Abstract
Acute SAH from a ruptured intracranial aneurysm contributes for 30% of all hemorrhagic strokes. The bleeding it-self occurs in the subarachnoid space. Nevertheless, injury to the brain parenchyma occurs as a consequence of the bleeding, directly, via several well-defined mechanisms and pathways, but also indirectly, or secondarily. This secondary brain injury following SAH has a variety of causes and possible mechanisms. Amongst others, inflammatory events have been shown to occur in parallel to, contribute to, or even to initiate programmed cell death (PCD) within the central nervous system (CNS) in human and animal studies alike.
Mechanisms of secondary brain injury are of utmost interest not only to scientists, but also to clinicians, as they often provide possibilities for translational approaches as well as distinct time windows for tailored treat-ment options.
In this article, we review secondary brain injury due to inflammatory changes, that occur on cellular, as well as on molecular level in the various different compartments of the CNS: the brain vessels, the subarachnoid space, and the brain parenchyma itself and hypothesize about possible signaling mechanisms between these compartments
Keywords: Subarachnoid hemorrhage, inflammation, secondary brain injury, microglia, neuronal cell death, secondary brain injury
1. Introduction
Brain injury following SAH is multimodal and occurs directly, as early brain injury, but also secondarily, as delayed brain injury. While early brain injury has been well described and occurs via a cascade of elevated intracranial pressure and a subsequent drop of the cerebral perfusion in the very instant of the bleeding, delayed brain injury has various causes that give rise to many different research targets as well as treatment options-especially due to possible time windows of various duration. Possible contributors to secondary brain injury are-beyond others-cerebral vasospasm, cortical spreading depolarization, compromised autoregulation, an opening of the blood brain barrier or inflammatory events, all of which can result in neuronal injury. The first reports of inflammation following SAH were published in the early nineties and focused very much on the possible relationships between cerebral vasospasm and inflammatory changes within the CSF, as at this time cerebral vasospasm was believed to be the main-if not the only-contributor to secondary brain injury following SAH [1-4]. The first reports on systemic inflammation were published towards the end of the nineties and early 2000s and summarized a peripheral immune modulation following SAH [5, 6]. Nevertheless, in those times-with cerebral vasospasm being believed to be the only-or at least biggest-contributor to secondary brain injury following SAH, inflammation was solely accounted to occur with, or aggravate cerebral vasospasm, instead of being understood as a distinct pathomechanism. It has also been around this time, when endothelin-A was first described to influence cerebral vasospasm in the time course of SAH [7, 8]. This finding led to a series of the biggest randomized controlled trials targeting cerebral vasospasm using the selective endothelin-A receptor antagonist clazosentan, the CONSCIOUS trials [9-13]. When the analysis of the outcome data in the randomized studies did not show a benefit for the patients concerning outcome, despite a significant reduction of cerebral vasospasm and consecutive infarctions, new ideas came into focus, seeking to unchain the – up to that time-very tight association of cerebral vasospasm and unfavorable outcome.
Uncoupling of secondary brain injury and cerebral vasospasm, thanks to several position papers, has only begun a few years ago and is an ongoing process, which is still controversially discussed [14-17].
Yet, thanks to the ongoing discussion, concepts like membrane pathology, cortical spreading depolarization or extracellular matrix homeostasis have since been followed [15, 18-24].
Inflammatory cell invasion or activation has been described in various other pathologies of the CNS and seems to have bidirectional effects, depending on timing, cytokine profile or the surrounding niche, in which they reside [25-33]. Integrating the detrimental and the protective effects of inflammation according to the various settings, tremendous progress has been made concerning the differentiation of the inflammatory cells involved in immune response within the CNS. Peripheral monocytes that invade the brain as macrophages with or without obvious opening of the blood brain barrier, or lymphocytes, the major enforcers of innate and adapted immunity, are the most commonly found inflammatory cells, especially in ischemic stroke, mostly but not exclusively initiating neuro-protective functions in that disease (recently reviewed by Anrather et al. [31]). Besides peripheral macrophages, secondarily invaded into the CNS, the brain’s innate immune cell, the microglia has been intensively studied, especially in neuro-inflammatory and neuro-degenerative diseases like EAE or Alzheimer’s [26, 27, 34, 35]. Microglia are of myeloid origin and develop from extra-embryonic yolk sac cells during ontogenesis only, while all other glia are of neuroectodermal origin [36]. A third type of immune cell within the CNS has recently been reported-the leptomeningeal/perivascular macrophage. Although small in absolute numbers, it seems to have a distinct singularity beyond the others [37, 38]. Whenever studying cellular inflammation, it is thus of utmost importance to differentiate between these cells [36]. Being already large in variety, the myeloid cells of the CNS are not the only contributors to the brain’s immune privileged site [39]. Comprehension of the different anatomical and functional spaces within different parts of the CNS, as well as knowledge barriers/borders can be crossed by elements which are crucial and are well-described by Engelhardt and co-workers [39-42].
2. Inflammation in the subarachnoid space – Outside-in or Inside-out?
The inflammatory events in the subarachnoid space can be divided into 1) cellular inflammation and 2) molecular inflammation. The cellular components enter the subarachnoid space from within the blood vessels, but both (cellular and molecular factors) act on the vascular walls, leading to the question whether inflammation in the CSF is an outside-in or an inside-out reaction.
The subarachnoid space, along with the peripheral blood, has been the first compartment in which inflammatory changes have been described after SAH. Being the first compartment involved in the bleeding, analysis of the CSF in patients after SAH has always been the obvious thing to do. Studies by multiple groups, inflammatory cells, inflammation associated cytokines as well as their corresponding receptors have been found to be upregulated in the CSF of patients suffering from SAH.
2.1. Cellular Components of Inflammation
Cells of the adaptive immune system (T-Cells and B-Cells) have only rarely been found to be up-regulated in the CSF after SAH [3, 43]. The cells of the innate immune system have in contrast been found in high numbers and highly activated states within post-hemorrhagic CSF. Neutrophils even more than NK cells and monocytes/macrophages contribute to an inflammatory niche within the CSF. A higher number of neutrophils as well as their enzymes myeloperoxidase and NADPH oxidase have been documented in patients suffering from vasospasm [43, 44]. Their depletion by a monoclonal antibody against Ly6G/C led to amelioration of vasospasm [45]. The cells of innate immunity are obviously not just passively spilled into the CSF, but extravasate actively from within the blood vessels, generating inflammation in the subarachnoid space in an inside-out fashion. Blockage of the CD11b/CD18 complex led to significantly lower numbers of inflammatory cells and ameliorated vasospasm [46, 47].
2.2. Molecular Components of Inflammation/Influence on Vasoconstriction
Molecular agents of inflammation have first been reported to be up-regulated within post-hemorrhagic CSF in the early nineties. IL-6, IL-1R antagonist and TNF-α were shown to have a correlation with poor clinical outcome [1-3]. A few years later, Fassbender et al. confirmed these findings and correlated them with cerebral vasospasm and poor outcome, respectively [8]. Within the following years, IL-6 has been further defined as a contributor to brain injury and/or cerebral vasospasm by our group and others, being correlated with poor clinical outcome, and proceeding in clinical diagnostics by implementation of bed-side testing methods [48, 49]. Elevated intracranial pressure was identified as cause of elevated IL-6 levels [50]. Within these times, a huge variety of other molecular agents has been identified to contribute to inflammation within post-hemorrhagic CSF, and thus within the subarachnoid space.
Directly surrounded and superfused by the pro-inflammatory CSF lies the cerebral blood vessels at the brain surface. A connection of inflammation and vasoconstriction has therefore always been highly likely. Due to the overwhelming focus on vasospasm research in the past, many studies have focused on functional effects of inflammation on cerebral blood flow or vascular kinetics (e.g. vasoconstriction) [43, 51-55]. Still following in the theory that cerebral vasospasm is one of the strongest contributors to secondary brain injury after SAH [56], many groups have concentrated on the fact that vasoconstriction can be initiated or aggravated by inflammatory cascades an outside effect. The multiple factors that have since been identified to initiate or aggravate cerebral vasoconstriction after SAH comprise interleukins (IL-6, IL-1α and IL-1β, IL-8), TNF-α, LFA-1, leukotrienes, arachidonic acid, vWF, matrix metalloproteases (MMP-9) or VEGF [1, 2, 8, 43, 49, 57-61]. All of these factors, acting as outside-in contributors to cerebral vasospasm, show how multi-factorial genesis is, and also point out how desperate its pharmacotherapy is.
In owr studies using bio-assays, we showed an increased reagibility of microvessels to posthemorrhagic CSF, as well as an increased leukocyte-endothelial interaction in the dorsal skinfold chamber in mice, confirming the previous findings of an association of vasospasm with posthemorrhagic CSF, and adding the information, that an intravascular inflammation is elicited by superfusion of microvessels with CSF from SAH patients. Furthermore, we showed an increased monocyte transmigration through an artificial endothelial cell layer, indicating an inflammatory stimulus, that induces inflammatory cell extravasation, creating or sustaining an inflammatory milieu within this compartment [62].
Modulation of inflammation within the CSF by administration of pro-inflammatory agents following SAH in experimental models led to a worsening of the clinical course, sustaining the previous experiments in a positive feedback fashion [63, 64]. Inflammatory cells are also involved in blood clearance from the subarachnoid space after SAH. In a recent study, Schallner and collaborators showed a microglia-dependent blood clearance, accelerated by higher levels of hemoxygenase-1 as well as carbon monoxide, offering a clinically applicable treatment method [65].
