Abstract
Background & objectives:
Respiratory tract infections are common among Hajj and Umrah pilgrims which pose a public health risk of spread of respiratory infections. Influenza has been reported from Indian Hajj and Umrah returning pilgrims, but data on other respiratory pathogens are sparse in India. Here we report the presence of common respiratory viral pathogens in returning Hajj and Umrah pilgrims suffering from acute respiratory illness (ARI) in 2014-2015.
Methods:
Respiratory specimens (nasopharyngeal and throat swabs) were collected from 300 consenting pilgrims with ARI in the past one week and tested for influenza and Middle East Respiratory Syndrome coronavirus (MERS-CoV) and other respiratory viruses using in-house standardized quantitative real-time reverse-transcription polymerase chain reaction. Clinical features among the pathogen positive and negative patients were compared. The patients received symptomatic treatment and antivirals where appropriate and were followed telephonically to collect data on illness outcome.
Results:
Ninety seven (32.3%) of the 300 participants were tested positive for any virus, most common being influenza viruses (n=33, 11%). Other respiratory viruses that were detected included human coronaviruses [n=26, 8.7%; OC43 (n=19, 6.3%) and C229E (n=7, 2.3%)], rhinovirus (n=20, 6%), adenoviruses (n=8, 2.6%), parainfluenza viruses (n=7, 2.3%), respiratory syncytial virus (n=3, 1%) and bocaviruses (n=2, 0.6%). Clinical features observed in pathogen positive and pathogen negative patients did not differ significantly. Eighteen influenza positive patients were treated with oseltamivir.
Interpretation & conclusions:
Pilgrims returning from mass gatherings are often afflicted with respiratory pathogens with a potential to facilitate transmission of respiratory pathogens across international borders. The study reinforces the need for better infection prevention and control measures such as vaccination, health education on cough etiquette and hand hygiene.
Keywords: Acute respiratory infection, coronavirus, Hajj pilgrims, influenza virus, respiratory syncytial virus, respiratory viruses
The annual pilgrimage of Hajj draws millions of pilgrims to Saudi Arabia during the 12th month of the lunar calendar, with pilgrims entering the country through any one of 16 ports of entry. The Hajj poses significant public health concern as a result of mandatory religious rituals that entail congregated situations for the pilgrims, particularly during the circumambulation of the holy Kaabah1 and the ritual of stoning the devil. A variety of bacterial and viral respiratory infections are common: 20-80 per cent of pilgrims may develop respiratory infection during the pilgrimage2,3,4. Investigators have reported an overall mean prevalence for influenza of 2.1 per cent (range, 0.6-7.5%) among all arriving pilgrims and 3.6 per cent (range, 0.5-7.8%) among visitors departing the Hajj5; however, some recent studies suggest influenza prevalence to be as high as 4-14 per cent among hajj returnees6. In the past few years, cases of Middle East Respiratory Syndrome coronavirus (MERS-CoV) have been reported from many countries7,8,9, however, MERS-CoV among pilgrims travelling to Saudi Arabia has not been reported. The rapid spread in nosocomial settings suggests that person-to-person transmission can be efficient in certain settings8,9. There are scanty data on the carriage of respiratory viruses among Indian pilgrims returning from Hajj and Umrah pilgrimage. We have recently reported on the absence of MERS-CoV and presence of influenza infection among symptomatic returning pilgrims from Hajj and Umrah of 2014-201510. In this study, the work was extended by testing the same Hajj pilgrims for additional pathogens of public health importance.
