Abstract
Background
Cryptococcal meningitis (CM) causes 10%–20% of HIV-related deaths in Africa. Due to limited access to liposomal amphotericin and flucytosine, most African treatment guidelines recommend amphotericin B deoxycholate (AmB-d) plus high-dose fluconazole; outcomes with this treatment regimen in routine care settings have not been well described.
Methods
Electronic national death registry data and computerized medical records were used to retrospectively collect demographic, laboratory, and 1-year outcome data from all patients with CM between 2012 and 2014 at Botswana’s main referral hospital, when recommended treatment for CM was AmB-d 1 mg/kg/d plus fluconazole 800 mg/d for 14 days. Cumulative survival was estimated at 2 weeks, 10 weeks, and 1 year.
Results
There were 283 episodes of CM among 236 individuals; 69% (163/236) were male, and the median age was 36 years. All patients were HIV-infected, with a median CD4 count of 39 cells/mm3. Two hundred fifteen person-years of follow-up data were captured for the 236 CM patients. Complete outcome data were available for 233 patients (99%) at 2 weeks, 224 patients (95%) at 10 weeks, and 219 patients (93%) at 1 year. Cumulative mortality was 26% (95% confidence interval [CI], 20%–32%) at 2 weeks, 50% (95% CI, 43%–57%) at 10 weeks, and 65% (95% CI, 58%–71%) at 1 year.
Conclusions
Mortality rates following HIV-associated CM treated with AmB-d and fluconazole in a routine health care setting in Botswana were very high. The findings highlight the inadequacies of current antifungal treatments for HIV-associated CM and underscore the difficulties of administering and monitoring intravenous amphotericin B deoxycholate therapy in resource-poor settings.
Keywords: amphotericin B, cryptococcal meningitis, HIV, resource-limited settings, sub-Saharan Africa
HIV-associated cryptococcal meningitis (CM) causes an estimated 181 100 deaths per year, 73% of which occur in sub-Saharan Africa (SSA) [1]. Ten-week mortality in developed country settings is as low as 10%–15% with amphotericin B deoxycholate (AmB-d)–based treatment [2–5]. In contrast, even under ideal clinical trial conditions, 10-week mortality with AmB-d-based therapies in Africa is around 30%–40% [6–8]. As AmB-d requires prolonged hospitalization with daily intravenous (IV) administration and is associated with hematological, renal, and metabolic toxicities [9], many under-resourced settings instead rely on oral fluconazole. Fluconazole monotherapy is associated with worse fungal clearance and an estimated 70% mortality within 3 months [10].
Short- and long-term outcomes with AmB-d under routine care settings in SSA are not well described. Several factors are important in CM management and may affect outcomes even with highly fungicidal AmB-d, including pretreatment hydration, electrolyte supplementation and regular laboratory monitoring for AmB-d-related toxicities [11], therapeutic lumbar punctures for management of intracranial pressure [12], and initiation and timing of antiretroviral therapy (ART) [6]. As countries in SSA increasingly adopt AmB-d, as recommended in international guidelines [13, 14], usually given with high-dose fluconazole due to lack of access to flucytosine in Africa [15], a better understanding of outcomes in routine hospital settings is needed to accurately characterize morbidity and mortality from cryptococcal disease and to improve management.
Botswana, a middle-income country in Southern Africa with a 25% adult HIV prevalence and high incidence of CM [16, 17], has been using AmB-d for over a decade [18]. National treatment guidelines recommend 14-day treatment with AmB deoxycholate 1 mg/kg/d and fluconazole 800 mg/d, followed by fluconazole maintenance [19]. Utilizing Botswana’s robust electronic national death registry and computerized medical records systems, we collected clinical, laboratory, and comprehensive 1-year outcome data of patients with microbiologically confirmed CM between 2012 and 2014 at the country’s major referral hospital to assess mortality with amphotericin-based therapy under usual care conditions and explore factors associated with poor outcomes.
METHODS
Participants and Procedures
Data were retrospectively collected on consecutive patients admitted to Princess Marina Hospital with laboratory-confirmed CM between January 1, 2012, and December 31, 2014. Princess Marina Hospital is a 530-bed public hospital in Gaborone that serves as 1 of 2 national referral centers for Botswana, providing free treatment to all citizens. The study was conducted in accordance with the ethical standards of the Helsinki Declaration (amended in 2008) and approved by institutional review boards at the University of Pennsylvania, University of Botswana, and Princess Marina Hospital, as well as the Botswana Ministry of Health’s Health Research and Development Committee. A waiver of informed consent was obtained as we collected routine retrospective data and as individual-level patient data were anonymized for the analysis. We included all patients who had laboratory-confirmed CM by either cerebrospinal fluid (CSF) India ink stain, cryptococcal antigen (CrAg) latex agglutination test, or fungal culture, with no exclusion criteria. Patients re-admitted with a recurrence of CM symptoms and laboratory-confirmed CM at any time point after their initial admission were classified as relapse episodes. During the study period, the recommended treatment for CM was amphotericin B deoxycholate 1 mg/kg/d IV plus fluconazole 800 mg/d orally for 14 days, followed by standard fluconazole consolidation and maintenance therapy. Electrolyte supplementation was given at the responsible physician’s dicretion. Antiretroviral therapy (ART) was freely available in the hospital and at public sector clinics, with tenofovir, emtricitabine, and efavirenz as firstline.
Evaluation and Outcomes
Cases of CM were identified through review of prospectively collected laboratory records. Demographic information and laboratory results, including blood tests, CSF results, HIV status and associated laboratory results, and basic clinical information including ART use and admission and discharge dates, were then retrospectively obtained for all patients from the electronic medical records system. More detailed clinical history and examination findings, CSF opening pressure recordings, and CM treatment details were obtained from detailed review of paper hospital records, where available, using a standardized data collection form. Time from hospital triage to initiation of antifungal therapy, duration and cumulative dosage of antifungal therapies, number of missed drug doses, frequency of toxicity monitoring, and details of management of raised intracranial pressure were recorded for each CM episode. Mortality data for all patients were collected by linking laboratory records to the electronic national death registry at the Botswana Ministry of Labour and Home Affairs using unique individual identifiers (name, date of birth, and Omang [national identification number]) at the end of December 2015. Cumulative mortality at 2 weeks, 10 weeks, and 1 year after hospital admission with CM were recorded.