Although inflammation within the CSF seems to start with a passive spilling of blood components into the subarachnoid space on one hand, it does obviously have an active component. Inflammatory cells extravasate from within the blood vessels in an inside-out fashion. Typical pro-inflammatory cytokines have been identified as contributors to secondary brain injury within the CSF, the origin of which, but also their mechanisms of action (many also via the induction or aggravation of cerebral vasospasm) will further have to be addressed. All of the pro-inflammatory factors within the CSF seem to act via an outside-in mechanism directly on the blood vessels, leading to vasoconstriction on one hand but also eliciting intravascular inflammation on the other hand.
3. Inflammation in the cerebral micro-vessels and the vascular wall-influence on the BBB
Therefore, the compartment of the cerebral vasculature as a focus of inflammation is the next logical step in a disease that has its primary cause in the rupture of a blood vessel, which depends on the weakening of the vessel wall and deploys its detrimental effects across the blood brain barrier. All the mechanisms of inflammation described previously derived from blood components being passively spilled into the CSF, or actively invading the perivascular space from within the – otherwise tightly closed – blood brain barrier.
Therefore, three questions arise concerning intravascular inflammation: 1) Characterization of the intravascular inflammation (cell type, time line, molecular mechanisms), 2) How is the blood brain barrier affected and 3) Do we see an intravascular couterpart to the extravascular cytokines acting on cerebral vasoconstriction?
3.1. Cellular and Molecular Mechanisms of Intravascular Inflammation
The characteristics of intravascular inflammation in SAH have been elucidated by our group. We have shown an increase of neutrophil recruitment to the endothelium as a reaction to superfusion of microvessels in the dorsal skinfold chamber with posthemorrhagic CSF in mice within the first few days after bleeding onset [62]. This intravascular inflammation has been further followed in vivo using intravital multi-fluorescence video microscopy through a chronic cranial window in mice suffering eSAH. Neutrophil endothelial interaction was shown to be dependent on ICAM-1 (on the endothelial side) and PSGL-1 (on the neutrophils) and was significantly upregulated within days 1-7 after the bleeding, while other basic hemodynamic parameters remained unchanged. Further, in the course of the disease, a significant general decline in neuronal cell number (not focal cell death) was found within brain samples of these mice as a sign of secondary brain injury. In this context we showed that the knockout of the two mentioned cell adhesion molecules led to a significant decrease of neuronal injury, giving proof that intravascular cellular inflammation contributes to delayed brain injury [66].
Other groups have unveiled additional inflammatory signaling within cerebral blood vessels following SAH. An enhanced expression of pro-inflammatory mediators (IL-1, IL-6 and MMP-9) has been documented as a result of increased activation of the MEK-ERK1/2 pathway in cerebral blood vessels isolated from rats [53]. On the surface of trafficking leukocytes, Toll-like receptor-4 (TLR-4), TLR-associated activator of interferon (TRIF) and the myeloid differentiation primary response gene (MyD88) (via NFkB and IRAK4 pathway) have been described to be important mediators for neuronal apoptosis and cerebral vasospasm, respectively [67]. Similar to our own experiments, Xu and colleagues reported neuroprotection through decreased neutrophil trafficking shortly after the bleeding by blocking vascular adhesion protein-1, another intravascular cell adhesion protein [55]. In a follow-up article on the topic, the same group found a decrease in cerebral vasospasm by attenuating leukocyte-endothelial interaction through blocking VAP-1 [54].
Taken together, we have evidenced intravascular mediators of inflammation on the endothelial as well as on leukocyte surface that comes into action within the first few days after the onset of bleeding, and that contribute to neuronal injury, decrease neurological outcome and increase the probability and/or severity of cerebral vasospasm, respectively. The latter thus being initiated, facilitated and/or aggravated via both, inside and outside (of the vascular wall) mechanisms.
3.2. Influence of Inflammation on the Blood-Brain Barrier
The molecular mechanisms of the blood brain barrier and how they might be compromised through SAH are of utmost importance. A tightly closed BBB contributes for the brain’s immune privilege. Thus, BBB dysfunction gives rise to inflammation in all described compartments of the CNS. Furthermore, the reverse conclusion also holds true: inflammation can lead to a BBB dysfunction and/ or breakdown.
An opening of the BBB through a loss of tightness in the endothelial lining is not only observed in SAH, but also in ischemic stroke, traumatic brain injury or brain tumors, and is always associated with a breakdown of tissue homeostasis (resulting in edema as an additional stress factor for an already injured brain) and the accumulation of potentially harmful substances. BBB dysfunction has early been linked to inflammation, as nicely reviewed by Abbott. Among others, IL-1, TNF-α, bradykinin and arachidonic acid, have been identified to destabilize the BBB significantly [68]. Alves and collaborators have gained further insight into the dependent downstream mechanisms, which are a significant loss of occludin, zonula occludens-1 and tight junction redistribution. Although shown in models of traumatic brain injury, rather than SAH, we can deduct that these mechanisms also play a role in this pathology [69]. In in vitro experiments with cultured cerebral microvascular endothelial cells, the pro-inflammatory cytokines IL-1, IL-6, IL-8 and TNF-α led to a breakdown of the endothelial lining, the latter of which through caspase-3-dependent pathways [59]. In another study, protecting effects of N-acetylcysteine, glucocortikoids and interferons were documented in similar artificial endothelial cultures. The immunomodulatory properties of these agents provide additional evidence on inflammation-mediated impairment of the BBB. Going further into the molecular details, adherens junction and tight junction transmembrane proteins (VE-cadherin, occluding, claudins) seem to be the most important factors in maintaining inter-cell stability in the physiological state, as well as under pathological conditions [70-72]. The cellular components of the BBB are pericytes, astrocytes, endothelial cells and choroid plexus epithelial cells, the distinct roles of which as well as their interaction and transmigration or transport through their closed layer have been well reviewed [41, 42]. During SAH, the upregulation of inflammatory cytokines, especially IL-6, leads to a destabilization of the BBB by a decrease in occluding, claudin-5 and VE-cadherin (own unpublished data, under revision). The source of IL-6 is microglia and possibly also endothelial cells [66, 73]. Other possible mechanisms of BBB destabilization after SAH are hypoxia-inducible factor-1α, aquaporin-4 and MMP-9, all of which were up-regulated in rat brains following SAH. Administration of the respective inhibitors, led to significant reduction of SAH-induced brain swelling [74]. Also hemoxygenase-1, a breakdown product of hemoglobin, induced BBB-breakdown-mediated cell death within the CNS in another animal model [75]. Furthermore, experimental treatment options have recently been discussed. Minocycline-treatment in rats suffering from SAH led to a stabilization of the BBB by inhibition of NLRP3 inflammasome activation and to an amelioration of neurological symptoms [76, 77]. A clinical study has meanwhile been initiated (ongoing, University of Buffalo, Siddiqui, AH).
Taken together, these results show severe impairment in BBB tightness as a reaction to a huge variety of inflammatory mediators, that accumulate in the early time-course of SAH. The main molecular mechanisms affected by inflammation, leading to a disruption of the BBB are tight junction and adherens junction transmembrane proteins-occluding, claudins and VE catherin. Impairment of the BBB leads to 1) alteration of fluid homeostasis within the brain, causing edema and facilitating secondary brain injury and 2) compromise of the immune privilege of the CNS, giving rise to further intraparenchymal inflammation and cell death, respectively [66, 73].
4. Inflammation within the brain tissue
Secondary brain injury following SAH has long since been described. Several mechanisms are supposed to contribute to neuronal injury, among them brain edema (as a consequence of BBB impairment), cerebral vasospasm (with consecutive infarctions), autoregulation changes (with consecutive hypo- or hyperperfusion), spreading depolarization, or the direct action of toxic noxae (e.g. hemoglobin end products, increased oxidative stress, etc.). All of these can act, be activated or be aggravated by inflammatory pathways, as summarized above.
The next question is, whether inflammation itself can elicit neuronal injury. Inflammation within the cerebral parenchyma proper, has only very recently been described. Compared to the knowledge on inflammatory processes within the other two compartments (subarachnoid space and vascular lumen), the inflammation within the brain tissue is the least understood and still holds most of the questions. It is important to 1) characterize the location and time-line of inflammation inside the brain parenchyma, 2) clearly define the underlying cellular mechanisms and gain insight into the molecular pathways, and 3) define and quantify the extent of brain injury attributable to inflammation.
4.1. Cerebral Spreading Inflammation/Microglia Accumulation
In a study by Hanafy and colleagues several molecular mechanism pathways (TLR-4, MyD88 and TRIF) have been described to contribute to neuronal cell death. They also found these pathways dependent on cultured Iba-1-positive cells, assuming these to be microglia and proposing immunotherapies like TLR-targeting as potential new therapies in SAH treatment [67]. These data inspired our group to characterizing the cellular immune response within the CNS more closely. In autopsies of human brains, we also found KiM1P-positive cells (potentially microglia) in a close spatial relationship to the site of aneurysm rupture. In experimental studies in mice, we showed a wave of Iba-1-positive cells spreading within the brain tissue between days 4 and 28 after experimental SAH. The wave of immune cells was chronologically correlated to neuronal cell death. We ruled out peripheral leukocyte recruitment by chimeric experiments, defining microglia to be the only underlying cell type of this newly described cerebral spreading inflammation. Furthermore, we unveiled the mechanism of microglia cells themselves inflicting neuronal cell death, instead of protecting the already stressed brain against further harm [73]. Our findings were in line with results by Greenhalgh and colleagues, who had also found microglia to accumulate near the site of vessel rupture during the first few weeks after SAH [75]. Why microglia act against the organ they are supposed to defend remains unclear. In microglia isolated from brains after SAH, we measured an increased level of all the aforementioned pro-inflammatory cytokines (IL-1, IL-6 and TNF-α), but also of their receptors, hinting on potential pathways of autocrine stimulation [73].