Material & Methods
The participants included pilgrims returning from Saudi Arabia after having completed Hajj or Umrah from October 2014 to April 2015. After in-flight announcement offering medical attention to those with fever or respiratory symptoms, consenting disembarking pilgrims were interviewed for respiratory symptoms, offered medical examination and testing for respiratory viruses by a medical team stationed in the arrival halls of the Srinagar International Airport, Jammu and Kashmir, India. The demographic and clinical features were recorded on a predefined case record form. Enrollment for testing was offered to those with respiratory symptoms (n=977); 300 consented. The study protocol was approved by the ethics committee of Sher-i-Kashmir Institute of Medical Sciences (SKIMS), Srinagar, India, and all 300 participants gave written informed consent. Twin swabs (nasopharyngeal and throat) were obtained from each participant, pooled in viral transport media; and then transported to the Influenza laboratory in SKIMS, within 3-4 h of collection. After testing for influenza and MERS-coV at SKIMS, the samples were transported to the ICMR-National Institute of Virology, Pune, where testing for respiratory viruses was performed using in-house standardized duplex real-time reverse transcriptase polymerase chain reaction (RT-PCR) using previously published primers and probes. Briefly, eight tubes duplex PCR was performed for respiratory syncytial virus (RSV), human metapneumovirus (hMPV)11,12, rhinovirus and ribonuclease protein (RNP)11, parainfluenzaviruses (PIV) 1 and 211,13, PIV 3 and 411,13, adenovirus and boca virus11,14,15, coronavirus OC 43 and NL 6511,16, corona virus 229E and enterovirus11,13,16. From 100 μl clinical specimen RNA was extracted using the MagMax automated RNA extractor as per the manufacturer details. Real-time RT PCR assays were carried out using the Invitrogen Superscript III one-step quantitative RT-PCR kit (Invitrogen, Thermo Fisher Scientific, USA). Real-time RT-PCR assays were performed on ABI 7500 machine (Applied Biosystems Inc, USA) in a 25 μl PCR reaction containing 10 picomol of each forward and reverse primer, 5 picomol of TaqMan probe, 12.5 μl ×2 buffer, 0.5 μl Superscript III enzyme and 5 μl RNA extract. RT-PCR thermal cycling conditions were as follows: 50°C for 30 min, initial denaturation at 94°C for 10 min, 45 cycles of 15 sec at 94°C, 45 sec at 55°C. In vitro transcribed RNAs for all viruses to be detected were used as a positive control except enterovirus and bocavirus for which known positive clinical samples previously confirmed by additional conventional PCR and monoplex RT-PCR were used.
The patients were managed with symptomatic treatment and antiviral drugs where appropriate. Patients were followed telephonically at weekly intervals until two weeks to collect data on illness outcome.
Statistical analysis: Descriptive statistics was used to assess various prevalence rates. Comparison of continuous variables was performed using Student's t test, whereas comparison of various proportions (clinical features and other variables among pathogen positive and pathogen negative participants) was performed by SPSS (Statistical Package for Social Sciences) Version 17 software (IBM Limited, USA). All values were expressed as percentages and P<0.05 was considered significant.
Results
Of the 300 participants 140 were males and 160 females with age ranging from 26 to 60 yr (median age 60 yr). Ninety seven (32.3%) were tested positive for any virus (Table), influenza viruses [n=33, A (H3N2)=13], A [(H1N1pdm09)=9 and B (Yamagata)=11] were the most frequent (Figure)10. Of the other viruses, human coronaviruses (n=26, 8.7%) were the most common detected consisting in OC43 (n=19, 6.3%) and C229E (n=7, 2.3%) subtypes. MERS-CoV was not detected in any of the patients10. Human coronavirus (HCOV)-positive patients (15 males, age 32-75 yr, median 60 yr) presented with symptoms of 1-3 days (median 2 days) duration, with no significant differences in the age distribution and the symptomatology between HCOV-positive and negative patients. Co-infections of HCOV with other respiratory viruses were seen in five patients (aged 38-75 yr, median 60 yr, 4 males). These included HCOV OC43+ adenovirus (n=1), HCOV 229E + adenovirus + influenza B (Victoria) (n=1), HCOV 229E + influenza A (H3N2) (n=1), HCOV 229E + adenovirus (n=1) and HCOV 229E + influenza A (H1N1pdm09) (n=1). Human rhinovirus (HRV) was detected in 20 patients, and there were no significant differences between age distribution, co-morbidities and clinical features of HRV-positive and negative patients. Other respiratory viruses that were detected included adenoviruses (n=8, 2.6%), parainfluenza viruses (n=7, 2.3%), RSV (n=3, 1%) and bocaviruses (n=2, 0.6%) (Figure). Co-infections were seen in two patients (aged 50 and 60 yr, both females): one each of enterovirus + adenovirus and adenovirus + PIV-1.
Table.
