10.1007/s12035-016-9788-3]. [PMID: 26894397]. [DOI] [PubMed] [Google Scholar]
- 153.Hwang J-Y., Aromolaran K.A., Zukin R.S. The emerging field of epigenetics in neurodegeneration and neuroprotection. Nat. Rev. Neurosci. 2017;18(6):347–361. doi: 10.1038/nrn.2017.46. [http://dx.doi.org/10.1038/nrn. 2017.46]. [PMID: 28515491]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Endres M., Meisel A., Biniszkiewicz D., Namura S., Prass K., Ruscher K., Lipski A., Jaenisch R., Moskowitz M.A., Dirnagl U. DNA methyltransferase contributes to delayed ischemic brain injury. J. Neurosci. 2000;20(9):3175–3181. doi: 10.1523/JNEUROSCI.20-09-03175.2000. [http://dx.doi.org/10. 1523/JNEUROSCI.20-09-03175.2000]. [PMID: 10777781]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Endres M., Fan G., Meisel A., Dirnagl U., Jaenisch R. Effects of cerebral ischemia in mice lacking DNA methyltransferase 1 in post-mitotic neurons. Neuroreport. 2001;12(17):3763–3766. doi: 10.1097/00001756-200112040-00032. [http://dx.doi.org/10.1097/00001756-200112040-00032]. [PMID: 11726790]. [DOI] [PubMed] [Google Scholar]
- 156.Dock H., Theodorsson A., Theodorsson E. DNA methylation inhibitor zebularine confers stroke protection in ischemic rats. Transl. Stroke Res. 2015;6(4):296–300. doi: 10.1007/s12975-015-0397-7. [http://dx.doi.org/10. 1007/s12975-015-0397-7]. [PMID: 25824538]. [DOI] [PubMed] [Google Scholar]
- 157.Jobe E.M., McQuate A.L., Zhao X. Crosstalk among epigenetic pathways regulates neurogenesis. Front. Neurosci. 2012;6:59. doi: 10.3389/fnins.2012.00059. [http://dx.doi.org/10.3389/fnins.2012.00059]. [PMID: 22586361]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Singh V., Sharma P., Capalash N. DNA methyltransferase-1 inhibitors as epigenetic therapy for cancer. Curr. Cancer Drug Targets. 2013;13(4):379–399. doi: 10.2174/15680096113139990077. [http://dx.doi.org/10.2174/ 15680096113139990077]. [PMID: 23517596]. [DOI] [PubMed] [Google Scholar]
- 159.Baltan S., Bachleda A., Morrison R.S., Murphy S.P. Expression of histone deacetylases in cellular compartments of the mouse brain and the effects of ischemia. Transl. Stroke Res. 2011;2(3):411–423. doi: 10.1007/s12975-011-0087-z. [http://dx.doi.org/10.1007/s12975-011-0087-z]. [PMID: 21966324]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Kim H.J., Rowe M., Ren M., Hong J-S., Chen P-S., Chuang D-M. Histone deacetylase inhibitors exhibit anti-inflammatory and neuroprotective effects in a rat permanent ischemic model of stroke: multiple mechanisms of action. J. Pharmacol. Exp. Ther. 2007;321(3):892–901. doi: 10.1124/jpet.107.120188. [http://dx.doi.org/10.1124/jpet.107.120188]. [PMID: 17371805]. [DOI] [PubMed] [Google Scholar]
- 161.Ren M., Leng Y., Jeong M., Leeds P.R., Chuang D.M. Valproic acid reduces brain damage induced by transient focal cerebral ischemia in rats: potential roles of histone deacetylase inhibition and heat shock protein induction. J. Neurochem. 2004;89(6):1358–1367. doi: 10.1111/j.1471-4159.2004.02406.x. [http://dx.doi.org/10.1111/j.1471-4159.2004.02406.x]. [PMID: 15189338]. [DOI] [PubMed] [Google Scholar]
- 162.Kassis H., Chopp M., Liu X.S., Shehadah A., Roberts C., Zhang Z.G. Histone deacetylase expression in white matter oligodendrocytes after stroke. Neurochem. Int. 2014;77:17–23. doi: 10.1016/j.neuint.2014.03.006. [http://dx.doi.org/10.1016/j.neuint.2014.03.006]. [PMID: 24657831]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Kim H.J., Leeds P., Chuang D.M. The HDAC inhibitor, sodium butyrate, stimulates neurogenesis in the ischemic brain. J. Neurochem. 2009;110(4):1226–1240. doi: 10.1111/j.1471-4159.2009.06212.x. [http://dx.doi.org/10.1111/j.1471-4159.2009.06212.x]. [PMID: 19549282]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Faraco G., Pancani T., Formentini L., Mascagni P., Fossati G., Leoni F., Moroni F., Chiarugi A. Pharmacological inhibition of histone deacetylases by suberoylanilide hydroxamic acid specifically alters gene expression and reduces ischemic injury in the mouse brain. Mol. Pharmacol. 2006;70(6):1876–1884. doi: 10.1124/mol.106.027912. [http://dx.doi.org/10.1124/mol.106.027912]. [PMID: 16946032]. [DOI] [PubMed] [Google Scholar]