4.2. Experimental Neuro-behavioural Testing
Besides structural changes of the brain tissue (e.g. neuronal cell death, disintegration of inter-cell connections or opening of the BBB), functional read-out parameters have to show a worsening of the clinical outcome, either clinically in patients or experimentally in animals, to determine, whether structural changes do indeed result in a clinically relevant problem. Rodent models are the most commonly used animal models for SAH research. While in rats, extensive neurological and mental testing has been established and serves as a good read-out parameter for clinical testing [78, 79]; this has not yet been the case for mouse models. Our group has performed extensive neurological and neuro-behavioural testing in mice suffering from SAH (data not published), that did not reveal any measurable changes between the groups. Neither have other groups succeeded in reporting useful neuro-behavioural testing in this field. Multimodal MRI-imaging can provide additional surrogate parameters for the clinical course, but cannot replace neurological testing [80]. One hypothesis causing this dilemma might be smaller subarachnoid spaces in mice, resulting in lesser blood volumes in single perforation-models. Using a double-hemorrhage model, most of the animals die, while the rest are severely disabled, also hinting at a more narrow- threshold of bleeding that is survivable by the much smaller animal.
Summarized, intraparenchymal inflammation following SAH is characterized by a wave of microglia, that inflicts neuronal injury via typical pro-inflammatory cytokines. Meticulous understanding of beneficial and detrimental effects of microglia under certain pathological conditions is crucial to further advance our knowledge on this very special kind of cell within the brain [26, 27, 32, 36, 81-84]. Yet, despite highly ranked publications in the field, we must not forget a significant difference between SAH and all of the other diseases mentioned earlier. The pathology in those lies within the brain tissue itself, while in SAH, the bleeding occurs mostly outside the brain parenchyma. Although being mediated into the brain, inflammatory pathways and mechanisms leading to neuronal injury might therefore differ substantially.
5. Integration of the current data and understanding of the inflammatory cascade
Our current understanding of the inflammatory processes following SAH within the subarachnoid space, the cerebral vessels and the brain parenchyma are a subsequent activation, that originates extraparenchymally by passive spilling, but much more by active transmigration of immune cells into the subarachnoid space, where they accumulate and produce pro-inflammatory mediators within the first few days after SAH. In an outside-in fashion these cells and cytokines exert unfavorable effects on 1) vascular structures, resulting in vasoconstriction and 2) the BBB, causing leakage and consecutive disturbance of the tissue homeostasis, as well as destabilizing the brains’s immune privilege.
At the same time, an intravascular inflammation (neutrophil recruitment to the vessel wall via several cell-adhesion molecules) is established inside the brain vessel. While these leukocytes do transmigrate into the subarachnoid space, they do not infiltrate the brain tissue.
Within the brain tissue, a wave of microglia activation runs through the brain starting at day 4 after the bleeding, thus, succeeding the subarachnoid and intravascular inflammation. This cerebral spreading inflammation causes neuronal cell death by production of pro-inflammatory cytokines (IL-1, IL-6, TNF-α).
The inflammatory milieu within the CSF is maintained for several days after the bleeding and might well be the first compartment affected by inflammation. Mechanistically, we have proven that intravascular inflammation is a pre-requisite for intracerebral inflammation, showing an outside-in activation. Whether inflammation within the CSF activates intravascular inflammation or vice versa, or if both occur in parallel and have common initiators is not fully understood. Clearly, typical pro-inflammatory cytokines play crucial roles in various pathways like vasoconstriction, disintegration of the BBB or direct neuronal injury.
Our current understanding of the inflammatory cascade following SAH in the different compartments of the CNS is illustrated in Figs. (1 and 2).
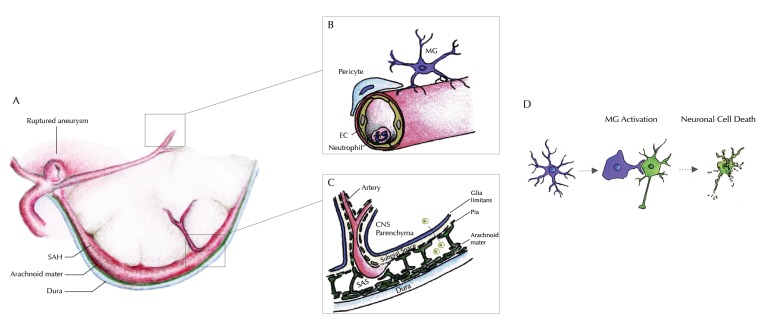
Fig. (1).
Illustration of our current understanding of inflammatory processes within the different compartments of the CNS. A: Rupture of an intracranial aneurysm within the subarachnoid space causes spilling of blood between the arachnoid mater and the pia mater – outside of the brain parenchyma. B: Within the cerebral microvessels, an increased gathering of neutrophil granulocytes at the endothelial surface is seen as a sign of intravascular inflammation, within the first 1-4 days after the bleeding. An extravasation (possibly into the subarachnoid space) has been reported, but transmigration into the brain parenchyma could be excluded. Around day 4 after the bleeding, an accumulation of microglia starts near the site of vessel rupture-i.e. near the large conductance vessels at the brain base. Knocking out of Neutrophil endothelial interaction inhibits microglia accumulation within the brain tissue. C: Signaling from the subarachnoid space into the brain parenchyma must therefore occur within the time window of vascular inflammation through the pia and the glia limitans. Profound understanding of the anatomical and molecular mechanisms of the blood brain barrier is therefore essential to understand this outside-in activation of innate immunity, that successively inflicts secondary brain injury. It remains unclear whether this signaling involves a cellular component (e.g. arachnoid lymphocytes) or occurs in a strictly non-cellular fashion. D: The inflammatory cascade continues through activation of innate immunity within the brain parenchyma. Microglia, the brain’s innate immune cells accumulate and are activated beginning from day four after the bleeding. Throughout the time interval of maximum accumulation (between days 9 and 14), microglia are also activated, expressing a variety of pro-inflammatory cytokines. Changing to their activated morphology, they gather around neurons, inflicting neuro-axonal injury, decimating the absolute number of surviving neurons significantly, hereby contributing substantially to secondary brain injury after SAH.
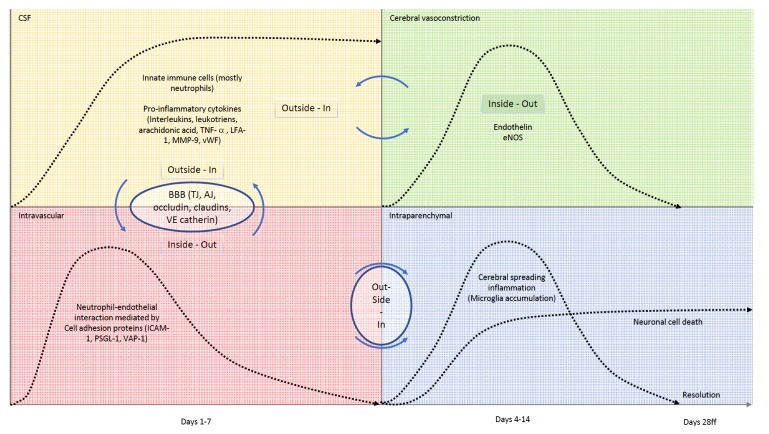
Fig. (2).
Schematic time-line and interactions of the inflammatory cascade within the different compartments of the CNS. Upper left (yellow): Within the CSF, accumulation of innate immune cells (mostly neutrophils) and pro-inflammatory factors is seen within the first few days after onset of the bleeding. The number of cells and burden of molecular inflammatory mediators stays high, even weeks after the bleeding. Inflammatory factors within the CSF contribute to cerebral vasoconstriction in an outside-in fashion, making cerebral vasospam a multi-factorial inflammation-co-triggered event. Inflammation of the subarachnoid space also acts on the cerebral microvessels, in which we see an intravascular neutrophil-endotheilal recruitment, mediated via different cell-adhesion molecules (lower left, red). Messaging pathways and transmigration of innate immune cells via the blood brain barrier are not completely uncovered, but to date it is clear, that occluding, claudins and VE-catherin play important roles in selective transmigration processes. Upper right, green: Being significantly influenced from the outside, cerebral vasospasm is also regulated from within the vessel lumen by substances secreted by activated endothelial cells like endogenous nitric oxide synthase or endothelin. If activation of the endothelium occurs via an outside-in stimulus or signal to the abluminal side of the vessel remains to be discovered. Lower right, blue: Neutrophil recruitment to the vascular wall initiates a wave of microglia within the brain tissue (cerebral spreading inflammation), that inflicts neuronal cell death. Microglia accumulation climaxes around day 14 and resolves after day 28. Correspondingly, neuronal injury occurs until day 14, with the number of remaining neurons staying on a constant level thereafter.
6. Further targets and concepts
Until now, it remains unclear which mechanism(s) initiate inflammation inside the blood vessels, or within the subarachnoid space. It further remains unclear, how intravascular neutrophil activation and recruitment to the endothelium transmits inflammation via the blood brain barrier into the brain parenchyma. The possible mechanisms need further research. Speculations on the initial processes of inflammation within the vasculature comprise mechanistic disturbances of the blood brain barrier, the tight junctions of the basal lamina or changes in shear stress or other blood flow macro- or microhemodynamics.
Besides initiation of inflammation, the resolution of inflammatory sequelae requires additional attention. An amelioration of secondary brain injury through acceleration of the resolution process is imaginable. Until now, neither time interval nor spatial dissolution have been investigated. It is unclear if the resolution process occurs organized (additional mechanism) or just passively (abatement of initiating process), or what the mediators are. Where do the redundant microglia go? How long is an activation status maintained? Do long-term changes within the blood brain barrier reside? In some of these questions, research on ischemic stroke or neuro-degenerative diseases might give some clues, yet, the unique features of SAH have to be respected.
