Abstract
Aim:
This study was to evaluate and compare the bone regeneration potential of autologous platelet-rich fibrin (PRF) placed in one of the extracted sockets after the surgical removal of bilateral impacted mandibular third molars.
Patients and Methods:
Twenty-five patients (10 females and 15 males; 18–35 years old) were taken for surgical removal of bilateral impacted mandibular third molar, performed in the same session. The autologous PRF was placed in one of the extracted sockets whereas the opposite side was taken as control side, and primary closure was done. Radiographic examination with orthopantomogram was done preoperatively and 1 month, 3 months, and 6 months postoperatively to assess the degree of bone regeneration at the extracted site and compare it with the control side using MATLAB software and the data are statistically analyzed using paired t-test.
Results:
PRF side had better healing and bone formation when compared with the control side as indicated by significant P values of (P = 0.06>5%) 1 month, (P = 0.00<1%) 3 month, and (P = 0.00<1%) 6 month postoperatively. The repeated-measures ANOVA showed a significant difference seen on 1st, 3rd, and 6th months postoperatively on PRF side (P = 0.001).
Conclusion:
The autologous PRF improves and fastens the bone regeneration and healing in the extracted sockets.
Keywords: Bilateral impacted third molar, bone regeneration, MATLAB software, orthopantomogram, paired t-test, platelet-rich fibrin
INTRODUCTION
Wound healing is a complex biological process which results in the restoration of tissue integrity. At the time of injury, multiple cellular and extracellular pathways are activated, in a tightly regulated and coordinated fashion.[1] Platelets release growth factors, which are required for wound healing and bone regeneration.
In 1974, Ross et al.[2] were the first to describe growth factor from the platelets trapped within a fibrin matrix, responsible for mitogenic response in the bone periosteum during normal wound healing.[3] In 1990, Gibble and Ness[4] introduced fibrin glue, alternatively referred as fibrin sealant or fibrin gel. This biomaterial was developed to improve the hemostatic agents with adhesive properties.[5] Consequently, the use of fibrin glue was replaced by platelet concentrates to improve healing as first described by Whitman et al.[6,7] Various uses and actions of platelet concentrates have been explored considerably during the last decade.
Platelets contain high quantities of key growth factors, such as platelet-derived growth factor AB (PDGF-AB), transforming growth factor β 1 (TGF-β1), and vascular endothelial growth factor (VEGF), which can stimulate cell proliferation, matrix remodeling, and angiogenesis.
Platelet-rich fibrin (PRF) is a fibrin matrix in which platelet cytokines, growth factors, and cells are trapped and released gradually over a period of time. PRF can serve as a resorbable membrane and improves bone healing.[8] It consists of a fibrin matrix polymerized in a tetra molecular structure, with incorporation of platelets, leukocytes, cytokines, and circulating stem cells into the matrix. Autologous PRF is considered to be a healing biomaterial which accelerates the physiologic wound healing and new bone formation. Platelet activation and fibrin polymerization are triggered without the addition of anticoagulant.[9]
PRF has been most widely used in cardiac surgery and vascular surgery to seal diffuse microvascular bleeding. It is also used to seal wound borders which facilitate the cutaneous reuse in general and plastic surgery. In oral and maxillofacial surgery, PRF is used in sinus lift procedures, implant procedures, alveolar osteitis, extracted sockets, and cyst enucleation procedures.[10]
This study evaluates the efficacy of PRF in wound healing by comparing bone healing in sockets packed with PRF to that of sockets which are allowed to heal normally, following the bilateral surgical removal of impacted third molar surgery.
PATIENTS AND METHODS
This clinical study was undertaken on outpatients, as a minor oral surgical procedure in the Department of Oral and Maxillofacial Surgery, Sri Ramakrishna Dental College and Hospital, who required prophylactic surgical removal of bilateral impacted mandibular third molars. The study was approved by the Institutional Ethical Committee.
