Abstract
Objective(s):
Circular RNAs (circRNAs), a new class of non-coding RNAs, have emerged as important regulators during tumorigenesis. However, the functions of circRNAs have not been completely clarified in the progression of cancers. In our study, a novel circRNA hsa_circ_0109291 was investigated in oral squamous cell carcinoma (OSCC) tissues and cell lines.
Materials and Methods:
The expression profile of circRNAs in OSCC tumor tissues was performed by high-throughput sequencing. The CCK-8 wound healing and apoptosis assay were measured in OSCC cell lines after transfection with si-0109291 or si-NC.
Results:
We discovered that hsa_circ_0109291 was significantly increased in OSCC tissues and cell lines compared with their corresponding control group. Knockdown of hsa_circ_0109291 inhibited proliferation and migration of OSCC cell lines in vitro. In addition, inhibition of hsa_circ_0109291 dramatically induced apoptosis of OSCC cells. We further found that high hsa_circ_0109291 levels in OSCC patients resulted in a poorer prognosis than in patients with low hsa_circ_0109291 levels.
Conclusion:
These findings indicated that hsa_circ_0109291 correlated with the progression of OSCC and might be a new therapeutic target for the treatment of OSCC.
Key Words: Apoptosis, CircRNA, Hsa_circ_0109291, Oral squamous cell – carcinoma, Prognosis
Introduction
Squamous cell carcinoma (OSCC) is one of the frequently occurring malignancies, with approximately 540,000 newly diagnosed cases annually and 5-year survival rate of less than 50% (1). The major reasons for the low survival rates of OSCC are lymph node metastasis and local or regional recurrence (2). Alcohol consumption, tobacco use, and human papillomavirus (HPV) infection are widely recognized as the main risk factors of OSCC (3). OSCC is histopathologically characterized by keratinization and squamous differentiation (4), which are complex pathological processes and may be related to the change of a variety of biological molecules such as growth factors (5), microRNAs (miRNAs) (6, 7) and long non-coding RNAs (lncRNAs) (8, 9), suggesting non-coding RNAs may play a crucial role in these process.
Circular RNAs (circRNAs) as a new class of non-coding RNAs are characterized by a covalently closed continuous loop, without 5’ to 3’ polarity and polyadenylated tail that endow the stable structure for circRNAs (10). CircRNAs are originally reported as the splicing products of endogenous RNA and considered as the byproducts of splicing errors (11). With the in-depth study of their pathophysiological features, circRNAs are widely expressed in mammals and participate in the pathogenesis of several types of diseases such as myocardial fibrosis (12), Alzheimer’s disease (13), preeclampsia (14), and osteoarthritis (15). Recent studies have revealed that circRNAs perform their function by acting as miRNA sponges (16, 17). It is well known that circRNA ciRS-7 functions as a miRNA-7 sponge and contains more than 70 binding sites for miR-7 (16). Moreover, circ-HIPK3 (18), circ-ITCH (19), and circMTO1 (20) are also able to act as miRNA sponges. Accumulating evidence demonstrates that circRNA targeting to miRNA, as a classical signal transduction pathway, participates in the initiation and development of cancer, including breast cancer (21), gastric cancer (22), colorectal cancer (23), and hepatocellular carcinoma (20). Recently, a handful of studies have revealed that circRNAs are involved in the development and progression of OSCC (24, 25). However, the potential regulatory mechanisms of circRNAs in OSCC have not been completely clarified, and their clinical and prognostic significance has yet to be reported.
In our study, high-throughput sequencing analysis was carried out to elaborate the differentially expressed circRNAs in OSCC and normal tissues, and the results demonstrated that hsa_circ_0109291 was significantly higher in OSCC tissues than in adjacent normal tissues. In addition, we examined the correlation between hsa_circ_0109291 expression in OSCC tissues and clinical outcomes. Furthermore, the association of hsa_circ_0109291 and cell proliferation, migration, and apoptosis were performed in OSCC cell lines.
Materials and Methods
Patients and specimens
Fifty-one pairs of OSCC tissues and adjacent normal tissues were collected from the Affiliated Stomatological Hospital of Nanchang University (Nanchang, China) between January 2014 and June 2017. All of OSCC patients were recruited according to the histopathological evaluation without radiotherapy or chemotherapy before a surgical operation. All OSCC tissues and normal tissues were immediately stored in liquid nitrogen after surgical operation. Written informed consent was obtained from the patients. This study was approved by the Ethics Committee of the Affiliated Stomatological Hospital of Nanchang University (Nanchang, China).