Transmission of inflammatory processes into the brain parenchyma from an extracerebral compartment (subarachnoid space or intravascular) is not mediated by inflammatory cells trafficking into the brain. By experiments in transgenic chimeric mice, we excluded peripheral cell invasion into the brain following subarachnoid hemorrhage [73]. This is in contrary to results after ischemic stroke, and may be explained by the fact, that while ischemic stroke leads to a direct damage of the brain parenchyma including a complete breakdown of the blood brain barrier, SAH does not. Therefore, to further understand the underlying mechanisms, it might be necessary to look beyond the “usual suspects” of inflammation. Cytokines (interleukines, tumor necrosis factor, etc.), fractalkine receptor, VEGF-receptor, or matrix metalloproteinases all have a known and recognized contribution to inflammatory processes. Nevertheless, relying on just those nearby targets will most probably not lead to “the next big idea”. Regarding the immune privilege of the brain, the lymphatic vessels of the meninges have very recently been discussed as a promising new line of thought by Engelhardt and collaborators [39]. Proposedly, their further analysis will require highly sophisticated intravital imaging methods in combination with genetically modified organisms, harboring conditional knock-in and knock-out possibilities.
Ischemic preconditioning has been en vogue during the last years, e.g. in tumor treatment, transplantation-, or (cardio-) vascular surgery [17, 31, 85-98]. The goal is to pre-sensitize the organ at risk to the impending situation, using a (remote) stimulus of the same kind, but of significantly less intensity. Inflammatory preconditioning has only scarcely been investigated in stroke or SAH, and has led to controversial results [31, 64, 99, 100].
Characterizing subtypes or activation states of microglia will help to explain how neuronal injury is inflicted by inflammatory cell accumulation [84]. Recently, a new development in microglia subtype characterization has been described. Applying electron microscopy, “dark microglia” have been detected and found associated with pathological states [101]. Although this term might sound a little fancy, at the moment it seems worthwhile to continue studies on that phenomenon-possibly through microglia isolation from compromised and inflamed brains to further analyze them in vitro (e.g. by FACS or in culture assays).
A methodological problem still seems to be the detection of subtle neuro-psychological impairments in mouse experiments. While neurological testing has been well established in rats, detecting all kinds of neurological as well as cognitive deficits following subarachnoid hemorrhage-possibly easier to detect due to a more severe bleeding than in mice-there have been many reports of neuro-psychological testing in mice after SAH, in which the gold-standard of testing could not yet be established [78, 79, 102, 103], including own unpublished data, in which we only detected minimal deficits in our animal population after extensive testing. In addition to neurological and neuro-psychological testing, cerebral imaging can act as supportive read-out parameter [80], but a reliable clinical assessment would be of major interest.
In the clinical setting of patient care, further application of immuno-modulating drugs in controlled trials might be helpful to test the various concepts in translational approaches. However, additional clinical diagnostic research still might yield helpful information – for example by intracerebral probes like microdialysis, intraoperative functional imaging like laser speckle or NIRS, or even by histological analyses in post mortem species.
Conclusion
Inflammatory processes of many kinds occur in various compartments of the CNS and contribute to secondary brain injury in the course of SAH. A lot is already known about the typical contributors to inflammation in general-mostly adapted from immunological research in other pathologies or conditions (e.g. EAE, ischemia, neuro-degenerative diseases). Yet, the “next big thing”-idea still seems to be missing. SAH holds a unique position among many other CNS pathologies, due to its origin outside the brain parenchyma and a bleeding that does not directly destroy brain tissue. This feature must not be ignored and might lead to substantially different results or reactions – especially in initiation, transmission and resolution of inflammation. Therefore, further research in the field must include established concepts and understanding of immunological principles, but also has to integrate SAH-specific characteristics.
Author Contribution
Schneider, U.C. Composition and writing of the manuscript. Xu, R. Composition and design of the artwork, revision of the manuscript. Vajkoczy, P. Revision and approval of the manuscript.
Acknowledgements
The work was funded by the following grant: SFB TR43, granted by Deutsche Forschungsgemeinschaft (DFG) to Peter Vajkoczy.
Consent for Publication
Not applicable.
Conflict of Interest
The authors declare no conflict of interest, financial or otherwise.
References
- 1.Mathiesen T., Andersson B., Loftenius A. Holst, von. H. J. Neurosurg. 1993;78(4):562–567. doi: 10.3171/jns.1993.78.4.0562. [http://dx.doi.org/10.3171/jns.1993. 78.4.0562]. [DOI] [PubMed] [Google Scholar]
- 2.Mathiesen T., Edner G., Ulfarsson E., Andersson B. Cerebrospinal fluid interleukin-1 receptor antagonist and tumor necrosis factor-alpha following subarachnoid hemorrhage. J. Neurosurg. 1997;87(2):215–220. doi: 10.3171/jns.1997.87.2.0215. [http://dx.doi.org/10.3171/jns.1997.87.2.0215]. [PMID: 9254084]. [DOI] [PubMed] [Google Scholar]
- 3.Mathiesen T., Lefvert A.K. Cerebrospinal fluid and blood lymphocyte subpopulations following subarachnoid haemorrhage. Br. J. Neurosurg. 1996;10(1):89–92. doi: 10.1080/02688699650040584. [http://dx.doi.org/10.1080/ 02688699650040584]. [PMID: 8672265]. [DOI] [PubMed] [Google Scholar]
- 4.Minami N., Tani E., Maeda Y., Yamaura I., Fukami M. Effects of inhibitors of protein kinase C and calpain in experimental delayed cerebral vasospasm. J. Neurosurg. 1992;76(1):111–118. doi: 10.3171/jns.1992.76.1.0111. [http://dx.doi.org/10.3171/jns.1992.76.1.0111]. [PMID: 1370069]. [DOI] [PubMed] [Google Scholar]
- 5.Yoshimoto Y., Tanaka Y., Hoya K. Acute systemic inflammatory response syndrome in subarachnoid hemorrhage. Stroke. 2001;32(9):1989–1993. doi: 10.1161/hs0901.095646. [http://dx.doi.org/10.1161/hs0901.095646]. [PMID: 11546886]. [DOI] [PubMed] [Google Scholar]
- 6.McGirt M.J., Mavropoulos J.C., McGirt L.Y., Alexander M.J., Friedman A.H., Laskowitz D.T., Lynch J.R. Leukocytosis as an independent risk factor for cerebral vasospasm following aneurysmal subarachnoid hemorrhage. J. Neurosurg. 2003;98(6):1222–1226. doi: 10.3171/jns.2003.98.6.1222. [http://dx.doi.org/10.3171/jns.2003.98.6.1222]. [PMID: 12816268]. [DOI] [PubMed] [Google Scholar]
- 7.Fassbender K., Hodapp B., Rossol S., Bertsch T., Schmeck J., Schütt S., Fritzinger M., Horn P., Vajkoczy P., Wendel-Wellner M., Ragoschke A., Kuehl S., Brunner J., Schürer L., Schmiedeck P., Hennerici M. Endothelin-1 in subarachnoid hemorrhage: An acute-phase reactant produced by cerebrospinal fluid leukocytes. Stroke. 2000;31(12):2971–2975. doi: 10.1161/01.str.31.12.2971. [http://dx.doi.org/ 10.1161/01.STR.31.12.2971]. [PMID: 11108758]. [DOI] [PubMed] [Google Scholar]
- 8.Fassbender K., Hodapp B., Rossol S., Bertsch T., Schmeck J., Schütt S., Fritzinger M., Horn P., Vajkoczy P., Kreisel S., Brunner J., Schmiedek P., Hennerici M. Inflammatory cytokines in subarachnoid haemorrhage: association with abnormal blood flow velocities in basal cerebral arteries. J. Neurol. Neurosurg. Psychiatry. 2001;70(4):534–537. doi: 10.1136/jnnp.70.4.534. [http://dx.doi.org/10.1136/jnnp. 70.4.534]. [PMID: 11254783]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vajkoczy P., Meyer B., Weidauer S., Raabe A., Thomé C., Ringel F., Breu V., Schmiedek P. Clazosentan (AXV-034343), a selective endothelin A receptor antagonist, in the prevention of cerebral vasospasm following severe aneurysmal subarachnoid hemorrhage: results of a randomized, double-blind, placebo-controlled, multicenter phase IIa study. J. Neurosurg. 2005;103(1):9–17. doi: 10.3171/jns.2005.103.1.0009. [http://dx.doi.org/10.3171/jns.2005.103.1.0009]. [PMID: 16121967]. [DOI] [PubMed] [Google Scholar]
- 10.Macdonald R.L., Kassell N.F., Mayer S., Ruefenacht D., Schmiedek P., Weidauer S., Frey A., Roux S., Pasqualin A., CONSCIOUS-1 Investigators Stroke. 2008;39(11):3015–3021. doi: 10.1161/STROKEAHA.108.519942. [http://dx.doi.org/10.1161/STROKEAHA.108.519942]. [PMID: 18688013]. [DOI] [PubMed] [Google Scholar]