- 165.Liu X.S., Chopp M., Zhang R.L., Zhang Z.G. MicroRNAs in cerebral ischemia-induced neurogenesis. J. Neuropathol. Exp. Neurol. 2013;72(8):718–722. doi: 10.1097/NEN.0b013e31829e4963. [http://dx.doi.org/10.1097/NEN.0b013 e31829e4963]. [PMID: 23860031]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Liu X.S., Chopp M., Zhang R.L., Tao T., Wang X.L., Kassis H., Hozeska-Solgot A., Zhang L., Chen C., Zhang Z.G. MicroRNA profiling in subventricular zone after stroke: MiR-124a regulates proliferation of neural progenitor cells through Notch signaling pathway. PLoS One. 2011;6(8):e23461. doi: 10.1371/journal.pone.0023461. [http://dx.doi. org/10.1371/journal.pone.0023461]. [PMID: 21887253]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Lapchak P.A., Zhang J.H. Neuroprotective Therapy for Stroke and Ischemic Disease. Springer; 2017. [http://dx.doi.org/10. 1007/978-3-319-45345-3] [Google Scholar]
- 168.Lee J-K., Kim J-E., Sivula M., Strittmatter S.M. Nogo receptor antagonism promotes stroke recovery by enhancing axonal plasticity. J. Neurosci. 2004;24(27):6209–6217. doi: 10.1523/JNEUROSCI.1643-04.2004. [http://dx.doi.org/10. 1523/JNEUROSCI.1643-04.2004]. [PMID: 15240813]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Murphy T.H., Corbett D. Plasticity during stroke recovery: from synapse to behaviour. Nat. Rev. Neurosci. 2009;10(12):861–872. doi: 10.1038/nrn2735. [http://dx.doi.org/10.1038/nrn2735]. [PMID: 19888284]. [DOI] [PubMed] [Google Scholar]
- 170.Lindenberg R., Renga V., Zhu L.L., Nair D., Schlaug G. Bihemispheric brain stimulation facilitates motor recovery in chronic stroke patients. Neurology. 2010;75(24):2176–2184. doi: 10.1212/WNL.0b013e318202013a. [http://dx.doi. org/10.1212/WNL.0b013e318202013a]. [PMID: 21068427]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Levy R., Ruland S., Weinand M., Lowry D., Dafer R., Bakay R. Cortical stimulation for the rehabilitation of patients with hemiparetic stroke: a multicenter feasibility study of safety and efficacy. 2008. [DOI] [PubMed]
- 172.Yamashima T. Implication of cysteine proteases calpain, cathepsin and caspase in ischemic neuronal death of primates. Prog. Neurobiol. 2000;62(3):273–295. doi: 10.1016/s0301-0082(00)00006-x. [http://dx.doi.org/10.1016/S0301-0082(00) 00006-X]. [PMID: 10840150]. [DOI] [PubMed] [Google Scholar]
- 173.Karatas H., Aktas Y., Gursoy-Ozdemir Y., Bodur E., Yemisci M., Caban S., Vural A., Pinarbasli O., Capan Y., Fernandez-Megia E., Novoa-Carballal R., Riguera R., Andrieux K., Couvreur P., Dalkara T. A nanomedicine transports a peptide caspase-3 inhibitor across the blood-brain barrier and provides neuroprotection. J. Neurosci. 2009;29(44):13761–13769. doi: 10.1523/JNEUROSCI.4246-09.2009. [http://dx.doi.org/ 10.1523/JNEUROSCI.4246-09.2009]. [PMID: 19889988]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Deng Y-h., He H-y. Yang, L-q. Zhang, P-y. Dynamic changes in neuronal autophagy and apoptosis in the ischemic penumbra following permanent ischemic stroke. Neural Regen. Res. 2016;11(7):1108–1114. doi: 10.4103/1673-5374.187045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Yu Z.F., Bruce-Keller A.J., Goodman Y., Mattson M.P. Uric acid protects neurons against excitotoxic and metabolic insults in cell culture, and against focal ischemic brain injury in vivo. J. Neurosci. Res. 1998;53(5):613–625. doi: 10.1002/(SICI)1097-4547(19980901)53:5<613::AID-JNR11>3.0.CO;2-1. [http://dx.doi.org/10.1002/ (SICI)1097-4547(19980901)53:5<613:AID-JNR11>3.0.CO;2-1]. [PMID: 9726432]. [DOI] [PubMed] [Google Scholar]
- 176.1999.