Cell culture
Normal human oral keratinocyte (NHOK) and five OSCC cell lines (SCC-1, SCC-4, SCC-9, CAL27, and TU183) were purchased from the Cell Bank of Type Culture Collection of Chinese Academy of Sciences (Shanghai, China). Cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM; Invitrogen, Carlsbad, CA, USA) with 5% fetal bovine serum (Thermo Scientific HyClone, Beijing, China), 5% CO2, and 95% air atmosphere in a humidified incubator (Thermo, USA).
High-throughput sequencing
CircRNAs high-throughput sequencing analysis was performed as described previously (26). According to Memczak et. al. methods (27), the clean reads were aligned to the reference genome by Bowtie2 (http://bowtie-bio.sourceforge.net/bowtie2/manual.shtml). For unmapped reads, the junctions were picked out using the back-splice algorithm. CircRNAs levels were calculated using “Mapped back-splicing junction reads per million mapped reads” (RPM).
Cell transfection
The small interfering RNA (si-RNA) was synthesized by GenePharma Co., Ltd. (Shanghai, China) to inhibit the expression of hsa_circ_0109291 in SCC4 and CAL27 cells. The targeted sequence of the functional si-0109291-1, -2, or -3 were 5’-AATCCCCAGGAGACGTTGACA-3’, 5’-CCCCAGGAGACGTTGACATTT-3’, or 5’-ATGAATCCCC-
AGGAGACGTTG-3’, respectively. Three si-RNAs were transfected into SCC4 and CAL27 cells using Lipofectamine 2000 (Invitrogen) according to the manufacturer’s protocol. Finally, si-0109291-1 was selected and was used in proliferation, migration, apoptosis, and apoptosis-related protein assays. All experiments were repeated three times.
CCK-8 assay
The cell viability of SCC4 and CAL27 cells (1×104) was detected using a CCK-8 assay kit (Dojindo, Japan). The absorbance was measured at 450 nm using a SpectraMax M5 plate reader (Molecular Devices, USA). The CCK-8 proliferation assay was performed as previously described (4).
Wound healing assay
SCC4 and CAL27 cells (2×105) were trypsinized and reseeded in new 6-well plates. With 24 hr incubation, the confluent cells monolayers were scratched with a 10 μl sterile pipette tip. The wound healing assay was determined as previously described (28). The migration rate was monitored by the inverted microscope (Olympus, Japan).
Flow cytometry for apoptosis
The cell apoptosis assay was performed as previously described (25). Annexin V-FITC apoptosis detection kit was purchased from Invitrogen (Carlsbad, Calif, USA). The apoptosis rate was analyzed using A flowcytometer (BD Biosciences, Franklin Lakes, NJ, USA) and CELL Quest 3.0 software (BD Biosciences).
Total RNA extraction
Total RNA was extracted from the OSCC tissues, normal tissues, and cell lines using Trizol reagent (Invitrogen, Carlsbad, CA, USA) and preserved at -80 °C until use.
RT-qPCR
Moloney Murine Leukemia Virus Reverse transcriptase (Promega Corporation, Madison, WI, USA) was used to synthesize cDNA. Divergent primers were designed and used to measure circRNA expression using an ABI7300 System (Applied Biosystems, Foster City, CA, USA) with SYBR Select Master Mix (Applied Biosystems). Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) served as an internal control gene to normalize the expression of hsa_circ_0109291. The PCR primers were used as follows: hsa_circ_0109291, forward 5’-TGCTGTCTCTAAGCAAGACCC-3’ and reverse 5’-AGGGTTCAGGCATTCCCACT-3’; GAPDH, forward 5’-GCACCGTCAAGCTGAGAAC-3’, and reverse 5’-TGGTGAAGACGCCAGTGGA-3’. The 2-ΔΔCt method was used to calculate the expression of hsa_circ_0109291 (29).