Clinical symptoms and respiratory viruses detected among enrolled Hajj returnees (2014-2015, n=300)
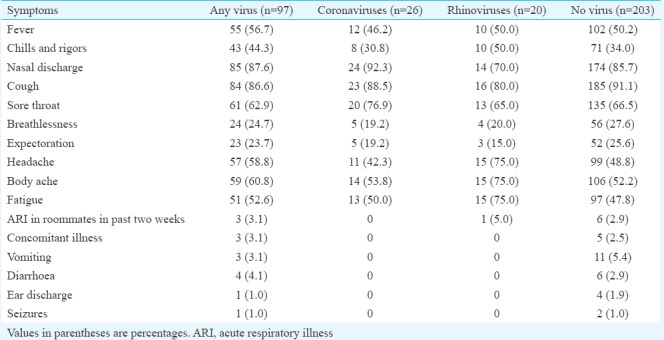
Figure.
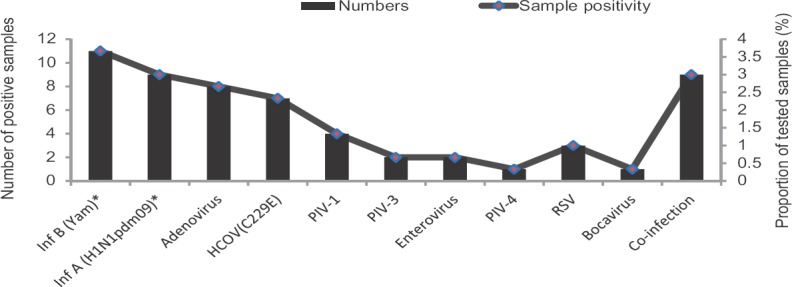
Frequency of respiratory pathogens detected in pilgrims returning from Hajj/Umrah (2014-2015). *Denotes data reported previously for these viruses10. HCOV, human coronavirus; PIV, para influenza virus; RSV, respiratory synctial virus; Inf, influenza virus.
All patients were given symptomatic treatment and advised about cough etiquette, hand hygiene and other infection control measures. Oseltamivir was used in 18 patients with laboratory-confirmed influenza viral infection who presented within 1-5 days (interquartile range 2-4 days) of the onset of the illness. None of the patients required hospitalization, and all had an uneventful recovery in 2-5 days.
Of the 300 participants, 216 (72%) had received trivalent inactivated influenza vaccine [comprising of A/California/7/2009 (H1N1)pdm09-like virus; A/Texas/50/2012 (H3N2)-like virus and B/Massachusetts/2/2012 (Yamagata)-like virus], 14-21 days (median 16 days) before embarking on the pilgrimage. The corresponding influenza infection detected included influenza A/H3N2 (n=13), A/H1N1pdm09 (n=9) and B/Yamagata (n=11)10. The participants had received the influenza vaccine as a part of their travel advice. Twenty one (10%) of these developed influenza-positive respiratory illness in comparison to 14.3 per cent (n=12) of those not vaccinated before the pilgrimage. None had received pneumococcal vaccination.
Discussion
Our data showed that apart from influenza, corona- and rhinoviruses were the most commonly detected viruses in Indian pilgrims returning from Hajj and Umrah in 2015. Coronaviruses OC43 and 229E have been known to be associated with upper respiratory infection17. A systematic review of 31 studies showed that rhinoviruses were the most commonly isolated viruses from symptomatic patients during the Hajj (5.9-48.8% prevalence), followed by influenza viruses (4.5-13.9%) and coronaviruses (2.7-13.2%) with coronavirus 229E being the most common; other viruses were less frequently detected17. The high prevalence of viruses such as influenza A (H1N1pdm09), influenza A (H3N2), rhinoviruses, coronaviruses (229E, HKU1 and OC43) in the study participants was similar to other such studies17, however, the aetiopathogenetic contribution was not clear in the absence of assessing the asymptomatic carriage in a control group. The crowded conditions during pilgrimage likely facilitate transmission of these viruses and could contribute to the high frequency of respiratory illness, the most common symptoms among the pilgrims3. Our data showed that respiratory virus infections were common among returning pilgrims with influenza, with corona and rhinoviruses being the most frequent and that clinical symptoms were not helpful in differentiating among respiratory infections.