- 177.Asano T., Mori T., Shimoda T., Shinagawa R., Satoh S., Yada N., Katsumata S., Matsuda S., Kagamiishi Y., Tateishi N. Arundic acid (ONO-2506) ameliorates delayed ischemic brain damage by preventing astrocytic overproduction of S100B. Curr. Drug Targets CNS Neurol. Disord. 2005;4(2):127–142. doi: 10.2174/1568007053544084. [http://dx.doi. org/10.2174/1568007053544084]. [PMID: 15857298]. [DOI] [PubMed] [Google Scholar]
- 178.Tateishi N., Mori T., Kagamiishi Y., Satoh S., Katsube N., Morikawa E., Morimoto T., Matsui T., Asano T. Astrocytic activation and delayed infarct expansion after permanent focal ischemia in rats. Part II: suppression of astrocytic activation by a novel agent (R)-(-)-2-propyloctanoic acid (ONO-2506) leads to mitigation of delayed infarct expansion and early improvement of neurologic deficits. J. Cereb. Blood Flow Metab. 2002;22(6):723–734. doi: 10.1097/00004647-200206000-00011. [http://dx.doi.org/10.1097/00004647-200206000-00011]. [PMID: 12045671]. [DOI] [PubMed] [Google Scholar]
- 179.Pettigrew L.C., Kasner S.E., Gorman M., Atkinson R.P., Funakoshi Y., Ishibashi H. Effect of arundic acid on serum S-100β in ischemic stroke. J. Neurol. Sci. 2006;251(1-2):57–61. doi: 10.1016/j.jns.2006.09.002. [http:// dx.doi.org/10.1016/j.jns.2006.09.002]. [PMID: 17092520]. [DOI] [PubMed] [Google Scholar]
- 180.Simão F, Matté A, Matté C, Soares FMS, Wyse ATS, Netto CA. 2011.
- 181.Canal C.C., Pagnussat A.S., Orlandi L., Worm P., Moura N., Etgen A.M. Coumestrol has neuroprotective effects before and after global cerebral ischemia in female rats. Brain Res. 2012;1474:82–90. doi: 10.1016/j.brainres.2012.07.025. [http://dx.doi.org/10.1016/j.brainres.2012.07.025]. [DOI] [PubMed] [Google Scholar]
- 182.Castro C.C., Pagnussat A.S., Moura N., da Cunha M.J., Machado F.R., Wyse A.T.S. Coumestrol treatment prevents Na+, K+-ATPase inhibition and affords histological neuroprotection to male rats receiving cerebral global ischemia. Neurol. Res. 2014;36(3):198–206. doi: 10.1179/1743132813Y.0000000286. [DOI] [PubMed] [Google Scholar]
- 183.Cheng B., Christakos S., Mattson M.P. Tumor necrosis factors protect neurons against metabolic-excitotoxic insults and promote maintenance of calcium homeostasis. Neuron. 1994;12(1):139–153. doi: 10.1016/0896-6273(94)90159-7. [http://dx.doi.org/10.1016/0896-6273(94)90159-7]. [DOI] [PubMed] [Google Scholar]
- 184.Nicole O., Ali C., Docagne F., Plawinski L., MacKenzie E.T., Vivien D., Buisson A. Neuroprotection mediated by glial cell line-derived neurotrophic factor: involvement of a reduction of NMDA-induced calcium influx by the mitogen-activated protein kinase pathway. J. Neurosci. 2001;21(9):3024–3033. doi: 10.1523/JNEUROSCI.21-09-03024.2001. [http://dx. doi.org/10.1523/JNEUROSCI.21-09-03024.2001]. [PMID: 11312287]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Kilic U., Kilic E., Dietz G.P.H., Bähr M. Intravenous TAT-GDNF is protective after focal cerebral ischemia in mice. Stroke. 2003;34(5):1304–1310. doi: 10.1161/01.STR.0000066869.45310.50. [http://dx.doi.org/10.1161/01.STR. 0000066869.45310.50]. [PMID: 12677018]. [DOI] [PubMed] [Google Scholar]
- 186.Sumbria R.K., Boado R.J., Pardridge W.M. Combination stroke therapy in the mouse with blood–brain barrier penetrating IgG–GDNF and IgG–TNF decoy receptor fusion proteins. Brain Res. 2013;1507:91–96. doi: 10.1016/j.brainres.2013.02.022. [DOI] [PubMed] [Google Scholar]