Western blotting
Total protein in SCC4 and CAL27 cells was extracted using RIPA Lysis Buffer (Beyotime Institute of Biotechnology, Haimen, China). 30 μg of total protein were separated by 10% SDS-PAGE gel and transferred to nitrocellulose membranes (Bio-Rad Laboratories, Inc., Hercules, CA, USA). Primary antibodies Bcl-2 (cat.no: sc-56015, dilution, 1:1,000) and Bax (cat.no: sc6236, dilution, 1:1,000) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Cleaved-Caspase3 (cat.no: 9661, dilution, 1: 1,000) was purchased from Cell Signaling Technology, Inc. (Massachusetts, USA). Then, the membranes were incubated with secondary antibody (cat.no: sc-516102; dilution: 1:10,000; Santa Cruz Biotechnology) at room temperature for 2 hr. GAPDH (Cat. no: 2118; dilution: 1:2,000; Cell Signaling Technology, Inc.) served as an internal control gene to normalize the protein expression.
Statistical analysis
Data were analyzed using GraphPad Prism Version 7.0 (GraphPad Software, Inc., La Jolla, CA, USA) and presented as the mean ± standard deviation (SD). Student t-test was used to analyze two-group differences. Inter-group differences were analyzed by one-way analysis of variance, followed by Tukey’s post hoc analysis. Survival analysis was performed using the Kaplan-Meier method. P-values less than 0.05 was considered to indicate a statistically significant difference.
Results
Expression pattern of circRNAs in OSCC
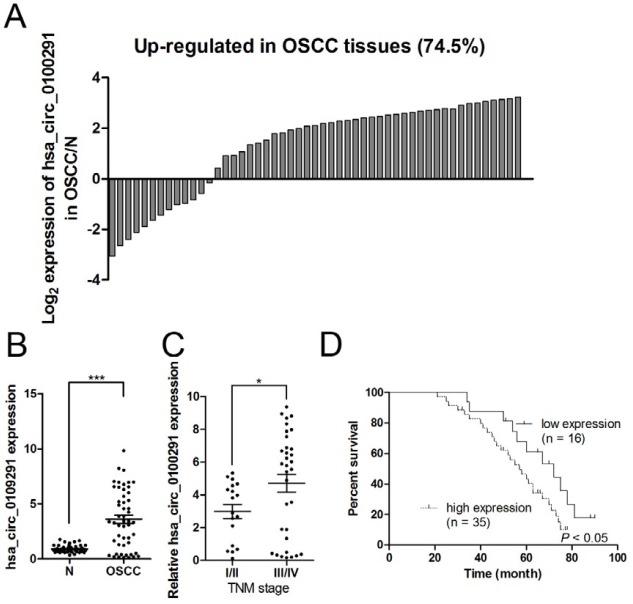
The expression profiles of circRNAs in 4 pairs of OSCC and corresponding adjacent non-cancerous tissues were detected using circRNAs high-throughput sequencing analysis. Based on FDR ≤ 0.001 and fold change ≥ 2 or fold change ≤ 0.5, the candidate circRNAs were filtered out, and the results demonstrated that 41 circRNAs were selected out, among which 17 circRNAs and 24 circRNAs were up-regulated and down-regulated, respectively (Figure 1). Among these up-regulated circRNAs, the fold change of hsa_circ_0109291 was up-regulated by nearly 5-fold and was the highest. To verify the sequencing results, RT-qPCR assay was performed in 51 pairs of OSCC and normal tissues. In accordance with the sequencing results, RT-qPCR assay demonstrated that hsa_circ_0109291 was up-regulated in 38 of 51 OSCC cases (74.5%) and was significantly increased (approximately 4-fold) in OSCC tissues compared with adjacent normal tissues (Figures 2A and 2B). Therefore, we focused on hsa_circ_0109291 in our study.
Figure 1.

CircRNAs expression profile in oral squamous cell carcinoma tumor tissues. OSCC-related circRNAs were selected out based on FDR ≤ 0.001 and fold change ≥ 2 or fold change ≤ 0.5. 41 circRNAs were differentially expressed between OSCC tissues and normal tissues. The heat map showed the 41 differentially expressed circRNAs, red indicating high expression and green indicating low expression
Figure 2.
Hsa_circ_0109291 expression was up-regulated in oral squamous cell carcinoma tumor tissues. Hsa_circ_0109291 expression was measured in 51 tumor tissues and matched adjacent non-tumorous tissues from OSCC patients, and the fold change was calculated (A and B). Hsa_circ_0109291 correlated to the advanced TNM stage (C). Kaplan-Meier survival curve was used to evaluate whether hsa_circ_0109291 expression levels were associated with overall survival in patients with OSCC (D). * P<0.05, *** P<0.001
To evaluate the clinical significance of hsa_circ_0109291 in OSCC patients, we found that hsa_circ_0109291 was positively correlated with the TNM stage in OSCC patients (Figure 2C). In addition, patients with low hsa_circ_0109291 expression (n = 16) had a better survival prognosis than those OSCC patients (n = 35) with high hsa_circ_0109291 levels in OSCC tissues (Figure 2D).