Patient selection
The study sample consisted of 25 patients, recruited (both women and men) between the age group of 18 and 35 years. Written consent was obtained from the patients/guardians who participated in the study. Patients with uncontrolled diabetes mellitus, immune diseases, or other systemic conditions were excluded from the study. Each patient was categorized into two groups as follows:
Group 1: PRF/case side – Surgical removal of third molar and placement of autologous PRF in socket followed by primary closure of the socket
Group 2: Control side – Surgical removal of third molar followed by primary closure of the socket. The side chosen as case side/control side was random.
Presurgical evaluation
A complete clinical examination and investigation with orthopantomogram (OPG) were carried out before the surgery. WAR lines and Pederson's difficulty index were performed using radiographs as a part of treatment planning.
Preparation of platelet-rich fibrin
The PRF was prepared in accordance with the protocol developed by Choukroun et al.[11] Under sterile aseptic conditions, about 20 ml of venous blood was taken from the patient. Blood was transferred to sterile 15 ml test tubes immediately without adding any anticoagulant. They were centrifuged at 3000 rpm for 10 min. The formed PRF gel was separated with the intermediate buffy coat from the red blood cell (RBC) [Figure 1], placed between two sterile gauzes and squeezed into a membrane [Figure 2]. The obtained PRF was then used in the extraction socket after the surgical removal of the third molars [Figures 3 and 4].
Figure 1.
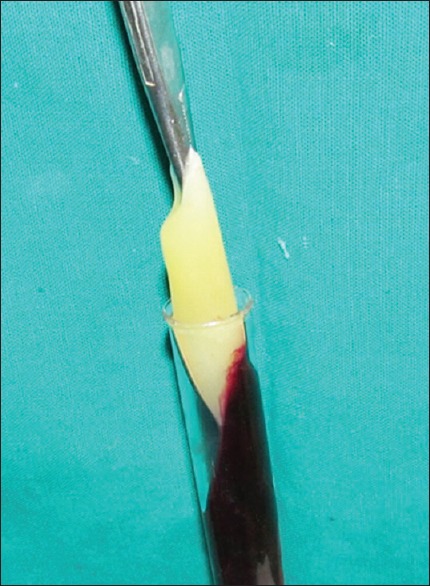
PRF taken using hemostat
Figure 2.
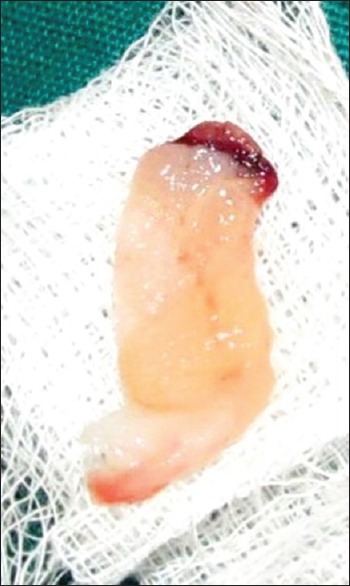
Squeezed PRF membrane
Figure 3.
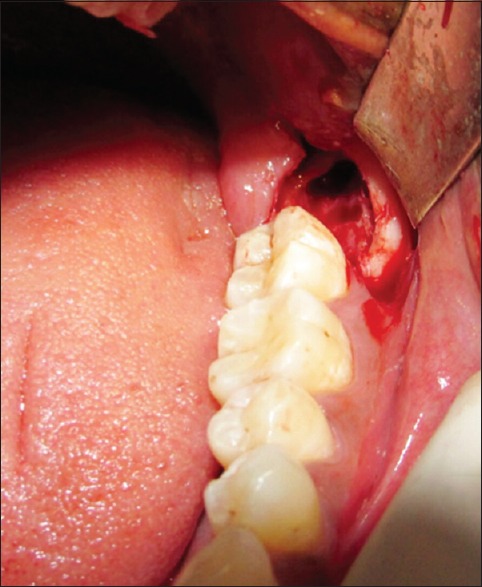
Extraction socket after removal of impacted third molar
Figure 4.