Statistical Analysis
Baseline demographic, clinical, and laboratory variables were summarized using descriptive statistics (counts and percentages, medians and interquartile ranges [IQRs]), and proportions were compared using χ2 testing. Cumulative survival was estimated at 2 weeks, 10 weeks, and 1 year after initial admission for all patients (ie, from the first admission if a patient had multiple episodes of CM) and displayed graphically on a Kaplan-Meier curve. Sensitivity analyses were performed assuming all missing patients either survived or died at the point of censoring. Cox proportional hazards models were constructed to explore factors associated with mortality. Unadjusted and adjusted hazard ratios were estimated for predictors of interest, and P values were derived using the likelihood ratio test. Statistical significance was defined as a P value ≤.05. Analyses were performed using Stata, version 13 (College Station, TX).
RESULTS
During the study period, there were 283 episodes of CM among 236 individuals (199 patients had a single episode, and 37 patients had 2 or more episodes a median [IQR] of 133 [23–614] days apart) (Table 1). The median age (IQR) was 36 (32–42) years, and 69% (163/236) of patients were male. All patients were HIV-infected, with a median baseline CD4 count (IQR) of 39 (17–83) cells/mm3. Seventy-five percent (177/236) of patients had documentation of HIV infection before their initial CM admission, diagnosed a median (IQR) of 14 (2–63) months earlier. Of the known HIV-infected individuals with treatment data available, 57% (81/141) were taking ART at admission, started a median (IQR) of 31 (6–156) weeks before. Baseline laboratory data were available for all patients (Table 1). Eighty-nine percent (210/236) were India ink–positive at first presentation. The median CSF white cell count (IQR) was 10 (0–62) cells/µL, with a median lymphocyte predominance (IQR) of 98% (90%–99%). Paper clinical records were retrieved for 64% (180/283) of CM episodes. Headache was the most frequently recorded presenting symptom, documented in 86% (n = 154) of all CM episodes and present for a median (IQR) of 7 (5–14) days before admission. Forty-two percent (n = 74) of individuals had abnormal mental status at presentation (defined as a Glasgow Coma Score [GCS] <15). CSF opening pressure (OP) was documented in 62% (n = 112) of episodes, with a median OP (IQR) of 39 (30–55) cm H2O. Other common documented presenting symptoms were vomiting (55%, n = 96) and visual disturbances (37%, n = 65). Comorbidities were common, with a history of treated tuberculosis (TB) in 27% of cases (n = 48) and concurrent TB treatment in 9% (n = 16).
Table 1.
Baseline Characteristics
| Cryptococcal Meningitis: Patients and Episodes | ||
|---|---|---|
| Total No. of patients | 236 | |
| Total No. of episodes | 283 | |
| 1 | 236 | (83%) |
| 2 | 37 |
(13%) |
| 3 | 8 | (3%) |
| 4 | 2 | (1%) |
| Variable | No. With Data (of 236 Patients) |
Value, Median (IQR) Unless Otherwise Stated |
| Baseline characteristics of patients (restricted to 1st episode of cryptococcal meningitis) | ||
| Age, y | 234 | 36 (32–42) |
| Male sex, % (No.) | 236 | 69 (163) |
| Headache, % (No.)a | 155 | 86 (134) |
| Vomiting, % (No.)a | 151 | 54 (81) |
| Visual disturbance, % (No.)a | 155 | 35 (54) |
| Symptom duration, da | 119 | 7 (5–14) |
| Glasgow Coma Scale score <15, % (No.)a | 155 | 40 (62) |
| Focal neurology, % (No.)a | 154 | 14 (21) |
| CSF opening pressure, cm H2Oa | 94 | 38 (28–48) |
| India ink, % positive (No.) | 235 | 89 (210) |
| CSF protein, g/dL | 194 | 0.74 (0.47–1.38) |
| CSF glucose, mmol/L | 195 | 2.3 (1.5–2.9) |
| CSF white cell count, cells/µL | 229 | 10 (0–62) |
| CSF lymphocyte, % | 105 | 98 (90–99) |
| HIV and TB status | ||
| Prior diagnosis of HIV, % (No.) | 236 | 75 (177) |
| Time from HIV diagnosis to CM, mo | 217 | 14 (2–63) |
| On ART at CM diagnosis, % (No.) | 181 | 45 (81) |
| Time from ART initiation to CM, d | 181 | 217 (40–1095) |
| Baseline CD4 cell count, cells/µL | 192 | 39 (17–82) |
| Previously treated for TB, % (No.)a | 152 | 26 (39) |
| Current on TB treatment, % (No.)a | 151 | 9 (13) |
Abbreviations: ART, antiretroviral therapy; CM, cyptococcal meningitis; CSF, cerebrospinal fluid; IQR, interquartile range; TB, tuberculosis.
aThese data were derived from paper records. Paper records were retrieved for 64% (180/283) of CM episodes, or 66% (156/236) of patients. Note that the results in the table are restricted to the first episode only in individuals who had more than 1 episode of CM. The overall results of these paper record variables for each episode of CM (ie, including both first and relapse episodes) are given in the text.
