Abstract
BACKGROUND & AIMS:
Endoscopists do not routinely follow guidelines to survey individuals with low-risk adenomas (LRAs; 1–2 small tubular adenomas, < 1 cm) every 5–10 years for colorectal cancer; many recommend shorter surveillance intervals for these individuals. We aimed to identify the reasons that endoscopists recommend shorter surveillance intervals for some individuals with LRAs and determine whether timing affects outcomes at follow-up examinations.
METHODS:
We collected data from 1560 individuals (45–75 years old) who participated in a prospective chemoprevention trial (of vitamin D and calcium) from 2004 through 2008. Participants in the trial had at least 1 adenoma, detected at their index colonos-copy, and were recommended to receive follow-up colonoscopy examinations at 3 or 5 years after adenoma identification, as recommended by the endoscopist. For this analysis we collected data from only participants with LRAs. These data included characteristics of participants and endoscopists and findings from index and follow-up colonoscopies. Primary endpoints were frequency of recommending shorter (3-year) vs longer (5-year) surveillance intervals, factors associated with these recommendations, and effect on outcome, determined at the follow-up colonoscopy.
RESULTS:
A 3-year surveillance interval was recommended for 594 of the subjects (38.1%). Factors most significantly associated with recommendation of 3-year vs a 5-year surveillance interval included African American race (relative risk [RR] to white, 1.41; 95% confidence interval [CI], 1.14–1.75), Asian/Pacific Islander ethnicity (RR to white, 1.7; 95% CI, 1.22–2.43), detection of 2 adenomas at the index examination (RR vs 1 adenoma, 1.47; 95% CI, 1.27–1.71), more than 3 serrated polyps at the index examination (RR=2.16, 95% CI, 1.59–2.93), or index examination with fair or poor quality bowel preparation (RR vs excellent quality, 2.16; 95% CI, 1.66–2.83). Other factors that had a significant association with recommendation for a 3-year surveillance interval included family history of colorectal cancer and detection of 1–2 serrated polyps at the index examination. In comparisons of outcomes, we found no significant differences between the 3-year vs 5-year recommendation groups in proportions of subjects found to have 1 or more adenomas (38.8% vs 41.7% respectively; P = .27), advanced adenomas (7.7% vs 8.2%;P = .73) or clinically significant serrated polyps (10.0% vs10.3%; P = .82) at the follow-up colonoscopy.
CONCLUSIONS:
Possibly influenced by patients’ family history, race, quality of bowel preparation, or number or size of polyps, endoscopists frequently recommend 3-year surveillance intervals instead of guideline-recommended intervals of 5 years or longer for individuals with LRAs. However, at the follow-up colonoscopy, similar proportions of participants have 1 or more adenomas, advanced adenomas, or serrated polyps. These findings support the current guideline recommendations of performing follow-up examinations of individuals with LRAs at least 5 years after the index colonoscopy.
Keywords: Colon Cancer, Detection, Tumor Development, Progression
It is well recognized that individuals with 1 or 2 small tubular adenomas are at low risk for subsequent colorectal cancer (CRC) incidence and mortality1,2 or even for advanced adenomas on follow-up exam.3–9 A pooled report that combined data from 8 prospective studies observed a lower risk for metachronous neoplasia in subjects with low-risk adenomas (LRAs; 6.9%; 95% confidence interval [CI], 6.2–7.6) as compared with those with advanced neoplasia on index exam (15.5%; 95% CI,14.5–16.6).6 A meta-analysis of 7 studies observed that the incidence of metachronous advanced neoplasia in individuals with low-risk adenomas on an index exam was small, ranging from 2.2 to 6.8%.10
However, despite this evidence, some endoscopists do not adhere to current guidelines that recommend surveillance interval of at least 5 years for individuals with low-risk findings. Studies conducted in various settings have observed that endoscopists often recommend intervals that are shorter than 5 years for low-risk patients.11–14 Possible explanations include a lack of knowledge about the guidelines, disagreement with the recommendations,15 or the presence of other factors unique to the individual or exam, such as inadequate bowel preparation.16–18
With both new technology and emphasis on lesion detection, greater numbers of small tubular adenomas are being detected during colonoscopy. Recent studies using high-definition colonoscopes have demonstrated adenoma detection rates of 40% to 60% in screenees.19–21 Thus, decisions about the timing of follow-up surveillance colonoscopy for individuals with these findings are made with increasing frequency and can significantly affect the cost effectiveness of CRC screening.
We used data from a recent adenoma prevention trial, the Vitamin D/Calcium Polyp Prevention Study,22 to examine the issue of follow-up of individuals with LRAs. To be enrolled, participants were required to have at least 1 adenoma removed shortly before study entry and a 3- or 5-year surveillance interval for follow-up colonoscopy. Endoscopists, at the time of the qualifying colonoscopy, determined the follow-up interval of either 3 or 5 years based on their own clinical discretion and before decisions about enrollment into the trial were made. We examined the frequency of recommending shorter (3-year) vs longer (5-year) intervals, as well as factors associated with recommending shorter intervals and the effect of that decision on outcome at the time of follow-up colonoscopy.
Methods
Data Source
The data used in our analysis were gathered during the Vitamin D/Calcium Polyp Prevention Study, a double-blind, placebo-controlled trial of 1000 IU daily vitamin D with or without 1200 mg daily calcium supplementation for the prevention of large bowel adenomas. The methods and results of the main trial are published elsewhere and demonstrate that the study agents did not confer a decreased recurrence of adenomas.22 Briefly, adults age 45 to 75 years were enrolled from 11 study centers in North America from July 2004–July 2008. Eligible subjects were required to have at least 1 histologically confirmed adenoma, 2 mm or greater in diameter as estimated by the endoscopist, removed during the 4 months prior to study entry. In addition, eligible participants were also required to have had a complete large bowel examination deemed free of any remaining polyps by the endoscopist completing the exam. In all cases, the qualifying colonoscopy (ie, detecting at least 1 adenoma ≥2 mm) and the clearing colonoscopy (ie, deeming the colon free of remaining polyps) were the same examination and will be simply referred to as the index exam below. Trial eligibility also required that prior to enrollment subjects receive either a 3- or 5-year recommended interval for surveillance colonoscopy, as determined by the endoscopist at the index exam. Participants were randomized in a modified 2 × 2 factorial design stratified by study center, sex and surveillance interval, to 4 of 4 study arms: daily 1000 IU vitamin D3, 1200 mg calcium as carbonate, both, or placebo (4-group randomization). Women who declined to forego calcium supplementation were randomized to calcium alone or with vitamin D (2-group randomization). In this analysis, we included subjects who had only 1 or 2 tubular adenomas <1 cm in estimated diameter (N = 1560).