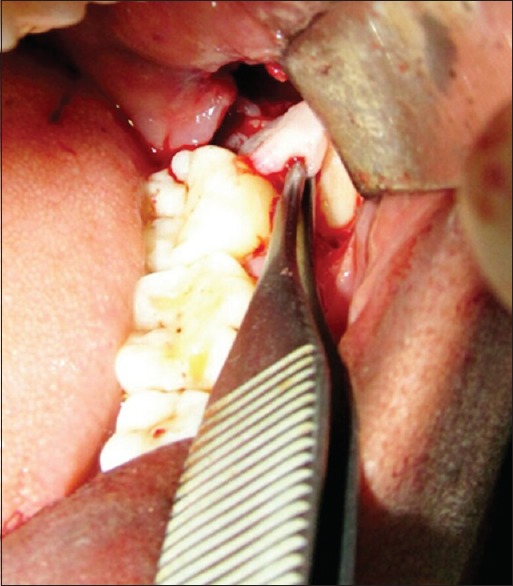
PRF placed in the extraction socket
Surgical procedure
All the surgical procedures were performed by the same surgeon on both the sides. Local anesthesia was achieved on both sides using 2% lignocaine hydrochloride with adrenaline bitartrate in 1:80,000. After surgical removal of mandibular third molar, PRF was placed in one of the sockets and the other side was taken as control. The flap was reapproximated and primary closure was achieved by suturing with nonabsorbable 3-0 black braided silk. Postoperative care was done with capsule amoxicillin 500 mg, tablet metronidazole 400 mg TDS for 3 days, and tablet aceclofenac 100 mg + paracetamol 325 mg b.i.d for 3 days was given.
Radiographic evaluation
OPG was taken before surgery using Sidexis software.[12] Similarly, OPGs were taken at 1 month, 3 months, and 6 months after the surgery [Figures 5–8]. The radiodensity of both the sockets was evaluated using image processing toolbox from MATLAB software.[13] This toolbox is used in many applications for processing and analyzing the images, and we used this toolbox to evaluate the density of PRF and non-PRF regions. OPG image taken from the patient is input into the image-processing toolbox; OPG image which is of red-green-blue format is converted to grayscale (black/white) image; pixel values from PRF region and non-PRF region are extracted separately. Average of the extracted pixel values from PRF region is computed.
Figure 5.
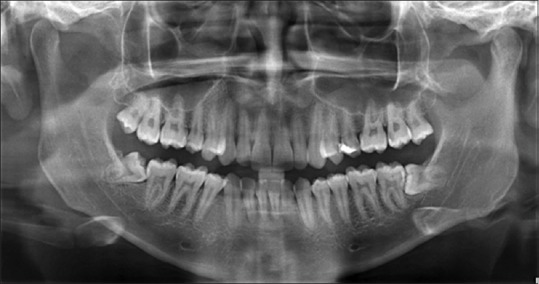
Pre-op OPG
Figure 8.
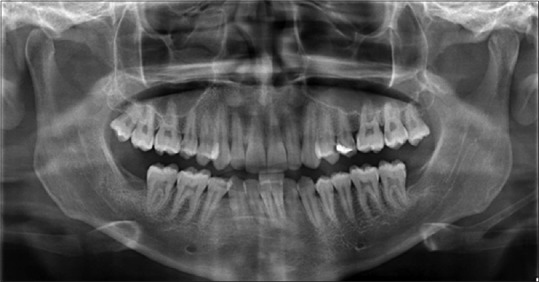
6th month OPG
Figure 6.