Treatment and Monitoring
All patients were treated with AmB-d (at a dose of approximately 0.7–1 mg/kg/d) plus fluconazole 800 mg/d during the study period. Patient weights were not reliably documented in the medical notes, and the majority of patients (81%) received a dose of 50 mg/d, equating to a single vial of AmB-d. There was a median (IQR) of 2 (1–3) days from presentation to administration of the first AmB-d dose (Table 2). Over half of patients missed at least 1 dose of AmB-d; 20% missed a single dose, 12% missed 2 doses, and 21% missed 3 or more doses. Baseline full blood count (FBC) and renal function were available for 91% (n = 257) and 87% (n = 245), respectively. Patients had a median (IQR) of 1 (0–1) monitoring (ie, post-baseline) FBC and 2 (1–3) monitoring renal function and electrolyte tests during admission. Even with this infrequent monitoring, Division of AIDS (DAIDS) [20] Grade 3 anemia (hemoglobin [Hb] < 7.5 g/dL) was observed in 46 cases (29% of those tested), and Grade 4 anemia (Hb < 6.5g/dL) was observed in 27 cases (17% of those tested). Grade 3 creatinine rises (216–400 µmol/L/2.47–4.42 mg/dL) were observed in 22 cases (10% of those tested), and Grade 4 creatinine rises (>400 µmol/L/>4.42 mg/dL) were observed in 3 cases (1%). Therapeutic lumbar punctures were documented in 46% (129/283) of cases.
Table 2.
Treatment and Outcomes
| Variable | No. With Data (of 283 Episodes) |
Value, Median (IQR) or % (No.) | |
|---|---|---|---|
| Duration of admission, d | 270 | 17 (11–22) | |
| Time from admission to 1st amphotericin dose, d | 159a | ||
| 0 | 11 (18) | ||
| 1 | 36 (57) | ||
| 2+ | 53 (84) | ||
| No. of missed amphotericin dosesa,b | 159a | ||
| 0 | 47 (74) | ||
| 1–2 | 32 (51) | ||
| 3+ | 21 (34) | ||
| Baseline CSF opening pressure recorded | 175 | 64 (112) | |
| No. of therapeutic lumbar puncturesc | |||
| 0 | 283 | 54 (154) | |
| 1 | 16 (46) | ||
| 2 | 11 (32) | ||
| 3 | 8 (23) | ||
| 4+ | 10 (28) | ||
| No. of monitoring full blood countsc | 283 | 1 (0–1) | |
| Median drop in hemoglobin, g/dLc | 146 | 2.0 (1.0–3.4) | |
| Median nadir hemoglobin, g/dLc | 161 | 8.9 (7.3–11.1) | |
| DAIDS Grade 3 anemia, <7.5 g/dLc | 161 | 29 (46) | |
| DAIDS Grade 4 anemia, <6.5 g/dLc | 161 | 17 (27) | |
| No. of monitoring electrolyte testsc | 283 | 2 (1–2) | |
| Median nadir serum potassium, mmol/Lc | 222 | 3.2 (2.8–3.9) | |
| DAIDS Grade 3 hypokalemiac | 222 | 9 (21) | |
| DAIDS Grade 4 hypokalemiac | 222 | 0 (0) | |
| No. of monitoring creatinine testsc | 283 | 2 (1–3) | |
| Median peak creatinine, µmol/Lc | 223 | 105 (73–147) | |
| Median % rise in creatininec | 194 | 53 (9–117) | |
| DAIDS Grade 3 creatinine risec | 223 | 10 (22) | |
| DAIDS Grade 4 creatinine risec | 223 | 1 (3) | |
| Outcomesd | |||
| Mortality at 2 wk, % (No.) | 233 | 26 (60) | |
| Mortality at 10 wk, % (No.) | 224 | 50 (112) | |
| Mortality at 1 y, % (No.) | 219 | 65 (142) | |
Abbreviations: CSF, cerebrospinal fluid; DAIDS, Division of AIDS; IQR, interquartile range.
aThese data were derived from paper records. Paper records were retrieved for 64% (180/283) of CM episodes, or 66% (156/236) of patients.
bThe number of missed doses was calculated from the first dose, by subtracting the actual number of doses given over 14 days from the recommended 14 doses, or, if the patient died before 14 doses, by subtracting the actual number of doses given from the number of days a patient was alive and should have received a dose.
cThese data were derived from electronic laboratory records. Note that the number of therapeutic lumbar punctures may be underestimated as CSF may not always have been sent to the laboratory for analysis, although it is routine hospital practice to do so.
dReported from date of first episode.
Missed AmB-d doses were more frequent in patients developing DAIDS Grade 4 anemia during treatment (47% [8/17] of whom missed 3 or more doses vs 15% [13/84] of those without; P < .01) and Grade 3 hypokalemia (44% [7/17] of whom missed 3 or more doses vs 17% [20/119] of those without; P = .01), but not in patients developing DAIDS Grade 3 or 4 renal impairment.
Mortality
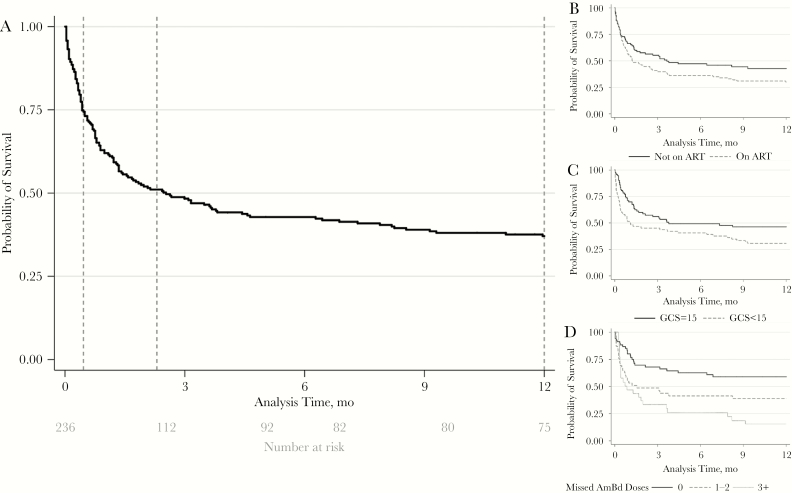
Two hundred fifteen person-years of follow-up data were captured for the 236 CM patients. Complete outcome data were available for 233 patients (99%) at 2 weeks, 224 patients (95%) at 10 weeks, and 219 patients (93%) at 1 year after initial presentation with CM. Overall mortality was 26% (95% confidence interval [CI], 20%–32%) at 2 weeks, 50% (95% CI, 43%–57%) at 10 weeks, and 65% (95% CI, 58%–71%) at 1 year (Figure 1). In sensitivity analysis, assuming all patients missing outcome data were either alive or dead, the point estimates for mortality were 25% (60/236) and 27% (63/236), respectively, at 2 weeks; 47% (112/236) and 53% (124/236), respectively, at 10 weeks; and 60% (142/236) and 67% (159/236), respectively, at 1 year (Table 2).