Exposure Data
Our first aim was to identify factors associated with shorter 3-year surveillance interval recommendations at the time of study entry among subjects with only 1 or 2 small tubular adenomas. Information on participants was obtained at enrollment by a trained study coordinator using standardized questionnaires and interview scripts, and included demographics, family history of CRC, diet, medical history, and lifestyle information such as smoking and alcohol use. Family history of CRC (self-reported) was defined as having a first-degree relative of any age diagnosed with CRC. Clinical data including the indication for index examination were abstracted from the colonoscopy and pathology reports and included information on the quality of colon preparation, indication for colonoscopy, and pathology findings (see outcome data). Index adenomas were stratified into 2 size categories (2–5 mm and 6–9 mm). While we did have information on the history of having a prior exam, we did not have detailed information regarding the findings in these exams. If the index colonoscopy was the first exam for an asymptomatic participant, it was considered a screening colonoscopy. Any exam performed in an asymptomatic participant with a previous colonoscopy was labeled as a follow-up exam. Finally, any exam that was performed for symptoms was considered diagnostic. We also included information gathered from publicly available Web sites regarding participating endoscopists’ characteristics (age at Index exam, sex, and specialty).
Outcome Data
All tissue removed during follow-up colonoscopies was sent for central study review by a single gastrointestinal trained study pathologist. While the majority (90%; data not shown) of the post-randomization colonoscopy exams were performed for routine surveillance, some subjects had interim follow-up exams performed for clinical indications. Our primary outcome of interest was the detection of advanced neoplasia at follow-up colonoscopy, including interim examinations. We also conducted analyses of any detected adenoma and clinically significant serrated polyps. An advanced adenoma was defined as any adenoma that was ≥ 1 cm in diameter, as estimated by the endoscopist, or having advanced pathology defined as villous or tubulovillous histology, high-grade dysplasia, or invasive cancer. A clinically significant serrated polyp included sessile serrated adenomas, traditional serrated adenomas, and hyperplastic or other serrated polyps that were ≥1 cm or occurred in the proximal colon (the cecum, ascending colon, hepatic flexure, or the transverse colon).
Analytic Plan
For our analysis, we included subjects with only 1 or 2 small tubular adenomas on the index exam. Participant, exam, and endoscopist-related characteristics were compared for those who were recommended a 3- vs a 5-year follow-up interval. A t-test was used to compare continuous variables and a χ2 test was used to compare categorical variables. A generalized linear model using a natural-logarithm link function and Poisson-distributed errors adjusted for over/under dispersion was used to estimate risk ratios of a 3-year vs a 5-year examination for each predictor separately. Variables with univariate P values < .05 were then put in a multivariate model. Variables with P > .05 were then removed successively until all variables remaining were statistically significant.
One potentially important factor that could influence follow-up decisions, but could not be adjusted for, was history of advanced neoplasia prior to the index colonoscopy for the study. To address this issue, we performed a restricted analysis including only participants with no prior colonoscopy to determine if risk factors for early recall in this restricted sample were similar to those identified in the entire sample.
To assess whether there were differences in findings on the follow-up exam according to the recommended surveillance interval, we determined the absolute risk and risk ratios for each of the following outcomes: at least 1 adenoma, at least 1 advanced adenoma, and at least 1 clinically significant serrated polyp on follow-up exam. For the risk ratios, we used 2 modeling strategies. First, for each outcome, comparing the recommended 3- to 5-year intervals, we computed risk ratios and 95% confidence limits as above and adjusted for factors related to trial participation including age, sex, clinical center, randomization groups (2- or 4-group) and study treatments (vitamin D vs no vitamin D and calcium vs no calcium). Second, we performed more fully adjusted models accounting for clinical factors that were associated with earlier recommended follow-up and outcome. These included race (white, African American, Asian/Pacific Islander, other), smoking status (never, former, and current), body mass index (BMI; continuous), family history of CRC (yes vs no), exam indication (as above), number of adenomas at index exam, presence of clinically significant serrated polyp on index exam, bowel preparation (excellent, good, adequate, fair, poor, and not stated in report) and endoscopist characteristics (age, gender, and specialty). Most participants completed their follow-up colonoscopy within a short window around the recommended follow-up date. However, we also performed a secondary analysis based on the actual surveillance intervals, irrespective of the recommended dates.