- 11.Beck J., Raabe A. Clazosentan: prevention of cerebral vasospasm and the potential to overcome infarction. Acta Neurochir. Suppl. (Wien) 2011;110(Pt 2):147–150. doi: 10.1007/978-3-7091-0356-2_26. [DOI] [PubMed] [Google Scholar]
- 12.Macdonald R.L., Higashida R.T., Keller E., Mayer S.A., Molyneux A., Raabe A., Vajkoczy P., Wanke I., Bach D., Frey A., Marr A., Roux S., Kassell N. Randomised trial of clazosentan, an endothelin receptor antagonist, in patients with aneurysmal subarachnoid hemorrhage undergoing surgical clipping (CONSCIOUS-2). Acta Neurochir. Suppl. (Wien) 2013;115:27–31. doi: 10.1007/978-3-7091-1192-5_7. [DOI] [PubMed] [Google Scholar]
- 13.Macdonald R.L., Higashida R.T., Keller E., Mayer S.A., Molyneux A., Raabe A., Vajkoczy P., Wanke I., Frey A., Marr A., Roux S., Kassell N.F. Preventing vasospasm improves outcome after aneurysmal subarachnoid hemorrhage: rationale and design of CONSCIOUS-2 and CONSCIOUS-3 trials. Neurocrit. Care. 2010;13(3):416–424. doi: 10.1007/s12028-010-9433-3. [http://dx.doi.org/10.1007/s12028-010-9433-3]. [PMID: 20838933]. [DOI] [PubMed] [Google Scholar]
- 14.Hansen-Schwartz J., Vajkoczy P., Macdonald R.L., Pluta R.M., Zhang J.H. Cerebral vasospasm: looking beyond vasoconstriction. Trends Pharmacol. Sci. 2007;28(6):252–256. doi: 10.1016/j.tips.2007.04.002. [http://dx.doi.org/ 10.1016/j.tips.2007.04.002]. [PMID: 17466386]. [DOI] [PubMed] [Google Scholar]
- 15.Pluta R.M., Hansen-Schwartz J., Dreier J., Vajkoczy P., Macdonald R.L., Nishizawa S., Kasuya H., Wellman G., Keller E., Zauner A., Dorsch N., Clark J., Ono S., Kiris T., Leroux P., Zhang J.H. Cerebral vasospasm following subarachnoid hemorrhage: time for a new world of thought. Neurol. Res. 2009;31(2):151–158. doi: 10.1179/174313209X393564. [http://dx.doi.org/10.1179/174313209X393564]. [PMID: 19298755]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sabri M., Ai J., Macdonald R.L. Dissociation of vasospasm and secondary effects of experimental subarachnoid hemorrhage by clazosentan. Stroke. 2011;42(5):1454–1460. doi: 10.1161/STROKEAHA.110.604728. [http://dx.doi.org/ 10.1161/STROKEAHA.110.604728]. [PMID: 21454820]. [DOI] [PubMed] [Google Scholar]
- 17.James R.F., Kramer D.R., Aljuboori Z.S., Parikh G., Adams S.W., Eaton J.C., Abou Al-Shaar H., Badjatia N., Mack W.J., Simard J.M. Novel treatments in neuroprotection for aneurysmal subarachnoid hemorrhage. Curr. Treat. Options Neurol. 2016;18(8):38. doi: 10.1007/s11940-016-0421-6. [http://dx.doi.org/10.1007/s11940-016-0421-6]. [PMID: 27325362]. [DOI] [PubMed] [Google Scholar]
- 18.Wellman G.C., Nathan D.J., Saundry C.M., Perez G., Bonev A.D., Penar P.L., Tranmer B.I., Nelson M.T. Ca2+ sparks and their function in human cerebral arteries. Stroke. 2002;33(3):802–808. doi: 10.1161/hs0302.104089. [http://dx.doi.org/10.1161/hs0302.104089]. [PMID: 11872907]. [DOI] [PubMed] [Google Scholar]
- 19.Wellman T.L., Jenkins J., Penar P.L., Tranmer B., Zahr R., Lounsbury K.M. Nitric oxide and reactive oxygen species exert opposing effects on the stability of hypoxia-inducible factor-1alpha (HIF-1alpha) in explants of human pial arteries. FASEB J. 2004;18(2):379–381. doi: 10.1096/fj.03-0143fje. [http://dx.doi.org/10.1096/fj.03-0143fje]. [PMID: 14657004]. [DOI] [PubMed] [Google Scholar]
- 20.Ishiguro M., Wellman T.L., Honda A., Russell S.R., Tranmer B.I., Wellman G.C. Emergence of a R-type Ca2+ channel (CaV 2.3) contributes to cerebral artery constriction after subarachnoid hemorrhage. Circ. Res. 2005;96(4):419–426. doi: 10.1161/01.RES.0000157670.49936.da. [http://dx.doi.org/10. 1161/01.RES.0000157670.49936.da]. [PMID: 15692089]. [DOI] [PubMed] [Google Scholar]
- 21.Budohoski K.P., Czosnyka M., Smielewski P., Varsos G.V., Kasprowicz M., Brady K.M., Pickard J.D., Kirkpatrick P.J. Cerebral autoregulation after subarachnoid hemorrhage: comparison of three methods. J. Cereb. Blood Flow Metab. 2013;33(3):449–456. doi: 10.1038/jcbfm.2012.189. [http://dx.doi.org/10.1038/jcbfm.2012.189]. [PMID: 23232948]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dreier J.P., Major S., Manning A., Woitzik J., Drenckhahn C., Steinbrink J., Tolias C., Oliveira-Ferreira A.I., Fabricius M., Hartings J.A., Vajkoczy P., Lauritzen M., Dirnagl U., Bohner G., Strong A.J. Cortical spreading ischaemia is a novel process involved in ischaemic damage in patients with aneurysmal subarachnoid haemorrhage. Brain. 2009;132(Pt 7):1866–1881. doi: 10.1093/brain/awp102. [http://dx. doi.org/10.1093/brain/awp102]. [PMID: 19420089]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Koide M., Sukhotinsky I., Ayata C., Wellman G.C. Subarachnoid hemorrhage, spreading depolarizations and impaired neurovascular coupling. Stroke Res. Treat. 2013;2013(3):819340–10. doi: 10.1155/2013/819340. [PMID: 23577279]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bosche B., Graf R., Ernestus R-I., Dohmen C., Reithmeier T., Brinker G., Strong A.J., Dreier J.P., Woitzik J. Members of the cooperative study of brain injury depolarizations (COSBID). Ann. Neurol. 2010;67(5):607–617. doi: 10.1002/ana.21943. [http://dx.doi.org/10.1002/ana. 21943]. [PMID: 20437558]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aungst S.L., Kabadi S.V., Thompson S.M., Stoica B.A., Faden A.I. Repeated mild traumatic brain injury causes chronic neuroinflammation, changes in hippocampal synaptic plasticity, and associated cognitive deficits. J. Cereb. Blood Flow Metab. 2014;34(7):1223–1232. doi: 10.1038/jcbfm.2014.75. [http://dx.doi.org/10.1038/jcbfm.2014.75]. [PMID: 24756076]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Berg, Vom, J.; Prokop, S.; Miller, K. R.; Obst, J.; Kälin, R. E.; Lopategui-Cabezas, I.; Wegner, A.; Mair, F.; Schipke, C. G.; Peters, O.; Winter, Y.; Becher, B.; Heppner, F. L. Nat. Med. 2012;18(12):1812–1819. doi: 10.1038/nm.2965. [PMID: 23178247]. [DOI] [PubMed] [Google Scholar]
- 27.Heppner F.L., Greter M., Marino D., Falsig J., Raivich G., Hövelmeyer N., Waisman A., Rülicke T., Prinz M., Priller J., Becher B., Aguzzi A. Experimental autoimmune encephalomyelitis repressed by microglial paralysis. Nat. Med. 2005;11(2):146–152. doi: 10.1038/nm1177. [http://dx.doi.org/10.1038/nm1177]. [PMID: 15665833]. [DOI] [PubMed] [Google Scholar]
- 28.Krabbe G., Halle A., Matyash V., Rinnenthal J.L., Eom G.D., Bernhardt U., Miller K.R., Prokop S., Kettenmann H., Heppner F.L. Functional impairment of microglia coincides with Beta-amyloid deposition in mice with Alzheimer-like pathology. PLoS One. 2013;8(4):e60921. doi: 10.1371/journal.pone.0060921. [http://dx.doi.org/10.1371/journal.pone. 0060921]. [PMID: 23577177]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Michaud J-P., Rivest S. Anti-inflammatory signaling in microglia exacerbates Alzheimer’s disease-related pathology. Neuron. 2015;85(3):450–452. doi: 10.1016/j.neuron.2015.01.021. [http://dx.doi.org/10.1016/j.neuron.2015.01.021]. [PMID: 25654250]. [DOI] [PubMed] [Google Scholar]
- 30.Spangenberg E.E., Lee R.J., Najafi A.R., Rice R.A., Elmore M.R.P., Blurton-Jones M., West B.L., Green K.N. Eliminating microglia in Alzheimer’s mice prevents neuronal loss without modulating amyloid-β pathology. Brain. 2016;139(Pt 4):1265–1281. doi: 10.1093/brain/aww016. [http://dx.doi.org/10.1093/brain/aww016]. [PMID: 26921617]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Anrather J., Iadecola C. Inflammation and stroke: An overview. Neurotherapeutics. 2016;13(4):661–670. doi: 10.1007/s13311-016-0483-x. [http://dx.doi.org/ 10.1007/s13311-016-0483-x]. [PMID: 27730544]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Varvel N.H., Grathwohl S.A., Baumann F., Liebig C., Bosch A., Brawek B., Thal D.R., Charo I.F., Heppner F.L., Aguzzi A., Garaschuk O., Ransohoff R.M., Jucker M. Microglial repopulation model reveals a robust homeostatic process for replacing CNS myeloid cells. Proc. Natl. Acad. Sci. USA. 2012;109(44):18150–18155. doi: 10.1073/pnas.1210150109. [http://dx.doi.org/10.1073/pnas.1210150109]. [PMID: 23071306]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Menasria R., Canivet C., Piret J., Gosselin J., Boivin G. Both cerebral and hematopoietic deficiencies in CCR2 result in uncontrolled herpes simplex virus infection of the central nervous system in mice. J. Gen. Virol. 2016;11(12):e0168034. doi: 10.1371/journal.pone.0168034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Grathwohl S.A., Kälin R.E., Bolmont T., Prokop S., Winkelmann G., Kaeser S.A., Odenthal J., Radde R., Eldh T., Gandy S., Aguzzi A., Staufenbiel M., Mathews P.M., Wolburg H., Heppner F.L., Jucker M. Formation and maintenance of Alzheimer’s disease beta-amyloid plaques in the absence of microglia. Nat. Neurosci. 2009;12(11):1361–1363. doi: 10.1038/nn.2432. [http://dx.doi.org/10.1038/ nn.2432]. [PMID: 19838177]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vainchtein I.D., Vinet J., Brouwer N., Brendecke S., Biagini G., Biber K., Boddeke H.W.G.M., Eggen B.J.L. In acute experimental autoimmune encephalomyelitis, infiltrating macrophages are immune activated, whereas microglia remain immune suppressed. Glia. 2014;62(10):1724–1735. doi: 10.1002/glia.22711. [http://dx.doi.org/10.1002/glia. 22711]. [PMID: 24953459]. [DOI] [PubMed] [Google Scholar]