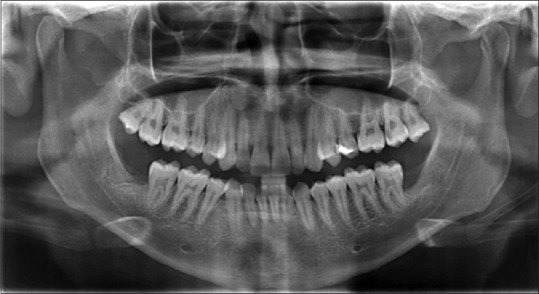
1st month OPG
Figure 7.
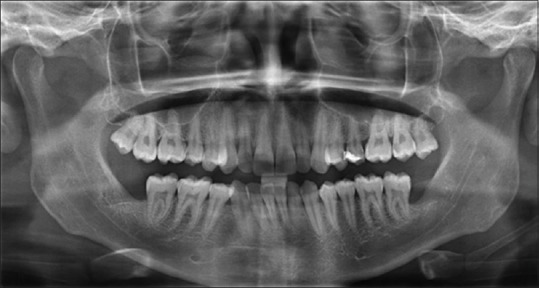
3rd month OPG
Similarly, average of the extracted pixel values from non-PRF region is computed. Each pixel value obtained conveys the intensity of that region. Intensity value ranges between 0 and 255, with 255 indicating the densest region. Hence, average of the pixel value conveys the density over that region [Figure 9]. Later, the density values of PRF region and non-PRF region in the 1st, 3rd, and 6th months were compared to note the improvement in bone healing, following placement of PRF in the extraction socket.
Figure 9.
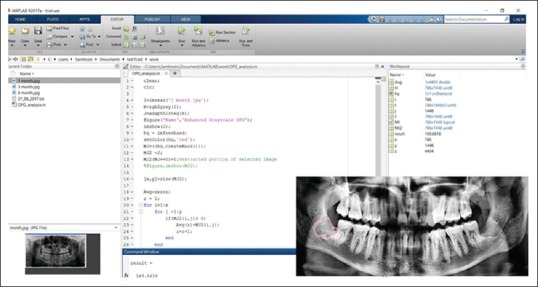
Measuring the density of the PRF region using Image processing Matlab Software
Statistical analysis
Data were analyzed with paired t-test at a significance level of 1% and 5% and it was expressed by statistical analysis: SPSS 17.0 software[14] as mean ± standard deviation.
RESULTS
In this study, five patients did not turn up for the 1st, 3rd, and 6th months of follow-up and hence they were excluded from the study. Of twenty patients, two patients had a postoperative complication of paresthesia on the control side and one patient had paresthesia on test side which subsequently reduced after 2 to 3 weeks of follow-up. Fifteen patients had postoperative complication of mild swelling and pain in the non-PRF region and five patients had diffuse swelling and pain in the PRF region for 2 days and it subsided after 3 days. We did not come across any other complications such as trismus, dry socket, or wound dehiscence.
P < 0.05 (5% and 1%) was considered statistically significant. Kolmogorov–Smirnov test was applied to continuous variables to check for normality distribution. Test results showed that the measurements follow normal distribution (all P > 5%). Therefore, the results were statistically analyzed by parametric methods such as repeated-measures ANOVA and paired “t-” test application of all and pair-wise comparison.
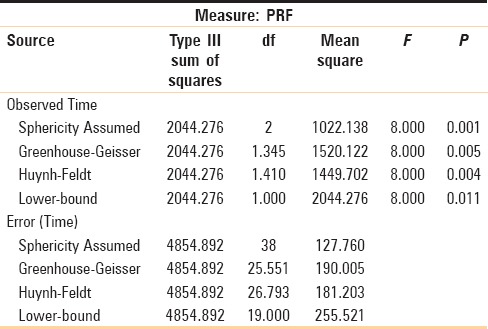
From paired “t-” test results, significant difference was noted on the 1st month postoperative follow-up (P = 0.061, >5%), with more radiodensity on PRF side compared to non-PRF side. From paired “t-” test results, a highly significant difference was noted on the 3rd and 6th months postoperative review (P = 0.000, <1% and P = 0.000, <1%), with more radiodensity on PRF side compared to non-PRF side [Table 1]. From the results of repeated-measures ANOVA, a significant difference was noted on the 1st, 3rd, and 6th months of PRF side postoperatively (P = 0.001) [Table 2].