- 187.Zhang Y., Pardridge W.M. Blood–brain barrier targeting of BDNF improves motor function in rats with middle cerebral artery occlusion. Brain Res. 2006;1111(1):227–229. doi: 10.1016/j.brainres.2006.07.005. [http://dx.doi.org/ 10.1016/j.brainres.2006.07.005]. [DOI] [PubMed] [Google Scholar]
- 188.Butovsky O., Ziv Y., Schwartz A., Landa G., Talpalar A.E. Pluchino, S Microglia activated by IL-4 or IFN-γ differentially induce neurogenesis and oligodendrogenesis from adult stem/progenitor cells. Mol. Cell. Neurosci. 2006;31(1):149–160. doi: 10.1016/j.mcn.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 189.Clausen B.H., Lundberg L., Yli-Karjanmaa M., Martin N.A., Svensson M., Alfsen M.Z. Fumarate decreases edema volume and improves functional outcome after experimental stroke. Exp. Neurol. 2017;295:144–154. doi: 10.1016/j.expneurol.2017.06.011. [http://dx.doi.org/10.1016/j.expneurol. 2017.06.011]. [DOI] [PubMed] [Google Scholar]
- 190.Taylor R.A., Chang C-F., Goods B.A., Hammond M.D., Grory B.M., Ai Y. TGF-β1 modulates microglial phenotype and promotes re-covery after intracerebral hemorrhage. Clin. Investig. (Lond.) 2017;127(1):280–292. doi: 10.1172/JCI88647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Dhungana H., Huuskonen M.T., Pihlajaniemi T., Heljasvaara R., Vivien D., Kanninen K.M., Malm T., Koistinaho J., Lemarchant S. Lack of collagen XV is protective after ischemic stroke in mice. Cell Death Dis. 2017;8(1):e2541. doi: 10.1038/cddis.2016.456. [http://dx.doi.org/10.1038/ cddis.2016.456]. [PMID: 28079884]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Mocco J., Choudhri T., Huang J., Harfeldt E., Efros L., Klingbeil C., Vexler V., Hall W., Zhang Y., Mack W., Popilskis S., Pinsky D.J., Connolly E.S., Jr HuEP5C7 as a humanized monoclonal anti-E/P-selectin neurovascular protective strategy in a blinded placebo-controlled trial of nonhuman primate stroke. Circ. Res. 2002;91(10):907–914. doi: 10.1161/01.res.0000042063.15901.20. [http://dx.doi.org/10.1161/01.RES. 0000042063.15901.20]. [PMID: 12433835]. [DOI] [PubMed] [Google Scholar]
- 193.Jin A.Y., Tuor U.I., Rushforth D., Kaur J., Muller R.N., Petterson J.L., Boutry S., Barber P.A. Reduced blood brain barrier breakdown in P-selectin deficient mice following transient ischemic stroke: a future therapeutic target for treatment of stroke. BMC Neurosci. 2010;11(1):12. doi: 10.1186/1471-2202-11-12. [http://dx.doi.org/10.1186/1471-2202-11-12]. [PMID: 20122276]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Yago T., Petrich B.G., Zhang N., Liu Z., Shao B., Ginsberg M.H., McEver R.P. Blocking neutrophil integrin activation prevents ischemia-reperfusion injury. J. Exp. Med. 2015;212(8):1267–1281. doi: 10.1084/jem.20142358. [http://dx.doi.org/10.1084/jem.20142358]. [PMID: 26169939]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Weise G., Pösel C., Möller K., Kranz A., Didwischus N., Boltze J. High-dosage granulocyte colony stimulating factor treatment alters monocyte trafficking to the brain after experimental stroke. Brain Behav. Immun. 2017;60:15–26. doi: 10.1016/j.bbi.2016.08.008. [http://dx.doi.org/10.1016/j.bbi. 2016.08.008]. [DOI] [PubMed] [Google Scholar]