- 36.Ransohoff R.M., Cardona A.E. The myeloid cells of the central nervous system parenchyma. Nature. 2010;468(7321):253–262. doi: 10.1038/nature09615. [http://dx.doi.org/10.1038/nature09615]. [PMID: 21068834]. [DOI] [PubMed] [Google Scholar]
- 37.Xin Z-L., Wu X-K., Xu J-R., Li X. Arachnoid cell involvement in the mechanism of coagulation-initiated inflammation in the subarachnoid space after subarachnoid hemorrhage. J. Zhejiang Univ. Sci. B. 2010;11(7):516–523. doi: 10.1631/jzus.B1000099. [http://dx.doi.org/10.1631/jzus. B1000099]. [PMID: 20593517]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xie X., Wu X., Cui J., Li H., Yan X. Increase ICAM-1 and LFA-1 expression by cerebrospinal fluid of subarachnoid hemorrhage patients: involvement of TNF-α. Brain Res. 2013;1512:89–96. doi: 10.1016/j.brainres.2013.03.041. [http://dx.doi.org/10.1016/j.brainres.2013.03.041]. [PMID: 23548604]. [DOI] [PubMed] [Google Scholar]
- 39.Engelhardt B., Vajkoczy P., Weller R.O. The movers and shapers in immune privilege of the CNS. Nat. Immunol. 2017;18(2):123–131. doi: 10.1038/ni.3666. [http://dx.doi.org/10.1038/ni.3666]. [PMID: 28092374]. [DOI] [PubMed] [Google Scholar]
- 40.Engelhardt B., Sorokin L. The blood-brain and the blood-cerebrospinal fluid barriers: function and dysfunction. Semin. Immunopathol. 2009;31(4):497–511. doi: 10.1007/s00281-009-0177-0. [http://dx.doi.org/10.1007/ s00281-009-0177-0]. [PMID: 19779720]. [DOI] [PubMed] [Google Scholar]
- 41.Engelhardt B., Coisne C. Fluids and barriers of the CNS establish immune privilege by confining immune surveillance to a two-walled castle moat surrounding the CNS castle. Fluids Barriers CNS. 2011;8(1):4. doi: 10.1186/2045-8118-8-4. [http://dx.doi.org/10.1186/2045-8118-8-4]. [PMID: 21349152]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Engelhardt B., Liebner S. Novel insights into the development and maintenance of the blood-brain barrier. Cell Tissue Res. 2014;355(3):687–699. doi: 10.1007/s00441-014-1811-2. [http://dx.doi.org/10.1007/s00441-014-1811-2]. [PMID: 24590145]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Provencio J. Inflammation in subarachnoid hemorrhage and delayed deterioration associated with vasospasm: a review J. Acta Neurochir. Suppl. (Wien) 2013;115:233–238. doi: 10.1007/978-3-7091-1192-5_42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Provencio J.J., Fu X., Siu A., Rasmussen P.A., Hazen S.L., Ransohoff R.M. CSF neutrophils are implicated in the development of vasospasm in subarachnoid hemorrhage. Neurocrit. Care. 2010;12(2):244–251. doi: 10.1007/s12028-009-9308-7. [http://dx.doi.org/10.1007/s12028-009-9308-7]. [PMID: 19967568]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Provencio J.J., Altay T., Smithason S., Moore S.K., Ransohoff R.M. Depletion of Ly6G/C(+) cells ameliorates delayed cerebral vasospasm in subarachnoid hemorrhage. J. Neuroimmunol. 2011;232(1-2):94–100. doi: 10.1016/j.jneuroim.2010.10.016. [http://dx.doi.org/10.1016/j.jneuroim.2010.10. 016]. [PMID: 21059474]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pradilla G., Wang P.P., Legnani F.G., Ogata L., Dietsch G.N., Tamargo R.J. Prevention of vasospasm by anti-CD11/CD18 monoclonal antibody therapy following subarachnoid hemorrhage in rabbits. J. Neurosurg. 2004;101(1):88–92. doi: 10.3171/jns.2004.101.1.0088. [http://dx.doi.org/ 10.3171/jns.2004.101.1.0088]. [PMID: 15255256]. [DOI] [PubMed] [Google Scholar]
- 47.Clatterbuck R.E., Gailloud P., Ogata L., Gebremariam A., Dietsch G.N., Murphy K.J., Tamargo R.J. Prevention of cerebral vasospasm by a humanized anti-CD11/CD18 monoclonal antibody administered after experimental subarachnoid hemorrhage in nonhuman primates. J. Neurosurg. 2003;99(2):376–382. doi: 10.3171/jns.2003.99.2.0376. [http:// dx.doi.org/10.3171/jns.2003.99.2.0376]. [PMID: 12924713]. [DOI] [PubMed] [Google Scholar]
- 48.Dengler J., Schefold J.C., Graetz D., Meisel C., Splettstösser G., Volk H-D., Schlosser H-G. Point-of-care testing for interleukin-6 in cerebro spinal fluid (CSF) after subarachnoid haemorrhage. Med. Sci. Monit. 2008;14(12):BR265–BR268. [PMID: 19043359]. [PubMed] [Google Scholar]
- 49.Sarrafzadeh A., Schlenk F., Gericke C., Vajkoczy P. Relevance of cerebral interleukin-6 after aneurysmal subarachnoid hemorrhage. Neurocrit. Care. 2010;13(3):339–346. doi: 10.1007/s12028-010-9432-4. [http://dx.doi.org/ 10.1007/s12028-010-9432-4]. [PMID: 20725805]. [DOI] [PubMed] [Google Scholar]
- 50.Graetz D., Nagel A., Schlenk F., Sakowitz O., Vajkoczy P., Sarrafzadeh A. High ICP as trigger of proinflammatory IL-6 cytokine activation in aneurysmal subarachnoid hemorrhage. Neurol. Res. 2010;32(7):728–735. doi: 10.1179/016164109X12464612122650. [http://dx.doi.org/10.1179/016164109 X12464612122650]. [PMID: 19682408]. [DOI] [PubMed] [Google Scholar]
- 51.Sabri M., Ai J., Knight B., Tariq A., Jeon H., Shang X., Marsden P.A., Loch Macdonald R. Uncoupling of endothelial nitric oxide synthase after experimental subarachnoid hemorrhage. J. Cereb. Blood Flow Metab. 2011;31(1):190–199. doi: 10.1038/jcbfm.2010.76. [http://dx.doi. org/10.1038/jcbfm.2010.76]. [PMID: 20517322]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sabri M., Ai J., Lass E., D’abbondanza J., Macdonald R.L. Genetic elimination of eNOS reduces secondary complications of experimental subarachnoid hemorrhage. J. Cereb. Blood Flow Metab. 2013;33(7):1008–1014. doi: 10.1038/jcbfm.2013.49. [http://dx.doi.org/10.1038/jcbfm. 2013.49]. [PMID: 23549379]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Maddahi A., Povlsen G.K., Edvinsson L. Regulation of enhanced cerebrovascular expression of proinflammatory mediators in experimental subarachnoid hemorrhage via the mitogen-activated protein kinase kinase/extracellular signal-regulated kinase pathway. J. Neuroinflammation. 2012;9(1):274. doi: 10.1186/1742-2094-9-274. [http://dx.doi.org/10.1186/ 1742-2094-9-274]. [PMID: 23259581]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Xu H., Testai F.D., Valyi-Nagy T., Pavuluri N.M., Zhai F., Nanegrungsunk D., Paisansathan C., Pelligrino D.A. Brain Res. 2015;1603:141–149. doi: 10.1016/j.brainres.2015.01.047. [http://dx.doi.org/10.1016/j.brainres.2015. 01.047]. [PMID: 25662771]. [DOI] [PubMed] [Google Scholar]
- 55.Xu H-L., Garcia M., Testai F., Vetri F., Barabanova A., Pelligrino D.A., Paisansathan C. Pharmacologic blockade of vascular adhesion protein-1 lessens neurologic dysfunction in rats subjected to subarachnoid hemorrhage. Brain Res. 2014;1586:83–89. doi: 10.1016/j.brainres.2014.08.036. [http://dx.doi.org/10.1016/j.brainres.2014.08.036]. [PMID: 25175836]. [DOI] [PubMed] [Google Scholar]
- 56.Macdonald R.L. Delayed neurological deterioration after subarachnoid haemorrhage. Nat. Rev. Neurol. 2014;10(1):44–58. doi: 10.1038/nrneurol.2013.246. [http://dx.doi.org/10.1038/nrneurol.2013.246]. [PMID: 24323051]. [DOI] [PubMed] [Google Scholar]
- 57.Osuka K., Suzuki Y., Tanazawa T., Hattori K., Yamamoto N., Takayasu M., Shibuya M., Yoshida J. Interleukin-6 and development of vasospasm after subarachnoid haemorrhage. Acta Neurochir. (Wien) 1998;140(9):943–951. doi: 10.1007/s007010050197. [http://dx.doi.org/10.1007/ s007010050197]. [PMID: 9842432]. [DOI] [PubMed] [Google Scholar]
- 58.Clatterbuck R.E., Oshiro E.M., Hoffman P.A., Dietsch G.N., Pardoll D.M., Tamargo R.J. Inhibition of vasospasm with lymphocyte function-associated antigen-1 monoclonal antibody in a femoral artery model in rats. J. Neurosurg. 2002;97(3):676–682. doi: 10.3171/jns.2002.97.3.0676. [http://dx.doi.org/10.3171/jns.2002.97.3.0676]. [PMID: 12296653]. [DOI] [PubMed] [Google Scholar]
- 59.Kimura H., Gules I., Meguro T., Zhang J.H. Cytotoxicity of cytokines in cerebral microvascular endothelial cell. Brain Res. 2003;990(1-2):148–156. doi: 10.1016/s0006-8993(03)03450-4. [http://dx.doi.org/10.1016/S0006-8993 (03)03450-4]. [PMID: 14568339]. [DOI] [PubMed] [Google Scholar]
- 60.Paoletti P., Gaetani P., Grignani G., Pacchiarini L., Silvani V., Rodriguez y Baena R. CSF leukotriene C4 following subarachnoid hemorrhage. J. Neurosurg. 1988;69(4):488–493. doi: 10.3171/jns.1988.69.4.0488. [http://dx.doi. org/10.3171/jns.1988.69.4.0488]. [PMID: 3418380]. [DOI] [PubMed] [Google Scholar]
- 61.McGirt M. J., Lynch J. R., Blessing R., Warner D. S., Friedman A. H., Laskowitz D. T. 2002.