Inhibition of hsa_circ_0109291 suppresses OSCC cells growth in vitro
The expression levels of hsa_circ_0109291 in NHOK and OSCC cell lines (SCC1, SCC4, SCC9, CAL27, and TU183) were detected by RT-qPCR. As expected, hsa_circ_0109291 was dramatically up-regulated in all OSCC cell lines as compared with NHOK cells. Intriguingly, hsa_circ_0109291 expression was markedly higher in SCC4 and CAL27 cells than in SCC1, SCC9, or TU183 (Figure 3A). Hence, SCC4 and CAL27 cells were focused on in our further experiments. After transfection with control si-RNA or si-RNAs against hsa_circ_0109291 (si-0109291-1, si-0109291-2, and si-0109291-3), the RT-qPCR results indicated that the inhibition efficiency of si-0109291-1 was markedly higher than si-0109291-2 and si-0109291-3 in SCC4 and CAL27 cells (Figure 3B). Introduction of si-0109291-1 notably suppressed SCC4 (Figure 3C) and CAL27 (Figure 3D) cells proliferation.
Figure 3.
Inhibition of hsa_circ_0109291 suppresses oral squamous cell carcinoma cell growth in vitro. Relative expression of hsa_circ_0109291 in NHOK cells and OSCC cell lines was measured by RT-qPCR (A). The efficiency of si-RNA to inhibit hsa_circ_0109291 expression was verified by RT-qPCR (B). The cell viability of SCC4 (C) and CAL27 (D) was measured by CCK-8 assay after transfection with si-0109291-1 and si-NC for 0-72 hr. * P<0.05, ** P<0.01 and *** P< 0.001
Inhibition of hsa_circ_0109291 suppresses OSCC cells migration and induces apoptosis in vitro
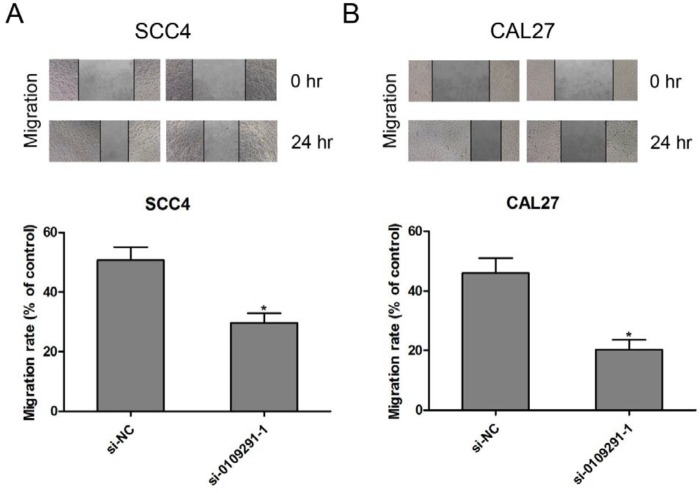
The effect of si-0109291-1 on OSCC cell migration was determined by wound healing assay. Compared with the si-NC group, the wound closing was significantly blunted in si-0109291-1 transfected SCC4 (Figure 4A) and CAL27 (Figure 4B) cells, suggesting that inhibition of hsa_circ_0109291 can block OSCC cell migration in vitro. Moreover, we performed flow cytometry analysis to further evaluate whether si-0109291-1 regulates OSCC cell proliferation by altering apoptosis. SCC4 and CAL27 cells were treated with si-NC or si-0109291-1 for 24 hr, the rate of apoptosis was significantly increased when SCC4 (Figure 5A) and CAL27 (Figure 5B) cells were transfected with si-0109291-1 compared with si-NC. Furthermore, we also found that hsa_circ_0109291 knockout significantly decreased the Bcl-2 protein expression and increased the protein expression of Bax and cleaved-caspase3 in SCC4 (Figure 6A) and CAL27 (Figure 6B) cells.
Figure 4.
Inhibition of hsa_circ_0109291 suppresses oral squamous cell carcinoma cell migration in vitro. After transfection with si-0109291-1 and si-NC, SCC4 (A) and CAL27 (B) cell migration was determined by wound healing assay for 24 hr. * P<0.05
Figure 5.