- 196.Sun Y., Jin K., Xie L., Childs J., Mao X.O., Logvinova A., Greenberg D.A. VEGF-induced neuroprotection, neurogenesis, and angiogenesis after focal cerebral ischemia. J. Clin. Invest. 2003;111(12):1843–1851. doi: 10.1172/JCI17977. [http://dx.doi.org/10.1172/JCI200317977]. [PMID: 12813020]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Soleman S., Yip P.K., Duricki D.A., Moon L.D. Delayed treatment with chondroitinase ABC promotes sensorimotor recovery and plasticity after stroke in aged rats. Brain. 2012;135(Pt 4):1210–1223. doi: 10.1093/brain/aws027. [http://dx.doi.org/10.1093/brain/aws027]. [PMID: 22396394]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Hill J.J., Jin K., Mao X.O., Xie L., Greenberg D.A. Intracerebral chondroitinase ABC and heparan sulfate proteoglycan glypican improve outcome from chronic stroke in rats. Proc. Natl. Acad. Sci. USA. 2012;109(23):9155–9160. doi: 10.1073/pnas.1205697109. [http://dx.doi.org/10.1073/ pnas.1205697109]. [PMID: 22615373]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Lee B., Clarke D., Al Ahmad A., Kahle M., Parham C., Auckland L., Shaw C., Fidanboylu M., Orr A.W., Ogunshola O., Fertala A., Thomas S.A., Bix G.J. Perlecan domain V is neuroprotective and proangiogenic following ischemic stroke in rodents. J. Clin. Invest. 2011;121(8):3005–3023. doi: 10.1172/JCI46358. [http://dx.doi.org/10.1172/ JCI46358]. [PMID: 21747167]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Deroide N., Li X., Lerouet D., Van Vré E., Baker L., Harrison J., Poittevin M., Masters L., Nih L., Margaill I., Iwakura Y., Ryffel B., Pocard M., Tedgui A., Kubis N., Mallat Z. MFGE8 inhibits inflammasome-induced IL-1β production and limits postischemic cerebral injury. J. Clin. Invest. 2013;123(3):1176–1181. doi: 10.1172/JCI65167. [http://dx.doi.org/10.1172/JCI65167]. [PMID: 23454767]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Zheng Y.Q., Liu J.X., Li X.Z., Xu L., Xu Y.G. RNA interference-mediated downregulation of Beclin1 attenuates cerebral ischemic injury in rats. Acta Pharmacol. Sin. 2009;30(7):919–927. doi: 10.1038/aps.2009.79. [http://dx.doi.org/10.1038/aps.2009.79]. [PMID: 19574998]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Xu X., Chua K-W., Chua C.C., Liu C-F., Hamdy R.C., Chua B.H.L. Synergistic protective effects of humanin and necrostatin-1 on hypoxia and ischemia/reperfusion injury. Brain Res. 2010;1355:189–194. doi: 10.1016/j.brainres.2010.07.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Chang P., Dong W., Zhang M., Wang Z., Wang Y., Wang T., Gao Y., Meng H., Luo B., Luo C., Chen X., Tao L. Anti-necroptosis chemical necrostatin-1 can also suppress apoptotic and autophagic pathway to exert neuroprotective effect in mice intracerebral hemorrhage model. J. Mol. Neurosci. 2014;52(2):242–249. doi: 10.1007/s12031-013-0132-3. [http://dx.doi.org/10.1007/s12031-013-0132-3]. [PMID: 24122153]. [DOI] [PubMed] [Google Scholar]
- 204.Chen F., Su X., Lin Z., Lin Y., Yu L., Cai J. Necrostatin-1 attenuates early brain injury after subarachnoid hemorrhage in rats by inhibiting necroptosis. Neuropsychiatr. Dis. Treat. 2017;13:1771–1782. doi: 10.2147/NDT.S140801. [http://dx.doi.org/10.2147/NDT.S140801]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Lu W., Sun J., Yoon J.S., Zhang Y., Zheng L., Murphy E., Mattson M.P., Lenardo M.J. Mitochondrial protein PGAM5 regulates mitophagic protection against cell necroptosis. PLoS One. 2016;11(1):e0147792. doi: 10.1371/journal.pone.0147792. [http://dx.doi.org/10.1371/journal.pone. 0147792]. [PMID: 26807733]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Turlova E., Bae C.Y.J., Deurloo M., Chen W., Barszczyk A., Horgen F.D., Fleig A., Feng Z.P., Sun H.S. TRPM7 regulates axonal outgrowth and maturation of primary hippocampal neurons. Mol. Neurobiol. 2016;53(1):595–610. doi: 10.1007/s12035-014-9032-y. [http://dx.doi.org/10.1007/ s12035-014-9032-y]. [PMID: 25502295]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Sun H-S., Jackson M.F., Martin L.J., Jansen K., Teves L., Cui H. Suppression of hippocampal TRPM7 protein prevents delayed neuronal death in brain ischemia. Nat. Neurosci. 2009;12(10):1300–1307. doi: 10.1038/nn.2395. [http://dx.doi.org/10.1038/nn.2395]. [DOI] [PubMed] [Google Scholar]