Table 1.
Comparing PRF and non PRF using paired sample statistics and measure the P
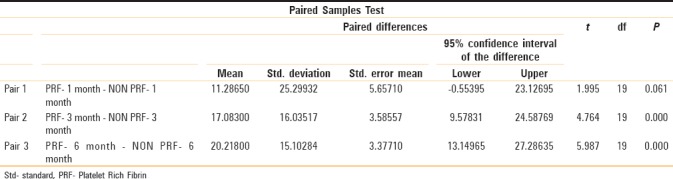
Table 2.
Repeated measure ANOVA-tests of within-subjects effects
DISCUSSION
Whenever there is an injury in the body, it prepares the site for healing by humoral and cellular reactions of inflammation. Cytokines and biologically active growth and differentiation factors control the cascade of biological events.[15] Classically, the process of wound healing is divided into four distinct phases: hemostasis, inflammation, proliferation, and tissue remodeling.
In hemostasis stage, fibrin and its degradation product along with macrophages and monocytes circulate in the wound site and release pro-inflammatory cytokines and growth factors such as TGF-β, PDGF, fibroblast growth factor, and epidermal growth factor until the repair is complete. Further blood loss at this stage is prevented. Once bleeding is controlled, inflammatory phase begins to release lipoxins and products of arachidonic acid metabolism that has anti-inflammatory properties, which dampens the immune response and allows the next phase of wound healing to arise. Once the injuring stimulus has ceased and hemostasis has been achieved, the inflammatory response is balanced and proliferative stage of healing begins to repair the wound. This complex process incorporates angiogenesis, granulation tissue formation, collagen deposition, epithelialization, and wound retraction occurring simultaneously. Wounds begin to contract about 7 days after injury. The final phase of wound healing is remodeling, which results in the development of normal epithelium and scar tissue maturation.[1]
Role of platelets and its growth factors
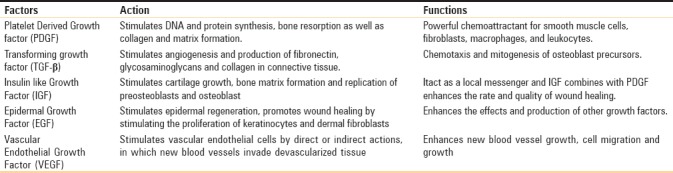
Platelets play a vital role not only in hemostasis but also in wound healing. Platelets contain α-granules and thirty bioactive proteins. When the activation phase starts, α-granules bind with the platelet plasma membrane and release numerous amount of growth factors as tabulated below [Table 3].[5]
Table 3.
Functions of the released growth factors
Platelet-rich plasma
Platelet-rich plasma (PRP) is prepared through double centrifugation method. Blood withdrawn from the patient is subjected to initial centrifugation at 2400 rpm for 10 min. After the separation of platelet-poor plasma (PPP) and PRP from the RBC fraction, both are again centrifuged at 3600 rpm for 15 min to separate the PRP from PPP.[16] The PRP coagulation process can be initiated with 10% calcium chloride and bovine thrombin. This effect causes rapid degranulation of platelets and liberation of growth factors into the surgical site.