Pilgrimage acquired viral infections are important for public health because these do not follow the traditional seasonality of any geography. We have earlier demonstrated that influenza and non-influenza respiratory viruses contribute significantly to acute exacerbations of COPD with a high level of activity during winter months in northern India, in a pattern that resembles the northern hemispherical pattern of circulation of influenza18,19,20. However, since Hajj and Umrah are performed throughout the year with changing dates, the congregation of potentially infected individuals from all across the world with individual seasonality of circulation of these viruses makes the acquisition and transmission of the viruses possible. International gatherings have a potential of acquisition of communicable disease outbreaks and exportation of these pathogens and initiation of local chain of spread in home countries triggering local outbreaks. Although not reported in the context of Hajj, transmission of MERS-CoV has been reported in home countries after travelling to a MERS-CoV-affected country in several instances like in the UK21, France22, and South Korea9.
Our study was limited by the fact that there was no information available about carriage of the viruses among those who did not have symptoms and the pre-pilgrimage status. However, all of the participants had been asymptomatic while before embarking on the pilgrimage. A control group of persons with a similar level of severity and without a recent history of travel, could have really brought out the difference in the viral spectrum, if any and given the quantitative impact of importation of viruses due to the pilgrimage. Furthermore, the patients represented a non-random selection that was, however, necessary in the unique circumstances of the recruitment.
Our study highlighted the potential for persons returning from mass gatherings to facilitate transmission of respiratory pathogens and reinforced the need for better infection prevention and control measures such as vaccination, health education on cough etiquette and hand hygiene, or use of facemasks23. There is a need for larger studies for surveillance for the viruses among the pilgrims returning from mass religious gatherings as also international and multi-sectorial coordination and communication for effective surveillance among Hajj and Umrah pilgrims and continued evaluation of the implementation of the recommended guidance4,18 for prevention of communicable health hazards among the pilgrims. An assessment of the burden of the viruses would also help public health and policy planners to devise appropriate public health response including infection control strategies during and after return from the pilgrimage.
Acknowledgment
The authors acknowledge the support of the Airport Authority of India, Srinagar, India.
Footnotes
Financial support & sponsorship: This study was supported in part by cooperative agreement U01IP000206 from the Centers for Disease Control and Prevention, Atlanta, USA.
Conflicts of Interest: None.
References
- 1.Memish ZA, Zumla A, Alhakeem RF, Assiri A, Turkestani A, Al Harby KD, et al. Hajj: Infectious disease surveillance and control. Lancet. 2014;383:2073–82. doi: 10.1016/S0140-6736(14)60381-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ahmed QA, Arabi YM, Memish ZA. Health risks at the Hajj. Lancet. 2006;367:1008–15. doi: 10.1016/S0140-6736(06)68429-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alzahrani AG, Choudhry AJ, Al Mazroa MA, Turkistani AH, Nouman GS, Memish ZA, et al. Pattern of diseases among visitors to Mina health centers during the Hajj season, 1429 H (2008 G) J Infect Public Health. 2012;5:22–34. doi: 10.1016/j.jiph.2011.10.003. [DOI] [PubMed] [Google Scholar]
- 4.Al-Tawfiq JA, Gautret P, Benkouiten S, Memish ZA. Mass gatherings and the spread of respiratory infections. Lessons from the Hajj. Ann Am Thorac Soc. 2016;13:759–65. doi: 10.1513/AnnalsATS.201511-772FR. [DOI] [PubMed] [Google Scholar]
- 5.Haworth E, Barasheed O, Memish ZA, Rashid H, Booy R. Prevention of influenza at Hajj: Applications for mass gatherings. J R Soc Med. 2013;106:215–23. doi: 10.1258/jrsm.2012.120170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alfelali M, Barasheed O, Badahdah AM, Bokhary H, Azeem MI, Habeebullah T, et al. Hajj Research Team. Influenza vaccination among Saudi Hajj pilgrims: Revealing the uptake and vaccination barriers. Vaccine. 2018;36:2112–8. doi: 10.1016/j.vaccine.2018.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Middle East respiratory syndrome coronavirus (MERS-CoV) Summary of Current Situation August 2018. Geneva, Switzerland: WHO; 2018. World Health Organization. WHO MERS-CoV Global Summary and Assessment of Risk, August 2018 (WHO/MERS/RA/August18) [Google Scholar]