Figure 1.
Kaplan-Meier survival curves showing the probability of survival following presentation with a first episode of HIV-associated cryptococcal meningitis in Botswana. A, Survival for all 236 patients, with vertical dotted lines indicating 2 weeks (26% mortality), 10 weeks (50% mortality), and 12 months (65% mortality). B, Survival stratified by antiretroviral therapy (ART) status at presentation. C, Survival stratified by mental status. D, According to number of missed amphotericin B deoxycholate doses.
Associations With Mortality
Baseline factors significantly associated with 2-week and 10-week mortality in univariable analysis were abnormal mental status (GCS < 15), older age (>50 years), anemia (<7.5 g/dL), raised peripheral white cell count (>10 × 109/L), renal impairment (creatinine >110 µmol/L/>1.24 mg/dL), hyponatremia (<125 mmol/L), and low CSF white cell count (<20 cells/µL) (Table 3A). There was no evidence for lower mortality in ART-experienced patients (Table 3A, Figure 1B) despite ART-experienced patients having significantly higher baseline CD4 counts (Supplementary Table 1). In multivariable analysis, abnormal mental status, older age, raised peripheral white cell count, hyponatremia, and low CSF white cell count remained independently associated with acute mortality (Table 3B). ART status was not associated with 2-week mortality, but ART-experienced patients had significantly higher hazards of death at 10 weeks and 1 year. Relapse episodes were associated with lower rates of India ink positivity, higher CSF white cell counts, higher CD4 counts, and lower acute mortality than initial episodes (0% vs 26% at 2 weeks and 9% vs 50% at 10 weeks; P < .01 for both comparisons) (Supplementary Table 2). Missing AmB-d doses during induction treatment was associated with significantly higher mortality at 2 weeks, 10 weeks, and 1 year (at 2 weeks: hazard ratio [HR], 3.20; 95% CI, 1.4–7.1; at 10 weeks: HR, 2.30; 95% CI, 1.4–3.9; at 1 year: HR, 2.33; 95% CI, 1.4–3.8) (Table 3 and Figure 1D). This association remained significant in an analysis limited to individuals who did not develop DAIDS Grade 3 anemia, hypokalemia, or renal impairment during treatment, that is, the 3 potential dose-limiting toxicities leading to AmB-d discontinuation (at 2 weeks: HR, 7.30; 95% CI, 0.9–60.0; at 10 weeks: HR, 4.55; 95% CI, 1.3–16.3; at 1 year: HR, 2.77; 95% CI, 1.1–7.3).
Table 3.
Associations With Mortality
| 3A. Univariable Associations | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variable | Category | Data, No. (%) | No. (%) | Mortality 2 wk, % (No.) |
HR (95% CI) | P Value | Mortality 10 wk, % (No.) |
HR (95% CI) | P Value | Mortality 1 y, % (No.) |
HR (95% CI) | P Value |
| Age | <50 y | 234 (99) | 213 (91) | 23 (49) | 1 | .05 | 47 (95) | 1 | <.01 | 61 (121) | 1 | <0.01 |
| ≥50 y | 21 (9) | 45 (9) | 2.02 (1.0–4.1) | 75 (15) | 2.18 (1.3–3.8) | 95 (19) | 2.53 (1.6–4.1) | |||||
| Sex | Male | 236 (100) | 163 (69) | 30 (48) | 1 | .04 | 53 (82) | 1 | .12 | 64 (96) | 1 | 0.75 |
| Female | 73 (31) | 17 (12) | 0.52 (0.3–1.0) | 43 (30) | 0.72 (0.5–1.8) | 67 (46) | 0.94 (0.7–1.3) | |||||
| Mental status | GCS = 15 | 155 (66) | 93 (60) | 20 (18) | 1 | <.01 | 46 (39) | 1 | .04 | 63 (52) | 1 | 0.02 |
| GCS < 15 | 62 (40) | 39 (24) | 2.35 (1.3–4.3) | 53 (32) | 1.64 (1.0–2.6) | 66 (90) | 1.64 (1.1–2.5) | |||||
| Concurrent TB | No | 151 (65) | 138 (91) | 26 (36) | 1 | .19 | 50 (64) | 1 | .25 | 66 (83) | 1 | 0.19 |
| Yes | 13 (9) | 46 (6) | 1.7 (0.7–4.2) | 54 (7) | 1.54 (0.74–3.2) | 69 (9) | 1.55 (0.8–3.0) | |||||
| ART status | No ART | 181 (77) | 100 (55) | 26 (26) | 1 | .77 | 46 (43) | 1 | .15 | 66 (59) | 1 | 0.10 |
| On ART | 81 (45) | 28 (23) | 1.09 (0.6–1.9) | 55 (43) | 1.36 (0.9–2.1) | 66 (51) | 1.37 (0.9–2.0) | |||||
| CD4 category | Per 50 cells/µL increase | 192 (81) | 192 | — | 0.93 (0.8–1.1) | .51 | — | 0.86 (0.7–1.0) | .08 | — | 0.87 (0.8–1.0) | 0.06 |