- 208.Chen W., Xu B., Xiao A., Liu L., Fang X., Liu R., Turlova E., Barszczyk A., Zhong X., Sun C.L., Britto L.R., Feng Z.P., Sun H.S. TRPM7 inhibitor carvacrol protects brain from neonatal hypoxic-ischemic injury. Mol. Brain. 2015;8(1):11. doi: 10.1186/s13041-015-0102-5. [http://dx.doi. org/10.1186/s13041-015-0102-5]. [PMID: 25761704]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Lule S., Wu L., McAllister L.M., Edmiston W.J., Chung J.Y., Levy E. Genetic inhibition of receptor interacting protein kinase-1 reduces cell death and improves functional outcome after intracerebral hemorrhage in mice. 2017. [DOI] [PMC free article] [PubMed]
- 210.Haugaard-Kedström L.M., Fernandes E.F.A., Strømgaard K. In: Targeting PSD-95 as a novel approach in the treatment of stroke. Neuroprotective Therapy for Stroke and Ischemic Disease; Lapchak, P.A. Zhang J.H., editor. Cham: Springer International Publishing; 2017. pp. 157–184. [http://dx.doi.org/10.1007/978-3-319-45345-3_6] [Google Scholar]
- 211.Sun H-S., Doucette T.A., Liu Y., Fang Y., Teves L., Aarts M., Ryan C.L., Bernard P.B., Lau A., Forder J.P., Salter M.W., Wang Y.T., Tasker R.A., Tymianski M. Effectiveness of PSD95 inhibitors in permanent and transient focal ischemia in the rat. Stroke. 2008;39(9):2544–2553. doi: 10.1161/STROKEAHA.107.506048. [http://dx.doi.org/10.1161/ STROKEAHA.107.506048]. [PMID: 18617669]. [DOI] [PubMed] [Google Scholar]
- 212.Xu B., Xiao A-J., Chen W., Turlova E., Liu R., Barszczyk A., Sun C.L.F., Liu L., Tymianski M., Feng Z.P., Sun H.S. Neuroprotective effects of a PSD-95 inhibitor in neonatal hypoxic-Ischemic brain injury. Mol. Neurobiol. 2016;53(9):5962–5970. doi: 10.1007/s12035-015-9488-4. [http://dx.doi.org/10.1007/s12035-015-9488-4]. [PMID: 26520452]. [DOI] [PubMed] [Google Scholar]
- 213.Stary C.M., Xu L., Li L., Sun X., Ouyang Y-B., Xiong X. Inhibition of miR-181a protects female mice from transient focal cerebral ischemia by targeting astrocyte estrogen receptor-α. Mol. Cell. Neurosci. 2017;82:118–125. doi: 10.1016/j.mcn.2017.05.004. [http://dx.doi.org/10.1016/j.mcn. 2017.05.004]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Xu L-J., Ouyang Y-B., Xiong X., Stary C.M., Giffard R.G. Post-stroke treatment with miR-181 antagomir reduces injury and improves long-term behavioral recovery in mice after focal cerebral ischemia. Exp. Neurol. 2015;264:1–7. doi: 10.1016/j.expneurol.2014.11.007. [http://dx.doi.org/10. 1016/j.expneurol.2014.11.007]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Mo J-L., Liu Q., Kou Z-W., Wu K-W., Yang P., Chen X-H., Sun F.Y. MicroRNA-365 modulates astrocyte conversion into neuron in adult rat brain after stroke by targeting Pax6. Glia. 2018;66(7):1346–1362. doi: 10.1002/glia.23308. [http://dx.doi.org/10.1002/glia.23308]. [PMID: 29451327]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Wang X., Chen S., Ni J., Cheng J., Jia J., Zhen X. miRNA-3473b contributes to neuroinflammation following cerebral ischemia. Cell Death Dis. 2018;9(1):11. doi: 10.1038/s41419-017-0014-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Yang X., Tang X., Sun P., Shi Y., Liu K., Hassan S.H., Stetler R.A., Chen J., Yin K.J. MicroRNA-15a/16-1 Antagomir ameliorates ischemic brain injury in experimental stroke. Stroke. 