Results
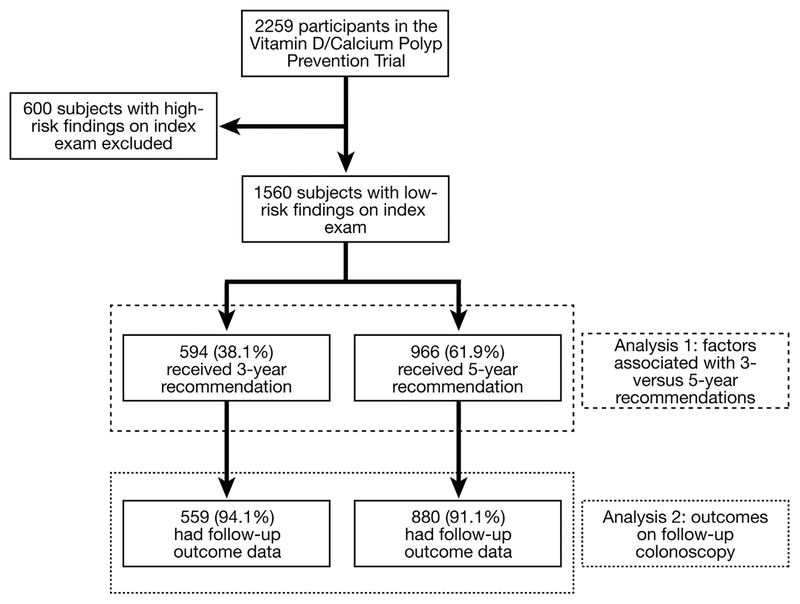
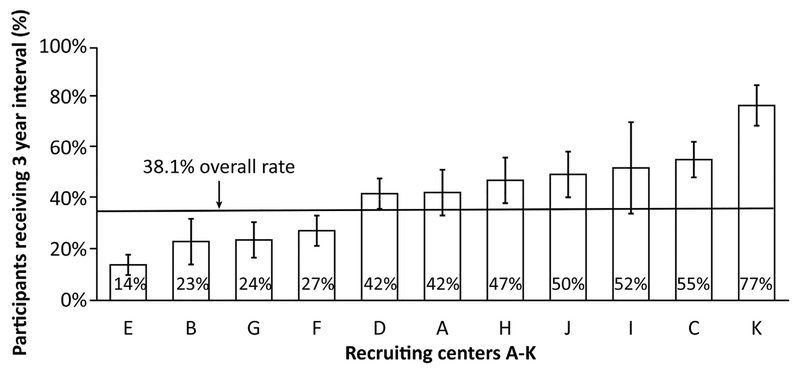
Of the 2259 randomized subjects, there were 1560 subjects with 1 to 2 small (< 1 cm) adenomas on index exam. In this sample, 594 (38%) had a recommendation for a 3-year follow-up surveillance interval (Figure 1). We observed that across the 11 recruitment centers there was a broad range with regard to the proportion of participants receiving 3-year surveillance recommendations (14%–77%; see Figure 2). Comparisons of subject, index exam, and endoscopist characteristics between individuals receiving 3-vs 5-year follow-up recommendations are shown in Table 1. There were no statistically significant differences between these 2 groups with regard to subject age, sex, Hispanic ethnicity, smoking history, BMI, or endoscopist gender. In comparison with subjects with a 5-year recommendation, those with a 3-year recommendation were more likely to be African American (10.9% vs 6.7%; P < .001) Asian/Pacific Islander (4.0% vs 1.6%; P < .0001), and have a family history of CRC (21.0% vs 15.6%; P =.01). Additionally, across 3 BMI categories (<25, 25–29.9, and >30) there was a non-significant trend for obese subjects to receive shorter intervals than non-obese participants (P =.09).
Figure 1.
Derivation of the low risk adenoma cohort from the parent Vitamin D/Calcium Polyp Prevention Trial.
Figure 2.
Variation of 3-year recommendation for subjects with LRAs on index exam by center.
Table 1.
Selected Study Participant, Colonoscopy Exam, and Endoscopist Characteristics by Recommended 3- vs 5-Year Follow-up Colonoscopies for Previous LRAs
| Category | Factor | 3-year (%) (N=594) | 5-year (%) (N=966) | P value |
|---|---|---|---|---|
| Subject | Age, mean ± SD | 58.0 ± 6.9 | 57.7 ± 6.7 | .37 |
| Sex | .77 | |||
| Male | 361 (60.8) | 580 (60.0) | ||
| Female | 233 (39.2) | 386 (40.0) | ||
| Race | ||||
| White | 482 (81.1) | 821 (85.0) | <.0001 | |
| African American | 65 (10.9) | 65 (6.7) | ||
| Asian/Pacific Islander | 24 (4.0) | 15 (1.6) | ||
| Other/multiple/unknown | 23 (3.9) | 65 (6.7) | ||
| Hispanic ethnicitya | ||||
| No | 554 (93.4) | 898 (93.0) | .73 | |
| Yes | 39 (6.6) | 68 (7.0) | ||
| Smoking status | .15 | |||
| Never | 303 (51.0) | 538 (55.7) | ||
| Former | 235 (39.6) | 355 (36.8) | ||
| Current | 56 (9.4) | 73 (7.6) | ||
| BMIa | .09 | |||
| <25 | 140 (23.6) | 224 (23.3) | ||
| 25–29.9 | 222 (37.4) | 410 (42.6) | ||
| ≥ 30 | 232 (39.1) | 329 (34.2) | ||
| Family history of CRCa | ||||
| No | 434 (79.1) | 763 (84.4) | .01 | |
| Yes | 115 (21.0) | 142 (15.6) | ||
| Exam | Indicationa | .01 | ||
| Screening | 208 (35.1) | 387 (40.2) | ||
| Follow-up exam | 298 (50.3) | 409 (42.5) | ||
| Diagnostic | 87 (14.7) | 166 (17.3) | ||
| Quality of preparation Pre-preparationa | <.0001 | |||
| Excellent | 138 (23.2) | 407 (42.1) | ||
| Good | 232 (39.1) | 348 (36.0) | ||
| Adequate | 47 (7.9) | 62 (6.4) | ||
| Fair | 50 (8.4) | 44 (4.6) | ||
| Poor | 2 (0.3) | 0 (0.0) | ||
| Not stated in report | 125 (21.0) | 105 (10.9) | ||
| Endoscopista | Age (mean years ± SD) | 47.3 ± 8.1 | 48.7 ± 9.6 | .003 |
| Gender | ||||
| Male | 519 (87.5) | 820 (84.9) | .15 | |
| Female | 74 (12.5) | 146 (15.1) | ||
| Specialty | <.0001 | |||
| Gastroenterology | 531 (89.5) | 909 (94.1) | ||
| Internal medicine | 29 (4.9) | 43 (4.5) | ||
| General surgery | 26 (4.4) | 12 (1.2) | ||
| Other | 7(1.2) | 2 (0.2) | ||
| Index findings | No. of adenomas | <.0001 | ||
| 1 | 431 (72.6) | 815 (84.4) | ||
| 2 | 163 (27.4) | 151 (15.6) | ||
| No. of serrated polyps | <.0001 | |||
| 0 | 414 (69.7) | 779 (80.6) | ||
| 1–2 | 150 (25.3) | 174 (18.0) | ||
| 3+ | 30 (5.1) | 13 (1.4) | ||
| Clinically significant serrated polypa | ||||
| No | 531 (90.8) | 897 (94.0) | .02 | |
| Yes | 54 (9.2) | 57 (6.0) |
BMI, body mass index; CRC, colorectal cancer; SD, standard deviation.