| Hemoglobin | ≥7.5 g/dL | 215 (91) | 198 (92) | 25 (49) | 1 | .13 | 49 (93) | 1 | .03 | 63 (119) | 1 | 0.09 |
| <7.5 g/dL | 17 (8) | 44 (7) | 1.84 (0.8–4.1) | 73 (11) | 1.99 (1.1–3.6) | 73 (11) | 1.68 (0.9–3.1) | |||||
| White blood cell count | ≤109/L | 215 (91) | 193 (90) | 24 (46) | 1 | .01 | 49 (90) | 1 | .03 | 64 (115) | 1 | 0.12 |
| >109/L | 22 (10) | 45 (10) | 2.44 (1.2–4.8) | 64 (14) | 1.87 (1.1–3.2) | 68 (15) | 1.52 (0.9–2.6) | |||||
| Creatinine | ≤110 µmol/L | 201 (85) | 181 (90) | 23 (41) | 1 | <.01 | 49 (84) | 1 | <.01 | 64 (108) | 1 | <0.01 |
| >110 µmol/L | 20 (10) | 50 (10) | 2.60 (1.3–5.2) | 79 (15) | 2.48 (1.5–4.2) | 84 (16) | 2.30 (1.4–3.8) | |||||
| Sodium | ≥125 mmol/L | 198 (84) | 166 (84) | 23 (38) | 1 | .03 | 48 (76) | 1 | .03 | 63 (97) | 1 | 0.01 |
| <125 mmol/L | 32 (16) | 44 (14) | 1.99 (1.1–3.7) | 66 (21) | 1.69 (1.0–2.7) | 77 (24) | 1.76 (1.1–2.7) | |||||
| CSF OP | <30 cmH2O | 94 (40) | 27 (29) | 30 (8) | 1 | .98 | 54 (14) | 1 | .95 | 72 (18) | 1 | 0.98 |
| ≥30 cmH2O | 67 (71) | 30 (20) | 1.01 (0.4–2.3) | 54 (34) | 1.01 (0.5–1.9) | 68 (42) | 0.99 (0.6–1.8) | |||||
| CSF WCC | ≥20 cells/µL | 236 (100) | 95 (40) | 19 (18) | 1 | .05 | 39 (34) | 1 | <.01 | 62 (52) | 1 | 0.08 |
| <20 cells/µL | 141 (60) | 30 (42) | 1.73 (1.0–3.0) | 57 (78) | 1.80 (1.2–2.7) | 67 (90) | 1.36 (1.0–1.9) | |||||
| Time to first AmB-d dose | 0 d | 157 (67) | 17 (11) | 17 (3) | 1 | .63 | 29 (5) | 1 | .29 | 53 (8) | 1 | 0.34 |
| 1 d | 56 (36) | 21 (12) | 1.18 (0.4–4.2) | 44 (24) | 1.83 (0.6–5.3) | 55 (30) | 1.24 (0.5–2.8) | |||||
| 2+ | 84 (53) | 22 (18) | 1.32 (0.4–4.5) | 44 (34) | 1.93 (0.7–5.5) | 65 (49) | 1.42 (0.6–3.2) | |||||
| Missed AmB-d dosesa | 0 | 158 (67) | 74 (47) | 11 (8) | 1 | <.01 | 31 (20) | 1 | <.01 | 49 (31) | 1 | <0.01 |
| 1–2 | 50 (32) | 28 (14) | 2.92 (1.2–7.0) | 48 (24) | 2.06 (1.1–3.8) | 61 (30) | 1.97 (1.1–3.4) | |||||
| 3+ | 34 (21) | 32 (11) | 3.67 (1.5–9.1) | 56 (19) | 2.71 (1.4–5.1) | 79 (26) | 2.99 (1.7–5.2) | |||||
| 3B. Multivariable Associations | ||||||||||||
| Variable | Category | 2-wk Mortality-Adjusted HR | P Value | 10-wk Mortality-Adjusted HR | P Value | 1-y Mortality-Adjusted HR | P Value | |||||
| Age | <50 y | 1 (base) | .05 | 1 (base) | .03 | 1 (base) | 0.04 | |||||
| ≥50 y | 2.82 (1.0–8.0) | 3.34 (1.1–9.7) | 2.43 (1.0–5.6) | |||||||||
| Mental status | GCS = 15 | 1 (base) | <.01 | 1 (base) | .02 | 1 (base) | 0.03 | |||||
| GCS < 15 | 2.83 (1.4–5.6) | 2.02 (1.1–3.7) | 1.66 (1.0–2.6) | |||||||||
| 3B. Multivariable Associations | ||||||||||||
| Variable | Category | 2-wk Mortality-Adjusted HR | P Value | 10-wk Mortality-Adjusted HR | P Value | 1-y Mortality-Adjusted HR | P Value | |||||
| ART status | No ART | — | — | 1 (base) | <.01 | 1 (base) | <0.01 | |||||
| On ART | — | 2.16 (1.2–3.9) | 1.88 (1.2–3.0) | |||||||||
| CD4 category | Per 50 cells/µL increase | — | — | 0.83 (0.7–1.1) | .07 | — | — | |||||
| White blood cell count | ≤109/L | 1 (base) | <.01 | 1 (base) | .04 | — | — | |||||
| >109/L | 4.23 (1.8–9.9) | 2.47 (1.1–5.8) | — | |||||||||
| Sodium | ≥125 mmol/L | 1 (base) | .05 | 1 (base) | .01 | 1 (base) | <0.01 | |||||
| <125 mmol/L | 2.24 (1.0–4.9) | 2.79 (1.3–6.2) | 2.74 (1.5–5.0) | |||||||||
| CSF WCC | ≥20 cells/µL | 1 (base) | .02 | — | — | — | — | |||||
| <20 cells/µL | 2.39 (1.1–5.0) | — | — | |||||||||
Abbreviations: AmB-d, amphotericin B deoxycholate; ART, antiretroviral therapy; CI, confidence interval; CSF, cerebrospinal fluid; GCS, Glasgow Coma Scale score; HR, hazard ratio; OP, opening pressure; WCC, white cell count.
aWhen restricted to individuals who did not develop either Grade 3 anemia, hypokalemia, or renal impairment during amphotericin B treatment, the hazard ratios were 7.30 (95% CI, 0.9–60.0) at 2 weeks, 4.55 (95% CI, 1.3–16.3) at 10 weeks, and 2.77 (95% CI, 1.1–7.3) at 1 year.