- 62.Schneider U.C., Schiffler J., Hakiy N., Horn P., Vajkoczy P. Functional analysis of Pro-inflammatory properties within the cerebrospinal fluid after subarachnoid hemorrhage in vivo and in vitro. J. Neuroinflammation. 2012;9(1):28. doi: 10.1186/1742-2094-9-28. [http://dx.doi.org/ 10.1186/1742-2094-9-28]. [PMID: 22316109]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Smithason S., Moore S.K., Provencio J.J. Systemic administration of LPS worsens delayed deterioration associated with vasospasm after subarachnoid hemorrhage through a myeloid cell-dependent mechanism. Neurocrit. Care. 2012;16(2):327–334. doi: 10.1007/s12028-011-9651-3. [http://dx.doi.org/10.1007/s12028-011-9651-3]. [PMID: 22090172]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Smithason S., Moore S.K., Provencio J. 2013. [Google Scholar]
- 65.Schallner N., Pandit R., LeBlanc R., Thomas A.J., Ogilvy C.S., Zuckerbraun B.S., Gallo D., Otterbein L.E., Hanafy K.A. J. Clin. Invest. 2015;125(125(7)):2609–2625. doi: 10.1172/JCI78443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Atangana E., Schneider U.C., Blecharz K., Magrini S., Wagner J., Nieminen-Kelhä M., Kremenetskaia I., Heppner F.L., Engelhardt B., Vajkoczy P. Transl. Stroke Res. 2016;8(2):1–13. doi: 10.1007/s12975-016-0485-3. [DOI] [PubMed] [Google Scholar]
- 67.Hanafy K.A. The role of microglia and the TLR4 pathway in neuronal apoptosis and vasospasm after subarachnoid hemorrhage. J. Neuroinflammation. 2013;10(1):83. doi: 10.1186/1742-2094-10-83. [http://dx.doi.org/10.1186/ 1742-2094-10-83]. [PMID: 23849248]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Abbott N.J. Inflammatory mediators and modulation of blood-brain barrier permeability. Cell. Mol. Neurobiol. 2000;20(2):131–147. doi: 10.1023/a:1007074420772. [http://dx.doi.org/10.1023/A:1007074420772]. [PMID: 10696506]. [DOI] [PubMed] [Google Scholar]
- 69.Alves J.L. Blood-brain barrier and traumatic brain injury. J. Neurosci. Res. 2014;92(2):141–147. doi: 10.1002/jnr.23300. [http://dx.doi.org/10.1002/jnr. 23300]. [PMID: 24327344]. [DOI] [PubMed] [Google Scholar]
- 70.Förster C., Burek M., Romero I.A., Weksler B., Couraud P-O., Drenckhahn D. Differential effects of hydrocortisone and TNFalpha on tight junction proteins in an in vitro model of the human blood-brain barrier. J. Physiol. 2008;586(7):1937–1949. doi: 10.1113/jphysiol.2007.146852. [http:// dx.doi.org/10.1113/jphysiol.2007.146852]. [PMID: 18258663]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Förster C. Tight junctions and the modulation of barrier function in disease. Histochem. Cell Biol. 2008;130(1):55–70. doi: 10.1007/s00418-008-0424-9. [http://dx.doi. org/10.1007/s00418-008-0424-9]. [PMID: 18415116]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Coisne C., Engelhardt B. Tight junctions in brain barriers during central nervous system inflammation. Antioxid. Redox Signal. 2011;15(5):1285–1303. doi: 10.1089/ars.2011.3929. [http://dx.doi.org/10.1089/ars.2011.3929]. [PMID: 21338320]. [DOI] [PubMed] [Google Scholar]
- 73.Schneider U.C., Davids A-M., Brandenburg S., Müller A., Elke A., Magrini S., Atangana E., Turkowski K., Finger T., Gutenberg A., Gehlhaar C., Brück W., Heppner F.L., Vajkoczy P. Microglia inflict delayed brain injury after subarachnoid hemorrhage. Acta Neuropathol. 2015;130(2):215–231. doi: 10.1007/s00401-015-1440-1. [http://dx.doi. org/10.1007/s00401-015-1440-1]. [PMID: 25956409]. [DOI] [PubMed] [Google Scholar]
- 74.Wang Z., Meng C-J., Shen X-M., Shu Z., Ma C., Zhu G-Q., Liu H-X., He W-C., Sun X-B., Huo L., Zhang J., Chen G. Potential contribution of hypoxia-inducible factor-1α, aquaporin-4, and matrix metalloproteinase-9 to blood-brain barrier disruption and brain edema after experimental subarachnoid hemorrhage. J. Mol. Neurosci. 2012;48(1):273–280. doi: 10.1007/s12031-012-9769-6. [http://dx.doi.org/10.1007/ s12031-012-9769-6]. [PMID: 22528459]. [DOI] [PubMed] [Google Scholar]
- 75.Greenhalgh A.D., Brough D., Robinson E.M., Girard S. ; Rothwell N.J., Allan S.M. Interleukin-1 receptor antagonist is beneficial after subarachnoid haemorrhage in rat by blocking haem-driven inflammatory pathology. Dis. Model. Mech. 2012;5(6):823–833. doi: 10.1242/dmm.008557. [http://dx.doi.org/10.1242/dmm.008557]. [PMID: 22679224]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sherchan P., Lekic T., Suzuki H., Hasegawa Y., Rolland W., Duris K., Zhan Y., Tang J., Zhang J.H. Minocycline improves functional outcomes, memory deficits, and histopathology after endovascular perforation-induced subarachnoid hemorrhage in rats. J. Neurotrauma. 2011;28(12):2503–2512. doi: 10.1089/neu.2011.1864. [http://dx.doi.org/10.1089/ neu.2011.1864]. [PMID: 22013966]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Li J., Chen J., Mo H., Chen J., Qian C., Yan F., Gu C., Hu Q., Wang L., Chen G. Minocycline protects against NLRP3 inflammasome-induced inflammation and P53-associated apoptosis in early brain injury after subarachnoid hemorrhage. Mol. Neurobiol. 2016;53(4):2668–2678. doi: 10.1007/s12035-015-9318-8. [http://dx.doi.org/10.1007/s12035-015-9318-8]. [PMID: 26143258]. [DOI] [PubMed] [Google Scholar]
- 78.Jeon H., Ai J., Sabri M., Tariq A., Macdonald R.L. Learning deficits after experimental subarachnoid hemorrhage in rats. Neuroscience. 2010;169(4):1805–1814. doi: 10.1016/j.neuroscience.2010.06.039. [http://dx.doi.org/10.1016/ j.neuroscience.2010.06.039]. [PMID: 20600651]. [DOI] [PubMed] [Google Scholar]
- 79.Jeon H., Ai J., Sabri M., Tariq A., Shang X., Chen G., Macdonald R.L. Neurological and neurobehavioral assessment of experimental subarachnoid hemorrhage. BMC Neurosci. 2009;10(1):103. doi: 10.1186/1471-2202-10-103. [http://dx.doi.org/10.1186/1471-2202-10-103]. [PMID: 19706182]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Atangana E.N., Homburg D., Vajkoczy P., Schneider U.C. Mouse cerebral magnetic resonance imaging fails to visualize brain volume changes after experimental subarachnoid hemorrhage. Acta Neurochir. (Wien) 2015;157(1):37–42. doi: 10.1007/s00701-014-2276-5. [http://dx.doi.org/10.1007/ s00701-014-2276-5]. [PMID: 25398554]. [DOI] [PubMed] [Google Scholar]
- 81.Prinz M., Priller J., Sisodia S.S., Ransohoff R.M. Heterogeneity of CNS myeloid cells and their roles in neurodegeneration. Nat. Neurosci. 2011;14(10):1227–1235. doi: 10.1038/nn.2923. [http://dx.doi.org/10.1038/ nn.2923]. [PMID: 21952260]. [DOI] [PubMed] [Google Scholar]
- 82.Goldmann T., Wieghofer P., Müller P.F., Wolf Y., Varol D., Yona S., Brendecke S.M., Kierdorf K., Staszewski O., Datta M., Luedde T., Heikenwalder M., Jung S., Prinz M. A new type of microglia gene targeting shows TAK1 to be pivotal in CNS autoimmune inflammation. Nat. Neurosci. 2013;16(11):1618–1626. doi: 10.1038/nn.3531. [http://dx.doi.org/10.1038/nn.3531]. [PMID: 24077561]. [DOI] [PubMed] [Google Scholar]