Inhibition of hsa_circ_0109291 induced oral squamous cell carcinoma cell apoptosis in vitro. After transfection with si-0109291-1 and si-NC, SCC4 (A) and CAL27 (B) cell apoptosis was detected by flowcytometry for 24 hr. * P<0.05
Figure 6.
Inhibition of hsa_circ_0109291 regulated apoptosis-related protein expression. After transfection with si-0109291-1 and si-NC, protein expression of Bcl-2, Bax, and Cleaved-caspase3 was measured by Western blotting in SCC4 (A) and CAL27 (B) cells for 24 hr. * P< 0.05
Discussion
The present study showed robust circRNA expression of hsa_circ_0109291 in OSCC, and silencing of hsa_circ_0109291 dramatically inhibited growth and migration and induced apoptosis in OSCC cells. These findings indicated that hsa_circ_0109291 might play an oncogenic role in the tumorigenesis of OSCC. Therefore, we deduced that hsa_circ_0109291 might be a potential therapeutic target for the clinical management of OSCC.
CircRNAs as a subset of non-coding RNAs are abundant in human cells and have recently emerged as a novel regulator of gene expression in a variety of cancers, including oral cancer (30). Based on high throughput microarray assay, circDOCK1 (has_circ_100721) is significantly up-regulated in OSCC tissues and suppresses OSCC cell apoptosis via regulating the miR196a5p/BIRC3 signaling pathway (31), suggesting that circDOCK1 may function as a competing endogenous RNA (ceRNA) in OSCC carcinogenesis. Recently, a circRNA microarray analysis indicates that hsa_circ_100290 is up-regulated in OSCC tissues and cell lines and induces CDK6 expression, while inactivation of circRNA_100290/CDK6 signaling can inhibit OSCC cell proliferation by releasing miR-29 expression (24). In our study, a new functional type circRNA, hsa_circ_0109291, was identified and found that overexpression of hsa_circ_0109291 was strongly linked to poor prognosis, hsa_circ_0109291 loss-of-function could significantly inhibit OSCC cell growth and induce apoptosis.
The further investigation found that the genomic length of hsa_circ_0109291 is 726 bp, and the spliced length is 226 bp, which is located in chr19:21280990-21281716, and its associated-gene symbol is zinc finger protein 714 (ZNF714; http://www.circbase.org/). ZNF proteins are a class of transcription factors that regulate multiple genes expression in transcriptional levels (32). Increasing evidence reveals that ZNF proteins have a double effect on cancer progression, including carcinogenesis and tumor suppressors (33). A recent study reveals that overexpressed ZNF703 contributes to cell proliferation and metastasis in OSCC (34). ZNF510 is significantly higher in OSCC tissues than in the OSCC free control tissues and has been discovered as a novel biomarker for detection of OSCC in the early stages (35). In contrast, ZNF750 as a tumor suppressor inhibits OSCC cell invasion and migration, suggesting that ZNF750 may inhibit cell metastasis during OSCC progression (36). Previous studies show a great interest in the underlying mechanism of ZNF proteins in the initiation and development of cancer, ZNF proteins mainly activate or suppress downstream genes via recruiting different interacting partners (33). Interestingly, cancer-related miRNAs, including miR-31, -199a-3p, and -525-3p can be regulated by ZNF proteins in a variety of tumors (33, 37), indicating that non-coding RNAs as post-translational regulatory mechanisms are involved in the development of cancers by regulating ZNF proteins expression. The present study indicated that circRNAs might be associated with ZNF proteins-related tumorigenesis, which provides a new theoretical insight to explore the underlying molecular mechanisms in OSCC.
Recent studies suggest that circRNAs can function as potential molecular markers of cancer to support diagnosis, which are more stable than other non-coding RNAs in vivo, due to their resistance to RNase activity (17, 30). In gastric cancer, hsa_circ_0000190 is found to be a potential biomarker and has better sensitivity and specificity than classic biomarkers, such as carcinoembryonic antigen (CEA) and CA19-9 (38). Overexpression of circRNA_100876 is positively related to lymph node metastasis and TNM stage in non-small cell lung cancer (NSCLC), indicating that it may be a potential prognostic marker for NSCLC (39). Hsa_circ_0001649, 0005075, and 0004018 are identified as valuable biomarkers for hepatocellular carcinoma diagnosis and prognosis (40-42). In our work, hsa_circ_0109291 was significantly up-regulated in OSCC and correlated with poor prognosis; further studies are required to confirm the diagnostic significance of hsa_circ_0109291 as a blood-based marker for OSCC.