Missing data not included above: Hispanic (n = 1), BMI (n=3), Family history of CRC (n = 106), Exam indication (n = 5)Endoscopist information (n=1), clinically significant serrated (n = 21).
Subjects for whom the indication for the index exam was determined to be follow-up were more likely to be given a 3-year rather than a 5-year recommendation (50.3% vs 42.5%; P = .01). Subjects with a 3-year recommendation were much less likely to have excellent bowel preparation on index(23.2% vs 42.1%; P < .0001). In about 20% of cases, no bowel prep quality was reported for the exam. One center accounted for over one third of all exams with missing bowel preparation (83/220). Endoscopists recommending shorter intervals were on average 1.4 years younger than those recommending 5-year intervals and came from specialties other than gastroenterology (10.5% vs 5.9%).
There were a number of specific polyp findings on index exam that predicted a shorter 3-year interval recommendation. Those with a 3-year interval recommendation were more likely to have 2 adenomas (27.4% vs 15.6%), at least 1 serrated polyp (30.3% vs 19.4%), and at least 1 clinically significant serrated polyp (9.2% vs 6.0%) than were those with 5-year interval recommendations (Table 1). Absolute size of the adenomas at index exam was also important in predicting the timing of follow-up. Subjects with at least 1 adenoma that was 6–9 mm in size were more likely to receive a 3-year recommendation than those with only adenomas that were 2–5 mm (41.7% vs 35.0% respectively; P = .01). There was no association between the location of adenoma and recommended follow-up interval (data not shown).
In the multivariate model, we identified factors that were statistically significantly independently associated with shorter interval recommendations (Table 2): African American race, Asian/Pacific Islanders, persons with a family history of CRC, those who had 2 adenomas (rather than 1) on their index exam, and those who had any serrated polyps. In addition, participants who had less than an excellent quality of preparation were all more likely to have a 3-year interval recommendation than those with an excellent quality of bowel preparation.
Table 2.
Relative Risk of Recommended 3 vs. 5-Year Follow-up in Subjects With index LRAs
| Unadjusted univariate | Multivariatea RR (95%CI) | |||
|---|---|---|---|---|
| Category | Factor | RR (95% CI) | (N=1453) | P value |
| Subject | Age (per 10 y) | 1.04 (0.95–1.15) | ||
| Sex | ||||
| Male | Reference | |||
| Female | 0.98 (0.86–1.12) | |||
| Race | ||||
| White | reference | reference | .004 | |
| Black | 1.35 (1.10–1.66) | 1.41 (1.14–1.75) | ||
| Asian/Pacific Islander | 1.66 (1.20–2.30) | 1.72 (1.22–2.43) | ||
| Other/multiple/unknown | 0.71 (0.51–0.98) | 0.73 (0.52–1.03) | ||
| Hispanic (% yes) | ||||
| No | reference | |||
| Yes | 0.96 (0.74–1.23) | |||
| Smoking status | ||||
| Never | reference | |||
| Former | 1.11 (0.97–1.26) | |||
| Current | 1.20 (0.96–1.51) | |||
| BMI | ||||
| <25 | reference | |||
| 25–29.9 | 0.91 (0.77–1.08) | |||
| ≥ 30 | 1.08 (0.91–1.27) | |||
| Family history of CRC | ||||
| No | reference | reference | .01 | |
| Yes | 1.23 (1.05–1.45) | 1.26 (1.07–1.48) | ||
| Exam | Indication | |||
| Screening | reference | |||
| Follow-up exam | 1.21 (1.05–1.39) | |||
| Diagnostic | 0.98 (0.81–1.20) | |||
| Quality of preparationa | <.0001 | |||
| Excellent | reference | reference | ||
| Good | 1.58 (1.34–1.87) | 1.50 (1.26–1.78) | ||
| Adequate | 1.70 (1.31–2.21) | 1.60 (1.23–2.10) | ||
| Fair/poor | 2.14 (1.66–2.75) | 2.16 (1.66–2.83) | ||
| Not stated in report | 2.15 (1.77–2.60) | 2.12 (1.74–2.60) | ||
| Endoscopist | Age (per 10 years) | 0.90 (0.84–0.97) | ||
| Gender | ||||
| Male | reference | |||
| Female | 0.87 (0.72–1.05) | |||
| Specialty | .001 | |||
| Gastroenterology | reference | reference | ||
| Internal medicine/General surgery/Other | 1.41 (1.15–1.74) | 1.45 (1.16–1.79) | ||
| Index findings | No. of adenomas | <.0001 | ||
| 1 | reference | reference | ||
| 2 | 1.50 (1.30–1.73) | 1.47 (1.27–1.71) | ||
| No. of serrated polyp | .0003 | |||
| 0 | reference | reference | ||
| 1–2 | 1.33 (1.15–1.55) | 1.29 (1.10–1.50) | ||
| 3+ | 2.01 (1.50–2.69) | 2.16 (1.59–2.93) | ||
| Clinically significant serrated polypb | ||||
| No | reference | |||
| Yes | 1.31 (1.05–1.63) |
BMI, body mass index; CI, confidence interval; CRC, colorectal cancer; RR, relative risk.
Multivariate model after removing variables stepwise with P < .05.
A clinically significant serrated polyp is defined as a sessile serrated adenoma, a traditional serrated adenoma, a proximal serrated polyp, or a serrated polyp ≥1 cm.
Because we did not have data on prior adenoma history, we performed a restricted analysis examining factors associated with 3- vs 5-year interval recommendations in subjects whose qualifying exam was their first lifetime examination (n = 606). We observed a similar proportion of exams with earlier recall (215/606; 35.5%) as in the total sample (594/1560; 38.1%). Findings were generally similar to those in the overall sample with regard to factors that were significantly associated with 3-year interval recommendations (see Supplementary Table 1). Analyses examining the risk for metachronous advanced neoplasia demonstrated no significant association with factors that were associated with earlier surveillance interval selection or other known colorectal neoplasia risk factors such as smoking or obesity (Supplementary Table 2).