- 83.Ransohoff R.M. Khoury, El. J. Cold Spring Harb Perspect Biol. 2015;8(1):a020560. doi: 10.1101/cshperspect.a020560. [http://dx.doi.org/10.1101/cshperspect.a020560]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ransohoff R.M. How neuroinflammation contributes to neurodegeneration. Science. 2016;353(6301):777–783. doi: 10.1126/science.aag2590. [http://dx.doi.org/ 10.1126/science.aag2590]. [PMID: 27540165]. [DOI] [PubMed] [Google Scholar]
- 85.Meybohm P., Hasenclever D., Zacharowski K. Remote ischemic Preconditioning and cardiac surgery. N. Engl. J. Med. 2016;374(5):491–492. doi: 10.1056/NEJMc1514509. [PMID: 26840140]. [DOI] [PubMed] [Google Scholar]
- 86.Tülü S., Mulino M., Pinggera D., Luger M., Würtinger P., Grams A., Bodner T., Beer R., Helbok R., Matteucci-Gothe R., Unterhofer C., Gizewski E., Schmutzhard E., Thomé C., Ortler M. Remote ischemic preconditioning in the prevention of ischemic brain damage during intracranial aneurysm treatment (RIPAT): study protocol for a randomized controlled trial. Trials. 2015;16(1):594. doi: 10.1186/s13063-015-1102-6. [http://dx.doi.org/10.1186/s13063-015-1102-6]. [PMID: 26714784]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hausenloy D.J., Candilio L., Yellon D.M. Remote ischemic preconditioning and cardiac surgery. N. Engl. J. Med. 2016;374(5):491–492. doi: 10.1056/NEJMc1514509. [PMID: 26848495]. [DOI] [PubMed] [Google Scholar]
- 88.Wagner N-M., Lu Y., Gross E.R. Remote ischemic preconditioning and cardiac surgery. N. Engl. J. Med. 2016;374(5):490. doi: 10.1056/NEJMc1514509. [PMID: 26840143]. [DOI] [PubMed] [Google Scholar]
- 89.Landoni G., Baiardo R.M., Votta C.D. Remote ischemic preconditioning and cardiac surgery. N. Engl. J. Med. 2016;374(5):489. doi: 10.1056/NEJMc1514509. [http://dx.doi.org/10.1056/NEJMc1514509]. [PMID: 26840141]. [DOI] [PubMed] [Google Scholar]
- 90.Limani P., Linecker M., Oberkofler C.E., Barmettler G., Kaech A., Graf R., Humar B., Clavien P-A. Remote ischemic preconditioning: A novel Strategy in rescuing older livers from ischemia-reperfusion injury in a rodent model. Ann. Surg. 2016;264(5):797–803. doi: 10.1097/SLA.0000000000001765. [http://dx.doi.org/10.1097/SLA.0000000000001765]. [PMID: 27584570]. [DOI] [PubMed] [Google Scholar]
- 91.Sardar P., Chatterjee S., Kundu A., Samady H., Owan T., Giri J., Nairooz R., Selzman C.H., Heusch G., Gersh B.J., Abbott J.D., Mukherjee D., Fang J.C. Remote ischemic preconditioning in patients undergoing cardiovascular surgery: Evidence from a meta-analysis of randomized controlled trials. Int. J. Cardiol. 2016;221:34–41. doi: 10.1016/j.ijcard.2016.06.325. [http://dx.doi.org/10.1016/j.ijcard.2016.06.325]. [PMID: 27400294]. [DOI] [PubMed] [Google Scholar]
- 92.Lanza G.A., Stazi A., Villano A., Torrini F., Milo M., Laurito M., Flego D., Aurigemma C., Liuzzo G., Crea F. Effect of Remote Ischemic Preconditioning on Platelet Activation Induced by Coronary Procedures. Am. J. Cardiol. 2016;117(3):359–365. doi: 10.1016/j.amjcard.2015.10.056. [http://dx.doi.org/10.1016/j.amjcard.2015.10.056]. [PMID: 26739396]. [DOI] [PubMed] [Google Scholar]
- 93.Healy D.A., Boyle E., McCartan D., Bourke M., Medani M., Ferguson J., Yagoub H., Bashar K., O’Donnell M., Newell J., Canning C., McMonagle M., Dowdall J., Cross S., O’Daly S., Manning B., Fulton G., Kavanagh E.G., Burke P., Grace P.A., Moloney M.C., Walsh S.R. Preconditioning shields against Vascular events in surgery (Preconditioning SAVES) trial group. Vasc. Endovascular Surg. 2015;49(8):220–227. doi: 10.1177/1538574415614404. [http://dx.doi. org/10.1177/1538574415614404]. [PMID: 26574485]. [DOI] [PubMed] [Google Scholar]
- 94.Zhao W., Meng R., Ma C., Hou B., Jiao L., Zhu F., Wu W., Shi J., Duan Y., Zhang R., Zhang J., Sun Y., Zhang H., Ling F., Wang Y., Feng W., Ding Y., Ovbiagele B., Ji X. Safety and efficacy of remote ischemic preconditioning in patients with severe carotid artery stenosis before carotid artery stenting: A proof-of-concept, randomized controlled trial. Circulation. 2017;135(14):1325–1335. doi: 10.1161/CIRCULATIONAHA.116.024807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Nikkola E., Laiwalla A., Ko A., Alvarez M., Connolly M., Ooi Y.C., Hsu W., Bui A., Pajukanta P., Gonzalez N.R. Remote ischemic conditioning alters methylation and expression of cell cycle genes in aneurysmal subarachnoid hemorrhage. Stroke. 2015;46(9):2445–2451. doi: 10.1161/STROKEAHA.115.009618. [http://dx.doi.org/10.1161/STROKEAHA. 115.009618]. [PMID: 26251247]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Watters O., O’Connor J.J. A role for tumor necrosis factor-α in ischemia and ischemic preconditioning. J. Neuroinflammation. 2011;8(1):87. doi: 10.1186/1742-2094-8-87. [http://dx.doi.org/10.1186/1742-2094-8-87]. [PMID: 21810263]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Altintas O., Ozgen Altintas M., Kumas M., Asil T. Neuroprotective effect of ischemic preconditioning via modulating the expression of cerebral miRNAs against transient cerebral ischemia in diabetic rats. Neurol. Res. 2016;38(11):1003–1011. doi: 10.1080/01616412.2016.1232013. [http://dx.doi. org/10.1080/01616412.2016.1232013]. [PMID: 27635859]. [DOI] [PubMed] [Google Scholar]
- 98.Muscari C., Giordano E., Bonafè F., Govoni M., Pasini A., Guarnieri C. Molecular mechanisms of ischemic preconditioning and postconditioning as putative therapeutic targets to reduce tumor survival and malignancy. Med. Hypotheses. 2013;81(6):1141–1145. doi: 10.1016/j.mehy.2013.10.022. [http://dx.doi.org/10.1016/j.mehy.2013.10.022]. [PMID: 24230458]. [DOI] [PubMed] [Google Scholar]
- 99.Schaafsma W., Zhang X., van Zomeren K.C., Jacobs S., Georgieva P.B., Wolf S.A., Kettenmann H., Janova H., Saiepour N., Hanisch U-K., Meerlo P., van den Elsen P.J., Brouwer N., Boddeke H.W.G.M., Eggen B.J.L. Long-lasting pro-inflammatory suppression of microglia by LPS-preconditioning is mediated by RelB-dependent epigenetic silencing. Brain Behav. Immun. 2015;48:205–221. doi: 10.1016/j.bbi.2015.03.013. [http://dx.doi.org/10.1016/j.bbi.2015.03.013]. [PMID: 25843371]. [DOI] [PubMed] [Google Scholar]
- 100.Gesuete R., Stevens S.L., Stenzel-Poore M.P. Acta Neurochir. Suppl. (Wien) 2016;121:39–44. doi: 10.1007/978-3-319-18497-5_7. [http://dx.doi.org/10.1007/978-3-319-18497-5_7]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Bisht K., Sharma K.P., Lecours C., Sánchez M.G. Hajj, El, H.; Milior, G.; Olmos-Alonso, A.; Gómez-Nicola, D.; Luheshi, G.; Vallières, L.; Branchi, I.; Maggi, L.; Limatola, C.; Butovsky, O.; Tremblay, M.-È. Glia. 2016;64(5):826–839. doi: 10.1002/glia.22966. [http://dx.doi.org/ 10.1002/glia.22966]. [PMID: 26847266]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Yamada K. Strain differences of selective attention in mice: effect of Kamin blocking on classical fear conditioning. Behav. Brain Res. 2010;213(1):126–129. doi: 10.1016/j.bbr.2010.04.037. [http://dx.doi.org/10.1016/j.bbr.2010. 04.037]. [PMID: 20434488]. [DOI] [PubMed] [Google Scholar]
- 103.Janus C., Welzl H. Mouse models of neurodegenerative diseases: criteria and general methodology. 2010. [DOI] [PubMed] [Google Scholar]