The immediate release of growth factors can only affect the immediate stages of wound healing, but it will not extend to the period of time needed for bone and soft-tissue regeneration.[17] The use of topical bovine thrombin in PRP preparation may lead to risk of life-threatening coagulopathies due to factor V deficiency caused by the cross-reactivity of antibovine factor V antibodies with human factor V following thrombin exposure.[5]
Due to the legal restrictions on blood handling, new family of platelet concentrate, which is neither fibrin glue nor a classical platelet concentrate, appeared in France. This new biomaterial called PRF looks like an autologous cicatricial matrix.[11]
Platelet-rich fibrin
PRF described by Choukroun et al. in France in 2001 is a second-generation platelet concentrate, which allows the formation of fibrin membrane enriched with platelets, growth factors, leukocytes, and cytokines. It is prepared by single-stage centrifugation without adding any additives or anticoagulant.[18]
Many growth factors are released from PRF, such as PDGF, TGF- β, and VEGF. PDGF and TGF-β are released from platelets on activation with thrombin. In combination with insulin-like growth factor, which is located in plasma, PRF supports bone regeneration. These growth factors also promote cell proliferation, cell differentiation, and motility and matrix synthesis either alone or upon binding with specific cell surface receptor.[16]
The PRF formation is a natural and progressive polymerization initiated during centrifugation. This polymerization signifies increased incorporation of the circulating cytokines into the fibrin meshes (intrinsic cytokines) and it increases the lifespan of cytokines, because it will be released and used only at the time of initial cicatricial matrix remodeling (long-term effect). The cytokines are maintained in convenient media till it is released.[19]
The released platelet cytokines are trapped in the colloidal suspension between the fibrin network meshes during gelling. Their physiologic elimination will be fast and it shares the cytokine fibrin synergies from PRF on healing process. PRF also organizes as a dense fibrin scaffold with a high number of leukocytes concentrated in one part of the clot, with a specific slow release of growth factors and glycoproteins for at least 1 week and up to 28 days. This means that the membrane stimulates its environment for a significant period of time during wound healing.[9,20]
The Statistical comparison of density between test side and control side are: The 1st month follow-up showed significant difference in regeneration in PRF side compared to non-PRF side for 15 patients with P = 0.06. During 3rd month of follow-up, significant regeneration was observed in PRF side over non-PRF side for 19 patients with P = 0.00. At the 6th month of follow-up, significant difference between PRF and non-PRF side is seen in all the 20 patients with P = 0.00.
CONCLUSION
PRF is a natural and optimized blood clot that appears to be adequate in improving the alveolar bone regeneration in the extracted sockets. The procedure for the preparation of PRF is simple, safe, and inexpensive for the patient. PRF improves and fastens the bone healing in the extracted socket of impacted mandibular third molar within 6 months after surgery when compared to non-PRF side. PRF still continues to be a biological tool which has a potential that calls for an extensive research in various types of surgeries. The clinical applications of PRF uses are numerous and should be further explored in the coming years in the surgical field.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Singh S, Young A, McNaught CE. The physiology of wound healing. Surgery-Oxford International Edition. 2017;35:473–7. [Google Scholar]
- 2.Ross R, Glomset J, Kariya B, Harker L. A Platelet dependent serum factor that stimulates the proliferation of arterial smooth muscles cells in vitro. Proceedings of the National Academy of Sciences. 1974;71:1207–10. doi: 10.1073/pnas.71.4.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Naik B, Karunakar P, Jayadev M, Marshal VR. Role of Platelet rich fibrin in wound healing: A critical review. J Conservative Dentistry: JCD. 2013;16:284. doi: 10.4103/0972-0707.114344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gibble JW, Ness PM. Fibrin glue: The perfect operative sealant. Transfusion. 1990;30:741–7. doi: 10.1046/j.1537-2995.1990.30891020337.x. [DOI] [PubMed] [Google Scholar]