Data regarding timing for follow-up exams is shown in Supplementary Table 3. The mean time to follow-up exam in the 3-year group was 39.6 months (standard deviation ±7.2 months) and 61.0 (± 8.5 months) in the 5-year group. While 81.4% of subjects in the 3-year group had their surveillance exam within 6 months of the recommended interval, 75% of those in the 5-year group had a repeat in that time frame. Subjects in the 5-year group were more likely to have an exam sooner than 6 months prior to the expected date (9.9% vs 1.1%) while those in the 3-year group were more likely to have an exam 6 months or longer after the expected date (19.9% vs 15.1%).
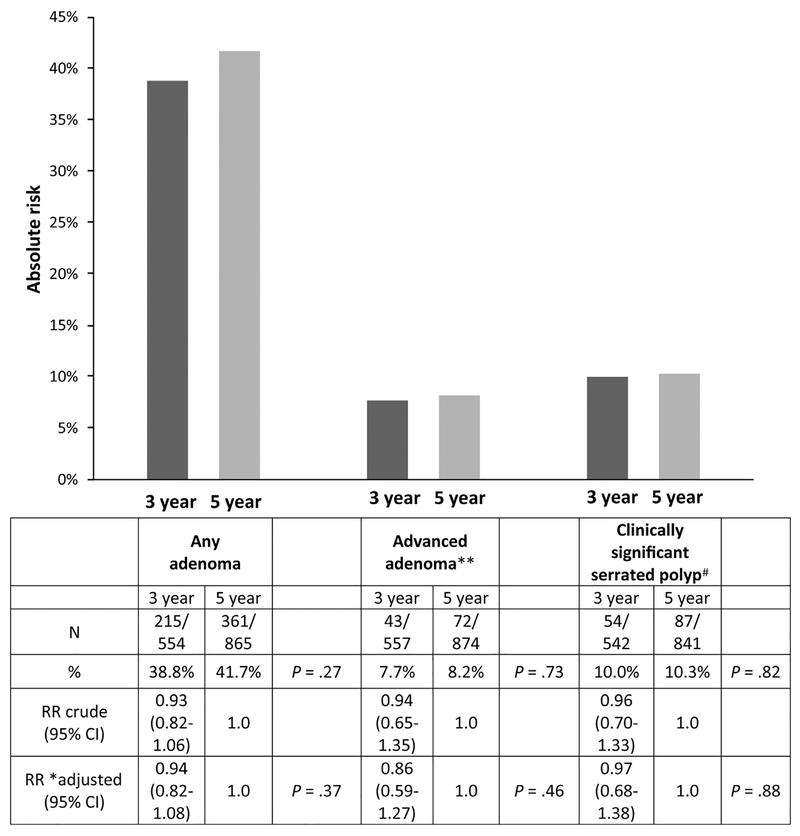
There were no significant differences between the 3- vs 5-year groups for risk of ≥1 adenomas (38.8% vs 41.7%;P =.27, respectively), advanced adenomas (7.7% vs 8.2%;P = .73), or a clinically significant serrated polyp (10.0% vs10.3%; P = .82) at follow-up colonoscopy (Figure 3). A large proportion of these metachronous clinically significant serrated polyps were proximal hyperplasic polyps ≤5 mm (92/213; 43.2%). There were 4 CRCs detected (3 in the 3-year group and 1 in the 5-year group (Supplementary Table 4).
Figure 3.
Outcomes at follow-up colonoscopy for participants with surveillance exams reommended at 3 vs 5 years.
Given the importance of prep quality to neoplasia detection, we performed an analysis examining recurrence in the 3- and 5-year groups in a sample restricted to those with good or excellent preparation, removing participants with no reported bowel preparation quality as well as those with poor or fair preparation. We observed that risk for advanced adenomas on surveillance exam was the same (8.4%) for the 3- and 5-year-groups (data not shown).
In our primary multivariate models, the recurrence risk ratio for a 3-year vs a 5-year interval was 0.86 (95% CI = 0.59–1.27) for advanced adenoma, 0.94 (95% CI = 0.82–1.08) for any adenoma, and 0.97 (95% CI = 0.68–1.38) for clinically significant serrated polyps (Figure 3). In a secondary model adjusting for clinical factors associated with a short interval recommendation, we also observed no significant differences between the 3- and 5-year groups with regard to 1 or more adenomas, advanced adenomas, or clinically significant serrated polyp on follow-up exam (Supplementary Table 5).
We repeated these analyses using the actual timing of the surveillance exams rather than the recommended interval. There were no significant differences between the absolute risks for participants with a 30- to 42-month follow-up vs those recalled at 54–66-months group for risk of ≥1 adenomas (37.0% vs 40.7%; P = .21, respectively), advanced adenomas (8.0% vs 6.8%; P = .42), or a clinically significant serrated polyp (11.1% vs 11.5%; P =.83). Adjusted model results are shown in Supplementary Table 6.
Discussion
Using data from a large chemoprevention trial, we observed 2 important findings regarding the surveillance of subjects with LRAs. In our sample, a significant proportion of subjects (38.1%) with 1 to 2 small adenomas on index exam were recommended to have a follow-up exam at 3 years. This 3-year interval was substantially shorter than the 5-year surveillance interval recommended by US Multi-Society Task Force (USMSTF) guidelines prevailing at the time of the study.23,24 Despite the difference in intervals, there were no significant differences in pathology between the 3- and 5-year groups on follow-up colonoscopy.
The 5-year post polypectomy colonoscopy interval recommendation by the USMSTF on CRC for individuals with LRAs was first published in 2003,23 a year before the start of the current study’s enrollment period, July 2004. The change in the recommended interval, an increase from 3 to 5 years, was based on published reports that found a lower risk for advanced lesions on follow-up examinations among individuals who had LRAs on a previous colonoscopy.25 The recommendation of 5 years was extended to 5 to 10 years in a subsequent version of the guidelines that was published in 2006, during the enrollment period.24 Thus, despite guideline recommendations as well as published data, a shorter recommendation of 3 years was made for a large proportion of subjects with LRAs.