2017;48(7):1941–1947. doi: 10.1161/STROKEAHA.117.017284. [http://dx.doi.org/10.1161/STROKEAHA.117. 017284]. [PMID: 28546328]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Zhao F., Qu Y., Zhu J., Zhang L., Huang L., Liu H., Li S., Mu D. miR-30d-5p plays an important role in autophagy and apoptosis in developing rat brains after hypoxic-ischemic injury. J. Neuropathol. Exp. Neurol. 2017;76(8):709–719. doi: 10.1093/jnen/nlx052. [http://dx.doi.org/10. 1093/jnen/nlx052]. [PMID: 28789480]. [DOI] [PubMed] [Google Scholar]
- 219.Kokaia Z., Tornero D., Lindvall O. Transplantation of reprogrammed neurons for improved recovery after stroke. 2017. [DOI] [PubMed] [Google Scholar]
- 220.Li Y., Chen J., Zhang C.L., Wang L., Lu D., Katakowski M., Gao Q., Shen L.H., Zhang J., Lu M., Chopp M. Gliosis and brain remodeling after treatment of stroke in rats with marrow stromal cells. Glia. 2005;49(3):407–417. doi: 10.1002/glia.20126. [http://dx.doi.org/10. 1002/glia.20126]. [PMID: 15540231]. [DOI] [PubMed] [Google Scholar]
- 221.Shen L.H., Li Y., Chen J., Zacharek A., Gao Q., Kapke A., Lu M., Raginski K., Vanguri P., Smith A., Chopp M. Therapeutic benefit of bone marrow stromal cells administered 1 month after stroke. J. Cereb. Blood Flow Metab. 2007;27(1):6–13. doi: 10.1038/sj.jcbfm.9600311. [http://dx. doi.org/10.1038/sj.jcbfm.9600311]. [PMID: 16596121]. [DOI] [PubMed] [Google Scholar]
- 222.Bang O.Y., Lee J.S., Lee P.H., Lee G. Autologous mesenchymal stem cell transplantation in stroke patients. Ann. Neurol. 2005;57(6):874–882. doi: 10.1002/ana.20501. [http://dx.doi.org/10.1002/ana.20501]. [PMID: 15929052]. [DOI] [PubMed] [Google Scholar]
- 223.Shen L.H. Li.; Chen, J.; Zhang, J.; Vanguri, P.; Borneman, J. Intracarotid transplantation of bone marrow stromal cells increases axon-myelin remodeling after stroke. Neuroscience. 2006;137(2):393–399. doi: 10.1016/j.neuroscience.2005.08.092. [http://dx.doi.org/10.1016/j.neuroscience.2005.08.092]. [DOI] [PubMed] [Google Scholar]
- 224.Hara K., Matsukawa N., Yasuhara T., Xu L., Yu G., Maki M., Kawase T., Hess D.C., Kim S.U., Borlongan C.V. Transplantation of post-mitotic human neuroteratocarcinoma-overexpressing Nurr1 cells provides therapeutic benefits in experimental stroke: in vitro evidence of expedited neuronal differentiation and GDNF secretion. J. Neurosci. Res. 2007;85(6):1240–1251. doi: 10.1002/jnr.21234. [http://dx.doi. org/10.1002/jnr.21234]. [PMID: 17335085]. [DOI] [PubMed] [Google Scholar]
- 225.Hara K., Yasuhara T., Maki M., Matsukawa N., Masuda T., Yu S.J. Neural progenitor NT2N cell lines from teratocarcinoma for transplantation therapy in stroke. Prog. Neurobiol. 2008;85(3):318–334. doi: 10.1016/j.pneurobio.2008.04.005. [DOI] [PubMed] [Google Scholar]
- 226.Banerjee S., Bentley P., Hamady M., Marley S., Davis J., Shlebak A., Nicholls J., Williamson D.A., Jensen S.L., Gordon M., Habib N., Chataway J. Intra-arterial immunoselected CD34+ stem cells for acute Ischemic Stroke. Stem Cells Transl. Med. 2014;3(11):1322–1330. doi: 10.5966/sctm.2013-0178. [http://dx.doi.org/10.5966/sctm.2013-0178]. [PMID: 25107583]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Jin W-N, Shi SX-Y, Li Z, Li M, Wood K, Gonzales R.J. Depletion of microglia exacerbates postischemic inflammation and brain injury. 2017. [DOI] [PMC free article] [PubMed]
- 228.Majmundar N., Kim B., Prestigiacomo C.J. Necroptosis pathway in treatment of intracerebral hemorrhage: Novel therapeutic target. World Neurosurg. 2016;89:716–717. doi: 10.1016/j.wneu.2016.03.071. [http://dx.doi.org/10. 1016/j.wneu.2016.03.071]. [PMID: 27020976]. [DOI] [PubMed] [Google Scholar]