- 5.Sánchez AR, Sheridan PJ, Kupp LI. Is platelet-rich plasma the perfect enhancement factor? A current review. International Journal of Oral and Maxillofacial Implants. 2003;18 [PubMed] [Google Scholar]
- 6.Whitman DH, Berry RL, Green DM. Platelet gel: An autologous alternative to fibrin glue with applications in oral and maxillifacial surgery. J Oral and Maxillofacial Surgery. 1997;55:1294–9. doi: 10.1016/s0278-2391(97)90187-7. [DOI] [PubMed] [Google Scholar]
- 7.Kaul RP, Godhi SS, Singh A. Autologous platelet rich plasma after third molar surgery: A comparative study. J Maxillofacial and Oral Surgery. 2012;11:200–5. doi: 10.1007/s12663-011-0311-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chang YC, Zhao JH. Effects of platelet-rich fibrin on human periodontal ligament fibroblasts and application for periodontal infrabony defects. Australian Dental J. 2011;56:365–71. doi: 10.1111/j.1834-7819.2011.01362.x. [DOI] [PubMed] [Google Scholar]
- 9.Kalburgi VC, Warad S, Jenefer HD, Ashok N, Kokatnur V. Application of platelet rich fibrin and osseomold bone graft in different intrabony defects–2 case reports. Intern J Clinical Dental Science. 2012;3 [Google Scholar]
- 10.Eshghpour M, Majidi MR, Nejat AH. Platelet-rich fibrin: An autologous fibrin matrix in surgical procedures: A case report and review of literature. Iranian J Otorhinolaryngology. 2012;24:197. [PMC free article] [PubMed] [Google Scholar]
- 11.Dohan DM, Choukroun J, Diss A, Dohan SL, Dohan AJ, Mouhyi J, et al. Platelet-rich fibrin (PRF): A second-generation platelet concentrate. Part I: Technological concepts and evolution. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology, and Endodontology. 2006;101:e37–44. doi: 10.1016/j.tripleo.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 12. [[Online] Last accessed on 2018 Jul 25]. Available from: http://www.dentsplysirona.com/en/products/imaginingsystems/software/image-processing.html .
- 13. [[Online] Last accessed on 2018 Jul 25]. Available from: http://www.mathworks.com/products/image.html .
- 14. [[Online] Last accessed on 2018 Jul 25]. Available from: http://www.jmp.com/en_us/offers/statistical-analysissoftware.html .
- 15.Del Fabbro M, Bortolin M, Taschieri S. Is autologous platelet concentrate beneficial for post-extraction socket healing? A systematic review. Intern J Oral and Maxillofacial Surgery. 2011;40:891–900. doi: 10.1016/j.ijom.2011.04.009. [DOI] [PubMed] [Google Scholar]
- 16.Zhu SJ, Choi BH, Jung JH, Lee SH, Huh JY, You TM, Lee HJ, Li J. A comparative histologic analysis of tissue-engineered bone using platelet-rich plasma and platelet-enriched fibrin glue. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology, and Endodontology. 2006;102:175–9. doi: 10.1016/j.tripleo.2005.08.034. [DOI] [PubMed] [Google Scholar]
- 17.Rao SG, Bhat P, Nagesh KS, Rao GH, Mirle B, Kharbhari L, Gangaprasad B. Bone regeneration in extraction sockets with autologous platelet rich fibrin gel. J Maxillofacial and Oral Surgery. 2013;12:11–6. doi: 10.1007/s12663-012-0370-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dohan Ehrenfest DM, Del Corso M, Diss A, Mouhyi J, Charrier JB. Three-dimensional architecture and cell composition of a Choukroun's platelet-rich fibrin clot and membrane. J Periodontology. 2010;81:546–55. doi: 10.1902/jop.2009.090531. [DOI] [PubMed] [Google Scholar]
- 19.Dohan DM, Choukroun J, Diss A, Dohan SL, Dohan AJ, Mouhyi J, Gogly B. Platelet-rich fibrin (PRF): A second-generation platelet concentrate. Part II: Platelet-related biologic features. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology, and Endodontology. 2006;101:e45–50. doi: 10.1016/j.tripleo.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 20.Moraschini V, Barboza ES. Effect of autologous platelet concentrates for alveolar socket preservation: A systematic review. International J Oral and Maxillofacial Surgery. 2015;44:632–41. doi: 10.1016/j.ijom.2014.12.010. [DOI] [PubMed] [Google Scholar]