Our findings demonstrate a large variation in recommendations for the timing of post polypectomy colonoscopy across study centers: While 77% of exams had a 3-year interval at 1 center, another reported only 14%. A large percentage of colonoscopies in our study were performed at intervals shorter than those endorsed in published guidelines, supporting results of previously published studies7,8,11–15,21,26–30 (see Supplementary Table 7). While most studies suggest that endoscopists are recommending shorter intervals, a recent analysis of Veterans Affairs Medical Center patients who had a colonoscopy in 2008 observed non-compliance for individuals with high-risk adenomas on index but not those with LRAs.31
The reasons for a recommendation disparate from the USMSTF are likely multifactorial. A survey of gastroenterologists conducted just after the current trial’s enrollment period observed that although 63.6% of gastrointestinal physicians were cognizant of the correct surveillance of 5 years for 2 small adenomas, a large proportion (28.8%) of the physicians disagreed with this interval.15 While it may not be possible to determine the exact motivation for the endoscopists in our population to recommend a shorter interval, we did identify some factors that were associated with a 3-year interval recommendation. Subjects with a family history of CRC were more likely to have a 3-year recommendation than those with no family history. Currently, the recommendation for colonoscopic surveillance intervals of patients with a family history of CRC but no adenomas is either 5 or 10 years, depending on the age of the CRC diagnosis in the relative; there are no specific surveillance recommendations for individuals who have a family history of CRC as well as an adenoma on colonoscopy.
Other factors that were associated with a 3-year interval recommendation in our study population included having 2 adenomas (vs 1) or at least 1 adenoma 6–9 mm in size (vs those with only adenomas 2–5 mm in size) or multiple serrated polyps on index exam. We also observed that African American adults and Asian/Pacific Islanders were more likely to receive 3-year intervals than white adults. This finding supports an analysis of Medicare data that also observed that African American adults were more likely than white individuals to have an early repeat colonoscopy after a normal exam.32 The American College of Gastroenterology CRC Screening Guidelines has also recommended that African American adults be screened at 45 years because of their increased risk of CRC.33 Thus, there may be a heightened concern among endoscopists regarding neoplasia risk in African American adults, though their risk for metachronous neoplasia after polypectomy may be similar to that for white adults.34 Thus, given the factors we identified in our analysis, it is likely that endoscopists may have provided shorter intervals to individuals whom they believed to be at higher risk for advanced neoplasia on the basis of race, family history of CRC, and findings at colonoscopy.
However, these factors were not associated with the risk of metachronous neoplasia, and despite the differences in characteristics at index exam between the 3-year and 5-year groups, there was no statistically significant difference in risk for being diagnosed with an advanced adenoma during follow-up (7.7% vs 8.2%, respectively). Of note, the prevalence of clinically significant serrated polyps was high at follow-up, approximately 10% for both groups. Over the time frame of the trial, serrated neoplasia was increasingly recognized and identified by endoscopists. We suspect that the high rate of follow-up serrated neoplasia, a large proportion of which were diminutive hyperplastic polyps (92/213; 43.2%), reflects missed lesions at the index exam.
In addition to patient-specific factors, we also identified some procedure-related predictors. In our study, we observed that individuals whose bowel preparation on index exam was not rated as excellent were more likely to receive a 3-year recommendation. Inadequate bowel preparation has previously been found to be a potential factor in endoscopists recommending shorter intervals.17,18,31,35 Our inclusion criteria, which required a complete inspection of the large bowel, minimized the number of subjects with a poor or fair preparation quality. Thus, our ability to examine poor or fair bowel preparation as a predictor of early surveillance recommendation may have been limited by low power because of the small numbers of exams with poor (n = 2) or fair (n = 94) preparation. In our study, we also observed that non-gastroenterologists were more likely to recommend 3-year intervals to individuals with LRAs than gastroenterologists. This finding is consistent with previous research showing that, in patients with LRAs, surgeons tended to recommend shorter call back intervals than those outlined in guidelines.28 A recent study of 25 Veterans Affairs Medical Center facilities found that general surgeons were more likely to overuse colonoscopy than gastroenterologists.36
A key finding of our paper is that neoplasia detection at follow-up was similar between the 3- and 5-year groups. In the National Polyp Study,37 similar detection of neoplasia at 1 and 3 years provided strong evidence to lengthen the interval to first surveillance and we believe our results should be interpreted similarly. However, because our study is not a clinical trial directly comparing the 2 intervals, we cannot exclude confounding from our analyses examining adenoma and advanced adenoma detection at follow-up. To the extent that the 3-year group is enriched with those at higher risk, comparison of follow-up findings would be potentially confounded. However, we observed no association between index exam factors that were associated with earlier recommendation and the risk of detecting metachronous advanced neoplasia on surveillance exams (See Supplementary Table 2). To further address the concern that endoscopists had identified significant factors, we examined our outcomes after adjusting for the covariates in Table 1 and observed no significant differences between these results and those from our analyses that adjusted only for trial specific factors (see Supplementary Table 5).
Finally, with regards to the impact of surveillance interval timing, we believe that 2 years would have made little difference with regard to adenoma development. An analysis of the Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial observed that all participants with LRAs on index exam had a similar risk for advanced neoplasia despite a follow-up period that ranged from 6 months to 10 years.38 In addition, the National Polyp Study observed no difference in adenomas detected on surveillance exams performed at 1 vs 3 years post index colonoscopy.37
One of the strengths of our study is that our findings represent “real life” experience because the practicing endoscopists selected the interval for the subjects with LRAs before study enrollment, thus avoiding biases associated with trial enrollment prior to interval selection. This allowed us to identify important possible predictors for shorter surveillance recommendations. In addition, this analysis presents follow-up data for subjects who had been recommended a 3- vs 5-year surveillance interval, allowing a direct comparison of these 2 intervals. Our study had a large sample size, and because the participants were enrolled in a chemoprevention trial, we had high quality, prospectively collected data for our exposures and outcomes of interest, and nearly complete follow-up of the study participants. There were 11 participating centers across the US, representing a broad representation of gastrointestinal practices, increasing the generalizability of our results.