Conclusion
Taken together, both circRNA high-throughput sequencing and RT-qPCR showed hsa_circ_0109291 was markedly increased in OSCC tissues. Hsa_circ_0109291 might exert regulatory functions in OSCC cell growth, migration, and apoptosis indicating that hsa_circ_0109291 might play a crucial role in OSCC tumorigenesis. These findings suggest that hsa_circ_0109291 can serve as a potential therapeutic target for the treatment of OSCC and may be a potential biomarker for OSCC diagnosis and prognosis.
Acknowledgment
This study was supported by the National Natural Science Foundation of China (grant no: 81660444), the Province Natural Science Foundation of Jiangxi Province (grant no: 20171BAB205050) and Jiangxi Key Research and Development Program (grant no: 20161BBG70149).
Conflicts of Interest
We have no conflicts of interest to declare.
References
- 1.Siegel R, Ward E, Brawley O, Jemal A. Cancer statistics, 2011: the impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA Cancer J Clin. 2011;61:212–236. doi: 10.3322/caac.20121. [DOI] [PubMed] [Google Scholar]
- 2.Yang CN, Deng YT, Tang JY, Cheng SJ, Chen ST, Li YJ, et al. MicroRNA-29b regulates migration in oral squamous cell carcinoma and its clinical significance. Oral Oncol. 2015;51:170–177. doi: 10.1016/j.oraloncology.2014.10.017. [DOI] [PubMed] [Google Scholar]
- 3.Kademani D. Oral cancer. Mayo Clin Proc. 2007;82:878–887. doi: 10.4065/82.7.878. [DOI] [PubMed] [Google Scholar]
- 4.Sun CC, Zhang L, Li G, Li SJ, Chen ZL, Fu YF, et al. The lncRNA PDIA3P interacts with miR-185-5p to modulate oral squamous cell carcinoma progression by targeting cyclin D2. Mol Ther Nucleic Acids. 2017;9:100–110. doi: 10.1016/j.omtn.2017.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Massano J, Regateiro FS, Januario G, Ferreira A. Oral squamous cell carcinoma: review of prognostic and predictive factors. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;102:67–76. doi: 10.1016/j.tripleo.2005.07.038. [DOI] [PubMed] [Google Scholar]
- 6.Lai YH, Liu H, Chiang WF, Chen TW, Chu LJ, Yu JS, et al. MiR-31-5p-ACOX1 axis enhances tumorigenic fitness in oral squamous cell carcinoma via the promigratory prostaglandin E2. Theranostics. 2018;8:486–504. doi: 10.7150/thno.22059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hashiguchi Y, Kawano S, Goto Y, Yasuda K, Kaneko N, Sakamoto T, et al. Tumor-suppressive roles of DeltaNp63beta-miR-205 axis in epithelial-mesenchymal transition of oral squamous cell carcinoma via targeting ZEB1 and ZEB2. J Cell Physiol. 2017 doi: 10.1002/jcp.26267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ding L, Ren J, Zhang D, Li Y, Huang X, Hu Q, et al. A novel stromal lncRNA signature reprograms fibroblasts to promote the growth of oral squamous cell carcinoma via LncRNA-CAF/interleukin-33. Carcinogenesis. 2018 doi: 10.1093/carcin/bgy006. [DOI] [PubMed] [Google Scholar]
- 9.Liang S, Zhang S, Wang P, Yang C, Shang C, Yang J, et al. LncRNA, TUG1 regulates the oral squamous cell carcinoma progression possibly via interacting with Wnt/beta-catenin signaling. Gene. 2017;608:49–57. doi: 10.1016/j.gene.2017.01.024. [DOI] [PubMed] [Google Scholar]
- 10.Chen LL, Yang L. Regulation of circRNA biogenesis. RNA Biol. 2015;12:381–388. doi: 10.1080/15476286.2015.1020271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hsu MT, Coca-Prados M. Electron microscopic evidence for the circular form of RNA in the cytoplasm of eukaryotic cells. Nature. 1979;280:339–340. doi: 10.1038/280339a0. [DOI] [PubMed] [Google Scholar]
- 12.Zhou B, Yu JW. A novel identified circular RNA, circRNA_010567, promotes myocardial fibrosis via suppressing miR-141 by targeting TGF-beta1. Biochem Biophys Res Commun. 2017;487:769–775. doi: 10.1016/j.bbrc.2017.04.044. [DOI] [PubMed] [Google Scholar]