DISCUSSION
Mortality following HIV-associated cryptococcal meningitis in a routine health care setting in Botswana was very high, with only 35% of patients surviving to 1 year. The comprehensive national death registration system in Botswana, combined with an electronic medical records system, provided the unique opportunity to accurately determine long-term outcomes in a large observational cohort of patients treated with amphotericin B deoxycholate therapy given with high-dose fluconazole under “real-world” routine care conditions in sub-Saharan Africa. These poor patient outcomes, in 1 of the better-resourced health care systems in Africa with good access to antiretroviral therapy, highlight the inadequacies of current antifungal treatments for HIV-associated cryptococcal meningitis. The findings also underscore the difficulties faced by busy clinicians in resource-poor settings in administering and monitoring 14 days of intravenous amphotericin B deoxycholate therapy and managing the frequent drug-associated toxicities, with the majority of patients receiving incomplete treatment courses.
Mortality rates in this cohort of patients with HIV-associated cryptococcal meningitis were substantially higher than those reported in recent randomized clinical trials (RCTs) from low-resource settings [8, 21, 22]. Ten-week mortality in patients treated with amphotericin B plus fluconazole in trial settings has ranged from 33% to 41% [8, 21, 22], compared with 50% (95% CI, 43%–57%) in this routine care cohort. Although some of this observed difference may reflect the selection and survival bias inherent in clinical trials, where the sickest patients may either die before study enrollment or be too unwell to consent to study inclusion, it is likely that the more intensive nursing, medical care, and toxicity monitoring in clinical trials leads to more regular drug administration, enhanced management of raised intracranial pressure, and earlier recognition and management of amphotericin B–related toxicities—all potentially contributing to improved survival [11, 12, 23, 24]. A large proportion of deaths occurred in the early posthospitalization period, emphasizing the need for close outpatient monitoring of patients following initial cryptococcal meningitis treatment.
In this routine care cohort, toxicity monitoring was performed less frequently than recommended in World Health Organization guidelines [14], with a median of 1 postbaseline full blood count and 2 postbaseline creatinine and electrolyte tests performed per admission, similar to recent laboratory-based surveillance data from Gauteng Province, South Africa, in which once-weekly laboratory monitoring of hemoglobin, potassium, and creatinine was performed in less than 50% of patients [25]. Even with this limited monitoring, which almost certainly led to a marked underascertainment of drug-related toxicities, amphotericin B–related adverse events were frequently detected, indicating the substantial toxicity of amphotericin B deoxycholate. As expected, the main toxicities were anemia (median 2-g/dL drop in hemoglobin during treatment), renal impairment, and hypokalemia. Amphotericin B doses were frequently missed, with more than half of patients missing at least 1 dose. Although we cannot ascertain for certain from our data, it is likely that while some missed amphotericin doses were intentionally withheld due to drug-related toxicities, the majority were missed due to the logistical challenges of administering daily intravenous therapy in an under-resourced health care setting. Unlike many African settings, Princess Marina Hospital has not had regular stock-outs of amphotericin B either during the study period or subsequently.
Importantly, short-term outcomes observed with amphotericin B deoxycholate plus fluconazole 800 mg/d treatment under usual care conditions were only marginally better than the survival described in prospective cohorts and RCTs from sub-Saharan Africa with high-dose (800–1200 mg/d) fluconazole monotherapy [26–29], likely reflecting the trade-off between more effective fungal clearance with amphotericin B deoxycholate [7, 30] and greater risk of severe drug-related toxicities and additional risks with extended intravenous therapy [9, 31].
The baseline predictors of mortality in this routine care cohort—older age, abnormal mental status, raised peripheral white blood cell count, hyponatremia, and low CSF white blood cell count—have previously been described in prospective cohort studies [7]. Seventy-five percent of patients had been diagnosed with HIV before admission, and almost half (45%) were on ART at the time of admission, reflecting recent shifts in the epidemiology of cryptococcal meningitis [8, 32, 33]. In contrast to a previous smaller study from Botswana [34], there was no evidence that being on ART at the time of initial presentation with cryptococcal meningitis led to improved outcomes. Conversely, although there was no significant difference in 2-week mortality between ART-experienced and ART-naïve patients, 10-week and 1-year outcomes were significantly worse in ART-experienced individuals in adjusted analysis. This may reflect the fact that many of these individuals were already failing or defaulting ART at the time of initial presentation and had worse adherence and outcomes once re-initiated on ART.
Given the high ongoing burden of HIV-associated cryptococcal meningitis in Africa [1, 16] and the extremely poor outcomes now described with amphotericin B deoxycholate plus fluconazole treatment, there is an urgent need to improve access to more effective, safe, and easily administered cryptococcal treatments in Africa. One potential strategy is shorter-course AmB-d treatment courses (7 days) [22], which may provide equivalent fungicidal activity to standard 14-day courses while avoiding many of the associated toxicities [35, 36]. In the recently completed ACTA trial [22], 1 week of AmB-d given with flucytosine was a highly effective and well-tolerated treatment, leading to lower mortality than standard 2-week treatment courses. One-week AmB-d given with fluconazole 800 mg/d was far less effective, leading to higher mortality than standard treatment, suggesting that such a short-course strategy is only viable with a more effective oral backbone therapy than fluconazole. Compatible with these trial findings, in this routine care cohort, missing any amphotericin doses was a strong predictor of mortality, even when accounting for related dose-limiting toxicities that may have led to the doses being withheld. This supports updated guidance recommending that in the context of fluconazole as the partner drug, AmB-d treatment should be given for a full 14-day course [14], and emphasizes the urgent need to establish access to flucytosine in Africa.
Another promising strategy to reduce mortality, currently being evaluated in a phase III study (ISRCTN 72509687), is administration of single high dose of liposomal amphotericin B. Phase II data have shown single 10-mg/kg doses to be safe, with similar early fungal clearance compared with 2 weeks of standard liposomal amphotericin B dosing [37]. In parallel with efforts to develop new improved drug treatment regimens and ensure access to these treatments, work is needed to ensure that patients who are receiving the currently available amphotericin B deoxycholate treatments in low-resource settings receive adequate toxicity monitoring and routine therapeutic lumbar punctures. These factors are often related to broader health system challenges and require addressing complex issues of equipment stock-out, physician training, and health care funding.