- 229.Gao L., Dong Q., Song Z., Shen F., Shi J., Li Y. NLRP3 inflammasome: a promising target in ischemic stroke. Inflamm. Res. 2017;66(1):17–24. doi: 10.1007/s00011-016-0981-7. [http://dx.doi.org/10.1007/s00011-016-0981-7]. [PMID: 27576327]. [DOI] [PubMed] [Google Scholar]
- 230.Underly R.G., Levy M., Hartmann D.A., Grant R.I., Watson A.N., Shih A.Y. Pericytes as Inducers of Rapid, Matrix Metalloproteinase-9-Dependent Capillary Damage during Ischemia. J. Neurosci. 2017;37(1):129–140. doi: 10.1523/JNEUROSCI.2891-16.2016. [http://dx.doi.org/10.1523/ JNEUROSCI.2891-16.2016]. [PMID: 28053036]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Siniscalchi A., Iannacchero R., Anticoli S., Pezzella F.R., De Sarro G., Gallelli L. Anti-inflammatory strategies in stroke: a potential therapeutic target. Curr. Vasc. Pharmacol. 2016;14(1):98–105. doi: 10.2174/1570161113666150923111329. [http://dx.doi.org/10.2174/1570161113666150923111329]. [PMID: 26411421]. [DOI] [PubMed] [Google Scholar]
- 232.Suzuki H., Hayashi T., Tojo S.J., Kitagawa H., Kimura K., Mizugaki M., Itoyama Y., Abe K. Anti-P-selectin antibody attenuates rat brain ischemic injury. Neurosci. Lett. 1999;265(3):163–166. doi: 10.1016/s0304-3940(99)00229-3. [http://dx.doi.org/10.1016/S0304-3940(99)00229-3]. [PMID: 10327156]. [DOI] [PubMed] [Google Scholar]
- 233.Suzuki H., Abe K., Tojo S.J., Kitagawa H., Kimura K., Mizugaki M., Itoyama Y. Reduction of ischemic brain injury by anti-P-selectin monoclonal antibody after permanent middle cerebral artery occlusion in rat. Neurol. Res. 1999;21(3):269–276. doi: 10.1080/01616412.1999.11740930. [http:// dx.doi.org/10.1080/01616412.1999.11740930]. [PMID: 10319335]. [DOI] [PubMed] [Google Scholar]
- 234.Ouyang Y.B., Stary C.M., Yang G.Y., Giffard R. microRNAs: innovative targets for cerebral ischemia and stroke. Curr. Drug Targets. 2013;14(1):90–101. doi: 10.2174/138945013804806424. [http://dx.doi.org/10.2174/ 138945013804806424]. [PMID: 23170800]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Gallelli L., Siniscalchi A., Carotenuto M., Caroleo M.C., Cione E., Guidetti V. microRNAs-based predictor factor in patients with migraine-ischemic stroke. MicroRNA. 2017;6(1):17–21. doi: 10.2174/2211536606666170104130101. [http:// dx.doi.org/10.2174/2211536606666170104130101]. [PMID: 28056747]. [DOI] [PubMed] [Google Scholar]
- 236.Ren C., Han R., Shi J., Ji X. In: Ischemic stroke pathophysiology and cell therapy.Bone marrow stem cell therapy for stroke; Jin, K.; Ji, X.; Zhuge, Q. Singapore S., editor. Singapore: 2017. pp. 1–36. [http://dx.doi.org/10.1007/978-981-10-2929-5_1] [Google Scholar]
- 237.Azad T.D., Veeravagu A., Steinberg G.K. Neurorestoration after stroke. Neurosurg. Focus. 2016;40(5):E2. doi: 10.3171/2016.2.FOCUS15637. [http://dx.doi.org/10. 3171/2016.2.FOCUS15637]. [PMID: 27132523]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Albers G.W., Goldstein L.B., Hess D.C., Wechsler L.R., Furie K.L., Gorelick P.B., Hurn P., Liebeskind D.S., Nogueira R.G., Saver J.L. Stroke Treatment Academic Industry Roundtable (STAIR) recommendations for maximizing the use of intravenous thrombolytics and expanding treatment options with intra-arterial and neuroprotective therapies. Stroke. 2011;42(9):2645–2650. doi: 10.1161/STROKEAHA.111.618850. [http://dx.doi.org/10.1161/STROKEAHA.111.618850]. [PMID: 21852620]. [DOI] [PubMed] [Google Scholar]