- 13.Zhao Y, Alexandrov PN, Jaber V, Lukiw WJ. Deficiency in the ubiquitin conjugating enzyme UBE2A in Alzheimer’s disease (AD) is linked to deficits in a natural circular miRNA-7 sponge (circRNA; ciRS-7) Genes (Basel) 2016:7. doi: 10.3390/genes7120116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Qian Y, Lu Y, Rui C, Qian Y, Cai M, Jia R. Potential significance of circular RNA in human placental tissue for patients with preeclampsia. Cell Physiol Biochem. 2016;39:1380–1390. doi: 10.1159/000447842. [DOI] [PubMed] [Google Scholar]
- 15.Liu Q, Zhang X, Hu X, Dai L, Fu X, Zhang J, et al. Circular RNA related to the chondrocyte ECM regulates MMP13 expression by functioning as a MiR-136 ‘Sponge’ in human cartilage degradation. Sci Rep. 2016;6:22572. doi: 10.1038/srep22572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hansen TB, Jensen TI, Clausen BH, Bramsen JB, Finsen B, Damgaard CK, et al. Natural RNA circles function as efficient microRNA sponges. Nature. 2013;495:384–388. doi: 10.1038/nature11993. [DOI] [PubMed] [Google Scholar]
- 17.Meng S, Zhou H, Feng Z, Xu Z, Tang Y, Li P, et al. CircRNA: functions and properties of a novel potential biomarker for cancer. Mol Cancer. 2017;16 doi: 10.1186/s12943-017-0663-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zheng Q, Bao C, Guo W, Li S, Chen J, Chen B, et al. Circular RNA profiling reveals an abundant circHIPK3 that regulates cell growth by sponging multiple miRNAs. Nat Commun. 2016;7:11215. doi: 10.1038/ncomms11215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li F, Zhang L, Li W, Deng J, Zheng J, An M, et al. Circular RNA ITCH has inhibitory effect on ESCC by suppressing the Wnt/beta-catenin pathway. Oncotarget. 2015;6:6001–6013. doi: 10.18632/oncotarget.3469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Han D, Li J, Wang H, Su X, Hou J, Gu Y, et al. Circular RNA circMTO1 acts as the sponge of microRNA-9 to suppress hepatocellular carcinoma progression. Hepatology. 2017;66:1151–1164. doi: 10.1002/hep.29270. [DOI] [PubMed] [Google Scholar]
- 21.Liang HF, Zhang XZ, Liu BG, Jia GT, Li WL. Circular RNA circ-ABCB10 promotes breast cancer proliferation and progression through sponging miR-1271. Am J Cancer Res. 2017;7:1566–1576. [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang J, Liu H, Hou L, Wang G, Zhang R, Huang Y, et al. Circular RNA_LARP4 inhibits cell proliferation and invasion of gastric cancer by sponging miR-424-5p and regulating LATS1 expression. Mol Cancer. 2017;16:151. doi: 10.1186/s12943-017-0719-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang XL, Xu LL, Wang F. Hsa_circ_0020397 regulates colorectal cancer cell viability, apoptosis and invasion by promoting the expression of the miR-138 targets TERT and PD-L1. Cell Biol Int. 2017;41:1056–1064. doi: 10.1002/cbin.10826. [DOI] [PubMed] [Google Scholar]
- 24.Chen L, Zhang S, Wu J, Cui J, Zhong L, Zeng L, et al. circRNA_100290 plays a role in oral cancer by functioning as a sponge of the miR-29 family. Oncogene. 2017;36:4551–4561. doi: 10.1038/onc.2017.89. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 25.Wang L, Wei Y, Yan Y, Wang H, Yang J, Zheng Z, et al. CircDOCK1 suppresses cell apoptosis via inhibition of miR196a5p by targeting BIRC3 in OSCC. Oncol Rep. 2017 doi: 10.3892/or.2017.6174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li L, Guo J, Chen Y, Chang C, Xu C. Comprehensive CircRNA expression profile and selection of key CircRNAs during priming phase of rat liver regeneration. BMC Genomics. 2017;18 doi: 10.1186/s12864-016-3476-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Memczak S, Jens M, Elefsinioti A, Torti F, Krueger J, Rybak A, et al. Circular RNAs are a large class of animal RNAs with regulatory potency. Nature. 2013;495:333–338. doi: 10.1038/nature11928. [DOI] [PubMed] [Google Scholar]