Our study has several important limitations, primarily relating to the retrospective data collection. Botswana’s electronic death registry and electronic laboratory records enabled near complete ascertainment of 1-year vital status data and laboratory results. However, we were still dependent on retrospective medical record review to collate several key clinical variables. Paper medical records were missing in a substantial proportion of patients, limiting clinical data availability. Patient weights were rarely available, precluding any assessment of potential weight-based underdosing or overdosing of amphotericin. The median weight in the cryptococcal meningitis patients in our clinical trials in these settings (IQR) was 52 (45–61) Kg [37], with a very small minority over 70 Kg, suggesting that a single 50-mg vial should provide a suitable weight-based dose of 0.7–1 mg/kg in most cases, with a higher probability of over- rather than underdosing. Documentation of therapeutic lumbar punctures and follow-up CSF opening pressures was poor, making a full analysis of the contribution of CSF pressure management difficult. Potential drug toxicities not captured through laboratory testing, such as thrombophlebitis, vomiting, and infusion reactions, were not routinely recorded; thus we can make no inference about their frequency or how often they contributed to discontinuation of amphotericin treatment. HIV viral load data were not available for the majority of ART-experienced patients at the time of CM presentation. In addition, data regarding ART initiation post–cryptococcal treatment were not available, precluding any assessment of the impact of ART timing on long-term outcome.
In conclusion, through analysis of registry-based data and electronic medical records, this study has demonstrated the extremely high mortality resulting from HIV-associated cryptococcal meningitis in patients treated with standard amphotericin B deoxycholate and high-dose fluconazole therapy in Africa. Much of this mortality occurs in the first months after hospital discharge and would be missed in hospital-based studies. These findings highlight the urgent need for effective implementation of cryptococcal prevention strategies such as cryptococcal antigen screening, the need for new, easy-to-administer, and effective treatment regimens for cryptococcal meningitis, and the importance of advocacy efforts to ensure that drugs such as flucytosine and liposomal amphotericin B, which will form the key components of these new treatment regimens, are made accessible and affordable in Africa.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Acknowledgments
We would like to thank the management and staff of Princess Marina Hospital.
Financial support. This work was supported by the Penn Center for AIDS Research, a National Institutes of Health–funded program (grant number P30 AI 045008).
Potential conflicts of interest. All authors report no conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Rajasingham R, Smith RM, Park BJ, et al. Global burden of disease of HIV-associated cryptococcal meningitis: an updated analysis. Lancet Infect Dis 2017; 17:873–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. van der Horst CM, Saag MS, Cloud GA, et al. Treatment of cryptococcal meningitis associated with the acquired immunodeficiency syndrome. National Institute of Allergy and Infectious Diseases Mycoses Study Group and AIDS Clinical Trials Group. N Engl J Med 1997; 337:15–21. [DOI] [PubMed] [Google Scholar]
- 3. Hamill RJ, Sobel JD, El-Sadr W, et al. Comparison of 2 doses of liposomal amphotericin B and conventional amphotericin B deoxycholate for treatment of AIDS-associated acute cryptococcal meningitis: a randomized, double-blind clinical trial of efficacy and safety. Clin Infect Dis 2010; 51:225–32. [DOI] [PubMed] [Google Scholar]
- 4. Pyrgos V, Seitz AE, Steiner CA, Prevots DR, Williamson PR. Epidemiology of cryptococcal meningitis in the US: 1997–2009. PLoS One 2013; 8:e56269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Saag MS, Powderly WG, Cloud GA, et al. Comparison of amphotericin B with fluconazole in the treatment of acute AIDS-associated cryptococcal meningitis. The NIAID Mycoses Study Group and the AIDS Clinical Trials Group. N Engl J Med 1992; 326:83–9. [DOI] [PubMed] [Google Scholar]
- 6. Boulware DR, Meya DB, Muzoora C, et al. ; COAT Trial Team Timing of antiretroviral therapy after diagnosis of cryptococcal meningitis. N Engl J Med 2014; 370:2487–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Jarvis JN, Bicanic T, Loyse A, et al. Determinants of mortality in a combined cohort of 501 patients with HIV-associated cryptococcal meningitis: implications for improving outcomes. Clin Infect Dis 2014; 58:736–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Beardsley J, Wolbers M, Kibengo FM, et al. ; CryptoDex Investigators Adjunctive dexamethasone in HIV-associated cryptococcal meningitis. N Engl J Med 2016; 374:542–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bicanic T, Bottomley C, Loyse A, et al. Toxicity of amphotericin B deoxycholate-based induction therapy in patients with HIV-associated cryptococcal meningitis. Antimicrob Agents Chemother 2015; 59:7224–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Park BJ, Wannemuehler KA, Marston BJ, et al. Estimation of the current global burden of cryptococcal meningitis among persons living with HIV/AIDS. AIDS 2009; 23:525–30. [DOI] [PubMed] [Google Scholar]
- 11. Bahr NC, Rolfes MA, Musubire A, et al. Standardized electrolyte supplementation and fluid management improves survival during amphotericin therapy for cryptococcal meningitis in resource-limited settings. Open Forum Infect Dis 2014; 1:ofu070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rolfes MA, Hullsiek KH, Rhein J, et al. The effect of therapeutic lumbar punctures on acute mortality from cryptococcal meningitis. Clin Infect Dis 2014; 59:1607–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Perfect JR, Dismukes WE, Dromer F, et al. Clinical practice guidelines for the management of cryptococcal disease: 2010 update by the Infectious Diseases Society of America. Clin Infect Dis 2010; 50:291–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guidelines on the diagnosis, prevention and management of cryptococcal disease in HIV-infected adults, adolescents and children: supplement to the 2016 consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection. Geneva: World Health Organization; 2018. Licence: CC BY-NC-SA 3.0 IGO. [PubMed] [Google Scholar]
- 15. Loyse A, Dromer F, Day J, et al. Flucytosine and cryptococcosis: time to urgently address the worldwide accessibility of a 50-year-old antifungal. J Antimicrob Chemother 2013; 68:2435–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tenforde MW, Mokomane M, Leeme T, et al. Advanced human immunodeficiency virus disease in Botswana following successful antiretroviral therapy rollout: incidence of and temporal trends in cryptococcal meningitis. Clin Infect Dis 2017; 65:779–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. UNAIDS. Botswana Country Factsheet (2017). UNAIDS AIDSinfo 2018. Available at: http://aidsinfo.unaids.org/. Accessed 31 October 2018.