A potential limitation of our study is that our main analyses were based on recommendation intervals and this may not be the same as the actual time period in which the follow-up exam was performed. However, nearly 80% of the subjects in each group (81.4% for 3-year and 75% for the 5-year group) received their follow-up exam within 6 months of the respective recommended interval. We examined the impact of the actual timing of the surveillance exam on the outcomes and observed no significant differences between these results and those obtained when examining the data by recommendation intervals. In addition, we did not have data regarding personal history of adenomas before the index or qualifying exam.39 Prior work from our group demonstrated that those with a history of advanced adenomas were at increased risk for advanced neoplastic findings both at the next exam and the subsequent (ie, third) colonoscopy exam.39 Although individuals with a previous history of CRC were excluded in our analysis, there may have been some subjects with a history of advanced adenomas, which may have influenced the endoscopist’s decision making regarding surveillance intervals. To address this limitation, we performed a restricted analysis in subjects whose qualifying exam was their first lifetime exam and found generally similar findings. Another limitation is that we had a relatively large percentage of exams with no reported quality of bowel preparation. However, we performed a sensitivity analysis by removing these exams as well as those with poor or fair preparation and observed that risk for advanced adenomas on surveillance exam was the same for the 3- and 5-year groups. Finally, our analysis does not examine 5- vs 10-year intervals as outlined in the current guidelines. However, we suspect that factors predicting short follow-up would likely be similar. To the extent that individuals just a few years ago were still selecting 3-year intervals, our data provides support for a 5-year minimum.
In summary, our findings suggest that a large percentage of endoscopists recommend follow-up colonoscopy intervals for LRAs that are shorter than those recommended by guidelines. In addition, there were statistically significant differences between study participants recommended 3- vs 5-year follow-up colonoscopies with regard to family history and number of adenomas and serrated polyps. Despite these differences, there was no substantial difference in outcome findings on follow-up exam. A survey conducted by the National Cancer Institute found that with regard to surveillance recommendations, physicians were more likely to be influenced by published evidence than by guidelines.28 Thus, our findings of similar rates of advanced adenomas detected after 3- or 5-year intervals in persons who had LRAs on their previous exam may help dissuade endoscopists from recommending shorter surveillance intervals for their patients.
Supplementary Material
EDITOR’S NOTES.
BACKGROUND AND CONTEXT
Endoscopists often recommend intervals for follow-up colonoscopy that are shorter than those in published guidelines for individuals with 1–2 small tubular adenomas less than 1 cm in size.
NEW FINDINGS
Patient factors such as race, family history and index exam findings such as number or size of polyps were associated with endoscopists recommending 3 versus 5-year intervals. However, when the follow up colonoscopy exams were completed, neoplastic findings were similar.
LIMITATIONS
The analysis did not examine 5 versus 10-year intervals as outlined in the current guidelines.
IMPACT
These data support current guideline recommendations for surveillance intervals of at least 5 years for individuals with low risk adenoma.
Acknowledgments
The contents of this work do not necessarily represent the views of the Department of Veterans Affairs or the United States Government.
Abbreviations used in this paper:
- BMI
body mass index
- CRC
colorectal cancer
- LRAs
low-risk adenomas
Footnotes
Supplementary Material
Note: To access the supplementary material accompanying this article, visit the online version of Gastroenterology at www.gastrojournal.org, and at http://dx.doi.org/10.1053/j.gastro.2017.02.010.
Conflicts of interest
The authors disclose no conflicts.
References
- 1.Atkin WS, Morson BC, Cuzick J. Long-term risk of colorectal cancer after excision of rectosigmoid adenomas. N Engl J Med 1992;326:658–662. [DOI] [PubMed] [Google Scholar]
- 2.Loberg M, Kalager M, Holme O, et al. Long-term colorectal-cancer mortality after adenoma removal. N Engl J Med 2014;371:799–807. [DOI] [PubMed] [Google Scholar]
- 3.Chung SJ, Kim YS, Yang SY, et al. Five-year risk for advanced colorectal neoplasia after initial colonoscopy according to the baseline risk stratification: a prospective study in 2452 asymptomatic Koreans. Gut 2011; 60:1537–1543. [DOI] [PubMed] [Google Scholar]
- 4.Laiyemo AO, Murphy G, Albert PS, et al. Postpolypectomy colonoscopy surveillance guidelines: predictive accuracy for advanced adenoma at 4 years. Ann Intern Med 2008;148:419–426. [DOI] [PubMed] [Google Scholar]
- 5.Lieberman DA, Weiss DG, Harford WV, et al. Five-year colon surveillance after screening colonoscopy. Gastroenterology 2007;133:1077–1085. [DOI] [PubMed] [Google Scholar]
- 6.Martinez ME, Baron JA, Lieberman DA, et al. A pooled analysis of advanced colorectal neoplasia diagnoses after colonoscopic polypectomy. Gastroenterology 2009; 136:832–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Laiyemo AO, Pinsky PF, Marcus PM, et al. Utilization and yield of surveillance colonoscopy in the continued followup study of the polyp prevention trial. Clin Gastroenterol Hepatol 2009;7:562–567; quiz 497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Radaelli F, Paggi S, Bortoli A, et al. Overutilization of post-polypectomy surveillance colonoscopy in clinical practice: a prospective, multicentre study. Dig Liver Dis 2012;44:748–753. [DOI] [PubMed] [Google Scholar]
- 9.Gupta S, Jacobs ET, Baron JA, et al. Risk stratification of individuals with low-risk colorectal adenomas using clinical characteristics: a pooled analysis. Gut 2017;66:446–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hassan C, Gimeno-Garcia A, Kalager M, et al. Systematic review with meta-analysis: the incidence of advanced neoplasia after polypectomy in patients with and without low-risk adenomas. Aliment Pharmacol Ther 2014;39:905–912. [DOI] [PubMed] [Google Scholar]