- 28.Fang S, Guo H, Cheng Y, Zhou Z, Zhang W, Han B, et al. circHECTD1 promotes the silica-induced pulmonary endothelial-mesenchymal transition via HECTD1. Cell Death Dis. 2018;9:396. doi: 10.1038/s41419-018-0432-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 30.Momen-Heravi F, Bala S. Emerging role of non-coding RNA in oral cancer. Cell Signal. 2018;42:134–143. doi: 10.1016/j.cellsig.2017.10.009. [DOI] [PubMed] [Google Scholar]
- 31.Wang L, Wei Y, Yan Y, Wang H, Yang J, Zheng Z, et al. CircDOCK1 suppresses cell apoptosis via inhibition of miR196a5p by targeting BIRC3 in OSCC. Oncol Rep. 2018;39:951–966. doi: 10.3892/or.2017.6174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kwon YW, Ahn HS, Park JY, Yang HM, Cho HJ, Kim HS. Imprinted gene Zinc finger protein 127 is a novel regulator of master pluripotency transcription factor, Oct4. BMB Rep. 2018;51:242–248. doi: 10.5483/BMBRep.2018.51.5.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jen J, Wang YC. Zinc finger proteins in cancer progression. J Biomed Sci. 2016;23 doi: 10.1186/s12929-016-0269-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang H, Deng X, Zhang J, Ou Z, Mai J, Ding S, et al. Elevated expression of zinc finger protein 703 promotes cell proliferation and metastasis through PI3K/AKT/GSK-3beta signalling in oral squamous cell carcinoma. Cell Physiol Biochem. 2017;44:920–934. doi: 10.1159/000485360. [DOI] [PubMed] [Google Scholar]
- 35.Jou YJ, Lin CD, Lai CH, Tang CH, Huang SH, Tsai MH, et al. Salivary zinc finger protein 510 peptide as a novel biomarker for detection of oral squamous cell carcinoma in early stages. Clin Chim Acta. 2011;412:1357–1365. doi: 10.1016/j.cca.2011.04.004. [DOI] [PubMed] [Google Scholar]
- 36.Yang H, Pan L, Xu C, Zhang Y, Li K, Chen S, et al. Overexpression of tumor suppressor gene ZNF750 inhibits oral squamous cell carcinoma metastasis. Oncol Lett. 2017;14:5591–5596. doi: 10.3892/ol.2017.6908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pang F, Zha R, Zhao Y, Wang Q, Chen D, Zhang Z, et al. MiR-525-3p enhances the migration and invasion of liver cancer cells by downregulating ZNF395. PLoS One. 2014;9:e90867. doi: 10.1371/journal.pone.0090867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen S, Li T, Zhao Q, Xiao B, Guo J. Using circular RNA hsa_circ_0000190 as a new biomarker in the diagnosis of gastric cancer. Clin Chim Acta. 2017;466:167–171. doi: 10.1016/j.cca.2017.01.025. [DOI] [PubMed] [Google Scholar]
- 39.Yao JT, Zhao SH, Liu QP, Lv MQ, Zhou DX, Liao ZJ, et al. Over-expression of CircRNA_100876 in non-small cell lung cancer and its prognostic value. Pathol Res Pract. 2017;213:453–456. doi: 10.1016/j.prp.2017.02.011. [DOI] [PubMed] [Google Scholar]
- 40.Qin M, Liu G, Huo X, Tao X, Sun X, Ge Z, et al. Hsa_circ_0001649: A circular RNA and potential novel biomarker for hepatocellular carcinoma. Cancer Biomark. 2016;16:161–169. doi: 10.3233/CBM-150552. [DOI] [PubMed] [Google Scholar]
- 41.Shang X, Li G, Liu H, Li T, Liu J, Zhao Q, et al. Comprehensive circular RNA profiling reveals that hsa_circ_0005075, a new circular RNA biomarker, is involved in hepatocellular crcinoma development. Medicine (Baltimore) 2016;95:e3811. doi: 10.1097/MD.0000000000003811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fu L, Yao T, Chen Q, Mo X, Hu Y, Guo J. Screening differential circular RNA expression profiles reveals hsa_circ_0004018 is associated with hepatocellular carcinoma. Oncotarget. 2017;8:58405–58416. doi: 10.18632/oncotarget.16881. [DOI] [PMC free article] [PubMed] [Google Scholar]