- 18. Government of Botswana Ministry of Health. Botswana National HIV/AIDS Treatment Guidelines 2008. Department of HIV/AIDS Prevention and Care, 1 November 2008. Available at: http://www.moh.gov.bw/Publications/HIVAIDS%20TREATMENT%20GUIDELINES.pdf. Accessed 31 October 2018.
- 19. Government of Botswana Ministry of Health. Botswana National HIV/AIDS Treatment Guidelines 2012. Department of HIV/AIDS Prevention and Care, 1 April 2012. Available at: https://aidsfree.usaid.gov/sites/default/files/tx_botswana_2012.pdf. Accessed 31 October 2018.
- 20. US Department of Health and Human Services, National Institutes of Health, National Institute of Allergy and Infectious Diseases, Division of AIDS. Division of AIDS (DAIDS) Table for Grading the Severity of Adult and Pediatric Adverse Events, Corrected Version 2.1. [July 2017]. Available at: https://rsc.niaid.nih.gov/sites/default/files/daidsgradingcorrectedv21.pdf. Accessed 31 October 2018.
- 21. Day JN, Chau TTH, Wolbers M, et al. Combination antifungal therapy for cryptococcal meningitis. N Engl J Med 2013; 368:1291–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Molloy SF, Kanyama C, Heyderman RS, et al. ACTA Trial Study Team Antifungal combinations for treatment of cryptococcal meningitis in Africa. N Engl J Med. 2018; 378:1004–17. [DOI] [PubMed] [Google Scholar]
- 23. Bicanic T, Brouwer AE, Meintjes G, et al. Relationship of cerebrospinal fluid pressure, fungal burden and outcome in patients with cryptococcal meningitis undergoing serial lumbar punctures. AIDS 2009; 23:701–6. [DOI] [PubMed] [Google Scholar]
- 24. Wijewardana I, Jarvis JN, Meintjes G, et al. Large volume lumbar punctures in cryptococcal meningitis clear cryptococcal antigen as well as lowering pressure. J Infect 2011; 63:484–6. [DOI] [PubMed] [Google Scholar]
- 25. Meiring S, Fortuin-de Smidt M, Kularatne R, et al. GERMS-SA Prevalence and hospital management of amphotericin B deoxycholate-related toxicities during treatment of HIV-associated cryptococcal meningitis in South Africa. PLoS Negl Trop Dis 2016; 10:e0004865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Nussbaum JC, Jackson A, Namarika D, et al. Combination flucytosine and high-dose fluconazole compared with fluconazole monotherapy for the treatment of cryptococcal meningitis: a randomized trial in Malawi. Clin Infect Dis 2009; 50:338–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Rothe C, Sloan DJ, Goodson P, et al. A prospective longitudinal study of the clinical outcomes from cryptococcal meningitis following treatment induction with 800 mg oral fluconazole in Blantyre, Malawi. PLoS One 2013; 8:e67311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gaskell KM, Rothe C, Gnanadurai R, et al. A prospective study of mortality from cryptococcal meningitis following treatment induction with 1200 mg oral fluconazole in Blantyre, Malawi. PLoS One 2014; 9:e110285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Longley N, Muzoora C, Taseera K, et al. Dose response effect of high-dose fluconazole for HIV-associated cryptococcal meningitis in southwestern Uganda. Clin Infect Dis 2008; 47:1556–61. [DOI] [PubMed] [Google Scholar]
- 30. Bicanic T, Meintjes G, Wood R, et al. Fungal burden, early fungicidal activity, and outcome in cryptococcal meningitis in antiretroviral-naive or antiretroviral-experienced patients treated with amphotericin B or fluconazole. Clin Infect Dis 2007; 45:76–80. [DOI] [PubMed] [Google Scholar]
- 31. Rajasingham R, Williams D, Meya DB, et al. Nosocomial drug-resistant bacteremia in 2 cohorts with cryptococcal meningitis, Africa. Emerg Infect Dis 2014; 20:722–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Scriven JE, Lalloo DG, Meintjes G. Changing epidemiology of HIV-associated cryptococcosis in sub-Saharan Africa. Lancet Infect Dis 2016; 16:891–2. [DOI] [PubMed] [Google Scholar]
- 33. Rhein J, Morawski BM, Hullsiek KH, et al. ASTRO-CM Study Team Efficacy of adjunctive sertraline for the treatment of HIV-associated cryptococcal meningitis: an open-label dose-ranging study. Lancet Infect Dis 2016; 16:809–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bisson GP, Nthobatsong R, Thakur R, et al. The use of HAART is associated with decreased risk of death during initial treatment of cryptococcal meningitis in adults in Botswana. J Acquir Immune Defic Syndr 2008; 49:227–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Muzoora CK, Kabanda T, Ortu G, et al. Short course amphotericin B with high dose fluconazole for HIV-associated cryptococcal meningitis. J Infect 2012; 64:76–81. [DOI] [PubMed] [Google Scholar]
- 36. Jackson AT, Nussbaum JC, Phulusa J, et al. A phase II randomized controlled trial adding oral flucytosine to high-dose fluconazole, with short-course amphotericin B, for cryptococcal meningitis. AIDS 2012; 26:1363–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Jarvis JN, Leeme TB, Molefi M, et al. Short course high-dose liposomal amphotericin B for HIV-associated cryptococcal meningitis: a phase-II randomized controlled trial. Clin Infect Dis. 2018; [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.