- 8.Soliman T, Cook AR, Coker RJ. Pilgrims and MERS-coV: What's the risk? Emerg Themes Epidemiol. 2015;12:3. doi: 10.1186/s12982-015-0025-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cho SY, Kang JM, Ha YE, Park GE, Lee JY, Ko JH, et al. MERS-coV outbreak following a single patient exposure in an emergency room in South Korea: An epidemiological outbreak study. Lancet. 2016;388:994–1001. doi: 10.1016/S0140-6736(16)30623-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Koul PA, Mir H, Saha S, Chadha MS, Potdar V, Widdowson MA, et al. Influenza not MERS coV among returning Hajj and Umrah pilgrims with respiratory illness, Kashmir, North India, 2014-15. Travel Med Infect Dis. 2017;15:45–7. doi: 10.1016/j.tmaid.2016.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brittain-Long R, Nord S, Olofsson S, Westin J, Anderson LM, Lindh M, et al. Multiplex real-time PCR for detection of respiratory tract infections. J Clin Virol. 2008;41:53–6. doi: 10.1016/j.jcv.2007.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maertzdorf J, Wang CK, Brown JB, Quinto JD, Chu M, de Graaf M, et al. Real-time reverse transcriptase PCR assay for detection of human metapneumoviruses from all known genetic lineages. J Clin Microbiol. 2004;42:981–6. doi: 10.1128/JCM.42.3.981-986.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang CY, Arden KE, Greer R, Sloots TP, Mackay IM. A novel duplex real-time PCR for HPIV-4 detects co-circulation of both viral subtypes among ill children during 2008. J Clin Virol. 2012;54:83–5. doi: 10.1016/j.jcv.2012.01.013. [DOI] [PubMed] [Google Scholar]
- 14.Wong S, Pabbaraju K, Pang XL, Lee BE, Fox JD. Detection of a broad range of human adenoviruses in respiratory tract samples using a sensitive multiplex real-time PCR assay. J Med Virol. 2008;80:856–65. doi: 10.1002/jmv.21136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Deng Y, Gu X, Zhao X, Luo J, Luo Z, Wang L, et al. High viral load of human bocavirus correlates with duration of wheezing in children with severe lower respiratory tract infection. PLoS One. 2012;7:e34353. doi: 10.1371/journal.pone.0034353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van Elden LJ, van Loon AM, van Alphen F, Hendriksen KA, Hoepelman AI, van Kraaij MG, et al. Frequent detection of human coronaviruses in clinical specimens from patients with respiratory tract infection by use of a novel real-time reverse-transcriptase polymerase chain reaction. J Infect Dis. 2004;189:652–7. doi: 10.1086/381207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gautret P, Benkouiten S, Al-Tawfiq JA, Memish ZA. Hajj-associated viral respiratory infections: A systematic review. Travel Med Infect Dis. 2016;14:92–109. doi: 10.1016/j.tmaid.2015.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Koul PA, Khan UH, Asad R, Yousuf R, Broor S, Lal RB, et al. Contribution of influenza to acute exacerbations of chronic obstructive pulmonary disease in Kashmir, India, 2010-2012. Influenza Other Respir Viruses. 2015;9:40–2. doi: 10.1111/irv.12291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koul PA, Mir H, Akram S, Potdar V, Chadha MS. Respiratory viruses in acute exacerbations of chronic obstructive pulmonary disease. Lung India. 2017;34:29–33. doi: 10.4103/0970-2113.197099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Koul PA, Broor S, Saha S, Barnes J, Smith C, Shaw M, et al. Differences in influenza seasonality by latitude, Northern India. Emerg Infect Dis. 2014;20:1723–6. doi: 10.3201/eid2010.140431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Health Protection Agency (HPA) UK Novel Coronavirus Investigation Team. Evidence of person-to-person transmission within a family cluster of novel coronavirus infections, United Kingdom, February 2013. Euro Surveill. 2013;18:20427. doi: 10.2807/ese.18.11.20427-en. [DOI] [PubMed] [Google Scholar]
- 22.Guery B, Poissy J, el Mansouf L, Séjourné C, Ettahar N, Lemaire X, et al. Clinical features and viral diagnosis of two cases of infection with Middle East respiratory syndrome coronavirus: A report of nosocomial transmission. Lancet. 2013;381:2265–72. doi: 10.1016/S0140-6736(13)60982-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ministry of Health – Kingdom of Saudi Arabia. Health requirements for travellers to Saudi Arabia for pilgrimage to Makkah (2016/1437H Hajj). Ministry of Health – Kingdom of Saudi Arabia. 2016. [accessed on April 30, 2017]. Available from: http://www.moh.gov.sa/en/hajj/pages/healthregulations.aspx .