- 11.Krist AH, Jones RM, Woolf SH, et al. Timing of repeat colonoscopy: disparity between guidelines and endoscopists’ recommendation. Am J Prev Med 2007; 33:471–478. [DOI] [PubMed] [Google Scholar]
- 12.Kruse GR, Khan SM, Zaslavsky AM, et al. Overuse of colonoscopy for colorectal cancer screening and surveillance. J Gen Intern Med 2015;30:277–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ransohoff DF, Yankaskas B, Gizlice Z, et al. Recommendations for post-polypectomy surveillance in community practice. Dig Dis Sci 2011;56:2623–2630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schoen RE, Pinsky PF, Weissfeld JL, et al. Utilization of surveillance colonoscopy in community practice. Gastroenterology 2010;138:73–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Saini SD, Nayak RS, Kuhn L, et al. Why don’t gastroenterologists follow colon polyp surveillance guidelines? Results of a national survey. J Clin Gastroenterol 2009; 43:554–558. [DOI] [PubMed] [Google Scholar]
- 16.Anderson JC, Butterly LF, Robinson CM, et al. Impact of fair bowel preparation quality on adenoma and serrated polyp detection: data from the New Hampshire colonoscopy registry by using a standardized preparation-quality rating. Gastrointest Endosc 2014;80:463–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chokshi RV, Hovis CE, Colditz GA, et al. Physician recommendations and patient adherence after inadequate bowel preparation on screening colonoscopy. Dig Dis Sci 2013;58:2151–2155. [DOI] [PubMed] [Google Scholar]
- 18.Lebwohl B, Kastrinos F, Glick M, et al. The impact of suboptimal bowel preparation on adenoma miss rates and the factors associated with early repeat colonoscopy. Gastrointest Endosc 2011;73:1207–1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Anderson JC, Stein B, Kahi CJ, et al. Association of smoking and flat adenomas: results from an asymptomatic population screened with a high-definition colonoscope. Gastrointest Endosc 2010;71:1234–1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kahi CJ, Anderson JC, Waxman I, et al. High-definition chromocolonoscopy vs. high-definition white light colonoscopy for average-risk colorectal cancer screening. Am J Gastroenterol 2010;105:1301–1307. [DOI] [PubMed] [Google Scholar]
- 21.Chen SC, Rex DK. Endoscopist can be more powerful than age and male gender in predicting adenoma detection at colonoscopy. Am J Gastroenterol 2007;102:856–861. [DOI] [PubMed] [Google Scholar]
- 22.Baron JA, Barry EL, Mott LA, et al. A trial of calcium and vitamin D for the prevention of colorectal adenomas. N Engl J Med 2015;373:1519–1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Winawer S, Fletcher R, Rex D, et al. Colorectal cancer screening and surveillance: clinical guidelines and rationale - update based on new evidence. Gastroenterology 2003;124:544–560. [DOI] [PubMed] [Google Scholar]
- 24.Winawer SJ, Zauber AG, Fletcher RH, et al. Guidelines for colonoscopy surveillance after polypectomy: a consensus update by the US Multi-Society Task Force on Colorectal Cancer and the American Cancer Society. Gastroenterology 2006;130:1872–1885. [DOI] [PubMed] [Google Scholar]
- 25.Noshirwani KC, van Stolk RU, Rybicki LA, et al. Adenoma size and number are predictive of adenoma recurrence: implications for surveillance colonoscopy. Gastrointest Endosc 2000;51:433–437. [DOI] [PubMed] [Google Scholar]
- 26.van Heijningen EM, Lansdorp-Vogelaar I, Steyerberg EW, et al. Adherence to surveillance guidelines after removal of colorectal adenomas: a large, community-based study. Gut 2015;64:1584–1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sohn DK, Colonoscopy Study Group of the Korean Society of C. A survey of colonoscopic surveillance after polypectomy. Ann Coloproctol 2014;30:88–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mysliwiec PA, Brown ML, Klabunde CN, et al. Are physicians doing too much colonoscopy? A national survey of colorectal surveillance after polypectomy. Ann Intern Med 2004;141:264–271. [DOI] [PubMed] [Google Scholar]
- 29.Menees SB, Elliott E, Govani S, et al. Adherence to recommended intervals for surveillance colonoscopy in average-risk patients with 1 to 2 small (<1 cm) polyps on screening colonoscopy. Gastrointest Endosc 2014; 79:551–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Boolchand V, Olds G, Singh J, et al. Colorectal screening after polypectomy: a national survey study of primary care physicians. Ann Intern Med 2006;145:654–659. [DOI] [PubMed] [Google Scholar]
- 31.Johnson MR, Grubber J, Grambow SC, et al. Physician Non-adherence to Colonoscopy Interval Guidelines in the Veterans Affairs Healthcare System. Gastroenterology 2015;149:938–951. [DOI] [PubMed] [Google Scholar]
- 32.Goodwin JS, Singh A, Reddy N, et al. Overuse of screening colonoscopy in the Medicare population. Arch Intern Med 2011;171:1335–1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rex DK, Johnson DA, Anderson JC, et al. American College of Gastroenterology guidelines for colorectal cancer screening 2009 [corrected]. Am J Gastroenterol 2009;104:739–750. [DOI] [PubMed] [Google Scholar]
- 34.Laiyemo AO, Doubeni C, Brim H, et al. Short- and long-term risk of colorectal adenoma recurrence among whites and blacks. Gastrointest Endosc 2013;77: 447–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Menees SB, Kim HM, Elliott EE, et al. The impact of fair colonoscopy preparation on colonoscopy use and adenoma miss rates in patients undergoing outpatient colonoscopy. Gastrointest Endosc 2013; 78:510–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Murphy CC, Sandler RS, Grubber JM, et al. Underuse and overuse of colonoscopy for repeat screening and surveillance in the Veterans Health Administration. Clin Gastroenterol Hepatol 2016;14:436–444e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Winawer SJ, Zauber AG, O’Brien MJ, et al. Randomized comparison of surveillance intervals after colonoscopic removal of newly diagnosed adenomatous polyps. The National Polyp Study Workgroup. N Engl J Med 1993; 328:901–906. [DOI] [PubMed] [Google Scholar]
- 38.Pinsky PF, Schoen RE, Weissfeld JL, et al. The yield of surveillance colonoscopy by adenoma history and time to examination. Clin Gastroenterol Hepatol 2009;7:86–92. [DOI] [PubMed] [Google Scholar]
- 39.Robertson DJ, Burke CA, Welch HG, et al. Using the results of a baseline and a surveillance colonoscopy to predict recurrent adenomas with high-risk characteristics. Ann Intern Med 2009;151:103–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.